ABSTRACT
Hemodialysis is associated with high morbidity and mortality rates as well as low quality of life. Altered nutritional status and protein-energy wasting are important indicators of these risks. Maintaining optimal nutritional status in patients with hemodialysis is a critical but sometimes overlooked aspect of care. Nutritional support strategies usually begin with dietary counseling and oral nutritional supplements. Patients may not comply with this advice or oral nutritional supplements, however , or compliance may be affected by other complications of progressive chronic kidney disease. Intradialytic parenteral nutrition (IDPN) may be a possibility in these cases, but lack of knowledge on practical aspects of IDPN delivery are seldom discussed and may represent a barrier. In this review, we, as a consensus panel of clinicians experienced with IDPN, survey existing literature and summarize our views on when to use IDPN, which patients may be best suited for IDPN, and how to effectively deliver and monitor this strategy for nutritional support.
Keywords: chronic kidney disease, hemodialysis, parenteral nutrition, protein-energy malnutrition
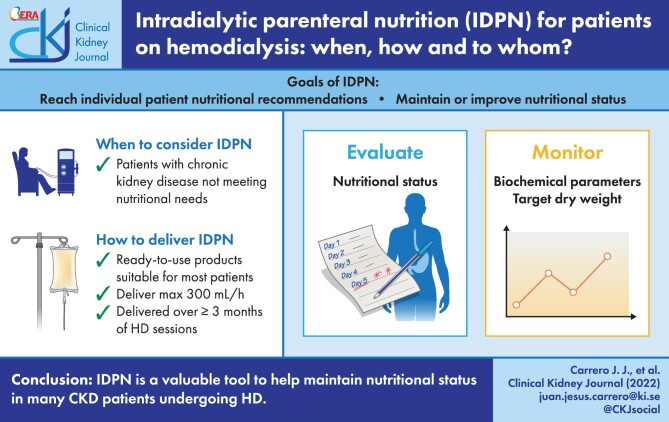
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is associated with high morbidity and mortality rates as well as low quality of life (QOL), with nutritional status being an important indicator of these risks [1–3]. Markers of nutritional status inevitably decline in CKD over time, resulting in progressive depletion of protein and energy stores (i.e. fat and muscle). This condition, termed ‘protein-energy wasting’ (PEW), is highly prevalent in patients on hemodialysis (HD) and is often associated with reduced functional capacity [4]. PEW is attributed to a variety of complex causes that can collectively be grouped into factors leading to undernutrition (poor appetite and low spontaneous nutrient intake) [5–8] and factors leading to catabolism of muscle protein (inflammation, resistance to anabolic drive, metabolic acidosis, and others) [9]. A variety of strategies are available to provide nutritional support to patients receiving HD who experience or are at risk of developing PEW. These include regular counseling by a dietitian, oral nutritional supplements (ONSs), and intradialytic parenteral nutrition (IDPN). Although narrative reviews [10–14] and clinical guidelines [15–17] discuss the role of IDPN in patients on HD, practical aspects of IDPN delivery are seldom discussed. As identified in a recent Australian survey, uneven and sometimes insufficient clinical guidance on the use of IDPN as a nutritional support strategy has resulted in uneven uptake [18], along with concerns regarding costs and insurance coverage [12].
To complement and add to the current guidance on the use of IDPN, we reviewed indications, clinical goals, indicators of clinical response, practical aspects, potential complications, and challenges of IDPN therapy.
Scope of the problem: protein energy wasting in patients on dialysis
As opportunities for kidney transplantation expand worldwide, remaining patients on chronic HD are more likely to have multiple comorbidities that may adversely affect nutritional status and loss of muscle mass and strength. Muscle mass loss and accelerated muscle protein breakdown have been reported in multiple studies of patients receiving HD, enhanced by precipitating factors such as inflammation [5], metabolic acidosis, resistance to anabolic drive, poor physical activity, and older age [6–9]. Diabetes, especially when suboptimally controlled, may be another condition leading to muscle protein breakdown in these patients [6–8]. These factors are exacerbated by fatigue, poor appetite, and inadequate protein and energy intake [9, 19]. As a result, PEW is common in patients on dialysis. In a meta-analysis of 90 studies including 16 434 patients on maintenance dialysis, most studies reported a prevalence of PEW between 28% and 54% [20]. Considering that those studies included clinically stable patients, the real prevalence may be even higher.
The HD procedure itself can result in infectious, inflammatory, or volume-related complications that contribute to PEW [9]. It also affects protein and energy homeostasis: Amino acid and protein loss during the HD session coupled with low nutrient intake result in low nutrient availability for muscle synthesis [9, 21–24]. HD has a profound catabolic effect, especially on protein homeostasis, that affects whole-body and skeletal protein homeostasis (Fig. 1) [9]. Nutritional support strategies during the dialysis session can counteract this negative protein balance and help maintain adequate nutritional status over time. This maintenance can be achieved through oral nutritional support, such as protein- and energy-enriched meals [25, 26] or oral ONSs [27] but also through IDPN [27, 28].
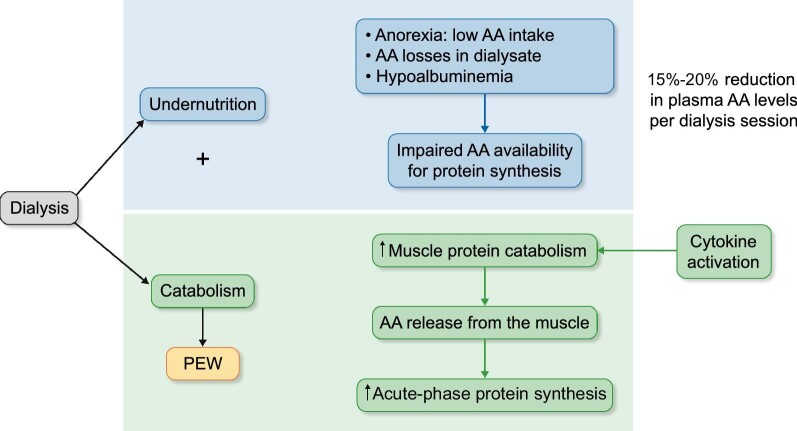
FIGURE 1:
The effects of hemodialysis coupled with inadequate nutrient intake on muscle loss. Dialysis contributes to underlying factors that result in protein energy wasting. The dialysis process induces the activation of defense mechanisms, and the body reacts by increasing acute phase protein synthesis and initiating the inflammation cascade. In this setting, chronic undernutrition and amino acid (AA) losses into dialysate lead to low availability of circulating AA for protein synthesis. The body, in turn, increases muscle protein catabolism to promote AA release as a substrate for acute-phase proteins; this muscle catabolism activates cytokine production in the muscle, which tends to perpetuate this process in a vicious cycle.
Defining intradialytic parenteral nutrition
The use of HD access for the delivery of PN eliminates the need for an additional permanent catheter placement or port system placement and is instead allows delivery through the venous drip chamber in the extracorporeal circuit. IDPN is administered throughout the HD session, which typically lasts about 4 hours and is usually done three times per week [15]. The IDPN admixture typically contains dextrose, amino acids, and lipids and may contain electrolytes, trace elements, and vitamins [29]. Because IDPN is time and volume restricted, it is considered a form of supplemental nutrition support adjuvant to any oral intake. Because it is intermittently delivered, IDPN can only provide up to 25% of a patient's targeted nutrient intake.
Although comprehensive data and guidance are still lacking, the current consensus is that until additional data on IDPN are available that demonstrate superiority to other strategies (i.e. dietary counselling or ONS), IDPN ought to be reserved for situations when other strategies fail to maintain nutritional status [30]. We feel, however, that it is important to consider the relative benefits and limitations of ONS and IDPN. ONS is a simple, low-cost strategy to maintain nutritional status over time. In our clinical experience, however, it is not uncommon that patients with sustained PEW require additional nutritional support beyond ONS. Meanwhile, in clinical practice, nonadherence is the main limitation to daily nutritional intake, including ONS [31]. We are not aware of any reports that quantify real-life compliance to ONS, but long-term use of ONS may create challenges in this regard because of loss of appetite; early satiety; and issues related to taste, flavor (less appealing or always the same taste), palatability, and food acceptability issues (habituation, social situation, culture, etc) [31].
Considering these potential hindrances to ONS, we believe that IDPN can be considered as a strategy to complement spontaneous oral intake that does not depend on patient compliance. Both IDPN and ONS have been proven to prevent derangements in protein uptake during HD [25], and, like ONS, IDPN helps maintain adequate nutrition status over time.
Indications and clinical goals for intradialytic parenteral nutrition
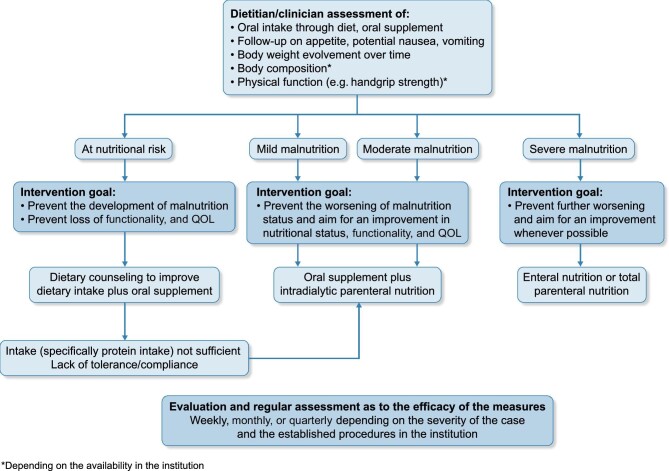
Reflecting our own evaluation of available evidence and guidelines, IDPN is indicated in malnourished, non-critically ill hospitalized patients with acute kidney injury or CKD on HD and in patients on chronic HD at risk for undernutrition who can safely feed orally but cannot meet nutritional guidelines with diet alone (Fig. 2) [15, 17, 32].
FIGURE 2:
A streamlined decision tree for nutritional support based on clinical guidelines and panel consensus.
We feel that IDPN should be considered an intervention reserved for situations when efforts to improve oral intake or effectively deliver regular ONS have been inadequate, but we stress that IDPN can also be a valuable tool in patients with CKD at risk for malnutrition [9, 28, 33].
Defining ‘inadequate intake’ or ‘at risk of malnutrition’ is, however, not straightforward, given the many factors that may underlie it. Because of this, the US National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) 2020 guidelines do not provide specific thresholds to define malnutrition [15], together with the potential confounding effect of inflammation and volume overload in most standard routine markers. KDOQI guidelines recommend evaluating complementary malnutrition markers and interpreting them together when making diagnosis. On the basis of this recommendation, we remain vague in our definition of ‘malnutrition’ as we feel that defining numerical thresholds may give a false impression of accuracy; specific thresholds are not supported by clinical evidence and should be decided on an individual patient basis.
IDPN should, in any case, be seen as a complement to established ONS so that dietary intake requirements are met, especially with respect to protein. We suggest that patients suitable for IDPN should demonstrate an oral intake of greater than 20 kcal/kg and 0.8 g of protein/kg/day. IDPN may also offer an opportunity to provide more balanced nutrition and reach individual patient nutritional goals when regular oral intake is imbalanced. Ultimately, the overarching goal of IDPN should be to improve nutritional status in patients receiving HD or, at the least, to avoid declines in nutritional status over time.
Clinical outcomes seen with intradialytic parenteral nutrition
Available evidence has reported multiple effects on clinical outcomes resulting from IDPN, as shown in Table 1 [34–41]. In the largest available randomized study of IDPN, 186 patients on HD were randomized to receive ONS with or without IDPN over the course of 1 year. Adding IDPN to ONS resulted in similar nutritional parameters compared with ONS alone. Regardless of the strategy, an improvement in nutritional status, defined by increases in prealbumin after the intervention, was associated with a higher 2-year survival and lower hospitalization rates [37]. It is not feasible to conduct a trial that randomized malnourished patients to treatment or placebo, but these findings confirm that restoring nutritional status, regardless of strategy, improves long-term patient outcomes.
Table 1.
Clinical outcomes reported with the use of IDPNa
| RCTs with control groups | Secondary outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | n | Design | Daysb | % Patients completed | IDPN content per session | Primary outcome | Body weight | Triceps skinfold | Serum pre-albumin, mg/L | Serum albumin, g/L | nPNA, g/kg/day | Serum creatinine, μmol/L |
| Guarnieri 1980 [34] | 18 | eAA vs eAA + non-eAA vs no treatment | 60 | 100 | 14 g/d (eAA); 10 g/d (eAA + non-eAA) | ↑ BW with eAA (P < .05); decrease in other groups |
eAA: 66 ± 6.5 to 68 ± 6.1 kg eAA + non-eAA: 73 ± 5.3 to 71 ± 5.7 kg Control: 61 ± 4.5 to 59 ± 5.3 kg |
No change | NR | No change | NR | No change |
| Cano 1990 [35] | 26 | IDPN vs no IDPN | 90 | 100 | Fat 16 kcal/kg + AA 0.08 g/kg | ↑ BW, s-albumin and s-creatinine with IDPN |
IDPN: +2 ± 0.47% Control: −1.5 ± 0.94% (P < .01) |
No change |
IDPN: +20 ± 11.6 Control: −18 ± 13.9 (P < .05) |
IDPN: +0.95 ± 0.63 Control: −0.43 ± 0.63 (P < .05) |
NR |
IDPN: +998 ± 444 Control: −725 ± 379 (P < .01) |
| Navarro 2000 [36] | 17 | IDPN vs no IDPN | 90 | 100 | 25.7 g AA | Prevention of reductions in plasma AA concentration with IDPN | NR | No change | NR |
IDPN: 36.2 ± 0.5 to 42.2 ± 0.5 P < .05) Control: no change |
IDPN; 1.07 ± 0.02 to 1.18 ± 0.02 P < .05) Control: no change |
No change |
| Cano 2007 [37] | 186 | IDPN + ONS vs ONS alone | 365 | 67 | Not specified | Mortality (NS) | Increase with both treatments (NS between groups) | NR | IDPN: 240 ± 5.1 to 265 ± 10 (NS between groups) | 32 ± 0.5 to 33.5 ± 0.5 with both treatments (NS between groups) | Increase with both treatments (NS between groups) | NR |
| Liu 2016 [38] | 32 | ONS vs ONS + glucose vs ONS + glucose + AA | 270 | 100 | 250 mL of 50% glucose solution; 21 g AA | ↑ S-albumin and prealbumin with AA; no difference in SGA between groups | NR | NR | AA: 330 (235–367) to 341 (288–348), no change in glucose and control groups, P < .05 for AA vs control | AA: 39 (37–41) to 39 (38–41), no change in glucose and control groups, P < .05 for AA | No change | No change |
| Marsen 2017 [39] | 107 | IDPN + standard nutritional counseling vs standard nutritional counseling only | 196 | 60.4 |
Glucose: 1.35 ± 0.36 g/kg AA: 0.68 ± 0.13 g/kg) |
↑ Prealbumin with IDPN | NR | NR |
IDPN: 209 ± 9.9 to 236 ± 11.5 Control: 226 ± 9.2 to 224 ± 10.1 (P = .02) |
No change | No change | No change |
| Thabet 2017 [40] | 40 | IDPN vs control | 180 | 100 | 16 mL/kg (not to exceed 1000 mL per session; composition not reported) | ↓ MIS with IDPN vs control (P = .001). IDPN: 12 ± 0.5 to 3 ± 0.3 Control: 10 ± 0.5 to 10 ± 0.5 |
No change | No change | NR |
IDPN: 30 ± 0.9 to 33 ± 0.7 Control: 30.±0.9 to 29 ± 0.9 (P = .003) |
NR | NR |
| RCTs without control groups | Body weight | Triceps skinfold | Serum pre-albumin, mg/L | Serum albumin, g/L | nPNA, g/kg/day | Serum creatinine, μmol/L | ||||||
| Cano 2006 [41] | 35 | Olive oil–based vs soybean oil–based IDPN | 35 | 100 | 16 ml/kg (AA, 50 g/L; glucose, 125 g/L; fat, 50 g/L) | In both groups: ↑ nPNA, s-albumin, prealbumin, s-creatinine | No change | NR |
Olive oil: 254 ± 3.3 to 259 ± 3.7 (NS) Soybean: 231 ± 3.1 to 254 ± 3.6 (P < .05); NS between groups |
Olive oil: 34 ± 0.2 to 35 ± 0.2 (P < .01) Soybean: 34 ± 0.2 to 36 ± 0.2 (P < .01); NS between groups |
Olive oil: 1.0 ± 0.02 to 1.2 ± 0.02 (P < .01) Soybean: 0.9 ± 0.02 to 1.2 ± 0.02 (P < .01); NS between groups |
Olive oil: 631 ± 10.7 to 630 ± 10.3 (NS) Soybean: 590 ± 8.5 to 627 ± 9.8 (P < .01); NS between groups |
a Data are provided as mean ± SEM or median (IQR) unless otherwise noted. AA: amino acid; BW: body weight; eAA: essential amino acid; IDPN: intradialytic parenteral nutrition; IQR: interquartile range; MIS: malnutrition inflammation score; nPCR: normalized protein catabolic rate; NR: not reported; NS: not significant; ONS: oral nutritional supplement RCT: randomized controlled trial; SGA: subjective global assessment.
Refers to the duration of the intervention.
Composition of intradialytic parenteral nutrition solutions
Available guideline recommendations are nonspecific in terms of the choice of IDPN solution and other practical aspects of IDPN administration, with suggested nutrition targets summarized in Table 2 [15–17, 42]. Available options for IDPN solutions include customized, compounded bags or commercially prepared ready-to-use (RTU) bags. IDPN requires a consideration of individual patient needs in terms of amino acids and energy. We agree that amino acid provision, rather than total calories, is the most important consideration of any IDPN solution. We therefore suggest that it would be reasonable to target an amino acid content that, at the least, overcomes anabolic resistance as well as intradialytic amino acid losses. The work done by Pupim and colleagues [28] evaluated IDPN containing 0.6 g amino acids/kg body weight administered in patients on HD without evidence of inflammation, which reversed the net protein degradation inherent with the HD session, and this protein content may be suitable for some patients. Meanwhile, we stress that the presence of significant inflammation or other factors that lead to anabolic resistance may necessitate a higher amino acid content. Ready-to-use (RTU) or all-in-one commercially produced IDPN products meet the nutritional needs of most patients in the setting of IDPN.
Table 2.
Current recommendations for IDPN composition
| AAs (per session) | Energy (kcal/session) | Glucose (g/session) | Fat (g/session) | |
|---|---|---|---|---|
| KDOQI Clinical Practice guideline for Nutrition in CKD: 2020 Update [15] | Not specified | |||
| ESPEN: Guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease (2021) [33] | NS | |||
| DGEM a : Guidelines on enteral and parenteral nutrition in patients with kidney disease (2015) [16] | At least 0.5 g/kg | 500–800 | Max. 50–80 | Max. 20–30 |
| ESPEN: Guidelines on parenteral nutrition: adult renal failure (2009) [17] | 30–60 g | 800–1200 | NS | NS |
Recommends limiting the applicable nutrient supply to avoid metabolic disorders. AA: amino acid; CKD: chronic kidney disease; DGEM: Deutsche Gesellschaft für Ernährungsmedizin (German Association for Nutritional Medicine); ESPEN: European Society for Parenteral and Enteral Nutrition; KDOQI: Kidney Disease Outcomes Quality Initiative; NS: not specified.
The amount of nutrients that can be provided depends on the length of the HD session because of the limitation of metabolic tolerance. Therefore, a 2-hour HD session allows for less nutrient supply than a 4-hour session. For the provision of an appropriate amount of amino acid and calories, considering a maximum infusion rate, we suggest that IDPN be delivered during a session lasting at least 3.5 hours. Although some clinicians calculate nutrient delivery of 0.8 g amino acids and 15 kcal/kg ideal body weight per session, others adapt the volume of the solution available at their institution, taking into consideration the patient's body weight.
Clinicians should consider strategies for balancing energy and protein delivery when using commercially available products. These strategies may also be used to tailor macronutrient delivery. For instance, IDPN can provide a higher-than-normal amino acid–to-energy product to compensate for regular poor oral intake [43]. Commercially available IDPN can also be used in a strategy to control carbohydrate delivery [13]. As for total nutrients provided during a dialysis session, the rational view is to consider individual amino acids, energy, and lipids needs and to supplement nutrients that are not met in IDPN with spontaneous oral intake or ONS.
Electrolytes are usually not part of the IDPN composition, which reflects our experience in this setting. Most manufacturers offer an electrolyte-free option, which may be preferred in these patients. Vitamins and trace elements are added when needed.
Compounded intradialytic parenteral nutrition solutions
Although compounding allows for tailoring of admixtures to specific patient needs, there are limitations related to process complexity when ordering and delivering IDPN during HD. These limitations include increased costs related to employee time for preparation, the need for a sterile compounding facility and all the related process regulations that apply to this preparation setting, complex logistical challenges related to the safe transportation and the requirement for refrigeration during storage of prepared products, and the limited time window between preparation and administration.
Commercial ready-to-use solutions
Unlike compounded solutions, commercial RTU bags can be safely stored at room temperature for long periods before use, in some cases up to 2 years. In general, solutions considered for IDPN usually do not contain electrolytes. Moreover, because of the limited time for delivery during a standard HD session, the total infused volume is constrained. RTU products can also be used to tailor macronutrient delivery, by selecting products with a comparably high amino acid–to energy ratio to compensate for poor spontaneous oral protein intake or to select products for a strategy to control carbohydrate delivery. Depending on specific patient needs, clinicians may also decide to provide or withhold components such as lipids.
All-in-one commercially produced RTU bags differ not only with regard to their nutrient content and available bag formats but also as to whether an IDPN application has been specifically applied for and included in the licensed dosing guidance for the respective product.
Aspects of administration decision-making with intradialytic parenteral nutrition
Depending on a given patient’s needs, specific IDPN components may be withheld, including lipids, vitamins, and trace elements. In our experience, vitamins or trace elements are rarely needed as an addition to IDPN, except for patients with specific known deficiencies. Although many patients with CKD may exhibit these deficiencies, IDPN should always be considered only an intermittent, supplemental nutrition strategy, and many patients possibly already receive routine oral supplements regularly [44].
Other clinically important factors for consideration include total volume to be delivered and infusion rate during the IDPN session. These will be dictated by the anticipated duration of the HD session and individual patient body weight, among other factors. In addition, amino acid delivery is an important consideration to maintain a positive protein balance and depends on individual patient dietary intake outside of HD.
Criteria for reconsidering or delaying intradialytic parenteral nutrition
There are no absolute contraindications for IDPN, but we have formulated several criteria for reconsidering or delaying IDPN (Box 1). Most of these were exclusion criteria in randomized controlled trials (RCTs). Uncontrolled diabetes, hypertension, or evidence of volume overload may also require a delay in a plan for regular IDPN administration, and the presence of severe malnutrition should prompt a more aggressive nutritional approach (Box 1). Some of these RCTs excluded patients with diabetes [34, 35, 38, 40] or patients with uncontrolled diabetes [39]. Although significant increases in serum triglycerides have not been demonstrated during IDPN, IDPN should not be started in cases where the baseline triglyceride level is already significantly elevated. Cano et al. excluded patients with triglyceride levels above 200 mg/dL [41], but we recommend a somewhat more liberal cutoff of 500 mg/dL based on our clinical experience. Although uncontrolled hypertension and fluid overload have not been used as exclusion criteria in published RCTs, we recommend exercising caution in these patients because of concerns regarding excessive ultrafiltration volume. Some trials excluded patients with recent enteral or parenteral tube feeding [37–39]. Similarly, although the lower boundaries of spontaneous intake we formulated have not been defined as exclusion criteria in existing studies, we expect IDPN to fail to reach the suggested targets mentioned in Table 4 in these patients, in whom enteral nutrition may lead to better outcomes.
Table 4.
Suggested parameters to assess the effectiveness of IDPN
| Parameter | Frequency of measurements | Suggested criteria for IDPN effectiveness. |
|---|---|---|
| Dry weight | • During and after each HD session | • A tendency to increase edema-free body weight during the past 3 months |
| Predialysis albumin and prealbumin levels | • Initial treatment• Weekly for 2 weeks• Then, every 4–6 weeks to coincide with regular dialysis blood work | • A tendency to increase mean albumin levels during the past 3 months• A tendency to increase mean prealbumin levels during the past 3 months |
| SGA | • Initial treatment• Then, every 3 months | • SGA improvement (SGA Score A, B) during the past 3 months |
| Body stores assessment by bioelectrical impedance | • Initial treatment• Then, every 4–6 weeks to coincide with regular dialysis blood work | • A tendency to increase/preserve lean tissue mass and fat during the past 3 months |
| Dietary interview or diarya | • Initial treatment• Then, every 3 months | • Caloric intake ≥25–30 kcal/kg/day• Protein intake ≥1.0 g/kg/day |
| Handgrip strength | • Initial treatment• Then, every 3 months | • A tendency to improve/preserve muscle strength, as assessed by handgrip strength during the past 3 months, with a minimum cutoff of 16 kg and 27 kg for women and men, respectively |
| Gait speed | • Initial treatment• Then, every 3 months | • A tendency to improve/preserve physical performance, as assessed by gait speed, during the past 3 months, with a minimum cutoff of 0.8 m/second.• Low physical performance gait speed >0.8 m/second |
Three-day dietary records followed by interviews and calculating nutrient intake by an experienced registered renal dietitian; should include intake derived from IDPN. HD: hemodynamic; IDPN: intradialytic parenteral nutrition; SGA: subjective global assessment.
Adapted from: García de Lorenzo A, Arrieta J, Ayúcar A et al. Nutrición parenteral intradiálisis en el enfermo renal crónico: consenso SEN-SENPE [Intra-dialysis parenteral nutrition in chronic renal patients: consensus SEN-SENPE]. Nutr Hosp 2010; 25 :375–377
Box 1.
Suggested clinical criteria for intradialytic parenteral nutrition
Considerations for IDPN:
Established risk of malnutrition
Failed attempt at intensive oral nutrition or poor compliance with ONS
Possible contraindications or factors requiring a delay in IDPN:
Severe malnutrition requiring more intensive intervention, including temporary parenteral nutrition
Baseline triglyceride level >500 mg/dl
Spontaneous intake of <20 kcal/kg and/or <0.8 g of protein/kg/day (consider enteral nutrition)
Uncontrolled diabetes or hypertension
Evidence of volume overload
Clinical aspects of intradialytic parenteral nutrition setup
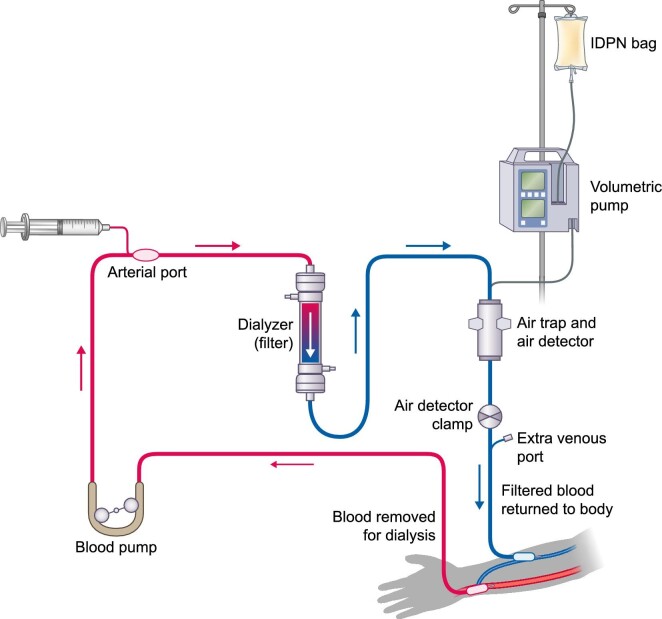
The addition of IDPN to an HD session requires careful planning and equipment setup by professionals specifically trained in HD administration [45]; we stress that the proper timing and process for bag preparation should also be considered. For reference, a general schematic of the HD circuit, including IDPN, is shown in Figure 3. The IDPN bag should be agitated before the administration set is inserted, and the infusion line should then be connected from the external infusion pump to the venous chamber of the machine. Pump flow rate should be set according to the IDPN formulation and at a rate that ensures individual patient safety, with the infusion rate determined according to a patient's ideal body weight. A typical pragmatic solution is to reduce the total amount infused (typically 1 L) only when the patient's body weight is less than 60 kg or when a shorter dialysis duration limits the infused volume.
FIGURE 3:
Schematic review of a hemodialysis circuit. Adapted from: National Institute of Diabetes and Digestive and Kidney Diseases, https://www.niddk.nih.gov/health-information/kidney-disease/kidney-failure/hemodialysis (December 3, 2021, date last accessed).
IDPN requires a stepwise approach to administration, with incremental increases occurring over the course of at least 1 week of HD sessions. For a typical RTU solution, we suggest initiating IDPN at 125 mL/hour in the first week, with the full dose of 250 mL/hour during a 4-hour session (max. 300 mL/hour during a session lasting at least 3.5 hours) achieved in the second week. If during a dialysis session IDPN is temporarily interrupted for any reason, then resumed, the infusion rate should not be increased to try to compensate for the loss of time. It is preferable to increase the length of the HD session or discard the excess IDPN volume after the HD session ends.
If transfusion of blood products or the use of intravenous iron is required during the HD session in addition to IDPN, these products should be administered through the arterial chamber, maintaining the IDPN through the venous chamber. The concomitant administration of antibiotics that require an extended infusion time (e.g. vancomycin) is discouraged. If these drugs are necessary, however, and a second venous port is not available, IDPN should be delivered through the arterial port while the venous chamber is used for antibiotics. Use of the arterial port for IDPN delivery may result in greater losses of its ingredients in the dialysate.
Important considerations and potential limitations to IDPN
The infusion rate depends on the composition of the IDPN solution. For instance, to avoid exceeding triglyceride clearance, infusion of a typical 1-L lipid-containing admixture should occur over at least 3.5 hours. Likewise, hyperglycemia can occur if a high amount of carbohydrates is delivered in the IDPN solution. This could be followed by hyperinsulinemia and hypoglycemia by the end of the HD session, especially if the IDPN infusion is terminated before the dialysis session is complete. Note that this clinical outcome is often the result of excessive exogenous insulin administration during dialysis, which should be avoided.
Patient monitoring during intradialytic parenteral nutrition treatment
General guidance has been published on patient monitoring during IDPN, both during the session and over the course of a long-term IDPN plan [14, 15, 45, 46]. We propose a variety of monitoring practices to detect complications during the first weeks of IDPN support, especially related to the potential risk for hyperglycemia. We also agree with available guidance on tracking the effectiveness of IDPN over time and strategies to detect any complications with prolonged treatment. These suggestions are summarized in Table 3. Blood pressure and volume status should also be monitored to detect possible volume overload during the infusion, and the ultrafiltration rate should be adjusted accordingly to remove the extra fluid provided by IDPN [15]. In general, patients should also be monitored for possible hemodynamic intolerance, such as nausea, vomiting, discomfort, hypotension, respiratory distress, and cardiac arrhythmias during the IDPN infusion [45]. It is imperative that the target dry weight is regularly reevaluated to accommodate changes in body composition.
Table 3.
Suggested parameters to assess the safety and tolerance of IDPN
| Parameter | Frequency of measurements | Comments and suggested management | Frequency of occurrence |
|---|---|---|---|
| Symptoms related to reaction to IDPN: Nausea, vomiting, discomfort, hypotension, respiratory distress, and cardiac arrhythmias (rare) | In each HD session and especially during the first week of IDPN | • If reaction suspected, stop IDPN. | Very rare |
| Hemodynamic monitoring (BP, heart rate) and volume status should be closely monitored during and after hemodynamic monitoring | During and after each HD session. | • Assess dry weight and adequate length of dialysis session for an adequate ultrafiltration rate (< 10 mL/kg/hour).• Ultrafiltration rate should be adjusted accordingly to remove the extra fluid IDPN provides. | Common (changes in dry weight) |
| Blood glucose levelsa | • In patients with diabetes, measure glucose before the start dialysis, after 2 hours, and before finishing dialysis treatment.• In patients who do not have diabetes, measure as in patients with diabetes during the first 3 dialysis sessions, and then discontinue monitoring unless results were outside of safe blood glucose ranges.b | In case of hyperglycemia:• Subcutaneous short-acting insulin when glucose >12 mmol/L (>220 mg/dL) in increasing doses depending on blood glucose levels. In case of hypoglycemia:• Teach patients about the signs and symptoms of hypoglycemia.• Encourage all patients receiving IDPN to bring 15- to 30-g carbohydrate snack to the dialysis session and consume it 20–30 minutes before the end of the dialysis session. | Common in patients with diabetes and prediabetesVery rare if insulin is not used |
| Liver tests (alkaline phosphatase, ALT, total bilirubin) and blood triglycerides | Before dialysis session during the first 2 weeks, and then every 4–6 weeks to coincide with regular dialysis blood work. | In case of significant disturbances, stop IDPN and resume it once solved. | Very rare |
Blood may be drawn by finger poke or from the HD line. If drawn from the HD line and the result is high, repeat the test using the finger poke method to verify results (recirculation may result in falsely elevated blood glucose levels). ALT: alanine aminotransferase; BP: blood pressure; HD: hemodialysis; IDPN: intradialytic parenteral nutrition.
After three dialysis sessions, a pattern of persistently raised blood glucose levels is apparent in patients without diabetes, and then monitoring may be interrupted if results are inside of safe blood glucose ranges {e.g. <12 mmol/L [<220 mg/dL]} and the patient does not require additional subcutaneous insulin. Otherwise, glucose monitoring before the start of dialysis, after 2 hours, and before finishing dialysis treatment should be maintained.
Patients with diabetes do not require any specific considerations during IDPN, although monitoring for both hyperglycemia and hypoglycemia during IDPN is an important aspect of the support of all patients during the HD session [14]. The addition or adjustment of insulin may be needed to address hyperglycemia. In the case of a new or additional insulin requirement, the use of insulin analogues should be individually tailored through consultation with an endocrinologist to avoid postdialytic hypoglycemia [15]. A carbohydrate-rich snack consumed around 30 minutes before the discontinuation of IDPN infusion can aid in preventing postinfusion hypoglycemia.
Laboratory monitoring between IDPN infusions may be warranted to track long-term nutritional parameters [41]. The composition of the IDPN solution should be considered to guide a schedule for routine monitoring of blood glucose, triglycerides, and liver tests [46]. In cases of severe hypertriglyceridemia, IDPN is withheld or a solution is infused that excludes lipids.
Optimal duration of intradialytic parenteral nutrition
Although the optimal duration of IDPN may depend on individual patient factors, it is reasonable to suggest that a course of IDPN during HD should continue for a minimum of at least 3 months to allow for meaningful evaluation [47]. After this period, nutritional status should be reassessed to guide the continued need for IDPN.
Determining clinical success with intradialytic parenteral nutrition
Monitoring response to therapy is a critical aspect of patient care, and a variety of measures may be proposed to assess the effectiveness of IDPN over time, as summarized in Table 4 [47]. The choice of markers in this table is based on our clinical experience and on the markers and tools usually available at the clinic. These are general markers of nutritional status, not specific to IDPN, and we believe that any improvement in them is likely to indicate a gain in nutritional status. In agreement with the 2020 KDOQI guidelines [15], we recommend assessing two or more biomarkers at a time and interpreting them together, as all of them are imperfect measures that may be influenced by processes such as inflammation or fluid overload. Some but not all of these parameters have been used as outcome measures in IDPN-related RCTs, including body weight [34, 35, 37, 40, 41], albumin [34–41], and prealbumin [35, 37, 39, 41] (Table 1), showing in general good response to the intervention. We suggest that other indicators of nutritional status that are commonly used to evaluate effectiveness of ONS, particularly subjective global assessment, protein intake, and body composition (e.g. by bioelectrical impedance) [15], may also offer important insights into the effectiveness of IDPN, despite not having been formally assessed in clinical trials. We also suggest that repeated assessments of muscle strength by handgrip strength as well as measurements of gait speed could help assess IDPN efficacy over time.
To summarize our suggestions based on current evidence, experience, and best practices, we have developed the suggested protocol shown in Box 2.
Box 2.
A suggested summary protocol for intradialytic parenteral nutrition support
Consider progression of support to IDPN:
Patients unable to maintain adequate oral intake or follow dietary counseling*
Patients unable to tolerate adequate oral intake because of comorbid gastrointestinal issues
Gastrointestinal issues related to poor tolerability of ONS (bloating, early satiety, diarrhea, nausea and regurgitation)
Choice of IDPN composition:
Consider an amino acid target >0.5 g/kg body weight (or ideal body weight)
Max 50–80 g glucose per session
Max 30–50 g lipids per session
No evidence for enrichment with n-3 PUFA
Addition of vitamins or trace elements is rarely needed
IDPN setup:
Pump flow 250–300 mL/hour for patients with body weight ≥60 kg, proportionally decreased rate for patients with lower body weight; start with pump flow 125 mL/hour in first week
Patient monitoring during IDPN:
-
Symptoms related to IDPN tolerance:
○ In each HD session and especially during the first week of IDPN
-
Hemodynamic monitoring:
○ Blood pressure, heart rate, and volume status should be closely monitored during and after the HD session
-
Blood glucose:
○ In patients with diabetes, measure glucose before the start dialysis, after 2 hours, and before the end of the HD session
○ In patients who do not have diabetes, measure glucose before the start dialysis, after 2 hours, and before the end of the HD session during the first three HD sessions, and then discontinue monitoring unless results are outside safe blood glucose ranges
-
Liver tests, triglyceride levels:
○ Before the HD session during the first 2 weeks, and then every 4–6 weeks to coincide with regular dialysis blood work
Length of IDPN intervention:
Minimum of 3 months, up to 6 months, barring the need to discontinue
Assessments of IDPN efficacy:
-
During and after every HD session:
○ Changes in patient dry weight
-
With initial session, then weekly for 2 weeks, then every 4–6 weeks to coincide with regular dialysis blood work:
○ Predialysis albumin and prealbumin levels
○ Body stores assessment by bioelectrical impedance
-
With initial session, then every 3 months:
○ Subjective global assessment
○ Dietary interview or diary
○ Handgrip strength
○ Gait speed
-
Monthly:
○ Normalized protein nitrogen appearance
-
Reassess IDPN plan at 6 months
*Consider enteral nutrition when spontaneous intake <20 kcal/kg/day or <0.8 g protein/kg/day.
Practical considerations and potential barriers to intradialytic parenteral nutrition
The use of IDPN is challenged not only by the outstanding clinical questions we have discussed but by operational and administrative challenges unique to this setting. For instance, in a recent survey of kidney dietitians in Australia, bureaucratic obstacles and misconceptions about the value of IDPN as a method for routine nutritional support have both been cited as hurdles to IDPN. Nevertheless, IDPN use was not reported as uncommon, and respondents cited the presence of a consistent care protocol, support from medical and administrative staff, and dietitian experience as factors facilitating the regular use of IDPN [18]. Staff availability and training are also critical factors in a facility's ability to consistently provide IDPN. This finding likely indicates a need for all stakeholders involved in IDPN, including clinicians, dietitians, nurses, patients, caregivers, and care coordinators, to ensure the success of this or any other nutritional intervention in patients with CKD.
Costs and regulatory concerns
Regulatory concerns and costs are other major factors that affect IDPN use in clinical practice, especially in the United States, because of variable insurance eligibility and resulting coverage of costs [30, 48, 49]. The costs of IDPN are often considerably greater than those associated with ONS or nutritional counseling, and comparative efficacy studies suggest similar benefits from both therapies [48]. Such conclusions often refer to a 1998 study that examined IDPN-related costs and did not demonstrate lasting overall cost savings with IDPN after 6 months of therapy [30, 48, 49]. The authors concluded that for IDPN to be considered a clinically superior and cost-effective treatment, data would be needed to demonstrate that IDPN consistently reduced the risk of mortality; improved patient function and QOL; and provided benefits that were specific to IDPN therapy and not the result of potential confounders in terms of patient population, baseline nutritional status, or other clinical factors [48]. We argue that such evidence is not available for any form of nutritional support in the context of CKD. Also, costs in 1998 may not reflect current costs, and reimbursement practices across countries may provide a different scenario to the US system. There is strong evidence, however, that treating malnutrition is cost-effective: In one 2007 regression modeling analysis of US dialysis patient data, researchers projected that 1400 lives could be saved and $36 million in Medicare costs could be avoided with increases in albumin levels of 0.2 g/dL as a result of nutritional support [50].
Guidance for future research
Future trials in IDPN should aim for a level of robustness that allows for the evaluation of important aspects of therapy, including the speed of restoration of nutritional status compared with other interventions, the capacity of IDPN to sustain this improvement over time, and the impact of IDPN on the prevention of muscle loss as well as muscle gain (Box 3). We recognize that the sample size and the duration of these trials may be challenging.
CONCLUSIONS
IDPN may be a useful nutritional strategy for patients with PEW, alone or in combination with ONS. The need for trained personnel for setup and patient monitoring and the costs associated with IDPN compared with ONS or counseling remain important challenges to its consistent use in practice.
Because adequate nutrition has such an important impact on patient mobility and function, future research is warranted to investigate whether improved nutritional support with a strategy like IDPN in patients with CKD may preserve nutritional status and muscle mass, which affects physical function and QOL over time. As current evidence is limited, we further suggest that future studies should better evaluate parameters such as the optimal patient population, duration of treatment, and composition of IDPN. In the meantime, our own clinical experience and current guidance suggests that IDPN is a valuable tool to help maintain nutritional status in many patients with CKD who hare undergoing HD.
Box 3.
Suggestions for future intervention studies
Topic: Effectiveness of IDPN in treating PEW
-
Comparator:
○ Other type of nutritional support (e.g. ONS)
-
Short-term outcomes of interest:
○ Speed of restoration of nutritional status
○ Muscle mass
○ Functional capacity
-
Possible trial duration:
○ 3–4 months
Topic: Effectiveness of IDPN in preventing deterioration of nutritional status
-
Study design:
○ Phase 1: effectiveness study as above (malnourished patients are treated for a short period until nutritional status is restored)
○ Phase 2: effect of sustained IPDN on prevention of new malnutrition event (patients are then randomized to remain on or discontinue IDPN)
-
Long-term outcomes:
○ Time to a new malnutrition event
-
Possible duration of such trial:
○ Phase 1: 3–4 months
○ Phase 2: ≥2 years
ACKNOWLEDGEMENTS
We would like to dedicate this work to the memory of Gabrielle Luft, Ph.D.
Contributor Information
Juan J Carrero, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
David Severs, Department of Internal Medicine, Division of Nephrology and Transplantation, Erasmus Medical Center, Rotterdam, The Netherlands.
Didier Aguilera, Centre Hospitalier de Vichy, Vichy, France.
Enrico Fiaccadori, Parma University Medical School Hospital, Parma, Italy.
Martin G Gonzalez, Hospital Universitario de La Princesa, Madrid, Spain.
Christoph C Haufe, KfH Kidney Center, Erfurt, Germany.
Daniel Teta, Hôpital du Valais, Sion, Switzerland.
Pablo Molina, Department of Nephrology, FISABIO, Hospital Universitari Doctor Peset, Universitat de València, Valencia, Spain.
Wesley Visser, Department of Internal Medicine, Division of Dietetics, Erasmus Medical Center, Rotterdam, The Netherlands.
FUNDING
The authors received no financial support for the research, authorship, and/or publication of this article. The organization of the Virtual Meeting that motivated this publication was funded by Baxter Healthcare SA.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed.
CONFLICT OF INTEREST STATEMENT
J.J. Carrero has received speaker fees from Baxter Healthcare SA. M.G. Gonzalez has engaged in expert testimony for Baxter Biopharma and has received speaker fees from AstraZeneca. C.C. Haufe has received speaker fees from Baxter Healthcare SA, AstraZeneca, and Astellas Pharma Inc, and consulting fees from CSL Vifor. P. Molina has received consulting and/or speaker fees from Fresnius-Kabi, CSL Vifor, Baxter, Abbott, Amgen, Palex Medical, and Sanofi. D. Severs has received consulting fees, speaker fees, and research grant funds from Baxter. W.J. Visser has received speaker fees and research grant funds from Baxter.
REFERENCES
- 1. Martinson M, Ikizler TA, Morrell Get al. Associations of body size and body composition with functional ability and quality of life in hemodialysis patients. Clin J Am Soc Nephrol 2014;9:1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rambod M, Bross R, Zitterkoph Jet al. Association of malnutrition-inflammation score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis 2009;53:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stenvinkel P. Inflammation in end-stage renal disease—a fire that burns within. In: Ronco C, Brendolan A, Levin NW (eds.), Cardiovascular Disorders in Hemodialysis. Basel: Karger Publishers, 2005; Vol. 149, 185–99. [DOI] [PubMed] [Google Scholar]
- 4. Fouque D, Kalantar-Zadeh K, Kopple Jet al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008;73:391–8. [DOI] [PubMed] [Google Scholar]
- 5. Visser WJ, de Mik-van Egmond AM, Timman Ret al. Risk factors for muscle loss in hemodialysis patients with high comorbidity. Nutrients 2020;12:2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcelli D, Brand K, Ponce Pet al. Longitudinal changes in body composition in patients after initiation of hemodialysis therapy: results from an international cohort. J Ren Nutr 2016;26:72–80. [DOI] [PubMed] [Google Scholar]
- 7. Pupim LB, Heimbürger O, Qureshi ARet al. Accelerated lean body mass loss in incident chronic dialysis patients with diabetes mellitus. Kidney Int 2005;68:2368–74. [DOI] [PubMed] [Google Scholar]
- 8. Pupim LB, Flakoll PJ, Majchrzak KMet al. Increased muscle protein breakdown in chronic hemodialysis patients with type 2 diabetes mellitus. Kidney Int 2005;68:1857–65. [DOI] [PubMed] [Google Scholar]
- 9. Carrero JJ, Stenvinkel P, Cuppari Let al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 2013;23:77–90. [DOI] [PubMed] [Google Scholar]
- 10. Ikizler TA, Wingard RL, Hakim RM.. Interventions to treat malnutrition in dialysis patients: the role of the dose of dialysis, intradialytic parenteral nutrition, and growth hormone. Am J Kidney Dis 1995;26:256–65. [DOI] [PubMed] [Google Scholar]
- 11. Cano NJ, Leverve XM.. Intradialytic nutritional support. Curr Opin Clin Nutr Metab Care 2008;11:147–51. [DOI] [PubMed] [Google Scholar]
- 12. Fuhrman MP. Intradialytic parenteral nutrition and intraperitoneal nutrition. Nutr Clin Pract 2009;24:470–80. [DOI] [PubMed] [Google Scholar]
- 13. Dukkipati R, Kalantar-Zadeh K, Kopple JD.. Is there a role for intradialytic parenteral nutrition? A review of the evidence. Am J Kidney Dis 2010;55:352–64. [DOI] [PubMed] [Google Scholar]
- 14. Sabatino A, Regolisti G, Antonucci Eet al. Intradialytic parenteral nutrition in end-stage renal disease: practical aspects, indications and limits. J Nephrol 2014;27: 377–83. [DOI] [PubMed] [Google Scholar]
- 15. Ikizler TA, Burrowes JD, Byham-Gray LDet al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 2020;76(3 suppl 1): S1–S107. [DOI] [PubMed] [Google Scholar]
- 16. Druml W, Contzen B, Joannidis Met al. , DGEM Steering Committee . S1-Guideline of the German Society for Nutritional Medicine (DGEM) in cooperation with the GESKES, the AKE and the DGfN: enteral and parenteral nutrition in patients with kidney disease [in German]. Aktuel Ernahrungsmed 2015;40:21–37. [Google Scholar]
- 17. Cano NJ, Aparicio M, Brunori Get al. ESPEN guidelines on parenteral nutrition: adult renal failure. Clin Nutr 2009;28:401–14. [DOI] [PubMed] [Google Scholar]
- 18. Lambert K, Conley MM.. Practice patterns relating to the use of intradialytic parenteral nutrition in Australian renal units: results from a survey of renal dietitians. J Ren Nutr 2020;30:163–7. [DOI] [PubMed] [Google Scholar]
- 19. Lopes AA, Elder SJ, Ginsberg Net al. Lack of appetite in haemodialysis patients—associations with patient characteristics, indicators of nutritional status and outcomes in the international DOPPS. Nephrol Dial Transplant 2007;22:3538–46. [DOI] [PubMed] [Google Scholar]
- 20. Carrero JJ, Thomas F, Nagy Ket al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr 2018;28:380–92. [DOI] [PubMed] [Google Scholar]
- 21. Lofberg E, Essen P, McNurlan Met al. Effect of hemodialysis on protein synthesis. Clin Nephrol 2000;54:284–94. [PubMed] [Google Scholar]
- 22. Mokrzycki MH, Kaplan AA.. Protein losses in continuous renal replacement therapies. J Am Soc Nephrol 1996;7:2259–63. [DOI] [PubMed] [Google Scholar]
- 23. Davies SP, Reaveley DA, Brown EAet al. Amino acid clearances and daily losses in patients with acute renal failure treated by continuous arteriovenous hemodialysis. Crit Care Med 1991;19:1510–15. [DOI] [PubMed] [Google Scholar]
- 24. Schepky AG, Bensch KW, Schulz-Knappe Pet al. Human hemofiltrate as a source of circulating bioactive peptides: determination of amino acids, peptides and proteins. Biomed Chromatogr 1994;8:90–94. [DOI] [PubMed] [Google Scholar]
- 25. Veeneman JM, Kingma HA, Boer TSet al. Protein intake during hemodialysis maintains a positive whole body protein balance in chronic hemodialysis patients. Am J Physiol Endocrinol Metab 2003;284:E954–65. [DOI] [PubMed] [Google Scholar]
- 26. Caglar K, Fedje L, Dimmitt Ret al. Therapeutic effects of oral nutritional supplementation during hemodialysis. Kidney Int 2002;62:1054–9. [DOI] [PubMed] [Google Scholar]
- 27. Bossola M, Tazza L, Giungi Set al. Artificial nutritional support in chronic hemodialysis patients: a narrative review. J Ren Nutr 2010;20:213–23. [DOI] [PubMed] [Google Scholar]
- 28. Pupim LB, Flakoll PJ, Brouillette JRet al. Intradialytic parenteral nutrition improves protein and energy homeostasis in chronic hemodialysis patients. J Clin Invest 2002;110:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sadu Singh BK, Abdul Gafor AH, Fiaccadori Eet al. Enabling intradialytic parenteral nutrition in maintenance haemodialysis patients in Malaysia: the what, who and how scenarios of implementation? Malays Appl Biol 2018;47:1–11. [Google Scholar]
- 30. Chan W. Chronic kidney disease and nutrition support. Nutr Clin Pract 2021;36:312–30. [DOI] [PubMed] [Google Scholar]
- 31. Hubbard GP, Elia M, Holdoway Aet al. A systematic review of compliance to oral nutritional supplements. Clin Nutr 2012;31:293–312. [DOI] [PubMed] [Google Scholar]
- 32. Ikizler TA. Nutrition support for the chronically wasted or acutely catabolic chronic kidney disease patient. Semin Nephrol 2009;29:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burrowes JD. Issues affecting dietary adherence. In: Byham-Gray LD, Burrowes JD, Chertow GM (eds.). Nutrition in Kidney Disease. 2nd ed.New York: Springer, 2014; 405–11. [Google Scholar]
- 34. Guarnieri G, Faccini L, Lipartiti Tet al. Simple methods for nutritional assessment in hemodialyzed patients. Am J Clin Nutr 1980;33:1598–1607. [DOI] [PubMed] [Google Scholar]
- 35. Cano N, Labastie-Coeyrehourq J, Lacombe Pet al. Perdialytic parenteral nutrition with lipids and amino acids in malnourished hemodialysis patients. Am J Clin Nutr 1990;52:726–30. [DOI] [PubMed] [Google Scholar]
- 36. Navarro JF, Mora C, León Cet al. Amino acid losses during hemodialysis with polyacrylonitrile membranes: effect of intradialytic amino acid supplementation on plasma amino acid concentrations and nutritional variables in nondiabetic patients. Am J Clin Nutr 2000;71:765–73. [DOI] [PubMed] [Google Scholar]
- 37. Cano NJ, Fouque D, Roth Het al. , French Study Group for Nutrition in Dialysis . Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: a 2-year multicenter, prospective, randomized study. J Am Soc Nephrol 2007;18:2583–91. [DOI] [PubMed] [Google Scholar]
- 38. Liu Y, Xiao X, Qin DPet al. Comparison of intradialytic parenteral nutrition with glucose or amino acid mixtures in maintenance hemodialysis patients. Nutrients 2016;8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marsen TA, Beer J, Mann H, German IDPN-Trial group . Intradialytic parenteral nutrition in maintenance hemodialysis patients suffering from protein-energy wasting. Results of a multicenter, open, prospective, randomized trial. Clin Nutr 2017;36:107–17. [DOI] [PubMed] [Google Scholar]
- 40. Thabet AF, Moeen SM, Labiqe MOet al. Could intradialytic nutrition improve refractory anaemia in patients undergoing haemodialysis? J Ren Care 2017;43:183–91. [DOI] [PubMed] [Google Scholar]
- 41. Cano NJ, Saingra Y, Dupuy AMet al. Intradialytic parenteral nutrition: comparison of olive oil versus soybean oil-based lipid emulsions. Br J Nutr 2006;95:152–9. [DOI] [PubMed] [Google Scholar]
- 42. Fiaccadori E, Sabatino A, Barazzoni Ret al. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin Nutr 2021;40:1644–68. [DOI] [PubMed] [Google Scholar]
- 43. Akner G, Cederholm T.. Treatment of protein-energy malnutrition in chronic nonmalignant disorders. Am J Clin Nutr 2001;74:6–24. [DOI] [PubMed] [Google Scholar]
- 44. Kopple JD. Trace elements and vitamins. In: Mitch WE, Ikizler TA (eds.) Handbook of Nutrition and the Kidney. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2010, 163–76. [Google Scholar]
- 45. BCRenal Provincial Health Services Authority . November 2019. Provincial Standards and Guidelines: Intradialytic Parenteral Nutrition (IDPN). http://www.bcrenal.ca/resource-gallery/Documents/Intradialytic_Parenteral_Nutrition%28IDPN%29.pdf (February 5, 2021, date last accessed). [Google Scholar]
- 46. Sarav M, Friedman AN.. Use of intradialytic parenteral nutrition in patients undergoing hemodialysis. Nutr Clin Pract 2018;33:767–71. [DOI] [PubMed] [Google Scholar]
- 47. García de Lorenzo A, Arrieta J, Ayúcar Aet al. Nutrición parenteral intradiálisis en el enfermo renal crónico: consenso SEN-SENPE [Intra-dialysis parenteral nutrition in chronic renal patients: consensus SEN-SENPE]. Nutr Hosp 2010;25:375–77. [PubMed] [Google Scholar]
- 48. Anderson J, Peterson K, Bourne Det al. Effectiveness of intradialytic parenteral nutrition in treating protein-energy wasting in hemodialysis: a rapid systematic review. J Ren Nutr 2019;29:361–9. [DOI] [PubMed] [Google Scholar]
- 49. Cranford W. Cost effectiveness of IDPN therapy measured by hospitalizations and length of stay. Nephrol News Issues 1998;12:33–5, 37–39. [PubMed] [Google Scholar]
- 50. Lacson E Jr, Ikizler TA, Lazarus JMet al. Potential impact of nutritional intervention on end-stage renal disease hospitalization, death, and treatment costs. J Ren Nutr 2007;17:363–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed.