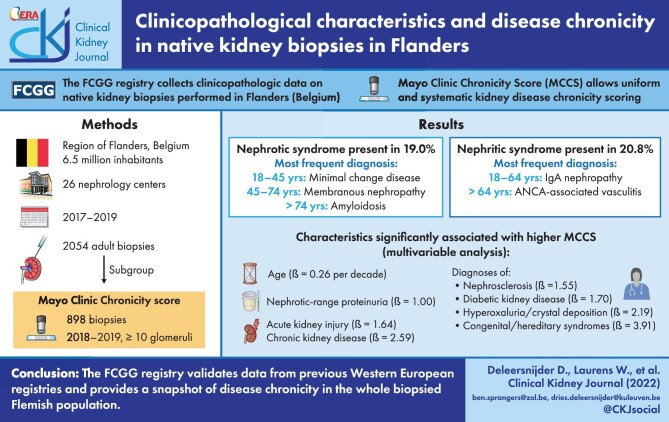
ABSTRACT
Background
The Flemish Collaborative Glomerulonephritis Group (FCGG) registry provides complete population data on kidney disease epidemiology in the region of Flanders (Belgium), as it captures all native kidney biopsies performed in its population of 6.5 million inhabitants.
Methods
From 2017 until 2019, 2054 adult kidney biopsies were included from 26 nephrology centers (one biopsy per patient). Data on nephrotic and nephritic syndrome were available in 1992 and 2026 biopsies, respectively. In a subgroup of 898 biopsies containing ≥10 glomeruli from 2018 to 2019, disease chronicity was graded using the Mayo Clinic Chronicity Score (MCCS). The association between clinical variables and MCCS was determined using simple and multiple linear regression models.
Results
Nephrotic syndrome (present in 378 patients, 19.0%) was most frequently caused by minimal change disease in younger patients (18–44 years), membranous nephropathy in older patients (45–74 years) and amyloidosis in the elderly (>75 years). Nephritic syndrome (present in 421 patients, 20.8%) was most frequently caused by immunoglobulin A nephropathy (IgAN) in younger patients (18–64 years) and ANCA-associated vasculitis (AAV) in older patients (>64 years). AAV and IgAN were the most frequent underlying diagnoses in biopsies in which crescents were identified. In multivariable analysis, acute and chronic kidney disease and diagnoses of diabetic kidney disease, nephrosclerosis and hyperoxaluria/hypercalcemic nephropathy were associated with the highest MCCS increases.
Conclusions
The FCGG registry validates data from previous Western European registries and provides a snapshot of disease chronicity in the whole biopsied Flemish population.
Keywords: chronicity, epidemiology, kidney biopsy, MCCS, registry
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Kidney biopsy registries enable both epidemiological research and patient selection for clinical trials [1]. The Flemish Collaborative Glomerulonephritis Group (FCGG) kidney biopsy registry was initiated in 2017 to characterize native kidney disease in Flanders, the Dutch-speaking Northern part of Belgium counting approximately 6.5 million inhabitants [2]. The registry involves all Flemish nephrology centers and covers all biopsies performed in Flanders, thus providing population data on kidney disease epidemiology in Western Europe.
Kidney histology is essential in diagnosis, but also provides valuable information on disease activity and chronicity, which is relevant for clinical decision making [3, 4]. Patterns of disease activity and chronicity have previously been implemented in disease-specific scores [5–7]. In 2017, Sethi et al. proposed the Mayo Clinic Chronicity Score (MCCS) as a uniform scoring system to grade chronic changes in native kidney biopsies, regardless of the underlying disease [4]. The FCGG registry is the first European multi-center registry to systematically collect the MCCS in nearly all included kidney biopsies through a standardized pathology report.
In this study, we report on clinicopathological characteristics of adult kidney biopsies in Flanders from 2017 until 2019. Next, we outline the MCCS of included biopsies, thereby providing information on the prevalence of kidney disease chronicity in the biopsied Flemish population.
MATERIALS AND METHODS
Ethics
This study complied with the principles of the Declaration of Helsinki, Good Clinical Practice Guidelines and all applicable regulatory requirements. The study was approved by the Ethical Committee of the University Hospitals Leuven (study reference S59182) and local committees of all participating centers. All study participants or their legal representative provided written informed consent.
Flemish population and participating centers
On 1 January 2017, Flanders counted 6 516 011 official inhabitants, of which 5 251 635 were adults [≥18 years, data from Statbel (https://statbel.fgov.be/en)]. Biopsies were analyzed by 17 (nephro)pathologists from 11 pathology departments (10 in Flanders, 1 in Brussels); two nephropathologists examined approximately 60% of all biopsies. All 24 nephrology centers in Flanders and two centers in Brussels participated in the registry.
Patient eligibility and biopsy inclusion
The FCGG registry includes all (non-tumor) native kidney biopsies from the participating centers. From 1 January 2017 until 31 December 2019, a total of 2419 adult and pediatric biopsies were available. For this study, additional exclusion criteria were defined (Fig. 1) and only one biopsy per patient was included. For analysis of MCCS, only biopsies from 2018 to 2019 containing ≥10 glomeruli were retained, as recommended [4]. As a result, 2054 adult kidney biopsies were included for epidemiological analysis and a subgroup of 898 biopsies for analysis of MCCS.
Figure 1:
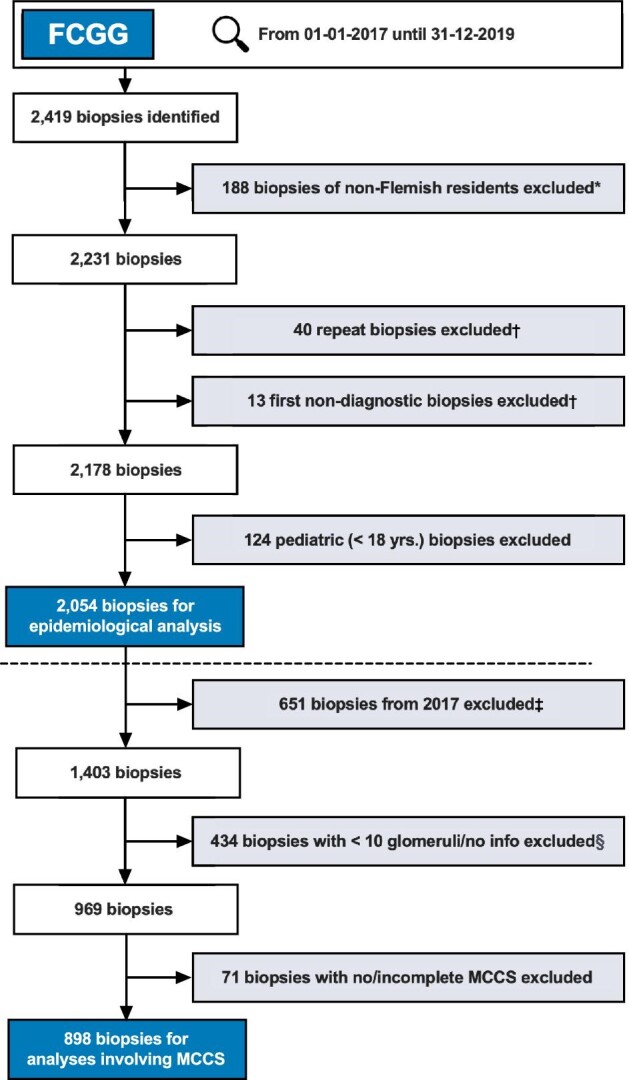
Flowchart of kidney biopsy selection for final analysis. *Based on ZIP-codes; †repeat biopsies were excluded, unless the first biopsy did not yield a diagnosis and a repeat biopsy was performed within the following 4 months, in which case the repeat biopsy was retained and the first biopsy excluded; ‡for analysis of the MCCS, biopsies from 2017 were excluded, as this score was only introduced by Sethi et al. in 2017 [4]; §37 biopsies with no info on glomeruli, 397 biopsies with 0 to 9 glomeruli.
Clinical data
Patient demographics and the following clinical parameters were provided by the attending nephrologist at time of biopsy: kidney injury, hematuria, proteinuria, presence of nephrotic syndrome (NS) (as reported by the clinician) and serum albumin (Table 1). Based on these data, the following clinical syndromes were retrospectively defined: NS, nephritic syndrome, isolated nephrotic-range proteinuria and isolated hematuria (Table 2).
Table 1:
Clinical parameters included in the FCGG registry.
| Clinical parameter | Options |
|---|---|
| Kidney injury | AKI |
| CKD | |
| No kidney injury | |
| Hematuria | Yes |
| No | |
| Proteinuria, UPCR (g/g) or 24 h collection (g/24 h) | <1.0 |
| ≥1.0 and <3.5 | |
| ≥3.5 | |
| NS (reported in data form) | Yes |
| No | |
| Serum albumin (g/L) | Continuous numerical variable |
Table 2:
Definitions of clinical nephrological syndromes.
| Nephrotic syndrome | |
| Proteinuria, UPCR (g/g) or 24 h collection (g/24 h) | ≥3.5 |
| Serum albumin (g/L) | <30.0 |
| Nephritic syndrome | |
| Hematuria | Yes |
| Proteinuria, UPCR (g/g) or 24 h collection (g/24 h) | ≥1.0 |
| Kidney injury | AKI or CKD |
| NS (reported in data form) | No |
| Isolated nephrotic-range proteinuria | |
| Hematuria | No |
| Proteinuria, UPCR (g/g) or 24 h collection (g/24 h) | ≥3.5 |
| Serum albumin (g/L) | ≥30.0 |
| NS (reported in data form) | No |
| Isolated hematuria | |
| Hematuria | Yes |
| Proteinuria, UPCR (g/g) or 24 h collection (g/24 h) | <1.0 |
| Kidney injury | No |
| NS (reported in data form) | No |
Histopathological data
Data on the following histopathological parameters were provided by the (nephro)pathologist. (i) Primary histopathological diagnosis, coded according to a proprietary coding system created for this registry (https://www.nbvn.be/blog/organisatie/fcgg-in-english). (ii) Histopathological pattern of glomerular injury (e.g. presence of crescents). (iii) The MCCS, which is a standardized pathology scoring system to uniformly score chronic changes in kidney biopsies [4]. It is derived from the sum of the degree of glomerulosclerosis (score 0–3), interstitial fibrosis (score 0–3), tubular atrophy (score 0–3) and arteriosclerosis (score 0–1), and is categorically classified into minimal (MCCS 0–1), mild (MCCS 2–4), moderate (MCCS 5–7) and severe (MCCS 8–10) chronic changes.
The number of glomeruli present in the biopsy sample for light microscopy was reported in 1743 adult biopsies (84.9% of total). Across all centers, the median number of glomeruli per biopsy was 14 [interquartile range (IQR) 8–21]. In 70.3% of biopsies, at least 10 glomeruli were present, which is considered adequate to reliably diagnose most glomerular diseases and MCCS [4, 8, 9].
Final nephrological diagnosis
The final nephrological diagnosis was provided by the attending nephrologist after the biopsy result had become available. This diagnosis resulted from the integration of clinical features, biochemical parameters, histopathology and results from genetics (if available). Nephrological diagnoses were coded according to the ERA coding system for Primary Renal Disease [10]. These coded diagnoses were pooled and categorized on two levels with decreasing detail (Supplementary data, Table S1). At the first level (ERA level 1), different classes or etiologies of the same kidney disease were pooled together. At the second level (ERA level 2), six categories were defined according to the affected kidney tissue compartment.
Data completeness
Every biopsy included in the analysis had received a final nephrological diagnosis. Complete data on all clinical parameters were present in 1985 of the 2054 adult biopsies (96.6%). Biopsies with missing clinical data were excluded from analysis of clinical characteristics (overview of data completeness in Supplementary data, Table S2).
Statistical analysis
Numerical variables were described using median and IQR, and categorical variables by proportions. Incidence rates were calculated using the sum of case biopsies in 2017, 2018 and 2019 in the numerator and the person-year follow-up from 2017 until 2019 in the denominator, and reported as biopsies per million persons per year (p.m.p./year). The association of sex, age, kidney injury, proteinuria and final nephrological diagnosis with MCCS was determined in univariate and multivariable analysis by using simple and multiple linear regression models, respectively. Potential predictors were chosen based on clinical plausibility. A linear mixed model with random effect for (nephro)pathologist was used to evaluate the heterogeneity of MCCS scoring between all (nephro)pathologists (model outlined in Supplementary data, Statistical Methods). The 95% confidence intervals (95% CIs) were calculated for the regression coefficients and P-values <.05 were considered significant. GraphPad Prism version 9.1.1 for Mac OS (GraphPad Software, www.graphpad.com) and R software version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/) were used for data analysis.
RESULTS
Characteristics of patients with NS and nephrotic-range proteinuria
Demographic, clinical and histopathological characteristics of most frequently diagnosed kidney diseases in Flemish adults are shown in Table 3. Nephrotic-range proteinuria was most often present in patients with membranous nephropathy (MN) (83.2%), minimal change disease (MCD) (77.7%), amyloidosis (72.1%) and focal segmental glomerulosclerosis (FSGS) (53.9%). Median serum albumin was <30 g/L in patients with MCD [23.6 g/L (IQR 19.0–34.4)], amyloidosis [25.0 g/L (IQR 20.3–30.0)] and MN [28.0 g/L (IQR 23.0–32.0)], and these patients also most often presented with NS (60.6%, 64.7% and 57.3% respectively).
Table 3:
Demographic, clinical and histopathological characteristics of the most frequently diagnosed kidney diseases.
| IgAN | TIN | FSGS | DKD | AAV | NScl | MN | MCD | LN | AMY | TMA | Alport/TMD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | 324 (15.8) | 220 (10.7) | 192 (9.3) | 154 (7.5) | 148 (7.2) | 127 (6.2) | 113 (5.5) | 96 (4.7) | 87 (4.2) | 69 (3.4) | 42 (2.0) | 25 (1.2) |
| Age (years), median (IQR) | 51.3 (33.4–63.5) | 62.9 (46.7–73.1) | 56.6 (44.0–70.1) | 63.7 (55.2–73.4) | 67.8 (59.3–75.3) | 69.4 (58.2–75.5) | 62.4 (54.3–69.8) | 48.7 (36.3–65.0) | 41.5 (28.3–56.2) | 71.1 (62.5–78.4) | 51.3 (40.4–64.7) | 41.0 (32.6–52.2) |
| Gender (% male) | 71.3 | 54.5 | 64.6 | 68.2 | 65.5 | 70.9 | 71.7 | 53.1 | 35.6 | 49.3 | 52.4 | 36.0 |
| Nephrotic-range proteinuria (%) | 19.2 | 12.4 | 53.9 | 49.7 | 14.3 | 18.3 | 83.2 | 77.7 | 31.0 | 72.1 | 29.3 | 8.0 |
| Serum albumin (g/L), median (IQR) | 38.0 (32.3–42.0) | 36.2 (31.0–41.6) | 35.0 (27.0–41.0) | 36.1 (29.0–40.9) | 34.0 (29.0–37.0) | 39.0 (34.3–42.2) | 28.0 (23.0–32.0) | 23.6 (19.0–34.4) | 31.2 (25.9–37.8) | 25.0 (20.3–30.0) | 33.0 (29.0–38.0) | 40.3 (37.2–44.0) |
| Hematuria present (%) | 84.6 | 38.8 | 44.5 | 33.1 | 96.6 | 32.3 | 54.9 | 37.9 | 75.9 | 36.8 | 54.8 | 84.0 |
| AKI (%) | 36.0 | 84.5 | 30.4 | 37.3 | 77.7 | 42.5 | 24.8 | 29.2 | 34.5 | 36.8 | 63.4 | 12.0 |
| CKD (%) | 36.0 | 12.3 | 46.6 | 53.6 | 11.5 | 55.1 | 23.9 | 8.3 | 13.8 | 33.8 | 31.7 | 12.0 |
| No kidney injury (%) | 28.0 | 3.2 | 23.0 | 9.2 | 10.8 | 2.4 | 51.3 | 62.5 | 51.7 | 29.4 | 4.9 | 76.0 |
| NS (%)a | 7.6 | 5.3 | 29.0 | 20.9 | 5.5 | 3.3 | 57.3 | 60.6 | 23.3 | 64.7 | 7.9 | 4.0 |
| Isolated nephrotic-range proteinuria (%)a | 2.2 | 3.4 | 14.0 | 16.3 | 0.0 | 7.5 | 7.3 | 3.3 | 0.0 | 1.5 | 2.6 | 0.0 |
| Nephritic syndrome (%)a | 32.1 | 13.4 | 17.9 | 13.8 | 50.3 | 11.9 | 8.8 | 3.3 | 17.2 | 8.8 | 32.5 | 8.0 |
| Isolated hematuria (%)a | 8.7 | 0.0 | 1.1 | 0.7 | 6.8 | 0.0 | 0.9 | 3.3 | 13.8 | 0.0 | 0.0 | 32.0 |
| Crescentic injury pattern (%) | 22.5 | 0.0 | 2.6 | 0.6 | 77.0 | 2.4 | 0.9 | 1.0 | 27.6 | 1.4 | 7.1 | 0.0 |
| MCCS, median (IQR) | 3.0 (0.0–6.0) | 2.0 (0.0–4.0) | 5.0 (2.0–7.0) | 6.0 (5.0–8.0) | 3.0 (1.0–5.0) | 6.0 (4.0–8.5) | 2.0 (0.0–4.0) | 0.0 (0.0–2.0) | 1.0 (0.0–2.8) | 3.5 (1.3–5.8) | 4.5 (2.5–6.3) | 2.0 (0.0–4.0) |
Final nephrological diagnoses are shown at ERA classification level 1.
According to definitions in Table 2.
AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; AMY: amyloidosis; Alport/TMD: Alport syndrome and thin basement membrane disease; NScl: nephrosclerosis; TMA: thrombotic microangiopathy.
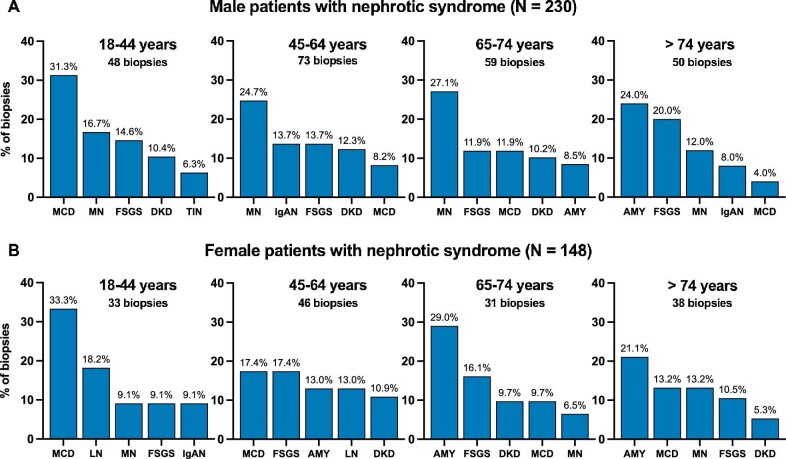
NS was present in 378 out of 1992 adult patients with available data (19.0%). The incidence rate of adult nephrotic patients undergoing kidney biopsy was 23.8 p.m.p./year. Overall, the top five most frequently diagnosed kidney diseases in nephrotic adults included MN (16.7%), followed by MCD (15.1%), FSGS (14.3%), amyloidosis (11.6%) and diabetic kidney disease (DKD) (8.5%) (Table 4). MCD was the most important etiology of NS in the age category of 18–44 years, while MN was most frequent in the category of 45–74 years and amyloidosis in the elderly (>74 years, Table 4). MN and immunoglobulin A nephropathy (IgAN) were more prevalent in adult nephrotic males, while MCD, amyloidosis and lupus nephritis (LN) were more frequent in nephrotic females (Fig. 2, Supplementary data, Tables S3 and S4). The median age of male and female nephrotic patients was similar (62.9 versus 62.4 years, respectively). Notably, amyloidosis appeared to occur more often in younger nephrotic females versus nephrotic males (Fig. 2).
Table 4:
Final nephrological diagnoses in patients with NS according to age category.
| Diagnosis | Overall (N = 378) | 18–44 years (N = 81) | 45–64 years (N = 119) | 65–74 years (N = 90) | >74 years (N = 88) |
|---|---|---|---|---|---|
| MN | 63 (16.7) | 11 (13.6) | 23 (19.3) | 18 (20.0) | 11 (12.5) |
| MCD | 57 (15.1) | 26 (32.1) | 14 (11.8) | 10 (11.1) | 7 (8.0) |
| FSGS | 54 (14.3) | 10 (12.4) | 18 (15.1) | 12 (13.3) | 14 (15.9) |
| AMY | 44 (11.6) | 1 (1.2) | 9 (7.6) | 14 (15.6) | 20 (22.7) |
| DKD | 32 (8.5) | 7 (8.6) | 14 (11.8) | 9 (10.0) | 2 (2.3) |
| IgAN | 24 (6.3) | 5 (6.2) | 10 (8.4) | 4 (4.4) | 5 (5.7) |
| LN | 20 (5.3) | 8 (9.9) | 9 (7.6) | 2 (2.2) | 1 (1.1) |
| GP, NOS | 17 (4.5) | 3 (3.7) | 6 (5.0) | 5 (5.6) | 3 (3.4) |
| TIN | 11 (2.9) | 3 (3.7) | 2 (1.7) | 4 (4.4) | 2 (2.3) |
| AAV | 8 (2.1) | 1 (1.2) | 2 (1.7) | 3 (3.3) | 2 (2.3) |
| Others | 48 (12.7) | 6 (7.4) | 12 (10.1) | 9 (10.0) | 21 (23.9) |
| Total | 378 (100.0) | 81 (100.0) | 119 (100.0) | 90 (100.0) | 88 (100.0) |
Data are presented as n (%).
Final nephrological diagnoses are shown at ERA classification level 1.
AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; AMY: amyloidosis; GP, NOS: glomerulopathy, not otherwise specified.
Figure 2:
Most frequent diagnoses in patients with NS according to age and sex category. The top five most frequent nephrological diagnoses (ERA level 1) are shown per age category in adult nephrotic males [(A), N = 230] and adult nephrotic females [(B), N = 148]. Disease proportions (%) were calculated in every age category separately. AMY: amyloidosis.
Isolated nephrotic-range proteinuria was present in 97 out of 1989 adult patients with available data (4.9%; Supplementary data, Table S5). In this group, FSGS and DKD were most prevalent (26.8% and 25.8%, respectively), while less frequent diagnoses included nephrosclerosis, MN and IgAN.
Characteristics of patients with nephritic syndrome and isolated hematuria
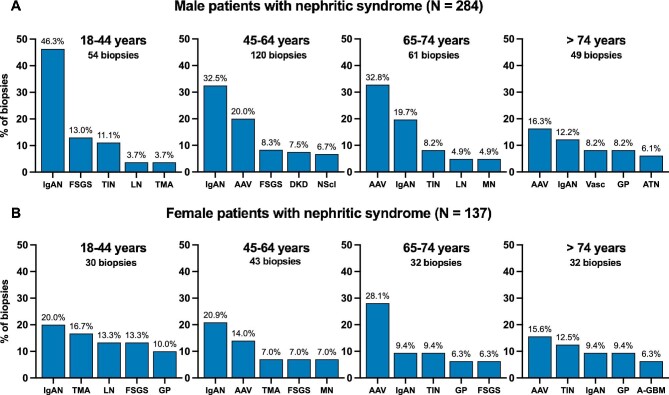
Nephritic syndrome was present in 421 out of 2026 adult patients with available data (20.8%). The incidence rate of adult nephritic patients undergoing kidney biopsy was 26.5 p.m.p./year. Overall, IgAN was the most frequent nephrological diagnosis (24.5% of total nephritic adults), especially in younger patients (18–64 years), while ANCA-associated vasculitis (AAV) was the most prevalent cause in patients aged >64 years (Table 5). The most frequent underlying etiologies in male and female nephritic patients were similar, although IgAN and AAV together covered approximately half of all male adult nephritic biopsies (47.6%), while this was only about one-third in female adult nephritic patients (30.6%), in which diagnoses were more various (Fig. 3, Supplementary data, Tables S6 and S7).
Table 5:
Final nephrological diagnoses in patients with nephritic syndrome according to age category.
| Diagnosis | Overall (N = 421) | 18–44 years (N = 84) | 45–64 years (N = 163) | 65–74 years (N = 93) | >74 years (N = 81) |
|---|---|---|---|---|---|
| IgAN | 103 (24.5) | 31 (36.9) | 48 (29.4) | 15 (16.1) | 9 (11.1) |
| AAV | 74 (17.6) | 2 (2.4) | 30 (18.4) | 29 (31.2) | 13 (16.1) |
| FSGS | 34 (8.1) | 11 (13.1) | 13 (8.0) | 5 (5.4) | 5 (6.2) |
| TIN | 29 (6.9) | 8 (9.5) | 8 (4.9) | 8 (8.6) | 5 (6.2) |
| AKI/CKD, NOS | 26 (6.2) | 4 (4.8) | 7 (4.3) | 5 (5.4) | 10 (12.4) |
| DKD | 21 (5.0) | 2 (2.4) | 12 (7.4) | 4 (4.3) | 3 (3.7) |
| GP, NOS | 16 (3.8) | 4 (4.8) | 3 (1.8) | 2 (2.2) | 7 (8.6) |
| LN | 15 (3.6) | 6 (7.1) | 3 (1.8) | 4 (4.3) | 2 (2.5) |
| NScl | 15 (3.6) | 0 (0.0) | 9 (5.5) | 3 (3.2) | 3 (3.7) |
| TMA | 13 (3.1) | 7 (8.3) | 5 (3.1) | 1 (1.1) | 0 (0.0) |
| MN | 10 (2.4) | 1 (1.2) | 4 (2.5) | 4 (4.3) | 1 (1.2) |
| Vasc | 9 (2.1) | 1 (1.2) | 1 (0.6) | 2 (2.2) | 5 (6.2) |
| Anti-GBM | 7 (1.7) | 3 (3.6) | 2 (1.2) | 0 (0.0) | 2 (2.5) |
| AMY | 6 (1.4) | 0 (0.0) | 1 (0.6) | 3 (3.2) | 2 (2.5) |
| ATN | 6 (1.4) | 0 (0.0) | 1 (0.6) | 1 (1.1) | 4 (4.9) |
| IgAV | 6 (1.4) | 2 (2.4) | 3 (1.8) | 1 (1.1) | 0 (0.0) |
| Others | 31 (7.4) | 2 (2.4) | 13 (8.0) | 6 (6.5) | 10 (12.3) |
| Total | 421 (100.0) | 84 (100.0) | 163 (100.0) | 93 (100.0) | 81 (100.0) |
Data are presented as n (%).
Final nephrological diagnoses are shown at ERA classification level 1.
AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; AKI/CKD, NOS: non-specific diagnoses of AKI or CKD; AMY: amyloidosis; anti-GBM: anti-glomerular basement membrane nephritis; ATN: acute tubular necrosis; GP, NOS: glomerulopathy, not otherwise specified; NScl: nephrosclerosis; Vasc: ANCA-negative systemic vasculitis excluding IgA vasculitis.
Figure 3:
Most frequent diagnoses in patients with nephritic syndrome according to age and sex category. The top five most frequent nephrological diagnoses (ERA level 1) are shown per age category in adult nephritic males [(A), N = 284] and adult nephritic females [(B), N = 137]. Disease proportions (%) were calculated in every age category separately. The non-specific nephrological diagnosis ‘AKI/CKD, NOS’ was omitted from the figure, and can be found in Supplementary data, Tables S6 and S7. AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; ATN: acute tubular necrosis; A-GBM: anti-glomerular basement membrane nephritis; GP: glomerulopathy, not otherwise specified; NScl: nephrosclerosis; TMA: thrombotic microangiopathy; Vasc: ANCA-negative systemic vasculitis excluding IgA vasculitis.
Isolated hematuria was present in 82 out of 2026 adult patients with available data (4.0%; Supplementary data, Table S8). The most frequent nephrological diagnoses were IgAN (34.1%), LN (14.6%), AAV (12.2%), Alport/thin basement membrane disease (9.8%) and IgA vasculitis (IgAV) (4.9%).
Etiology of glomerular disease with crescentic pattern of injury
A total of 278 biopsies (13.5% of total) showed a crescentic glomerular injury pattern on kidney biopsy. In these biopsies, the most frequent final nephrological diagnoses were AAV, IgAN, LN, IgAV, ANCA-negative systemic vasculitis and anti-glomerular basement membrane (GBM) nephritis (Fig. 4). AAV showed the highest proportion of biopsies with a crescentic pattern of injury (77.0%), followed by ANCA-negative systemic vasculitis (72.7%), anti-GBM nephritis (60.0%), IgAV (32.3%), LN (27.6%) and IgAN (22.5%).
Figure 4:
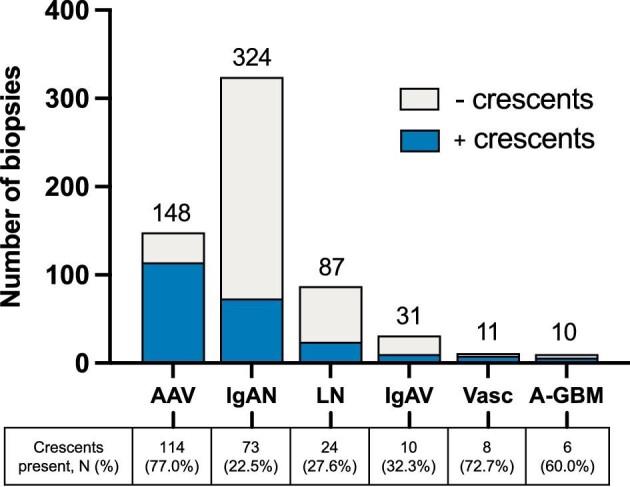
Etiologies most frequently associated with a crescentic pattern of kidney injury. The top six nephrological diagnoses (ERA level 1) of biopsies with a crescentic pattern of kidney injury are shown. The bar chart shows the absolute number of biopsies with crescents (blue bar), biopsies without crescents (grey bar) and total number of biopsies per disease (annotation above bar). The table shows the absolute number and proportion (%) of biopsies with a crescentic pattern of injury. AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; A-GBM: anti-glomerular basement membrane nephritis; Vasc: ANCA-negative systemic vasculitis excluding IgA vasculitis.
Disease chronicity in kidney biopsies graded by the MCCS
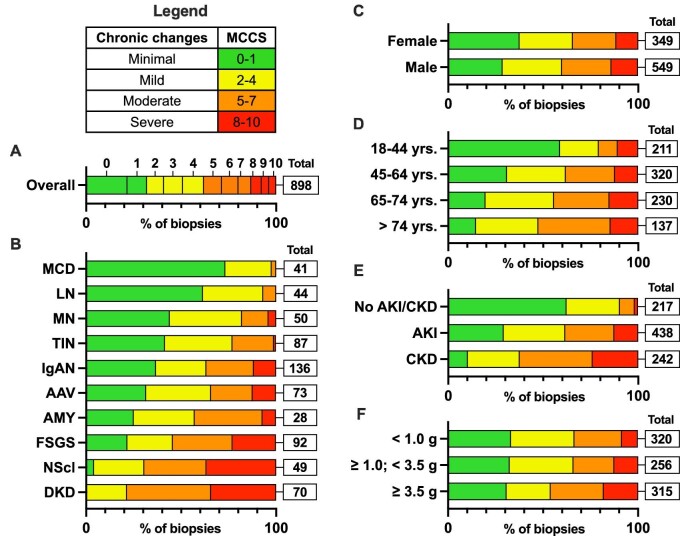
From January 2018 until December 2019, the MCCS was available for 898 biopsies (92.7% of biopsies with ≥10 glomeruli; Fig. 5A). The effect of (nephro)pathologist on the MCCS was evaluated with a linear mixed-effect model and showed no evidence that differences between scores could be attributed to the (nephro)pathologist (Supplementary data, Statistical Methods). Biopsies with a diagnosis of MCD and LN showed mostly minimal to mild disease chronicity (Fig. 5B). Biopsies with MN, tubulointerstitial nephritis (TIN), AAV and IgAN showed an increasing proportion of moderate to severe chronic changes (18.0%, 23.0%, 34.2% and 36.8%, respectively). In FSGS, nephrosclerosis and DKD, the proportion of biopsies with moderate to severe chronic changes exceeded 50% (54.3%, 69.4% and 78.6%, respectively). The MCCS appeared to be higher in biopsies from older patients, patients with chronic kidney disease (CKD) and nephrotic-range proteinuria (Fig. 5D–F).
Figure 5:
MCCS in adult biopsies. Chronicity grade (MCCS) in adult biopsies in Flanders (2018–19, ≥10 glomeruli), ‘total’ refers to the absolute number of biopsies in the subgroups: (A) overall chronicity (N = 898); (B) chronicity according to most frequently diagnosed kidney diseases (ERA level 1 shown, N = 670); (C) chronicity according to sex category (N = 898); (D) chronicity according to age category (N = 898); (E) chronicity according to kidney injury (N = 897; one biopsy with no data on kidney function excluded); (F) chronicity according to proteinuria, UPCR (g/g) or 24 h collection (g/24 h) (N = 891; seven biopsies with no data on proteinuria excluded). AAV: ANCA-associated vasculitis and pauci-immune glomerulonephritis; AMY: amyloidosis; NScl: nephrosclerosis.
Clinical characteristics associated with higher MCCS
The association between MCCS and sex, age, kidney injury, proteinuria and final nephrological diagnosis was determined using univariate and multivariable analyses (Table 6, Supplementary data, Tables S9 and S10). Male sex was associated with significantly higher MCCS in univariate analysis (MCCS increase of 0.438, 95% CI 0.034, 0.842, P = .033), but not in multivariable analysis (Table 6). Increasing patient age was associated with significantly higher MCCS, although estimates were rather small (MCCS increase of 0.507 (univariate, 95% CI 0.396, 0.618, P < .001) and 0.261 (multivariable, 95% CI 0.152, 0.371, P < .001 per decade]. When compared with patients with normal kidney function, both acute kidney injury (AKI) and CKD were associated with large MCCS increases in univariate and multivariable analyses (Table 6). CKD was associated with an MCCS increase of 3.704 (univariate, 95% CI 3.210, 4.199, P < .001) and 2.585 (multivariable, 95% CI 2.070, 3.100, P < .001). When compared with patients with proteinuria <1.0 g/g (UPCR) or g/24 h, only nephrotic-range proteinuria was significantly associated with higher MCCS [MCCS increase of 0.759 (univariate, 95% CI 0.294, 1.225, P = .001) and 0.997 (multivariable, 95% CI 0.536, 1.459, P < .001]. When compared with IgAN, diagnoses of nephrosclerosis, DKD, crystal/cylinder deposition (i.e. hyperoxaluria/hypercalcemic nephropathy) and congenital/hereditary syndromes (i.e. Fabry disease and tuberous sclerosis) were associated with significantly higher MCCS in multivariable analysis (Table 6). Diagnoses of TIN, LN, medication-induced nephropathy (i.e. non-specific nephrotoxicity and nephropathy due to analgesic drugs, lithium or tacrolimus), MN, IgAV, MCD, infection-related immune-complex glomerulonephritis and NS without a definite histopathological diagnosis were associated with significantly lower MCCS (Table 6). The number of biopsies with diagnoses of congenital/hereditary syndromes (N = 2) and NS without a definite histopathological diagnosis (N = 4) were very low, warranting caution in the interpretation of their significant estimates in the model.
Table 6:
Simple and multiple linear regression analysis of clinical characteristics and MCCS.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | β | 95% CI | P-value | β | 95% CI | P-value |
| Sex category | ||||||
| Female (reference) | ||||||
| Male | 0.438 | 0.034, 0.842 | .033 | 0.078 | −0.265, 0.420 | .657 |
| Age (decades) | 0.507 | 0.396, 0.618 | <.001 | 0.261 | 0.152, 0.371 | <.001 |
| Kidney injury | ||||||
| No kidney injury (reference) | ||||||
| AKI | 2.153 | 1.713, 2.593 | <.001 | 1.640 | 1.156, 2.124 | <.001 |
| CKD | 3.704 | 3.210, 4.199 | <.001 | 2.585 | 2.070, 3.100 | <.001 |
| Proteinuria (g/g or g/24 h) | ||||||
| <1.0 (reference) | ||||||
| ≥1.0 and <3.5 | 0.255 | −0.236, 0.747 | .308 | 0.411 | −0.018, 0.840 | .061 |
| ≥3.5 | 0.759 | 0.294, 1.225 | .001 | 0.997 | 0.536, 1.459 | <.001 |
| Final nephrological diagnosis (ERA level 1)a | ||||||
| IgAN (reference) | ||||||
| Congenital/hereditary syndromesb | 2.978 | −0.754, 6.710 | .118 | 3.910 | 0.493, 7.326 | .025 |
| Crystal/cylinder depositionc | 2.478 | 0.675, 4.281 | .007 | 2.192 | 0.531, 3.853 | .010 |
| DKD | 2.942 | 2.167, 3.716 | <.001 | 1.699 | 0.962, 2.436 | <.001 |
| FSGS | 1.250 | 0.542, 1.957 | .001 | 0.599 | −0.072, 1.269 | .080 |
| IgAV | −1.522 | −3.164, 0.120 | .069 | −1.727 | −3.232, −0.222 | .025 |
| Infection-related immune-complex GN | −1.522 | −3.164, 0.120 | .069 | −2.074 | −3.589, −0.558 | .007 |
| LN | −2.045 | −2.953, −1.136 | <.001 | −1.348 | −2.202, −0.495 | .002 |
| MCD | −2.449 | −3.382, −1.515 | <.001 | −2.015 | −2.921, −1.110 | <.001 |
| Medication-induced nephropathyd | −1.165 | −2.635, 0.306 | .120 | −1.523 | −2.883, −0.162 | .028 |
| MN | −1.102 | −1.969, −0.236 | .013 | −1.560 | −2.406, −0.714 | <.001 |
| Nephrosclerosis | 2.539 | 1.666, 3.412 | <.001 | 1.547 | 0.722, 2.372 | <.001 |
| NS, no histology | −2.022 | −4.680, 0.636 | .136 | −3.095 | −5.540, −0.650 | .013 |
| TIN | −0.855 | −1.582, −0.128 | .021 | −1.205 | −1.916, −0.495 | .001 |
β indicates change in estimated MCCS when category is compared with the reference category (for age variable, β indicates MCCS increase with one decade). In multivariable analysis, all other variables in the model remain constant. P-values <.05 are considered significant (bold). Multivariable analysis included 890 biopsies (8 out of 898 biopsies excluded because of missing proteinuria or kidney injury data).
Only diagnoses with a significant result for β shown (all diagnoses shown in Supplementary data, Table S9).
Includes Fabry disease and tuberous sclerosis.
Includes enteric hyperoxaluria and hypercalcemic nephropathy.
Includes non-specific nephrotoxicity and nephropathy due to analgesic drugs, lithium or tacrolimus.
DISCUSSION
In this study, we present the clinicopathological features of all native kidney biopsies performed in the Flemish adult population from 2017 until 2019. The etiology of NS differed across age and sex categories, with MN being the overall most prevalent underlying diagnosis. Etiologies of nephritic syndrome mostly varied across age categories, with IgAN being most frequent in younger and AAV in older patients. In patients with a crescentic glomerular injury pattern on kidney biopsy, AAV was the most frequent underlying cause. Finally, chronic damage on kidney biopsies, summarized by the MCCS, most importantly differed with kidney function and underlying diagnosis.
In Flanders, NS was present in 19.0% of biopsied patients and the overall biopsy rate was high (129.3 biopsies p.m.p./year).2 Previously published European registries with a lower overall biopsy rate generally reported higher proportions of nephrotic patients. In registries from Italy [11] and Spain [12–14] (<50 biopsies p.m.p./year), NS was present in 27.1% and 35.0%, respectively, while studies from Serbia [15, 16] and Romania [17] (<15 biopsies p.m.p./year) observed even higher proportions (48.4%–53.6%). In contrast, a Scottish registry with an equally high overall biopsy rate when compared with Flanders, reported a similar biopsy rate in nephrotic patients (18.3–27.4 versus 23.8 p.m.p./year in Flanders) [18]. This suggests that registries with low overall biopsy rates mainly include biopsies from patients with severe nephrological symptoms, such as NS. In Flanders, the threshold for kidney biopsy in patients with less severe symptoms may be lower, explaining the high overall biopsy rate with lower proportion of nephrotic patients.
MN was the overall most frequent etiology in nephrotic adults, followed by MCD and FSGS. This corresponds with most previous European studies, in which MN was also the most frequent cause of NS or nephrotic-range proteinuria (in Spain [13], Italy [11], Serbia [15, 16] and Poland [19]). In contrast, IgAN was most important in adults with nephrotic-range proteinuria in Czech Republic [20] and membranoproliferative glomerulonephritis in nephrotic adults in Romania [17]. In our study, MCD was most frequent in the youngest adult nephrotic population (18–44 years), while MN was most prevalent in older patients (45–74 years) and amyloidosis in the nephrotic elderly (>75 years). Previous studies that specifically analyzed elderly nephrotic patients (>60 years) also found a predominance of MN and amyloidosis in this age category [13, 19].
The proportion of adult patients with nephritic syndrome (20.8%) in our study was considerably higher than most European studies [12, 13, 15, 16, 19] (3.9%–13.8% in adults), although percentages were also high in the Czech Republic [20] (22.3%–33.9%) and Romania [17] (21.9%). The high proportion in Flanders is likely explained by our less specific definition of nephritic syndrome, which does not incorporate presence of arterial hypertension, oliguria or edema. The etiology of nephritic syndrome in Flanders resembles data from most European registries, with IgAN being the most important cause in younger adults and AAV in older adults [11, 13, 19, 20]. Both etiologies were also the most frequent underlying kidney diseases in biopsies with a crescentic pattern of injury. Although IgAN has a relatively lower risk of presenting with crescents on kidney biopsy, its overall high frequency in the registry explains why it is the second most frequently diagnosed kidney disease when crescents are identified on kidney biopsy.
Regardless of the underlying kidney disease, disease chronicity on native kidney biopsy predicts renal outcomes and may have treatment implications [3, 4]. As an alternative to disease-specific chronicity scores [5–7], the MCCS was proposed as a standardized pathology scoring system to address chronic changes in native kidney biopsies in a more uniform way, regardless of the underlying diagnosis. The prognostic value of MCCS was validated in a large cohort of various native kidney diseases [3] and has shown to predict outcomes in individual cohorts of patients with C3 glomerulopathy (C3GP) [21, 22], AAV [23], MN [24], FSGS [25] and MCD [26]. Due to its prognostic value, the MCCS was implemented in the FCGG registry from 2018 across all pathology centers. The MCCS was scored in 92.7% of biopsies with ≥10 glomeruli and we found no evidence that the MCCS values might be confounded by the scoring (nephro)pathologist. This proves that it is feasible to implement this robust score in a multi-center clinical and research setting.
Male sex was significantly associated with higher MCCS in univariate, but not multivariable analysis. This is likely explained by the fact that patients with FSGS, diabetic kidney disease and nephrosclerosis (i.e. diagnoses with high degrees of chronicity) were predominantly males (65%, 68% and 71%, respectively) [2]. Older age appeared to be associated with a higher degree of chronicity, but after correction for potential confounders in multivariable analysis, chronicity only increased slightly with older age (approximately 1 MCCS point increase per 40 years). Proteinuria may reflect both disease activity and/or chronicity, likely explaining the relatively small estimates observed in our model. As expected, CKD was associated with a large MCCS increase, although AKI was also associated with an important increase in chronic damage. Diagnoses of nephrosclerosis and DKD were associated with a significant increase in MCCS, as well as a diagnosis of oxalate nephropathy or hypercalcemic nephropathy. Although the estimate for this last group should be interpreted with caution due to small sample size (N = 9), its high chronicity corresponds with previous studies that have demonstrated that a high proportion of patients with oxalate nephropathy progress to kidney failure [27]. Chronicity of IgAV was significantly lower when compared with IgAN in multivariable analysis, which is in line with previous studies [28, 29].
Following the broader application of the MCCS in recent studies, additional research questions arise. Studies in AAV and C3GP have identified an MCCS value of ≥4 as an ideal cut-off for the risk of progression to kidney failure: hazard ratios for progression to kidney failure in patients with an MCCS value of ≥4 versus <4 ranged from 3.3 to 5.2 depending on the statistical model and follow-up period [22, 23]. However, it is still uncertain whether such a predictive cut-off value is similar in other kidney diseases. Moreover, the value of MCCS in predicting therapy response and its potential to tailor treatment should be further established. It also remains questionable whether the MCCS should be applied to biopsies with <10 glomeruli, since these biopsies are generally less representative and the predictive value of MCCS in these cases may be inaccurate.
Our study has several strengths. The FCGG registry provides data on a Western European population of 5.3 million adults and has implemented a standardized pathology reporting protocol incorporating the pattern of glomerular injury and disease chronicity, according to recent guidelines [30, 31]. We not only showed that it is feasible to use the MCCS in a large multi-center registry, but also generated highly useful population data that can be correlated with long-term follow-up outcome studies for further validation. Our study is mainly limited by the fact that biopsy indications were not collected at the moment of biopsy and therefore only retrospectively defined, likely causing our definition of nephritic syndrome to be less specific. In conclusion, the FCGG registry validates data from previous Western European registries and provides a snapshot of disease chronicity in the whole biopsied Flemish population.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank all collaborating nephrologists in Flanders and Brussels and responsible persons at the data entry centers (Elsie De Man, Sabine Verhofstede, Ben Sprangers) for their participation in the FCGG registry. The FCGG registry was initiated in collaboration with the Nederlandstalige Belgische Vereniging voor Nefrologie (NBVN), the organization that represents the majority of nephrologists in the region of Flanders. The study was approved by the Ethical Committee of the University Hospitals Leuven (study reference S59182) and local committees of all participating centers.
Appendix I.
Appendix I. ‘FCGG reference nephrologists’ and collaborating pathologists of participating centers
| Reference nephrologist | Center |
|---|---|
| An De Vriese | AZ Sint-Jan, Brugge |
| Anja De Rycke | AZ Sint-Blasius, Dendermonde |
| Anne-Marie Bogaert | AZ Glorieux, Ronse |
| Annemie Woestenburg | AZ Voorkempen, Malle |
| Bart Denys | Onze-Lieve-Vrouwziekenhuis, Aalst |
| Bart Maes | AZ Delta, Roeselaere |
| Domien Peeters | Sint-Trudo Ziekenhuis, Sint-Truiden |
| Hilde Vanbelleghem | Jan Yperman ziekenhuis, Ypres |
| Jan Donck | AZ Sint-Lucas, Ghent |
| Johan Scharpé, Nele De Clippeleir | GZA, Antwerp |
| Ann Colson | Kliniek Sint-Jan, Brussels |
| Karen Meyvis | AZ Monica, Antwerp |
| Kurt Vandepitte | Heilig-Hartziekenhuis, Lier |
| Liza-Maria Reyns | AZ Sint-Lucas, Brugge |
| Jacques Peeters | Ziekenhuis Oost-Limburg, Genk |
| Marc Decupere | AZ Groeninge, Kortrijk |
| Mark Helbert | ZNA, Antwerp |
| Miranda Zeegers | AZ Turnhout, Turnhout |
| Nathalie Neirynck | VITAZ, Sint-Niklaas |
| Pascale Bernaert | AZ Maria Middelares, Ghent |
| Tom Dejagere | Jessa Ziekenhuis, Hasselt |
| Wim Lemahieu | Imeldaziekenhuis, Bonheiden |
| Ben Sprangers | University Hospitals Leuven, Leuven |
| Lissa Pipeleers | University Hospital Brussels, Brussels |
| Rachel Hellemans | Antwerp University Hospital, Antwerp |
| Steven Van Laecke | Ghent University Hospital, Ghent |
| Noël Knops, Elena Levtchenko | Pediatric Nephrology Department, University Hospitals Leuven, Leuven |
| Johan Vande Walle, Sevasti Karamaria | Pediatric Nephrology Department, Ghent University Hospital, Ghent |
| Koen Van Hoeck, Dominique Trouet | Pediatric Nephrology Department, Antwerp University Hospital, Antwerp |
| Reiner Mauel | Pediatric Nephrology Department, University Hospital Brussels, Brussels |
| Pathologist | Center |
| Amélie Dendooven | Antwerp University Hospital, Antwerp |
| Ghent University Hospital, Ghent | |
| Anne Hoorens, Jo Van Dorpe, Marleen Praet | Ghent University Hospital, Ghent |
| Caroline Geers | University Hospital Brussels, Brussels |
| Evelyne Lerut, Priyanka Koshy, Tania Roskams | University Hospitals Leuven, Leuven |
| Selda Aydin | Cliniques Universitaires Saint-Luc, Brussels |
| Vasiliki Siozopoulou | Antwerp University Hospital, Antwerp |
| Anne-Marie Schelfhout, Hendrik De Raeve | Onze-Lieve-Vrouwziekenhuis, Aalst |
| Edwin Steenkiste, Francesca Dedeurwaerdere | AZ Delta, Roeselaere |
| Ignace Dalle | AZ Sint-Lucas, Brugge |
| Kristof Cokelaere, Stijn Deloose | Jan Yperman ziekenhuis, Ypres |
| Pascale De Paepe | AZ Sint-Jan, Brugge |
| Peter Van Eyken | Ziekenhuis Oost-Limburg, Genk |
Notes
Members of the FCGG collaborative group are listed in Appendix I. These members are collaborators, unless also mentioned in the author list above, in which case they are authors.
Contributor Information
Dries Deleersnijder, Department of Microbiology, Immunology and Transplantation, Laboratory of Molecular Immunology, Rega Institute, KU Leuven, Leuven, Belgium; Department of Nephrology, University Hospitals Leuven, Leuven, Belgium.
Wim Laurens, Department of Nephrology and Dialysis, VITAZ Hospital, Sint-Niklaas, Belgium; Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium.
Johan De Meester, Department of Nephrology and Dialysis, VITAZ Hospital, Sint-Niklaas, Belgium.
Evert Cleenders, Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium.
Amélie Dendooven, Division of Pathology, University Hospital Ghent, Ghent, Belgium; Laboratory of Experimental Medicine and Pediatrics, University of Antwerp, Wilrijk, Belgium.
Evelyne Lerut, Department of Imaging and Pathology, KU Leuven, Leuven, Belgium; Department of Pathology, University Hospitals Leuven, Leuven, Belgium.
An S De Vriese, Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium; Department of Nephrology and Infectious Diseases, AZ Sint-Jan, Brugge, Belgium.
Tom Dejagere, Department of Nephrology, Jessa Hospital, Hasselt, Belgium.
Mark Helbert, Department of Nephrology, ZNA Middelheim Hospital, Antwerp, Belgium.
Rachel Hellemans, Laboratory of Experimental Medicine and Pediatrics, University of Antwerp, Wilrijk, Belgium; Department of Nephrology, Antwerp University Hospital, Edegem, Belgium.
Priyanka Koshy, Department of Pathology, University Hospitals Leuven, Leuven, Belgium.
Bart Maes, Department of Nephrology, AZ Delta, Roeselare, Belgium.
Lissa Pipeleers, Department of Nephrology, University Hospital Brussels, Brussels, Belgium.
Amaryllis H Van Craenenbroeck, Department of Nephrology, University Hospitals Leuven, Leuven, Belgium; Department of Microbiology, Immunology and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium.
Steven Van Laecke, Renal Division, Department of Internal Medicine, Ghent University Hospital, Ghent, Belgium.
Johan Vande Walle, Department of Internal Medicine and Pediatrics, Ghent University, Ghent, Belgium; Department of Pediatric Nephrology, Ghent University Hospital, Ghent, Belgium.
Marie M Couttenye, Laboratory of Experimental Medicine and Pediatrics, University of Antwerp, Wilrijk, Belgium; Department of Nephrology, Antwerp University Hospital, Edegem, Belgium.
Gert Meeus, Department of Nephrology, AZ Groeninge Hospital, Kortrijk, Belgium.
Ben Sprangers, Department of Microbiology, Immunology and Transplantation, Laboratory of Molecular Immunology, Rega Institute, KU Leuven, Leuven, Belgium; Department of Nephrology, University Hospitals Leuven, Leuven, Belgium.
the FCGG collaborative group:
An De Vriese, Anja De Rycke, Anne-Marie Bogaert, Annemie Woestenburg, Bart Denys, Bart Maes, Domien Peeters, Hilde Vanbelleghem, Jan Donck, Johan Scharpé, Nele De Clippeleir, Ann Colson, Karen Meyvis, Kurt Vandepitte, Liza-Maria Reyns, Jacques Peeters, Marc Decupere, Mark Helbert, Miranda Zeegers, Nathalie Neirynck, Pascale Bernaert, Tom Dejagere, Wim Lemahieu, Ben Sprangers, Lissa Pipeleers, Rachel Hellemans, Steven Van Laecke, Noël Knops, Elena Levtchenko, Johan Vande Walle, Sevasti Karamaria, Koen Van Hoeck, Dominique Trouet, Reiner Mauel, Amélie Dendooven, Anne Hoorens, Jo Van Dorpe, Marleen Praet, Caroline Geers, Evelyne Lerut, Priyanka Koshy, Tania Roskams, Selda Aydin, Vasiliki Siozopoulou, Anne-Marie Schelfhout, Hendrik De Raeve, Edwin Steenkiste, Francesca Dedeurwaerdere, Ignace Dalle, Kristof Cokelaere, Stijn Deloose, Pascale De Paepe, and Peter Van Eyken
FUNDING
D.D. is supported by a PhD Fellowship grant fundamental research from the Research Foundation Flanders (F.W.O., grant number 11L5622N). B.S. is a senior clinical investigator of The Research Foundation Flanders (F.W.O., grant number 1 842 919 N). The FCGG registry is funded by the Nederlandstalige Belgische Vereniging voor Nefrologie (NBVN).
CONFLICT OF INTEREST STATEMENT
B.S. is a member of the CKJ editorial board. The results presented in this paper have not been published previously in whole or part, except in abstract format.
AUTHORS’ CONTRIBUTIONS
D.D., W.L., J.D.M., A.D., E.L., A.S.D.V., T.D., M.H., R.H., P.K., B.M., L.P., A.H.V.C., S.V.L., J.V.W., M.M.C., G.M. and B.S. were responsible for the conception, design and data acquisition of the study. D.D., W.L., J.D.M., E.C., A.D., A.H.V.C. and B.S. were responsible for analysis and interpretation of the data. D.D., W.L., J.D.M., E.C., A.D., E.L., A.S.D.V., T.D., M.H., R.H., P.K., B.M., L.P., A.H.V.C., S.V.L., J.V.W., M.M.C., G.M. and B.S. drafted the work and revised it critically for important intellectual content. D.D., W.L., J.D.M., E.C., A.D., E.L., A.S.D.V., T.D., M.H., R.H., P.K., B.M., L.P., A.H.V.C., S.V.L., J.V.W., M.M.C., G.M. and B.S. approved the submitted version of the manuscript. D.D., W.L., J.D.M., E.C., A.D., E.L., A.S.D.V., T.D., M.H., R.H., P.K., B.M., L.P., A.H.V.C., S.V.L., J.V.W., M.M.C., G.M. and B.S. agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
REFERENCES
- 1. Fiorentino M, Bolignano D, Tesar Vet al. Renal biopsy in 2015 - From epidemiology to evidence-based indications. Am J Nephrol 2016;43:1–19. 10.1159/000444026 [DOI] [PubMed] [Google Scholar]
- 2. Laurens W, Deleersnijder D, Dendooven Aet al. Epidemiology of native kidney disease in Flanders: results from the FCGG kidney biopsy registry. Clin Kidney J 2022;15:1361–72. 10.1093/ckj/sfac033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srivastava A, Palsson R, Kaze ADet al. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol 2018;29:2213–24. 10.1681/ASN.2017121260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sethi S, D'Agati VD, Nast CCet al. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int 2017;91:787–9. 10.1016/j.kint.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 5. Cattran DC, Coppo R, Cook HTet al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009;76:534–45. 10.1038/ki.2009.243 [DOI] [PubMed] [Google Scholar]
- 6. Bajema IM, Wilhelmus S, Alpers CEet al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018;93:789–96. 10.1016/j.kint.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 7. Berden AE, Ferrario F, Hagen ECet al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010;21:1628–36. 10.1681/ASN.2010050477 [DOI] [PubMed] [Google Scholar]
- 8. Fogo AB. Approach to renal biopsy. Am J Kidney Dis 2003;42:826–36. 10.1016/j.ajkd.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 9. Hogan JJ, Mocanu M, Berns JS.. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol 2016;11:354–62. 10.2215/CJN.05750515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venkat-Raman G, Tomson CRV, Gao Yet al. New primary renal diagnosis codes for the ERA-EDTA. Nephrol Dial Transplant 2012;27:4414–9. 10.1093/ndt/gfs461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schena FP. Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. Nephrol Dial Transplant 1997;12:418–26. 10.1093/ndt/12.3.418 [DOI] [PubMed] [Google Scholar]
- 12. Rivera F, López-Gómez JM, Pérez-García R.. Frequency of renal pathology in Spain 1994–1999. Nephrol Dial Transplant 2002;17:1594–602. 10.1093/ndt/17.9.1594 [DOI] [PubMed] [Google Scholar]
- 13. Rivera F, López-Gómez JM, Pérez-García R.. Clinicopathologic correlations of renal pathology in Spain. Kidney Int 2004;66:898–904. 10.1111/j.1523-1755.2004.00833.x [DOI] [PubMed] [Google Scholar]
- 14. López-Gómez JM, Rivera F.. Spanish Registry of glomerulonephritis 2020 revisited: past, current data and new challenges. Nefrología 2020;40:371–83. 10.1016/j.nefro.2020.04.012 [DOI] [PubMed] [Google Scholar]
- 15. Naumovic R, Pavlovic S, Stojkovic Det al. Renal biopsy registry from a single centre in Serbia: 20 years of experience. Nephrol Dial Transplant 2008;24:877–85. 10.1093/ndt/gfn564 [DOI] [PubMed] [Google Scholar]
- 16. Brkovic V, Milinkovic M, Kravljaca Met al. Does the pathohistological pattern of renal biopsy change during time? Pathol Res Pract 2018;214:1632–7. 10.1016/j.prp.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 17. Covic A, Schiller A, Volovat Cet al. Epidemiology of renal disease in Romania: a 10 year review of two regional renal biopsy databases. Nephrol Dial Transplant 2006;21:419–24. 10.1093/ndt/gfi207 [DOI] [PubMed] [Google Scholar]
- 18. McQuarrie EP, Mackinnon B, Young Bet al. Centre variation in incidence, indication and diagnosis of adult native renal biopsy in Scotland. Nephrol Dial Transplant 2009;24:1524–8. 10.1093/ndt/gfn677 [DOI] [PubMed] [Google Scholar]
- 19. Perkowska-Ptasinska A, Bartczak A, Wagrowska-Danilewicz Met al. Clinicopathologic correlations of renal pathology in the adult population of Poland. Nephrol Dial Transplant 2017;32:ii209–18. 10.1093/ndt/gfw365 [DOI] [PubMed] [Google Scholar]
- 20. Maixnerova D, Jancova E, Skibova Jet al. Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994–2011. J Nephrol 2015;28:39–49. 10.1007/s40620-014-0090-z [DOI] [PubMed] [Google Scholar]
- 21. Bomback AS, Santoriello D, Avasare RSet al. C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int 2018;93:977–85. 10.1016/j.kint.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 22. Caravaca-Fontán F, Trujillo H, Alonso Met al. Validation of a histologic scoring index for C3 glomerulopathy. Am J Kidney Dis 2021;77:684–695.e1. 10.1053/j.ajkd.2020.11.011 [DOI] [PubMed] [Google Scholar]
- 23. Casal Moura M, Fervenza FC, Specks U, Sethi S. Kidney biopsy chronicity grading in antineutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2021;37:gfab250.(published online, 26 August 2021, https://doi.org/10.1093/ndt/gfab250). 10.1093/ndt/gfab250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yin P, Wang J, Liang Wet al. Outcomes of primary membranous nephropathy based on serum anti-phospholipase A2 receptor antibodies and glomerular phospholipase A2 receptor antigen status: a retrospective cohort study. Ren Fail 2020;42:675–83. 10.1080/0886022X.2020.1792315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chávez-Mendoza CA, Niño-Cruz JA, Correa-Rotter Ret al. Calcineurin inhibitors with reduced-dose steroids as first-line therapy for focal segmental glomerulosclerosis. Kidney Int Rep 2019;4:40–47. 10.1016/j.ekir.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stefan G, Busuioc R, Stancu Set al. Adult-onset minimal change disease: the significance of histological chronic changes for clinical presentation and outcome. Clin Exp Nephrol 2021;25:240–50. 10.1007/s10157-020-01985-7 [DOI] [PubMed] [Google Scholar]
- 27. Rosenstock JL, Joab TMJ, DeVita MVet al. Oxalate nephropathy: a review. Clin Kidney J 2022;15:194–204. 10.1093/ckj/sfab145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pillebout E. IgA vasculitis and IgA nephropathy: same disease? J Clin Med 2021;10:1–14. 10.3390/jcm10112310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oh HJ, Ahn SV, Yoo DEet al. Clinical outcomes, when matched at presentation, do not vary between adult-onset Henöch-Schönlein purpura nephritis and IgA nephropathy. Kidney Int 2012;82:1304–12. 10.1038/ki.2012.302 [DOI] [PubMed] [Google Scholar]
- 30. Sethi S, Haas M, Markowitz GSet al. Mayo Clinic/Renal Pathology Society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol 2016;27:1278–87. 10.1681/ASN.2015060612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sethi S, Fervenza FC.. Standardized classification and reporting of glomerulonephritis. Nephrol Dial Transplant 2019;34:193–9. 10.1093/ndt/gfy220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.