Abstract
Addiction is now recognized as a neurobiological and cognitive brain disorder and is generally viewed as a switch from recreational or voluntary to compulsive substance use despite aversive consequences. The habenula, composed of medial (MHb) and lateral (LHb) domains, has been implicated in regulating behavioral flexibility and anxiety-related behaviors and is considered a core component of the brain “anti-reward” system. These functions position the habenula to influence voluntary behaviors. Consistent with this view, emerging evidence points to alterations in habenula activity as important factors to contributing the loss of control over the use of drugs of abuse and the emergence of compulsive drug seeking behaviors. In this review, we will discuss the general functions of the MHb and LHb and describe how these functional properties allow this brain region to promote or suppress volitional behaviors. Then, we highlight mechanisms by which drugs of abuse may alter habenular activity, precipitating the emergence of addiction-relevant behavioral abnormalities.
Keywords: Substance use disorder, Drugs of abuse, Habenula, Motivated behavior, Compulsive drug use, Reward, Aversion, Avoidance, Dopamine, Serotonin, Acetylcholine
1. Introduction
According to the 2015 National Survey on Drug Use and Health (NSDUH) report, an estimated 27.1 million people in the United States, aged 12 or older, used illicit drugs. This represented over 10 percent of the US population in that age group. The European Drug Report (2017) estimates similarly high levels of drug use. Approximately 17.1 million young adult Europeans aged 15–34 years old used cannabis and 2.3 million consumed cocaine in 2015–2016. Differences exist between European and US drug users with respect to classes, levels and patterns of drug use, likely influenced in part by differences in local drug-relevant laws. For instance, cannabis use is less commonly reported than tobacco use in Europe relative to the USA, where cannabis use exceeds tobacco use. Conversely, European students report more frequent and intense patterns of alcohol consumption than their American counterparts. Despite the high prevalence of drug use in the US, Europe and beyond, not all casual users will transition to habitual patterns of consumption and, ultimately, develop a drug addiction (Wagner and Anthony, 2002). Factors that influence vulnerability versus resilience to addiction remain unclear, but genetic and environmental factors are thought to play key roles. For instance, genome-wide association studies (GWAS and twin studies have revealed that the CHRNA5/CHRNA3/CHRNB4 gene cluster on chromosome 15 contains alleles that predispose to nicotine dependence (Amos et al., 2008; Berrettini and Doyle, 2012; Hung et al., 2008; Thorgeirsson et al., 2008). Nevertheless, heritability appears specific to the population under study, and some studies of twins suggest that environmental and epigenetic mechanisms are also likely to influence the of addiction for review, (Agrawal and Lynskey, 2008; Agrawal et al., 2014). A better understanding of the factors that influence vulnerability to addiction, and how these factors modify the actions of drugs of abuse on reward- and motivation-relevant brain circuits, is likely to lead to new strategies for therapeutic intervention for the treatment of addiction.
1.1. Definitions and conceptual framework
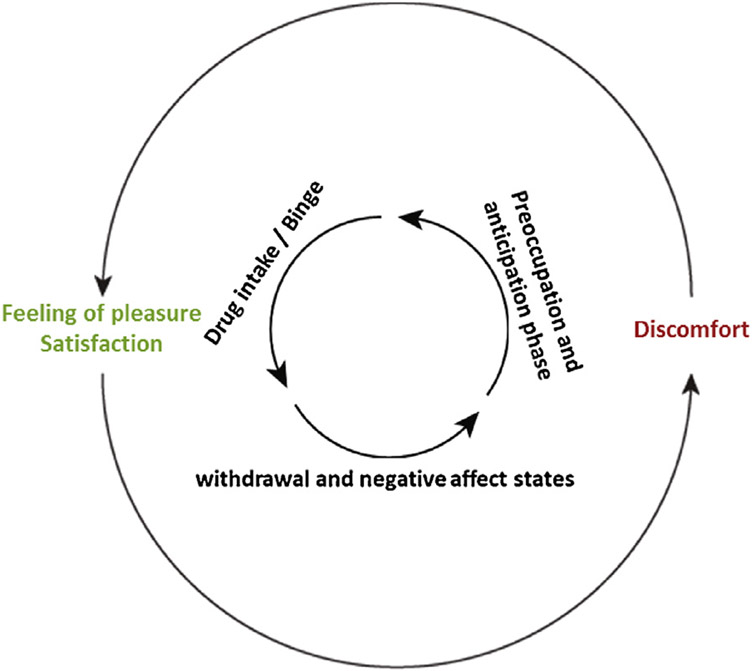
In this review, we will consider drug addiction as a brain disease (Volkow et al., 2016; Koob and Volkow, 2010) that involves pre-existing and/or drug-induced alterations in anatomical, chemical and physiological functions of brain systems relevant to motivated behaviors (Lüscher and Malenka, 2011; Nestler, 2005; Robinson and Kolb, 2004). As the term addiction is complex, controversial, confounded and continuously evolving, and differs depending on personal and professional perspective, we will instead use the term substance use disorder (SUD), as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) (American Psychiatric Association, 2013). SUDs are classically defined as chronically relapsing disorders, characterized by a compulsion to seek and obtain a drug (or drugs in the case of polydrug use), a loss of control over the amount of drug consumed, and the emergence of negative emotional states when the substance is no more accessible (Koob and Volkow, 2016). SUDs are often described by a three-stage cycle comprising a binge/intoxication phase, followed by a withdrawal and negative affect state, which results in a preoccupation and anticipation phase, ultimately leading to the next intoxication and the start of a new cycle (Volkow et al., 2016; Fig.1). This model accords well with the DSM-5 criteria of SUDs, since the phases of the cycle capture the features used to define SUDs.
Fig. 1. The three stages cycle of addiction.
Substance use disorders are characterized by different stages. After a first experience of drug intake, accompanied by a feeling of pleasure, individuals enter the withdrawal phase characterized by negative affects states. During this second phase, the drug is not available, leading the subject to the third phase, the stage of preoccupation and anticipation where individuals seek the drug and will start a new cycle.
The natural history of SUDs can be traced to a progressive failure to exert control over drug-taking behavior (Everitt and Robbins, 2016; Volkow and Baler, 2012; Volkow et al., 2013). Specifically, a switch from controlled and volitional to compulsive and increasingly automatic drug use is at the heart of all SUDs. Hence, identifying brain systems that regulate volitional drug use, and understanding how they are impacted by a history of drug consumption, may reveal how control over intake can be progressively lost in those who develop compulsive patterns of use.
The aim of this review is twofold. First, we will briefly discuss how drug use may transition from controlled to compulsive and describe brain systems implicated in this process. Second, we will highlight the importance of the habenular complex in the control of motivated behaviors and review emerging evidence supporting a prominent role for this brain structure in the development of SUDs.
2. Reward and aversion systems
In 1911, Thorndike described the law of effect, which stipulates that the probability of a response (here, resulting in the delivery of a drug of abuse) is increased when it is followed by “satisfaction” (reward) or alleviation of “discomfort” (aversion); what he called the “after-effect” (Thorndike, 1933; Edward and Thorndike, 1898). This simple but elegant law explains to large degree how goal-directed behaviors are acquired. Olds and Milner were the first to demonstrate that, in rats, electrical brain stimulation of brain regions, particularly the septal area, nucleus accumbens and other components of the medial forebrain bundle (MFB), may be intrinsically rewarding, reflected by the fact that rats will work vigorously to obtain such stimulation (Olds and Milner, 1954). Subsequently, MFB inputs to the mesocorticostriatal dopamine system were heavily implicated in reward-relevant behaviors, with the evidence suggesting that activation of this system by a rewarding stimulus serves as a major substrate for the acquisition of goal-directed behaviors (Wise, 1998; Wise and Koob, 2014), including the acquisition of drug-taking behaviors (Koob and Volkow, 2016). In addition to their rewarding effects, drugs of abuse also have aversive or noxious properties. Specifically, acute exposure to drugs of abuse can have aversive effects, particularly at higher doses for nicotine (Fowler and Kenny, 2014), or after their initial (transient) rewarding effects have dissipated (Jhou et al., 2013). Withdrawal from chronic exposure is also known to precipitate an aversive withdrawal syndrome. In contrast to the intense focus on understanding the pleasurable actions of drugs of abuse, much less is known about the brain systems and neurobiological mechanisms involved in their noxious actions. Recently, the habenula has been described as a key brain area involved in the aversive actions of drugs of abuse (Fowler et al., 2011; Lecca et al., 2014, 2016; Matsumoto and Hikosaka, 2009; Valentinova et al., 2015). Importantly, the habenula can exert inhibitory control over mesocorticolimbic dopamine systems (Barrot et al., 2012; Hong et al., 2011; Ji and Shepard, 2007; Omelchenko et al., 2009), and other major neurotransmitter systems in the midbrain, suggesting that the habenula may represent an important nexus in the brain that influences reward-seeking or avoidance behaviors. This is consistent with conceptualizations of substance use disorder proposing that opponent brain systems, requiring in part overlapping but also distinct cerebral regions, control the positive and negative effects of drugs of abuse and that perturbations in the balance between these opponent systems precipitates pathological drug-seeking behaviors (Wise and Koob, 2014; Koob and Moal, 2008).
As noted above, the mesocorticolimbic system, comprising dopamine neurons that arise in the ventral tegmental area (VTA) and project to the nucleus accumbens (NAc) and cortex (Koob and Volkow, 2010, 2016; Hyman et al., 2006; Nestler and Malenka, 2004), plays an important role in reward-seeking behaviors. In general, this system responds to rewarding stimuli, particularly unexpected rewards or cues that predict reward delivery, with phasic increases in activity and consequently dopamine release into the NAc (Baik, 2013; Roberts and Koob, 1982; Schultz, 2013; Tobler et al., 2005; Volkow and Morales, 2015; Willuhn et al., 2010). Schultz and colleagues described two types of reward-relevant “prediction error” encoded by these neurons; a positive error of prediction, when the reward is better than expected, or the converse, when the reward is worse than expected or omitted entirely (Tobler et al., 2005; Fiorillo et al., 2003; Schultz, 2016). Thus, by modulating the dopamine signal from midbrain dopamine neurons, information in the NAc related to whether delivery of expected reward matches what was expected.
Conversely, an “anti-reward” system is recruited as a result of excessive activation of the reward system and provides a source of negative hedonic valence (Koob and Moal, 2008). This anti-reward system involves some of the same structures belonging to the reward system described above. Indeed, decreases in the activity of midbrain dopamine neurons are thought to encode negative reward events. In addition, other region such as the lateral habenula (LHb) are considered core brain regions involved in transmitting negative-reward signals (Matsumoto and Hikosaka, 2007). In the most straightforward conceptualization, the LHb acts in a manner exactly opposite to midbrain dopamine reward neurons (Brown and Shepard, 2016; Brown et al., 2017; Stopper et al., 2014). Indeed, in primate studies, Matsumoto and Hikosaka showed that when a reward is obtained (delivery of juice), activity of the LHb decreases while that of dopamine neurons increases. By contrast, LHb activity increases when a punishing stimulus is delivered (an air puff) and dopamine neurons show a concomitant decrease in activity (Matsumoto and Hikosaka, 2009, 2007). Finally, if an expected reward is omitted, or a reward is better than expected, LHb activity is increased or decreased, respectively. These findings are consistent with the hypothesis that LHb neurons act as a “negative reward” prediction error signal (Matsumoto and Hikosaka, 2009; Bromberg-Martin et al., 2010; Hikosaka, 2010). Thus, by these two systems, the brain can integrate and process the reward-relevant stimuli and events to guide approach/avoidance behaviors. Consequently, SUDs could be conceptualized as a cycle of spiraling dysregulation of the brain reward/anti-reward systems leading to compulsive use of drugs.
3. From controlled to compulsive drug use: brain-wide failure approach/avoidance systems
Volkow and colleagues have proposed that five major brain regions/circuits involved in the behavioral selection, adaptation and seeking behaviors are particularly vulnerable to dysregulation in response to continued drug use. These brain regions, comprising the mesocorticolimbic dopamine system, amygdala, hippocampus, habenula and prefrontal cortex (PFC) circuits (Uylings et al., 2003), regulate reward, learning/memory and habit formation, inhibitory control and executive function (Volkow and Baler, 2014). Drug-induced alterations in these circuits contribute to the evolution of specific features of SUDs. Indeed, studies implicate the NAc, dorsal striatum (DS), and globus pallidus (GP) components of the mesocorticolimic dopamine system in the binge/intoxication stage of SUDs; the extended amygdala, including the central nucleus of the amygdala, bed nucleus of the stria terminals (BNST), and a transition area in the shell of the NAc as key elements of the withdrawal/negative affect stage; and the frontal cortex, including the PFC, orbitofrontal cortex (OFC), insula and cortical inputs to the hippocampus, as key elements of the preoccupation/anticipation stage (Volkow et al., 2016; Koob and Volkow, 2016; Wise and Koob, 2014).
Key findings in animal studies have implicated the brain regions described above in addiction-relevant behavioral abnormalities. For example, the striatum is one of the most studied brain area in the context of switching between volitional to habitual/compulsive behaviors. Indeed, Everitt and Robbins have advanced the view that a progressive transition from initial voluntary drug use to stimulus-evoked “habitual” drug use is driven by dysregulation of DS circuits that receive inputs from midbrain dopamine neurons and higher-order cortical sites (Everitt and Robbins, 2016; Robbins and Everitt, 1999). This transition is characterized by a progressive loss of control of substance use progressively greater control over drug-taking by drug-paired environmental stimuli such that drug use becomes progressively more automated. At the neural level, they propose that the control of the substance use progressively shift from the ventral to the dorsal part of the striatum as behavior becomes more habitual (Everitt and Robbins, 2013), as shown in learning tasks (Packard and McGaugh, 1992).
Dysfunction in cortical regions has also been implicated in behaviors in animal studies that are relevant to substance use disorders in humans. For example, it has been proposed that executive control over drug-taking becomes progressively weaker as drug experience increases and compulsive-like responding emerges in rodents (Everitt and Robbins, 2013; Chen et al., 2013). Chen et al. described a decrease of the PFC excitability in rats following cocaine self-administration. Those rats with decreased PFC activity also showed greater compulsive-like drug seeking behaviors, reflected by drug-responding that was resistant to punishment. Using PFC photoinhibition to mimic drug-induced “hypo-frontality”, they precipitated compulsive-like drug seeking in rats (Chen et al., 2013). In the same study, optogenetic activation of the PFC was shown to rescue compulsive-like cocaine seeking. Based on these findings, the authors proposed that cortical areas promote compulsive intake in the presence of a challenge such as an aversive situation. In contrast to striatal areas that sustain habitual intake in the absence of such challenges. Moreover, the importance of dopamine transmission in the PFC in flexible decision making has also been discussed (Floresco, 2013). The dopamine action through dopamine D1 in PFC likely promotes the persistence of a particular choice whereas the D2 receptors enable flexible decision making. Thus, altered dopamine transmission, and the balance between D1 and D2 receptor signaling, in the PFC in response to drug use may disturb cortical control over drug use, which in turn predisposes to the emergence of compulsive drug-taking behaviors.
Another important brain region implicated in substance use disorder is the amygdalar complex, well-described in the fields of emotional learning and cue conditioning (Stamatakis et al., 2014; Wassum and Izquierdo, 2015). It has been shown that projections from the amygdala to the NAc promotes reward seeking behavior (Stuber et al., 2011;) The amygdala seems necessary for attributing emotional value to cues that predict salient events and has an integral role in the processing of affective states (LeDoux, 2003; Phelps and LeDoux, 2005). Lesion studies of the basolateral amygdala (BLA) have shown the disruption of cue-induced reinstatement of otherwise extinguished cocaine seeking behaviors in rodents (Meil and See, 1997). However, BLA lesions alone do not disrupt cocaine self-administration (Meil and See, 1997), suggesting the BLA plays an important role in the motivational properties of cues associated with natural reinforcers and drugs of abuse (Stamatakis et al., 2014).
Thus, those different brain networks facilitating the selection of appropriate behavioral strategies are profoundly altered by drug use, particularly those sites known to be controlled by dopamine transmission. Such alterations are likely to contribute to discrete aspects of SUD symptoms, such as compulsion, negative affective states and preoccupation. Intriguingly, the habenula (Hb) receives afferents from almost every major brain region implicated in SUDs. In turn, the LHb can plays an important role in regulating the activity of midbrain dopamine neurons, most notably those dopamine neurons that project to the NAc and mPFC (Lecourtier et al., 2008). These features suggest that the Hb is an important brain site to consider in the emergence of SUDs. Considering its ability to modulate the activity of almost all circuits involved in substance use disorder, the LHb is strategic located to play a “driver” role in the emergence of SUDs. This possibility is considered in more detail below.
4. The habenula: a key node in substance use disorder?
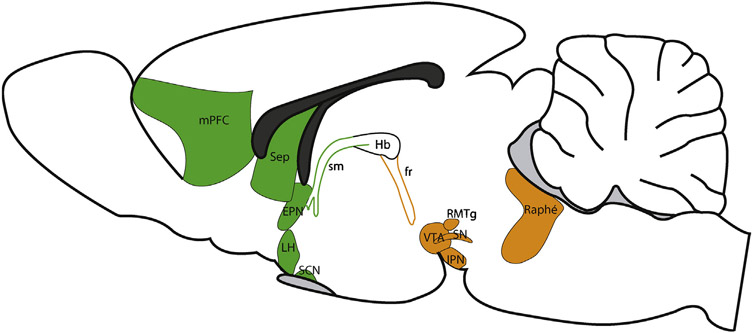
The habenula (Hb) is a diencephalic structure, belonging to the dorsal diencephalic conduction system (Sutherland, 1982), composed of medial (MHb) and a lateral (LHb) domains for review (Geisler and Trimble, 2008; Hikosaka et al., 2008). The habenular complex is conserved across phylogeny, as it can be identified from lamprey, considered as the oldest living-vertebrate, to human (Aizawa et al., 2011; Bianco and Wilson, 2009; Concha and Wilson, 2001; Villalón et al., 2012). Because of its small size and its anatomical position close to the third ventricle, most studies of the habenular complex until recently focused almost entirely on its neuroanatomy. These studies described notable differences in afferent and efferent connectivity between the MHb and LHb (Geisler and Trimble, 2008; Herkenham and Nauta, 1977, 1979; Viswanath et al., 2014). In brief, the major source of inputs to the MHb originates from the septum and, in turn, the MHb projects almost exclusively to the interpeduncular nucleus (IPN) (Fig. 2). By contrast, the LHb receives a far more distributed network of afferent inputs. Indeed, the LHb is a major point of convergence of information flow originating from the basal ganglia (more precisely the entopedoncular nucleus, EPN), the lateral hypothalamus, the BNST, and the medial PFC. In turn, the LHb projects to major midbrain monoaminergic centers for a more exhaustive review of its connectivity see (Geisler and Trimble, 2008; Hikosaka et al., 2008; Root et al., 2014a; Shabel et al., 2014). Based on this anatomical connectivity, Geisler and Trimble hypothesized that the LHb serves a point of converging for forebrain “macrosystems”, where information from this higher-order brain sites are integrated and processed, then transmitted to downstream dopamine and serotonin effector systems (Geisler and Trimble, 2008).
Fig. 2. The connectivity of the habenular complex.
The habenular complex is also called dorsal diencephalic conduction system, conveying information from the limbic forebrain to limbic midbrain areas (Sutherland 1989). The main habenular inputs (green) comprised the medial prefrontal cortex (mPFC), the septum (Sep), the entopedoncular nucleus (EPN), the lateral hypothalamus (LH) and the suprachiasmatic nucleus (SCN). This complex sends axons to different midbrain areas (orange) such as the ventral tegmental area (VTA), substancia nigra (SN), the rostromedial tegmentum (RMTg), the raphe nuclei and the interpeduncular nucleus (IPN).
4.1. Functions of the habenular complex
Until recently, many studies investigating the role of the habenular complex in behavior used lesions. Because such lesions often included both the MHb and the LHb, this prevented firm conclusions regarding the specific contributions of these subnuclei to behaviors under consideration. Nevertheless, whole-structure habenular lesions result in learning and memory deficits, attention deficits, hyper-reactivity to stress, and schizophrenia-like behavioral abnormalities in laboratory animals (Thornton et al., 1990; Wang et al., 2013).
The majority of the studies focused on MHb functions have used zebrafish as a model system. These studies hypothesize that the dorsal habenula in zebrafish is equivalent to the MHb in mammals (Amo et al., 2010). Based on this work, it has been hypothesized that the MHb serves as a “switchboard” for controlling emotional behaviors (Mathuru and Jesuthasan, 2013a; Okamoto et al., 2012a), particularly fear- and anxiety-related behaviors (Molas et al., 2017a; Yamaguchi et al., 2013). In rodents, specific inhibition of MHb neuronal activity accomplished using elegant genetic targeting strategies, induces maladaptive responses to anxiogenic environments in mice (Kobayashi et al., 2013). These animals also showed deficits in impulsivity (Viswanath et al., 2014; Kobayashi et al., 2013), suggesting that the MHb plays a role in response selection and behavioral flexibility. Interestingly, optogenetic activation of MHb neurons supports intracranial self-stimulation behavior, suggesting that activation of this structure can, in some circumstances, trigger reward-related experiences (Hsu et al., 2014). Conversely, inhibition of this structure induces aversion (Hsu et al., 2014). In the same study, genetic ablation of the MHb reduced wheel running, suggesting a deficit in responsiveness to reward-relevant stimuli. More recently, Molas and colleagues described a role for the MHb-IPN circuit in novelty preference and familiarity. First, the authors showed that GABAergic neurons in the IPN are recruited as a stimulus becomes more familiar (Molas et al., 2017b). Then, using an optogenetic approach, they demonstrated that activation of the MHb-IPN pathway signals familiarity and induces a reduction in exploratory behaviors. They also demonstrated that a VTA-IPN pathway participates in signaling of novelty and facilitates exploratory behavior. Yamaguchi and colleagues have generated data suggesting that two discrete MHb-regulated circuits are involved in active and passive coping behaviors (Yamaguchi et al., 2013). Using transgenic mice, they elegantly described a projection from the triangular nucleus of the septum to the ventral part of the MHb (TS→vMHb) that is selectively involved in anxiety-related behaviors. By contrast, the bed nucleus of the anterior commissure (BAC) is shown to project to the dorsal part of the MHb (BAC→dMHb), and is involved in regulating fear learning. Finally, in addition to fear and anxiety, studies in rodents have explored the role of MHb in regulating the motivational properties of nicotine (Fowler et al., 2011; Molas et al., 2017a; Antolin-Fontes et al., 2015; Fowler and Kenny, 2012; Tuesta et al., 2017). The role of the MHb in substance use disorder is considered in more detail below. Together, these studies suggest that the MHb plays an important role in behaviors relevant to fear and anxiety and may control the avoidance of nicotine and other drugs of abuse (Molas et al., 2017a; Okamoto and Aizawa, 2013).
Over the past decade, most studies investigating habenular functions have focused on the role of the LHb in basal ganglia-mediated functions. The seminal work of Matsumoto and Hikosaka in primates (Matsumoto and Hikosaka, 2009, 2007), showing that LHb neurons increase their activity in response to punishing stimuli and decrease their activity in response to rewarding stimuli, catalyzed major interest in this brain region. As mentioned above, they described the LHb as a negative reward center that encodes an aversion-related “prediction-error signal”, which is activated when there is a negative discrepancy between predicted and actual value of reward-related outcomes (Matsumoto and Hikosaka, 2009, 2007; Hikosaka, 2010). Further work showed that the firing rate of LHb neurons is inhibited by unexpected reward and by presentation of reward-predicting cues, with the magnitude of this effect being directly proportional to the discrepancy between expected and delivered reward. Based on these and related findings, the LHb is considered as a major integrating and relaying structure for striatal and limbic reward-relevant information toward midbrain monoaminergic nuclei (Hikosaka, 2010; Geisler and Trimble, 2008), which plays a major role in coding negative motivational value and consequently influencing subjective decision-making biases (Stopper et al., 2014; Bromberg-Martin et al., 2010; Bromberg-Martin and Hikosaka, 2011). This role for the LHb in reward signal (and also in punishment) identifies the LHb an attractive brain structure to investigate in the context of substance use disorders and other psychiatric disorders characters by negative emotional states, such as depression and anxiety (Lecca et al., 2014; Valentinova et al., 2015; Li et al., 2013; Sartorius and Henn, 2007). Many studies have also described the involvement of the LHb in approach/avoidance behaviors. Indeed, activation of the LHb→VTA pathway is aversive and supports avoidance behavior (Root et al., 2014b). Similarly, activation of the inputs from the EPN to the LHb is also aversive (Shabel et al., 2012). Interestingly, other studies suggest that the LHb plays a role in behavioral adaptation and outcome evaluation (Mizumori and Baker, 2017; Stephenson-Jones et al., 2016a). In rats, Baker and Mizumori generated evidence that the LHb guides response flexibility to maximize the effectiveness of goal-directed behaviors (Baker et al., 2015, 2017). Using a T-maze task, in which rats learned to turn left or right when a low or high tone was played, respectively, the authors found that pharmacological inactivation of the LHb impaired the ability of rats to use the tone to choose the correct arm when the rule was changed. This hypothesis was also proposed by Stopper and colleagues using a model of risk/reward decision-making task in rodents (Stopper et al., 2014). In this study, the authors showed that electrical stimulation of the LHb influenced the strategy used by the animal. If the LHb stimulation occurs after a “large risky choice” (low probability to obtain a large reward), rats will prefer to use a “small certain choice” for the next trials (high probability to obtain a small reward), regardless the results of the previous action. The authors described the exact opposite switch in choice if the LHb stimulation occurs after the “small certain choice”. Thus, the LHb seems to be involved in the ability to switch strategies to maximize rewarding outcomes.
Using functional magnetic resonance imaging (fMRI) in humans engaging in a difficult judgment task that results in high rate of errors (with or without feedbacks for the participants), Shepard and colleagues demonstrated that errors with negative feedback prompted significantly greater hemodynamic signals in the habenula than errors without feedback. Interestingly, correct choices without feedback also showed a tendency to promote greater habenular activity than correct choices with feedback. These data suggest that the habenula provides a signal that facilitates the association of actions with negative feedback when the subject is unaware of whether the correct behavioral strategy was employed. The authors hypothesized that this interplay between error likelihood and feedback plays an important role in learning tasks associated with high levels of ambiguity.
In rodents, the LHb has been implicated in spatial learning and working memory (Baker et al., 2015; Mathis et al., 2015, 2016), highlighting its potential role in the storage and the use of different strategies to use depending on the situation. Consistent with a role in the integration of the ongoing actions and their outcomes, the LHb is known to be activated after application of stressful stimuli (Chastrette et al., 1991; Lecca et al., 2017a; Wirtshafter et al., 1994; Zhang et al., 2016a). More precisely, it was shown that aversive stimuli induce the activation of the lateral hypothalamus projections to the LHb, which promotes escape behavior (Lecca et al., 2017b). Zhang and colleagues also demonstrated that a decrease in innate fear induced by water deprivation may be due to inhibition of the LHb from resulting from vasopressin-expressing neurons of the paraventricular nucleus of the hypothalamus (Zhang et al., 2016b). Thus, the LHb appears able to flexibly promote or suppress behaviors depending on internally generated drives (for instance, the thirst) to help guide behavior that and thereby reduce these noxious behavioral states (Molas et al., 2017a; Okamoto and Aizawa, 2013; Mizumori and Baker, 2017; Fore et al., 2017; Mathis and Lecourtier, 2017). Together, these studies suggest that the LHb plays an important role in adaptive behaviors (Hikosaka, 2010; Stephenson-Jones et al., 2016b) in response to negative emotional states. This is consistent with recent conceptualizations suggesting that the habenular complex may be involved in behavioral abnormalities associated with schizophrenia and aggression-related disorders (Golden et al., 2016; van Kerkhof et al., 2013; Shepard et al., 2006).
Finally, it is important to note that the precise functional relationship between the MHb and LHb is currently unclear. Although a unidirectional projection from the MHb to the LHb has been reported (Kim and Chang, 2005), little is known about how, or even if, information is processed and transferred between these nuclei and, if so, what is the behavioral relevance of such intra-habenular information flow.
4.2. Role for the medial habenula in regulating drug use
The role of the habenular complex as a hub for relaying reward-relevance information from forebrain to downstream midbrain effector systems, and monitoring the effectiveness of subsequent behaviors, suggests that this structure is likely to play a key role in drug seeking behaviors (Baldwin et al., 2011; Meye et al., 2017; Salaberry and Mendoza, 2015; Velasquez et al., 2014). Evidence linking the MHb in to the actions of drugs of abuse, particularly nicotine and opioid addiction, comes from the fact that it has some of the highest densities of nicotinic acetylcholine receptor subunits, particularly α3, α5 and β3 subunits (Fowler and Kenny, 2012; Shih et al., 2014) as well as μ opioid receptors (Gardon et al., 2014), in the brain. Indeed, approximately ~90–100% of MHb neurons express α5, α3, α4, β2 and/or β4 nAChR subunits (Shih et al., 2014). As noted above, the MHb-IPN circuit plays a role in regulate avoidance of noxious stimuli and adaptive behaviors in response to the familiarity or novelty of a particular stimulus (Yamaguchi et al., 2013), (Molas et al., 2017b; Okamoto and Aizawa, 2013; Mathuru and Jesuthasan, 2013b; Okamoto et al., 2012b). Our laboratory has shown that deletion of α5 nAChR subunits decreases the function of nAChRs in the MHb and IPN of mice, as measured using rubidium efflux (Fowler et al., 2011). Based on these observations, we hypothesized that α5-containing (and perhaps other subtypes of) nAChRs in the MHb-IPN circuit regulate nicotine avoidance behaviors, such that deficits in nAChR transmission in this circuit results in greater nicotine intake. Consistent with this hypothesis, we found that levels of intravenous nicotine self-administration behavior were far higher in α5 subunit knockout (Chrna5−/−) mice compared with their wild-type littermates (Fowler et al., 2011). This effect in Chrna5−/− mice was most apparent when higher unit doses of nicotine, which can support avoidance behaviors in place conditioning procedures, were available for self-administration. We also investigated the role for α5* nAChRs in the MHb-IPN circuit in regulating this effect in the Chrna5−/− mice. Specifically, we developed a lentivirus vector to express α5 subunits (Lenti-Chrna5), and used this virus to ‘rescue’ α5 subunit expression in the mHb-IPN circuit of the Chrna5−/− mice (Fowler et al., 2011). When treated with a control virus (Lenti-Control), we again found that Chrna5−/− mice consumed greater amounts of nicotine than wild-type mice. By contrast, Chrna5−/− mice treated with the Lenti-Chrna5” rescue” virus showed nicotine intake that was indistinguishable from their wild-type littermates.
The α5 nAChR subunit is not the only actor in MHb nAChR transmission that regulates nicotine intake. A series of elegant experiments from the laboratory of Ibanez-Tallon and colleagues demonstrated that nAChRs containing β4 subunits exert a profound influence over nicotine intake in a manner similar to α5-containing nAChRs (Antolin-Fontes et al., 2015; Ślimak et al., 2014) They conclude that increased β4-mediated nAChR currents increase aversion to nicotine, while decreases in these currents attenuates nicotine aversion. These findings further support the hypothesis that the MHb-IPN pathway controls nicotine avoidance behaviors (Antolin-Fontes et al., 2015; Ślimak et al., 2014), highlighting the potential importance of α5- and β4-containing receptors as targets for the development of novel smoking cessation (Biasi and Salas, 2008).
In addition to the MHb-IPN system, α5 and β4 nAChR subunits are also densely expressed in the nucleus of the solitary tract (NTS). Based on this expression pattern, which resembles the MHb-IPN circuit, we investigated the contribution of the NTS in the regulation of nicotine intake. We found the nicotine induced robust dose-dependent increases in Fos immunoreactivity in NTS neurons, with this effect prominent in NTS neurons that express the neuropeptide glucagon-like peptide-1 (GLP-1) (Tuesta et al., 2017). Using a line of Chrna5-tdTomato reporter mice, we confirmed that GLP-1 neurons express α5 nAChR subunits. We found that systemic delivery of the GLP-1 receptor agonist exendin-4 (Ex-4), or the dipeptidyl peptidase 4 (DPP-4) inhibitor sitagliptin, to inhibit GLP-1 breakdown, decreased responding for nicotine but not food rewards in mice. Used two separate Cre-expressing mouse lines (Phox2b-Cre and glucagon-Cre mice) to target chemogenetically stimulate GLP-1 neurons in the NTS resulted in decreased responding for nicotine but not food in these mice. Conversely, nicotine but not food responding was increased in GLP-1 receptor knockout (Glp1r−/−) mice. Together, these findings suggest that GLP-1 signaling in the brain derived from NTS neurons decreases the motivation to consume nicotine.
Next, we investigated the mechanisms by which GLP-1 neurons in the NTS control nicotine-taking behavior. Some of the highest densities of GLP-1 receptor binding sites in the brain are detected in the IPN (Göke et al., 1995). Therefore, we investigated the possibility that GLP-1 released from NTS neurons may enhance activity of the MHb-IPN avoidance circuit to control nicotine intake. We detected GLP-1-immunoreactive fibers in the IPN of mice (Tuesta et al., 2017), suggesting that GLP-1 neurons in the NTS project to the IPN. Next, we injected Cre-inducible channelerhodopsin-2 (DIO-ChR2-GFP) into the NTS of Gcg-Cre mice, in which Cre in the NTS is expressed exclusively in GLP-1 neurons. We found that opto-stimulation of GLP-1 neuron terminals in the IPN markedly increased the frequency but not the amplitude of excitatory post-synaptic currents (EPSCs) in IPN neurons (Tuesta et al., 2017). The IPN receives massive cholinergic innervation from MHb (Ren et al., 2011), with MHb cholinergic neurons co-releasing glutamate and providing the major source of glutamatergic input to IPN. The GLP-1 agonist Ex-4 markedly enhanced EPSCs in IPN neurons optically evoked from terminals the terminals of MHb cholinergic neurons. These findings show that GLP-1, released from NTS inputs to IPN, acts on the terminals of MHb neurons to enhance excitatory transmission in IPN neurons.
Finally, we investigated the role for GLP-1 transmission in the MHb-IPN circuit in regulating nicotine intake. shRNA-mediated knockdown of Glp1r in the MHb increased nicotine intake in rats, particularly when higher doses of nicotine were available. Also, we found that infusion of the GLP-1 receptor agonist exendin-4 (Ex-4) into the IPN dramatically decreased nicotine intake in rats but not food responding. Conversely, IPN infusion of the GLP-1 receptor antagonist exendin-(9–39)-amide (Ex-9) increased nicotine intake in rats. These data show that GLP-1 acts on the MHb- IPN circuit to regulate nicotine avoidance behaviors.
More recently, the role for discrete populations of α5 nAChR sub-unit-expressing neurons in the IPN in regulating nicotine reward was explored. Using translating ribosome affinity purification (TRAP) technology, two non-overlapping populations of Chrna5-expressing neurons were identified in the IPN; those that co-expressed Amigo and those that co-expressed Epyc (Ables et al., 2017). Chronic nicotine with treatment maerkedly perturbed the transcriptome of α5-Amigo1 neurons, whereas the α5-Epyc neurons were relatively resistance to the transcriptional actions of nicotine (Ables et al., 2017). Closer examination of the genes whose expression was altered in α5-Amigo1 neurons suggested that somatostatin and genes related to nitric oxide (NO) signaling were impacted by nicotine (Ables et al., 2017). Moreover, somatostatin and NO were shown to inhibit optically stimulated excitatory currents derived from MHb terminals in the IPN. Moreover, inhibition of neurotransmitter release from the α5-Amigo1 but not from the α5-Epyc neurons in the IPN blocked nicotine reward, as measured using a place conditioning procedure. Similarly, shRNA-mediated knockdown of Nos1 in the IPN also blocked nicotine reward. These findings suggest that retrograde control of MHb terminals by IPN neurons play an important role in regulating the motivational properties of nicotine and perhaps other drugs of abuse that act on the MHb-IPN circuit.
In addition to the states of aversion induced by consumption of nicotine, the MHb-IPN circuit has also been implicated in the aversion state associated with withdrawal from nicotine after a period of chronic exposure to the drug. Görlich and colleagues demonstrated that cholinergic neurons in the MHb display a pacemaker activity and that nicotine withdrawal is associated with profound alterations in this pacemaker activity (Görlich et al., 2013). Infusion of the nAChR antagonist mecamylamine into the MHb or the IPN precipitates a withdrawal-like behavioral syndrome in nicotine-dependent rodents (Salas et al., 2009). This suggests that adaptive responses in nAChR signaling in the MHb-IPN circuit contributes to the development of nicotine dependence. In addition, Zhao-Shea and colleagues demonstrated that pharmacological blockade of corticotropin release factor (CRF) receptor 1 in the IPN, or optogenetic silencing of MHb inputs to the IPN, attenuated the otherwise increased IPN activation seen during nicotine withdrawal and alleviated withdrawal-associated increases in anxiety-related behaviors (Zhao-Shea et al., 2015). Conversely, CRF infusion into the IPN of nicotine-dependent mice increased anxiety-related behaviors. The data described above reveal a key role for the MHb, and the MHb-IPN circuit, in regulating nicotine intake and nicotine withdrawal (Fowler and Kenny, 2012; Görlich et al., 2013; Dao et al., 2014).
Together, these findings suggest that the MHb-IPN circuit is likely to play a critical role in the establishment and maintenance of the nicotine-taking habit in human smokers. The role of the MHb-IPN circuit in the actions of other drugs of abuse is less clear, but emerging evidence suggests that this circuit may play a role in regulating cocaine seeking behaviors (López et al., 2018).
4.3. Role for the lateral habenula in regulating drug use
The activation of the LHb in response to punishment or omission of expected rewards has catalyzed interest in this structure in the negative affective state that accompanies withdrawal from many classes of drugs of abuse. Using patch-clamp electrophysiological recordings in mice, Zuo et al. have shown that nicotine application induces a fast but transient decrease, then a persistent increase, in LHb activity (Zuo et al., 2016) suggesting that, in addition to the MHb, the LHb may also regulate the motivational properties of nicotine. The μ-opioid agonist DAMGO hyperpolarizes a small subset of LHb neurons and inhibits glutamate release from presynaptic terminals (Margolis and Fields, 2016), suggesting that opioids inhibit the activity of this aversion-relevant brain site. In addition, opioids have also been shown to inhibit the release of GABA in LHb from inhibitory inputs in a large subset of LHb neurons (Margolis and Fields, 2016). This suggests that opioids exert a complex patter of effects LHb neurons. It will be important to determine if the inhibitory and excitatory effects of opioids on LHb activity show a dissociable temporal profile of effect in vivo. This is based on the bimodal actions that cocaine is known to exert on LHb activity. Specifically, Jhou and colleagues demonstrated that the LHb shows an initial decrease in activity in response to cocaine injections in rats that coincides with the expression of reward-related behaviors (Jhou et al., 2013). This effect lasts ~10-15 min after cocaine delivery and is followed by a persistent increase in the firing rate of LHb neurons lasting at least 30 min and coinciding with the expression of cocaine-induced states of aversion (Jhou et al., 2013). Hence, it will be interesting to determine if opioids induce a similar pattern of LHb inhibition followed by LHb activation that coincides with opioid reward and aversion, respectively.
Neumann and colleagues, using patch-clamp preparations, have also recorded the activity of LHb neurons after cocaine self-administration behavior in rats. They describe an increase of the LHb membrane excitability when assessed 24 to 48 h following self-administration of cocaine, which persists for at least 7 days after the last cocaine exposure (Neumann et al., 2014). This suggests that increased LHb activity could contribute to the long-lasting decreases in mood and insensitivity to rewarding stimuli detected in rats underling cocaine withdrawal that are thought to play a key role in precipitating compulsive cocaine use (Ahmed and Kenny, 2011; Kenny et al., 2003, 2018). Mahler and Aston-Jones described that the LHb neurons projecting to the VTA are more active during 7 days of extinction from intravenously self-administered cocaine infusions compared with their activity after cue-induced reinstatement of responding (Mahler and Aston-Jones, 2012). This observation suggests that exposure to cocaine-paired cues, and engaging in cocaine-seeking behaviors, can at least temporarily decrease the firing rate of LHb neurons, perhaps contributing to the motivation to seek and obtain the drug during periods of abstinence. Mameli and colleagues have described an important series of studies in which the LHb becomes hyperactive during periods of cocaine withdrawal in mice (Meye et al., 2015, 2016), with this hyperactivity contributing to a negative affect state (Lecca et al., 2016; Valentinova et al., 2015). Furthermore, they found that projections from the EPN to the LHB can co-release both glutamate and GABA, which is consistent with previous observations (Root et al., 2014a; Shabel et al., 2014). During withdrawal, this co-release process is impacted such that the LHb receives a greater component of excitatory drive from EPN inputs, which leads to negative affective states (Meye et al., 2016). Moreover, normalizing dysregulated balance between excitatory and inhibitory transmission in the EPN-LHb circuit not only rescued the negative state induced by cocaine withdrawal but also prevented stress-induced reinstatement (Meye et al., 2016). Thus, the LHb seems to first, signals the rewarding effect of a drug of abuse by decreasing its firing rate. Then, as a self-protective mechanism, the LHb activity seems to increase, likely in order to suppress the consumption, and even drives the aversive effects of the drug.
Moreover, during withdrawal periods the LHb drug-induced hyperactivity (LHb-DIH) could play a key role in the aversive states experienced by drug users. This LHb-DIH may still be a protective mechanism protecting the individual to retake a drug of abuse.
4.4. Neurodegeneration of the habenular complex in response to drugs of abuse
It has been reported that cocaine can trigger degeneration of the fasciculus retroflexus (FR) (the main efferent LHb pathway) in rodents after continuous cocaine or amphetamine treatment (Ellison, 1992, 2002; Lax et al., 2013). Nicotine induces striking degeneration of the FR in rats, but in this case it is axons from neurons that originate in the MHb that are affected (Carlson et al., 2001). These findings suggest that the habenular complex is uniquely sensitive to neurotoxic actions of drugs of abuse. Degeneration of FR inputs to downstream dopamine and monoaminergic brain centers may induce long-lasting disruption in brain homeostasis in response to the consumption of drugs of abuse that contributes to persistent vulnerability to relapse. This raises the intriguing possibility that substance use disorders may not be purely behavioral disorders but may also reflect the impact of highly selective neurodegenerative processes in the brains of drug users. This possibility will require intensive investigation.
4.5. The habenular complex in substance use disorders
Based on what we can surmise from the emerging literature on the role of the habenular complex in the actions of drugs of abuse we can begin to identify some basic principles on its likely involvement in addiction-relevant behavioral abnormalities. First, the MHb and LHb are responding robustly after consumption of doses of nicotine or cocaine, respectively, that induce aversive states. Hence, these structures are likely involved in the noxious responses to these drugs that limit their consumption. These observations raise some important questions: First, is the habenula complex involved in noxious responses to other addictive drugs? Second, how precisely that these aversive states transduced in downstream brain circuits, such as the IPN, VTA and raphe nuclei and beyond (Fig. 2), such that this information is processed in parallel with reward-relevant information in the brain and behavioral output modified accordingly? Third, can this information be leveraged to generate novel medications for substance use disorders that are based on modifying avoidance responses to drug of abuse rather than modifying approach behaviors?
As noted above, the habenula complex also plays a role in the aversive behavioral state that is experienced during withdrawal from drugs of abuse after a period of chronic exposure. Once, again, this raises questions about the broader circuit thigh which these effects are relayed and whether a network of habenula-regulated brain systems can be targeted to develop novel treatments for those suffering from substance use disorders.
It is important to note that some of the strongest human genetics evidence linking allelic variation to vulnerability to substance use disorders relate to genes that are densely expressed in the habenular complex. Indeed, allelic variation in the CHRNA5/CHRNA3/CHRNB4 gene cluster, which encodes the α5, α3 and β4 subunits, respectively, increases vulnerability to tobacco and alcohol dependence, and these genes are densely expressed in the habenula. This raises the intriguing possibility that other genes that regulate habenular function may also influence genetic vulnerability to addiction. Moreover, environmental factors that alter habenular function could also predispose to substance use disorders, and chromatin modifiers that control habenular gene expression could likewise influence vulnerability.
Finally, it is worth pointing out that neurons in the MHb and the LHb demonstrate marked pacemaker activity (Görlich et al., 2013; Sakhi et al., 2014a, b; Zhao and Rusak, 2005). Further investigation of this particular capacity of the habenular complex to generate complex intrinsic patterns of activity is likely to better reveal how drugs of abuse impact the habenula and how this structure controls drug intake. Görlich and colleagues already proposed that the pacemaker activity of cholinergic neurons in MHb may be modified during withdrawal from nicotine, which may contribute to relapse vulnerability (Görlich et al., 2013).
5. Conclusions
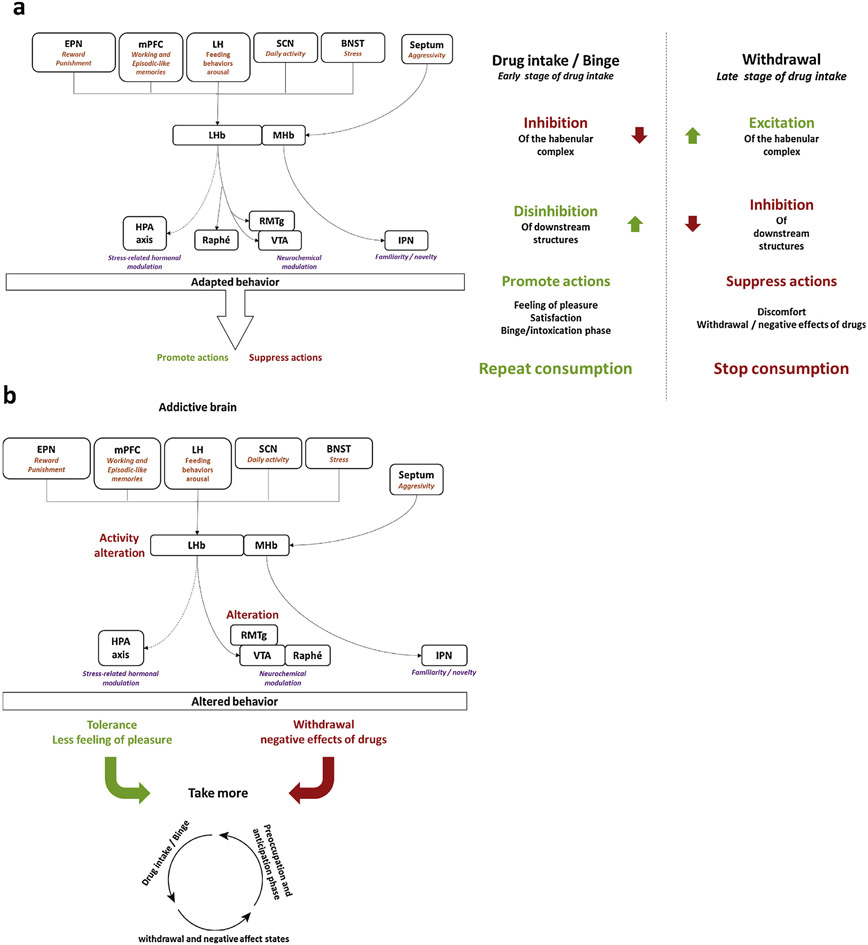
In summary, the habenular complex plays a key role in processing aversive and emotionally negative stimuli to influence action selection to optimize adaptive behavioral responses (Hikosaka, 2010; Mizumori and Baker, 2017; Stephenson-Jones et al., 2016a; Mathis and Lecourtier, 2017). Indeed, the habenula integrates external as well as internal stimuli (Baker et al., 2015; Zhang et al., 2016b) to regulate adaptations to aversive-related stimuli (Mathis et al., 2015, 2016; Lammel et al., 2012; Stamatakis and Stuber, 2012). Thus, the habenula appears to be at the center of a brain network involved in reward- and aversion- relevant behaviors. Drugs of abuse appear to profoundly alter the activity neurons in the medial and lateral habenula. We propose that drug-induced alterations in habenular activity contribute to the spiraling dysregulation in brain hedonic homeostasis that drives the development of the compulsive drug use that characterizes substance use disorders (Fig. 3). Future studies on precisely how the activity in the habenula complex is impacted by drug use, and identification of strategies to reverse such alterations, may lead to new therapeutics for the treatment of compulsive drug use.
Fig. 3. The habenular complex plays a role in the reward and anti-reward systems.
Drugs of abuse can modify the habenular activit y. (a) In general, after drug intake the habenular complex receives information from several brain areas leading to its inhibition which participate in the positive effect of drug (pleasure). Then, after a period of withdrawal this complex shows an increased activity, as the reflection of the activation of an “opponent process” of the “anti-reward system”. The aim of the activation of such a process is likely to suppress the drug intake by inducing the aversive effects of drugs of abuse.
In the context of substance use disorders (b), activity alterations of the habenular complex likely lead to midbrain areas, such as VTA, RMTg and IPN, dysfunctions. These areas control the monoaminergic release, which will therefore be altered, impairing the subject abilities to control his drug seeking, the starting point of the addiction cycle.
Acknowledgements
This study was partially supported by a grant from the Fondation Fyssen (VM) and by NIDA grant DA020686 (PJK).
References
- Ables JL, et al. , 2017. Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference. Proc. Natl. Acad. Sci. U. S. A 114 (December (49)), 13012–13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, 2008. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addict. Abingdon Engl 103 (July (7)), 1069–1081. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Madden PAF, Bucholz KK, Heath AC, Lynskey MT, 2014. Initial reactions to tobacco and cannabis smoking: a twin study. Addict. Abingdon Engl 109 (April (4)), 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, 2011. Cracking the molecular code of cocaine addiction. ILAR J. 52 (January (3)), 309–320. [DOI] [PubMed] [Google Scholar]
- Aizawa H, Amo R, Okamoto H, 2011. Phylogeny and ontogeny of the habenular structure. Front. Neurosci 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, fifth edition. American Psychiatric Association. [Google Scholar]
- Amo R, et al. , 2010. Identification of the Zebrafish Ventral Habenula As a Homolog of the Mammalian Lateral Habenula. J. Neurosci 30 (January (4)), 1566–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos CI, et al. , 2008. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat. Genet 40 (May (5)), 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolin-Fontes B, Ables JL, Görlich A, Ibañez-Tallon I, 2015. The habenulo-interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology 96 (September (Pt B)), 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik J-H, 2013. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Oh SE, Kidder KS, Mizumori SJY, 2015. Ongoing behavioral state information signaled in the lateral habenula guides choice flexibility in freely moving rats. Front. Behav. Neurosci, vol. 9, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Raynor SA, Francis NT, Mizumori SJY, 2017. Lateral habenula integration of proactive and retroactive information mediates behavioral flexibility. Neuroscience 345 (March), 89–98. [DOI] [PubMed] [Google Scholar]
- Baldwin PR, Alanis R, Salas R, 2011. The role of the habenula in nicotine addiction. J. Addict. Res. Ther, vol. S1 (October 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC, 2012. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J. Neurosci. Off. J. Soc. Neurosci 32 (October (41)), 14094–14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini WH, Doyle GA, 2012. The CHRNA5-A3-B4 gene cluster in nicotine addiction. Mol. Psychiatry 17 (September (9)), 856–866. [DOI] [PubMed] [Google Scholar]
- Bianco IH, Wilson SW, 2009. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos. Trans. R. Soc. B Biol. Sci 364 (April (1519)), 1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasi MD, Salas R, 2008. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp. Biol. Med 233 (no. August (8)), 917–929. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O, 2011. Lateral habenula neurons signal errors in the prediction of reward information. Nat. Neurosci 14 (August (9)), 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Nakahara H, Hikosaka O, 2010. Multiple timescales of memory in lateral habenula and dopamine neurons. Neuron 67 (August (3)), 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Shepard PD, 2016. Functional evidence for a direct excitatory projection from the lateral habenula to the ventral tegmental area in the rat. J. Neurophysiol 116 (September (3)), 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Palacorolla H, Brady D, Riegger K, Elmer GI, Shepard PD, 2017. Habenula-induced inhibition of midbrain dopamine neurons is diminished by lesions of the rostromedial tegmental nucleus. J. Neurosci. Off. J. Soc. Neurosci 37 (January (1)), 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J, Noguchi K, Ellison G, 2001. Nicotine produces selective degeneration in the medial habenula and fasciculus retroflexus. Brain Res., vol. 906 (July (1–2)), 127–134. [DOI] [PubMed] [Google Scholar]
- Chastrette N, Pfaff DW, Gibbs RB, 1991. Effects of daytime and nighttime stress on fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 563 (November (1–2)), 339–344. [DOI] [PubMed] [Google Scholar]
- Chen BT, et al. , 2013. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496 (April (7445)), 359–362. [DOI] [PubMed] [Google Scholar]
- Concha ML, Wilson SW, 2001. Asymmetry in the epithalamus of vertebrates. J. Anat 199 (1–2), 63–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DQ, Perez EE, Teng Y, Dani JA, De Biasi M, 2014. Nicotine enhances excitability of medial habenular neurons via facilitation of neurokinin signaling. J. Neurosci. Off. J. Soc. Neurosci 34 (March (12)), 4273–4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward EL, Thorndike L, 1898. Animal Intelligence : an Experimental Study of the Associative Processes in Animals. Macmillan, New York. [Google Scholar]
- Ellison G, 1992. Continuous amphetamine and cocaine have similar neurotoxic effects in lateral habenular nucleus and fasciculus retroflexus. Brain Res., vol. 598 (December (1–2)), 353–356. [DOI] [PubMed] [Google Scholar]
- Ellison G, 2002. Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol 12 (August (4)), 287–297. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2013. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev 37 (November (9) Pt A), 1946–1954. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2016. Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W, 2003. Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299 (March (5614)), 1898–1902. [DOI] [PubMed] [Google Scholar]
- Floresco SB, 2013. Prefrontal dopamine and behavioral flexibility: shifting from an ‘inverted-U’ toward a family of functions. Front. Neurosci 7 p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fore S, Palumbo F, Pelgrims R, Yaksi E, 2017. Information processing in the vertebrate habenula. Semin. Cell. Dev. Biol(August). [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ, 2012. Habenular signaling in nicotine reinforcement. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 37 (January (1)), 306–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ, 2014. Nicotine aversion: neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology, vol. 76 (January (Pt B)), 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ, 2011. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471 (March (7340)), 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardon O, Faget L, Chu Sin Chung P, Matifas A, Massotte D, Kieffer BL, 2014. Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience 277 (September), 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Trimble M, 2008. The lateral habenula: no longer neglected. CNS Spectr. 13 (June (6)), 484–489. [DOI] [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP, 1995. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur. J. Neurosci 7 (November (11)), 2294–2300. [DOI] [PubMed] [Google Scholar]
- Golden SA, et al. , 2016. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 534 (June (7609)), 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich A, et al. , 2013. Reexposure to nicotine during withdrawal increases the pace-making activity of cholinergic habenular neurons. Proc. Natl. Acad. Sci. U. S. A 110 (October (42)), 17077–17082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ, 1977. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J. Comp. Neurol 173 (May (1)), 123–146. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ, 1979. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol 187 (no. 1), 19–47. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, 2010. The habenula: from stress evasion to value-based decision-making. Nat. Rev. Neurosci 11 (July (7)), 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD, 2008. Habenula: crossroad between the Basal Ganglia and the Limbic system. J. Neurosci 28 (November (46)), 11825–11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O, 2011. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J. Neurosci. Off. J. Soc. Neurosci 31 (August (32)), 11457–11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-WA, et al. , 2014. Role of the dorsal medial habenula in the regulation of voluntary activity, motor function, hedonic state, and primary reinforcement. J. Neurosci. Off. J. Soc. Neurosci 34 (August (34)), 11366–11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung RJ, et al. , 2008. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452 (April (7187)), 633–637. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ, 2006. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci 29, 565–598. [DOI] [PubMed] [Google Scholar]
- Jhou TC, et al. , 2013. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J. Neurosci. Off. J. Soc. Neurosci 33 (April (17)), 7501–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD, 2007. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABAA receptor-mediated mechanism. J. Neurosci 27 (June 26), 6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A, 2003. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur. J. Neurosci 17 (January (1)), 191–195. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Hoyer D, Koob GF, 2018. Animal models of addiction and neuropsychiatric disorders and their role in drug discovery: honoring the legacy of athina markou. Biol. Psychiatry (February). [DOI] [PubMed] [Google Scholar]
- Kim U, Chang S-Y, 2005. Dendritic morphology, local circuitry, and intrinsic electrophysiology of neurons in the rat medial and lateral habenular nuclei of the epithalamus. J. Comp. Neurol 483 (March (2)), 236–250. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, et al. , 2013. Genetic dissection of medial habenula-interpeduncular nucleus pathway function in mice. Front. Behav. Neurosci, vol. 7 p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Moal Le M., 2008. Addiction and the brain antireward system. Annu. Rev. Psychol 59, 29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 35 (January (1)), 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3 (August (8)), 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, et al. , 2012. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491 (October 7423), 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax E, et al. , 2013. Neurodegeneration of lateral habenula efferent fibers after intermittent cocaine administration: implications for deep brain stimulation. Neuropharmacology 75 (December), 246–254. [DOI] [PubMed] [Google Scholar]
- Lecca S, Meye FJ, Mameli M, 2014. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur. J. Neurosci 39 (April (7)), 1170–1178. [DOI] [PubMed] [Google Scholar]
- Lecca S, et al. , 2016. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat. Med 22 (March (3)), 254–261. [DOI] [PubMed] [Google Scholar]
- Lecca S, et al. , 2017a. Aversive stimuli drive hypothalamus-to-habenula excitation to promote escape behavior. eLife 6 (September). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, et al. , 2017b. Aversive stimuli drive hypothalamus-to-habenula excitation to promote escape behavior. eLife 6 (September) p. e30697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, DeFrancesco A, Moghaddam B, 2008. Differential tonic influence of lateral habenula on prefrontal cortex and nucleus accumbens dopamine release. Eur. J. Neurosci 27 (April (7)), 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, 2003. Emotion Circuits in the Brain. [DOI] [PubMed] [Google Scholar]
- Li K, et al. , 2013. βCaMKII in lateral habenula mediates core symptoms of depression. Science 341 (August (6149)), 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López AJ, et al. , 2018. Medial habenula cholinergic signaling regulates cocaine-associated relapse-like behavior. Addict. Biol vol. 0, no. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC, 2011. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69 (February (4)), 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS, 2012. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J. Neurosci. Off. J. Soc. Neurosci 32 (September (38)), 13309–13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, 2016. Mu opioid receptor actions in the lateral habenula. PloS One 11 (no. 7) p. e0159097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis V, Lecourtier L, 2017. Role of the lateral habenula in memory through online processing of information. Pharmacol. Biochem. Behav(July). [DOI] [PubMed] [Google Scholar]
- Mathis V, Cosquer B, Avallone M, Cassel J-C, Lecourtier L, 2015. Excitatory transmission to the lateral habenula is critical for encoding and retrieval of spatial memory. Neuropsychopharmacology 40 (no. 12), 2843–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis V, Barbelivien A, Majchrzak M, Mathis C, Cassel J-C, Lecourtier L, 2016. The lateral habenula as a relay of cortical information to process working memory. Cereb. Cortex(October). [DOI] [PubMed] [Google Scholar]
- Mathura AS, Jesuthasan S, 2013a. The medial habenula as a regulator of anxiety in adult zebrafish. Front. Neural Circ, vol. 7, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathura AS, Jesuthasan S, 2013b. The medial habenula as a regulator of anxiety in adult zebrafish. Front. Neural Circuits, vol. 7, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2007. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447 (June (7148)), 1111–1115. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2009. Representation of negative motivational value in the primate lateral habenula. Nat. Neurosci 12 (January (1)), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE, 1997. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav. Brain Res 87 (September (2)), 139–148. [DOI] [PubMed] [Google Scholar]
- Meye FJ, et al. , 2015. Cocaine-evoked negative symptoms require AMPA receptor trafficking in the lateral habenula. Nat. Neurosci 18 (March (3)), 376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Soiza-Reilly M, Smit T, Diana MA, Schwarz MK, Mameli M, 2016. Shifted pallidal co-release of GABA and glutamate in habenula drives cocaine withdrawal and relapse. Nat. Neurosci 19 (August (8)), 1019–1024. [DOI] [PubMed] [Google Scholar]
- Meye FJ, Trusel M, Soiza-Reilly M, Mameli M, 2017. Neural circuit adaptations during drug withdrawal – spotlight on the lateral habenula. Pharmacol. Biochem. Behav (August). [DOI] [PubMed] [Google Scholar]
- Mizumori SJY, Baker PM, 2017. The lateral habenula and adaptive behaviors. Trends Neurosci.(July). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molas S, DeGroot SR, Zhao-Shea R, Tapper AR, 2017a. Anxiety and nicotine dependence: emerging role of the habenulo-interpeduncular axis. Trends Pharmacol. Sci 38 (February (2)), 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molas S, Zhao-Shea R, Liu L, DeGroot SR, Gardner PD, Tapper AR, 2017b. A circuit-based mechanism underlying familiarity signaling and the preference for novelty. Nat. Neurosci 20 (September (9)), 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, 2005. The neurobiology of cocaine addiction. Sci. Pract. Perspect 3 (December (1)), 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Malenka RC, 2004. The addicted brain. Sci. Am 290 (March (3)), 78–85. [DOI] [PubMed] [Google Scholar]
- Neumann PA, Ishikawa M, Otaka M, Huang YH, Schlüter OM, Dong Y, 2014. Increased excitability of lateral habenula neurons in adolescent rats following cocaine self-administration. Int. J. Neuropsychopharmacol 18 (December (6)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Aizawa H, 2013. Fear and anxiety regulation by conserved affective circuits. Neuron 78 (May (3)), 411–413. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Agetsuma M, Aizawa H, 2012a. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev. Neurobiol 72 (March (3)), 386–394. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Agetsuma M, Aizawa H, 2012b. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev. Neurobiol 72 (March (3)), 386–394. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P, 1954. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol 47 (December (6)), 419–427. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR, 2009. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area: habenula input to VTA cell populations. Eur. J. Neurosci 30 (October (7)), 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL, 1992. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav. Neurosci 106 (June (3)), 439–446. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE, 2005. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48 (October (2)), 175–187. [DOI] [PubMed] [Google Scholar]
- Ren J, et al. , 2011. Habenula ‘Cholinergic’ neurons corelease glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron 69 (February (3)), 445–452. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, 1999. Drug addiction: bad habits add up. Nature 398 (April (6728)), 567–570. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, 1982. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol. Biochem. Behav 17 (November (5)), 901–904. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B, 2004. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology, vol. 47 (Suppl. 1), 33–46. [DOI] [PubMed] [Google Scholar]
- Root DH, et al. , 2014a. Single rodent mesohabenular axons release glutamate and GABA. Nat. Neurosci 17 (November (11)), 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Qi J, Morales M, 2014b. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J. Neurosci 34 (October (42)), 13906–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi K, Belle MDC, Gossan N, Delagrange P, Piggins HD, 2014a. daily variation in the electrophysiological activity of mouse medial habenula neurones: daily variation in electrical activity of mouse medial habenula neurones. J. Physiol 592 (February (4)), 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi K, et al. , 2014b. Intrinsic and extrinsic cues regulate the daily profile of mouse lateral habenula neuronal activity. J. Physiol 592 (November (22)), 5025–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaberry NL, Mendoza J, 2015. Insights into the role of the habenular circadian clock in addiction. Front. Psychiatry, vol. 6, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M, 2009. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. Off. J. Soc. Neurosci 29 (March (10)), 3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius A, Henn FA, 2007. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med. Hypotheses 69 (6), 1305–1308. [DOI] [PubMed] [Google Scholar]
- Schultz W, 2013. Updating dopamine reward signals. Curr. Opin. Neurobiol 23 (April (2)), 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, 2016. Dopamine reward prediction-error signalling: a two-component response. Nat. Rev. Neurosci 17 (March (3)), 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Trias A, Murphy RT, Malinow R, 2012. Input to the lateral habenula from the basal ganglia Is excitatory, aversive, and suppressed by serotonin. Neuron 74 (May (3)), 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Piriz J, Malinow R, 2014. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science 345 (September (6203)), 1494–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard PD, Holcomb HH, Gold JM, 2006. Schizophrenia in translation: the presence of absence: habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophr. Bull 32 (July (3)), 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih P-Y, et al. , 2014. Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J. Neurosci. Off. J. Soc. Neurosci 34 (July (29)), 9789–9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ślimak MA, et al. , 2014. Habenular expression of rare missense variants of the β4 nicotinic receptor subunit alters nicotine consumption. Front. Hum. Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD, 2012. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci 15 (June 8), 1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD, 2014. Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology 76 (January (Pt B)), 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson-Jones M, et al. , 2016a. A basal ganglia circuit for evaluating action outcomes. Nature 539 (November (7628)), 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson-Jones M, et al. , 2016b. A basal ganglia circuit for evaluating action outcomes. Nature 539 (7628), 289–293 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Tse MTL, Montes DR, Wiedman CR, Floresco SB, 2014. Overriding phasic dopamine signals redirects action selection during risk/reward decision making. Neuron 84 (October (1)), 177–189. [DOI] [PubMed] [Google Scholar]
- Stuber GD, et al. , 2011. “Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 75 (June (7356)), 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, 1982. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci. Biobehav. Rev 6 (1), 1–13. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, et al. , 2008. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452 (April (7187)), 638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL, 1933. A proof of the law of effect. Science 77 (February (1989)), 173–175. [DOI] [PubMed] [Google Scholar]
- Thornton EW, Bradbury GE, Davies C, 1990. Increased immobility in an automated forced swimming test following lesion of the habenula in rats: absence of evidence for a contribution from motor impairment. Behav. Neurosci 104 (February (1)), 37–43. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W, 2005. Adaptive coding of reward value by dopamine neurons. Science 307 (March (5715)), 1642–1645. [DOI] [PubMed] [Google Scholar]
- Tuesta LM, et al. , 2017. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat. Neurosci 20 (May (5)), 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B, 2003. Do rats have a prefrontal cortex? Behav. Brain Res 146 (November (1–2)), 3–17. [DOI] [PubMed] [Google Scholar]
- Valentinova K, Tchenio A, Meye FJ, Lecca S, Mameli M, 2015. Hell after the pleasure: drug-induced negative symptoms involve lateral habenula. Med. Sci. MS 31 (May (5)), 478–481. [DOI] [PubMed] [Google Scholar]
- van Kerkhof LWM, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJMJ, 2013. Functional integrity of the habenula is necessary for social play behaviour in rats. Eur. J. Neurosci 38 (November (10)), 3465–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez KM, Molfese DL, Salas R, 2014. The role of the habenula in drug addiction. Front. Hum. Neurosci, vol. 8, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalón A, Sepúlveda M, Guerrero N, Meynard MM, Palma K, Concha ML, 2012. Evolutionary plasticity of habenular asymmetry with a conserved efferent connectivity pattern. PLoS ONE 7 (April (4)) p. e35329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath H, Carter AQ, Baldwin PR, Molfese DL, Salas R, 2014. The medial habenula: still neglected. Front. Hum. Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, 2012. Neuroscience. To stop or not to stop? Science 335 (February (6068)), 546–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, 2014. Addiction science: uncovering neurobiological complexity. Neuropharmacology 76 (January (Pt B)), 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Morales M, 2015. The brain on drugs: from reward to addiction. Cell 162 (August (4)), 712–725. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Tomasi D, Baler RD, 2013. Unbalanced neuronal circuits in addiction. Curr. Opin. Neurobiol 23 (August (4)), 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT, 2016. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med 374 (Janury (4)), 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC, 2002. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 26 (April (4)), 479–488. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang L, Yamamoto R, Sugai T, Kato N, 2013. Role of the lateral habenula in shaping context-dependent locomotor activity during cognitive tasks. NeuroReport 24 (April (6)), 276–280. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A, 2015. The basolateral amygdala in reward learning and addiction. Neurosci. Biobehav. Rev 57 (October), 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PEM, 2010. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr. Top. Behav. Neurosci 3, 29–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE, Pitzer MR, 1994. Dopamine agonists and stress produce different patterns of fos-like immunoreactivity in the lateral habenula. Brain Res. 633 (January (1–2)), 21–26. [DOI] [PubMed] [Google Scholar]
- Wise RA, 1998. Drug-activation of brain reward pathways. Drug Alcohol Depend. 51 (July (1–2)), 13–22. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF, 2014. The development and maintenance of drug addiction. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 39 (January (2)), 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S, 2013. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron 78 (May (3)), 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hernández VS, Vázquez-Juárez E, Chay FK, Barrio RA, 2016a. Thirst is associated with suppression of habenula output and active stress coping: Is there a role for a non-canonical vasopressin-glutamate pathway? Front. Neural Circ 10 (March). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hernández VS, Vázquez-Juárez E, Chay FK, Barrio RA, 2016b. Thirst is associated with suppression of habenula output and active stress coping: Is there a role for a non-canonical vasopressin-glutamate pathway? Front. Neural Circ, vol.10, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Rusak B, 2005. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience 132 (January 2), 519–528. [DOI] [PubMed] [Google Scholar]
- Zuo W, et al. , 2016. Nicotine regulates activity of lateral habenula neurons via presynaptic and postsynaptic mechanisms. Sci. Rep, vol. 6 (September), 32937. [DOI] [PMC free article] [PubMed] [Google Scholar]