ABSTRACT
Cardiorenal syndromes (CRS) are broadly defined as disorders of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other. CRS are currently classified into five categories, mostly based on disease-initiating events and their acuity or chronicity. CRS types 3 and 4 (also called renocardiac syndromes) refer to acute and chronic kidney dysfunction resulting in acute and chronic heart dysfunction, respectively. The notion of renocardiac syndromes has broadened interest in kidney–heart interactions but uncertainty remains in the nephrological community's understanding of the clinical diversity, pathophysiological mechanisms and optimal management approaches of these syndromes. This triple challenge that renocardiac syndromes (and likely other cardiorenal syndromes) pose to the nephrologist can only be faced through a specific and demanding training plan to enhance his/her cardiological scientific knowledge and through an appropriate clinical environment to develop his/her cardiological clinical skills. The first must be the objective of the subspecialty of cardionephrology (or nephrocardiology) and the second must be the result of collaboration with cardiologists (and other specialists) in cardiorenal care units. This review will first consider various aspects of the challenges that renocardiac syndromes pose to nephrologists and, then, will discuss those aspects of cardionephrology and cardiorenal units that can facilitate an effective response to the challenges.
Keywords: acute kidney injury, cardionephrology, cardiorenal syndromes, chronic kidney disease, renocardiac syndromes
INTRODUCTION
Cardiorenal syndrome (CRS) encompasses a spectrum of disorders involving both the heart and kidneys. The Acute Dialysis Quality Initiative outlined a consensus approach in 2008 that phenotyped CRS into two major groups, cardiorenal and renocardiac syndromes, based on the primum movens of the disease process [1]. This was further grouped into five subtypes based on disease acuity and sequential organ involvement. A recent Scientific Statement from the American Heart Association developed the central notion that in CRS, acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ [2].
Although the notion of CRS has stimulated the research of the cross-talk between the heart and the kidney across several clinical scenarios, some aspects deserve to be considered [3]. Mainly due to the influence of aging and increased incidence of common cardiac and renal metabolic and hemodynamic risk factors, the interactions between heart and kidney diseases are of increasing complexity and include important epidemiological, diagnostic, preventive and therapeutic aspects which are not fully addressed in the limited context of simultaneous acute or chronic organ dysfunction. Furthermore, patients with the dual burden of heart and kidney disease continue to experience unacceptably high rates of clinical complications, hospitalization and mortality. On the other hand, the pathophysiological framework underlying the classification of CRS has been challenged by recent mechanistic advances.
Therefore, this review article is based on the consideration that the time has come for the nephrologist to advance the view of heart–kidney interactions proposed in the CRS classification. In this conceptual framework, and by way of example, we will focus on some clinical, mechanistic and therapeutic aspects of types 4 and 3 of CRS (i.e., chronic and acute renocardiac syndromes, respectively) that underlie the complex and broad kidney–heart relationship in these two syndromes. Finally, we will consider how to address the growing clinical and scientific challenge that patients with renocardiac syndromes pose to nephrologists.
DIVERSITY OF CLINICAL MANIFESTATIONS IN RENOCARDIAC SYNDROMES
Both chronic and acute renocardiac syndromes have multiple clinical manifestations, beyond the classically acknowledged and researched conditions.
Chronic renocardiac syndrome
This syndrome is characterized by primary chronic kidney disease (CKD) leading to an increased risk of chronic impairment of cardiac function [1, 2] (Fig. 1). Decreased kidney function worsens chronic heart failure (CHF) prognosis [4]. Adverse left ventricular (LV) remodeling both macroscopic [i.e., progressive development of left ventricular hypertrophy (LVH) with diastolic dysfunction, evolving lately to LV dilatation and systolic dysfunction] and microscopic (e.g., cardiomyocyte hypertrophy and apoptosis, and myocardial interstitial inflammation and fibrosis) is involved in the development and progression of CHF in CKD patients [5].
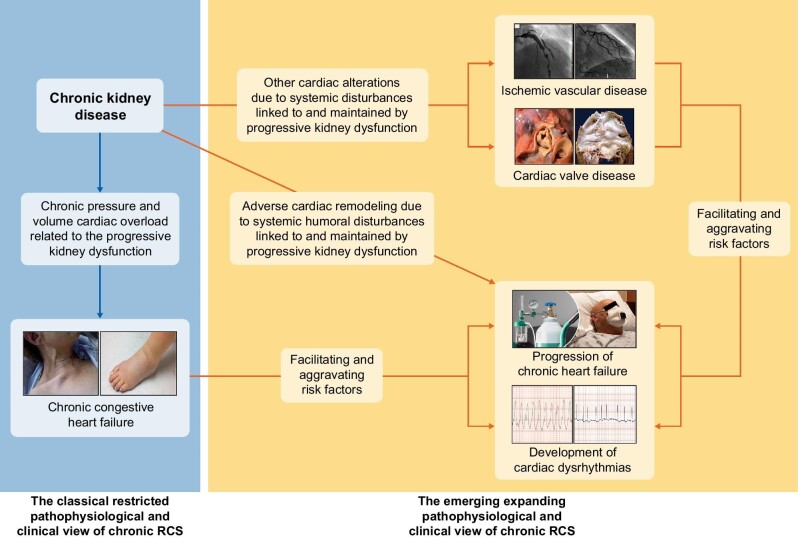
FIGURE 1:
Schematic view of the two pathophysiological and clinical relationships participating in views of chronic renocardiac syndrome (RCS) or type 4 cardiorenal syndrome: the classical restricted one (left part of the figure) and the emerging expanding one (right part of the figure). The depicted photographs are the following: ischemic vascular disease: angiographic view of obstructive coronary artery disease (left) and diminished density of coronary microvessels (right). Cardiac valve disease: macroscopic appearance of aortic stenosis (left) and mitral regurgitation (right). Chronic congestive heart failure: clinical aspect of jugular engorgement (left) and peripheral oedema (right). Progression of chronic heart failure: oxygen therapy in a patient suffering from stage D heart failure. Cardiac dysrhythmias: electrocardiographic image of ventricular tachycardia (left) and atrial fibrillation (right).
Increasing interest is being devoted to the role of disturbances of the right ventricle in chronic renocardiac syndrome. In fact, CKD is independently associated with the risk of pulmonary hypertension [6, 7] and right ventricular–pulmonary artery uncoupling [8], which it is recognized as a major mechanism of right ventricular systolic dysfunction [9]. Interestingly, CKD is associated with severe right ventricular systolic dysfunction, which is independently associated with mortality [10].
Recently, a group of experts identified several aspects of CKD that fit criteria of unmet medical needs, among them the prevention and management of cardiac complications beyond CHF [11, 12]. Furthermore, cardiac complications in patients with CKD are more prevalent, with higher complexity and severity compared with the non-CKD population, and are associated with larger economic and societal burden [13–15].
Although classically the cardiac risk of CKD has been related to coronary atherosclerosis [16] and vascular calcification [17], it is now accepted that the risk of other cardiac complications (including coronary microvascular dysfunction, cardiac valve disease, dysrhythmias and sudden cardiac death) is also increased in CKD patients (Fig. 1) [18].
Coronary microvascular dysfunction results from different structural, functional and/or dynamic alterations in the coronary microcirculation associated with CKD that may result in angina even in the absence of atherosclerotic coronary artery disease or coronary calcifications [19]. Coronary microvascular dysfunction is characterized by a reduced coronary flow reserve [20, 21] and is independently associated with adverse cardiovascular events [22, 23].
There is an epidemiological collinearity of the prevalence and incidence of CKD with aortic and mitral valve diseases, which are present in 88–99% of stage 5 CKD patients [24]. Calcification plays an important role in CKD-associated valve disease occurring 10–20 years earlier in CKD patients compared with the general population, with an increase in the incidence and prevalence in parallel to the progression of CKD stage [25]. The presence of aortic and/or mitral valve disease has a strong unfavorable impact on the outcome in patients with CKD, namely in those on dialysis [26]. Compared with the bibliography focusing on left-sided valves and CKD, evidence on tricuspid and pulmonary valve disease is much less. However, tricuspid regurgitation is prevalent in CKD, namely in patients on dialysis (in whom the prevalence is of 63%) and mostly due to pulmonary hypertension [27].
CKD patients have a significant increased burden from atrial fibrillation (AF) compared with subjects without CKD, with a prevalence reaching up to 20% in non-dialysis CKD patients and up to 40% in patients on dialysis [28]. CKD and AF share many risk factors, making it difficult to discern the contributions of individual factors to either condition or associated outcomes (e.g., stroke) [29].
There is an increased risk of sudden cardiac death (SCD) in CKD. While the annual incidence rate of SCD is around 0.1% in the general population, it rises to 1.5%–2.7% in non-dialysis CKD patients, reaching 7% in patients initiating dialysis [28]. There is a significant gap of knowledge in the understanding of electrical and hemodynamic mechanisms underlying SCD. In a retrospective study of hemodialysis patients who were prescribed a wearable cardioverter defibrillator, 80% of cardiac arrests were recorded as ventricular tachyarrhythmias (ventricular tachycardia or ventricular fibrillation) compared with 20% bradyarrhythmias, and most events occurred during or immediately after dialysis sessions [30]. In contrast, in a recent prospective study with continuous electrocardiogram monitoring, bradyarrhythmias and asystole, rather than ventricular tachyarrhythmias, were important determinants of SCD during the long interdialytic period [31].
Taken together, the above considerations indicate that nephrologists should be encouraged and educated to discuss cardiac risks and potential cardiac diagnostic and treatment options for CKD patients in a broader manner than is currently the case from the perspective of chronic renocardiac syndrome, which focuses primarily on CHF attributable to LV failure.
Acute renocardiac syndrome
This syndrome occurs when acute kidney injury (AKI) contributes to and/or precipitates the development of acute cardiac injury or dysfunction [1, 2] (Fig. 2). In particular, AKI is associated with increased risk of acute decompensated HF (ADHF). In fact, in a population-based study involving a large cohort of patients who survived a hospitalization complicated by AKI, 20% of patients were readmitted within 30 days, most often with ADHF [32]. In another study of a large cohort of hospitalized adults, during the first year after discharge the risk of hospitalization for ADHF was increased by 44% [33] in the group with AKI as compared with the group that did not have AKI.
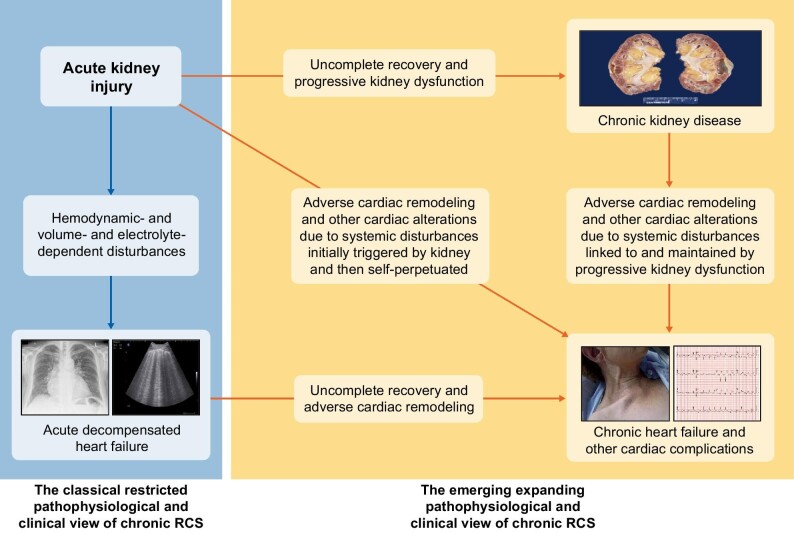
FIGURE 2:
Schematic view of the two pathophysiological and clinical views of acute renocardiac syndrome (RCS) or type 3 cardiorenal syndrome: the classical restricted one (left part of the figure) and the emerging expanding one (right part of the figure). The depicted photographs are the following: chronic kidney disease: macroscopic appearance of end-stage kidney disease. Acute decompensated kidney failure: chest X-ray image of pulmonary oedema (left) and lung ultrasound image of B-lines (right). Chronic heart failure and other cardiac complications: clinical aspect of jugular engorgement (left) and electrocardiographic image of acute myocardial infarction (right).
In addition, AKI is also associated with increased risk of other long-term cardiovascular complications (Fig. 2). A 2017 meta-analysis of 25 studies involving a total of 254 408 patients, including 55 150 with AKI, showed that AKI was associated with an 86% increase in the risk of death from cardiovascular causes during a median follow-up of 2.6 years [34]. There was a 58% increase in the risk of CHF during 2.9 years of follow-up, a 40% increase in the risk of acute myocardial infarction during 2.3 years of follow-up, and a 15% increase in the risk of stroke over a period of 2.7 years [34]. Other studies have shown that the increased risks did not differ between AKI patients with and without previous CKD and neither status with respect to recovery of renal function nor severity of AKI [35–37]; there are also no differences between patients with and without previous cardiovascular conditions, including CHF [38, 39], suggesting that the long-term risk of cardiovascular events is associated with AKI itself.
A new category of AKI diagnosed by elevations of tubular damage biomarkers alone [e.g., neutrophil gelatinase-associated lipocalin (NGAL) also known as lipocalin 2], which might evolve into a clinically manifest syndrome characterized by a rise in serum creatinine levels and a decrease in estimated glomerular filtration rate (eGFR), has been described and termed subclinical AKI [40]. In a pooled data analysis from 2 322 critically ill patients, increased urine or plasma NGAL detects patients with likely subclinical AKI who have an increased risk of adverse outcomes including need for renal replacement therapy (primary endpoint), hospital mortality, their combination and duration of stay in intensive care and in-hospital, in absence of elevated serum creatinine [41]. As increased urinary or blood NGAL levels are an independent risk factor for future CHF and atherosclerotic coronary disease [42], the link between clinically overt AKI and cardiac dysfunction might also extend to subclinical AKI.
Therefore, nephrologists must be aware that AKI is associated not only with increased risk of CKD and ADHF but also with increased risk of future long-term adverse cardiovascular sequelae, especially CHF. These sequelae lead to other complications and poor outcomes, independent of or intertwined with the risks associated with the development of CKD [43].
COMPLEXITY OF MECHANISMS IN RENOCARDIAC SYNDROMES
Recent research has illustrated the growing complexity of the interrelated cellular and molecular mechanisms underlying chronic and acute renocardiac syndromes.
Insights from experimental models
Animal models have been established that induce primary renal damage/dysfunction and allow the assessment of the impact of kidney injury on the initiation and development of cardiac acute and chronic alterations simulating human renocardiac syndromes [44] (Table 1).
Table 1.
Some examples of candidates identified as potential mediators and/or biomarkers of the mechanisms involved in renocardiac syndromes
| Type of observation | Type of RCS | Potential mediators | References |
|---|---|---|---|
| Experimental | Chronic | microRNA-21 | [47] |
| TGF-β1 | [48] | ||
| Renal sympathetic nerve activity | [49] | ||
| TWEAK-Fn14 | [50, 51] | ||
| α-Klotho | [52] | ||
| Acute | Toll-like receptors 2 and 4 | [53] | |
| Dynamin-related protein-1 | [54] | ||
| TWEAK-Fn14 | [55, 56] | ||
| “Omics” | Chronic | Indoxyl sulfate, p-cresyl sulfate | [57] |
| TMAO | [58, 59] | ||
| FGF23 | [57] | ||
| Acute | ADMA | [60] | |
| FGF23 | [61] | ||
| Clinical | Chronic | CRP, IL-6, IL-1β | [62, 63] |
| PICP, CITP:MMP-1 | [64] | ||
| α-Klotho | [65, 66] | ||
| Soluble TWEAK | [67, 68] | ||
| Acute | High blood pressure | [69] |
Abbreviations: ADMA, asymmetric dimethylarginine; CRP, C reactive protein; FGF23, fibroblast growth factor 23; Fn14, fibroblast growth factor-inducible 14; IL-1β, interleukin-1β; IL-6, interleukin-6; PICP, C-terminal propeptide of procollagen type I; CITP:MMP-1, C-terminal telopeptide of procollagen type I to matrix metalloproteinase-1 ratio; RCS, renocardiac syndrome; TGF-β, transforming growth factor-β; TMAO, trimethylamine-N-oxide; TWEAK, tumor necrosis factor-like weak inducer of apoptosis.
The most widely used model of severe CKD is induced by subtotal nephrectomy (STNx) consisting of complete removal of one kidney and upper and lower pole resections of the remnant kidney. STNx-induced CKD was associated with uremic cardiomyopathy, characterized by adverse LV remodeling, and enhanced susceptibility to myocardial ischemia [45, 46]. Of note, an excess of cardiac microRNA-21 has been reported in STNx rats and microRNA-21 inhibition prevented LV remodeling, through changes in the peroxisome proliferator-activated receptor-α signaling pathway [47].
Effects of mild-to-moderate CKD on the heart have been investigated in a unilateral nephrectomy model by which one kidney is removed, whereas the contralateral kidney is left intact. Unilateral nephrectomy caused early LV microscopic remodeling (i.e., apoptosis and fibrosis) with mild LVH and LV diastolic dysfunction, which later progressed to LV dilatation and a reduction in LV ejection fraction [48]. Changes in genes related to transforming growth factor-β1 and apoptosis pathways in the heart were involved in this kidney–heart interaction in mild-to-moderate CKD [48]. Whether these changes also occur in the remaining kidney from living kidney donors remains to be investigated.
Recent findings obtained in an animal model of CKD due to adenine show that renal dysfunction is associated with left atrial dilation, hyperinnervation, fibrosis and arrhythmogenesis, which are attenuated by renal denervation [49]. While they do not indicate causality, they support the implication of kidney-mediated sympathetic overactivity in left atrial structural and electrical remodeling and in the pathogenesis of AF. Furthermore, these observations add experimental support to the ERADICATE-AF study [70], a single blind randomized clinical trial that demonstrated improved freedom from atrial arrhythmias at 12 months when renal denervation was added to catheter ablation of AF.
Warm ischemia–reperfusion is the most widely used model of hypoxia-induced AKI and is characterized by an abrupt decline in renal function and severe injury in the straight segment of proximal tubules. In this model, kidney injury caused LV macroscopic (i.e., hypertrophy and dilation) and myocardial microscopic (i.e., apoptosis) remodeling accompanied by impairment of LV systolic function [71]. Pathways linked to Toll-like receptors 2 and 4 [53] and maladaptive mitochondrial dynamics mediated by dynamin-related protein-1 [54] may be critically involved in these alterations.
There is also the possibility that some cardiac damaging mechanisms act in conditions of either CKD or AKI, depending on the timing and duration of their actions. In this regard, the potential pathogenic role of the cytokine tumor necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor, fibroblast growth factor inducible 14 (Fn14) signaling in cardiac and vascular injury accompanying CKD and AKI deserves some attention. The TWEAK–Fn14 axis promotes tissue (either renal, vascular or cardiac) remodeling such as apoptosis, inflammation and fibrosis, while restraining the expression of tissue protective factors such as the antiaging factor α-Klotho and the master regulator of mitochondrial biogenesis peroxisome proliferator-activated receptor-γ coactivator-1α [72]. Increased tissue expression and activity of Fn14 and, to a lesser extent, TWEAK, have been reported both in experimental CKD [50, 51] and AKI [55, 56] and shown to lead to decreased α-Klotho [52]. Kidney α-Klotho is also lost very early in the course of CKD as, in addition to inflammation, albuminuria itself downregulates α-Klotho before glomerular filtration rate decreases [73].
Insights from systems medicine studies
Limited data exist on genomics, epigenomics, transcriptomics, proteomics and metabolomics in the setting of renocardiac syndromes [74]. However, some examples may illustrate the potential of systems medicine (also termed “omics”) studies to gain insight into the heart–kidney interactions in CRS and, more specifically, to identify novel mediators and/or biomarkers of these interactions (Table 1).
Recent findings derived from “multi-omics” approaches have provided a deeper insight into the pathogenesis and diagnosis of CKD-related atherosclerosis beyond traditional and nontraditional risk factors [75]. Combined metabolomics and proteomics approaches added a piece of a puzzle to the knowledge of atherosclerotic endothelial dysfunction in CKD and mainly attributed to inflammation and oxidative stress [57]. The relationship between indoxyl sulfate and p-cresyl sulfate with 181 cardiovascular-related proteins involved in endothelial dysfunction and inflammation was recently analyzed in patients on dialysis [57]. Both metabolites were positively associated with the increased risk of atherosclerotic events and with fibroblasts growth factor 23 (FGF23), a factor produced in bones that participates in the maintenance of mineral homeostasis regulating phosphaturia through the interaction with α-Klotho–FGF receptor complexes expressed in renal tubule cells [76]. However, FGF23 also exerts direct actions on the cardiovascular system. For instance, FGF23 experimentally impairs endothelial function apparently via activation of a FGF receptor-dependent, α-Klotho-independent signaling pathway resulting in oxidative stress [77]. Plasma FGF23 is increased in CKD patients due to a maladaptive compensatory response to acquired α-Klotho deficiency, and is associated with atherosclerotic (e.g., ischemic events) and non-atherosclerotic (e.g., CHF) complications in this patient population [78, 79].
In a proteomics proof-of-concept study, known biomarkers for AKI were integrated to underlying disease conditions in pathway and protein interaction analyses [61]. Both a GeneMania network analysis and a term cluster analysis of AKI-modulated molecules allowed to identify AKI biomarker patterns for molecular pathways potentially involved in extrarenal damage (e.g., cardiac damage). One of these molecular pathways is related to FGF23 that induces LVH and myocardial fibrosis in animals apparently through a FGF receptor-dependent, α-Klotho-independent mechanism resulting in activation of calcineurin-nuclear factor of activated T-cells and upregulation of active β-catenin and transforming growth factor-β1 [80, 81]. Of note, increased serum FGF23 is associated with LVH, LV dysfunction and incident HF in patients with CKD [82–84].
Metabolomics has identified an excess of trimethylamine-N-oxide as a predictor of cardiovascular events [85] and several groups have confirmed the association between trimethylamine-N-oxide and cardiovascular disease among individuals with CKD [58, 59, 86, 87]. Metabolomics also identified novel features of AKI. Increases in acylcarnitines and certain amino acids (methionine, homocysteine, pyroglutamate, asymmetric dimethylarginine and phenylalanine) and a reduction in serum levels of arginine and several lysophosphatidyl cholines were observed in patients with AKI compared with healthy subjects [60]. Of interest, several studies have demonstrated that asymmetric dimethylarginine is an important risk factor for the increase of cardiovascular diseases and CHF in CKD [88].
Insights from clinical observations
Several clinical observations are providing novel insight on the potential primary drivers of pathophysiology in renocardiac syndromes (Table 1).
Systemic inflammation is a key process in the pathophysiology of CKD with relevant involvement in cardiovascular complications [89–91]. In fact, inflammatory biomarkers [e.g., C reactive protein (CRP), interleukins-6 and -1β, and tumor necrosis factor-α] progressively increase as kidney function declines and predict cardiovascular events in CKD patients [62, 63]. In accordance, the CANTOS trial (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) focusing on 10 061 stable postmyocardial infarction patients with high high-sensitivity CRP levels demonstrated a benefit of inhibiting interleukin-1β with canakinumab on the incidence of cardiovascular events, which was larger in patients with estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 than in those with eGFR ≥60 mL/min/1.73 m2 [92]. Recently, two studies performed in stages 3–5 CKD patients [93] and patients on hemodialysis [94] demonstrated that blockade of interleukin-6 with ziltivekimab markedly reduced biomarkers of inflammation (e.g., CRP) relevant to atherosclerosis. In addition, ziltivekimab also reduced a biomarker of thrombosis [93] and erythropoiesis-stimulating agent requirements and increased serum albumin [94].
Myocardial fibrosis is a frequent finding in endomyocardial biopsies and necropsy studies in patients with CKD [95, 96]. Interestingly, recent studies have identified causal connections between CKD and myocardial fibrosis [97]. Furthermore, it has been proposed that myocardial fibrosis may play a key role in the development and progression of CHF in CKD patients [98]. Of notice, CKD patients with CHF exhibit a pattern of myocardial fibrosis circulating biomarkers that differs from non-CKD patients with CHF, and characterized by increased C-terminal propeptide of procollagen type I and by a low C-terminal telopeptide of collagen type I to matrix metalloproteinase type 1 ratio [64]. This pattern is thought to reflect extensive deposition of highly stiff collagen type I fibers and is associated with severe adverse macroscopic LV remodeling [64].
It has been proposed that AKI induces structural cardiac damage characterized by myocardial inflammation and cellular apoptosis and necrosis developing within days and myocardial fibrosis developing months or years later [99]. Of note, analyses of claims databases show that patients with AKI are more likely than patients without AKI to develop subsequent hypertension [69]. Chronic pressure overload associated to systemic hypertension is a major inducer of myocardial microscopic adverse remodeling (namely, fibrosis) associated with CHF [100].
Data suggest that FGF23 excess might be future sensitive and specific marker for cardiovascular disease associated with both CKD and AKI. It is not clear, however, that this is the case for its co-receptor α-Klotho. Currently, CKD is considered as a state of α-Klotho deficiency [101] and results from animal experiments showed that α-Klotho deficiency causes vascular calcification, and cardiac hypertrophy and fibrosis [102–104]. Furthermore, intravenous delivery of a transgene encoding soluble α-Klotho ameliorated cardiac hypertrophy in CKD mice with Klotho deficiency [104]. Circulating levels of soluble α-Klotho (which results either from the release of the extracellular domain of membrane α-Klotho after cleavage by the A Disintegrin and metalloproteinases 10 and 17 or by alternative splicing) are directly correlated with eGFR and inversely correlated with circulating FGF23 in CKD patients [105]. Interestingly, a recent clinical study in subjects with established cardiovascular disease and preserved renal function showed that lower soluble α-Klotho levels are associated with a proinflammatory status [65]. Moreover, lower soluble α-Klotho levels are independently associated with subclinical atherosclerosis in patients with moderate-to-severe CKD [66]. However, a cohort study of 444 patients with CKD stages 2–4 showed that plasma-soluble α-Klotho did not predict atherosclerotic events, CHF or cardiovascular death after 2.6 years of follow-up [106]. Further studies are required to elucidate the role of α-Klotho in cardiovascular disease that develops in kidney disease. In this regard, technology to assess α-Klotho levels should be further optimized [107].
Low circulating soluble TWEAK, which results from the proteolytic processing of the full-length protein by furin, is independently associated with both atherosclerosis burden [67] and progression [68] in CKD patients. This is consistent with the development of hypersensitivity to TWEAK related to increased cell membrane Fn14 [108]. Indeed, Fn14 upregulation during tissue stress or injury is the main mechanism driving TWEAK–Fn14 signaling. Thus, decreased soluble TWEAK levels may reflect activation of the TWEAK–Fn14 axis, analogous to low complement levels reflecting complement activation. Alternatively, soluble TWEAK may also bind to the scavenger receptor CD163, which might be a compensatory mechanism to protect from excessive TWEAK–Fn14 signaling [108]. More research is necessary to characterize and clinically validate soluble TWEAK as a biomarker of cardiovascular risk in CKD patients.
ADDRESSING THE CHALLENGE OF RENOCARDIAC SYNDROMES
The aspects developed in the two preceding sections are just some examples supporting the concept that a new comprehensive approach to renocardiac syndromes in particular, and likely to CRS in general, warrants a subspecialty that combines scientific knowledge and clinical skills from both nephrology and cardiology (i.e., cardionephrology) [109–111] and that is developed in a physical and organizational context of multidisciplinarity (i.e., cardiorenal units) [112, 113].
Aspects related to cardionephrology
There is an extensive relationship between nephrology and cardiology in a variety of aspects, including epidemiology, risk factors, pathophysiology, diagnosis, prognosis, prevention, treatment, monitoring and research, that involve both the kidney and the heart in cardiorenal patients, particularly in those presenting with renocardiac syndromes (Table 2) [114]. The subspecialty of cardionephrology is aimed at the study of the multidirectional interplay of kidney and heart disease from all these standpoints to provide high-quality care in the vulnerable cardiorenal population [114].
Table 2.
Some examples of aspects related to renocardiac syndromes that remain to be investigated and developed in the context of cardionephrology (adapted from Hatamizadeh [114])
| Aspects | Examples |
|---|---|
| Epidemiology | High prevalence of CV-related conditions beyond CHF or ADHF in CKD and AKI patients, respectively |
| Risk factors | Common classical and emerging risk factors to CKD and chronic CV disease, and to AKI and acute CV disease |
| Pathophysiology | Emerging pathogenic connections between the kidney and the heart when CKD or AKI are present |
| Diagnosis | Interference of CKD or AKI on clinical presentation, and indication and interpretation of biomarkers of CV injury and/or dysfunction |
| Prognosis | Influence of coexisting kidney and CV injury/dysfunction on mutual worsening function and clinical outcomes |
| Prevention | Modified prophylactic targets of CV disease when CKD or AKI are the initiating conditions |
| Treatment | Interference of CKD or AKI on the indication of certain modalities of CV therapy |
| Monitoring | Influence of CKD and AKI on follow-up strategies of associated chronic and acute CV complications |
| Research | Identify differential phenotypes of renocardiac syndromes using personalized medicine-based approaches |
Abbreviations: ADHF, acute decompensated HF; AKI; acute kidney injury; CHF, chronic heart failure; CKD, chronic kidney disease; CV, cardiovascular.
As is illustrated by renocardiac syndromes, the interactions between nephrology and cardiology are broad, complex, and include subtleties that are not routinely discussed in either nephrology or cardiology [111]. Any nephrologist or cardiologist should be familiar with those topics, and a cardionephrologist must master them. Cardionephrologists should also lead additional translational research to further discover the extent of those interactions and to establish the optimal clinical approaches to those complexities.
In this regard, it is relevant to emphasize that although the field of oncology has made significant steps toward individualized precision medicine, cardiology and nephrology still often use a “one size fits all” approach. This applies to the intersection of the heart–kidney interaction and the CRS as well [115]. As reviewed here, the pathophysiologic and clinical heterogeneity of renocardiac syndromes is so extensive that more research is needed to bring precision medicine into routine clinical practice for the care of patients with CRS.
Aspects related to cardiorenal units
The clinical rationale of the cardiorenal units is to provide coordinated multidisciplinary care for patients hospitalized with concomitant kidney and heart disease, thereby improving patient outcomes and optimizing utilization of resources. Preliminary data recently published on the impact of a cardiorenal unit on the clinical course and outcomes of patients with AKI and ADHF are encouraging, although they need to be verified in larger series of patients and over a longer period of follow-up [116].
Inpatient cardiorenal units provide support for nephrologists and cardiologists on regular medical floors, telemetry units and intensive care units. Cardiorenal units should allow for a more consistent dialogue between the two specialties, thus providing nephrologists and cardiologists with the clinical and educational environment and activities that allow one to gain substantial experience in solving cardiovascular and renal problems in cardiorenal patients.
In addition, cardiorenal units should contribute to building a foundation to advance research in CRS in general and renocardiac syndromes in particular. Collecting longitudinal electronic medical record data on cardiorenal patients and recruiting directly these patients into clinical research studies are the cornerstones of the scientific component of cardiorenal units. In this regard, it is mandatory to remember that there is an unmet need for evidence-based therapy for patients with chronic renocardiac syndrome, particularly for those with advanced CKD and CHF [117].
CONCLUSIONS
The time has come to move from a taxonomic approach to the concurrence of kidney and heart diseases to a broader approach based on the view of patients primarily diagnosed with either kidney disease or heart disease as cardiorenal patients. This means moving from the limited opportunities for diagnosis and treatment offered by the CRS classification to the growing possibilities for knowledge and clinical development inherent in the subspecialty of cardionephrology.
That said, nephrologists (and cardiologists) should always be grateful to the pioneers who coined and developed the classification of renocardiac syndromes (and other CRS) because thanks to them, today we can realistically consider the new subspecialty of cardionephrology aimed at stimulating us as clinicians and scientists and, above all, to improve the care, prognosis and quality of life of cardiorenal patients.
Contributor Information
Borja Quiroga, IIS-La Princesa, Nephrology Department, Hospital Universitario de la Princesa, Madrid, Spain.
Alberto Ortiz, Division of Nephrology IIS-Fundacion Jimenez Diaz, Department of Medicine, Universidad Autónoma de Madrid, Madrid, Spain; RICORS2040, Carlos III Institute of Health, Madrid, Spain.
Juan F Navarro-González, RICORS2040, Carlos III Institute of Health, Madrid, Spain; Division of Nephrology and Research Unit, University Hospital Nuestra Señora de Candelaria, and University Institute of Biomedical Technologies, University of La Laguna, Santa Cruz de Tenerife, Spain.
Rafael Santamaría, RICORS2040, Carlos III Institute of Health, Madrid, Spain; Division of Nephrology, University Hospital Reina Sofia, Cordoba, Spain; Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Cordoba, Spain.
Patricia de Sequera, Department of Nephrology, University Hospital Infanta Leonor, University Complutense of Madrid, Madrid, Spain.
Javier Díez, Center of Applied Medical Research and School of Medicine, University of Navarra, Pamplona, Spain; Centro de Investigación Biomédica en Red de la Enfermedades Cardiovasculares (CIBERCV), Carlos III Institute of Health, Madrid, Spain.
AUTHORS’ CONTRIBUTIONS
B.Q., A.O. and J.D. developed the concept and design of the manuscript, and J.D. drafted and wrote it. J.F.N.-G, R.S. and P.S. revised and edited the manuscript. All authors approved the final version.
CONFLICT OF INTEREST STATEMENT
B.Q. has received honoraria for conferences, consulting fees and advisory boards from Vifor Pharma, Astellas, Amgen, Bial, Ferrer, Novartis, AstraZeneca, Sandoz, Esteve, Sanofi-Genzyme and Otsuka. A.O. has received consultancy or speaker fees or travel support from AstraZeneca, Amicus, Amgen, Fresenius Medical Care, Bayer, Sanofi-Genzyme, Menarini, Kyowa Kirin, Alexion, Otsuka and Vifor Fresenius Medical Care Renal Pharma, and is Director of the Cátedra Mundipharma-UAM of diabetic kidney disease and the Cátedra AstraZeneca–UAM of chronic kidney disease and electrolytes. A.O. is the until recently was the Editor-in-Chief of CKJ. J.F.N.-G. has served as a consultant and has received speaker fees or travel support from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Esteve, Eli Lilly, MSD, Mundipharma, Novartis, NovoNordisk, Sanofi-Genzyme, Servier, Shire and Vifor Fresenius Medical Care Renal Pharma. R.S. has received consultancy or speaker fees or travel support from AstraZeneca, Vifor Fresenius Medical Care Renal Pharma and Boehringer Ingelheim. P.S. has received consultancy or speaker fees or travel support from Vifor Pharma, Amgen, Fresenius, AstraZeneca, Nipro, Alexion, Astellas, Sandoz, Braun and Baxter. J.D. has received consultancy or speaker fees or travel support from AstraZeneca, Bayer and Vifor Pharma. This article has not been published previously in whole or part.
REFERENCES
- 1. Ronco C, McCullough P, Anker SDet al. Cardio-renal syndromes: report from the Consensus Conference of the Acute Dialysis Quality Initiative. Eur Heart J 2010; 31: 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rangaswami J, Bhalla V, Blair JEAet al. Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation 2019; 139: e840–e878 [DOI] [PubMed] [Google Scholar]
- 3. Zannad F, Rossignol P.. Cardiorenal syndrome revisited. Circulation 2018; 138: 929–944 [DOI] [PubMed] [Google Scholar]
- 4. House AA, Wanner C, Sarnak MJet al. Heart failure in chronic kidney disease: conclusions from a kidney disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 95: 1304–1317 [DOI] [PubMed] [Google Scholar]
- 5. Schefold JC, Filippatos G, Hasenfuss Get al. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol 2016; 12: 610–623 [DOI] [PubMed] [Google Scholar]
- 6. O'Leary JM, Assad TR, Xu Met al. Pulmonary hypertension in patients with chronic kidney disease: invasive hemodynamic etiology and outcomes. Pulm Circ 2017; 7: 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sise ME, Courtwright AM, Channick RN.. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int 2013; 84: 682–692 [DOI] [PubMed] [Google Scholar]
- 8. Fortuni F, Butcher SC, Dietz MFet al. Right ventricular-pulmonary arterial coupling in secondary tricuspid regurgitation. Am J Cardiol 2021; 148: 138–145 [DOI] [PubMed] [Google Scholar]
- 9. Sanz J, Damián Sánchez-Quintana D, Bossone Eet al. Anatomy, function, and dysfunction of the right ventricle. JACC State-of-the-Art Review. J Am Coll Cardiol 2019; 73: 1463–1482 [DOI] [PubMed] [Google Scholar]
- 10. Butcher SC, Fortuni F, Dietz MFet al. Renal function in patients with significant tricuspid regurgitation: pathophysiological mechanisms and prognostic implications. J Intern Med 2021; 290: 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bello AK, Alrukhaimi M, Ashuntantang GEet al. Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl 2017; 7: 122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levin A, Tonelli M, Bonventre Jet al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–1917 [DOI] [PubMed] [Google Scholar]
- 13. Go AS, Chertow GM, Fan Det al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 14. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. United States Renal Data System . USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 16. Valdivieso JM, Rodriguez-Pujol D, Pascual Jet al. Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol 2019; 39: 1938–1966 [DOI] [PubMed] [Google Scholar]
- 17. Schlieper G, Schurgers L, Brandenburg Vet al. Vascular calcification in chronic kidney disease: an update. Nephrol Dial Trasplant 2016; 31: 31–39 [DOI] [PubMed] [Google Scholar]
- 18. Herzog CA, Asinger RW, Berger AKet al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011; 80: 572–586 [DOI] [PubMed] [Google Scholar]
- 19. Konst RE, Guzik TJ, Kaski J-Cet al. The pathogenic role of coronary microvascular dysfunction in the setting of other cardiac or systemic conditions. Cardiovasc Res 2020: 116: 817–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chade AR, Brosh D, Higano STet al. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int 2006; 69: 266–271 [DOI] [PubMed] [Google Scholar]
- 21. Nelson AJ, Dundon BK, Worthley SGet al. End-stage renal failure is associated with impaired coronary microvascular function. Coron Artery Dis 2019; 30: 520–527 [DOI] [PubMed] [Google Scholar]
- 22. Charytan DM, Skali H, Shah NRet al. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int 2018; 93: 501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah NR, Charytan DM, Murthy VLet al. Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol 2016; 27: 1823–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baumgartner H, Falk V, Bax JJet al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791 [DOI] [PubMed] [Google Scholar]
- 25. Urena-Torres P, D'Marco L, Raggi Pet al. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dial Transplant 2019; 1: 1–8 [DOI] [PubMed] [Google Scholar]
- 26. Wang Z, Jiang A, Wei Fet al. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: a meta-analysis. BMC Cardiovasc Disord 2018; 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Ding X-H, Pang Fet al. The prevalence and independent risk factors of significant tricuspid regurgitation jets in maintenance hemodialysis patients with ESRD. Front Physiol 2020; 11: 568812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turakhia MP, Blankestijn PJ, Carrero J-Jet al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 2018; 39: 2314–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yew WY, Gupta D, Wong CFet al. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc Res 2021; 117: 1046–1059 [DOI] [PubMed] [Google Scholar]
- 30. Wan C, Herzog CA, Zareba Wet al. Sudden cardiac arrest in hemodialysis patients with wearable cardioverter defibrillator. Ann Noninvasive Electrocardiol 2014; 19: 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong MC, Kalman JM, Pedagogos Eet al. Temporal distribution of arrhythmic events in chronic kidney disease: highest incidence in the long interdialytic period. Heart Rhythm 2015; 12: 2047–2055 [DOI] [PubMed] [Google Scholar]
- 32. Silver SA, Harel Z, McArthur Eet al. 30-Day readmissions after an acute kidney injury hospitalization. Am J Med 2017; 130: 163.e4–172.e4 [DOI] [PubMed] [Google Scholar]
- 33. Go AS, Hsu C-Y, Yang Jet al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol 2018; 13: 833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Odutayo A, Wong CX, Farkouh Met al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 2017; 28: 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gallagher M, Cass A, Bellomo Ret al. Long-term survival and dialysis dependency following acute kidney injury in intensive care: extended follow-up of a randomized controlled trial. PLoS Med 2014; 11: e1001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Linder A, Fjell C, Levin Aet al. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med 2014; 189: 1075–1081 [DOI] [PubMed] [Google Scholar]
- 37. Hsu C, Chertow GM, McCulloch CEet al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol 2009; 4: 891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bansal N, Matheny ME, Greevy RA Jret al. Acute kidney injury and risk of incident heart failure among US veterans. Am J Kidney Dis 2018; 71: 236–245 [DOI] [PubMed] [Google Scholar]
- 39. Tecson KM, Hashemi H, Afzal Aet al. Community acquired acute kidney injury as a risk factor of de novo heart failure hospitalization. Cardiorenal Med 2019; 9: 252–260 [DOI] [PubMed] [Google Scholar]
- 40. Haase M, Kellum JA, Ronco C.. Subclinical AKI-an emerging syndrome with important consequences. Nat Rev Nephrol 2012; 8: 735–739 [DOI] [PubMed] [Google Scholar]
- 41. Haase M, Devarajan P, Haase-Fielitz Aet al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury. J Am Coll Cardiol 2011; 57: 1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sivalingam Z, Larsen SB, Grove ELet al. Neutrophil gelatinase-associated lipocalin as a risk marker in cardiovascular disease. Clin Chem Lab Med 2017; 56: 5–18 [DOI] [PubMed] [Google Scholar]
- 43. Bansal N, Zelnick L, Bhat Zet al. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol 2019; 73: 2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu S. Heart-kidney interactions: mechanistic insights from animal models. Am J Physiol Renal Physiol 2019; 316: F974–F985 [DOI] [PubMed] [Google Scholar]
- 45. Lekawanvijit S, Kompa AR, Manabe Met al. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a nondialysable uremic toxin, indoxyl sulfate. PLoS One 2012; 7: e41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Song Y, Yu Q, Zhang Jet al. Increased myocardial ischemia-reperfusion injury in renal failure involves cardiac adiponectin signal deficiency. Am J Physiol Endocrinol Metab 2014; 306: E1055–E1064 [DOI] [PubMed] [Google Scholar]
- 47. Chuppa S, Liang M, Liu Pet al. MicroRNA-21 regulates peroxisome proliferator-activated receptor-α, a molecular mechanism of cardiac pathology in cardiorenal syndrome type 4. Kidney Int 2018; 93: 375–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin FL, McKie PM, Cataliotti Aet al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am J Physiol Regul Integr Comp Physiol 2012; 302: R292–R299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hohl M, Selejan SR, Wintrich Jet al. Renal denervation prevents atrial arrhythmogenic substrate development in CKD. Circ Res 2022; 130: 814–828 [DOI] [PubMed] [Google Scholar]
- 50. Muñoz-García B, Moreno JA, López-Franco Oet al. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) enhances vascular and renal damage induced by hyperlipidemic diet in ApoE-knockout mice. Arterioscler Thromb Vasc Biol 2009; 29: 2061–2068 [DOI] [PubMed] [Google Scholar]
- 51. Ucero AC, Benito-Martin A, Fuentes-Calvo Iet al. TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim Biophys Acta 2013; 1832: 1744–1755 [DOI] [PubMed] [Google Scholar]
- 52. Moreno JA, Izquierdo MC, Sanchez-Niño MDet al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 2011; 22: 1315–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trentin-Sonoda M, da Silva RC, Kmit FVet al. Knockout of Toll-like receptors 2 and 4 prevents renal ischemia-reperfusion-induced cardiac hypertrophy in mice. PLoS One 2015; 10: e0139350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sumida M, Doi K, Ogasawara Eet al. Regulation of mitochondrial dynamics by dynamin-related protein-1 in acute cardiorenal syndrome. J Am Soc Nephrol 2015; 26: 2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sanz AB, Justo P, Sanchez-Niño MDet al. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 2008; 19: 695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hotta K, Sho M, Yamato Iet al. Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int 2011; 79: 179–188 [DOI] [PubMed] [Google Scholar]
- 57. Wu P-H, Lin Y-T, Chiu Y-Wet al. The relationship of indoxyl sulfate and p-cresyl sulfate with target cardiovascular proteins in hemodialysis patients. Sci Rep 2021; 11: 3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stubbs JR, House JA, Ocque AJet al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 2016; 27: 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim RB, Morse BL, Djurdjev Oet al. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int 2016; 89: 1144–1152 [DOI] [PubMed] [Google Scholar]
- 60. Sun J, Shannon M, Ando Yet al. Serum metabolomic profiles from patients with acute kidney injury: A pilot study. J Chromatogr B Analyt Technol Biomed Life Sci 2012; 893-894: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marx D, Metzger J, Pejchinovski Met al. Proteomics and metabolomics for AKI diagnosis. Semin Nephrol 2017; 38: 63–87 [DOI] [PubMed] [Google Scholar]
- 62. Kielstein JT, Veldink H, Martens-Lobenhoffer Jet al. Unilateral nephrectomy causes an abrupt increase in inflammatory mediators and a simultaneous decrease in plasma ADMA: a study in living kidney donors. Am J Physiol Renal Physiol 2011; 301: F1042–F1046 [DOI] [PubMed] [Google Scholar]
- 63. Amdur RL, Feldman HI, Dominic EAet al. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: fiindings from the CRIC Study. Am J Kidney Dis 2019; 73: 344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eiros R, Romero-González G, Gavira JJet al. Does chronic kidney disease facilitate malignant myocardial fibrosis in heart failure with preserved ejection fraction of hypertensive origin? J Clin Med 2020; 9: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martín-Núñez E, Donate-Correa J, Ferri Cet al. Association between serum levels of Klotho and inflammatory in cardiovascular disease: a case-control study. Aging (Albany NY) 2020; 12: 1952–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Donate-Correa J, Ferri CM, Martín-Núñez Eet al. Klotho as a biomarker of subclinical atherosclerosis in patients with moderate to severe chronic kidney disease. Sci Rep 2021; 11: 15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Valdivielso JM, Coll B, Martín-Ventura JLet al. Soluble TWEAK is associated with atherosclerotic burden in patients with chronic kidney disease. J Nephrol 2013; 26: 1105–1113 [DOI] [PubMed] [Google Scholar]
- 68. Fernández-Laso V, Menéndez-Barbero N, Valdivielso JMet al. Soluble TWEAK and atheromatosis progression in patients with chronic kidney disease. Atherosclerosis 2017; 260: 130–137 [DOI] [PubMed] [Google Scholar]
- 69. Hsu C, Hsu RK, Yang Jet al. Elevated BP after AKI. J Am Soc Nephrol 2016; 27: 914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steinberg JS, Shabanov V, Ponomarev Det al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA 2020; 323: 248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 2003; 14: 1549–1558 [DOI] [PubMed] [Google Scholar]
- 72. Blanco-Colio LM. TWEAK/Fn14 axis: A promising target for the treatment of cardiovascular diseases. Front Immunol 2014; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fernandez-Fernandez B, Izquierdo MC, Valiño-Rivas Let al. Albumin downregulates Klotho in tubular cells. Nephrol Dial Transplant 2018; 33: 1712–1722 [DOI] [PubMed] [Google Scholar]
- 74. Virzì GM, Clementi A, Battaglia GGet al. Multi-omics approach: New potential key mechanisms implicated in cardiorenal syndromes. Cardiorenal Med 2019; 9: 201–211 [DOI] [PubMed] [Google Scholar]
- 75. Tracz J, Luczak M.. Applying proteomics and integrative “omics” strategies to decipher the chronic kidney disease-related aterosclerosis. Int J Mol Sci 2021; 22: 7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ho BB, Bergwitz C.. FGF23 signalling and physiology. J Mol Endocrinol 2021; 66: R23–R32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Silswal N, Touchberry CD, Daniel DRet al. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab 2014; 307: E426–E436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Seiler S, Reichart B, Roth Det al. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant 2010; 25: 3983–3989 [DOI] [PubMed] [Google Scholar]
- 79. Scialla JJ, Xie H, Rahman Met al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 2014; 25: 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Faul C, Amaral AP, Oskouei Bet al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hao H, Li X, Li Qet al. FGF23 promotes myocardial fibrosis in mice through activation of β-catenin. Oncotarget 2016; 7: 64649–64664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Faul C, Amaral AP, Oskouei Bet al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gruson D, Ferracin B, Ahn SAet al. Comparison of fibroblast growth factor 23, soluble ST2 and Galectin-3 for prognostication of cardiovascular death in heart failure patients. Int J Cardiol 2015; 189: 185–187 [DOI] [PubMed] [Google Scholar]
- 84. Poelzl G, Trenkler C, Kliebhan Jet al. FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur J Clin Invest 2014; 44: 1150–1158 [DOI] [PubMed] [Google Scholar]
- 85. Wang Z, Klipfell E, Bennett BJet al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tang WH, Wang Z, Kennedy DJet al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015; 116: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Missailidis C, Hallqvist J, Qureshi ARet al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One 2016; 11: e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu X, Xu X, Shang Ret al. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide 2018; 78: 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tinti F, Lai S, Noce Aet al. Chronic kidney disease as a systemic inflammatory syndrome: Update on mechanisms involved and potential treatment. Life (Basel) 2021; 11: 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zoccali C, Vanholder R, Massy ZAet al. The systemic nature of CKD. Nat Rev Nephrol 2017; 13: 344–358 [DOI] [PubMed] [Google Scholar]
- 91. Jankowski J, Floege J, Fliser Det al. Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation 2021; 143: 1157–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ridker PM, MacFadyen JG, Glynn RJet al. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol 2018; 71: 2405–2414 [DOI] [PubMed] [Google Scholar]
- 93. Ridker PM, Devalaraja M, Baeres FMMet al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021; 397: 2060–2069 [DOI] [PubMed] [Google Scholar]
- 94. Pergola PE, Devalaraja M, Fishbane Set al. Ziltivekimab for treatment of anemia of inflammation in patients on hemodialysis: Results from a phase 1/2 multicenter, randomized, double-blind, placebo-controlled trial. J Am Soc Nephrol 2021; 32: 211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kaesler N, Babler A, Floege Jet al. Cardiac remodeling in chronic kidney disease. Toxins (Basel) 2020; 12: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang X, Shapiro JI.. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol 2019; 15: 159–175 [DOI] [PubMed] [Google Scholar]
- 97. Hulshoff MS, Rath S, Xu Xet al. Causal connections from chronic kidney disease to cardiac fibrosis. Semin Nephrol 2018; 38: 629–636 [DOI] [PubMed] [Google Scholar]
- 98. Romero-González G, González A, López Bet al. Heart failure in chronic kidney disease: the emerging role of myocardial fibrosis. Nephrol Dial Transplant 2020; 37: 817–824 [DOI] [PubMed] [Google Scholar]
- 99. Legrand M, Rossignol P.. Cardiovascular consequences of acute kidney injury. N Engl J Med 2020; 382: 2238–2247 [DOI] [PubMed] [Google Scholar]
- 100. González A, Ravassa S, López Bet al. Myocardial remodeling in hypertension. Hypertension 2018; 72: 549–558 [DOI] [PubMed] [Google Scholar]
- 101. Zou D, Wu W, He Yet al. The role of klotho in chronic kidney disease. BMC Nephrol 2018; 19: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hu MC, Shi M, Zhang Jet al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011; 22: 124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hu MC, Shi M, Cho HJet al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol 2015; 26: 1290–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xie J, Yoon J, An SWet al. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 2015; 26: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang Q, Su W, Shen Zet al. Correlation between soluble α-Klotho and renal function in patients with chronic kidney disease: a review and meta-analysis. Biomed Res Int 2018; 2018: 9481475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Seiler S, Rogacev KS, Roth HJet al. Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2-4. Clin J Am Soc Nephrol 2014; 9: 1049–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Neyra JA, Moe OW, Pastor Jet al. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin Kidney J 2019; 13: 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Poveda J, Vázquez-Sánchez S, Sanz ABet al. TWEAK-Fn14 as a common pathway in the heart and the kidneys in cardiorenal syndrome. J Pathol 2021; 254: 5–19 [DOI] [PubMed] [Google Scholar]
- 109. Díez J, Ortiz A.. The need for a cardionephrology subspecialty. Clin Kidney J 2021; 14: 1491–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Burlacu A, McCullough PA, Covic A.. Cardionephrology from the point of view of the cardiologist: no more agree to disagree-getting to ‘yes’ for every patient. Clin Kidney J 2021; 14: 1995–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hatamizadeh P. Introducing nephrocardiology. Clin J Am Soc Nephrol 2022; 17: 311–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. De la Espriella R, González M, Górriz JLet al. Setting up a cardiorenal clinic. Consensus document of the cardiorenal working groups of the Spanish Society of Cardiology and the Spanish Society of Nephrology. CardioClinics 2021; 56: 284–295 [Google Scholar]
- 113. Uppal NN, Jhaveri KD.. Cardio-renal service, time for a change. Clin Kidney J 2021; 14: 2278–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hatamizadeh P. Introduction to nephrocardiology. Cardiol Clin 2021; 39: 295–306 [DOI] [PubMed] [Google Scholar]
- 115. Wettersten N, Maisel AS, Cruz DN.. Toward precision medicine in the cardiorenal syndrome. Adv Chronic Kidney Dis 2018; 25: 418–424 [DOI] [PubMed] [Google Scholar]
- 116. Bansal N, Arora N, Mariuma Det al. Mission and one-year outcomes of a cardio-renal subspecialty consultation service. Kidney360, 2022; 3: 749–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ortiz A, Navarro-González J, Núñez Jet al. The unmet need of evidence-based therapy for patients with advanced chronic kidney disease and heart failure: Position paper from the Cardiorenal Working Groups of the Spanish Society of Nephrology and the Spanish Society of Cardiology. Clin Kidney J 2021; 15: 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]