Abstract
We isolated a Vibrio vulnificus mutant that was deficient in both metalloprotease and cytolysin by allelic exchange. The virulence of this mutant in mice and its cytotoxicity for HEp-2 cells were comparable to those of the wild-type strain, indicating that neither factor was essential for these properties. The cytolysin, but not the protease, seemed to be important for causing damage in the alimentary tract of the mice.
Vibrio vulnificus, a gram-negative estuarine bacterium, causes wound infections and septicemia in humans, mostly in immunocompromised people and those with underlying conditions such as hemochromatosis, liver cirrhosis, and alcoholism (2, 3, 5, 23). Infection is usually acquired via direct contact or the gastrointestinal (GI) route; in both cases, skin lesions with ulcer and edema are common (5, 12, 32).
Strains of V. vulnificus secrete a variety of products that have been implicated in bacterial virulence and pathogenesis, including capsular polysaccharide (34), cytolysin (7, 16), metalloprotease (protease) (15, 19), phospholipases (31), and siderophores (25). The purified protease of V. vulnificus has been shown to increase vascular permeability and induce edema by activating the plasma kallikrein-kinin cascade (18, 21, 22) and to cause hypodermic hemorrhage (20). It also facilitates bacterial acquisition of iron by digesting heme proteins, transferrin and lactoferrin (24). The cytolysin, a pore-forming cytotoxin and hemolysin (13), is lethal to mice at a submicrogram level (26). It damages mast cells, resulting in release of histamine (36), and causes hypotension, tachycardia (14), and skin (9) and pulmonary (26) damage in animals. Collectively, the cytolysin and the protease are thought to be important for the pathogenesis of V. vulnificus. The presence of cytolysin in V. vulnificus-infected mice (10) and the detection of anticytolysin antibodies in sera from mice and a human that survived V. vulnificus disease (8) further support the role of cytolysin in disease development.
Genes encoding the protease (4) and cytolysin (35) of V. vulnificus have been cloned, and isogenic mutants deficient in either gene product have been isolated by an allelic exchange technique (29, 33). Although purified cytolysin and protease exhibited a variety of biological activities that seemed to be detrimental to the animals, elimination of either factor did not attenuate the pathogenicity in mice, as assayed with several animal models (29, 33). In fact, the protease-deficient (PD) mutant was even more virulent than the wild-type strain in mice challenged orally (29). As mentioned above, both the cytolysin and the protease are able to increase vascular permeability and cause tissue damage, which may enhance bacterial invasiveness. Therefore, deficiency in either factor may not be sufficient to reduce the bacterial virulence, since compensation of the other factor may occur. We therefore reasoned that elimination of both factors may be necessary for attenuation of bacterial virulence. To test this hypothesis, we generated a double mutant deficient in both the cytolysin and protease and compared its cytotoxicity for cultured epithelial cells and its virulence and potential to cause tissue damage with those of both the wild-type strain and single mutants deficient in either protease or cytolysin.
Bacterial strains and plasmids.
The V. vulnificus and Escherichia coli strains and the plasmids used in this study are listed in Table 1. All strains were routinely grown in Luria-Bertani (LB) medium at 37°C with aeration.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| V. vulnificus strains | ||
| YJ016 | Clinical isolate | 11 |
| CP080 | YJ016 Δvvp | This study |
| FJ201 | YJ016 Δvvh | This study |
| FJ202 | YJ016 Δvvp Δvvh | This study |
| E. coli SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpir Kmr Nalr | 17 |
| Plasmids | ||
| pUC19 | Cloning vector; Apr | Laboratory collection |
| pFJ004 | pUC19 inserted with a SalI-BamHI fragment containing vvhA and vvhB | This study |
| pFJ005 | PFJ004 with a 792-bp deletion in vvhA (Δvvh) | This study |
| pCVD442 | Suicide vector; Apr | 6 |
| pFJ006 | pCVD442 inserted with a SalI-BamHI fragment that contains Δvvh cloned from pFJ005 | This study |
Apr, Kmr, and Nalr, resistance to ampicillin, kanamycin, and nalidixic acid, respectively.
Cloning of the vvhA gene.
Recombinant clones containing sequences of the cytolysin gene, vvhA, were identified in a V. vulnificus gene library (4) by colony hybridization (1, 28). The probe (Fig. 1A), which was derived from the known vvhA sequence (35), was labeled with [α-32P]dCTP by random priming with a kit (Megaprime DNA labeling system; Amersham, Little Chalfont, United Kingdom) using a PCR product as the template. Among the identified clones, one exhibited hemolysis of murine red blood cells and was shown to contain both vvhA and vvhB (35) by comparing the restriction pattern of the insert with that derived from the published restriction map of this locus (35). A SalI-BamHI fragment containing vvhA, vvhB, and the flanking sequences was then excised from the recombinant plasmid of this clone and inserted into pUC19 to create pFJ004.
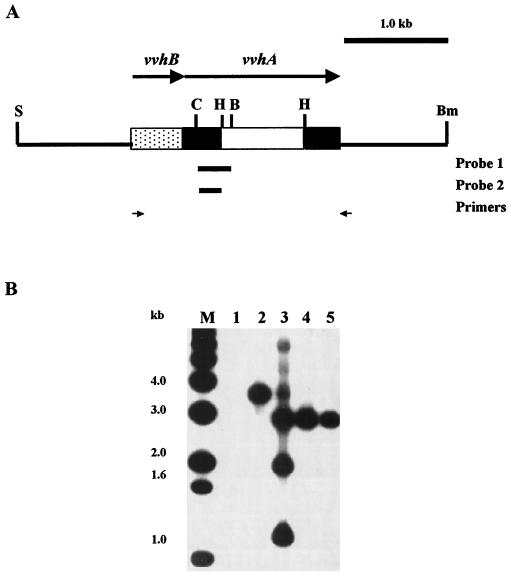
FIG. 1.
Detection of deletion in vvhA in the chromosome of V. vulnificus. (A) Restriction map of the insert in pFJ004, which was used to construct V. vulnificus mutants FJ201 and FJ202. The deletion (white bar), the probes used in colony (probe 1) and Southern hybridization (probe 2), and the PCR primers used in detecting the deletion in the mutants are indicated. S, SalI; C, ClaI; H, HpaI; B, BglII; Bm, BamHI. (B) Southern hybridization of the ClaI digests of the plasmid or chromosomal DNA fractionated by electrophoresis on a 1.2% agarose gel. Lanes: 1, pCVD442; 2, YJ016; 3, FJ008 (YJ016 integrated with pFJ006); 4, FJ201; 5, FJ202; M, molecular size standards.
Isolation of V. vulnificus cytolysin-deficient (CD) and protease- and cytolysin-deficient (double-deficient [DD]) mutants.
V. vulnificus mutants with disruptions in vvhA and in both vvhA and the protease gene (vvp) were isolated from clinical strain YJ016 and its PD mutant (CP080), respectively, by allelic exchange. CP080, which contained a 426-bp deletion in vvp, was isolated by allelic exchange as described previously for isolating another PD mutant (29).
To introduce an in-frame deletion in vvhA, an HpaI-HpaI fragment (792 bp) was removed from the vvhA gene in pFJ004 (Fig. 1A) to generate pFJ005. Correct deletion was confirmed by nucleotide sequence determination. The whole insert of pFJ005 was then subcloned into pCVD442 (6) to generate pFJ006. pCVD442 is a suicide plasmid containing the sacB gene of Bacillus subtilis which allows positive selection with sucrose for loss of the vector. pFJ006 was transferred from SM10λpir to either YJ016 or CP080 by conjugation. The transconjugants, which had the plasmid integrated into the chromosome via homologous recombination, were selected by ampicillin (100 μg/ml) and polymyxin B (50 U/ml) and tested for sensitivity to 10% sucrose. One of the sucrose-sensitive transconjugants was grown in LB medium with 10% sucrose overnight and spread onto a 10% sucrose-containing LB plate for selecting the sucrose-resistant clones, which were cured of the plasmid via a second homologous recombination. The resultant strains were further tested for ampicillin sensitivity. Some of the sucrose-resistant, ampicillin-sensitive strains were then screened by PCR with a pair of primers (Fig. 1A) flanking the deletion for those that had the deletion in vvhA and, therefore, gave rise to a shorter product.
The presence of the vvhA deletion in the mutants was confirmed by Southern hybridization (1, 28) with a probe derived from the vvhA gene (Fig. 1A). As shown in Fig. 1B, a deletion of approximately 0.8 kb was detected in both the CD mutant, FJ201, and the DD mutant, FJ202. The colonies of the CD and DD mutants were as opaque as those of the parent strains, indicating that both were encapsulated.
Protease and cytolysin activities in the culture supernatant.
The protease and cytolysin activities in the culture supernatant were assayed as described previously (29). Briefly, protease activity in bacterial culture supernatants was assayed with azocasein, and the amount of digested azocasein which was not precipitated by 3.2% trichloroacetic acid was measured as optical density at 450 nm (OD450). Cytolysin activity was assayed based on its ability to lyse the murine blood cells (0.9% in phosphate-buffered saline [PBS]). The level of hemolysis was estimated by measuring the optical density at 545 nm (OD545) of the supernatant after pelleting of the unlysed cells and cell debris by centrifugation. Cytolysin activity was expressed as [(OD545 of specimen/OD545 of complete hemolysis)] × 100.
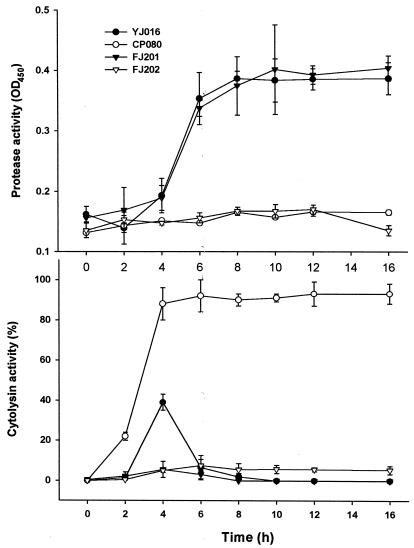
As expected, the DD mutant exhibited neither protease nor cytolysin activity, while the PD and CD mutants expressed strong cytolysin and protease activities, respectively (Fig. 2). On the other hand, the cytolysin activity of the wild-type strain reached a maximum that was only one-half of that of the PD mutant at 4 h of growth and then declined rapidly when the protease activity was increased (Fig. 2).
FIG. 2.
Protease and cytolysin activities in the culture supernatants of various V. vulnificus strains. Bacteria were grown at 37°C after a 1:100 dilution of an overnight culture in fresh medium. The culture supernatant was then collected at intervals, and the protease and cytolysin activities were determined (n = 3).
Virulence in mice.
Six- to eight-week-old C3H/HeNCrj mice purchased from the animal center of College of Medicine at the National Cheng-Kung University were used to determine the virulence of the various V. vulnificus strains. The mice were infected either by intraperitoneal (i.p.) injection or by force-feeding. For infection by i.p. injection, mice were either untreated or pretreated with 5 mg of iron dextran per mouse by intramuscular injection 2 h before challenge. The animals were then given a 10-fold serially diluted bacterial suspension in PBS, and mortality was monitored 48 h postinfection. For infection by force-feeding, each mouse was fed by intragastric intubation with 500 μl of a 10-fold serially diluted bacterial suspension in PBS, and the mortality was recorded 72 h after challenge. To enhance their susceptibility to V. vulnificus, the force-fed animals were pretreated with 3.75 mg of cyclophosphamide (Sigma, St. Louis, Mo.) per mouse by i.p. injection 72 h before challenge in addition to iron dextran pretreatment as described above (11). The animals also fasted for 12 h, starting from 8 h before till 4 h after the feeding of bacteria. The dose lethal to 50% of the mice (LD50) of each strain was calculated by the method of Reed and Muench (27).
As shown in Table 2, the LD50s of all the mutants were comparable to that of the wild-type strain in mice challenged by i.p. injection, whether the animals were iron overloaded or not. On the other hand, in mice challenged by force-feeding, the PD mutant appeared to be more virulent than the wild-type strain. Specifically, the LD50 of the PD mutant was sevenfold less than that of the wild-type strain. This finding agreed with our previous report (29). At least two lines of evidence suggest that an increase in virulence in the absence of protease may be associated with increased amounts of some other bacterial factors. First, we observed that a 9-h culture supernatant of the PD mutant exhibited more protein bands than that of the parent strain in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (unpublished data). Second, we demonstrated that the cytolysin activity in the culture supernatant of the PD mutant was twofold higher and sustained much longer than that of the parent strain (29). Surprisingly, the LD50s of the CD and DD mutants were not remarkably increased and were only approximately four- to fivefold higher than that of the wild-type strain. The virulence of the CD and DD mutants suggested that cytotoxicity was not required for bacterial virulence in mice or, alternatively, that there is another unidentified cytotoxic factor(s) in the mutants.
TABLE 2.
Virulence of V. vulnificus strains in mice
| Route of challengea | LD50 (CFU/mouse)b
|
|||
|---|---|---|---|---|
| YJ016 | CP080 | FJ201 | FJ202 | |
| ip. injection | ||||
| Normal | 1.1 × 106 | 1.2 × 106 | 3.1 × 106 | 1.3 × 106 |
| Fe overloaded | <101 | <101 | <101 | <101 |
| Force-feeding | 7.0 × 106 | 1.0 × 106 | 3.5 × 107 | 3.1 × 107 |
C3H/HeN mice (n = 5 for each dilution of bacterial suspension) challenged by i.p. injection were either untreated (normal) or pretreated (Fe overloaded) with iron dextran (5 mg/mouse) 2 h before challenge. Those challenged by force-feeding were pretreated with iron dextran (5 mg/mouse) and cyclophosphamide (3.75 mg/mouse) 2 h and 72 h, respectively, before challenge.
YJ016, wild-type strain; CP080, PD mutant; FJ201, CD mutant; FJ202, DD mutant.
Cytotoxicity assay.
Our results prompted us to determine whether other cytotoxic factors were present. Cytotoxicity of the various V. vulnificus strains was determined with HEp-2 cells, a human laryngeal carcinoma cell line. The cells were maintained in Earle's minimal essential medium (MEM) (Gibco BRL Life Technologies Inc., Gaithersburg, Md.) containing 10% fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel) and 40 μg of gentamicin (Gibco BRL Life Technologies) per ml.
Cytotoxicity was assayed after treatment of cells with bacterial culture supernatant, which was sterilized by filtration through a 0.45-μm-pore-size membrane filter, or bacterial suspension. HEp-2 cell suspension (0.2 ml) was plated at a density of 3 × 105/ml in each well of a 24-well tissue culture plate coated with 0.1% bovine serum albumin, and the cells were grown at 37°C to confluence. The cell monolayer was washed and then added with 180 μl of Earle's MEM. In one experiment, bacterial culture supernatant collected from a 4-h culture in LB medium was added in 20 μl to the cell monolayer in the well. In the other experiment, the monolayer was infected with 20 μl of washed bacteria (suspended in PBS) at a multiplicity of infection (MOI) of 0.1, 1, or 10. After incubation at 37°C for 3 h, the cytotoxicity was determined by measuring the activity of lactate dehydrogenase (LDH), a cytosolic enzyme released upon cell lysis, in the supernatant. LDH activity was assayed with a commercial kit (CytoTox 96 nonradioactive cytotoxicity assay; Promega) and was expressed as [(activity of monolayer treated with sample − activity of that treated with vehicle)/(LDH activity of monolayer completely lysed by 1% Triton X-100 − activity of that treated with vehicle)] × 100.
Table 3 shows that the culture supernatant of only the PD mutant was cytotoxic to the HEp-2 cells. Disruption of the cytolysin gene in the PD mutant abolished the cytotoxicity of the culture supernatant (compare the DD mutant with the PD mutant), indicating that the cytolysin was the only cytotoxin secreted. Little cytotoxicity was detected in the 4-h culture supernatant of the wild-type strain. This was not surprising, because the cytolysin activity declined rapidly to the basal level when the protease was produced (Fig. 2B).
TABLE 3.
Cytotoxicity of culture supernatant and bacterial suspension of various V. vulnificus strains for HEp-2 cells
| Strainb | Cytotoxicity (%)a
|
|||
|---|---|---|---|---|
| Culture supernatantc | Bacterial suspension at an MOI ofd:
|
|||
| 0.1 | 1 | 10 | ||
| YJ016 | −15.9 ± 1.4 | 6.4 ± 0.6 | 42.4 ± 5.2 | 95.2 ± 1.7 |
| CP080 | 77.2 ± 0.7 | 4.3 ± 0.8 | 43.3 ± 1.8 | 96.2 ± 0.5 |
| FJ201 | −17.5 ± 0.7 | 5.7 ± 1.2 | 39.3 ± 7.7 | 95.1 ± 1.4 |
| FJ202 | −2.5 ± 3.0 | 4.1 ± 1.4 | 44.8 ± 1.4 | 95.0 ± 0.5 |
Calculated as [(LDH activity of monolayer treated with sample − that treated with vehicle)/(LDH activity of completely lysed monolayer − that treated with vehicle)] × 100. The vehicles used were the LB medium and PBS for the culture supernatants and bacterial suspensions, respectively. Data are means ± standard deviations (n = 3).
YJ016, wild-type strain; CP080, PD mutant; FJ201, CD mutant; FJ202, DD mutant.
Bacterial culture supernatant was collected from a 4-h culture in LB medium and added to the HEp-2 monolayer after mixing with an equal volume of Earle's MEM. Cytotoxicity was determined 3 h after incubation at 37°C.
Bacterial suspension in PBS was prepared from a 4-h culture in LB medium and was added to the HEp-2 monolayer at the indicated MOIs. Cytotoxicity was determined 3 h after infection at 37°C.
On the other hand, whole cells of all the mutants as well as the wild-type strain exhibited cytotoxicity for the HEp-2 cells in a dose-dependent manner (Table 3), indicating that another cytotoxin(s) might exist in this organism. This factor(s) may not be secreted into the medium, at least not to a detectable level, upon bacterium-host cell interaction, because the conditioned medium harvested from the infected cells was not cytotoxic (data not shown). In addition, UV-killed bacteria of all strains were not cytotoxic (data not shown), suggesting that cytotoxicity may be mediated by an energy-dependent mechanism which is likely activated upon contact with the host cells.
It is intriguing that the LB medium resulted in a higher level of cytotoxicity than the culture supernatants of the wild-type strain and CD mutant (Table 3). Because strains producing the protease (wild-type and CD mutant) had a lower cytotoxicity than those deficient in the protease (PD and DD mutants) or the LB medium alone, it is suspected that the protease may degrade some of the toxic components in the LB medium. Alternatively, the protease may act directly on the cells via some unknown mechanism to protect them from death.
Tissue damage.
The roles of the protease and cytolysin in causing damage of the GI tract were examined after infection of mice with the various strains by force-feeding. Mice (n = 3) pretreated with cyclophosphamide and iron dextran were challenged with 1.5 × 107 bacteria per mouse by force-feeding as described above. Two of those challenged by the wild-type strain, three of those challenged by the PD mutant, one of those challenged by the CD mutant, and none of those challenged by the DD mutant died within 24 h after infection. No diarrhea was observed in either the dead or the surviving mice. The alimentary tract, from stomach to rectum, was removed and examined immediately after death or 24 h postinfection. Macroscopically, severe GI congestion and hemorrhage, particularly in the upper intestine, were observed with the wild-type strain or the PD mutant (including in the mouse that survived the infection). In contrast, in mice infected with the CD or DD mutant, whether they died or survived infection, mild congestion was occasionally observed. The severity of the GI lesions was correlated with the mortality rate for each strain.
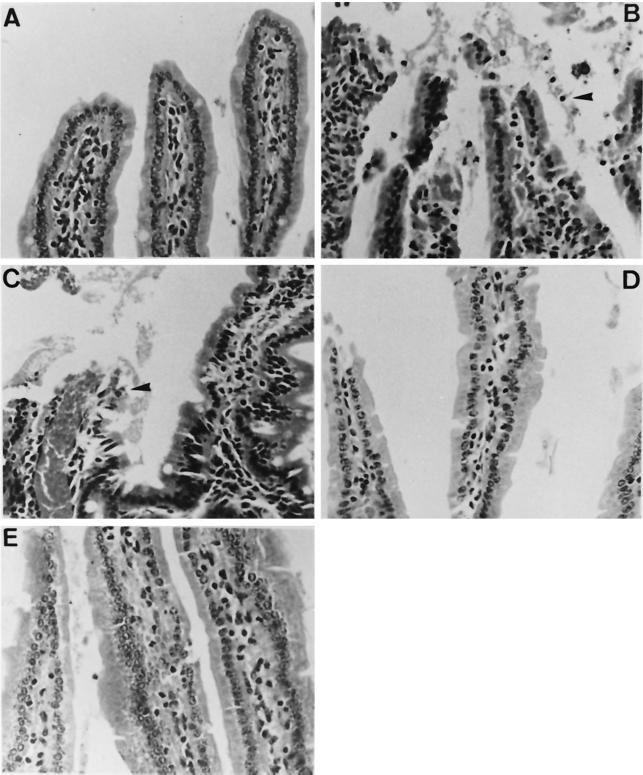
Excised portions of stomach and small intestine were fixed in 10% buffered-formalin, embedded in paraffin, sliced into 4-μm sections, and stained with hematoxylin-eosin for histological examination. No microscopic lesions were observed in the sections of the stomach examined. However, various pathological manifestations were seen in the intestines of the challenged mice. Mice infected with the wild-type strain had a severe necrotizing enteritis characterized by mucosal and submucosal congestion, shortening of villi, sloughed and necrotic epithelial cells with condensed or pyknotic nuclei, and hypercellularity in the lamina propria (Fig. 3). Infiltration of inflammatory cells was not present, which should be due to the pretreatment with cyclophosphamide. Congestion and hemorrhage were more severe in mice challenged with the PD mutant than in mice infected with the wild-type strain. No remarkable pathological change, except for a very mild, occasion congestion, was present in mice infected with the CD or DD mutant (Fig. 3). Damage of the intestine, therefore, appeared to be associated with the amounts of cytolysin produced.
FIG. 3.
Micrographs of upper intestines of mice challenged by force-feeding with the wild-type strain (B), the PD mutant (C), the CD mutant (D), and the DD mutant (E). Mice inoculated with PBS were used as the negative control (A). Note the sloughed and necrotic epithelial cells with condensed and pyknotic nuclei (arrowheads) and hypercellularity and congestion in lamina propria (B and C). Intestinal tissues from mice infected with the CD or DD mutant appear normal. Hematoxylin-eosin stain was used. Magnification, ×400.
Conclusions.
A number of conclusions can be drawn from this study. First, neither the protease nor the cytolysin of V. vulnificus was essential for the virulence in mice, particularly in those challenged by i.p. injection, because mutants deficient in either one or both factors were as virulent as the wild-type strain. Second, cytolysin was the only cytotoxin secreted, because disruption of the cytolysin gene in the PD mutant abolished the cytotoxicity of the culture supernatant. However, there was probably another factor(s) present that enabled the DD mutant to lyse the epithelial cells upon bacterium-cell interaction. Third, the cytolysin was more potent than the protease in destruction of the intestine, because the wild type and the PD mutant, but not the CD and DD mutants, resulted in remarkable tissue damage. The damage probably accounts for the slightly higher mortality rates in mice challenged orally with the wild type and the PD mutant than in those challenged with the CD and DD mutants.
V. vulnificus is a highly invasive pathogen, being able to reach the bloodstream and cause septicemia via translocation across the intact intestinal wall. However, how the organism crosses the epithelial wall is not known. Our unpublished observation showed that this organism could hardly invade the epithelial cells. Therefore, one possibility is that the organisms spread to the bloodstream via open wounds caused by the bacterial products, as our data showed that the cytolysin caused remarkable damage to the enterocytes. Alternatively, the organisms may invade through the M cells, which are specialized epithelial cells lining the intestinal tract. A number of bacteria have been shown to use the M cells as an invasion route to enter the host (30). The fact that the CD and DD mutants were still invasive, lethal to mice, and cytotoxic strongly suggests the presence of some unidentified cytotoxin(s). Identification and characterization of the additional cytotoxin(s) would be crucial for unraveling the invasion mechanism of V. vulnificus.
Acknowledgments
This work was supported by grants DOH 87-HR-606 from the National Health Research Institute and NSC 89-2320-B-006-018 from the National Science Council, Taiwan, and a summer research grant from Schering-Plough Limited for Ya-Chi Ho.
We are grateful to H. M. Sheu and K. C. Huang for their valuable comments.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Short protocols in molecular biology. 3rd ed. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 2.Brennt C E, Wright A C, Dutta S K, Morris J G., Jr Growth of Vibrio vulnificus in serum from alcoholics: association with high transferrin iron saturation. J Infect Dis. 1991;164:1030–1032. doi: 10.1093/infdis/164.5.1030. [DOI] [PubMed] [Google Scholar]
- 3.Bullen J J, Spaldin P B, Ward C G, Gutteridge J M. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–1609. [PubMed] [Google Scholar]
- 4.Cheng J C, Shao C P, Hor L I. Cloning and nucleotide sequencing of the protease gene of Vibrio vulnificus. Gene. 1996;183:255–257. doi: 10.1016/s0378-1119(96)00488-x. [DOI] [PubMed] [Google Scholar]
- 5.Chuang Y C, Yuan C Y, Liu C Y, Lan C K, Huang A H M. Vibrio vulnificus infection in Taiwan: report of 28 cases and review of clinical manifestations and treatment. Clin Infect Dis. 1992;15:1–6. doi: 10.1093/clinids/15.2.271. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray L D, Kreger A S. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect Immun. 1985;48:62–72. doi: 10.1128/iai.48.1.62-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray L D, Kreger A S. Detection of anti-Vibrio vulnificus cytolysin antibodies in sera from mice and a human surviving V. vulnificus disease. Infect Immun. 1986;51:964–965. doi: 10.1128/iai.51.3.964-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray L D, Kreger A S. Mouse skin damage caused by cytolysin from Vibrio vulnificus and by V. vulnificus infection. J Infect Dis. 1987;155:236–241. doi: 10.1093/infdis/155.2.236. [DOI] [PubMed] [Google Scholar]
- 10.Gray L D, Kreger A S. Detection of Vibrio vulnificus cytolysin in V. vulnificus-infected mice. Toxicon. 1989;27:459–464. doi: 10.1016/0041-0101(89)90208-0. [DOI] [PubMed] [Google Scholar]
- 11.Hor L-I, Chang Y-K, Chang C-C, Lei H-Y, Ou J T. Mechanism of high susceptibility of iron-overloaded mouse to Vibrio vulnificus infection. Microbiol Immunol. 2000;44:871–878. doi: 10.1111/j.1348-0421.2000.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 12.Ianda J M, Powers C, Bryant R G, Abbott S L. Current perspectives on the epidemiology and pathogenesis of clinical significant Vibrio spp. Clin Microbiol Rev. 1988;1:245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H R, Rho H W, Jeong M H, Park J W, Kim J S, Park B H, Kim U H, Park S D. Hemolytic mechanism of cytolysin produced from V. vulnificus. Life Sci. 1993;53:571–577. doi: 10.1016/0024-3205(93)90714-e. [DOI] [PubMed] [Google Scholar]
- 14.Kook H, Lee S E, Baik Y H, Chung S S, Rhee J H. Vibrio vulnificus hemolysin dilates rat thoracic aorta by activating guanylate cyclase. Life Sci. 1996;59:41–47. doi: 10.1016/0024-3205(96)00292-5. [DOI] [PubMed] [Google Scholar]
- 15.Kothary M H, Kreger A S. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J Gen Microbiol. 1987;133:1783–1791. doi: 10.1099/00221287-133-7-1783. [DOI] [PubMed] [Google Scholar]
- 16.Kreger A, Lockwood D. Detection of extracellular toxin(s) produced by Vibrio vulnificus. Infect Immun. 1981;33:583–590. doi: 10.1128/iai.33.2.583-590.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires ToxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyoshi N, Miyoshi S, Sugiyama K, Suzuki Y, Furuta H, Shinoda S. Activation of the plasma kallikrein-kinin system by Vibrio vulnificus protease. Infect Immun. 1987;55:1936–1939. doi: 10.1128/iai.55.8.1936-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyoshi N, Shimizu C, Miyoshi S I, Shinoda S. Purification and characterization of Vibrio vulnificus protease. Microbiol Immunol. 1987;31:13–25. doi: 10.1111/j.1348-0421.1987.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 20.Miyoshi S, Nakazawa H, Kawata K, Tomochika K, Tobe K, Shinoda S. Characterization of the hemorrhagic reaction caused by Vibrio vulnificus metalloprotease, a member of the thermolysin family. Infect Immun. 1998;66:4851–4855. doi: 10.1128/iai.66.10.4851-4855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyoshi S, Shinoda S. Role of the protease in the permeability enhancement by Vibrio vulnificus. Microbiol Immunol. 1988;32:1025–1032. doi: 10.1111/j.1348-0421.1988.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi S, Shinoda S. Activation mechanism of human Hageman factor-plasma kallikrein-kinin system by Vibrio vulnificus metalloprotease. FEBS Lett. 1992;308:315–319. doi: 10.1016/0014-5793(92)81301-2. [DOI] [PubMed] [Google Scholar]
- 23.Muench K H. Hemachromatosis and infection: alcohol and iron, oysters and sepsis. Am J Med. 1989;87:40N–43N. [PubMed] [Google Scholar]
- 24.Okujo N, Akiyama T, Miyoshi S, Shinoda S, Yamamoto S. Involvement of vulnibactin and exocellular protease in utilization of transferrin- and lactoferrin-bound iron by Vibrio vulnificus. Microbiol Immunol. 1996;40:595–598. doi: 10.1111/j.1348-0421.1996.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 25.Okujo N, Saito M, Yamamoto S, Yshida T, Miyoshi S, Shinoda S. Structure of vulnibactin, a new polyamine-containing siderophore from Vibrio vulnificus. BioMetals. 1994;7:109–116. doi: 10.1007/BF00140480. [DOI] [PubMed] [Google Scholar]
- 26.Park J W, Ma S N, Song E S, Song C H, Chae M R, Park B H, Rho H W, Park S D, Kim H R. Pulmonary damage by Vibrio vulnificus cytolysin. Infect Immun. 1996;64:2873–2876. doi: 10.1128/iai.64.7.2873-2876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Shao C P, Hor L I. Metalloprotease is not essential for Vibrio vulnificus virulence in mice. Infect Immun. 2000;68:3569–3573. doi: 10.1128/iai.68.6.3569-3573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siebers A, Finlay B B. M cells and pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4:22–29. doi: 10.1016/0966-842x(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 31.Testa J, Daniel L W, Kreger A S. Extracellular phospholipase A2 and lysophospholipase produced by Vibrio vulnificus. Infect Immun. 1984;45:458–463. doi: 10.1128/iai.45.2.458-463.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warnock E W, MacMath T L. Primary Vibrio vulnificus septicemia. J Emerg Med. 1993;11:153–156. doi: 10.1016/0736-4679(93)90510-e. [DOI] [PubMed] [Google Scholar]
- 33.Wright A C, Morris J G., Jr The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect Immun. 1991;59:192–197. doi: 10.1128/iai.59.1.192-197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright A C, Simpson L M, Oliver J D, Morris J G., Jr Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect Immun. 1990;58:1769–1773. doi: 10.1128/iai.58.6.1769-1773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto K, Wright A C, Kaper J B, Morris J G., Jr The cytolysin gene of Vibrio vulnificus: sequence and relationship to the Vibrio cholerae El Tor hemolysin gene. Infect Immun. 1990;58:2706–2709. doi: 10.1128/iai.58.8.2706-2709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamanaka H, Sugiyama K, Furuta H, Miyoshi S, Shinoda S. Cytolytic action of Vibrio vulnificus haemolysin on mast cells from rat peritoneal cavity. J Med Microbiol. 1990;32:39–43. doi: 10.1099/00222615-32-1-39. [DOI] [PubMed] [Google Scholar]