ABSTRACT
Since the breakthrough of kidney replacement therapy, increases in life expectancy for patients with end-stage kidney disease have been limited. However, patients have become increasingly vocal that, although mortality and life expectancy matter to them, the quality of their life, and particularly the relief of symptoms associated with their treatment, are in many cases more important. The majority of dialysis-associated symptoms and adverse effects do not currently have any approved treatments in this patient population, with the few treatments that are available used off-label, frequently without proven efficacy, yet still potentially adding further adverse effects to patients’ current symptom burden. This article will illustrate how understanding the pathophysiology of a single, particularly burdensome symptom of dialysis (chronic kidney disease-associated pruritus) resulted in the design, development and regulatory approval of a treatment for that symptom. The pathway described here can be applied to other symptoms associated with dialysis, meaning that if we cannot add years to patients’ lives, we can at least add life to their remaining years.
Keywords: chronic kidney disease-associated pruritus, dialysis, difelikefalin, quality of life, symptom relief
INTRODUCTION
This article will illustrate the design, development and regulatory approval of a treatment for a single, particularly burdensome symptom of dialysis [chronic kidney disease-associated pruritus (CKD-aP)]. This was achieved through acknowledging the importance of CKD-aP symptom management to patients and understanding the symptom's underlying pathophysiology. This enabled the development of a treatment to target CKD-aP, utilizing clinical trials specifically designed to measure the impact and improvement of CKD-aP. Patient-reported outcomes (PROs) were used in all trials, enabling regulators to assess the value of this treatment in this patient population and therefore resulting in regulatory approval—a key point in the pathway to patient access (Fig. 1) [1].
Figure 1:
Outline of the difelikefalin pathway, from symptom identification to regulatory approval.
SYMPTOM BURDEN AND ITS IMPORTANCE TO PATIENTS
Compared with the general population, the physical health-related quality of life (QoL) in patients with CKD [2] and in those with CKD on kidney replacement therapy [3] is substantially diminished; furthermore, the symptom burden is high [4, 5], with up to half of patients reporting at least one symptom as severe or overwhelming [6]. A growing recognition for the need to relieve symptoms in patients on dialysis offers an opportunity to address this in a scientifically robust way.
This lack of incorporation of patient priorities into treatment plans resulted in the development of the Standardized Outcomes in Nephrology (SONG) initiative [7], in which dialysis outcomes were aligned to the needs of all stakeholders (including patients, caregivers, clinicians, researchers and policy makers), with SONG-HD [8] and SONG-PD [9] focusing specifically on core outcomes relevant to hemodialysis (HD) and peritoneal dialysis (PD) stakeholders, respectively. Symptoms identified by SONG-HD as important include fatigue (a core outcome), depression, pain, anxiety, cramps, itching, nausea, restless legs syndrome, anemia, sexual function and sleep disturbance; the lack of food enjoyment, mobility, dialysis-free time or the ability to work or travel; as well as reduced cognitive function, the impact on family/friends, hospitalization and feeling washed out after diagnosis. Other initiatives, such as the Kidney Health Initiative [10], a public–private partnership between the American Society of Nephrology and US Food and Drug Administration, have also sought to develop a conceptual framework for symptom-based PROs, contributing to the development of PROs and electronic PROs (ePROs) to specifically assess HD treatment-related physical symptoms [11].
However, most dialysis-associated symptoms and adverse effects do not currently have any approved treatments in this patient population, resulting in a clear unmet need (Table 1). Health-related QoL in older or more frail patients has even been reported to be similar in patients who choose not to enter the dialysis pathway, due to the significant burden of symptoms associated with dialysis [12]. The clear exception to the unmet need of treatments for dialysis adverse effects and symptoms is CKD-aP (and the associated impact on sleep disturbance), for which a specific treatment has recently been approved [13].
Table 1:
Overview of bothersome symptoms associated with dialysis and current treatment options.
| Symptom | Treatment | Efficacy/safety | Approval/off-label for treatment of symptom? |
|---|---|---|---|
| Fatigue | Non-pharmacologic interventions: sleep hygiene, energy conservation, acupressure | Limited evidence of efficacy in small-scale studies [54] | NA |
| Pharmacologic interventions: hematopoietics, antidepressants, anxiolytics, levocarnitine, human growth hormone, more frequent dialysis | Hematopoietics and antidepressants show some efficacy in patients with underlying anemia or depression | Treatments approved for underlying conditions such as anemia and depression [54] | |
| Levocarnitine and human growth hormone have limited evidence of efficacy in small-scale studies | |||
| Increased dialysis frequency has demonstrated efficacy but also increases overall time on dialysis [54] | |||
| Depression | Psychotherapy | Some evidence of efficacy, although quality of evidence is low [55] | NA |
| SSRIs | Limited evidence of efficacy in the dialysis population [55] | Approved in general population | |
| Pain | Conservative management, e.g. exercise, massage, heat/cold therapy, cognitive behavioral therapy | Some evidence of efficacy, although quality of evidence is low [56] | NA |
| Analgesics: opioid analgesics are indicated if pain control is not optimal with other methods | Evidence of efficacy in the general population, limited evidence in dialysis populations [56] | Approved in general population | |
| Gabapentin/pregabalin | Demonstrated efficacy in several small, short-term randomized trials conducted in patients on HD [57] | Recommended for the treatment of neuropathic pain in patients with kidney failure [58] | |
| Associated with increased risk of mental state changes and falls [57] | |||
| Anxiety | Psychotherapy | Some evidence of efficacy [59] | NA |
| Pharmacologic agents, including SSRIs and benzodiazepines | Evidence of efficacy in the general population, limited evidence in dialysis populations | Approved in general population | |
| Treatment with benzodiazepines is not suitable for long-term treatment [59] | |||
| Cramps | Hypertonic solutions | Evidence of efficacy in patients on HD | NA |
| Mild post-dialysis hyperglycemia and hypernatremia have been reported [60] | |||
| Pharmacologic agents | Limited evidence of efficacy of quinine, vitamin E supplementation and L-carnitine [60, 61] | Off-label treatment | |
| Restless legs syndrome | Non-pharmacologic: exercise, near-infrared light, vibration and massage | Limited evidence of efficacy in small-scale studies [62] | NA |
| Pharmacologic: dopamine agonists, levodopa and iron supplements | Limited evidence of efficacy in small-scale studies [62] | Off-label treatment | |
| Parathyroidectomy | Limited evidence of efficacy in small-scale studies [62] | NA | |
| Nausea | Ondansetron, metoclopramide and haloperidol | Evidence of efficacy for uremia-associated nausea [63] | Approved in general population |
| Sleep disturbance | Non-pharmacologic: exercise and sleep hygiene | Evidence of efficacy in the general population, limited evidence in dialysis populations [64] | NA |
| Pharmacologic: treatment of underlying disorders, e.g. restless legs syndrome, pruritus or use of hypnotics | Evidence of efficacy in the general population, limited evidence in dialysis populations [64] | Several medications approved for insomnia in the general population—only eszopiclone is approved for longer-term use [64] | |
| Pruritus | Difelikefalin | Robust clinical efficacy and safety data from large well-designed Phase 3 RCTs [13, 46, 48] | Only treatment that is FDA-approved by regulatory authorities for treatment of CKD-aP |
| Gabapentinoids (pregabalin and gabapentin) | Effective for reduction of itch intensity | Not approved for CKD-aP, off-label treatment | |
| Risk of potentially serious adverse effects, particularly at higher doses, including altered mental status, falls and fractures [57] |
FDA, Food and Drug Administration; NA, not applicable; RCT, randomized controlled trial; SSRI, selective serotonin reuptake inhibitor.
OVERVIEW OF CKD-aP (EPIDEMIOLOGY, ETIOLOGY, UNMET TREATMENT NEED)
CKD-aP is a prevalent and distressing condition for patients with kidney failure undergoing dialysis [14]. Recent data from the international observational Dialysis Outcomes and Practice Patterns Study (DOPPS) (phases 4–6; 2009–18) found that 67% of HD patients surveyed reported that they were bothered by pruritus, with 37% reporting moderate to extreme symptoms [15].
Pruritus as a symptom of HD has a unique underlying etiology compared with pruritus in the general population, meaning that common interventions for pruritus, such as moisturizers and topical corticosteroids [16], are often ineffective in the patients with kidney failure [17].
CKD-aP can significantly reduce the QoL of patients undergoing HD and can inhibit their ability to work or maintain an active social life [14, 17]. It is also associated with worse clinical outcomes, including an increased risk of infections and a higher rate of hospitalizations and mortality [15]. However, despite these negative aspects, CKD-aP still remains under-recognized by physicians [17].
CKD-aP frequently co-occurs with other physical and psycho-emotional symptoms experienced by HD patients, including poor sleep quality, depression, fatigue and pain [17–19], which together represent an important symptom cluster that is inadequately managed in clinical practice [20].
PATHOPHYSIOLOGY OF CKD-aP—THE CENTRAL ROLE OF THE OPIOID PATHWAY
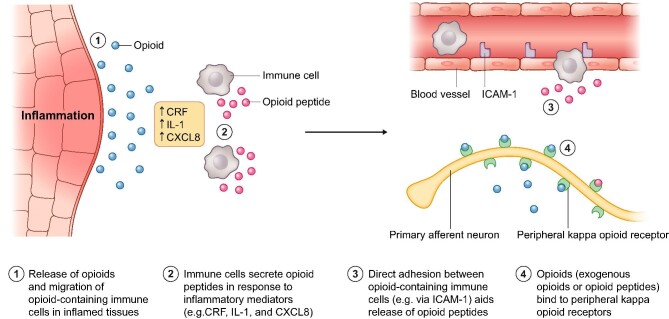
Understanding the pathophysiology of CKD-aP was the essential first step before therapies could be developed. Although the pathophysiology is not yet fully understood, a combination of several mechanisms appears to contribute to its occurrence (Fig. 2). These include the accumulation of uremic toxins in the skin and activation of the non-histaminergic itch pathway [21], as a result of peripheral neuropathy, immune system dysregulation and/or opioid imbalance, with subsequent microinflammation and xerosis [22–24].
Figure 2:
Pathophysiology of the itch mechanism through activation of kappa-opioid receptors in response to inflammation in CKD-aP. CRF, corticotropin-releasing factor; CXCL8, C-X-C motif chemokine ligand 8; ICAM-1, intercellular adhesion molecule 1; IL-1, interleukin-1.
Elucidation of each of these mechanisms has provided a potential treatment pathway to alleviate CKD-aP; however, for many years, treatments with sufficient efficacy remained elusive. For example, toxin accumulation and deposition are now thought to cause CKD-aP in only a subset of patients, because increasing dialysis efficiency (with resulting decreasing Kt/V) and reducing serum calcium, parathyroid hormone or phosphorus all alleviate itching in only a small proportion of patients [23].
Peripheral neuropathy has been demonstrated to cause itching when diseased neurons are activated independently to the presence of pruritogens, with peripheral neuropathy highly prevalent in dialysis patients [23]. However, large-scale clinical trials of treatments that reduce pain associated with peripheral neuropathy have yet to show clear efficacy in CKD-aP, and although they have shown some efficacy in small-scale trials [25], they may also be associated with neurological adverse effects [26].
Immune system dysregulation still remains as a potential modulator of CKD-aP, as increased levels of eosinophils, mast cells, histamine and tryptase have all been reported; however, anti-histamines have limited efficacy against pruritus [23]. It is recognized that inflammation plays a key role in sensitizing the small nerve fibers in the skin that carry the itching sensation to the brain, producing the uncomfortable symptom of itching. Furthermore, high levels of markers of systemic inflammation are observed in patients with CKD-aP, including high levels of T cells and white blood cells, C-reactive protein, interleukins-6 and -2, and ferritin, alongside low levels of albumin [27].
ROLE OF OPIOID RECEPTORS IN THE PATHOGENESIS OF ITCHING
Imbalance in the endogenous opioid system, characterized by overexpression of mu-opioid receptors (MOR) signaling and downregulation of kappa-opioid receptor (KOR) signaling, has been implicated in the pathogenesis of itch in CKD-aP [28]. Furthermore, MOR and KOR expression imbalance has been observed in the skin of patients with CKD-aP in comparison with those free from itch [29]. Several drugs that act on the MOR and/or KOR have been evaluated for the treatment of CKD-aP, such as naltrexone, loratadine and nalbuphine; however, in clinical trials these have mostly reported limited efficacy [30–34]. There are some promising studies with the partial MOR agonist and KOR agonist, nalfurafine hydrochloride, which at higher doses demonstrated significant reductions in itch intensity, as measured through the Worst Itch Numeric Rating Scale (WI-NRS) [34] and visual analogue scale [33]. Nalfurafine is currently approved for the treatment of CKD-aP in Japan and South Korea. The MOR and KOR pathways therefore remain a promising target for the systemic relief of CKD-aP, resulting in the development of difelikefalin.
THE DEVELOPMENT OF DIFELIKEFALIN
Difelikefalin is a selective, peripherally restricted KOR agonist recently approved in the USA and Europe for the treatment of moderate-to-severe pruritus in patients undergoing HD [13, 35]. Difelikefalin was developed as an analogue of an endogenous opioid peptide, Dynorphin A, which is known to be a neuromodulator of pruritus [36]. Similar to Dynorphin A and other KOR agonists, difelikefalin is thought to alleviate itch by activating KORs on peripheral sensory neurons and immune cells [36, 37]. In order to limit the potential for psychotomimetic and dysphoric effects observed with some other KOR agonists, the chemical structure of difelikefalin was chosen to ensure it was not a substrate for drug uptake transporters, and would not be significantly metabolized. The half-life of difelikefalin is 23–31 h in patients undergoing HD; plasma concentrations of difelikefalin were reduced by 70%–80% following HD, with difelikefalin undetectable in plasma following two dialysis cycles [38].
THE ASSESSMENT OF CKD-aP
Nephrologists are familiar with measurements of multiple variables tracked over time so they can accurately quantify disease progression. For example, we have measurements of kidney injury through urine albumin to urine creatinine ratio and measurement of kidney failure through measurement of estimated glomerular filtration rate, and together they are used to stage kidney disease. Such a staging system allows for the appropriate use of diagnostic procedures and the institution of treatments, and provides a prognosis of future progression of kidney disease and cardiovascular events in such patients. Conversely, symptoms have not been subjected to this scrutiny.
Because there is no test for a symptom chain, such as serum creatinine or a urine test, a tool is necessary to monitor the symptom over time. The clinical impact of CKD-aP can be quantified in two domains by the severity of the symptom and the impact of the symptom on the patient's wellbeing. The assessment of symptoms in a bidimensional framework—severity and impact—lends itself well to the examination of the response to therapy. Although many PROs have been tried to gauge the severity of CKD-aP, few have been rigorously tested, and using such a tool was the primary mechanism for regulatory approval.
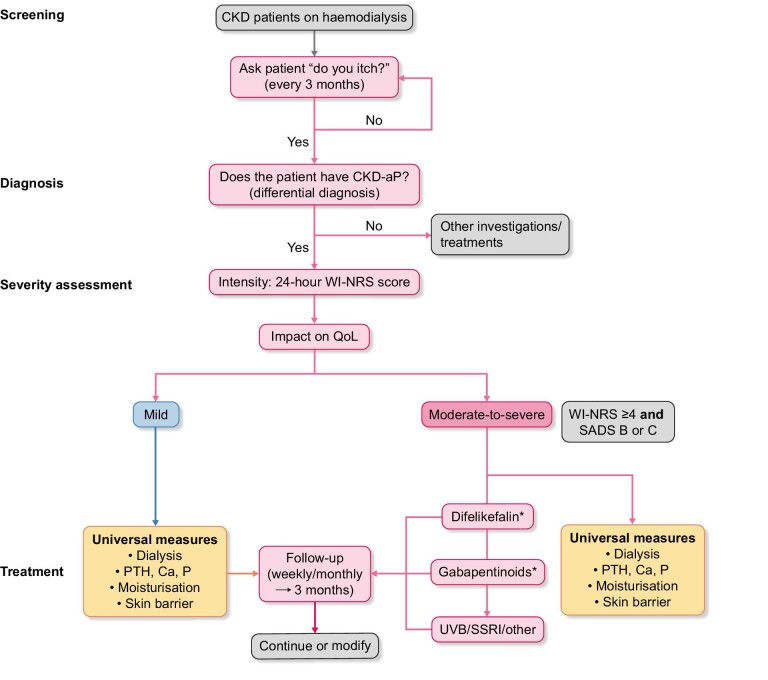
For patients diagnosed with CKD-aP, the assessment of pruritus severity should therefore incorporate an evaluation of both the level of itch intensity and the impact of itch on patient QoL (Fig. 3).
Figure 3:
Proposed algorithm for the screening, diagnosis, assessment and treatment of CKD-aP among patients undergoing HD. *Treatment selection dependent on the availability of difelikefalin. Ca, calcium; NRS, numerical rating scale; P, phosphorus; PTH, parathyroid hormone; SSRI, selective serotonin reuptake inhibitor; UVB, ultraviolet B; VRS, verbal rating scale.
QUANTIFYING ITCH INTENSITY AND THE EFFECT OF ITCHING ON A PATIENT'S QoL
Several validated unidimensional and multidimensional PRO scales are available for the assessment of itch intensity and itch-related QoL in patients with CKD-aP [39].
For example, The Self-Assessed Disease Severity (SADS) questionnaire is validated for assessing the impact of itch severity [40, 41]. This is a simple and appropriate tool that enables patients rapidly to self-assess the similarity of three descriptions to symptoms experienced by the patients. Thus, the SADS questionnaire allows patients to classify themselves into one of three “types” of patient (A, B or C), depending on the presence of scratch marks on the skin, the impact of itching on sleep and the presence of agitation/sadness due to itching, ranging from type A (mild) to type C (severe). Although this tool has not been used in the difelikefalin clinical studies to date, its use is proposed in the treatment algorithm; in clinical trials we can obtain relatively high completion rates for more complex, multi-question QoL tools which may also take substantial time and resources to analyze, whereas the use of single-question (unidimensional) scales are the easiest and most time-efficient measures for assessing patient-reported itch intensity in clinical practice. The use of the 24-h WI-NRS has also been developed as a useful, single-question tool to assess rapidly the severity of itch. The WI-NRS consists of a validated 11-point scale ranging from 0 (“no itch”) to 10 (“worst itch imaginable”), and patients rate the intensity of their worst itching during the previous 24-h period [40, 42, 43], with a 3-point improvement on the scale validated as a meaningful improvement [39].
Based on the WI-NRS score and SADS category, pruritus can be categorized as “mild” or “moderate-to-severe” (WI-NRS score ≥4; SADS patient type B or C). This could be useful to plot pruritus trajectories in individual patients.
Other appropriate validated unidimensional scales for measurement of itch intensity that may be used include the visual analogue scale (100-mm line) and verbal rating scale (4-point scale) [40, 42].
The impact of itch on the patient's QoL can also be evaluated by asking the patient about how itch affects their sleep or mood, through the use of a validated PRO measure.
Several available validated dermatological PRO questionnaires can be used to assess the impact of itch on QoL (Table 2), including the Skindex-10 [40], 5-D itch [44] and Dermatology Life Quality Index [45]. These instruments are frequently utilized in clinical research but may not be as convenient to use in clinical practice due to their length, complexity and time required to complete and evaluate the responses.
Table 2:
Overview of itch-related PRO measures.
| Measure | Description |
|---|---|
| WI-NRS | 11-point scale of itch intensity over the previous 24-h period (range 0 to 10; higher scores indicate greater itch intensity), which has been validated in patients with CKD-aP [40, 42, 43]. Reduction of ≥3 points on the WI-NRS has been shown to be associated with a clinically meaningful change in itch intensity for patients with moderate-to-severe pruritus undergoing HD [43]. |
| SADS | SADS categorizes patients into one of three self-designated types (A, B or C), depending on the presence of scratch marks on the skin, the impact of itching on sleep, and the presence of agitation/sadness due to itching [40, 41]. |
| Skindex-10 | Multidimensional tool to evaluate the impact on itch-related QoL of CKD-aP across three separate itch-related domains: Disease, Mood/Emotional Distress and Social Functioning [65]. Skindex-10 total scores range from 0 to 60, with worsening itch-related QoL indicated by higher scores. Analysis of Phase 2 clinical trial data in adult HD patients with moderate-to-severe pruritus indicated that a ≥15-point reduction (improvement) from baseline in the total Skindex-10 score represents a clinically meaningful change [52]. |
| 5-D itch | Multidimensional tool that assesses itch-related QoL and itch intensity across five separate itch-related domains (Duration, Degree, Direction, Disability and Distribution) over a 2-week recall period [44]. 5-D itch scale total scores range from 5 to 25, with worsening itch intensity and itch-related QoL indicated by higher scores. Analysis of Phase 2 clinical trial data indicates that a clinically meaningful improvement was represented by a reduction from baseline of ≥5-point in the total 5-D itch score [52]. |
| Sleep Quality Numerical Rating Scale | 11-point scale to indicate how much itch interfered with sleep over the preceding 24 h, with responses ranging from 0 (“did not interfere”) to 10 (“completely interfered”). |
| Patient self-categorization of pruritus severity | Patients select which of three patient profiles they are most like according to occurrence of scratch marks on skin, problems sleeping because of itching and feelings of agitations or sadness: Patient A (mild signs and symptoms), Patient B (moderate signs and symptoms) or Patient C (severe signs and symptoms). |
| MOS sleep scale | 6-point scale ranging from “all of the time (0)” to “none of the time (6)” to indicate the frequency of various aspects of sleep disruption. Also records estimates of average hours of sleep during the past week and length of time taken to fall asleep. Higher scores reflect better sleep-related HRQoL. |
| PGI-S | Single-item scale with five possible values ranging from none to very severe to assess patient impression of itch severity; higher scores reflect worse severity. |
| PGI-C | Single-item measure with values ranging from “1” (Very Much Improved) to “7” (Very Much Worse); higher scores reflect worse status to assess patient impression of change (improvement or worsening) in overall status relative to the start of the study. |
| M-PGIC | Single-item measure with four response options: “My itch got worse,” “No change,” “My itch got better but the amount of improvement was not meaningful to me” and “My itch got better and the amount of improvement was meaningful to me” to assess patients’ overall impression of change in itch during the course of the clinical trial and whether the amount of improvement was meaningful to them. |
| Dermatology Life Quality Index | 10 questions concerning patients’ perception of the impact of skin diseases on different aspects of their HRQoL over the last week, calculated by summing the score of each question resulting in a maximum of 30 and a minimum of 0. The higher the score, the more quality of life is impaired [45]. |
HRQoL, health-related quality of life; MOS, Medical Outcomes Study; PGI-S, Patient Global Impression of Worst Itch Severity; PGI-C, Patient Global Impression of Change; M-PGIC, modified Patient Global Impression of Change.
Questionnaires may not be suitable for some patients (e.g. individuals with severe visual/cognitive impairment); instead, these patients can be asked verbally about how itch impacts their QoL. However, it is important to recognize that questionnaires may not be a viable method to assess disease severity and QoL in all patients; language barriers or literacy barriers, as well as lack of patient interest in completing surveys, can all impact the use of PROs, although this may be improved with the use of ePROs [11].
Among therapies to treat symptoms in patients on HD, to our knowledge, difelikefalin has the largest clinical development program of any kappa agonist for CKD-aP in HD, with 1306 patients receiving active treatment in Phase 3 trials, of whom 400 received at least 1 year of continuous treatment [13, 46, 47].
However, it was the early phases of the drug development program that formed the basis for the later success of the clinical trials. Of particular importance for difelikefalin was the demonstration of its lack of mu-agonist activity or metabolism and minimal peripheral restriction, with a subsequent abuse assessment study confirming that it has low potential as a drug of abuse [38] and is therefore not considered a controlled substance [13].
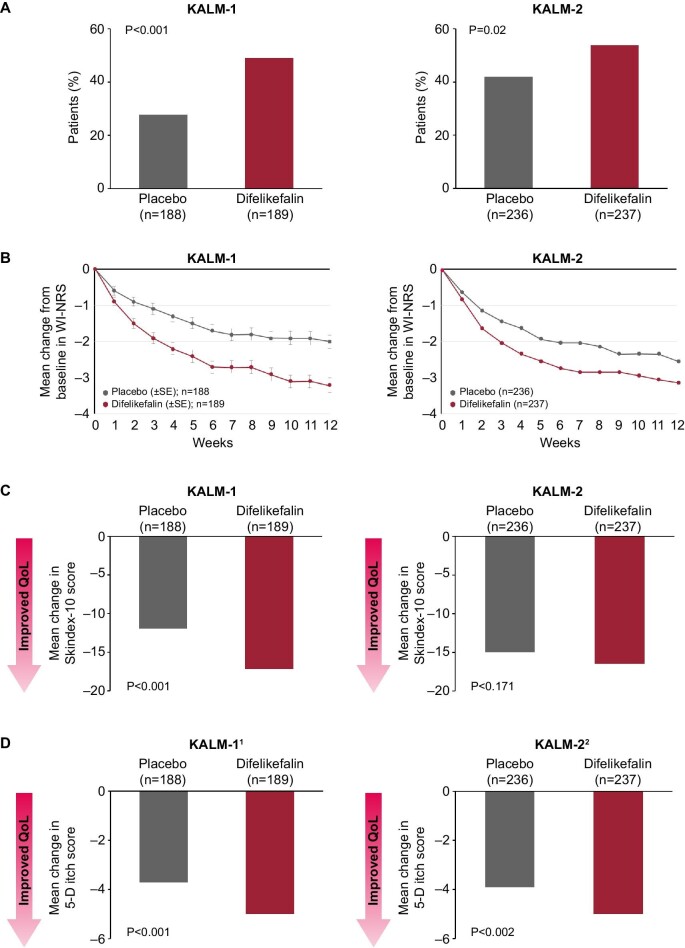
The efficacy and safety of intravenous difelikefalin were evaluated by two pivotal randomized, placebo-controlled, Phase 3 trials (KALM-1 and KALM-2) [13, 47, 48], which enrolled a total of 851 adult patients on HD with moderate-to-severe pruritus. In each of the two trials, patients received intravenous difelikefalin 0.5 µg/kg of dry body weight or placebo after HD sessions three times per week for 12 weeks. As well as clinical measures of efficacy, these trials utilized validated PRO measures of disease severity and itch-related QoL, enabling direct measurement of the improvement of CKD-aP symptom burden in this specific patient population. The proportion of patients achieving a ≥3-point greater decrease from baseline in daily 24-h WI-NRS scores was significantly greater with difelikefalin versus placebo at week 12 (KALM-1: 49% versus 28%, P < .001; KALM-2: 54% versus 42%, P = .02), as well as at each week throughout the study, with significant improvements in itch-related QoL also reported at Week 12 for Skindex-10 and 5-D itch PROs (Fig. 4) [46, 47]. Pooled safety analysis of KALM-1 and KALM-2 showed that frequent adverse events occurring at an incidence of ≥2% with difelikefalin and ≥1% higher than in the placebo group included: diarrhea (9.0% versus 5.7% with placebo), dizziness (6.8% versus 3.8%), nausea (6.6% versus 4.5%), gait disturbances, including falls (6.6% versus 5.4%) and hyperkalemia (4.7% versus 3.5%) [49].
Figure 4:
Improvement in itch severity and itch-related QoL with difelikefalin versus placebo, measured by (a) ≤3-point improvement in WI-NRS, (b) weekly improvement in WI-NRS, (c) Skindex-10 and (d) 5-D itch scale. SE, standard error. References: Fishbane et al. N Engl J Med 2020;382:222–32; Wooldridge et al. J Am Soc Nephrol 2020;31:22–23.
Additionally, in conjunction with improving itch severity, treatment with difelikefalin has been shown to significantly improve sleep quality compared with placebo (evaluated through the Sleep Quality Questionnaire and 5-D itch scale sleep disability question), both of which are key burdensome symptoms for patients [8, 50–52].
In the KALM studies, patients continued any existing anti-itch medications. Although around one-third of patients were prescribed concomitant anti-itch medications, the majority were using antihistamines, which are unlikely to be effective in CKD-aP, because CKD-aP is not thought to involve a histaminergic pathway [21]. Only 1.2% of patients were prescribed gabapentoids as an anti-itch medication (although some patients were also prescribed gabapentoids for non-itch-related conditions). The treatment algorithm for CKD-aP (Fig. 3) therefore proposes initiating treatment with difelikefalin, as the only approved therapy for this condition in the US and Europe as well as the only treatment with several large-scale clinical trials in this patient population. However, the safety data from the clinical studies has demonstrated that patients may continue concomitant anti-itch medications already prescribed, if desired.
If patients are contraindicated for difelikefalin, it is not available, or resistant disease is reported, the use of gabapentoids is suggested as alternative or additional treatment, followed by other therapies such as phototherapy or selective serotonin reuptake inhibitors, which have some evidence of efficacy in small, uncontrolled clinical trials [53].
SUMMARY
Patients with CKD undergoing kidney replacement therapy report many particularly burdensome symptoms. Treatments for these symptoms, where available at all, are frequently used off-label, with limited evidence of efficacy and safety in the CKD population.
The development of difelikefalin to treat CKD-aP demonstrates a pathway from symptom identification and understanding of the underlying pathophysiology, through drug development and demonstration of efficacy and safety, to regulatory approval and patient access, and therefore, ultimately, symptom relief and associated improvements in QoL.
However, there are many symptoms of dialysis with an unacceptable unmet need remaining; it is hoped that the example of difelikefalin can be followed to successfully treat or relieve other symptoms and side effects of dialysis—in the absence of adding years to patients’ lives, we can at least add life into their remaining years.
NEXT STEPS
The example of CKD-aP illustrates that treatment of symptoms using a double-blind controlled trial is possible. Such treatments can now be applied to other unmet need areas, such as cramping, depression, fatigue and pain, among patients with CKD to alleviate symptoms and improve QoL.
Contributor Information
Rajiv Agarwal, Richard L. Roudebush VA Medical Center and Indiana University, Indianapolis, IN, USA.
James Burton, Department of Cardiovascular Sciences, University Hospitals of Leicester NHS Trust, Leicester, UK.
Maurizio Gallieni, Department of Biomedical and Clinical Sciences “Luigi Sacco”, Università Di Milano, Milano, Italy.
Kamyar Kalantar-Zadeh, Division of Nephrology, Hypertension and Kidney Transplantation, University of California, Irvine, CA, USA.
Gert Mayer, Department of Internal Medicine IV (Nephrology and Hypertension), Medical University Innsbruck, Innsbruck, Austria.
Carol Pollock, Renal Research Laboratory, Kolling Institute, University of Sydney, Royal North Shore Hospital, St Leonards, Sydney, Australia.
Jacek C Szepietowski, Department of Dermatology, Venereology and Allergology, Medical University, Wroclaw, Poland.
FUNDING
Medical writing support was provided by AXON Communications and funded by Vifor Pharma.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
R.A. reports personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals Inc., during the conduct of the study; he also reports personal fees and nonfinancial support from Akebia Therapeutics, Relypsa, Vifor Pharma, Boehringer Ingelheim and Eli Lilly; he has received personal fees from Lexicon, Reata and Diamed; he is a member of data safety monitoring committees for Chinook and Vertex; he is a member of steering committees of randomized trials for Akebia Therapeutics and Bayer; he is a member of adjudication committees for AbbVie, Bayer, Boehringer Ingelheim and Janssen; he has served as associate editor of the American Journal of Nephrology and Nephrology Dialysis Transplantation, and has been an author for UpToDate; and he has received research grants from the US Veterans Administration and the National Institutes of Health. J.B. reports fees from Vifor Pharma. M.G. reports fees from Vifor Pharma. K.K.-Z. reports fees from Vifor Pharma, Kabi and AstraZeneca. G.M. reports speaker or advisory board honoraria from AbbVie, Astellas, Boehringer Ingelheim, Novo Nordisk and Sanofi. C.P. has no conflicts to disclose. J.C.S. has no conflicts to disclose.
REFERENCES
- 1. US Food and Drug Administration . Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims, Available at: https://www.fda.gov/media/77832/download (10 February 2022, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal R. Developing a self-administered CKD symptom assessment instrument. Nephrol Dial Transplant 2010;25:160–6. 10.1093/ndt/gfp426 [DOI] [PubMed] [Google Scholar]
- 3. Mittal SK, Ahern L, Flaster Eet al. . Self-assessed physical and mental function of haemodialysis patients. Nephrol Dial Transplant 2001;16:1387–94. 10.1093/ndt/16.7.1387 [DOI] [PubMed] [Google Scholar]
- 4. van der Willik EM, Hemmelder MH, Bart HAJet al. . Routinely measuring symptom burden and health-related quality of life in dialysis patients: first results from the Dutch registry of patient-reported outcome measures. Clin Kidney J 2021;14:1535–44. 10.1093/ckj/sfz192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lowney AC, Myles HT, Bristowe Ket al. . Understanding what influences the health-related quality of life of hemodialysis patients: a collaborative study in England and Ireland. J Pain Symptom Manage 2015;50:778–85. 10.1016/j.jpainsymman.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 6. Moskovitch JT, Mount PF, Davies MRP. Changes in symptom burden in dialysis patients assessed using a symptom-reporting questionnaire in clinic. J Palliat Care 2020;35:59–65. 10.1177/0825859719827315. [DOI] [PubMed] [Google Scholar]
- 7. SONG Executive Committee . Standardised outcomes in nephrology initiative, Available at: https://songinitiative.org/ (16 November 2021, date last accessed). [Google Scholar]
- 8. SONG Executive Committee . SONG-HD, available at: https://songinitiative.org/projects/song-hd/ (16 November 2021, date last accessed). [Google Scholar]
- 9. SONG Executive Committee . SONG-PD, available at: https://songinitiative.org/projects/song-pd/ (16 November 2021, date last accessed). [Google Scholar]
- 10. Flythe JE, Hilliard TS, Ikeler Ket al. . Toward patient-centered innovation: a conceptual framework for patient-reported outcome measures for transformative kidney replacement devices. Clin J Am Soc Nephrol 2020;15:1522–30. 10.2215/cjn.00110120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pérez-Morales R, Buades-Fuster JM, Esteve-Simó Vet al. . Electronic patient-reported outcomes in nephrology: focus on hemodialysis. J Clin Med 2022;11:861. 10.3390/jcm11030861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verberne WR, van den Wittenboer ID, Voorend CGNet al. . Health-related quality of life and symptoms of conservative care versus dialysis in patients with end-stage kidney disease: a systematic review. Nephrol Dial Transplant 2021;36:1418–33. 10.1093/ndt/gfaa078 [DOI] [PubMed] [Google Scholar]
- 13. US Food and Drug Administartion . Korsuva prescribing information, Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214916s000lbl.pdf (16 November 2021, date last accessed) [Google Scholar]
- 14. Shirazian S, Aina O, Park Yet al. . Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis 2017;10:11–26. 10.2147/ijnrd.S108045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sukul N, Karaboyas A, Csomor PAet al. . Self-reported pruritus and clinical, dialysis-related, and patient-reported outcomes in hemodialysis patients. Kidney Med 2021;3:42–53. 10.1016/j.xkme.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nowak DA, Yeung J. Diagnosis and treatment of pruritus. Can Fam Physician 2017;63:918–24 [PMC free article] [PubMed] [Google Scholar]
- 17. Rayner HC, Larkina M, Wang Met al. . International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clin J Am Soc Nephrol 2017;12:2000–7. 10.2215/cjn.03280317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pisoni RL, Wikström B, Elder SJet al. . Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2006;21:3495–505. 10.1093/ndt/gfl461 [DOI] [PubMed] [Google Scholar]
- 19. Weiss M, Mettang T, Tschulena Uet al. . Health-related quality of life in haemodialysis patients suffering from chronic itch: results from GEHIS (German Epidemiology Haemodialysis Itch Study). Qual Life Res 2016;25:3097–106. 10.1007/s11136-016-1340-4 [DOI] [PubMed] [Google Scholar]
- 20. Ahdoot RS, Kalantar-Zadeh K, Burton JOet al. . Novel approach to unpleasant symptom clusters surrounding pruritus in patients with chronic kidney disease and on dialysis therapy. Curr Opin Nephrol Hypertens 2022;31:63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddy VB, Iuga AO, Shimada SGet al. . Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 2008;28:4331–5. 10.1523/JNEUROSCI.0716-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanot A, Kottler D, Béchade C. [Pruritus associated chronic kidney disease]. Nephrol Ther 2021;17:488–95. 10.1016/j.nephro.2021.07.002 [DOI] [PubMed] [Google Scholar]
- 23. Verduzco HA, Shirazian S. CKD-associated pruritus: new insights into diagnosis, pathogenesis, and management. Kidney Int Rep 2020;5:1387–402. 10.1016/j.ekir.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Namer B, Carr R, Johanek LMet al. . Separate peripheral pathways for pruritus in man. J Neurophysiol 2008;100:2062–9. 10.1152/jn.90482.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aquino TMO, Luchangco KAC, Sanchez EVet al. . A randomized controlled study of 6% gabapentin topical formulation for chronic kidney disease-associated pruritus. Int J Dermatol 2020;59:955–61. 10.1111/ijd.14953 [DOI] [PubMed] [Google Scholar]
- 26. Agarwal P, Garg V, Karagaiah Pet al. . Chronic kidney disease-associated pruritus. Toxins 2021;13:527. 10.3390/toxins13080527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shirazian S, Aina O, Park Yet al. . Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis 2017;10:11–26. 10.2147/IJNRD.S108045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mettang T, Kremer AE. Uremic pruritus. Kidney Int 2015;87:685–91. 10.1038/ki.2013.454 [DOI] [PubMed] [Google Scholar]
- 29. Wieczorek A, Krajewski P, Kozioł-Gałczyńska Met al. . Opioid receptors expression in the skin of haemodialysis patients suffering from uraemic pruritus. J Eur Acad Dermatol Venereol 2020;34:2368–72. 10.1111/jdv.16360 [DOI] [PubMed] [Google Scholar]
- 30. Legroux-Crespel E, Clèdes J, Misery L. A comparative study on the effects of naltrexone and loratadine on uremic pruritus. Dermatology 2004;208:326–30. 10.1159/000077841 [DOI] [PubMed] [Google Scholar]
- 31. Pauli-Magnus C, Mikus G, Alscher DMet al. . Naltrexone does not relieve uremic pruritus: results of a randomized, double-blind, placebo-controlled crossover study. J Am Soc Nephrol 2000;11:514–9. 10.1681/ASN.V113514 [DOI] [PubMed] [Google Scholar]
- 32. Peer G, Kivity S, Agami Oet al. . Randomised crossover trial of naltrexone in uraemic pruritus. Lancet 1996;348:1552–4. 10.1016/S0140-6736(96)04176-1 [DOI] [PubMed] [Google Scholar]
- 33. Kumagai H, Ebata T, Takamori Ket al. . Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 2010;25:1251–7. 10.1093/ndt/gfp588 [DOI] [PubMed] [Google Scholar]
- 34. Mathur VS, Kumar J, Crawford PWet al. . A multicenter, randomized, double-blind, placebo-controlled trial of nalbuphine ER tablets for uremic pruritus. Am J Nephrol 2017;46:450–8. 10.1159/000484573 [DOI] [PubMed] [Google Scholar]
- 35. Electronic Medicines Compendium . Kapruvia summary of product characteristics, Available at: https://www.medicines.org.uk/emc/product/13735/smpc (16 May 2022, date last accessed). [Google Scholar]
- 36. Kardon AP, Polgár E, Hachisuka Jet al. . Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014;82:573–86. 10.1016/j.neuron.2014.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cowan A, Kehner GB, Inan S. Targeting itch with ligands selective for κ opioid receptors. Handb Exp Pharmacol 2015;226:291–314. 10.1007/978-3-662-44605-8_16 [DOI] [PubMed] [Google Scholar]
- 38. Shram MJ, Spencer RH, Qian Jet al. . Evaluation of the abuse potential of difelikefalin, a selective kappa-opioid receptor agonist, in recreational polydrug users. Clin Transl Sci 2022;15:535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vernon MK, Swett LL, Speck RMet al. . Psychometric validation and meaningful change thresholds of the Worst Itching Intensity Numerical Rating Scale for assessing itch in patients with chronic kidney disease-associated pruritus. J Patient Rep Outcomes 2021;5:134. 10.1186/s41687-021-00404-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mathur VS, Lindberg J, Germain Met al. . A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010;5:1410–9. 10.2215/CJN.00100110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manenti L, Leuci E. Do you feel itchy? A guide towards diagnosis and measurement of chronic kidney disease-associated pruritus in dialysis patients. Clin Kidney J 2021;14:i8–15. 10.1093/ckj/sfab143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phan NQ, Blome C, Fritz Fet al. . Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012;92:502–7. 10.2340/00015555-1246 [DOI] [PubMed] [Google Scholar]
- 43. Vernon M, Ständer S, Munera Cet al. . Clinically meaningful change in itch intensity scores: an evaluation in patients with chronic kidney disease-associated pruritus. J Am Acad Dermatol 2021;84:1132–4. 10.1016/j.jaad.2020.06.991 [DOI] [PubMed] [Google Scholar]
- 44. Elman S, Hynan LS, Gabriel Vet al. . The 5-D itch scale: a new measure of pruritus. Br J Dermatol 2010;162:587–93. 10.1111/j.1365-2133.2009.09586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basra MK, Fenech R, Gatt RMet al. . The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008;159:997–1035. [DOI] [PubMed] [Google Scholar]
- 46. Fishbane S, Wen W, Munera Cet al. . Long-term safety and efficacy of difelikefalin in patients with chronic kidney disease-associated pruritus: analysis from KALM-1 and KALM-2. Am J Kidney Dis 2021;77:593–4. [Google Scholar]
- 47. Wooldridge TD, Mccafferty K, Schoemig Met al. . Efficacy and safety of difelikefalin for moderate-to-severe CKD–associated pruritus: a global phase 3 study in hemodialysis patients (KALM-2). J Am Soc Nephrol 2020;31:22–3. [Google Scholar]
- 48. Fishbane S, Jamal A, Munera Cet al. . A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med 2019;382:222–32. 10.1056/NEJMoa1912770 [DOI] [PubMed] [Google Scholar]
- 49. Cara Therapeutics Inc . KORSUVA (difelikefalin) injection for intravenous use. Highlights of Prescribing Information. Available at: https://korsuva.com/sites/g/files/brlbcj1286/files/2021-08/korsuva-prescribing-information.pdf (22 November 2021, date last accessed) [Google Scholar]
- 50. Ahdoot RS, Kalantar-Zadeh K, McCafferty Ket al. . Improvement in sleep quality from reduction of itch intensity in patients with moderate-to-severe pruritus undergoing hemodialysis. World Congress on Itch. Virtual 2021. [Google Scholar]
- 51. Weiner DE, Walpen S, Schaufler Tet al. . Itch reduction with difelikefalin correlates with improved sleep quality in hemodialysis patients with pruritus. American Society of Nephrology 2021 Virtual, 2021; [Google Scholar]
- 52. Fishbane S, Mathur V, Germain MJet al. . Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep 2020;5:600–10. 10.1016/j.ekir.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lipman ZM, Paramasivam V, Yosipovitch Get al. . Clinical management of chronic kidney disease-associated pruritus: current treatment options and future approaches. Clin Kidney J 2021;14:i16–22. 10.1093/ckj/sfab167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jhamb M, Weisbord SD, Steel JLet al. . Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis 2008;52:353–65. 10.1053/j.ajkd.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nadort E, Schouten RW, Witte SHSet al. . Treatment of current depressive symptoms in dialysis patients: a systematic review and meta-analysis. Gen Hosp Psychiatry 2020;67:26–34. 10.1016/j.genhosppsych.2020.07.012 [DOI] [PubMed] [Google Scholar]
- 56. Raina R, Krishnappa V, Gupta M. Management of pain in end-stage renal disease patients: short review. Hemodial Int 2018;22:290–6. 10.1111/hdi.12622 [DOI] [PubMed] [Google Scholar]
- 57. Ishida JH, McCulloch CE, Steinman MAet al. . Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol 2018;29:1970–8. 10.1681/asn.2018010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Davison SN. The prevalence and management of chronic pain in end-stage renal disease. J Palliat Med 2007;10:1277–87. 10.1089/jpm.2007.0142 [DOI] [PubMed] [Google Scholar]
- 59. Cohen SD, Cukor D, Kimmel PL. Anxiety in patients treated with hemodialysis. Clin J Am Soc Nephrol 2016;11:2250–5. 10.2215/cjn.02590316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mujais SK. Muscle cramps during hemodialysis. Int J Artif Organs 1994;17:570–2. 10.1177/039139889401701102 [DOI] [PubMed] [Google Scholar]
- 61. Lynch KE, Feldman HI, Berlin JAet al. . Effects of L-carnitine on dialysis-related hypotension and muscle cramps: a meta-analysis. Am J Kidney Dis 2008;52:962–71. 10.1053/j.ajkd.2008.05.031 [DOI] [PubMed] [Google Scholar]
- 62. Salib M, Memon AN, Gowda ASet al. . Dialysis patients with restless leg syndrome: can we relieve their suffering? Cureus 2020;12:e10053. 10.7759/cureus.10053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. O'Connor NR, Corcoran AM. End-stage renal disease: symptom management and advance care planning. Am Fam Physician 2012;85:705–10. [PubMed] [Google Scholar]
- 64. Novak M, Shapiro CM, Mendelssohn Det al. . Diagnosis and management of insomnia in dialysis patients. Semin Dial 2006;19:25–31. 10.1111/j.1525-139X.2006.00116.x [DOI] [PubMed] [Google Scholar]
- 65. Mathur VS, Lindberg J, Germain Met al. . Investigators INR . A longitudinal study of uremic pruritus in hemodialysis patients. Clin J Am Soc Nephrol 2010;5:1410–9. 10.2215/CJN.00100110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.