Abstract
Background
Postherpetic neuralgia (PHN) is a common, complex, and refractory type of neuropathic pain. Several systematic reviews support the efficacy of acupuncture and related treatments for PHN. Nevertheless, the efficacy of various acupuncture-related treatments for PHN remains debatable.
Objective
We aimed to assess the efficacy and safety of acupuncture-related treatments for PHN, identify the most effective acupuncture-related treatments, and expound on the current inadequacies and prospects in the applications of acupuncture-related therapies.
Methods
We searched PubMed, Cochrane Central Register of Controlled Trials, Embase, Web of Science, Google Scholar, four Chinese databases (China National Knowledge Infrastructure, China Biomedical, Chongqing VIP, and Wan Fang databases), clinical research registration platform (World Health Organization International Clinical Trial Registration platform, China Clinical Trial Registration Center) for relevant studies. We also examined previous meta-analyses; gray literature; and reference lists of the selected studies. We then evaluated the risk of bias in the included studies and performed a Bayesian multiple network meta-analysis.
Results
We included 29 randomized controlled trials comprising 1,973 patients, of which five studies showed a high risk of bias. The pairwise meta-analysis results revealed that the efficacy of all acupuncture-related treatments for pain relief related to PHN was significantly better than antiepileptics. The network meta-analysis results showed that pricking and cupping plus antiepileptics were the most effective treatment, followed by electroacupuncture (EA) plus antiepileptics for pain relief in patients with PHN. EA plus antiepileptics ranked the best regarding reduced Pittsburgh Sleep Quality Index (PSQI) and Self-Rating Depression Scale (SDS) scores in patients with PHN. No results were found regarding the total response rate or quality of life in this study. Acupuncture-related treatments showed a lower incidence of adverse events than that of antiepileptics.
Conclusion
Acupuncture-related therapies are potential treatment options for PHN and are safe. Pricking and cupping plus antiepileptics, are the most effective acupuncture-related techniques for pain relief, while EA plus antiepileptics is the best acupuncture-related technique for improving PHN-related insomnia and depression symptoms. However, owing to the limitations of this study, these conclusions should be cautiously interpreted, and future high-quality studies are needed.
Systematic review registration
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021226422, identifier CRD42021226422.
Keywords: acupuncture, acupuncture analgesia, postherpetic neuralgia, systematic review, network meta-analysis
1. Introduction
Postherpetic neuralgia (PHN) is a complex and refractory type of neuropathy (Baron and Wasner, 2006), and is the most frequent chronic complication of herpes zoster (shingles) infection (Johnson and Rice, 2014). Although there are multiple definitions of PHN, the most conventionally used criterion is dermatomal pain persisting for at least 90 days after acute herpes zoster rash onset (Cohen, 2013; Scholz et al., 2019). The case definition of PHN in clinical trials often includes a minimum threshold of clinically significant pain intensity, which is usually a score of 40 or higher (sometimes ≥ 30) on a Likert scale of 0–100 (Oxman et al., 2005; Dworkin et al., 2010). Pain associated with PHN can be classified into three categories: persistent spontaneous pain (e.g., persistent burning pain), paroxysmal shooting or electric shock-like pain, evoked pain that includes allodynia (pain triggered from the innocuous stimulus), and hyperalgesia that may persist for several months or even years (Jensen and Finnerup, 2014; Finnerup et al., 2021).
The incidence and prevalence of PHN vary according to the definition used; 19.5 and 13.7% of patients with herpes zoster developed PHN at least 1 month and 3 months after symptom onset, respectively (Gauthier et al., 2009). One meta-analysis showed an annual incidence rate for PHN of 3.9–42.0 per 100,000 people (van Hecke et al., 2014). Like herpes zoster, a positive correlation has been seen between PHN severity or prevalence and age (Zin et al., 2008; Mallick-Searle et al., 2016), and the proportion of patients with spontaneous symptom remission decreases with age (Johnson and Rice, 2014). Other than older age, PHN risk factors may include the clinical features of acute herpes zoster infection [including prodromal pain, severe acute pain, severe skin lesions, and herpes rash in specific areas (e.g., ophthalmic symptoms)], chronic diseases, diabetes mellitus, and severe immunosuppression (Drolet et al., 2010; Forbes et al., 2016a,b; Wei et al., 2019). Family members of patients with PHN are also prone to fatigue, stress, insomnia, and emotional distress (Weinke et al., 2014). PHN causes considerable pain, resulting in a global healthcare burden for individuals and society (Doth et al., 2010; Friesen et al., 2016).
Unfortunately, PHN management remains challenging. The only well-documented means of PHN prevention is herpes zoster prevention (Johnson and Rice, 2014). However, once a patient develops PHN, the primary goal is symptom control, including pain reduction and emotional and sleep regulation. Existing evidence supports the use of oral drugs, including antiepileptics (e.g., pregabalin and gabapentin), tricyclic antidepressants (e.g., amitriptyline), or topical treatments (e.g., 5% lidocaine or capsaicin patches) as first-line treatments for PHN (Johnson and Rice, 2014; Moore et al., 2014, 2015; Onakpoya et al., 2019). Some clinical data suggest that opioids (e.g., tramadol, oxycodone, morphine) and neurointerventional surgery (e.g., nerve block, pulsed radiofrequency) are also effective (Binder and Baron, 2008; Argoff, 2011; Ke et al., 2013; Li et al., 2018), although the evidence is sparse, inconsistent, or lacking long-term follow-up (Petersen and Rowbotham, 2007; Whitley et al., 2010; Dworkin et al., 2013; Ke et al., 2013; McNicol et al., 2013). Nevertheless, oral drugs used to treat PHN can cause systemic and cognitive adverse effects (Nalamachu and Morley-Forster, 2012; Yu et al., 2016; Onakpoya et al., 2019). In addition, pain may last for years or a lifetime, and long-term medication is usually required. Patients with PHN are often older adults with underlying diseases who take other drugs, and patients who use oral drugs have an increased risk of adverse reactions and side effects (Schmader et al., 2010; Nalamachu and Morley-Forster, 2012). Therefore, identifying an effective treatment for PHN with fewer side effects is crucial.
Acupuncture therapy, an essential part of traditional Chinese medicine, is valuable in pain management (Vickers and Linde, 2014; Kelly and Willis, 2019) and has the advantages of quick effects and minimal adverse reactions (Melchart et al., 2004; Cui et al., 2022). One systematic review found insufficient evidence regarding whether acupuncture was more effective than pharmacological therapy but concluded that acupuncture was safer (Wang Y. et al., 2018). Nevertheless, another systematic review showed that the efficacy of acupuncture combined with cupping was significantly higher than that of conventional Western medicine therapies (Zhou et al., 2021). A systematic review by Wu et al. (2021) suggested that the efficacy of moxibustion was higher than that of control groups (pharmacological therapies, herbal medicine, or no treatment), despite the high heterogeneity of the included studies, and a systematic review by Pei et al. (2019) suggested that acupuncture is comparable to the effects of Western medicines, but with no reported adverse effects. Therefore, evidence-based medical evidence supports the efficacy of acupuncture-related therapies on PHN. Various treatments related to acupuncture may have different therapeutic effects, but no studies have compared them. The above factors contribute to the complexityin choosing clinical acupuncture treatment plans.
To compare the analgesic effects of various acupuncture therapies on PHN, we conducted a pairwise and network meta-analysis (NMA) to comprehensively and critically evaluate all available and eligible clinical evidence to provide evidence-based recommendations for selecting the best therapeutic acupuncture schedule. Additionally, we expound on the current insufficiency and prospects in the applications of acupuncture-related therapies, to provide a reference for subsequent research.
2. Methods
This study strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al., 2009) and PRISMA-NMA guidelines (Hutton et al., 2015). It was registered with the International Prospective Register of Systematic Reviews (CRD42021226422).
2.1. Data sources and search strategies
We searched eight electronic databases, including PubMed, Embase, Cochrane Library, Web of Science, Google Scholar, China National Knowledge Infrastructure, China Biomedical, Chongqing VIP, and Wan Fang, from inception to December 7, 2022; language was restricted to English and Chinese.
English search terms included: “neuralgia, postherpetic”, “postherpetic neuralgia”, “post-herpetic pain,”; “acupuncture analgesia”, “acupuncture therapy”, “acupuncture”, “manual acupuncture”, “surrounding acupuncture”, “surround needling”, “acupuncture, ear”, “auricular acupuncture”, “ear acupuncture”, “electroacupuncture”, “electro-acupuncture”, “fire needling”, “fire needle”, “warm needling”, “warm needle”, “moxibustion”, “thermal moxibustion”, “medicated thread moxibustion”, “pricking and cupping”, “pricking blood and cupping”, “blood-letting puncture and cupping”, “Fu’s subcutaneous needling”, “Fu’s acupuncture”, “acupoint catgut embedding”, “catgut embedding”, “dry needling”, “dry needle”, “laser acupuncture”.
Chinese search terms included: “带状疱疹后神经痛”, “带状疱疹后疼痛”, “针灸”, “针刺”, “围刺”, “耳针”, “耳穴”, “电针”, “火针”, “温针”, “艾灸”, “热敏灸”, “药线灸”, “刺络拔罐”, “刺血拔罐”, “梅花针”, “浮针”, “埋线”, “干针”, “激光针”.
The complete search strategy used for the Cochrane Central Register of Controlled Trials and other databases is shown in Table 1.
TABLE 1.
CENTRAL: Search strategy.
| Query | Search terms | Results | Date |
| #1 | MeSH descriptor: [neuralgia, postherpetic] explode all trees | 306 | December 7, 2022 |
| #2 | (Postherpetic neuralgia): ti, ab, kw | 1,145 | December 7, 2022 |
| #3 | (Postherpetic pain):ti, ab, kw | 279 | December 7, 2022 |
| #4 | #1 OR #2 OR #3 | 1,189 | December 7, 2022 |
| #5 | MeSH descriptor: [acupuncture analgesia] explode all trees | 302 | December 7, 2022 |
| #6 | MeSH descriptor: [acupuncture Therapy] explode all trees | 5,393 | December 7, 2022 |
| #7 | MeSH descriptor: [acupuncture] explode all trees | 167 | December 7, 2022 |
| #8 | MeSH descriptor: [acupuncture, ear] explode all trees | 222 | December 7, 2022 |
| #9 | MeSH descriptor: [electroacupuncture] explode all trees | 900 | December 7, 2022 |
| #10 | MeSH descriptor: [moxibustion] explode all trees | 531 | December 7, 2022 |
| #11 | (Acupuncture analgesia): ti, ab, kw | 1,211 | December 7, 2022 |
| #12 | (Acupuncture therapy): ti, ab, kw | 9,588 | December 7, 2022 |
| #13 | (Acupuncture): ti, ab, kw | 17,576 | December 7, 2022 |
| #14 | (Manual acupuncture): ti, ab, kw | 665 | December 7, 2022 |
| #15 | (Surrounding acupuncture): ti, ab, kw | 63 | December 7, 2022 |
| #16 | (Surrounding needling): ti, ab, kw | 28 | December 7, 2022 |
| #17 | (Auricular acupuncture): ti, ab, kw | 861 | December 7, 2022 |
| #18 | (Ear acupuncture): ti, ab, kw | 751 | December 7, 2022 |
| #19 | (Electroacupuncture): ti, ab, kw | 3,118 | December 7, 2022 |
| #20 | (Electro-acupuncture): ti, ab, kw | 663 | December 7, 2022 |
| #21 | (Fire needling): ti, ab, kw | 57 | December 7, 2022 |
| #22 | (Warm needling): ti, ab, kw | 114 | December 7, 2022 |
| #23 | (Fire needle): ti, ab, kw | 133 | December 7, 2022 |
| #24 | (Warm needle): ti, ab, kw | 137 | December 7, 2022 |
| #25 | (Moxibustion): ti, ab, kw | 2,238 | December 7, 2022 |
| #26 | (Thermal moxibustion): ti, ab, kw | 43 | December 7, 2022 |
| #27 | (Medicated thread moxibustion): ti, ab, kw | 13 | December 7, 2022 |
| #28 | (Pricking and cupping): ti, ab, kw | 69 | December 7, 2022 |
| #29 | (Pricking blood and cupping): ti, ab, kw | 21 | December 7, 2022 |
| #30 | (Blood-letting puncture and cupping): ti, ab, kw | 26 | December 7, 2022 |
| #31 | (Fu’s subcutaneous needling): ti, ab, kw | 35 | December 7, 2022 |
| #32 | (Fu’s acupuncture): ti, ab, kw | 52 | December 7, 2022 |
| #33 | (Acupoint catgut embedding): ti, ab, kw | 158 | December 7, 2022 |
| #34 | (Catgut embedding): ti, ab, kw | 203 | December 7, 2022 |
| #35 | (Dry needling): ti, ab, kw | 931 | December 7, 2022 |
| #36 | (Dry needle): ti, ab, kw | 421 | December 7, 2022 |
| #37 | (Laser acupuncture): ti, ab, kw | 675 | December 7, 2022 |
| #38 | #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 | 20,699 | December 7, 2022 |
| #39 | #4 AND #38 (in trials) | 81 | December 7, 2022 |
ti, title; ab, abstract; kw, keywords.
Additionally, we searched the World Health Organization International Clinical Trial Registration platform,1 China Clinical Trial Registration Center,2 previous meta-analyses, and gray literature.3
Two researchers crosschecked the search results and searched the reference lists of the included studies.
2.2. Eligibility criteria
We predefined inclusion criteria following the PICOS protocol (Cumpston et al., 2019):
-
(1)
Participants: to address the inconsistent diagnostic criteria for PHN are inconsistent, we referred to the International Association for the Study of Pain Classification of Chronic Pain for the International Classification of Diseases-11, which defines PHN as pain persisting for over 3 months after the onset of rash (Scholz et al., 2019), and the Chinese Expert Consensus on Diagnosis and Treatment of Postherpetic Neuralgia, which defines PHN as pain persisting for over 1 month after the rash healed (Yu et al., 2016). Patients who met either of the above criteria were included, regardless of age, sex, or race.
-
(2)
Treatment group: treatment groups involving a single type of acupuncture combined with pharmacotherapy were included. Acupuncture techniques were defined as manual, electro- (EA), and other acupuncture used in clinical practice (e.g., fire needling and warm needling); however, acupoint injections were excluded. No limitations on needle material, acupoint matching, duration of needle retention, and treatment course were imposed.
-
(3)
Control group: controls received pharmacotherapy, a different single type of acupuncture than the treatment group, or sham acupuncture. To ensure homogeneity, pharmacotherapies were limited to evidence-based effective agents for PHN, including antiepileptics (pregabalin, gabapentin), tricyclic antidepressants (e.g., amitriptyline, nortriptyline), opioids (morphine, oxycodone, and tramadol), and topical treatments (5% lidocaine or capsaicin patches) (Johnson and Rice, 2014; Song et al., 2018). When antiepileptics were used, mecobalamin could be used as adjunctive therapy, reducing the adverse events of antiepileptics with no significant effect on pain relief. The same drug should be administered to the treatment and control groups when a combination of pharmacotherapy is used.
-
(4)
Outcome indicators: primary outcome was reduced pain intensity, indicated by the change from baseline. The pain was measured using a visual analog scale (VAS) or numerical rating scale. Secondary outcomes were improvement in pain-related outcomes in Pittsburgh Sleep Quality Index (PSQI) score, Self-Rating Depression Scale (SDS) score, clinical response rate (defined as the ratio of the number of effective cases to the total number of patients), quality of life, and adverse events (AEs).
-
(5)
Study design: studies based on needle insertion or stimulation of acupoints for therapeutic purposes, including randomized controlled trials (RCTs) that evaluated acupuncture and related therapies for PHN, were included.
Exclusion criteria were:
-
(1)
Secondary analysis or duplicate publication (with multilingual publications, only the earliest was chosen).
-
(2)
Full text was unavailable after contacting the corresponding author.
-
(3)
Flawed study design, such as not strictly following the principle of randomization (e.g., randomization performed based on the order in which patients were admitted to the hospital, odd and even case numbers, or birth dates).
-
(4)
Missing baseline data.
-
(5)
Unspecified diagnostic criteria.
-
(6)
Outcome indicators are unobtainable or not applicable to the NMA.
-
(7)
Interventions included acupuncture combined with other acupuncture therapies.
-
(8)
Interventions were single drug treatment with no evidence-based efficacy (e.g., antiviral drugs, Chinese herbal medicine) (Johnson and Rice, 2014; Song et al., 2018).
2.3. Trial selection
After deleting duplicate records using EndNote X9 (Clarivate Analytics, London, UK), two researchers independently reviewed all titles and abstracts based on the inclusion and exclusion criteria; studies that did not meet the inclusion criteria were excluded. Subsequently, full texts of the studies that met the inclusion criteria were carefully reviewed to determine if they should be included. Differences of opinion were resolved through discussions. If no agreement could be reached, a decision arbitrator was consulted. All researchers are licensed physicians with more than 2 years of clinical acupuncture experience.
2.4. Data extraction
The following information was extracted according to a fixed protocol. After the final selection, two researchers extracted the data independently and created summary tables. Data were extracted according to a template, including the study (first author, publication year, location) and participant characteristics (patient source, sample size, mean age, PHN course), intervention (type, acupoint, specific drug, dose, frequency, treatment course), and outcome indicators. The results were then cross-checked.
2.5. Quality assessment
Under the Cochrane Collaboration recommendation (Cumpston et al., 2019), two researchers independently assessed these seven major risks of bias for all included studies using RevMan version 5.4 (The Cochrane Collaboration, London, UK): random sequence generation, allocation hiding, blinding of participating researchers, blinding of outcome evaluation, incomplete outcome data, selective reporting, and other types of bias. Outcomes were classified into (as high, low, or unclear risk).
2.6. Data synthesis and analysis
A pairwise meta-analysis was performed using STATA software version 15.1 (StataCorp LLC, College Station, TX, US). Weighted mean differences (WMDs) and corresponding 95% confidence intervals (CIs) were used to analyze continuous variables. P < 0.05 indicated statistical significance. A fixed-effects model was used for analysis (obvious heterogeneity; I2 < 50%); otherwise, a random-effects model was used.
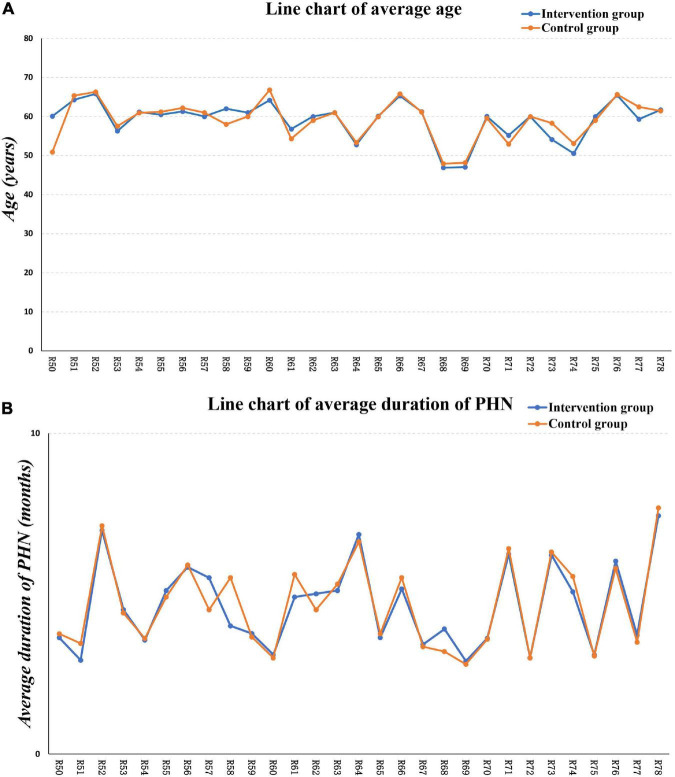
The similarity hypothesis is one of the most important assumptions in NMA, which states that factors affecting the effect size are similar among all studies and different control groups. Only when the similarity hypothesis is satisfied can NMA produce reliable results; otherwise, bias is present. In this study, descriptive statistical analysis was performed on the RCTs’ population characteristics, and participants’ baseline levels were evaluated, including mean age and disease duration. Line graphs were drawn for visualization.
A Bayesian multiple treatment NMA was performed using R (version 4.0.5; R Foundation for Statistical Computing, Vienna, Austria) and the “gemtc” package, which interfaces with OpenBUGS. Network plots were created in STATA (StataCorp LLC). A random-effects model was computed using Markov chain Monte Carlo (Gelman and Rubin, 1996) methods, based on simulations of 50,000 iterations in each of the four chains. The node-splitting method was used to test the consistency of the assumptions (Dias et al., 2010). P > 0.05, indicated that direct and indirect comparisons had better consistency. A convergence diagnosis plot was used to monitor the convergence of the model (Gelman and Rubin, 1996) by calculating the median value of the shrink factor and the value of 97.5% in each iteration and observing the consistency of the curves after 50,000 iterations. The surface under the cumulative ranking (SUCRA) curve was used for probability sorting (Salanti et al., 2011). A rank histogram was drawn, and the SUCRA value was 0 when the intervention was 0 or 1 for the worst and best intervention, respectively. Finally, publication bias was tested using comparison-adjusted funnel plots in STATA (StataCorp LLC).
3. Results
3.1. Literature retrieval and characteristics
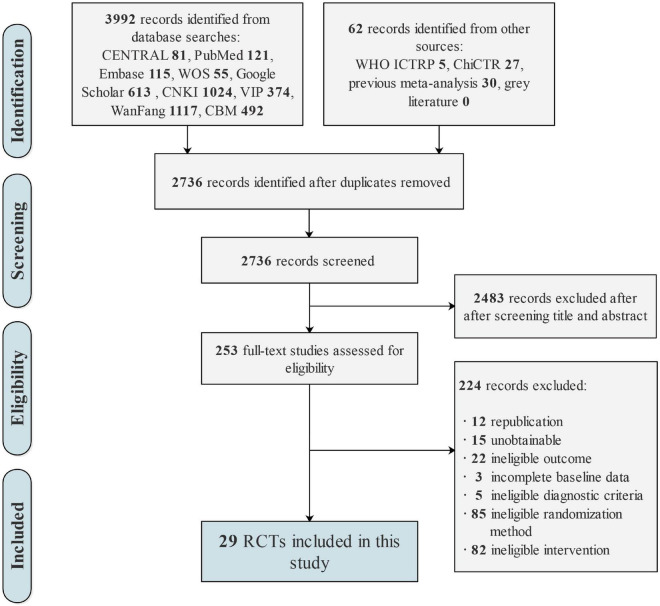
The initial search resulted in 3,992 pending records, and we selected 253 potentially eligible articles for full-text review. After further screening, 224 studies were excluded; the reasons for excluding each study are detailed in Supplementary Appendix 1. Ultimately, 29 RCTs (Wang, 2006; Huang et al., 2011; Chen et al., 2012, 2018; Tian, 2013; Xie and Wen, 2013; Zhang, 2013, 2015; Duan, 2014; Sheng et al., 2014; Lei, 2015; Sun and Li, 2015; Hong, 2016; Wu and Zhao, 2016; Yang and Pan, 2016; Han et al., 2018; Xie et al., 2018; Xu, 2018, 2020; Zhou and Huang, 2018; Chen, 2019; Mo, 2019; Zhong, 2019; Pang, 2020; Wang et al., 2020; Zhang H. L. et al., 2020; Zhao et al., 2020; Ding et al., 2021; Zhang et al., 2021) met the inclusion criteria and were included in the quality evaluation and statistical analysis; all Chinese references for the included trials are detailed in Supplementary Appendix 2. The flowchart illustrating the selection process is shown in Figure 1.
FIGURE 1.
Flow chart of study selection.
All included studies were conducted in China, published in Chinese, and included a total of 1,973 patients (mean age: 46.91–66.79 years); 54.45% (n = 1010) were women. The average sample size of included studies was 64 (range: 37–115), and included seven types of acupuncture-related therapies: manual acupuncture, EA, fire needling, pricking and cupping, Fu’s acupuncture, medicated thread moxibustion, and acupoint catgut embedding in addition to pharmacotherapy. Only comparisons between acupuncture-related therapies and antiepileptic drugs were found for the pharmacotherapy group, and no eligible studies compared acupuncture with tricyclic antidepressants, opioids, or topical treatments. The Ashi point (82.76%) was the most frequently used acupoint among the included studies, followed by the Jiaji point (51.72%). Interestingly, pain reduction in all studies was reported according to a VAS. The details of each trial are provided in Table 2.
TABLE 2.
Characteristics of the included studies.
| References | Sample size (female rate) | Group | Types of intervention | Details of intervention (specific drug/acupoint, frequency, course of treatment) | Follow-up (weeks) | Age (years) | Duration of PHN (months) | Outcome indicator | Cochrane Collaboration Risk of Bias |
| Wang, 2006 | 34 (58.8%) | Treatment | Fire needling | Ashi point, Jiaji point, qod; 14 days | 1&4 | 60.06 ± 7.34 | 3.63 ± 2.31 | ① ④ | Low; unclear; unclear; unclear; low; low; unclear |
| 34 (52.9%) | Control | Manual acupuncture | Ashi point, Jiaji point, qod; 14 days | 59.91 ± 7.46 | 3.75 ± 2.77 | ||||
| Huang et al., 2011 | 49 (42.9%) | Treatment | Pricking and cupping | Ashi point, GB21, qod; 30 days | / | 64.28 | 2.93 | ① ④ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 47 (46.8%) | Control | Manual acupuncture | Ashi point, LI4, LI11, LR3, SP6, qod; 30 days | 65.35 | 3.45 | ||||
| Chen et al., 2012 | 35 (51.4%) | Treatment | Medicated thread moxibustion | Ashi point, LI10, PC6, SP6, ST36, LR3, qd; 14 days | / | 65.81 ± 4.45 | 6.98 ± 2.58 | ① ④ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 35 (54.3%) | Control | Fire needling | Ashi point, qd; 14 days | 66.31 ± 4.05 | 7.12 ± 2.54 | ||||
| Zhang, 2013 | 26 (50.0%) | Treatment | Electroacupuncture | Ashi point, LI4, LJ6, LR3, ST36, qd; 20 days | 4 | 56.30 ± 12.54 | 4.5 | ① ③ ④ ⑤ | Low; low; high; low; low; low; unclear |
| 27 (55.6%) | Control | Antiepileptics | Gabapentin, maximum dose 3600 mg per day; 20 days | 57.55 ± 7.69 | 4.4 | ||||
| Tian, 2013 | 32 (56.3%) | Treatment | Pricking and cupping | Ashi point, qod; 16 days | / | 61.15 ± 7.13 | 3.56 ± 2.68 | ① ③ ④ ⑤ | Low; high; unclear; unclear; low; low; unclear |
| 32 (50.0%) | Control | Antiepileptics | Pregabalin, 150mg, bidpo; 16 days | 60.93 ± 7.45 | 3.61 ± 2.12 | ||||
| Xie and Wen, 2013 | 35 (45.7%) | Treatment | Manual acupuncture | Ashi point, Jiaji point, SP6, ST36, KI3, SP9, 20 min, qd; 21 days | 4 | 60.5 ± 8.2 | 5.10 ± 3.30 | ① ② ④ | Low; unclear; unclear; unclear; low; low; unclear |
| 30 (34.3%) | Control | Electroacupuncture | Ashi point, Jiaji point, SP6, ST36, KI3, SP9, 3/90 Hz dilatational wave, 20 min, qd; 21 days | 61.2 ± 7.8 | 4.90 ± 3.50 | ||||
| Duan, 2014 | 30 (56.7%) | Treatment | Electroacupuncture | Ashi point, Jiaji point, 30 mins, bid; 18 days | 4 | 61.30 ± 8.35 | 5.83 ± 10.0 | ① ④ | Low; unclear; unclear; unclear; low; low; unclear |
| 30 (60.0%) | Control | Manual acupuncture | Ashi point, Jiaji point, 30 min, bid; 18 days | 62.20 ± 9.76 | 5.90 ± 9.29 | ||||
| Sheng et al., 2014 | 20 (55.5%) | Treatment | Electroacupuncture | Ashi point, Jiaji point, 30 min, bid; 18 days | / | 46 to 77 | 3 to 8 | ① ④ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 20 (65.5%) | Control | Manual acupuncture | Ashi point, Jiaji point, 30 min, bid; 18 days | 42 to 80 | 3 to 6 | ||||
| Sun and Li, 2015 | 45 (57.8%) | Treatment | Electroacupuncture | Ashi point, Jiaji point, 30 mins, qd; 10 days | / | 20 to 69 | 1 to 7 | ① ④ | Low; unclear; unclear; unclear; low; low; unclear |
| 45 (51.1%) | Control | Manual acupuncture | Ashi point, Jiaji point, 30 mins, qd; 10 days | 33 to 58 | 2 to 9 | ||||
| Zhang, 2015 | 20 (60.0%) | Treatment | Fire needling | Ashi point, qod; 30 days | 4 | 60.0 ± 7.0 | 3.62 ± 0.54 | ① ④ ⑤ | Low; high; unclear; unclear; low; low; unclear |
| 20 (35.5%) | Control | Manual acupuncture | Ashi point, 30 min, qd; 30 days | 60.0 ± 7.0 | 3.76 ± 0.43 | ||||
| 20 (40.0%) | Control | Pricking and cupping | Ashi point, qod; 30 days | 61.0 ± 7.0 | 3.59 ± 0.68 | ||||
| 20 (55.0%) | Control | Antiepileptics | Pregabalin, 150mg, bidpo; 30 days | 60.0 ± 8.0 | 3.65 ± 0.51 | ||||
| Lei, 2015 | 20 (75.0%) | Treatment | Electroacupuncture plus Antiepileptics | Ashi point, SJ6, GB34, ST36 30 min, 5 times per 7 days plus Gabapentin; 14 days | 4 | 62.55 ± 7.48 | 3.1 | ① ② ③ ④ ⑤ | Low; unclear; unclear; unclear; low; low; unclear |
| 20 (55.0%) | Control | Electroacupuncture | Ashi point, SJ6, GB34, 30 min, 5 times per 7 days; 14 days | 64.20 ± 10.81 | 3.2 | ||||
| 19 (42.1%) | Control | Antiepileptics | Gabapentin, maximum dose 1800 mg per day; 14 days | 66.79 ± 11.25 | 3 | ||||
| Yang and Pan, 2016 | 34 (35.3%) | Treatment | Electroacupuncture plus Antiepileptics | Ashi point, Jiaji point, 50 Hz, 30 min, qd plus Gabapentin; 28 days | 4 | 56.8 ± 14.9 | 4.90 ± 3.80 | ① ④ ⑤ | Low; unclear; unclear; unclear; low; low; unclear |
| 34 (32.4%) | Control | Antiepileptics | Gabapentin, maximum dose 1800 mg per day; 28 days | 54.3 ± 12.4 | 5.60 ± 3.40 | ||||
| Wu and Zhao, 2016 | 25 (64.0%) | Treatment | Fu’s subcutaneous needling | Ashi point; 14 days | / | 40 to 70 | 2 to 8 | ① ⑤ | Low; high; unclear; unclear; unclear; unclear; unclear |
| 25 (56.0%) | Control | Antiepileptics | Pregabalin, 150 mg, bidpo; 14 days | 38 to 70 | 2 to 7 | ||||
| Hong, 2016 | 29 | Treatment | Pricking and cupping | Ashi point, qod; 28 days | / | 61 | 5.1 | ① ⑤ | Low; unclear; unclear; unclear; low; low; unclear |
| 29 | Control | Antiepileptics | Gabapentin, maximum dose 1800 mg per day; 28 days | 61 | 5.3 | ||||
| Zhou and Huang, 2018 | 30 | Treatment | Fire needling plus Antiepileptics | BL15, BL17, qod plus Pregabalin; 10 days | / | 52.78 ± 8.12 | 6.85 ± 4.48 | ① ④ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 30 | Control | Antiepileptics | Pregabalin, 150 mg, bidpo; 10 days | 53.34 ± 7.60 | 6.62 ± 4.13 | ||||
| Xu, 2018 | 34 (58.8%) | Treatment | Fire needling | Ashi point, qod; 14 days | 1&4 | 60.06 ± 7.34 | 3.63 ± 2.31 | ① ④ ⑤ | Low; unclear; unclear; unclear; low; low; unclear |
| 34 (52.9%) | Control | Manual acupuncture | Ashi point, Jiaji point, 30 min, qd; 14 days | 59.91 ± 7.46 | 3.75 ± 2.77 | ||||
| Xie et al., 2018 | 40 (47.5%) | Treatment | Electroacupuncture plus antiepileptics | Ashi point, Jiaji point, 50 Hz, 60 min, 1 time per 3 days plus Gabapentin; 42 days | / | 65.3 ± 10.4 | 5.15 | ① ④ ⑤ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 40 (55.0%) | Control | Antiepileptics | Gabapentin, maximum dose 1800 mg per day; 42 days | 65.8 ± 10.8 | 5.50 | ||||
| Chen et al., 2018 | 45 (31.1%) | Treatment | Fire needling | Ashi point, Jiaji point, qod; 20 days | / | 61.24 ± 3.65 | 3.42 ± 0.39 | ① ② ④ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 45 (32.0%) | Control | Manual acupuncture | Ashi point, Jiaji point, 30 min, qd; 20 days | 61.15 ± 3.74 | 3.35 ± 0.37 | ||||
| Han et al., 2018 | 32 (50.0%) | Treatment | Pricking and cupping | Jiaji point, 15 min, 1 time per 3 days; 60 days | / | 46.91 ± 13.88 | 3.90 ± 3.70 | ① | Low; low; unclear; low; unclear; unclear; low |
| 32 (53.1%) | Control | Antiepileptics | Gabapentin, maximum dose 1800 mg per day; 60 days | 47.91 ± 13.94 | 3.2 ± 4.10 | ||||
| Mo, 2019 | 19 (52.6%) | Treatment | Pricking and cupping | Jiaji point, 10 min, 1 time per 3 days; 60 days | / | 47.05 ± 12.75 | 2.9 | ① ④ | Low; low; unclear; unclear; low; low; unclear |
| 18 (55.6%) | Control | Antiepileptics | Gabapentin, maximum dose 1800 mg per day; 60 days | 48.17 ± 11.17 | 2.8 | ||||
| Zhong, 2019 | 33 (51.5%) | Treatment | Fire needling | Ashi point, Jiaji point, qod; 28 days | / | 60.03 ± 3.09 | 3.61 ± 1.00 | ① ② ③ ④ ⑤ | Low; low; unclear; unclear; low; low; unclear |
| 33 (54.5%) | Control | Antiepileptics | Pregabalin, 75 mg, bidpo; 28 days | 59.55 ± 5.36 | 3.58 ± 1.00 | ||||
| Chen, 2019 | 30 (50.0%) | Treatment | Catgut embedding plus antiepileptics | Jiaji point, 1 time per 14 days plus Antiepileptics; 28 days | / | 55.17 ± 10.88 | 6.23 ± 1.61 | ① ② ④ | Low; unclear; unclear; unclear; low; low; unclear |
| 28 (53.6%) | Control | Antiepileptics | Pregabalin, 75 mg, bidpo; 28 days | 52.93 ± 10.04 | 6.41 ± 1.92 | ||||
| Pang, 2020 | 34 (55.9%) | Treatment | Fire needling plus antiepileptics | Wrist ankle point, 1 time per 4 days plus Gabapentin; 20 days | 4 | 60 | 3 | ① ② ④ ⑤ | Low; low; high; low; low; low; low |
| 33 (45.5%) | Control | Antiepileptics | Gabapentin, maximum dose 900 mg; 20 days | 60 | 3 | ||||
| Zhang et al., 2020 | 54 (46.3%) | Treatment | Fire needling | Ashi point, qod; 14 days | / | 54.1 ± 13.7 | 6.20 ± 2.60 | ① ④ | Low; high; unclear; unclear; low; low; unclear |
| 53 (49.1%) | Control | Fu’s subcutaneous needling | Ashi point, qod; 14 days | 58.3 ± 11.4 | 6.30 ± 2.70 | ||||
| Xu, 2020 | 29 (55.2%) | Treatment | Manual acupuncture | Regulating mind points, 30 min, qd; 14 days | 4&12 | 50.55 ± 10.02 | 5.06 ± 2.39 | ① ④ | Low; high; unclear; unclear; low; low; unclear |
| 28 (50.0%) | Control | Electroacupuncture | Ashi point, Jiaji point, dilatational wave, 2/100 Hz, 30 min, qd; 14 days | 53.07 ± 9.49 | 5.54 ± 2.83 | ||||
| Wang et al., 2020 | 34 (51.4%) | Treatment | Pricking and cupping | Ashi point, 20 min, qod; 16 days | / | 40 to 70 | 3.09 | ① ④ | Low; unclear; unclear; unclear; low; low; unclear |
| 32 (54.3%) | Control | Antiepileptics | Gabapentin, maximum dose 900 mg; 16 days | 40 to 69 | 3.06 | ||||
| Zhao et al., 2020 | 28 (60.7%) | Treatment | Pricking and cupping | Ashi poinrt, 30 min, qod; 40 days | / | 65.45 ± 2.23 | 6.02 ± 2.50 | ① ② ④ ⑤ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 28 (64.3%) | Control | Manual acupuncture | Ashi poinrt, 5 min, qod; 40 days | 65.67 ± 2.32 | 5.79 ± 2.45 | ||||
| Ding et al., 2021 | 57 (56.1%) | Treatment | Pricking and cupping plus antiepileptics | Ashi point, Jiaji point, 2 to 3 times per 7 days plus Gabapentin plus Mecobalamine 0.5 mg tidpo; 28 days | / | 59.33 ± 6.25 | 3.71 ± 0.49 | ① ④ ⑤ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 58 (53.4%) | Control | Antiepileptics | Gabapentin, maximum dose 1800 mg per day plus Mecobalamine 0.5 mg, tidpo; 28 days | 62.45 ± 9.23 | 3.49 ± 0.63 | ||||
| Zhang et al., 2021 | 31 (48.4%) | Treatment | Pricking and cupping plus antiepileptics | Ashi point, 20 min, qod plus Pregabalin; 30 days | / | 61.69 ± 8.43 | 7.43 ± 1.49 | ① ④ ⑤ | Low; unclear; unclear; unclear; unclear; unclear; unclear |
| 30 (43.3%) | Control | Antiepileptics | Pregabalin, 150 mg, bidpo; 28 days | 61.42 ± 7.96 | 7.68 ± 1.52 |
① = VAS; ② = PSQI; ③ = SDS; ④ = response rate; ⑤ = adverse events.
3.2. Study quality assessment
Twenty-nine studies implemented the correct randomization method. Five studies (Tian, 2013; Zhang, 2015; Wu and Zhao, 2016; Xu, 2020; Zhang H. L. et al., 2020) were classified as high-risk for allocation concealment because they sequentially assigned patients to treatment in the order of inpatient admissions after patients were correctly randomly assigned to treatment or control groups, which increased the possibility of exposure allocation information. Three studies (Zhang, 2013; Han et al., 2018; Pang, 2020) performed outcome measure blindness, whereas the remaining studies did not. Eighteen studies (Wang, 2006; Tian, 2013; Xie and Wen, 2013; Zhang, 2013, 2015; Duan, 2014; Lei, 2015; Sun and Li, 2015; Hong, 2016; Yang and Pan, 2016; Xu, 2018, 2020; Chen, 2019; Mo, 2019; Zhong, 2019; Pang, 2020; Wang et al., 2020; Zhang H. L. et al., 2020) reported detailed information on shedding and loss to follow-up. Regarding other biases, no studies were classified as high-risk. Table 2 summarizes all included studies’ quality assessments and Cochrane risk of bias (Supplementary Appendix 3).
3.3. Pairwise meta-analysis
Pairwise meta-analyses were performed to compare the two interventions with a combined effect size. The results are shown in Tables 3–5. Compared with antiepileptics for PHN, manual acupuncture (one RCT; WMD = 1.73, 95% CI = 0.10–2.47, P = 0.000 < 0.001), EA (two RCTs; WMD = 1.50, 95% CI = 1.03–1.97, P = 0.000 < 0.001), fire needling (two RCTs; WMD = 1.42, 95% CI = 0.91–1.93, P = 0.000 < 0.001), pricking and cupping (six RCTs; WMD = 1.50, 95% CI = 0.52–2.47, P = 0.003 < 0.01), Fu’s acupuncture (one RCT; WMD = 0.83, 95% CI = 0.80–2.86, P = 0.000 < 0.001), EA plus antiepileptics (three RCTs; WMD = 1.79, 95% CI = 1.03–2.56, P = 0.000 < 0.001), fire needling plus antiepileptics (two RCTs; WMD = 1.47, 95% CI = 1.11–1.84, P = 0.000 < 0.001), pricking and cupping plus antiepileptics (two RCTs; WMD = 2.12, 95% CI = 1.77–2.48, P = 0.000 < 0.001), and acupoint catgut embedding plus antiepileptics (one RCT; WMD = 0.66, 95% CI = 0.18–1.14, P = 0.007 < 0.01) were all more efficacious in reducing VAS scores. However, EA plus antiepileptics had a greater effect than EA alone (one RCT; WMD = 1.25, 95% CI = 0.24–2.26, P = 0.015 < 0.05). Nevertheless, in terms of VAS score reduction, no significant differences were observed between manual acupuncture and fire needling, manual acupuncture and pricking and cupping, medicated thread moxibustion and fire needling, and pricking and cupping and fire needling. No significant differences were observed between EA and antiepileptics in reducing PSQI (Table 4) or SDS scores (Table 5), or between manual acupuncture and pricking and cupping in reducing PSQI scores (Table 4). Tables 3–5 present the results of the pairwise meta-analysis and heterogeneity.
TABLE 3.
Visual analog scale (VAS) pairwise meta-analysis results.
| Comparison | Number | WMD [95% CI] | I2 (%) | P |
| B VS. A | 1 | 1.73 [0.10, 2.47] | – | 0 |
| C VS. A | 2 | 1.50 [1.03, 1.97] | 0 | 0 |
| D VS. A | 2 | 1.42 [0.91, 1.93] | 0 | 0 |
| *E VS. A | 6 | 1.50 [0.52, 2.47] | 95.1 | 0.003 |
| F VS. A | 1 | 1.83 [0.80, 2.86] | – | 0 |
| *H VS. A | 3 | 1.79 [1.03, 2.56] | 75.5 | 0 |
| I VS. A | 2 | 1.47 [1.11, 1.84] | 0 | 0 |
| J VS. A | 2 | 2.12 [1.77, 2.48] | 0 | 0 |
| K VS. A | 1 | 0.66 [0.18, 1.14] | – | 0.007 |
| *D VS. B | 4 | 0.25 [−0.25, 0.75] | 52.6 | 0.323 |
| *E VS. B | 3 | 0.83 [−0.19, 1.85] | 85.0 | 0.112 |
| H VS. C | 1 | 1.25 [0.24, 2.26] | – | 0.015 |
| F VS. D | 1 | 0.42 [0.08, 0.77] | – | 0.017 |
| G VS. D | 1 | 0.37 [−0.68, 1.42] | – | 0.488 |
| D VS. E | 1 | 0.05 [−0.70, 0.80] | – | 0.897 |
The bold font indicates a statistical difference; *random effect model was used; A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; F = Fu’s acupuncture; G = medicated thread moxibustion; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; J = pricking and cupping plus antiepileptics; K = acupoint catgut embedding plus antiepileptics.
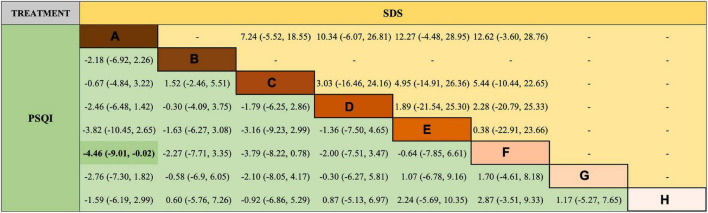
TABLE 5.
Self-Rating Depression Scale (SDS) pairwise meta-analysis results.
| Comparison | Number | WMD [95% CI] | I2 (%) | P |
| *C VS. A | 2 | 6.73 [−3.61, 17.07] | 88.1 | 0.202 |
| D VS. A | 1 | 10.37 [8.95, 11.79] | – | 0 |
| E VS. A | 1 | 12.24 [8.64, 15.84] | – | 0 |
| H VS. A | 1 | 10.09 [4.19, 15.99] | – | 0.001 |
| H VS. C | 1 | 9.15 [1.33, 16.97] | – | 0.022 |
The bold font indicates a statistical difference; *random effect model was used; A = antiepileptics; C = electroacupuncture; D = fire needling; E = pricking and cupping; H = electroacupuncture plus antiepileptics.
TABLE 4.
Pittsburgh Sleep Quality Index (PSQI) pairwise meta-analysis results.
| Comparison | Number | WMD [95% CI] | I2 (%) | P |
| C VS. A | 1 | 1.04 [−0.51, 2.59] | – | 0.190 |
| D VS. A | 1 | 2.28 [1.51, 3.06] | – | 0 |
| H VS. A | 1 | 4.64 [3.16, 6.12] | – | 0 |
| I VS. A | 1 | 2.76 [1.77, 3.75] | – | 0 |
| K VS. A | 1 | 1.60 [0.29, 2.91] | – | 0.017 |
| C VS. B | 1 | −1.79 [−2.96,−0.62] | – | 0.003 |
| D VS. B | 1 | 0.40 [0.06, 0.74] | – | 0.021 |
| E VS. B | 1 | 1.63 [−0.05, 3.31] | – | 0.058 |
| H VS. C | 1 | 3.60 [2.06, 5.14] | – | 0 |
The bold font indicates a statistical difference; A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; K = acupoint catgut embedding plus antiepileptics.
3.4. Network meta-analysis
The similarity was evaluated by assessing the baseline differences in the enrolled patients’ mean age and disease duration. As shown in Figure 2A, the average patient age in various studies showed few differences and high degrees of similarity. As shown in Figure 2B, the disease course of patients in various studies showed few differences and high degrees of similarity, satisfying the similarity assumption and obtaining reliable NMA results.
FIGURE 2.
Baseline assessment. (A) The average age assessment, (B) the average PHN duration assessment.
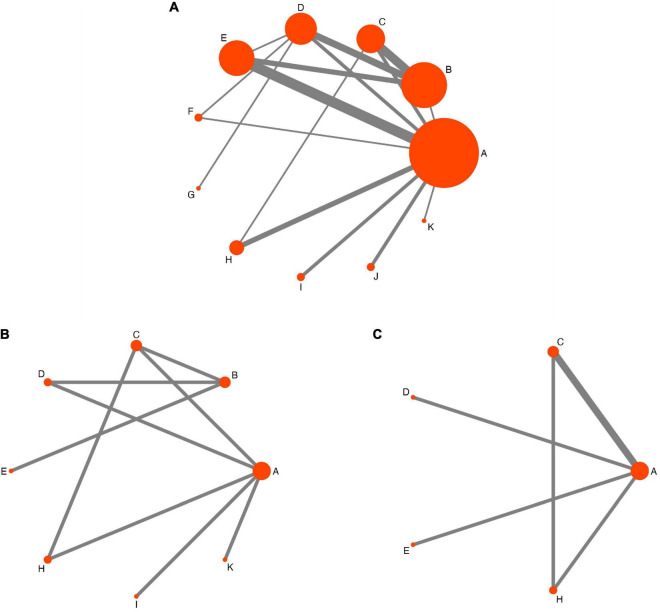
Figure 3 shows the NMA network evidence diagrams. All included studies with 1,973 participants and 11 therapy techniques had VAS data (Figure 3A), including one three-arm (Lei, 2015) and one four-arm (Zhang, 2015) study; the remaining studies were two-arm RCTs. Of these, the largest sample size was in the antiepileptic group, and studies comparing pricking and cupping with antiepileptics were the most common. PSQI scores were reported in seven studies (Xie and Wen, 2013; Lei, 2015; Chen et al., 2018; Chen, 2019; Zhong, 2019; Pang, 2020; Zhao et al., 2020) involving 461 participants and eight interventions (Figure 3B). Four studies (Tian, 2013; Zhang, 2013; Lei, 2015; Zhong, 2019) with 242 participants and five therapies reported SDS scores. Similarly, most participants were in the antiepileptic group (Figure 3C).
FIGURE 3.
Network evidence diagram. A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; F = Fu’s acupuncture; G = medicated thread moxibustion; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; J = pricking and cupping plus antiepileptics; K = acupoint catgut embedding plus antiepileptics. (A) The visual analog scale, (B) the Pittsburgh Sleep Quality Index (PSQI), and (C) the Self-Rating Depression Scale (SDS) network evidence diagram.
Effective NMA results depend on the internal consistency of the evidence network; direct evidence and various sources of indirect evidence should be consistent (Chaimani et al., 2013). We used the node-splitting method was used to test inconsistencies in the NMA. The results (Supplementary Appendix 4) showed that direct or indirect comparisons of each segmentation node had no statistical significance (P > 0.05), which demonstrated no evidence of inconsistency. A convergence diagnosis plot was used to test the convergence of the model. The results showed that the shrink factor’s median and 97.5% value tended to be 1 and stabilized after 50,000 iterations, with continued stability of the curve fitting and merging, indicating that the convergence of the model was excellent (Supplementary Appendix 5).
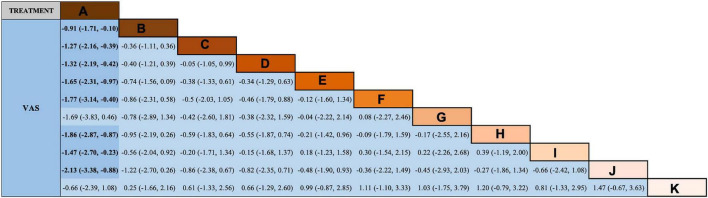
Regarding VAS score reduction, the results (Figure 4) show that all therapy techniques were advantageous over antiepileptics alone, except medicated thread moxibustion, acupoint catgut embedding plus antiepileptics. Additionally, EA plus antiepileptics was superior to antiepileptics alone in reducing PSQI scores (Figure 5).
FIGURE 4.
Network meta-analysis (NMA) results of visual analog scale (VAS). A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; F = Fu’s acupuncture; G = medicated thread moxibustion; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; J = pricking and cupping plus antiepileptics; K = acupoint catgut embedding plus antiepileptics.
FIGURE 5.
Network meta-analysis results of Pittsburgh Sleep Quality Index (PSQI) and Self-Rating Depression Scale (SDS). A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; K = acupoint catgut embedding plus antiepileptics.
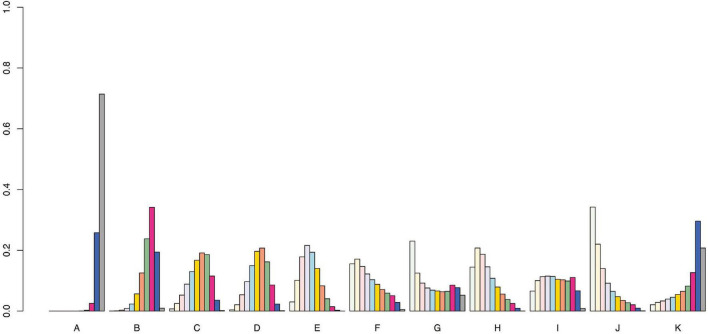
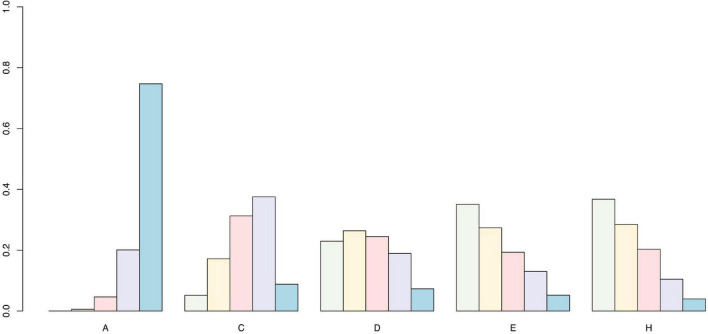
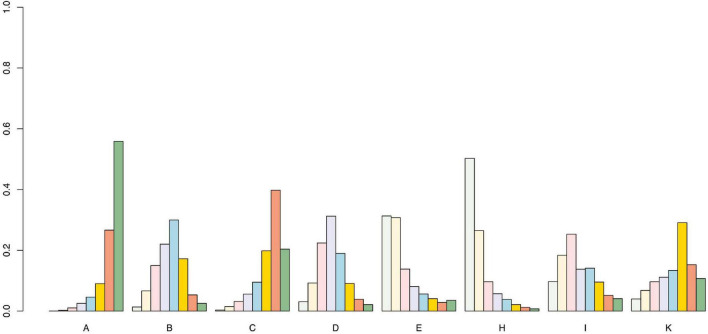
We used the SUCRA curve for probability sorting to plot probability sorting histograms (Figures 6–8) using R software. Figure 6 shows that antiepileptic drugs ranked lowest for VAS score reduction. Of the 11 treatments, the top three techniques for VAS score reduction were: pricking and cupping plus antiepileptics, EA plus antiepileptics, and pricking and cupping. While EA plus antiepileptics, pricking and cupping, and fire needling plus antiepileptics were the three best interventions for reducing PSQI scores (Figure 7). Among the five therapies, EA plus antiepileptics, pricking and cupping, and EA ranked highest in reducing SDS scores (Figure 8).
FIGURE 6.
Visual analog scale probability ranking diagram. A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; F = Fu’s acupuncture; G = medicated thread moxibustion; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; J = pricking and cupping plus antiepileptics; K = acupoint catgut embedding plus antiepileptics.
FIGURE 8.
Self-Rating Depression Scale probability ranking diagram. A = antiepileptics; C = electroacupuncture; D = fire needling; E = pricking and cupping; H = electroacupuncture plus antiepileptics.
FIGURE 7.
Pittsburgh Sleep Quality Index probability ranking diagram. A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; K = acupoint catgut embedding plus antiepileptics.
3.5. Adverse events
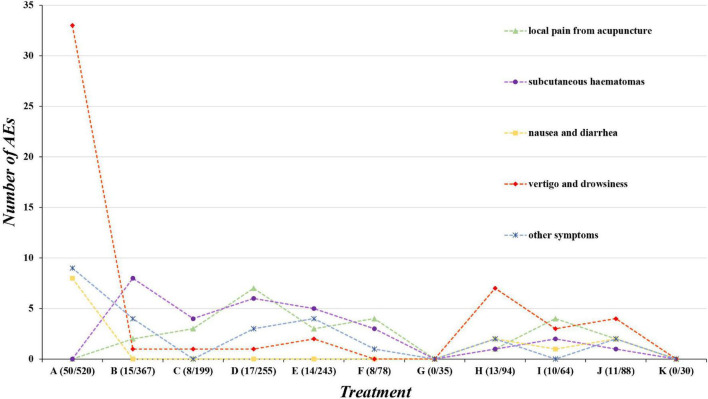
Of the 29 studies, 16 reported AEs (Tian, 2013; Zhang, 2013, 2015; Sheng et al., 2014; Lei, 2015; Hong, 2016; Wu and Zhao, 2016; Yang and Pan, 2016; Xie et al., 2018; Xu, 2018; Zhou and Huang, 2018; Zhong, 2019; Pang, 2020; Zhao et al., 2020; Ding et al., 2021; Zhang et al., 2021), while the remaining studies did not describe AEs. We divided the AEs into five categories: local pain from acupuncture, subcutaneous hematoma, nausea and diarrhea, vertigo, drowsiness, and others, such as influenza, during treatment. Pricking and cupping plus antiepileptics and EA plus antiepileptics were associated with the highest number of AEs. Fire needling plus antiepileptics had the highest incidence of AEs (14.06%). Additionally, the highest incidences of AEs were vertigo and drowsiness, subcutaneous hematoma, and local pain from acupuncture (35.62, 20.55, and 17.81%, respectively). Briefly, acupuncture and related therapies are associated with an increased incidence of subcutaneous hematoma and local pain from acupuncture pain, whereas antiepileptics and their combined therapies are more associated with reports of vertigo and drowsiness. No severe AEs occurred in any study (Figure 9).
FIGURE 9.
Adverse events. A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; F = Fu’s acupuncture; G = medicated thread moxibustion; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; J = pricking and cupping plus antiepileptics; K = acupoint catgut embedding plus antiepileptics.
3.6. Publication bias
Publication bias was examined using an adjusted funnel plot (Figure 10); most points were evenly distributed on both sides of the midline and concentrated in the middle region. The reasonable assumption is that the sample size of most included studies was moderate, and therefore the degree of bias in these studies was low. Nevertheless, several points were beyond the two dotted lines, indicating heterogeneity among studies.
FIGURE 10.
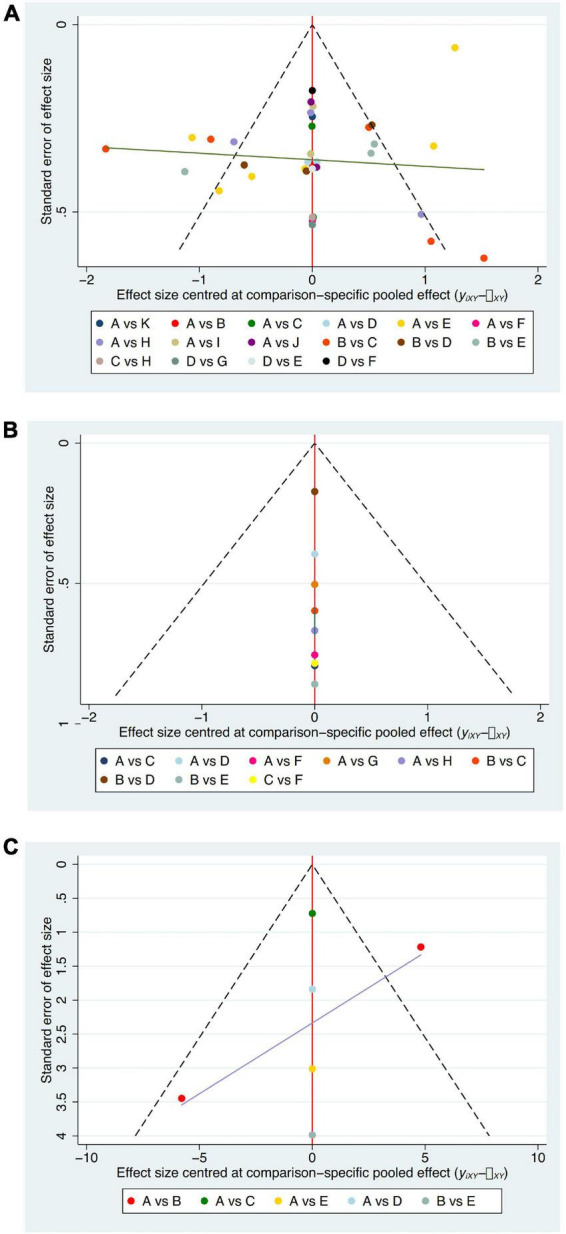
Adjusted funnel plot. (A) VAS; (B) PSQI; (C) SDS; A = antiepileptics; B = manual acupuncture; C = electroacupuncture; D = fire needling; E = pricking and cupping; F = Fu’s acupuncture; G = medicated thread moxibustion; H = electroacupuncture plus antiepileptics; I = fire needling plus antiepileptics; J = pricking and cupping plus antiepileptics; K = acupoint catgut embedding plus antiepileptics.
4. Discussion
There is currently no disease-relieving therapy for PHN; therefore, treatment should focus on controlling symptoms. Besides the physical pain symptoms of PHN patients, the negative emotions caused by pain often lead to symptoms like depression and anxiety (Johnson and Rice, 2014). Most clinical attention with existing therapies is often paid to pain treatment but ignores its negative emotional management, which is difficult to safely and effectively address with state-of-the-art treatments (Boersma et al., 2019). In addition, a previous study confirmed that spontaneous PHN pain has a specific pattern of brain activity, and the regions involved in emotion best reflect the changes in pain after treatment (Geha et al., 2007). Therefore, improved therapeutic approaches are needed to affect patients’ emotional states in the context of pain management therapy.
This is the first NMA for acupuncture and related therapies for PHN. Although evidence has shown that acupuncture is effective in treating PHN (Pei et al., 2019; Zhou et al., 2021), methods vary, and techniques with poor efficacy affect the patient’s condition and waste medical resources. Accordingly, we attempted to identify the optimal PHN acupuncture treatment. The original protocol divided the intervention into four categories: acupuncture techniques alone, sham placebo acupuncture, effective pharmacotherapy, and single acupuncture plus effective pharmacotherapy. Unfortunately, studies regarding effective drug treatments related to acupuncture did not include criteria. Thus, the only pharmacotherapy included in this study, was antiepileptics. Comparisons between acupuncture and antiepileptics are meaningful because antiepileptics are the recommended first-line treatment for PHN with fewer side effects than other effective drugs. No suitable sham-placebo acupuncture was included in this study, and the placebo effect of acupuncture in PHN was not demonstrated. Moreover, because the PHN diagnostic criteria are not standardized, we included two sets of representative PHN criteria in English and Chinese to include a broader collection of literature. Different diagnostic criteria may lead to different clinical implications, but both criteria are generally based on persistent chronic neuropathic pain after the rash healed. However, it also may increase the heterogeneity, affecting the strength of the evidence. Subsequent studies should unify PHN diagnostic criteria, update the expert consensus to facilitate comparisons, and improve the quality of evidence.
Our quality assessment of the 29 RCTs on acupuncture and related therapies for PHN revealed that five RCTs were at high risk of bias. Allocation concealment resulted in the highest risk, which may have led to selection bias. We did not consider studies that did not blind researchers high risk because of the unique clinical features of acupuncture techniques. Unlike clinical drug studies, researchers cannot properly use blinding in acupuncture. Therefore, further high-quality and multicenter clinical trials are required to improve the quality of the evidence.
A total of 1,973 patients were included in this study. VAS, SDS, and PSQI scores and adverse events were evaluated for 10 acupuncture and related techniques and compared with those of antiepileptics for PHN, including manual acupuncture, EA, fire needling, pricking and cupping, Fu’s subcutaneous needling, medicated thread moxibustion, EA plus antiepileptics, fire needling plus antiepileptics, pricking and cupping plus antiepileptics, and acupoint catgut embedding plus antiepileptics. Although 25 studies reported the clinical response rate, it was not included in the comprehensive analysis because most studies were based on different self-designed methods of measuring efficacy. Guidelines or expert consensus for acupuncture treatment of PHN should use unified efficacy criteria to standardize outcome reporting and help with evidence comparisons.
Our NMA results generally followed those of the pairwise meta-analysis when comparing antiepileptics with all types of acupuncture. These treatments reduced VAS scores more effectively than antiepileptics. Regarding PSQI score reduction, only EA plus antiepileptics was more effective than antiepileptics, while the reduction was not significantly different for the other six acupuncture-related therapies. Notably, there was only one study for each intervention. No significant difference among the four acupuncture-related treatments was observed for SDS score reduction compared with antiepileptics. Similarly, only four studies included the SDS outcomes. Some comparisons in the pairwise meta-analysis results were heterogeneous, mainly in EA and pricking and cupping interventions. However, due to our strict inclusion and exclusion criteria, we believe that this heterogeneity does not come from the study designs but rather from clinical heterogeneity. Because of the particularity of acupuncture therapy, it is difficult for acupuncturists to accurately use fixed acupuncture depth and stimulation intensity, which we believe is the main source of clinical heterogeneity. Future studies should adopt unified and standardized techniques, reducing the heterogeneity of acupuncture clinical research. Therefore, these results should be interpreted with caution.
Our ranking probability results suggested that pricking and cupping plus antiepileptics are the most effective pain relief, followed by EA plus antiepileptics; EA plus antiepileptics seems to be most effective in PSQI and SDS score reduction. Additionally, the other eight methods have moderate pain relief effects, although it is difficult to determine which single acupuncture treatment is the best, considering the complexity of the results. Nevertheless, pricking and cupping plus antiepileptics and EA plus antiepileptics have better effects on PHN. We can assume that antiepileptics combined with acupuncture techniques enhance analgesic effects. Currently, which parts play a key role, or which interact to amplify efficacy is unknown. Indeed, this potential hypothesis must be confirmed by basic follow-up research. This provides a new direction for clinical treatment of PHN and, perhaps, a novel approach for treating other types of chronic neuropathic pain using acupuncture.
Modern studies have shown that the analgesic mechanism of EA stimulation with different frequencies differs. It is essential to select the appropriate parameters of EA to optimize the therapeutic effect of EA. Some of the included studies did not detail the frequency parameters used by EA, but most of the reported RCTs with EA used dilatational waves. In rat experiments, 2-Hz electrical stimulation at acupoints can cause the release of high levels of enkephalins and endorphins in the brain and spinal cord. In contrast, 100-Hz electrical stimulation can cause the release of a high level of dynorphin in the spinal cord. Both have analgesic effects, but each has specific characteristics. If a dilatational wave alternating between 2 and 100 Hz is used, the above three peptides can be released simultaneously and play a synergistic analgesic effect (Han, 2011). However, there is still controversy regarding the parameters of EA (Sun et al., 2022). More research is needed on which EA parameters are most effective in treating PHN. In addition, we also summarized the acupoints for treating PHN: the Ashi point was the most frequently used acupoint, followed by the Jiaji point (EX-B2). Ashi acupoints are located in the most painful area of the skin, which can be understood as points located in the sensitized state in the pathological state, and acupoint sensitization can be regarded as a modern supplement to Ashi acupoints. Jiaji acupoints (EX-B2) are located 0.5 cun bilateral to the posterior midline in the dorsal thoracic and lumbar region, ranging from the first thoracic vertebra to the fifth lumbar vertebra. Stimulation can inhibit the expression of pro-inflammatory cytokines and Nogo-NgR signaling pathway, promote nerve growth, and regulate related nerves and organs (Sun et al., 2022). This perspective explains why these two acupoints were used most frequently. However, the research on the complex mechanism of the acupoint effect and the difference in curative impact caused by different acupoint selections is still incomplete and needs further exploration.
The mechanism of acupuncture analgesia for PHN has not yet been clarified despite advances in neurobiology and pain signal transmission. Acupuncture analgesia in PHN is a complex network regulation mechanism involving the entire nervous system from the periphery to the central, and many biologically active substances are involved in acupuncture’s inhibitory effect on PHN. Identifying the mechanism of acupuncture in the treatment of PHN from a pathophysiological perspective is essential. Animal models are used to explain the curative effect of acupuncture on PHN, although there is a dearth of published studies.
Therefore, we summarized current mainstream acupuncture analgesic mechanisms into seven aspects, to provide ideas and a basis for further exploration of acupuncture analgesia in PHN treatment. (1) Acupuncture modulates the interaction between nociceptive receptors and the inflammatory immune response, inhibiting the release of inflammatory cytokines, lipids, prostaglandin E2, 5-hydroxytryptamine, histamine, and nerve growth factor by immune cells, inhibiting peripheral receptor nociceptor sensitization and transient receptor potential cation channel subfamily V member 1 phosphorylation, alleviating peripheral sensitization resulting in pain relief (Zhang et al., 2014; Zhang Y. et al., 2020; Hsu et al., 2019). (2) Sodium channels are involved in the overexcitation of sensory neurons leading to neuropathic pain, and acupuncture can change sodium ion channel expression to produce analgesic effects (Lu et al., 2016; Yen et al., 2018; Li et al., 2020). (3) Although PHN is caused by peripheral nervous system injury, neuropathic pain is maintained by central sensitization, that is, plasticity changes in the central nervous system (Meacham et al., 2017). Acupuncture can inhibit central sensitization and the formation of synaptic plasticity, achieving analgesic effects (Ji et al., 2018; Lai et al., 2019; Qiao et al., 2019). (4) Spinal glial cells (microglia and astrocytes) are involved in inflammatory responses and neuropathic pain, and glial cells—especially microglia—play key roles in the pathogenesis of neuropathic pain. Acupuncture reduces pain by inhibiting this activation (Lin et al., 2016; Wang J. Y. et al., 2018). (5) Acupuncture can regulate the entire brain area network of pain stimulation by adjusting the interaction between various brain functional areas and neurotransmitters, producing analgesic effects (Yuan et al., 2018; Zhao et al., 2018). (6) Acupuncture exerts analgesic effects by mediating mitogen-activated protein kinase (MAPK) signaling pathways, including the spinal cord p38/MAPK (Fang et al., 2013; Liang et al., 2016), extracellular-signal-regulated kinase (Choi et al., 2012; Park et al., 2014), c-Jun N-terminal kinase (Lee et al., 2013), and nuclear factor kappa-B pathways (Park et al., 2002). (7) Acupuncture exerts analgesic effects through epigenetic regulation of genes in tissues and cells, including DNA methylation (Jang et al., 2021), histone modification (Lai et al., 2012), and microRNA regulation (Liu and Wu, 2017; Zou et al., 2019). These theories have provided a theoretical basis for evaluating the effectiveness of acupuncture in PHN treatment and have laid the foundation for follow-up research.
We analyzed 16 RCTs that reported AEs—summarized into five aspects: local pain from acupuncture, subcutaneous hematoma, nausea and diarrhea, vertigo and drowsiness, and other symptoms. Vertigo and drowsiness, subcutaneous hematoma, and local pain from acupuncture were the most common AEs. Antiepileptics- (including combined acupuncture treatments) mainly relate to vertigo and drowsiness, and acupuncture-related techniques mainly caused subcutaneous hematoma and local pain. Therefore, we do not recommend antiepileptics for patients with PHN comorbid with neurological diseases or older people, although combined therapy is better than acupuncture alone for pain relief. Patients with renal deficiency should also avoid antiepileptics due to drug contraindications. Patients should be informed of the possibility of mild pain and subcutaneous hematoma before acupuncture-related treatment; if they are intolerant, acupuncture should also be avoided. Concisely, acupuncture is relatively safe compared to antiepileptics because acupuncture pain is mild and transient.
In conclusion, for the sole purpose of relieving pain, the clinician should choose pricking and cupping plus antiepileptics, while PHN patients with negative emotions and insomnia should use electroacupuncture plus antiepileptics. Nevertheless, these two treatments both had the most AEs, combining the side effects of both antiepileptic and acupuncture treatments. This presents a thought-provoking approach to personalized precision therapy. In future clinical research, patients should be divided according to degrees of pain; for example, combined treatment is recommended for severe and moderate pain, and acupuncture alone is recommended for mild pain. This indicates the future direction of combined acupuncture and Western medicine treatments for PHN and may inspire other combined acupuncture and Western medicine treatment programs. However, this is currently only a hypothesis that requires further testing.
4.1. Strengths
First, this review was strictly conducted using PRISMA and PRISMA-NMA guidelines. Second, to ensure adequate retrieval, we searched eight Chinese and English electronic databases and searched two clinical trial registration platforms, previous meta-analyses, gray literature, and reference lists of selected studies. Third, because RCTs are the gold standard for clinical trials, we excluded unqualified RCTs or non-RCTs, which might have affected our outcomes. Fourth, we tried to divide Western drugs by category to improve the quality of evidence comparison, although only one drug met the criteria. Fifth, we used Bayesian multiple-treatment NMA to acquire more accurate estimates than the frequentist framework method. Finally, the network results of our study are reliable because the P-values of the inconsistent node-splitting analysis were all greater than 0.05, and all curves of the diagnostic convergence graph approach 1.
4.2. Limitations
First, high-quality RCTs were rare, and the sample sizes in most studies were small; therefore, this finding’s power is limited. Second, we used two diagnostic criteria, which increased heterogeneity, affecting the power of evidence. Third, the long-term efficacy of the interventions involved in this study has not been verified. Fourth, all included studies were performed in China; thus, language bias is possible. Fifth, there is no evidence to guide the choice of optimal acupoints, treatment frequency, or duration in this study. Finally, the study lacked clinical response rate and quality-of-life indicators.
4.3. Future prospects
In the future, basic studies should explore the potential mechanism of acupuncture, fully utilizing modern neurobiological technology to explore the complex network mechanism of acupuncture treatment for PHN. Second, basic research should focus on more than just animal models, as research on human biochemical indicators is lacking. Multiomics technology can conduct in vitro experiments to detect changes in related metabolites and signal pathway molecules at all levels in patients with PHN before and after acupuncture, further clarifying the mechanism of action and exploring the potential value of acupuncture therapy for PHN. Third, subsequent studies should unify the diagnostic criteria of PHN and modify the expert consensus to facilitate the comparison, and improve the quality, of evidence. Fourth, existing studies lack quality-of-life outcomes and unified evaluation criteria for the clinical response rates. Follow-up studies should adopt unified clinical efficacy evaluation criteria and gradually establish a set of homogeneous outcome indicators directly related to patient interests. Fifth, long-term follow-up and acupuncture placebo groups should be in future studies to clarify thelong-term efficacy and placebo effect of acupuncture. Sixth, there are no comparative studies on the long-term efficacy of different acupuncture techniques for chronic pain; therefore a relevant NMA should be conducted to find the best acupuncture-related technique. Seventh, given the mixed nature and complexity of acupuncture techniques and antiepileptics for PHN, further research should focus on classifying the degree of pain in patients with PHN into discrete subtypes, ultimately providing the basis for more personalized clinical treatment schemes. Eighth, the clinical treatment of acupuncture and moxibustion should adopt unified and standardized techniques, such as the same basic points, acupuncture depth, acupuncture frequency, and treatment course. This is not contradictory to individualized treatment, although it is more convenient for evidence comparisons, thus reducing the heterogeneity of acupuncture clinical research and improving the quality of evidence. Finally, most Chinese studies on acupuncture do not share original research data. Data sharing and transparency in clinical studies should be supported to facilitate data mining and reusing clinical data for acupuncture therapy.
4.4. Comparison
Coincidentally, one study on a similar topic (Wang et al., 2022) was published just a few days ago. Therefore, we decided to compare our analysis with theirs. First, NMA is mainly based on two theoretical frameworks: frequency and Bayesian. We used R software to conduct NMA based on the Bayesian framework, while Wang et al. (2022) used STATA software to conduct a frequency-based NMA. Notably, the frequency method estimates the maximum likelihood function by constantly iterating during parameter estimation, and the result is more likely to be biased. The Bayesian approach does not have this problem, and the estimation value is more accurate (Hutton et al., 2015). Second, we not only analyzed the outcome indicators of pain reduction and AEs but also conducted NMA on improvement in pain-related outcomes (sleep quality and depression), whereas Wang et al. (2022) only analyzed pain reduction and AEs. Third, our study search strategy was more comprehensive, searching not only entire electronic databases but also clinical trial registries and gray literature, ensuring a comprehesive literature search’. In addition, we also recorded the exact reasons for the elimination of each study (Supplementary Appendix 2). Fourth, the method for measuring clinical response rates is based on two main categories: (1) objective measures based on the percentage reduction in pain scores and (2) subjective measures based on improvement in patients’ self-perceived pain. Therefore, our study could not comprehensively analyze of this outcome. Although Wang et al. (2022) analyzed the efficiency rate outcome, they used two different efficacy evaluation criteria. There is heterogeneity in this method, reducing the evidence’s power for this outcome. Notably, we pointed out the solution to this problem in the discussion. Fifth, we evaluated the similarity, consistency, and convergence of the results among the included RCTs to ensure the feasibility of NMA and the stability of its results, while Wang et al. (2022) did not. Sixth, we believe that acupoint injection is a combination of needle and medicine, a unique injection therapy in which drugs inject into specific acupoints through a syringe. According to our investigation, the types and dosages of drugs used for acupoint injection in RCTs are also inconsistent. Therefore, we did not include acupoint injection technique as opposed to Wang et al. (2022), and we believe that the significance of this treatment is limited. Seventh, we made a refined classification of first-line PHN drugs, although our study included only antiepileptics. Nonetheless, the control group in the study by Wang et al. (2022) combined different types of drugs into the same Western medicine category, reducing the strength of the evidence. Eighth, we discussed and summarized the analgesic mechanism of acupuncture in the treatment of PHN, as well as deficiencies and prospects of study on acupuncture-related therapies, to clarify its analgesic mechanism and provide ideas for subsequent research.
In addition, we compared the protocol of Bian et al. (2022) with our study, although its formal study has yet to be accomplished. First, the treatment was limited to a single acupuncture technique in our study, while Bian et al. (2022) allowed multiple acupuncture treatments to be used in combination. In clinical practice, physicians are more inclined to use a single, effective acupuncture treatment, which can reduce patients’ cost burden and the fear and pain of acupuncture stimulation. Therefore, the results of their protocol may lead to confusion regarding the clinician’s selection. Furthermore, due to the mixing and complexity of multiple acupuncture combinations, Bian et al. (2022) are unable to specify which acupuncture technique plays the most important role, which cannot provide a reference for subsequent clinical and basic research. Second, we made a refined classification of first-line PHN drugs, although our study included only antiepileptics. Nonetheless, the control group for Bian et al. (2022) only selects conventional drugs vaguely, without limiting the use of recognized first-line PHN treatment drugs and without precise classification of drugs, which reduces the strength of the quality of evidence for comparison. Finally, we discussed and summarized the analgesic mechanism of acupuncture in the treatment of PHN as well as deficiencies and prospects of study on acupuncture-related therapies to clarify its analgesic mechanism and provide ideas for subsequent research.
5. Conclusion
Based on the evidence from our analysis, acupuncture-related therapies may be potential treatment options for PHN and are relatively safe with only mild side effects. Pricking and cupping plus antiepileptics is the most effective acupuncture-related technique for PHN pain reduction, followed by EA plus antiepileptics. Moreover, EA plus antiepileptics is the best acupuncture-related technique for improving PHN-related insomnia and depression symptoms. Nevertheless, owing to the limitations of this study, these conclusions should be cautiously interpreted, and future high-quality studies are needed.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YC: conception and design and drafting and writing. XYZ, QL, DW, and JZ: retrieval and screening of studies and extraction of data. XXZ and QH: evaluation of the methodological quality of included studies. RY, SX, and DZ: statistical analysis. XM and SZ: revision of chart. HY and ZS: review and revision. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors acknowledge all authors of the original studies used. Additionally, we would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
This work was supported by the National Administration of Traditional Chinese Medicine Evidence-based Capacity Building Project (2019XZZX-ZJ005).
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2022.1056102/full#supplementary-material
References
- Argoff C. E. (2011). Review of current guidelines on the care of postherpetic neuralgia. Postgrad. Med. 123 134–142. 10.3810/pgm.2011.09.2469 [DOI] [PubMed] [Google Scholar]
- Baron R., Wasner G. (2006). Prevention and treatment of postherpetic neuralgia. Lancet 367 186–188. 10.1016/S0140-6736(06)68010-0 [DOI] [PubMed] [Google Scholar]
- Bian Z., Yu J., Tu M., Liao B., Huang J., Izumoji G., et al. (2022). Acupuncture therapies for postherpetic neuralgia: A protocol for a systematic review and Bayesian network meta-analysis. BMJ Open 12:e056632. 10.1136/bmjopen-2021-056632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder A., Baron R. (2008). Postherpetic neuralgia–fighting pain with fire. Lancet Neurol. 7 1077–1078. 10.1016/S1474-4422(08)70242-4 [DOI] [PubMed] [Google Scholar]
- Boersma K., Södermark M., Hesser H., Flink I. K., Gerdle B., Linton S. J. (2019). Efficacy of a transdiagnostic emotion-focused exposure treatment for chronic pain patients with comorbid anxiety and depression: A randomized controlled trial. Pain 160 1708–1718. 10.1097/j.pain.0000000000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaimani A., Higgins J. P., Mavridis D., Spyridonos P., Salanti G. (2013). Graphical tools for network meta-analysis in STATA. PLoS One 8:e76654. 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. (2019). Clinical observation on Jia-ji acupoint catgut embedding combined with pregabalin on treatment for postherpetic neuralgia. Master Dissertation. Harbin: Heilongjiang University of Chinese Medicine. [Google Scholar]
- Chen P., Lin C., Yang J. P. (2012). Comparative analysis of effects between acupuncture of fire needle and medicated thread moxibustion on postherpetic neuralgia. Chin. J. Tradit. Chin. Med. 7 1847–1849. [Google Scholar]
- Chen S. Y., Li J. J., Zhou P. (2018). Comparison of the efficacy of fire acupuncture and acupuncture needle in the treatment of postherpetic neuralgia. Chin. Med. Innov. 6 50–53. [Google Scholar]
- Choi D. C., Lee J. Y., Lim E. J., Baik H. H., Oh T. H., Yune T. Y. (2012). Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp. Neurol. 236 268–282. 10.1016/j.expneurol.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Cohen J. I. (2013). Clinical practice: Herpes zoster. N. Engl. J. Med. 369 255–263. 10.1056/NEJMcp1302674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Li Q., Wang D., Bao R., Li L., Zhu J., et al. (2022). Does electroacupuncture benefit mixed urinary incontinence? A systematic review and meta-analysis with trial sequential analysis. Int. Urogynecol. J. 33 751–766. 10.1007/s00192-021-05057-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumpston M., Li T., Page M. J., Chandler J., Welch V. A., Higgins J. P., et al. (2019). Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev. 10:ED000142. 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias S., Welton N. J., Caldwell D. M., Ades A. E. (2010). Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 29 932–944. 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- Ding X. Y., Yang Y. M., Ding Y., Zhang X. (2021). Efficacy of bleeding and cupping combined with gabapentin in the treatment of moderate to severe postherpetic neuralgia patients and impact on pain-related neuropeptides and inflammatory cytokines. Chin. J. Dermatol. Venereol. Integr. Tradit. West. Med. 4:346. [Google Scholar]
- Doth A. H., Hansson P. T., Jensen M. P., Taylor R. S. (2010). The burden of neuropathic pain: A systematic review and meta-analysis of health utilities. Pain 149 338–344. 10.1016/j.pain.2010.02.034 [DOI] [PubMed] [Google Scholar]
- Drolet M., Brisson M., Schmader K., Levin M., Johnson R., Oxman M., et al. (2010). Predictors of postherpetic neuralgia among patients with herpes zoster: A prospective study. J. Pain 11 1211–1221. 10.1016/j.jpain.2010.02.020 [DOI] [PubMed] [Google Scholar]
- Duan L. L. (2014). The clinical efficacy observation of electroacupuncture Jia-ji for treatment of postherpetic intercostals neuralgia. Master Dissertation. Harbin: Heilongjiang University of Chinese Medicine. [Google Scholar]
- Dworkin R. H., O’Connor A. B., Kent J., Mackey S. C., Raja S. N., Stacey B. R., et al. (2013). Interventional management of neuropathic pain: NeuPSIG recommendations. Pain 154 2249–2261. 10.1016/j.pain.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin R. H., Turk D. C., Peirce-Sandner S., McDermott M. P., Farrar J. T., Hertz S., et al. (2010). Placebo and treatment group responses in postherpetic neuralgia vs. painful diabetic peripheral neuropathy clinical trials in the REPORT database. Pain 150 12–16. 10.1016/j.pain.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Fang J. Q., Du J. Y., Liang Y., Fang J. F. (2013). Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats. Mol. Pain 9:13. 10.1186/1744-8069-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup N. B., Kuner R., Jensen T. S. (2021). Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 101 259–301. 10.1152/physrev.00045.2019 [DOI] [PubMed] [Google Scholar]
- Forbes H. J., Bhaskaran K., Thomas S. L., Smeeth L., Clayton T., Mansfield K., et al. (2016a). Quantification of risk factors for postherpetic neuralgia in herpes zoster patients: A cohort study. Neurology 87 94–102. 10.1212/WNL.0000000000002808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes H. J., Thomas S. L., Smeeth L., Clayton T., Farmer R., Bhaskaran K., et al. (2016b). A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain 157 30–54. 10.1097/j.pain.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen K. J., Falk J., Alessi-Severini S., Chateau D., Bugden S. (2016). Price of pain: Population-based cohort burden of disease analysis of medication cost of herpes zoster and postherpetic neuralgia. J. Pain Res. 9 543–550. 10.2147/JPR.S107944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier A., Breuer J., Carrington D., Martin M., Rémy V. (2009). Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol. Infect. 137 38–47. 10.1017/S0950268808000678 [DOI] [PubMed] [Google Scholar]
- Geha P. Y., Baliki M. N., Chialvo D. R., Harden R. N., Paice J. A., Apkarian A. V. (2007). Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain 128 88–100. 10.1016/j.pain.2006.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A., Rubin D. B. (1996). Markov chain Monte Carlo methods in biostatistics. Stat. Methods Med. Res. 5 339–355. 10.1177/096228029600500402 [DOI] [PubMed] [Google Scholar]
- Han H. T., Li M. K., Li J., Li X. J., Lai J. J., Li W. Q., et al. (2018). Analgesic effect and serum IL-18 and TNF-α of Zhuang-yi Lotus needle cupping for postherpetic neuralgia. J. Guangxi Univ. Tradit. Chin. Med. 4:8. [Google Scholar]
- Han J. S. (2011). Acupuncture analgesia: Areas of consensus and controversy. Pain 152(Suppl. 3) S41–S48. 10.1016/j.pain.2010.10.012 [DOI] [PubMed] [Google Scholar]
- Hong Y. B. (2016). Clinical effect of blood pricking and cupping therapy on postherpetic neuralgia of the head and face. China J. Tradit. Chin. Med. 1:1424. [Google Scholar]
- Hsu H. C., Hsieh C. L., Wu S. Y., Lin Y. W. (2019). Toll-like receptor 2 plays an essential role in electroacupuncture analgesia in a mouse model of inflammatory pain. Acupunct. Med. 37 356–364. 10.1136/acupmed-2017-011469 [DOI] [PubMed] [Google Scholar]
- Huang J. M., Han H. T., Li J. J., Chen L., Lu X. L., Ge C. L. (2011). Effect of lotus needle cupping therapy on postherpetic neuralgia. Guangxi Tradit. Chin. Med. 1 31–32. [Google Scholar]
- Hutton B., Salanti G., Caldwell D. M., Chaimani A., Schmid C. H., Cameron C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 777–784. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- Jang J. H., Song E. M., Do Y. H., Ahn S., Oh J. Y., Hwang T. Y., et al. (2021). Acupuncture alleviates chronic pain and comorbid conditions in a mouse model of neuropathic pain: The involvement of DNA methylation in the prefrontal cortex. Pain 162 514–530. 10.1097/j.pain.0000000000002031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. S., Finnerup N. B. (2014). Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 13 924–935. 10.1016/S1474-4422(14)70102-4 [DOI] [PubMed] [Google Scholar]
- Ji R. R., Nackley A., Huh Y., Terrando N., Maixner W. (2018). Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology 129 343–366. 10.1097/ALN.0000000000002130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. W., Rice A. S. (2014). Clinical practice. Postherpetic neuralgia. N. Engl. J. Med. 371 1526–1533. 10.1056/NEJMcp1403062 [DOI] [PubMed] [Google Scholar]
- Ke M., Yinghui F., Yi J., Xeuhua H., Xiaoming L., Zhijun C., et al. (2013). Efficacy of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: A randomized, double-blinded, controlled trial. Pain Physician 16 15–25. [PubMed] [Google Scholar]
- Kelly R. B., Willis J. (2019). Acupuncture for pain. Acupunct. Pain 100 89–96. [PubMed] [Google Scholar]
- Lai H. C., Lin Y. W., Hsieh C. L. (2019). Acupuncture-analgesia-mediated alleviation of central sensitization. Evid. Based Complement. Alternat. Med. 2019:6173412. 10.1155/2019/6173412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X., Wang J., Nabar N. R., Pan S., Tang C., Huang Y., et al. (2012). Proteomic response to acupuncture treatment in spontaneously hypertensive rats. PLoS One 7:e44216. 10.1371/journal.pone.0044216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Choi D. C., Oh T. H., Yune T. Y. (2013). Analgesic effect of acupuncture is mediated via inhibition of JNK activation in astrocytes after spinal cord injury. PLoS One 8:e73948. 10.1371/journal.pone.0073948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y. T. (2015). Clinical study on postherpetic neuralgia by acupuncture treatment of Sheng’s “remove blood stasis and righting” and its effect on serum 5–HT. Master Dissertation. Nanjing: Nanjing University of Chinese Medicine. [Google Scholar]
- Li D., Sun G., Sun H., Wang Y., Wang Z., Yang J. (2018). Combined therapy of pulsed radiofrequency and nerve block in postherpetic neuralgia patients: A randomized clinical trial. PeerJ 6:e4852. 10.7717/peerj.4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Liu B., Wu W., Hong Y., Zhang J., Liu Y., et al. (2020). Upregulation of transcription factor 4 downregulates NaV1.8 expression in DRG neurons and prevents the development of rat inflammatory and neuropathic hypersensitivity. Exp. Neurol. 327:113240. 10.1016/j.expneurol.2020.113240 [DOI] [PubMed] [Google Scholar]
- Liang Y., Du J. Y., Qiu Y. J., Fang J. F., Liu J., Fang J. Q. (2016). Electroacupuncture attenuates spinal nerve ligation-induced microglial activation mediated by p38 mitogen-activated protein kinase. Chin. J. Integr. Med. 22 704–713. 10.1007/s11655-015-2045-1 [DOI] [PubMed] [Google Scholar]
- Lin L., Skakavac N., Lin X., Lin D., Borlongan M. C., Borlongan C. V., et al. (2016). Acupuncture-induced analgesia: The role of microglial inhibition. Cell Transplant. 25 621–628. 10.3727/096368916X690872 [DOI] [PubMed] [Google Scholar]
- Liu J., Wu Y. (2017). Electro-acupuncture-modulated miR-214 prevents neuronal apoptosis by targeting Bax and inhibits sodium channel Nav1.3 expression in rats after spinal cord injury. Biomed Pharmacother. 89 1125–1135. 10.1016/j.biopha.2017.02.077 [DOI] [PubMed] [Google Scholar]
- Lu K. W., Hsu C. K., Hsieh C. L., Yang J., Lin Y. W. (2016). Probing the effects and mechanisms of electroacupuncture at ipsilateral or contralateral ST36-ST37 acupoints on CFA-induced inflammatory pain. Sci. Rep. 6:22123. 10.1038/srep22123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick-Searle T., Snodgrass B., Brant J. M. (2016). Postherpetic neuralgia: Epidemiology, pathophysiology, and pain management pharmacology. J. Multidiscip. Healthc. 9 447–454. 10.2147/JMDH.S106340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol E. D., Midbari A., Eisenberg E. (2013). Opioids for neuropathic pain. Cochrane Database Syst. Rev. 2013:CD006146. 10.1002/14651858.CD006146.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham K., Shepherd A., Mohapatra D. P., Haroutounian S. (2017). Neuropathic pain: Central vs. peripheral mechanisms. Curr. Pain Headache Rep. 21:28. 10.1007/s11916-017-0629-5 [DOI] [PubMed] [Google Scholar]
- Melchart D., Weidenhammer W., Streng A., Reitmayr S., Hoppe A., Ernst E., et al. (2004). Prospective investigation of adverse effects of acupuncture in 97 733 patients. Arch. Intern. Med. 164 104–105. 10.1001/archinte.164.1.104 [DOI] [PubMed] [Google Scholar]
- Mo Y. (2019). Zhuang medical lotus acupuncture and cupping method for PHN patients effects of Wnt3a and TNF alpha. Master Dissertation. Nanning: Guangxi University of Chinese Medicine. [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. A., Derry S., Aldington D., Cole P., Wiffen P. J. (2015). Amitriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015:CD008242. 10.1002/14651858.CD008242.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. A., Wiffen P. J., Derry S., Toelle T., Rice A. S. (2014). Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2014:CD007938. 10.1002/14651858.CD007938.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalamachu S., Morley-Forster P. (2012). Diagnosing and managing postherpetic neuralgia. Drugs Aging 29 863–869. 10.1007/s40266-012-0014-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpoya I. J., Thomas E. T., Lee J. J., Goldacre B., Heneghan C. J. (2019). Benefits and harms of pregabalin in the management of neuropathic pain: A rapid review and meta-analysis of randomised clinical trials. BMJ Open 9:e023600. 10.1136/bmjopen-2018-023600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman M. N., Levin M. J., Johnson G. R., Schmader K. E., Straus S. E., Gelb L. D., et al. (2005). A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352 2271–2284. 10.1056/NEJMoa051016 [DOI] [PubMed] [Google Scholar]
- Pang H. X. (2020). Clinical observation of “wrist-ankle acupuncture with filiform-fire needle” on postherpetic neuralgia. Master Dissertation. Chengdu: Chengdu University of Traditional Chinese Medicine. [Google Scholar]
- Park H. J., Lee H. S., Lee H. J., Yoo Y. M., Lee H. J., Kim S. A., et al. (2002). Decrease of the electroacupuncture-induced analgesic effects in nuclear factor-kappa B1 knockout mice. Neurosci. Lett. 319 141–144. 10.1016/s0304-3940(01)02582-4 [DOI] [PubMed] [Google Scholar]
- Park J. Y., Park J. J., Jeon S., Doo A. R., Kim S. N., Lee H., et al. (2014). From peripheral to central: The role of ERK signaling pathway in acupuncture analgesia. J. Pain 15 535–549. 10.1016/j.jpain.2014.01.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei W., Zeng J., Lu L., Lin G., Ruan J. (2019). Is acupuncture an effective postherpetic neuralgia treatment? A systematic review and meta-analysis. J. Pain Res. 12 2155–2165. 10.2147/JPR.S199950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K. L., Rowbotham M. C. (2007). Relief of post-herpetic neuralgia by surgical removal of painful skin: 5 years later. Pain 131 214–218. 10.1016/j.pain.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L. N., Yang Y. S., Liu J. L., Zhu J., Tan L. H., Shi Y. N., et al. (2019). Contribution of GABAergic modulation in DRGs to electroacupuncture analgesia in incisional neck pain rats. J. Pain Res. 12 405–416. 10.2147/JPR.S180165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanti G., Ades A. E., Ioannidis J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 64 163–171. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- Schmader K. E., Baron R., Haanpää M. L., Mayer J., O’Connor A. B., Rice A. S., et al. (2010). Treatment considerations for elderly and frail patients with neuropathic pain. Mayo Clin. Proc. 85 S26–S32. 10.4065/mcp.2009.0646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J., Finnerup N. B., Attal N., Aziz Q., Baron R., Bennett M. I., et al. (2019). The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 160 53–59. 10.1097/j.pain.0000000000001365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng G. B., Duan L. L., Cai Y. M., Tang Y., Zhu T. (2014). Clinical observation of Jia-ji electroacupuncture for postherpetic neuralgia. Inf. Tradit. Chin. Med. 6 85–86. [Google Scholar]
- Song D., He A., Xu R., Xiu X., Wei Y. (2018). Efficacy of pain relief in different postherpetic neuralgia therapies: A network meta-analysis. Pain Physician 21 19–32. [PubMed] [Google Scholar]
- Sun R., Li S., Ren L., Xia Y., Wang Y., Bian Z., et al. (2022). Efficacy of electroacupuncture for the treatment of postherpetic neuralgia: study protocol for a multicenter randomized controlled trial. J. Pain Res. 15 959–968. 10.2147/JPR.S357435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Z., Li L. (2015). Clinical observation of electroacupuncture cleft points combined with peri-acupuncture in the treatment of postherpetic neuralgia. Zhongguo Zhen Jiu 1:4. [Google Scholar]
- Tian H. (2013). Efficacy and mechanism research on treating postherpetic neuralgia by blood-letting puncturing and cupping therapy. Ph.D. Dissertation. Beijing: China Academy of Traditional Chinese Medicine. [Google Scholar]
- van Hecke O., Austin S. K., Khan R. A., Smith B. H., Torrance N. (2014). Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 155 654–662. 10.1016/j.pain.2013.11.013 [DOI] [PubMed] [Google Scholar]
- Vickers A. J., Linde K. (2014). Acupuncture for chronic pain. JAMA 311 955–956. 10.1001/jama.2013.285478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Wan R., Chen S., Qin H., Cao W., Sun L., et al. (2022). Comparison of efficacy of acupuncture-related therapy in the treatment of postherpetic neuralgia: A network meta-analysis of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2022:3975389. 10.1155/2022/3975389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. (2006). Fire needling of blood stasis type of post-herpetic neuralgia clinical research. Ph.D. Dissertation. Chaoyang: Beijing University of Chinese Medicine. [Google Scholar]
- Wang J. Y., Gao Y. H., Qiao L. N., Zhang J. L., Duan-Mu C. L., Yan Y. X., et al. (2018). Repeated electroacupuncture treatment attenuated hyperalgesia through suppression of spinal glial activation in chronic neuropathic pain rats. BMC Complement. Altern. Med. 18:74. 10.1186/s12906-018-2134-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Fang Y. F., Li Q. J., Li J. W., Li W. L. (2020). Effect of pricking and cupping on serum substance P and IL-6 in patients with postherpetic neuralgia. Chin. Mod. Distance Educ. Tradit. Chin. Med. 16:92. [Google Scholar]
- Wang Y., Li W., Peng W., Zhou J., Liu Z. (2018). Acupuncture for postherpetic neuralgia: Systematic review and meta-analysis. Medicine (Baltimore) 97:e11986. 10.1097/MD.0000000000011986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Li X., Wang H., Liu Q., Shao L. (2019). Analysis of the risk factors for postherpetic neuralgia. Dermatology 235 426–433. 10.1159/000500482 [DOI] [PubMed] [Google Scholar]
- Weinke T., Glogger A., Bertrand I., Lukas K. (2014). The societal impact of herpes zoster and postherpetic neuralgia on patients, life partners, and children of patients in Germany. ScientificWorldJournal 2014:749698. 10.1155/2014/749698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R. J., Volpi A., McKendrick M., van Wijck A., Oaklander A. L. (2010). Management of herpes zoster and post-herpetic neuralgia now and in the future. J. Clin. Virol. 48 S20–S28. 10.1016/S1386-6532(10)70005-6 [DOI] [PubMed] [Google Scholar]
- Wu L. L., Zhao H. (2016). Clinical observation on postherpetic neuralgia with blood pricking and cupping. Hubei J. Tradit. Chin. Med. 11:57. [Google Scholar]
- Wu Q., Hu H., Han D., Gao H. (2021). Efficacy and safety of moxibustion for postherpetic neuralgia: A systematic review and meta-analysis. Front. Neurol. 12:676525. 10.3389/fneur.2021.676525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. H., Wen N. (2013). Clinical observation of reinforcing and reducing technique at Jia-ji and Ashi points for post-herpetic neuralgia. Beijing Tradit. Chin. Med. 1:23. 10.16025/J.1674-1307.2013.01.017 [DOI] [Google Scholar]
- Xie Z. J., Zhao J. J., Jiang M., Yuan P., Ma Y. H., Gao Q. Y., et al. (2018). Clinical study of surround needling combined with gabapentin treating PHN. Chin. J. Acupunct. Moxibustion 9:20. [Google Scholar]
- Xu X. P. (2020). Clinical observation on acupuncture treatment of postherpetic neuralgia based on the theory of “regulating the spirit”. Master Dissertation. Harbin: Heilongjiang University of Chinese Medicine. [Google Scholar]
- Xu Y. Y. (2018). Clinical research on treatment of postherpetic neuralgia with filiform-fire needle. Master Dissertation. Wuhan: Hubei University of Chinese Medicine. [Google Scholar]
- Yang Z. G., Pan C. L. (2016). Clinical effect of gabapentin combined with electro-acupuncture therapy for post-herpetic neuralgia. Clin. Med. Eng. 1 43–44+47. [Google Scholar]
- Yen L. T., Hsu Y. C., Lin J. G., Hsieh C. L., Lin Y. W. (2018). Role of ASIC3, Nav1.7 and Nav1.8 in electroacupuncture-induced analgesia in a mouse model of fibromyalgia pain. Acupunct. Med. 36 110–116. 10.1136/acupmed-2016-011244 [DOI] [PubMed] [Google Scholar]
- Yu S., Wan Y., Wan Q. (2016). Chinese expert consensus on the diagnosis and treatment of postherpetic neuralgia. Chin. J. Pain Med. 22 161–167. [Google Scholar]
- Yuan X. C., Zhu B., Jing X. H., Xiong L. Z., Wu C. H., Gao F., et al. (2018). Electroacupuncture potentiates cannabinoid receptor-mediated descending inhibitory control in a mouse model of knee osteoarthritis. Front. Mol. Neurosci. 11:112. 10.3389/fnmol.2018.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. (2013). Clinical study on the postherpetic neuralgia with round acupuncture and its effect on IL. Master Dissertation. Nanjing: Nanjing University of Chinese Medicine. [Google Scholar]
- Zhang H. L., Yin H. N., Yan M., Wang J. Z., Liu G. (2020). Clinical research of Fu’s subcutaneous needling combined with “countercurrent supplement of Ying” fire acupuncture in treating PHN. Clin. J. Acupunct. Moxibustion 11 38–41. [Google Scholar]
- Zhang L. Q., Ding F., Jiao Y. (2021). Clinical efficacy of pricking and cupping combined with pregabalin in patients with postherpetic neuralgia. Chin. Patent Med. 2:560. [Google Scholar]
- Zhang R., Lao L., Ren K., Berman B. M. (2014). Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 120 482–503. 10.1097/ALN.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X. (2015). Comparative study on therapeutic efficacies of different acupuncture methods for post-herpetic neuralgia. Shanghai J. Acupunct. Moxibustion 34:620. 10.1002/central/CN-01434782/full [DOI] [Google Scholar]
- Zhang Y., Huang L., Kozlov S. A., Rubini P., Tang Y., Illes P. (2020). Acupuncture alleviates acid- and purine-induced pain in rodents. Br. J. Pharmacol. 177 77–92. 10.1111/bph.14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Y., Liu L. Y., Cai J., Cui Y. J., Xing G. G. (2018). Electroacupuncture treatment alleviates the remifentanil-induced hyperalgesia by regulating the activities of the ventral posterior lateral nucleus of the thalamus neurons in rats. Neural Plast. 2018:6109723. 10.1155/2018/6109723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xu P., Bi J., Zhang S. N. (2020). Clinical effects of plum blossom needle acupuncture and cupping on patients with postherpetic neuralgia. Master Dissertation. Chengdu: Chengdu University of Traditional Chinese Medicine. [Google Scholar]
- Zhong H. (2019). Clinical observation on the milli-fire-needle in treating postherpetic neuralgia. Master Dissertation. Guangzhou: Guangzhou University of Chinese Medicine. [Google Scholar]
- Zhou Q., Wei S., Zhu H., Hu Y., Liu Y., Yang H., et al. (2021). Acupuncture and moxibustion combined with cupping for the treatment of post-herpetic neuralgia: A meta-analysis. Medicine (Baltimore) 100:e26785. 10.1097/MD.0000000000026785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. L., Huang Y. J. (2018). Clinical observation on the treatment of postherpetic neuralgia by acupuncture xin-shu and ge-shu points with fire needle. Tianjin Tradit. Chin. Med. 1:28. [Google Scholar]
- Zin C. S., Nissen L. M., Smith M. T., O’Callaghan J. P., Moore B. J. (2008). An update on the pharmacological management of post-herpetic neuralgia and painful diabetic neuropathy. CNS Drugs 22 417–442. 10.2165/00023210-200822050-00005 [DOI] [PubMed] [Google Scholar]
- Zou J., Dong X., Li Y., Tong S., Wang J., Liao M., et al. (2019). Deep sequencing identification of differentially expressed miRNAs in the spinal cord of resiniferatoxin-treated rats in response to electroacupuncture. Neurotox. Res. 36 387–395. 10.1007/s12640-019-00052-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.