Glomerular diseases with organized deposits are classified as amyloidosis, diabetic fibrillosis, fibrillary glomerulopathy, immunotactoid glomerulopathy, fibronectin glomerulopathy, collagenofibrotic glomerulopathy, cryoglobulinemic glomerulonephritis and various advanced kidney diseases [1]. We herein report a case of glomerular disease with unusual, organized deposits.
An 84-year-old man with a 4-year history of microhematuria and proteinuria was referred to our hospital because of the exacerbation of hypertension and nephrotic-range proteinuria. He had developed left glaucoma 11 years previously and suffered a cerebral lacunar infarction 2 years previously. His medications included clopidogrel (75 mg/day) for stroke prevention; amlodipine (5 mg/day), enalapril (5 mg/day), and azosemide (30 mg/day) for hypertension; atorvastatin (5 mg/day) for hyperlipidemia; and naftopidil (50 mg/day) for benign prostatic hypertrophy. He had also been diagnosed with prostate cancer, which had not shown progression at regular outpatient visits. He was admitted to our hospital for further evaluation.
His blood pressure was 189/95 mmHg. Pitting edema was found on his bilateral lower legs, without any purpura.
A urinalysis revealed microhematuria and proteinuria (the urinary protein:creatinine ratio was 5.67 g/g Cr; Supplementary data, Table S1). His cystatin C–based estimated glomerular filtration rate (eGFR) was 53.4 mL/min/1.73 m2. His serum albumin level was decreased and his serum total cholesterol level was increased. Antinuclear antibody, cryoglobulin and cryofibrinogen were all negative with normal complement levels. Serum and urine immunoelectrophoresis showed negative results. Chest computed tomography (CT) showed bilateral pleural effusions and pericardial fluid and abdominal CT showed several simple cysts in both kidneys without atrophy.
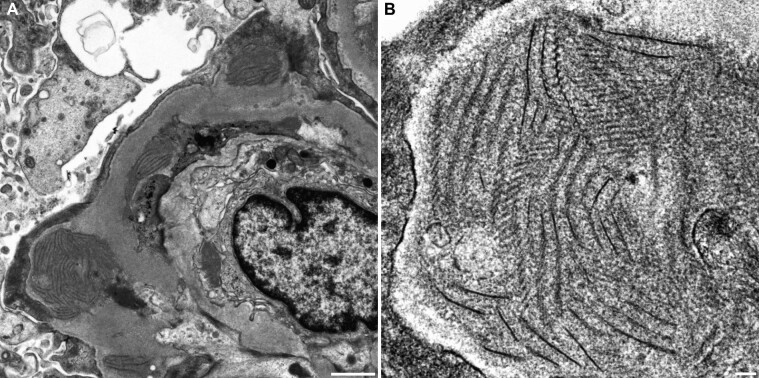
A kidney biopsy showed lobulated glomeruli with thickening and duplication of the glomerular basement membranes (GBMs), mesangial hypercellularity and mild endocapillary hypercellularity (Supplementary data, Figure S1). An immunofluorescence study revealed granular deposition of immunoglobulin M (IgM) and complement 3 (C3) along the glomerular capillary wall and in the mesangial area (Supplementary data, Figure S2). There was a light chain restriction for κ. Direct fast scarlet staining was negative under polarized light microscopy. Electron-dense deposits were observed in the subepithelial, intramembranous and subendothelial area under transmission electron microscopy (Figure 1A). A high-magnification view of the deposits revealed fibrillary structures of 8–14 nm in width that were associated with ladder formation, with a periodicity of 25–30 nm (Figure 1B). Glomeruli on paraffin sections were analyzed with laser-capture microdissection followed by liquid chromatography–tandem mass spectrometry. The results identified an increase in collagen α1 (VI), α2 (VI) and α3 (VI) chains; IgM; C3; κ light chain; fibrinogen α, β and γ chains and apolipoprotein B-100 (Supplementary data, Figure S3). An immunofluorescence study using rabbit polyclonal antibody against collagen α1 (VI) chain showed positive findings on capillary wall segments (Supplementary data, Figure S4).
FIGURE 1:
(A) Electron-dense deposits were observed in the subepithelial, intramembranous and subendothelial areas of the GBM under transmission electron microscopy. Extensive foot process effacement of the podocytes and mesangial interposition were also observed. Bar = 1 µm. (B) The deposits at high magnification revealed fibrillary structures with a width of 8–14 nm associated with ladder formation with a periodicity of 25–30 nm. Bar = 100 nm.
Intravenous methylprednisolone (500 mg/day) was administered for 3 consecutive days, as proteinuria did not improve with azilsartan and the urinary protein:creatinine ratio and serum albumin value were 0.73 g/g Cr and 3.2 g/dl, respectively, under treatment with prednisolone at a dose of 7.5 mg/day.
While the present case resembled cryofibrinogen-associated glomerulonephritis [2–4], cryofibrinogen was not identified in the plasma. There have been two similar cases with unusual, organized deposits [5, 6]. However, a proteomic analysis was not performed in one report [5] and immunohistochemical staining of type VI collagen was negative in the other report [6]. In contrast, a proteomic analysis in the case of cryofibrinogen-associated glomerulonephritis identified collagen α3 (VI) chain [4]. Although the details concerning the mechanism underlying the glomerular deposition of type VI collagen are unclear, the diagnosis in the present case was type VI collagen-related nephropathy.
Supplementary Material
Contributor Information
Mutsuki Mori, Department of Nephrology, Saiseikai Matsusaka General Hospital, Matsusaka, Japan; Department of Cardiology and Nephrology, Mie University Graduate School of Medicine, Tsu, Japan.
Kan Katayama, Department of Cardiology and Nephrology, Mie University Graduate School of Medicine, Tsu, Japan.
Kensuke Joh, Department of Pathology, Jikei University School of Medicine, Tokyo, Japan.
Eiji Ishikawa, Department of Nephrology, Saiseikai Matsusaka General Hospital, Matsusaka, Japan.
Kaoru Dohi, Department of Cardiology and Nephrology, Mie University Graduate School of Medicine, Tsu, Japan.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests in association with the present study. The results presented in this article have not been published previously in whole or part. Written informed consent was obtained from the patient. A proteomic analysis of glomeruli was approved by Mie University (H2020-184).
REFERENCES
- 1. Herrera GA, Turbat-Herrera EA. Renal diseases with organized deposits: an algorithmic approach to classification and clinicopathologic diagnosis. Arch Pathol Lab Med 2010; 134: 512–531 [DOI] [PubMed] [Google Scholar]
- 2. Sethi S, Yachoui R, Murray DLet al. Cryofibrinogen-associated glomerulonephritis. Am J Kidney Dis 2017; 69: 302–308 [DOI] [PubMed] [Google Scholar]
- 3. Ibuki E, Shiraishi A, Sofue Tet al. Characteristic electron-microscopic features of cryofibrinogen-associated glomerulonephritis: a case report. BMC Nephrol 2020; 21: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kakeshita K, Yamazaki H, Imamura Tet al. Cryofibrinogen-associated glomerulonephritis accompanied by advanced gastric cancer. CEN Case Rep 2021; 10: 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hara S, Tsukaguchi H, Oka Tet al. Monoclonal immunoglobulin-associated proliferative glomerulonephritis characterized by organized deposits of striated ultra-substructures: a case report. Ultrastruct Pathol 2017; 41: 301–307 [DOI] [PubMed] [Google Scholar]
- 6. Ohtani H, Wakui H, Komatsuda Aet al. Progressive glomerulopathy with unusual deposits of striated structures: a new disease entity? Nephrol Dial Transplant 2010; 25: 2016–2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.