Abstract
Cannabis is the most widely used recreational drug in the world. Cannabis sativa and Cannabis indica have been selectively bred to develop their psychoactive properties. The increasing use in many countries has been accelerated by the COVID-19 pandemic. Cannabis can provoke both type 1 and type 4 allergic reactions. Officially recognized allergens include a pathogenesis-related class 10 allergen, profilin, and a nonspecific lipid transfer protein. Other allergens may also be relevant, and recognition of allergens may vary between countries and continents. Cannabis also has the potential to provoke allergic cross-reactions to plant foods. Since cannabis is an illegal substance in many countries, research has been hampered, leading to challenges in diagnosis since no commercial extracts are available for testing. Even in countries such as Canada, where cannabis is legalized, diagnosis may rely solely on the purchase of cannabis for prick-to-prick skin tests. Management consists of avoidance, with legal issues hindering the development of other treatments such as immunotherapy. Education of healthcare professionals is similarly lacking. This review aimed to summarize the current status of cannabis allergy and proposes recommendations for the future management of this global issue.
Keywords: allergy diagnosis, allergy treament, cannabis allergy, IgE, occupational allergies
1 ∣. INTRODUCTION
Approximately 192 million people, 3.9% of the global population use Cannabis sativa (Can s) for medical and/or recreational purposes.1-4 The legal status of cannabis varies, but one recent key driver of consumption is the COVID-19 pandemic, with rising intakes during lockdown, especially when self-isolating.5-7 Adverse effects associated with cannabis use include respiratory, dermatologic, gastrointestinal and cardiovascular symptoms, and mental health issues.8 These effects are often ignored or overlooked in favor of the positive benefits of reduced anxiety, improved sleep, pain relief, and decreased nausea.9 First described 50 years ago, allergic reactions to cannabis can present with symptoms of rhinitis, conjunctivitis, asthma, cutaneous reactions due to industrial contact and anaphylaxis to hemp seed.10-12 As well as exposure through ingestion, inhalation, or skin contact, cannabis can also provoke occupational allergies.13
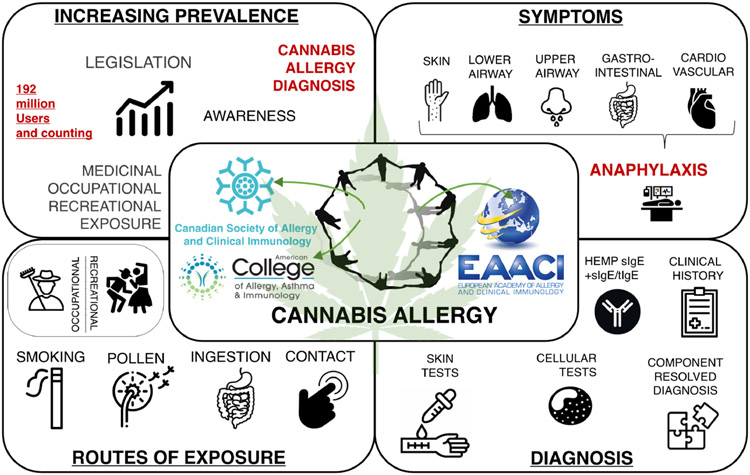
Rising consumption underlines the need for greater awareness of the spectrum of cannabis allergy, its diagnosis and management. In recognition of this unmet need, members of the American College of Asthma Allergy and Immunology (ACAAI), the European Academy of Allergy and Clinical Immunology (EAACI) and the Canadian Society of Allergy and Clinical Immunology (CSACI) formed a joint Cannabis Allergy Interest Group (CAIG) (Appendix I in Appendix S1). This group developed a plan of action, which includes a survey of the membership of the three societies, the development of an international registry and biobank, and the publication of this joint paper on cannabis allergy (Figure 1).
FIGURE 1.
Current status of cannabis Allergy
2 ∣. BACKGROUND AND EPIDEMIOLOGY OF CANNABIS USE
Cannabis has been used for five millennia for spiritual, medicinal, and recreational purposes, with recent archaeological evidence of ritualized consumption dating from 500 BC.14,15 Cannabis use varies worldwide and rates of consumption do always correlate with legalization status; the illicit use of cannabis can be similar to that reported in countries where it has been decriminalized or fully legalized. Although there is some variation between countries and continents, the highest use is in young adults, although the age range of cannabis users is increasing and is projected to continue to do so.16-18
2.1 ∣. United States
Cannabis was prohibited in the United States by the 1937 Marijuana Tax Act, and further restricted in 1970 following its classification as a Schedule I substance.19 In 1996, medical cannabis was legalized in California, with recreational cannabis legalized in Colorado and Washington states in 2012, Colorado being the first to roll out commercial sales for adult non-medical use in 2014. Currently, 36 states, and the District of Columbia (DC), have moved to legalize medical cannabis, 15 states, and DC, legalizing recreational cannabis, and 16 states decriminalizing its use.20 However, cannabis remains illegal under US Federal Law.21 In the United States, roughly one in 7–8 individuals has engaged in medical cannabis use.22 Since the early 2000s, there has been little change in adolescent use, but an increase in adult cannabis use, especially in those aged 50 years and over.23 Cannabis use across most sociodemographic groups is more common in states where it has been fully legalized, whereas the effect of medical legalization is heterogeneous by age and socioeconomic status.24 A study evaluating cannabis attitudes and patterns of use among followers of the Allergy & Asthma Network, reported that 88/489 (18%) of respondents used cannabis, 66% for medical reasons.9 Just over half (58%) of these individuals reported asthma, especially uncontrolled asthma, but asthma control, quality of life, and frequency of exacerbations were no different in respondents with asthma who did and did not report cannabis use. Of the non-cannabis users, 2.5% reported cannabis allergy.
2.2 ∣. Canada
In Canada, the Cannabis Act of 2018 legalized cannabis, giving it a similar status to that of alcohol.25 Cannabis use has been assessed since 1989 by the annual Canadian Cannabis Survey (Health Canada), and the quarterly National Cannabis Survey (Statistics Canada).26-35 The prevalence of lifetime cannabis use has gradually increased from 23.2% (1989) to 45.3% (2019).16,26 At the end of 2020, 20% of Canadians reported Cannabis use within the past 3 months, compared with 14% immediately preceding its legalization.35,36 Surveys suggest 25% of Canadians consumed cannabis in 2020, mainly those aged 18–44 years, with 7.9% reporting almost daily use.9,36 Most Canadians consume cannabis via smoking (79%), followed by ingestion (52%), with the use of edible cannabis products increasing, and dried flower or leaf use decreasing.34,36 It is reported that 14% of Canadians used cannabis for medical purposes in the past year, with 321,539 active medical cannabis federal license holders in Canada as of December 2020.34,37 A cross-sectional survey of adults in a Canadian allergy clinic found that 107/179 had a lifetime past use of cannabis, with 42/107 reporting symptoms upon exposure that may be explained by cannabis allergy.38
2.3 ∣. Europe
Cannabis is the most widely used illicit drug in Europe, with a higher prevalence in Mediterranean and Central-Western European countries.39 Some cannabinoids are approved for medicinal use in some European countries, while in others the recreational use of cannabis has been decriminalized.40 In the 15–64 age group, approximately 90.2 million people (27%) in the European Union have used cannabis at least once in their lifetime, ranging from over 33% of adults in Denmark, France, and the United Kingdom, to 8% or less in Bulgaria, Romania, Turkey, and Malta.37,41 Long-term trends show that, apart from Sweden, many countries have a modest increase in prevalence, although only a small percentage (1.8%) of adults in the EU are daily/almost daily users.42,43 The lifetime prevalence of cannabis use in Europe is projected to grow from 28.3% in 2015 to 42.0% in 2045, but 30 days use is projected to only increase slightly (2.7%–3.4%) accompanied by an increase in the age of users.17
3 ∣. MEDICAL CANNABIS USE
Medical cannabis generally refers to the use of cannabis to treat disease or alleviate symptoms.44 In ancient Chinese, Greek, and Middle Eastern culture, cannabis was used for its psychoactive and medical effects.45 In western culture, there was more disease-targeted treatment beginning in the 19th century and cannabis was utilized by physicians world-wide in the pharmacopeia of the time.46,47 Regulation of the medical use of cannabis started in the 20th century, and the widespread use and acceptance of medical cannabis often depends on its legal status in the nation or state.44 Medical cannabis is used in various preparations, including smoking, vaporizing, ingestion of raw plant products, or consumption of capsules or oils in manufactured extracts. In the US, 3 cannabinoids, dronabinol, nabilone, and cannabidiol (CBD) are approved for medical use by the US Food and Drug Administration.48 In Europe, some countries have introduced specific laws to permit the use of cannabis preparations to treat the symptoms of severe diseases.49 The extent of the possible medical uses of cannabis is quite exhaustive and include, multiple sclerosis, arthritis, epilepsy, some psychiatric conditions, and chemotherapy-induced nausea and vomiting in cancer patients.50 The highest quality of evidence exists for its use in chronic pain spasticity and the anti-epileptic activity in the therapy-resistant Dravet and Lennox–Gastaut syndromes.44,51 The use of cannabis for pain relief may also be common; in one survey, over one-third of Danish patients with spinal cord injury had tried cannabis at least once and 9% were current users, although most of these patients also used cannabis before their injury.52
4 ∣. BOTANY OF CANNABIS AND PROPOSED ALLERGENS
Cannabis belongs to the Cannabaceae family, can grow in diverse climates, and is found in most parts of the world. Cannabis is an annual herb, largely dioecious with separate male and female plants. It is easily recognized by the distinctive arrangement of leaves. The plant is marked by hair-like glandular projections called trichomes, which serve as a rich source of cannabinoids. Cannabis sativa and Cannabis indica (and to a lesser extent Cannabis ruderalis) are the most common botanical varieties, but there is significant diversity in the biological and vernacular use of terms to identify different strains.53,54 This is largely due to the fact that since the 1970s, the plant has undergone significant selective hybridization and crossbreeding, leading to the development of strains with poorly characterized botanical backgrounds. Consequently, a wide variety of cannabis strains are in circulation, selected for their unique biochemical and mood-altering profile. However, genetic studies have shown that there is a merging of Cannabis sativa and Cannabis indica and that the separation of the two is becoming less common.55 Another class of Cannabis sativa is hemp, the cultivars of which are grown specifically for industrial or medicinal use. It is unrelated to water hemp (Amaranthus tuberculatus) which is a dioecious plant native to North America.
Over 140 phytocannabinoids have been identified in cannabis, although only those with a terpenyl residue including geranyl (CBG-type), menthyl (CBD-type and THC-type), or prenylchromanyl (CBC-type) are present in any significant amount.56-58 Delta-9-Tetrahydrocannabinol (THC) is the most well-known cannabinoid that leads to psychoactive effects and, due to selective crossbreeding, the proportion of THC in cannabis has risen from 5% to 15% or more, leading to increases in psychoactivity and adverse effects.59,60 The US federal government defines cannabis with more than 0.3% THC as a Class I Controlled Substance. However, derivatives of cannabis containing less than 0.3% THC, such as CBD oil and hemp may be legal in some states or countries.61
Cannabis proteins can act as high-molecular weight allergens and contribute to type I allergic reactions.62 Multiple cannabis allergens have been sequenced, four of which are accepted by the WHO/IUIS Allergen Nomenclature Subcommittee (www.allergen.org). (Table 1). Molecular analysis has suggested the presence of other diverse allergenic proteins in cannabis including ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCo), adenosine triphosphate synthase, glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, heat shock protein 70,63 thaumatin-like protein,64 peptinesterases and polygalactouranases.65 Plant carbohydrates may also play a role in IgE-binding and perceived allergy, and glycoproteins that cross-react with other carbohydrates, known as cross-reactive carbohydrate domains (CCDs) may partly explain cross-reactivity.63,66 Additional studies are needed to clarify the relative contribution of glycosylation sites on cannabis proteins to the allergic sensitization potential of the plant.
TABLE 1.
Cannabis allergens
| Allergen | IUIS ID |
|---|---|
| Profilin67 | Can s 2 |
| Nonspecific lipid transfer protein75,76 | Can s 3 |
| Oxygen-evolving enhancer protein 263,122 | Can s 4 |
| Pathogenesis related protein 10 homologue67 | Can s 5 |
Note: Details sourced from WHO/IUIS Allergen Nomenclature Subcommittee (www.allergen.org).
5 ∣. LINKS BETWEEN CANNABIS ALLERGY AND FOOD ALLERGY
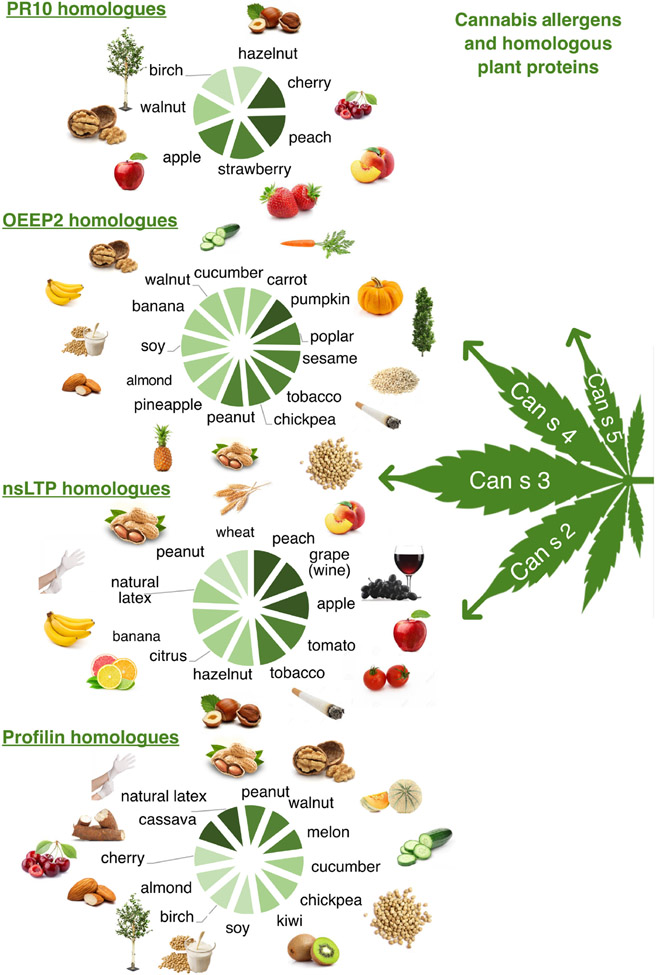
The relevance of different cannabis allergens may vary, depending on geographical location, prior sensitization to food or/pollen, and other factors.67 Nonspecific lipid transfer proteins are a common food allergen in Europe, and are stable to heat and digestion, thus provoking reactions to both raw and cooked plant foods.68-74 Sensitization to Can s 3, the nsLTP in cannabis, is a feature of European patients with cannabis allergy.75,76 It was first shown in 2007 that a patient sensitized to the peach nsLTP Pru p 3 reacted to Cannabis sativa; further research confirmed that Can s 3 was a relevant cause of sensitization and reactions to other nsLTP, even via passive inhalation.64,75,77-79 Thus Cannabis sativa could act as a primary sensitizing allergen, with a significant percentage of those allergic to cannabis reporting severe allergy to plant foods.75,80-83 Due to cross-reactivity between nsLTPs, patients sensitized to Can s 3 may develop sensitization and relevant clinical symptoms to a wide range of fruits, vegetables, and cereals, and also to wine, beer, Hevea latex, and tobacco (Figure 2).68,81,82,84,85 A feature of reactions to nsLTP is that they can occur in combination with co-factors (exercise, non-steroidal anti-inflammatory drugs or alcohol).72,83,86 Cannabis sativa may also act as a co-factor of reactions to foods in cannabis-allergic individuals.87 Sensitization to the Bet v 1 homologous cannabis allergen Can s 5 occurs in European individuals with cannabis allergy, but it is not clear whether these patients also have Bet v 1-related food allergy, otherwise known as pollen food syndrome (PFS) or oral allergy syndrome (OAS). Similarly, it is also unknown how prevalent sensitization is to other Cannabis sativa allergens such as the profilin Can s 2, the oxygen-evolving enhancer protein Can s 4, or the thaumatin-like protein, and whether they are relevant allergens in relation to food cross-reactions.63,64,67
FIGURE 2.
Cannabis Allergens and homologous plant proteins. There is proven cross-reactivity between Can s 3 and other nsLTP, whereas for the other sequenced cannabis allergens, this has not been investigated. However, there are protein amino acid identity matches across species suggesting possible IgE cross-reactivity
6 ∣. CLINICAL MANIFESTATIONS OF CANNABIS ALLERGY
Cannabis hypersensitivity spans the spectrum of allergic response and can provoke both type 1 and type 4 reactions.38,88 Cannabis pollen and/or cannabis smoke has been implicated in allergic rhinoconjunctivitis, allergic keratoconjunctivitis, hypersensitivity pneumonitis, and exacerbations of asthma symptoms.11,89 Additionally, patients may experience cutaneous reactions in the form of generalized pruritus, contact urticaria, angioedema, upper and lower respiratory tract symptoms, and anaphylaxis from cannabis use. Early reports of allergic reactions were of contact dermatitis after touching cannabis leaves or flowers, and toxico-dermatitis after smoking hemp.90,91 More recently, contact dermatitis has been described in a patient harvesting Cannabis sativa and Cannabis indica, which was proven by positive skin patch testing to the involved cannabis species.88 A case report detailed the development of sensitization and occupational contact urticaria to cannabis in a forensic sciences technician due to regular and repeated handling of cannabis.92 Erythema multiforme has also been associated with recreational consumption.93 Anaphylaxis to Cannabis sativa with hemp seed ingestion, smoking, and injection have also been reported.10,94,95 It is not only users or those at high occupational risk who may be affected by cannabis exposure; there are case reports of cannabis allergy, or allergies to foods which cross-react with cannabis, due to passive inhalation of cannabis smoke at home.96,97 Exposure to cannabis has expected, but undesirable physiologic effects including conjunctival injection, sinus tachycardia, orthostatic hypotension, anxiety or panic reactions, and dysphoria. If reported, such symptoms should not be ignored or misattributed if the index of suspicion for a serious reaction or anaphylaxis to cannabis is high.11
7 ∣. OCCUPATIONAL CANNABIS ALLERGY
Cannabis varieties such as hemp contain very low or undetectable levels of THC and have been used for many years as an agricultural commodity and source of fiber, with the seeds used in food products. In recent years, occupational sensitization to cannabis has mostly centered on the cannabis exposures of law enforcement officers and forensic investigators.92,98-100 However, increased access to cannabis for medicinal and recreational use has led to an emerging cannabis industry, currently valued at $11 billion, with a projected growth rate of approximately 15% through the next 5 years.101 Consequently, a considerable workforce is now engaged in growing, processing, and distributing cannabis for commercial use, thus allergic sensitization to cannabis is an emerging topic of interest.12,13 However, little is known of the potential for occupational allergic reactions in those with close contact and prolonged exposure to the plant and its derived materials. The Colorado Department of Public Health and Environment (CDPHE), and the National Institute for Occupational Safety and Health (NIOSH), have identified multiple biological hazards in cannabis work environments with potential to drive allergic reactions.102,103
Most allergic symptoms observed from direct handling of cannabis are typically marked by respiratory symptoms, ranging from rhinoconjunctivitis to bronchial hyperresponsiveness and chest tightness.13 Prolonged occupational exposure to hemp dust results in respiratory irritation, airflow obstruction, and inflammation called “byssinosis”.104-106 Byssinosis is marked by severe bronchial hyperresponsiveness, but occurs in absence of any specific IgE antibodies, suggesting lack of allergic sensitization. High levels of endotoxin in occupational hemp dust exposures have been consistently associated with adverse respiratory outcomes in exposed workers.107 Cutaneous reactions such as urticaria and angioedema are also commonly reported through occupational exposure.88 Although these symptoms resemble those observed in symptomatic recreational cannabis users, cross-reactivity does not appear to be a driver of symptoms. For example, Can s 3, a mediator of cross-reactive allergies related to cannabis, has not been established as a relevant allergen in the context of occupational exposures.13 Detailed studies into occupational cohorts are needed, including whether specific strains are more likely to provoke allergic reactions or if those with a previous allergic history have a higher rate of cannabis allergy.108
8 ∣. OTHER RISKS ASSOCIATED WITH CANNABIS CONSUMPTION
Cannabis consumption presents a risk to immunosuppressed patients via exposure to microbiological contaminants, particularly when inhaled. Aspergillus has been isolated from cannabis samples.109,110 In one observational study, the majority of cannabis users had antibody evidence of Aspergillus exposure, compared with a minority of controls.111 There is also an association between cannabis use and sensitization to Alternaria.112,113 Regarding the pulmonary effects of cannabis smoking on respiratory symptoms, a recent review found that, prevalence of chronic cough, sputum and wheeze were similar to those who smoke tobacco but the effects from habitual cannabis use on progressive obstructive lung disease and emphysema are less clear.114
9 ∣. DIAGNOSIS
The most important test for diagnosing IgE-dependent cannabis allergy is the clinical history, although patients may be reluctant to admit consumption where cannabis use is legally restricted. Determining whether symptoms are attributable to cannabis is challenging, especially in pollen allergic individuals.115 An example is a birch pollen allergic patient who experiences pronounced seasonal rhinoconjunctivitis while smoking cannabis outdoors during the pollen season. New-onset reactions to plant foods in teens and adults could also be linked to a cannabis allergy, but establishing whether a food allergy represents cross-reactivity with a pollen allergen (e.g., the Bet v 1 homologue) or cannabis allergen (e.g., the cannabis nsLTP Can s 3) may be difficult. Although younger patients may also be potentially exposed, cannabis allergy should be considered in those aged 16 years and over, presenting with symptoms of cough and wheeze indicating a new onset of asthma, or difficult to control existing asthma despite medication adherence.116 The creation of a standardized intake form on cannabis-related questions can further guide the physician/healthcare professional, such as the one developed by the ACAAI.117
After establishing the clinical history, it may not always be possible to undertake standard allergy tests. There are no commercially available extracts, so unstandardized prick–prick tests (PPT) with the buds, leaves, or seeds of the cannabis plant is usually the only option, if available.63,118 If possible, several different strains of the cannabis plant (sativa, indica and hybrid) should be used to ensure a wide variety of potential allergens are included.108 Unfortunately, those sensitized to pollens or plant foods can have clinically insignificant positive results, due to ubiquitous plant proteins such as profilins, decreasing the specificity of the cannabis PPT test. An alternative option is to perform SPTs with pre-prepared cannabis extracts, which can be better standardized, and designed to concentrate known allergen components such as Can s 3.75,82,115 Such extracts can achieve a negative predictive value (NPV) of 91%, but the positive predictive value (PPV) is low, around 50%, increasing to 80% in those reporting anaphylaxis and/or respiratory symptoms although the NPV is reduced to 58% (Table 2).75 Unfortunately such extracts may not be available in routine clinical practice, and also the same issue of cross-sensitization may occur in those individuals sensitized to nsLTP allergens.
TABLE 2.
Performance of different tests in Cannabis-sensitized and Cannabis allergic patients
| SPT nCan s 3-rich extract |
sIgE hemp (ImmunoCAP) |
sIgE rCan s 3 (CBA) |
CD63-BAT crude CS extract |
CD63-BAT Can S 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | A | B | |
| Sensitivity | 72% (51–89) | 58% (49–67) | 86% (66–97) | 82% (74–89) | 63% (41–81) | 47% (38–56) | 63% (38–84) | 49% (37–60) | 71% (48–89) | 45% (35–55) |
| Specificity | 81% (71–88) | 81% (71–88) | 32% (20–45) | 32% (20–45) | 87% (78–93) | 87% (78–93) | 67% (55–78) | 67% (55–78) | 85% (76–92) | 85% (76–92) |
| PPV | 51% (39–63) | 80% (72–86) | 33% (28–38) | 70% (66–74) | 56% (40–70) | 82% (72–89) | 35% (25–47) | 64% (54–73) | 54% (39–67) | 78% (67–86) |
| NPV | 91% (84–95) | 58% (53–64) | 86% (66–95) | 47% (34–61) | 90% (84–94) | 56% (51–60) | 87% (78–92) | 52% (46–59) | 93% (86–96) | 57% (52–62) |
| LHR+ | 3.7 (2.3–6.0) | 3.0 (1.9–4.7) | 1.3 (1.0–1.6) | 1.2 (1.0–1.5) | 4.7 (2.6–8.7) | 3.5 (2.0–6.2) | 1.9 (1.2–3.1) | 1.5 (1.0–2.2) | 4.8 (2.7–8.6) | 3.0 (1.8–5.2) |
| LHR− | 0.4 (0.2–0.7) | 0.5 (0.4–0.7) | 0.4 (0.1–1.3) | 0.6 (0.3–1.0) | 0.40 (0.3–0.7) | 0.60 (0.5–0.7) | 0.6 (0.3–1.0) | 0.8 (0.6–1.0) | 0.3 (0.2–0.7) | 0.7 (0.5–0.8) |
Note: A: Cannabis-anaphylaxis group vs. cannabis-tolerant individuals sensitized to either pollen alone, or to pollen and nsLTP.
B: Complete cannabis allergy group (both anaphylaxis and/or cutaneous symptoms) vs. cannabis-tolerant individuals sensitized to either pollen alone, or to pollen and nsLTP.
Test performance for both CD63-BAT's was calculated by considering both responders and non-responders to anti-IgE (positive control).
PPV and NPV = positive and negative predictive values, respectively. LHR+ and LHR− = positive and negative likelihood ratio's, respectively.
Adapted from75:
Abbreviations: CBA, cytometric bead assay; CD63, lysosomal membrane protein used as basophil degranulation marker; sIgE, specific IgE; SPT, skin prick test.
Currently, there are no commercial sIgE tests for Cannabis sativa or Cannabis indica, and a possible alternative, the sIgE hemp assay using a crude industrial hemp extract (FEIA ImmunoCAP® Phadia Thermofisher Scientific), is only available for research purposes (Table 2). Although the sensitivity exceeds 80% (Table 2), sIgE hemp is not a reliable diagnostic tool, because a significant proportion of positive results occur in cannabis-tolerant individuals due to sensitization to pollen and/or nsLTPs. Although CCDs rarely trigger clinically irrelevant SPT results, they can decrease the specificity of sIgE tests due to their structural similarity to allergens originating from both taxonomically related and distant plants, e.g., cannabis, pollen, plant-derived foods, Hevea latex and Hymenoptera venom.75,115,119 However, the specificity of sIgE hemp can be improved; using a cut-off of 0.02 for sIgE hemp-to-total IgE, achieves a specificity of 93% (95% confidence interval (CI), 85–98%).120
Molecular diagnostics using purified and/or recombinant (non-glycosylated) species-specific allergen components could be useful. However, a single component rarely covers the entire sensitization profile, and test results are influenced by age and/or population.121 Although four cannabis allergens are officially recognized (Table 1), only Can s 3 merits the status of a major allergen. In some European regions, Can s 3 covers approximately 70% of the sensitization profile, but its value in the US or Canada is unknown.75 Thus, Can s 3 tests cannot be used as a sole substitute for a whole extract test (Table 2), if component allergens are utilized. Unfortunately, sIgE to rPru p 3 from peach (Prunus persica), a biomarker often used to depict sensitization to nsLTP, frequently fails to detect sIgE to rCan s 3.75 It is unknown whether further studies with the more recently identified cannabis allergens Can s 2, Can s 4, and Can s 5,63,67,122 or allergens such as ribulose-1,5-bisphospate carboxylase/oxygenase63 and thaumatin-like protein64 will advance the molecular diagnosis of cannabis allergy. It is also unknown whether ratios between whole extract sIgE/total IgE and/or component sIgE/whole extract sIgE might benefit the diagnosis, as might be achieved for hemp.120 Should all component tests be negative, sensitization to CCDs can be detected with commercially available assays.119
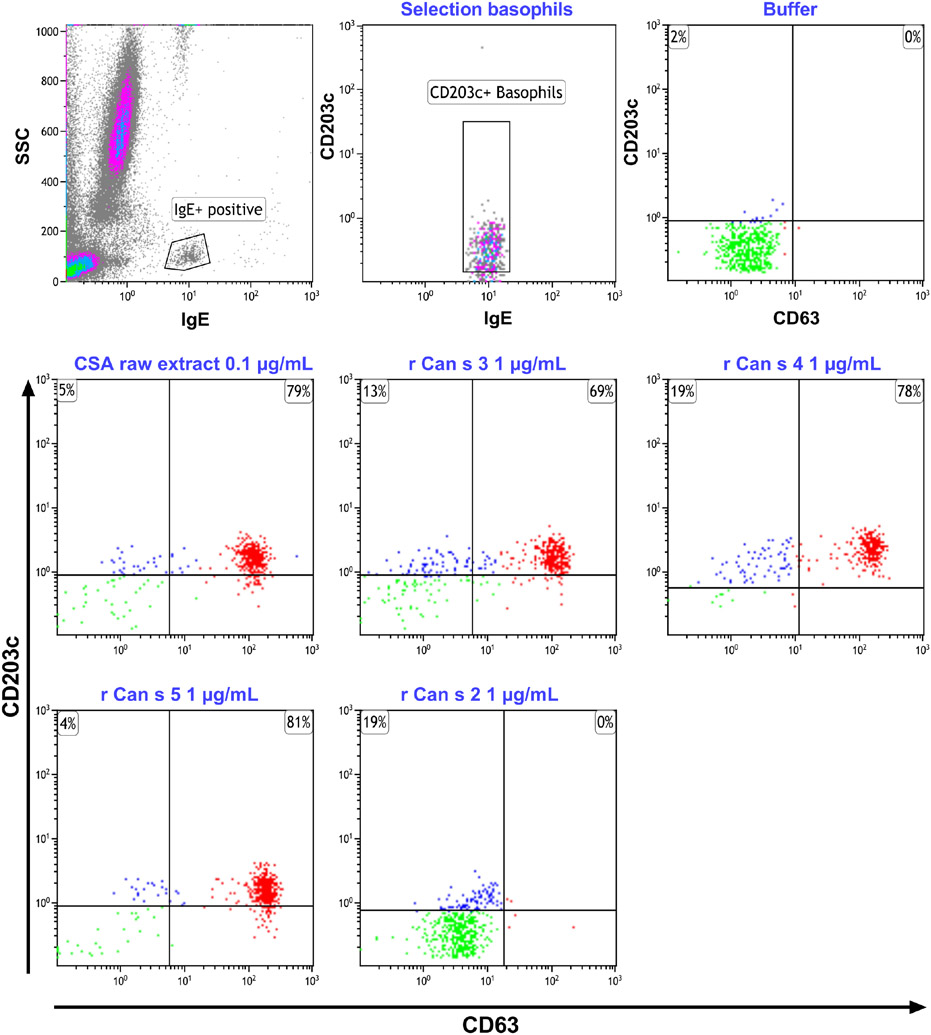
Cellular tests such as ex vivo basophil activation tests (BAT), and mast cell activation tests (pMAT) may also be useful (see Fig S1).67,123,124 Unlike, traditional sIgE binding assays, they more closely reflect the in vivo situation, and in addition to SPTs, can help to detect sensitization to CCDs, and enable safe evaluation of recombinant components (Figure 3).75,115 BAT using rCan s 3 has predictive values similar to SPTs with a Can s 3-rich extract (Table 2). Thus, a negative SPT or BAT with a crude extract, and negative sIgE to hemp, have a good negative predictive value, but a positive result for one of these tests warrants complementary diagnostics (Figure 4). This is especially important when diagnosis is complicated by PR-10, profilin, nsLTP, or CCD sensitization, resulting in cross-reactivity between cannabis and other allergens. BAT and pMAT have also been used to study other cannabis allergens, but larger collaborative studies are needed to verify whether these tests could enter mainstream application.
FIGURE 3.
Basophil activation plots of a patient with CSA with sIgE reactivity to Can s 3, Can s 4, and Can s 5. Cells are responsive to a raw extract, Can s 3, Can s 4, Can s 5, but not to Can s 2. The percentages in the plots denote the net percentages of CD63+ basophils after stimulation with relevant allergen
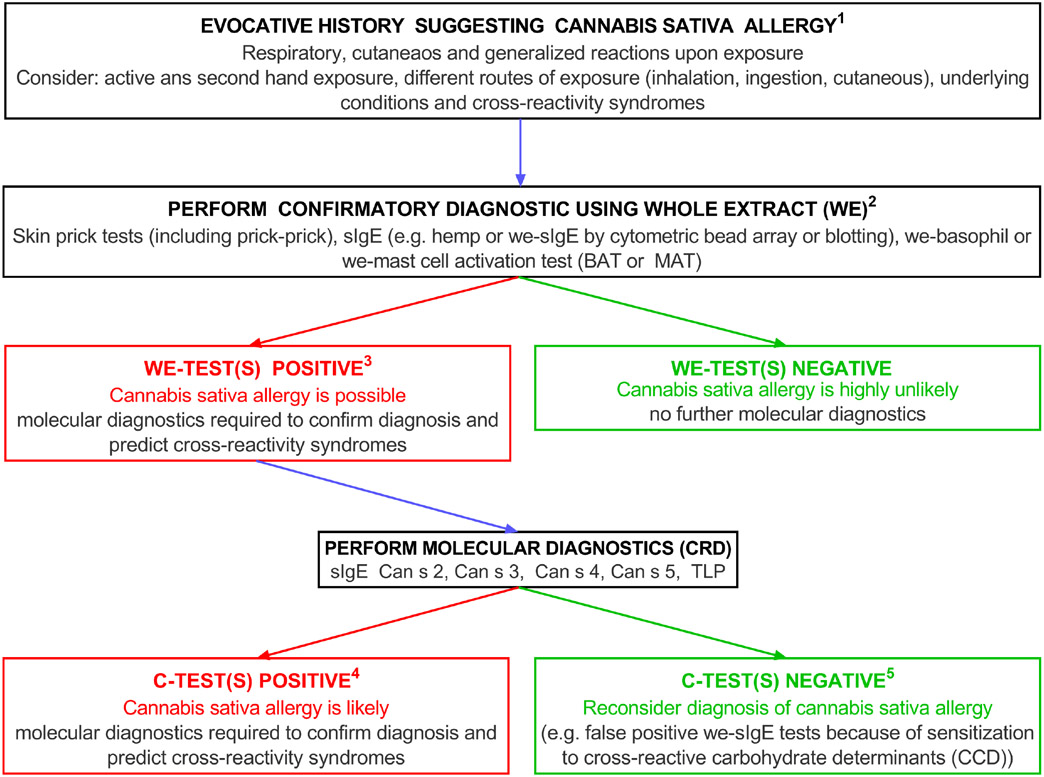
FIGURE 4.
Diagnostic algorithm for Cannabis Allergy.13,87 Molecular diagnostics—the Can s allergens have all been sequenced except for TLP (Thaumatin-like protein), a potential new cannabis allergen
Where there is clinical suspicion of a cannabis allergy, our diagnostic algorithm (Figure 4) proposes starting the work-up with SPTs using a native extract and/or quantification of sIgE hemp. In difficult cases, diagnosis can be complemented by calculation of the sIgE/total IgE ratio, molecular diagnostics and/or BAT/pMAT, if available. If the results are negative, cannabis allergy is highly unlikely. Given the lack of standardized extracts, and non-availability of commercial BAT and pMAT tests, it is currently not possible to optimize the process. A provocation challenge with inhaled cannabis is not recommended, as apart from the legal issues this may cause in many countries, inhalation of cannabis fumes can trigger nonspecific hyperresponsiveness without confirming allergy.125 It is unknown whether an oral challenge to edible cannabis products or hemp seed would be useful. As with any allergy diagnostic work-up, establishing sensitization to other relevant allergens including molds, pollens, and foods may be helpful, utilizing the most appropriate country-specific tests.
10 ∣. MANAGEMENT STRATEGIES
Currently, the only available treatment for cannabis allergy is avoidance. When avoidance is difficult or impossible, such as occupational exposure, treatment of symptoms is identical to that of other allergens and based on the clinical phenotype upon exposure. Medications could include non-sedating, second generation antihistamines, intranasal and inhaled corticosteroids, and ophthalmic antihistamine/mast cell stabilizers.11,126 Patients with a history of severe systemic symptoms or anaphylaxis to cannabis should be supplied with auto-injectable epinephrine. For occupational exposure, while a combination of administrative, engineering, and protective controls may mitigate some of the exposures, most occupational settings have limited options to remedy the situation. Treatment with omalizumab has been tried successfully in one case of unavoidable occupational allergy with anaphylaxis.127
In established cannabis allergy it is important to understand the cross-reactivity profile to foods. Treatment for cannabis-related cross-reactivity to fruits, vegetables, and latex (due either to pollens or nsLTP) entails avoidance, not only of cannabis, but also of the reported food triggers.128,129 The history of those presenting with more severe reactions needs to be analyzed for the presence of co-factors such as exercise, non-steroidal inflammatory drugs (NSAIDs) and alcohol.
Besides avoidance and symptomatic treatments, there is limited evidence for other therapeutic approaches. The seminal paper on the potential of immunotherapy in cannabis allergy, published in a veterinary journal, described a dog successfully desensitized with cannabis pollen extract.130 Kumar et al. successfully adopted subcutaneous immunotherapy to reduce a patient's symptoms of allergic rhinitis and asthma.131 With increasing legalization likely to entail a rise of cannabis sativa use, and consequent increasing occupational exposure, safe desensitization and/or tolerance induction protocols need to be developed.12,13,126
11 ∣. EDUCATIONAL NEEDS OF PATIENTS AND HEALTHCARE PROFESSIONALS
For many allergy healthcare professionals, determining cannabis use is not part of routine history-taking. Initiating such a discussion is vital, and questions should address cannabis exposure in a non-judgmental manner.132 Confidential discussions on issues surrounding cannabis allergy, within a medical facility, can ease concerns of patients. Patient-oriented information, both web-based, and written handouts can supplement the patient consultation. Healthcare professionals who see allergy patients would benefit from continuous professional development initiatives on cannabis allergy; for example, the development of competencies addressing practical approaches to its diagnosis and management. These could be adapted by individual countries to link in with existing competencies such as the Canadian Medical Education Directives for Specialists (CanMEDs) that address Communicator, Advocate, and Medical Expert roles in Canada.133 While the viewpoints and regulations pertaining to cannabis vary globally, it is important to recognize the issues surrounding its use. Continued education can increase the clinician's understanding and comfort level and improve the chances of them initiating an open discussion on cannabis use with each patient. Furthermore, it gives the clinician the opportunity to discuss the potential harm of recreational or non-evidence based medical use of cannabis.
12 ∣. CONCLUSION
The global use of cannabis, either for medical reasons, and/or where legalized for recreational purposes, is only likely to increase, and with it the prevalence of cannabis allergy. The diagnosis of any allergy can be problematic, and there are added difficulties with cannabis allergy, not least due to legal issues. There are currently no commercial allergy tests for the diagnosis of cannabis allergy in a clinical setting. Whole cannabis extracts could be purchased for SPT, depending on the legal status of cannabis in that area. Specific IgE to hemp is another option, but usually only available for use in research settings. Where possible, a connection should be made with laboratories that can perform IgE, molecular and cellular tests. Where cannabis is allowed for medical/recreational use, there needs to be recognition of cannabis allergy and support from allergy organizations, to address what might become a significant public health issue. A large survey demonstrated that people with asthma or allergies may not wish to discuss cannabis use with their physicians, but also physicians often did not inquire about cannabis use.9 This highlights the need to raise awareness and improve communication between patient and physician on cannabis use and/or exposure.
The CAIG aims to emphasize the need for more data to establish the significance of cannabis allergies in context of increasing access. Current projects include a survey of knowledge, attitudes and practices related to cannabis allergy among members of the three CAIG societies (ACAAI, CSACI and EAACI). This collaboration also plans to identify challenges that currently limit studies on cannabis allergy for example, Schedule I drug limitations, access to patients, lack of guidance on management, poor validation of diagnostic assays. Another goal is to clarify the importance of the context of exposures and the need to collect details on strain diversity and complexity of use. Key aspects of cannabis allergy need to be addressed, including the relevant allergens, diagnostic work-up, surveillance programs for occupational and environmental exposures and the prevention of cannabis allergy. To collect more real-world data, the CAIG seeks to establish a registry and biobank to collect samples from Europe, USA, and Canada. The intention is that these workstreams lead to the development of international guidelines on the diagnosis and management of cannabis allergy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the support of all members of the International Cannabis Allergy Collaboration, including Donald H Beezhold, Leonard Bielory, Michael S. Blaiss, Rohit Katial, David R. Naimi, Robert S. Zeiger, Mina Abbaslou, Harold Kim, Matteo Bonini, Francisca (Paqui) Gómez, Oscar Palomares Gracia, Hans Peter Rihs, Khaldon Abbas,Tara Croston, Sylvie Franken, Shmuel Kivity, Christel Mertens, Karin Pacheco, Thomas Rustemeyer, and Umesh Singh. The authors would also like to express their very great appreciation to Miriam Standish from the ACAAI, who has supported the work of the group, arranged meetings and facilitated the progress of this document. The authors would also like to thank and acknowledge the very generous financial support for the work of the International Cannabis Allergy Collaboration from the American College of Allergy, Asthma & Immunology, the Canadian Society of Allergy & Clinical Immunology, and the European Academy of Allergy, and Clinical Immunology.
Abbreviations:
- BAT
basophil activation test
- Bet v
Betula verrucosa
- CAIG
Cannabis Allergy Interest Group
- CBD
Cannabidiol
- CCDs
cross-reactive carbohydrate determinants
- CRD
component resolved diagnosis
- MAT
mast cell activation test
- pMAT
passive MAT
- nCan s
native Cannabis sativa component
- NPV
negative predictive value
- nsLTP
nonspecific lipid transfer protein
- PPV
positive predictive value
- rCan s
recombinant Cannabis sativa component
- rPru p 3
recombinant ns-LTP from peach (Prunus persica)
- THC
Tetrahydrocannabinol
- sIgE
specific IgE antibody
- SPTs
skin prick tests
- WE
whole extract
Footnotes
CONFLICT OF INTEREST
The following authors declare they have no conflicts of interest in relation to this paper; Hannelore Brucker, Didier Ebo, Richard Goodman, Ine Decuyper, Jolanta Walusiak-Skorupa, Anil Nanda, Amin Kanani, and Shun Chi Ryan Lo. The following authors have institutional positions or honoraria to declare; Isabel Skypala has received honoraria from ThermoFisher and is a member of the Executive Committee of the European Academy of Allergy & Clinical Immunology (EAACI); Ajay P. Nayak's institution has received grant funding from National Institutes of Health (NIH/NIAID R21AI140411) and National Institute of Allergy & Infectious Diseases; Samira Jeimy has received honoraria for speaking engagements from Sanofi Regeneron, AstraZeneca, Novartis, Aralez, Medexus Pharmaceuticals Inc and registration fees for the European Academy of Allergy & Clinical Immunology annual meetings in 2020, 2021 reimbursed by Medexus Pharmaceuticals Inc; Jonathan Bernstein has received PI, consultant, and speaker fees from—Sanofi Regeneron, AstraZeneca, Novartis, Genentech, Takeda/Shire, CSL Behring, Pharming, Biocryst, GSK; ALK; PI, consultant—Amgen, Biomarin, Kalvista; Jill Poole has received grants from NIH, Department of Defense, National Institute for Occupational Safety and Health, AstraZeneca and Takeda, is co-editor/board member of the journal Current Allergy and Asthma Reports, receiving ~$300/year for editorial role and has had a waived registration fee for speaking at the American Academy of Asthma, Allergy and Immunology (AAAAI); William Silvers is Chief Scientific Officer of the Canna Research Foundation; Joanna S. Zeiger is the CEO of Canna Research Foundation and has received payments from EAACI, Canadian Society of Allergy & Clinical Immunology and the American College of Asthma, Allergy and Immunology for other projects run by the Cannabis Allergy Interest Group; Lori Connors has received honoraria from AstraZeneca, ALK, Novartis, has participated on advisory boards of AbbVie, GSK, Novartis, Sanofi and is the CPD Chair of the CSACI; Anne Ellis is vice president of CSACI, has participated in advisory boards for Abbvie, ALK-Abello, AstraZeneca, Aralez, Bausch Health, Circassia, GSK, LEO Pharma, Merck, Novartis, Pfizer, and has been a speaker for ALK-Abello, Aralez, AstraZeneca, CSL Behring, Medexus, Novartis, Mylan, Pfizer, Sanofi and Takeda, and her institution has received research grants from ALK Abelo, Aralez, AstraZeneca, Bayer LLC, Circassia, Green Cross, Merck, Medexus, Pfizer, Novartis, Sanofi and Regeneron, she has also served as an independent consultant to Bayer LLC and Regeneron and Ludger Klimek reports grants and personal fees from Allergopharma, grants and personal fees from MEDA/Mylan, personal fees from HAL Allergie, grants from ALK-Abelló, grants and personal fees from LETI Pharma, grants from Stallergenes, grants from Quintiles, grants and personal fees from Sanofi, grants from ASIT biotech, grants from Lofarma, personal fees from Allergy Therapeutic, grants from AstraZeneca, grants from GSK, grants from Inmunotk, personal fees from Cassella med, outside the submitted work; and Membership of the following organisations: AeDA DGHNO, Deutsche Akademie für Allergologie und klinische Immunologie, HNO-BV, GPA and EAACI. Gordon Sussman has received research support from Aimmune, ALK, Amgen, AstraZeneca, DBV technologies, Genentech, Leo Pharma Inc., Novartis, and Sanofi. He is a medical advisor and/or has received payment for lectures from AbbVie, Novartis, CSL Behring, Pfizer, and the Allergy and Immunology Society of Ontario; Kevin Murphy reports receiving consulting fees from AstraZeneca, Sanofi, Genzyme, Genentech, Novartis, speaker honoraria from AstraZeneca, Sanofi, Genzyme, Genentech, Novartis, GlaxoSmithKline, TEVA, Amgen, participates on Data Safety Monitoring Board or Advisory Boards for AstraZeneca and TAKEDA, and is a Board Member of the ACAAI.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.United Nations Office on Drugs and Crime. UNODC World Drug Report 2018. https://www.unodc.org/wdr2018/. Accessed March 28, 2021.
- 2.World Health Organization. Facts and figures - cannabis. 2017. http://www.who.int/substance_abuse/facts/cannabis/en/. Accessed March 28, 2021.
- 3.Peacock A, Leung J, Larney S, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. 2018;113(10):1905–1926. [DOI] [PubMed] [Google Scholar]
- 4.Hall W, Stjepanović D, Caulkins J, et al. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet. 2019;394(10208):1580–1590. [DOI] [PubMed] [Google Scholar]
- 5.Imtiaz S, Wells S, Rehm J, et al. Cannabis use during the COVID-19 pandemic in Canada: a repeated cross-sectional study. J Addict Med. 2020;15(6):484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel SJ, Sherry SB, Stewart SH. Self-isolation: a significant contributor to cannabis use during the COVID-19 pandemic. Subst Abus. 2020;41(4):409–412. [DOI] [PubMed] [Google Scholar]
- 7.van Laar MW, Oomen PE, van Miltenburg CJA, Vercoulen E, Freeman TP, Hall WD. Cannabis and COVID-19: reasons for concern. Front Psychiatry. 2020:11:601653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu V, Leung J, Hall W, Stjepanović D, Degenhardt L. Public health impacts to date of the legalisation of medical and recreational cannabis use in the USA. Neuropharmacology. 2021;193:108610. doi: 10.1016/j.neuropharm.2021.108610 [DOI] [PubMed] [Google Scholar]
- 9.Zeiger JS, Silvers WS, Winders TA, Hart MK, Zeiger RS. Cannabis attitudes and patterns of use among followers of the Allergy & Asthma Network. Ann Allergy Asthma Immunol. 2021;126(4):401–410.e1. [DOI] [PubMed] [Google Scholar]
- 10.Liskow B, Liss JL, Parker CW. Allergy to marihuana. Ann Intern Med. 1971;75:571–573. [DOI] [PubMed] [Google Scholar]
- 11.Ocampo TL, Rans TS. Cannabis sativa: the unconventional "weed" allergen. Ann Allergy Asthma Immunol. 2015;114(3):187–192. [DOI] [PubMed] [Google Scholar]
- 12.Sussman GL, Beezhold DH, Cohn JR, Silvers WS, Zeiger JS, Nayak AP. Cannabis: an emerging occupational allergen? Ann Work Expo Health. 2020;7:679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decuyper II, Green BJ, Sussman GL, et al. Occupational allergies to cannabis. J Allergy Clin Immunol Pract. 2020;8(10):3331–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisanti S, Bifulco M. Medical cannabis: a plurimillennial history of an evergreen. J Cell Physiol. 2019;234(6):8342–8351. doi: 10.1002/jcp.27725 [DOI] [PubMed] [Google Scholar]
- 15.Ren M, Tang Z, Wu X, et al. The origins of cannabis smoking: chemical residue evidence from the first millennium BCE in the Pamirs. Sci Adv. 2019;5(6):eaaw1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Statistics Canada. National Cannabis Survey, third quarter 2019. 2019. https://www150.statcan.gc.ca/n151/en/daily-quotidien/191030/dq191030a-eng.pdf?st=191032jr191056HMc. Accessed May 29, 2021. [Google Scholar]
- 17.Vogel M, Nordt C, Bitar R, et al. Cannabis use in Switzerland 2015–2045: a population survey based model. Int J Drug Policy. 2019;69:55–59. [DOI] [PubMed] [Google Scholar]
- 18.Stewert C Cannabis usage in the Netherlands 2019, by age and frequency. 2021. https://www.statista.com/statistics/631197/cannabis-usage-in-the-netherlands-by-age/. Accessed October 5, 2021. [Google Scholar]
- 19.United States Drug Enforcement Administration. The controlled substances act. https://www.dea.gov/drug-information/csa. Accessed June 27, 2021. [Google Scholar]
- 20.Legality of cannabis by U.S. jurisdiction. Wikipedia. https://en.wikipedia.org/wiki/Legality_of_cannabis_by_U.S.jurisdiction. Accessed June 2021.
- 21.Van Keymeulen E. Cannabis legal & regulatory update: October–December 2018. https://www.jdsupra.com/legalnews/cannabis-legal-regulatory-update-53730. Accessed June 27, 2021. [Google Scholar]
- 22.Anthony JC, Lopez-Quintero C, Alshaarawy O. Cannabis epidemiology: a selective review. Curr Pharm Des. 2017;22(42):6340–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carliner H, Brown QL, Sarvet AL, Hasin DS. Cannabis use, attitudes, and legal status in the U.S.: a review. Prev Med. 2017;104:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodwin RD, Kim JH, Cheslack-Postava K, et al. Trends in cannabis use among adults with children in the home in the United States, 2004–2017: impact of state-level legalization for recreational and medical use. Addiction. 2021;116(10):2770–2778. [DOI] [PubMed] [Google Scholar]
- 25.Government of Canada. Cannabis Act. Justice Laws Website. https://lawslois.justice.gc.ca/Search/Search.aspx?&ddC0nt3ntTyp3=Statutes&h1dd3nPag3Num=1&txtT1tl3=%22Cannabis+Act%22&txtS23archA11=Cannabis&h21ts20n21y=20#results. Accessed June 27, 2021. [Google Scholar]
- 26.Statistics Canada. National Alcohol and Drug Survey. 1989. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3873. Accessed July 9, 2021. [Google Scholar]
- 27.Statistics Canada. Canada's Alcohol and Other Drugs Survey. 1994. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=4408. Accessed July 9, 2021. [Google Scholar]
- 28.Statistics Canada. Canadian Community Health Survey - Mental Health and Well-being (CCHS). 2002. https://www23.statcan.gc.ca/imdb/p22SV.pl?Function=getSurvey&Survld=1632&InstaId=5285&SDDS=5015. Accessed July 9, 2021. [Google Scholar]
- 29.Canadian Centre of Substance Use and Addiction. Canadian Addiction Survey (CAS): A National Survey of Canadians' Use of Alcohol and Other Drugs: Prevalence of Use and Related Harms: Detailed Report. 2005. https://www.ccsa.ca/canadian-addiction-survey-cas-national-survey-canadians-use-alcohol-and-other-drugs-prevalence-0. Accessed July 9, 2021. [Google Scholar]
- 30.Statistics Canada. Canadian Community Health Survey - Mental Health (CCHS). 2012. https://www23.statcan.gc.ca/imdb/p22SV.pl?Function=getSurvey&SDDS=5015. Accessed July 9, 2021. [Google Scholar]
- 31.Statistics Canada. Canadian Tobacco, Alcohol and Drugs Survey (CTADS). 2013. https://www23.statcan.gc.ca/imdb/p22SV.pl?-Function=getSurvey&Id=136981. Accessed July 9, 2021. [Google Scholar]
- 32.Statistics Canada. Canadian Tobacco, Alcohol and Drugs Survey (CTADS). 2015. https://www23.statcan.gc.ca/imdb/p22SV.pl?-Function=getSurvey&Id=299299. Accessed July 9, 2021. [Google Scholar]
- 33.Statistics Canada. Canadian Tobacco, Alcohol and Drugs Survey (CTADS). 2017. https://www23.statcan.gc.ca/imdb/p22SV.pl?-Function=getSurvey&Id=333871. Accessed July 9, 2021. [Google Scholar]
- 34.Government of Canada. Canadian Cannabis Survey 2020: Summary. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2020-summary.html. Accessed July 9, 2021. [Google Scholar]
- 35.Statistics Canada. National Cannabis Survey 2020. https://www23.statcan.gc.ca/imdb/p22SV.pl?Function=getSurvey&SDDS=5262. Accessed July 9, 2021. [Google Scholar]
- 36.Rotermann M. Looking back from 2020, how cannabis use and related behaviours changed in Canada. Health Rep. 2021;32(4):3–14. [DOI] [PubMed] [Google Scholar]
- 37.Government of Canada. Data on cannabis for medical purposes. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/medical-purpose.html. Accessed July 9, 2021. [Google Scholar]
- 38.Lo SCR, Abbas K, Kobric D, Silvers W, Sussman G. Cannabis hypersensitivity prevalence and presentation – A survey-based study of an allergic adult population in a Toronto Clinic at Onset of Canadian Legalization of Recreational Marijuana. J Allergy Clin Immunol. 2020;145(2):AB129. [Google Scholar]
- 39.European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2017. https://www.emcdda.europa.eu/publications/edr/trends-developments/2017. Accessed June 27, 2021. [Google Scholar]
- 40.Abuhasira R, Shbiro L, Landschaft Y. Medical use of cannabis and cannabinoids containing products - Regulations in Europe and North America. Eur J Intern Med. 2018;49:2–6. [DOI] [PubMed] [Google Scholar]
- 41.European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2020. https://www.emcdda.europa.eu/system/files/publications/13236/TDAT20001ENN_web.pdf. Accessed June 27, 2021. [Google Scholar]
- 42.Manthey J. Cannabis use in Europe: current trends and public health concerns. Int J Drug Policy. 2019;68:93–96. [DOI] [PubMed] [Google Scholar]
- 43.European Monitoring Centre for Drugs and Drug Addiction European Drug Report 2021: Trends and Developments. 2021. https://www.emcdda.europa.eu/system/files/publications/13838/TDAT21001ENN.pdf. Accessed June 27, 2021. [Google Scholar]
- 44.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473. [DOI] [PubMed] [Google Scholar]
- 45.Russo EB, Jiang HE, Li X, et al. Phytochemical and genetic analyses of ancient cannabis from Central Asia. J Exp Bot. 2008;59(15):4171–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandra S, Lata H, Elsohly MA. Cannabis Sativa L. - Botany and Biotechnology. Springer International Publishing; 2017. [Google Scholar]
- 47.Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD. Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J Opioid Manag. 2009;5(3):153–168. [DOI] [PubMed] [Google Scholar]
- 48.Hill KP. Medical use of Cannabis in 2019. JAMA. 2019;322(10): 974–975. [DOI] [PubMed] [Google Scholar]
- 49.Bifulco M, Pisanti S. Medicinal use of cannabis in Europe: the fact that more countries legalize the medicinal use of cannabis should not become an argument for unfettered and uncontrolled use. EMBO Rep. 2015;16(2):130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Health Canada Information for Health Care Professionals: Cannabis (marihuana, marijuana) and the cannabinoids. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids.html. Accessed June 27, 2021. [Google Scholar]
- 51.Szaflarski JP, Bebin EM. Cannabis, cannabidiol, and epilepsy–from receptors to clinical response. Epilepsy Behav. 2014;41:277–282. [DOI] [PubMed] [Google Scholar]
- 52.Andresen SR, Biering-Sørensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB. Cannabis use in persons with traumatic spinal cord injury in Denmark. J Rehabil Med. 2017;49(2):152–160. [DOI] [PubMed] [Google Scholar]
- 53.McPartland J Cannabis sativa and Cannabis indica versus “Sativa” and “Indica”. In: Chandra S, Lata H, ElSohly MA, eds, Cannabis sativa L. - Botany and Biotechnology. Springer; 2017. [Google Scholar]
- 54.Chandra S, Lata H, ElSohly MA. Propagation of Cannabis for clinical research: an approach towards a modern herbal medicinal products development. Front Plant Sci. 2020;11:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reimann-Philipp U, Speck M, Orser C, et al. Cannabis Chemovar nomenclature misrepresents chemical and genetic diversity; survey of variations in chemical profiles and genetic markers in Nevada Medical Cannabis Samples. Cannabis Cannabinoid Res. 2020;5(3):215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hill KP. Medical Marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474–2483. [DOI] [PubMed] [Google Scholar]
- 57.Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33(12):1357–1392. [DOI] [PubMed] [Google Scholar]
- 58.Aizpurua-Olaizola O, Soydaner U, Öztürk E, et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J Nat Prod. 2016;79(2):324–331. [DOI] [PubMed] [Google Scholar]
- 59.Room R, Fischer B, Hall WD, Lenton S, Reuter P. Cannabis Policy: Moving Beyond Stalemate. Oxford University Press. [Google Scholar]
- 60.Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur Arch Psychiatry Clin Neurosci. 2019;269(1):5–15. [DOI] [PubMed] [Google Scholar]
- 61.Abernethy A. Hemp Production and the 2018 Farm Bill. Food and Drug Administration, Department of Health And Human Services. https://www.fda.gov/news-events/congressional-testimony/hemp-production-and-2018-farm-bill-07252019 [Google Scholar]
- 62.Gell PGH, Coombs RRA. The classification of allergic reactions underlying disease. In Gell PGH, Coombs RRA, eds. Clinical Aspects of Immunology. Blackwell Science; 1963. [Google Scholar]
- 63.Nayak AP, Green BJ, Sussman G, et al. Characterization of Cannabis sativa allergens. Ann Allergy Asthma Immunol. 2013;111(1):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larramendi CH, López-Matas M, Ferrer A, et al. Prevalence of sensitization to Cannabis sativa. Lipid-transfer and thaumatin-like proteins are relevant allergens. Int Arch Allergy Immunol. 2013;162(2):115–122. [DOI] [PubMed] [Google Scholar]
- 65.de Larramendi CH, Carnés J, García-Abujeta JL, et al. Sensitization and allergy to Cannabis sativa leaves in a population of tomato (Lycopersicon esculentum)-sensitized patients. Int Arch Allergy Immunol. 2008;146(3):195–202. [DOI] [PubMed] [Google Scholar]
- 66.Armentia A, Herrero M, Martín-Armentia B, Rihs HP, Postigo I, Martínez-Quesada J. Molecular diagnosis in cannabis allergy. J Allergy Clin Immunol Pract. 2014;2(3):351–352. [DOI] [PubMed] [Google Scholar]
- 67.Ebo DG, Decuyper II, Rihs HP, et al. IgE-binding and mast cell-activating capacity of the homologue of the major birch pollen allergen and profilin from Cannabis sativa. J Allergy Clin Immunol Pract. 2021;9(6):2509–2512.e3. [DOI] [PubMed] [Google Scholar]
- 68.García-Olmedo F, Molina A, Segura A, Moreno M. The defensive role of nonspecific lipid-transfer proteins in plants. Trends Microbiol. 1995;3(2):72–74. [DOI] [PubMed] [Google Scholar]
- 69.Sun JY, Gaudet DA, Lu ZX, Frick M, Puchalski B, Laroche A. Characterization and antifungal properties of wheat non-specific lipid transfer proteins. Mol Plant Microbe Interact. 2008;21(3):346–360. [DOI] [PubMed] [Google Scholar]
- 70.Guo L, Yang H, Zhang X, Yang S. Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J Exp Bot. 2013;64(6):1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez-Rivas M, Gonzalez-Mancebo E, Rodriguez-Perez R, et al. Clinically relevant peach allergy is related to peach lipid transfer protein, Pru p 3, in the Spanish population. J Allergy Clin Immunol. 2003;112(4):789–795. [DOI] [PubMed] [Google Scholar]
- 72.Pascal M, Munoz-Cano R, Reina Z, et al. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy. 2012;42(10):1529–1539. [DOI] [PubMed] [Google Scholar]
- 73.Munoz-Garcia E, Luengo-Sanchez O, Moreno-Perez N, Cuesta-Herranz J, Pastor-Vargas C, Cardona V. Lettuce allergy is a lipid transfer syndrome-related food allergy with a high risk of severe reactions. J Investig Allergol Clin Immunol. 2017;27(2):98–103. [DOI] [PubMed] [Google Scholar]
- 74.Palacin A, Cumplido J, Figueroa J, et al. Cabbage lipid transfer protein Bra o 3 is a major allergen responsible for cross-reactivity between plant foods and pollens. J Allergy Clin Immunol. 2006;117(6):1423–1429. [DOI] [PubMed] [Google Scholar]
- 75.Decuyper II, Van Gasse AL, Faber MA, et al. Exploring the diagnosis and profile of Cannabis allergy. J Allergy Clin Immunol Pract. 2019;7(3):983–989.e985. [DOI] [PubMed] [Google Scholar]
- 76.Rihs HP, Armentia A, Sander I, Brüning T, Raulf M, Varga R. IgE-binding properties of a recombinant lipid transfer protein from Cannabis sativa. Ann Allergy Asthma Immunol. 2014;113(2):233–234. [DOI] [PubMed] [Google Scholar]
- 77.Gamboa P, Sanchez-Monge R, Sanz ML, Palacín A, Salcedo G, Diaz-Perales A. Sensitization to Cannabis sativa caused by a novel allergenic lipid transfer protein, Can s 3. J Allergy Clin Immunol. 2007;120(6):1459–1460. [DOI] [PubMed] [Google Scholar]
- 78.Rojas Perez-Ezquerra P, Sanchez-Morillas L, Davila-Ferandez G, et al. Contact urticaria to Cannabis sativa due to a lipid transfer protein (LTP). Allergol Immunopathol (Madr). 2015;43(2):231–233. [DOI] [PubMed] [Google Scholar]
- 79.Decuyper II, Faber MA, Sabato V, et al. Where there's smoke, there's fire: cannabis allergy through passive exposure. J Allergy Clin Immunol Pract. 2017;5(3):864–865. [DOI] [PubMed] [Google Scholar]
- 80.Metz-Favre C, Pauli G, Bessot JC, De Blay F. Molecular allergology in practice: an unusual case of LTP allergy. Eur Ann Allergy Clin Immunol. 2011;43(6):193–195. [PubMed] [Google Scholar]
- 81.Drouet M, Hoppe A, Moreau AS, Bonneau JC, Leclere JM, Le Sellin J. Cannabis and crossed allergy with food. Rev Pneumol Clin. 2017;73(6):290–293. [DOI] [PubMed] [Google Scholar]
- 82.Ebo DG, Swerts S, Sabato V, et al. New food allergies in a European non-Mediterranean region: is Cannabis sativa to blame? Int Arch Allergy Immunol. 2013;161(3):220–228. [DOI] [PubMed] [Google Scholar]
- 83.Pastorello EA, Robino AM. Clinical role of lipid transfer proteins in food allergy. Mol Nutr Food Res. 2004;48(5):356–362. [DOI] [PubMed] [Google Scholar]
- 84.Faber M, Van Gasse A, Sabato V, et al. Marihuana allergy: beyond the joint. J Investig Allergol Clin Immunol. 2015;25(1):70–72. [PubMed] [Google Scholar]
- 85.Faber MA, Sabato V, Bridts CH, Nayak A, Beezhold DH, Ebo DG. Clinical relevance of the Hevea brasiliensis lipid transfer protein Hev b 12. J Allergy Clin Immunol. 2015;135(6):1645–1648. [DOI] [PubMed] [Google Scholar]
- 86.Cardona V, Luengo O, Garriga T, et al. Co-factor-enhanced food allergy. Allergy. 2012;67(10):1316–1318. [DOI] [PubMed] [Google Scholar]
- 87.Decuyper II, Rihs HP, Van Gasse AL, et al. Cannabis allergy: what the clinician needs to know in 2019. Expert Rev Clin Immunol. 2019;15(6):599–606. [DOI] [PubMed] [Google Scholar]
- 88.Foster E, Nguyen C, Norris P. Contact buzz: allergic contact dermatitis to Cannabis. Dermatitis. 2018;29(4):223–224. [DOI] [PubMed] [Google Scholar]
- 89.Mayoral M, Calderón H, Cano R, Lombardero M. Allergic rhinoconjunctivitis caused by Cannabis sativa pollen. J Investig Allergol Clin Immunol. 2008;18(1):73–74. [PubMed] [Google Scholar]
- 90.Allen P. Poisonous and injurious plants of panama. Am J Trop Med Hyg. 1943;s1-23(1_Suppl):3–76. [Google Scholar]
- 91.Beliaev NV. General toxicodermatitis following acute poisoning caused by smoking Indian Hemp. Vestn Dermatol Venerol. 1964;38:77–78. [PubMed] [Google Scholar]
- 92.Williams C, Thompstone J, Wilkinson M. Work-related contact urticaria to Cannabis sativa. Contact Dermatitis. 2008;58(1): 62–63. [DOI] [PubMed] [Google Scholar]
- 93.Ozyurt S, Muderrisoglu F, Ermete M, Afsar F. Cannabis-induced erythema multiforme-like recurrent drug eruption. Int J Dermatol. 2014;53(1):e22–e23. [DOI] [PubMed] [Google Scholar]
- 94.Gilbert JD, Grabowski M, Byard RW. Intravenous administration of cannabis and lethal anaphylaxis. Med Sci Law. 2017;57(2):91–94. [DOI] [PubMed] [Google Scholar]
- 95.Stadtmauer G, Beyer K, Bardina L, Sicherer SH. Anaphylaxis to ingestion of hempseed (Cannabis sativa). J Allergy Clin Immunol. 2003;112(1):216–217. [DOI] [PubMed] [Google Scholar]
- 96.Hoffman BC, Kuhl M, Harbeck RJ, Rabinovitch N. Cannabis allergy in a child with asthma chronically exposed to marijuana. J Allergy Clin Immunol Pract. 2020;8(1):422–423. [DOI] [PubMed] [Google Scholar]
- 97.Drouet M, Moreau AS, Hoppe A. Eight-year-old children with numerous food allergy inducer by passive exposition to cannabis. Rev Pneumol Clin. 2017;73(6):323–325. [DOI] [PubMed] [Google Scholar]
- 98.Wiegand DM, Methner MM, Grimes GR, et al. Occupational exposure to secondhand cannabis smoke among law enforcement officers providing security at outdoor concert events. Ann Work Expo Health. 2020;64(7):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herzinger T, Schöpf P, Przybilla B, Ruëff F. IgE-mediated hypersensitivity reactions to cannabis in laboratory personnel. Int Arch Allergy Immunol. 2011;156(4):423–426. [DOI] [PubMed] [Google Scholar]
- 100.Lindemayr H, Jäger S. Occupational immediate type allergy to hemp pollen and hashish (author's transl). Derm Beruf Umwelt. 1980;28(1):17–19. [PubMed] [Google Scholar]
- 101.Research GV. U.S. Cannabis market size, share & trends analysis report by cannabis type (medical, recreational), by product type (buds, oils, tincture), by medical application, (chronic pain, mental disorder, cancer), and segment forecasts, 2019–2025. https://www.grandviewresearch.com/industry-analysis/us-cannabis-market. Accessed June 13, 2021. [Google Scholar]
- 102.Couch JR, Grimes GR, Green BJ, Wiegand DM, King B, Methner MM. Review of NIOSH cannabis-related health hazard evaluations and research. Ann Work Expo Health. 2020;64(7):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marijuana Occupational Health and Safety Work Group. Guide to Worker Safety and Health in the Marijuana Industry. 2017. https://cdphe.colorado.gov/workplace-safety/resources-and-links/marijuana-occupational-safety-and-health. Accessed June 27, 2021. [Google Scholar]
- 104.Valić F, Zuskin E, Walford J, Kersić W, Pauković R. Byssinosis, chronic bronchitis, and ventilatory capacities in workers exposed to soft hemp dust. Br J Ind Med. 1968;25(3):176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barbero A, Flores R. Dust disease in hemp workers. Arch Environ Health. 1967;14(4):529–532. [DOI] [PubMed] [Google Scholar]
- 106.Bouhuys A, Barbero A, Lindell SE, Roach SA, Schilling RS. Byssinosis in hemp workers. Arch Environ Health. 1967;14(4):533–544. [DOI] [PubMed] [Google Scholar]
- 107.Er M, Emri SA, Demir AU, et al. Byssinosis and COPD rates among factory workers manufacturing hemp and jute. Int J Occup Med Environ Health. 2016;29(1):55–68. [DOI] [PubMed] [Google Scholar]
- 108.Stepaniuk P, Kanani A. Selective cannabis strain allergy in a patient presenting with a local allergic reaction. Allergy Asthma Clin Immunol. 2021;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thompson GR 3rd, Tuscano JM, Dennis M, et al. A microbiome assessment of medical marijuana. Clin Microbiol Infect. 2017;23(4):269–270. [DOI] [PubMed] [Google Scholar]
- 110.Szyper-Kravitz M, Lang R, Manor Y, Lahav M. Early invasive pulmonary aspergillosis in a leukemia patient linked to aspergillus contaminated marijuana smoking. Leuk Lymphoma. 2001;42(6):1433–1437. [DOI] [PubMed] [Google Scholar]
- 111.McLaren J, Swift W, Dillon P, Allsop S. Cannabis potency and contamination: a review of the literature. Addiction. 2008;103(7):1100–1109. [DOI] [PubMed] [Google Scholar]
- 112.Min JY, Min KB. Marijuana use is associated with hypersensitivity to multiple allergens in US adults. Drug Alcohol Depend. 2018;182:74–77. [DOI] [PubMed] [Google Scholar]
- 113.Martyny JW, Serrano KA, Schaeffer JW, Van Dyke MV. Potential exposures associated with indoor marijuana growing operations. J Occup Environ Hyg. 2013;10(11):622–639. [DOI] [PubMed] [Google Scholar]
- 114.Tashkin DP, Roth MD. Pulmonary effects of inhaled cannabis smoke. Am J Drug Alcohol Abuse. 2019;45(6):596–609. [DOI] [PubMed] [Google Scholar]
- 115.Decuyper II, Faber MA, Lapeere H, et al. Cannabis allergy: a diagnostic challenge. Allergy. 2018;73(9):1911–1914. [DOI] [PubMed] [Google Scholar]
- 116.Underner M, Peiffer G, Perriot J, Jaafari N. Asthma and cannabis, cocaine or heroin use. Rev Mal Respir. 2020;37(7):572–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.George M, Avila M, Speranger T, Bailey HK, Silvers WS. Conducting an integrative health interview. J Allergy Clin Immunol Pract. 2018;6(2):436–439.e433. [DOI] [PubMed] [Google Scholar]
- 118.Tessmer A, Berlin N, Sussman G, Leader N, Chung EC, Beezhold D. Hypersensitivity reactions to marijuana. Ann Allergy Asthma Immunol. 2012;108(4):282–284. [DOI] [PubMed] [Google Scholar]
- 119.Ebo DG, Hagendorens MM, Bridts CH, De Clerck LS, Stevens WJ. Sensitization to cross-reactive carbohydrate determinants and the ubiquitous protein profilin: mimickers of allergy. Clin Exp Allergy. 2004;34(1):137–144. [DOI] [PubMed] [Google Scholar]
- 120.Decuyper II, Rihs HP, Mertens CH, et al. In search of the golden ratio for cannabis allergy: utility of specific allergen-to-total IgE ratios. Allergy. 2021;76(11):3522–3525. [DOI] [PubMed] [Google Scholar]
- 121.Van Gasse AL, Mangodt EA, Faber M, Sabato V, Bridts CH, Ebo DG. Molecular allergy diagnosis: status anno 2015. Clin Chim Acta. 2015;444:54–61. [DOI] [PubMed] [Google Scholar]
- 122.Decuyper II, Rihs HP, Mertens CH, et al. A new cannabis allergen in Northwestern Europe: the oxygen-evolving enhancer protein 2 (OEEP2). J Allergy Clin Immunol Pract. 2020;8(7):2421–2424. e2422. [DOI] [PubMed] [Google Scholar]
- 123.Ebo DG, Bridts CH, Mertens CH, Sabato V. Principles, potential, and limitations of ex vivo basophil activation by flow cytometry in allergology: a narrative review. J Allergy Clin Immunol. 2021;147(4):1143–1153. [DOI] [PubMed] [Google Scholar]
- 124.Bahri R, Custovic A, Korosec P, et al. Mast cell activation test in the diagnosis of allergic disease and anaphylaxis. J Allergy Clin Immunol. 2018;142(2):485–496.e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vidal C, Fuente R, Iglesias A, Sáez A. Bronchial asthma due to Cannabis sativa seed. Allergy. 1991;46(8):647–649. [DOI] [PubMed] [Google Scholar]
- 126.Jackson B, Cleto E, Jeimy S. An emerging allergen: cannabis sativa allergy in a climate of recent legalization. Allergy Asthma Clin Immunol. 2020;16:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Engler DBMA Saraf SK, Dargel LA. Severe marijuana allergy controlled with omalizumab. J Allergy Clin Immunol. 2013;131:1. [Google Scholar]
- 128.Gunawardana NCR-GH, Skypala IJ. Nutritional management of patients with pollen food syndrome: is there a need? Curr Treat Options Allergy. 2018;5:500–514. [Google Scholar]
- 129.Skypala IJ, Bartra J, Ebo DG, et al. The diagnosis and management of allergic reactions in patients sensitized to non-specific lipid transfer proteins. Allergy. 2021;76(8):2433–2446. [DOI] [PubMed] [Google Scholar]
- 130.Evans AG. Allergic inhalant dermatitis attributable to marijuana exposure in a dog. J Am Vet Med Assoc. 1989;195(11):1588–1590. [PubMed] [Google Scholar]
- 131.Kumar R, Gupta N. A case of bronchial asthma and allergic rhinitis exacerbated during Cannabis pollination and subsequently controlled by subcutaneous immunotherapy. Indian J Allergy Asthma Immunol. 2013;27:143–146. [Google Scholar]
- 132.Silvers WS. A Colorado allergist's experience with marijuana legalization. Ann Allergy Asthma Immunol. 2016;116(2):175–177. [DOI] [PubMed] [Google Scholar]
- 133.Royal College of Physicians and Surgeons of Canada. CanMEDS: Better standards, better physicians, better care. https://www.royalcollege.ca/rcsite/canmeds/canmeds-framework-e. Accessed July 26, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.