Abstract
RBM46 is a germ cell-specific RNA-binding protein required for gametogenesis, but the targets and molecular functions of RBM46 remain unknown. Here, we demonstrate that RBM46 binds at specific motifs in the 3ʹUTRs of mRNAs encoding multiple meiotic cohesin subunits and show that RBM46 is required for normal synaptonemal complex formation during meiosis initiation. Using a recently reported, high-resolution technique known as LACE-seq and working with low-input cells, we profiled the targets of RBM46 at single-nucleotide resolution in leptotene and zygotene stage gametes. We found that RBM46 preferentially binds target mRNAs containing GCCUAU/GUUCGA motifs in their 3ʹUTRs regions. In Rbm46 knockout mice, the RBM46-target cohesin subunits displayed unaltered mRNA levels but had reduced translation, resulting in the failed assembly of axial elements, synapsis disruption, and meiotic arrest. Our study thus provides mechanistic insights into the molecular functions of RBM46 in gametogenesis and illustrates the power of LACE-seq for investigations of RNA-binding protein functions when working with low-abundance input materials.
Keywords: RBM46, LACE-seq, RNA-binding protein, meiosis, cohesin
Introduction
The RNA-binding motif protein (RBM) family is a subgroup of RNA-binding proteins (RBPs). RBMs contain multiple RNA-binding domains, among which RNA recognition motifs are the most common (Qin et al., 2020). Like other RBPs, RBM proteins are known to function in various RNA processes, including pre-mRNA splicing, RNA stability, mRNA translation, and RNA editing, thereby influencing multiple physiological processes (Li et al., 2021). As one example, RBM10 is a splicing factor responsible for SAT1 exon 4 skipping. Further, as it prevents SAT1 splicing changes in infected cells, RBM10 can limit viral replication (Pozzi et al., 2020). Studies of the RBM24 protein have shown that it modulates hepatitis B virus replication by targeting the viral RNA and inhibits core protein translation by targeting the terminal redundancy sequence (Yao et al., 2018). RBM47 is known to function in a variety of biological processes by regulating alternative splicing and RNA stability: it can inhibit breast cancer progression by stabilizing tumor suppressor mRNAs (Vanharanta et al., 2014) and has been shown to regulate multiple splicing events in nasopharyngeal carcinoma based on binding with another RBP-hnRNPM (Xu et al., 2021).
The term “cohesin” describes a variety of multisubunit complexes that are essential for sister chromatid cohesion. Cohesin complexes form ring structures to hold sister chromatids together, and this occurs in both mitosis and meiosis. However, there are both compositional and functional distinctions for cohesin complexes between meiosis and mitosis (Klein et al., 1999; Haering and Jessberger, 2012): five universal cohesin subunits (SMC1α, SMC3, RAD21, SA1/SA2) are required for proper cohesin complex function in both meiosis and mitosis (Eijpe et al., 2000; Xu et al., 2004). In mammalian meiosis, there are four meiosis-specific cohesin subunits, including one SMC protein (SMC1β), two kleisins (RAD21L and REC8), and a stromal antigen protein (STAG3) (Prieto et al., 2001; Revenkova et al., 2001; Lee et al., 2003; Lee and Hirano, 2011). The differential combination of specific cohesin subunits enables the formation of at least six cohesin complexes (Jessberger, 2011; Haering and Jessberger, 2012). In meiosis, cohesin complexes load onto sister chromatids during the premeiotic S phase and early meiosis I (leptotene stage) (McNicoll et al., 2013; Biswas et al., 2016), and studies have demonstrated how the meiosis-specific subunits confer specific functions in meiosis-related chromosomal events such as assembly of chromosome axial elements (AEs), DNA double-strand breaks (DSBs), and chromosome synapsis (Xu et al., 2005; Adelfalk et al., 2009; Herran et al., 2011; Llano et al., 2012; Fukuda et al., 2014; Ward et al., 2016).
Rbm46 is conserved among jawed vertebrates. In mice and most animals, its gene product comprises three RNA recognition motif domains and one C-terminal double-stranded RNA-binding motif (DSRM) domain (Fig. S1A). Microarray-based profiling study of 9- to 18-week human fetal ovaries revealed an increasing trend in RBM46 expression that steadily increases with gestational age (Houmard et al., 2009; Gerstberger et al., 2014). Single-cell RNA-sequencing analysis of juvenile and aged cynomolgus monkey ovaries found that RBM46 is highly expressed in oocytes of early follicle stages (Wang et al., 2020b). Dai et al., (2021) used TALENs to generate two rbm46 mutant zebrafish lines and reported that both rbm46 homozygous mutant zebrafish lines are infertile, failed to initiate meiosis, and did not produce any sperm, concluding that RBM46 may be involved in post-transcriptional regulation during spermatogenesis (Dai et al., 2021). However, these studies have not investigated the in vivo binding targets of RBM46. In this study, we performed high-resolution linear amplification of complementary DNA ends and sequencing (LACE-seq) to identify the targets of RBM46 in mice leptotene and zygotene spermatocytes. We show that RBM46 binding at specific motifs in the 3ʹUTRs of mRNAs encoding four meiosis-specific cohesin complex subunits that regulate the synaptonemal complex (SC) formation, thus explaining the shortened AEs and abnormal synapsis we observed in Rbm46 KO animals. Our study uncovers a post-transcriptional role for RBM46 in meiosis and characterizes RBM46 as a regulator of both male and female fertility.
Results
RBM46 is a germ-cell-specific cytoplasmic RBP required for gametogenesis
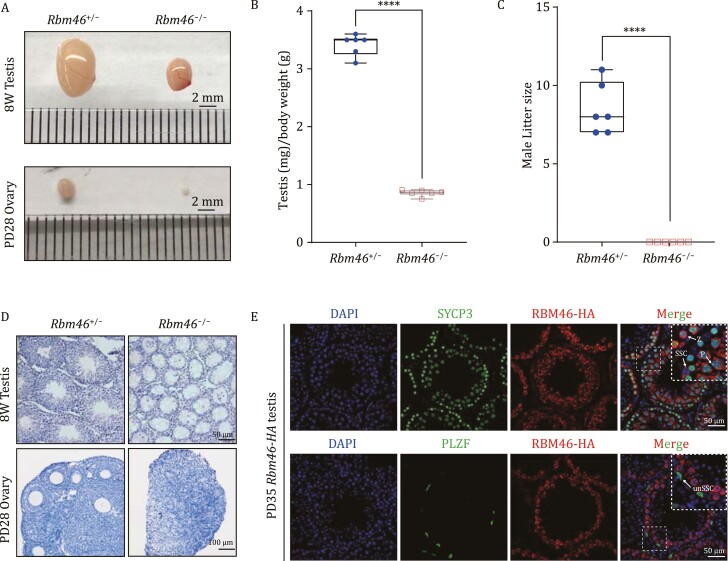
We used CRISPR/Cas9 to generate Rbm46 knockout (Rbm46−/−) mice, and immunoblotting of Rbm46−/− testes confirmed successful knockout (Fig. S1B). Upon examining reproductive organs, it was immediately obvious that the Rbm46−/− mice testes and ovaries were smaller than these organs in their respective littermate controls (Rbm46+/−), and the Rbm46−/− testes and ovaries weights were significantly lower than the Rbm46+/− organs (Fig. 1A and 1B). Consistent with these striking reproductive morphologies, fertility testing showed that neither male nor female Rbm46−/− mice were fertile (Figs. 1C and S3B). Further, histological analysis showed that the Rbm46−/− ovaries lacked oocytes and that the Rbm46−/− testes lacked spermatids in seminiferous tubules (Fig. 1D). These results support that RBM46 is essential for normal reproductive development and that RBM46 is required for both male and female fertility.
Figure 1.
Germ-cell-specific RBM46 is required for male and female fertility. (A) Morphological analysis of testes and ovaries showed that the Rbm46−/− mice testes and ovaries were smaller than respective littermate controls (Rbm46+/−). The scale bar is 2 mm. (B) The scatter plot showed the testis/body weight from 8-week-old Rbm46−/− mice compared with their littermate controls (n = 6). Student’s t-tests were performed, ****P < 0.0001. (C) Number of pups per litter from male mice (>8 weeks old) naturally crossed with WT female mice for 6 months (n = 6). Rbm46−/− male mice showed complete sterility. Student’s t-tests were performed, ****P < 0.0001. (D) Hematoxylin and eosin (H&E) staining of testes and ovaries in control and Rbm46−/− mice. Spermatids were absent in the seminiferous tubules of the adult Rbm46−/− mice and only a few spermatocytes were present. Oocytes and follicle structures were completely absent in the 1-month-old mice ovaries. (E) Immunofluorescence costaining RBM46-HA-tag (red) with the AR marker SYCP3 and undifferentiated SSC marker PLZF showing that RBM46 localization in the cytoplasm of germ cells in testis sections of RBM46-HA-tag mice. White arrows indicate germ cell stages: Z, zygotene spermatocytes; P, pachytene spermatocytes; unSSC, undifferentiated SSCs. The scale bar is 50 µm.
Immunoblotting of extracts from major organs of wild-type C57BL/6 mice supported the reproductive-organ-specific accumulation of the RBM46 protein. We also examined RBM46 in testes samples from postnatal day 3 up to 12 weeks, which revealed strong accumulation starting from postnatal day 8 (PD8), the developmental point at which spermatogonial stem cells (SSCs) begin to differentiate (Bellve et al., 1977). Note that we also detected RBM46 accumulation in wild-type E17.5 mice ovaries (Fig. S1B). These findings support that RBM46 is a reproductive-organ-specific protein that apparently functions in meiotic I. To profile the spatiotemporal expression and subcellular localization of RBM46 we used CRISPR/Cas9 to generate mice harboring an HA-tag fusion variant of RBM46. Immunofluorescence staining against the HA-tag, the meiotic AE marker SYCP3, and the undifferentiated SSC marker PLZF showed that RBM46 is a cytoplasmic RBP with weak accumulation in SSCs, with high accumulation in meiotic I spermatocytes, and with no detectable expression in spermatids (Fig. 1E).
The germ cells from Rbm46-null mice fail to progress through meiosis I
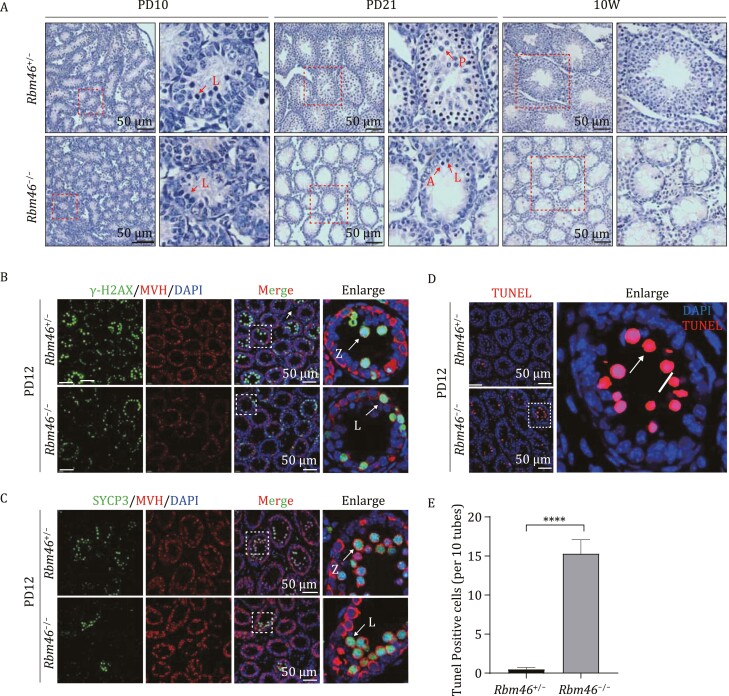
Given the observed fertility and spermatogenesis defects of the Rbm46−/− mice, we were interested in determining when the apparent gametogenesis dysfunction starts. Mice begin producing spermatocytes around postnatal day 10 (Feng et al., 2014); we sampled Rbm46+/− and Rbm46−/− testes at time points including PD8, PD10, PD12, PD21, and PD70 (10 weeks) and conducted histological analysis. We did not observe any obvious between Rbm46−/− testes and Rbm46+/− testes in PD8 mice. Defects were evident throughout this time course, starting with an obviously reduced number of spermatocytes entering meiosis in PD10 Rbm46−/− testes. And the reduction of meiotic spermatocytes became more severe in PD12 Rbm46−/− testes. At PD21, Rbm46+/− testes had normal round spermatids, whereas no round spermatids were observed in the Rbm46−/− mice. And these changes were even more pronounced in the adult (PD70) testes (Figs. 2A and S2A).
Figure 2.
RBM46 deletion causes meiosis arrest at the leptotene stage. (A) H&E staining of control and Rbm46−/− mice testes at PD10 (postnatal day 10), PD21, and at 10 weeks, showing inhibition of meiosis in Rbm46−/− testes. Red arrows indicate the representative stages of the spermatocytes, L, leptotene spermatocytes; P, pachytene spermatocytes; A, apoptotic cell. The scale bars are shown in the image. (B) Immunofluorescence staining for the DSB marker γ-H2AX (green) and the germ cell marker MVH (red) in controls (Rbm46+/−) and Rbm46−/− testes at PD12. Insets show an enlarged version of one seminiferous tubule. L, leptotene spermatocytes; Z, zygotene spermatocytes. The scale bar is 50 µm. (C) Immunofluorescence staining for the AE marker SYCP3 (green) and the MVH (red) in controls (Rbm46+/−) and Rbm46−/− testes at PD12. Insets show an enlarged version of one seminiferous tubule. The scale bar is 50 µm. (D) Tunnel staining showed that there were more apoptotic cells in the seminiferous tubule of Rbm46−/− testis than in control testis; the enlarged region highlights that all of the apoptotic cells are apparently leptotene or zygotene spermatocytes. Arrow, an apoptotic cell. (E) Quantification of the number of apoptotic cells in seminiferous tubules in PD12 testes. Ten adjacent tubules were examined as one group, and three testis sections were examined from control and Rbm46−/− mice. Student’s t-tests were performed, ****P < 0.0001.
We also conducted histological analysis of Rbm46+/− and Rbm46−/− ovaries sampled across a developmental series and found that Rbm46+/− ovaries underwent normal development, whereas few meiotic cells were present in E17.5 Rbm46−/− ovaries, and no oocytes were observed in postnatal mice ovaries (Fig. S3A). To observe the potential effect(s) of RBM46 on SSC maintenance and/or differentiation we performed immunofluorescence staining of PD6 and PD10 testes against the undifferentiated SSC marker PLZF and the differentiated SSCs marker LIN28. The intensity of the PLZF signal did not change between Rbm46+/− and Rbm46−/− testes at PD6 and PD10, whereas the LIN28 signal was slightly decreased in PD10 Rbm46−/− testes (Figs. S4A and 4B). These results indicate that RBM46 has a slight effect on differentiated SSCs, but no significant effect on the maintenance of undifferentiated SSCs.
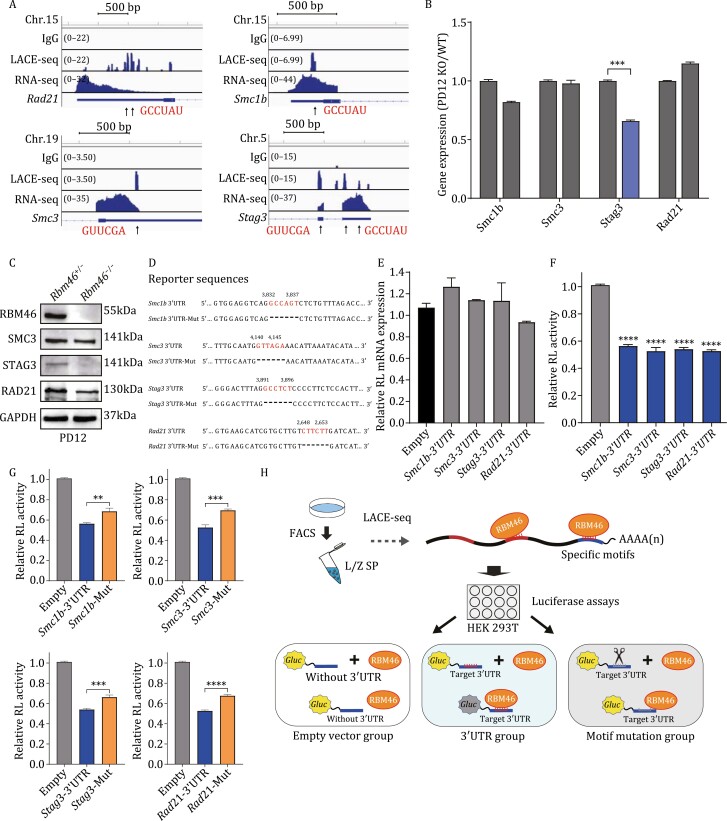
Figure 4.
RBM46 binds at specific motifs in the 3ʹUTRs of mRNAs encoding multiple subunits of meiotic cohesin complexes and regulates their translation. (A) Genome browser tracks showing LACE-seq-binding peak distributions and transcript levels of the four meiotic cohesin mRNAs encoded by the mouse genome. Predicted RBM46-binding sites are present in the 3ʹUTRs of Rad21, Smc3, and Smc1b; Stage3 have binding sites both in 3ʹUTRs and CDS region. (B) qPCR to measure the expression of the four indicated genes encoding meiotic cohesin complex subunits in PD12 Rbm46−/−mice and in their respective littermate Rbm46+/− controls. Data are shown as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001; Student’s t-tests. (C) Immunoblotting showing that the protein levels of the three indicated meiotic cohesin complex subunits are significantly reduced in PD12 Rbm46−/− testes compared with Rbm46+/− controls. (D) Predicted RBM46-binding motifs and sequences at the 3ʹUTRs of Smc1b, Smc3, Stag3, and Rad21 and mutated 3ʹUTR renilla luciferase reporter sequences. (E) qPCR showing that the mRNA levels of luciferase reporter genes were not changed. **P < 0.01, ***P < 0.001, ****P < 0.0001; Student’s t-tests. (F) The relative luciferase reporter activity of target cohesin genes showed that RBM46 could specifically bind to their 3ʹUTRs. The luciferase reporter mRNAs containing the 3ʹUTR sequence of target cohesin genes and RBM46 vehicle were cotransfected into the HEK 293 T cells. The renilla luciferase reporter activities were normalized to the SEAP activities. The empty luciferase vehicle cotransfected with RBM46 vehicle served as the control. (G) The decreased luciferase activities were partially rescued in cells cotransfected with the deletion variant control vectors for the Rad21, Smc1b, Smc3, and Stag3 3ʹUTRs. (H) Schematic illustration of the luciferase experimental workflow.
We subsequently conducted immunofluorescence staining of seminiferous tubule sections of PD12 testes with the DSB marker γ-H2AX and the AE marker SYCP3. Both markers were detected starting from the early stage of meiosis I (Scherthan et al., 1996; Mahadevaiah et al., 2001), and revealed an obvious reduction in the number meiotic spermatocytes in the Rbm46−/− testes compared with the Rbm46+/− testes (Fig. 2B and 2C). Additionally, the distribution of SYCP3 in Rbm46−/− spermatocytes was diffuse compared with the linear SYCP3 pattern in Rbm46+/− spermatocytes (Fig. 2C). An obvious reduction in the number of meiotic oocytes was also observed in a comparison of Rbm46−/− and Rbm46+/− E17.5 ovaries (Fig. S5A). Consistent with the dearth of spermatocytes in PD12 Rbm46−/− testes, TUNEL staining with quantification showed a significant increase in the number of apoptotic cells in the seminiferous tubules of the Rbm46−/− mice compared with the Rbm46+/− mice (Fig. 2D and 2E). Moreover, staining with the aforementioned γ-H2AX and SYCP3 markers revealed that all of the TUNEL-positive cells were leptotene or zygotene stage spermatocytes (Fig. 2B and 2C). Thus, Rbm46−/− gametes fail to progress through meiosis I.
Profiling RBM46-binding sites by LACE-seq
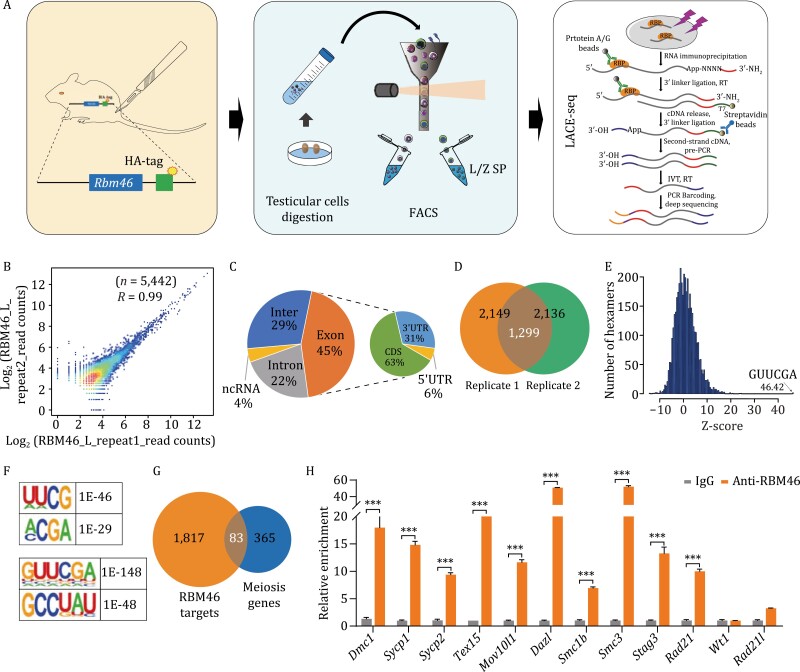
Given the predicted function of RBM46 as an RBP (Gerstberger et al., 2014; Table S1), and empowered by very recent methodological progress in the field of RBP target profiling (Su et al., 2021), we pursued the mechanistic basis of the observed meiotic arrest by conducting LACE-seq analysis. Very briefly, this technique exploits an in vitro transcription (IVT) approach to amplify trace amounts of cDNAs from single or dozens of cells and can identify the target molecules of RBPs based on ultraviolet (UV) light crosslinking and reverse transcription (RT) of immunopurified protein–RNA complexes (Fig. 3A). We examined leptotene/zygotene spermatocytes isolated by flow cytometry from PD12 testes of the HA-tagged RBM46 mice (i.e., the time point when meiosis I advances to the zygotene stage) (Bellve et al., 1977). The two testes of each mouse were pooled together as one sample, and the single-cell samples isolated by flow cytometry were divided into three groups (each with two biological replicates): an RNA-seq group to establish the background RNA levels, a control group (treated with IgG), and the RBM46 experimental group (treated with HA antibody to pull-down RNAs bound by HA-tagged RBM46).
Figure 3.
Identification of RBM46 targets of mice testes in L/Z spermatocytes by LACE-seq. (A) Schematic illustration of the experimental workflow. Testes from PD12 RBM46-HA-tagged mice were collected and digested into single cells, after which flow cytometry was used to isolate L- and Z-stage spermatocytes. We subsequently conducted UV crosslinking of these cells and used LACE-seq to identify target RNA molecules (and putative binding sites) of RBM46. L/Z SP, leptotene and zygotene spermatocytes. (B) The LACE-seq was highly correlated in two biological replicates, R = 0.99. (C) Genomic distribution of the RBM46 LACE-seq reads in L/Z spermatocytes. Data are plotted using the mean value from two biological replicates. (D) Venn diagram of two biological replicates of RBM46-binding peaks. (E) Histogram showing overrepresented RBM46-binding motifs of identified by LACE-seq using a K-mer algorithm. The Z-score of the top hexamer is indicated. (F) RBM46-binding motifs identified by LACE-seq using HOMER. (G) 83 genes were identified upon assessing the intersection of candidate LACE-seq target RNA molecules in L/Z spermatocytes and genes with annotated functions in meiosis in the Ingenuity Pathway Analysis (IPA) software. (H) RIP-qPCR confirmed that RBM46 binds to RNA transcripts of 10 putative target mRNAs expressed during meiosis. Among the mRNAs examined the RIP-qPCR data for 10 putative targets showed a >5-fold changed (calculated by 2−ΔΔCt) compared with the IgG control, and were therefore considered as “confirmed targets.” In contrast, no enrichment was detected for two nontarget mRNAs that showed a <5-fold changed. Student’s t-tests were performed and data are shown as the mean ± SEM of three independent experiments. ***P < 0.001.
The two biological replicates of the RBM46 experimental group showed strong correlations in the detected putative binding target RNA molecules (R = 0.99) (Fig. 3B). We examined the genomic distribution of the RBM46 LACE-seq reads and found that 45% of the putative binding sites were located in the CDS and 3ʹUTR regions of mRNAs (63% of the RBM46 LACE-seq reads mapped to the CDS; 31% mapped to 3ʹUTR regions) (Fig. 3C). There were a total of 1,299 candidate target RNA molecules common to the two examined replicates (from among 2,149 and 2,136 in each sample) (Fig. 3D and Table S3). Note that this number of target molecules is similar to a previously reported LACE-seq-based analysis of the RBP AGO2 in murine oocytes, and the number of reads for each RBM46 sample (more than 107) is similar to a LACE-seq-based analysis of PTBP1 in HeLa cells (Su et al., 2021).
We employed a K-mer algorithm (Berger et al., 2006) to identify the consensus sequence of RBM46-binding sites and discovered the mRNA motifs were enriched in RBM46-binding sites. The top hexamer was GUUCGA, with a Z-score of 46.42 (Fig. 3E). We also used HOMER (Heinz et al., 2010) to identify the sequence, and GUUCGA also ranked highest among the most enriched 6-mer motif with a P value of 1 × 10−148 (Fig. 3F). GCCUAU ranks second with P = 1 × 10−48. Of particular note, 36.91% of all RBM46 peaks contain a GCCUAU site, and 6.77% of RBM46 peaks contain an GUUCGA site. Among the most enriched 4-mer motifs, UUCG ranked highest with a P value of 1 × 10−46 (Fig. 3F).
RBM46-binding targets are enriched for meiosis-related functions
Given our finding that RBM46 deletion results in meiosis arrest at the meiosis I, we focused on meiosis-related molecules among the putative RBM46-target molecules. An analysis using Ingenuity Pathway Analysis (IPA) software (Kramer et al., 2014; Wang et al., 2021) identified 83 molecules with meiosis-related functional annotations among the 1,900 mRNA hits from the LACE-seq analysis for RBM46-binding targets (Fig. 3G). Among these putative targets, we selected 10 molecules for binding validation in PD12 wild-type C57 mouse testes using RNA immunoprecipitation (RIP) assays (Keene et al., 2006); we also measured the binding capacity of two control molecules, a Sertoli cell-specific marker (Wt1) and a cohesin subunit (Rad21l, i.e., which was not among the putative RBM46-binding targets) (Fig. 3H). All 10 of the putative targets were enriched in the anti-RBM46 immunoprecipitant compared with the IgG control; no enrichment was detected for Wt1 or Rad21l. These findings support that RBM46 interacts in vivo with the meiosis-related mRNAs identified in the LACE-seq analysis.
RBM46 binds at the 3ʹUTR of mRNAs encoding multiple subunits of meiotic cohesin complexes and regulates their translation
Cohesin complexes form ring structures to hold sister chromatids together during mitosis and meiosis, and studies have shown that abnormal formation of cohesin complexes can block meiosis I (from leptotene to pachytene) (Klein et al., 1999; Haering and Jessberger, 2012; Biswas et al., 2016). Among the validated RMB46-binding targets were mRNAs encoding four known cohesin subunits that were enriched in an IPA analysis of Canonical Pathways (Fig. 3H and Table S4). Two of these are meiosis-specific cohesin subunits (Smc1b and Stag3), while the other two are subunits of ubiquitous cohesin complexes (Rad21 and Smc3). Previous studies have shown that the absence of the meiosis-specific cohesin subunit Smc1b results in meiotic arrest in pachytene (Revenkova et al., 2001) and that the absence of Stag3 results in meiotic arrest in leptotene (Prieto et al., 2001).
We analyzed the LACE-seq-binding peaks and transcript levels of the four RBM46-target cohesin mRNAs (Fig. 4A), and the nontarget cohesin Rad21l mRNA as a negative control (Fig. S4A). Rad21l had no obvious RBM46-binding peaks; Stag3 had binding peaks in both its 3ʹUTR and CDS; Rad21, Smc3, and Smc1b had binding peaks in their 3ʹUTRs. Integrative Genomics Viewer (IGV) (Robinson et al., 2011) based analysis of the preferential binding sequences located within the binding peaks identified GCCUAU and GUUCGA as apparent RBM46-binding motifs (Fig. 4A). Our results thus support that RBM46 preferentially interacts with GCCUAU/GUUCGA motifs in the 3ʹUTRs of mRNA encoding cohesin subunits, suggesting that RBM46 functions in regulating sister chromatid cohesion and synapsis.
To investigate the potential post-transcriptional function of RBM46 in regulating targets in mice gametogenesis we examined RBM46-target mRNAs encoding meiotic cohesin complex subunits at both the mRNA and protein levels. qPCR analysis of PD12 testes (when meiosis advances to the zygotene stage) from Rbm46+/− and Rbm46−/− mice detected no differences in the levels of mRNA transcripts for Smc1b, Smc3, or Rad21, while Stag3 mRNA levels in Rbm46−/− testes displayed a significant reduction (Fig. 4B). Immunoblotting revealed obvious differences between the Rbm46+/− and Rbm46−/− PD12 testes: the protein levels of SMC3, RAD21, and STAG3 were decreased in the RBM46 KO samples (Fig. 4C). These results indicate RBM46 is a positive regulator of mRNA translation and that deletion of RBM46 in mice testes results in a decrease in the protein levels of its mRNA targets.
RBM46 binds cohesin subunit mRNAs at the 3ʹUTR via GCCUAU/GUUCGA motifs
Recalling the predicted RBM46-binding motifs in the cohesin subunits mRNAs (Fig. 4A), we conducted dual-luciferase reporter assays to help delineate the regulatory impacts of RBM46 for these specific mRNAs. Dual-luciferase assays were conducted to assess the RBM46 motifs in vitro; we inserted the cloned 3ʹUTR regions containing the RBM46-binding motifs into the MCS2 region of the pEZX-GA02 vector (downstream of the sequence encoding a luciferase reporter). These assays examined 3ʹUTR fragments of four cohesin subunit mRNAs, including Smc3 and Rad21 (containing GUUCGA motifs) and Smc1b and Stag3 (GCCUAU motifs) (Fig. 4D); we also prepared deletion variant control plasmids for each examined 3ʹUTR fragment (e.g., “Smc1b 3ʹUTR-Mut”). HEK 293T cells were then cotransfected with an RBM46 overexpression vector and one of the vectors bearing the 3ʹUTR fragment of the cohesin subunit (or an empty vector control).
While we observed no difference in the expression levels of the reporter mRNAs (Fig. 4E), the luciferase activities in the cells with the RBM46-binding motifs were reduced by between 50% and 60% compared with the empty vector control cells (Fig. 4F). Moreover, the decreased luciferase activities were partially rescued in cells cotransfected with the deletion variant control vectors for the Rad21, Smc1b, Smc3, and Stag3 3ʹUTRs (Fig. 4G). These findings demonstrate that RBM46 binds to GCCUAU/GUUCGA motifs in 3ʹUTRs in vivo and indicate that such binding disrupts the translation of mRNA transcripts bearing these motifs (Fig. 4H).
Rbm46 −/− arrested spermatocytes have shortened AEs and display partial synapsis
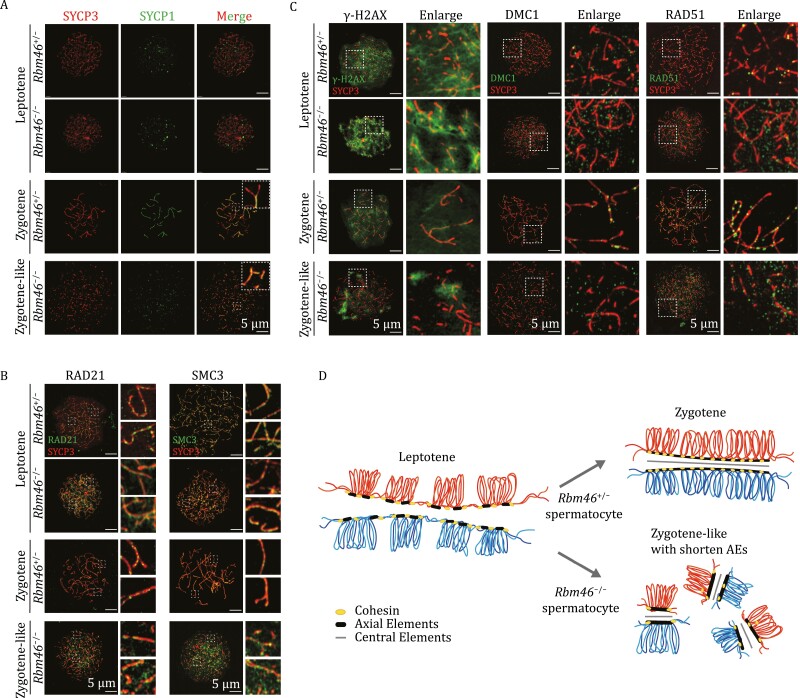
We next investigated how loss of RBM46 results in spermatocyte arrest during meiosis I. We narrowed our focus to specific meiotic stages in chromosome spreads prepared from PD35 Rbm46+/− and Rbm46−/− mice testes using immunofluorescence staining against SYCP1 and SYCP3. For context, cohesin complexes together with other axial proteins such as the SC protein SYCP3 form the two lateral elements of the SC (also referred to as AEs upon completion of synapsis); these are bridged together by the central element protein, SYCP1, and this bridging is known to stimulate synapsis of homologous chromosomes (Meuwissen et al., 1992; Yuan et al., 2000). SYCP3 is expressed starting from the preleptotene stage (Scherthan et al., 1996), while SYCP1 marks synapsed chromosomes and are expressed starting from the early zygotene stage (de Vries et al., 2005).
A previous study conducted costaining of Rbm46+/− spermatocyte chromosome spreads and observed that chromosome axes were positive for both SYCP1 and SYCP3 in zygotene spermatocytes, whereas leptotene spermatocytes were only positive for SYCP3 (Meuwissen et al., 1992; Cahoon and Hawley, 2016). In Rbm46−/− mouse spermatocytes, we observed leptotene spermatocytes with the expected SYCP3 localization, but no spermatocytes developed beyond the zygotene stage (Fig. 5A). Note that we also observed that oocytes arrest at leptotene stage in E17.5 Rbm46−/− mice ovaries (Fig. S5A). We did observe many abnormal spermatocytes displaying shortened chromosome axes (indicated by a fragmented SYCP3 signal), and some of these abnormal spermatocytes had a signal for SYCP1 (Fig. 5A). We refer to these spermatocytes present in the Rbm46−/− testes as “zygotene-like” spermatocytes. Specifically, the presence of these spermatocytes indicates that a partially synapsed, SC-like structure was initiated, but failed to progress normally toward the zygotene stage.
Figure 5.
Rbm46 −/− arrested spermatocytes have shortened AEs and display partial synapsis. (A) Chromosome spreads of PD35 Rbm46+/− and Rbm46−/− spermatocytes costained with the chromatid central element marker SYCP1 (green) and the AE marker SYCP3 (red), showing that Rbm46−/− spermatocytes arrest at the leptotene stage and present “zygotene-like” spermatocytes. The scale bar is 5 µm. (B) Chromosome spread of Rbm46+/− and Rbm46−/− mice spermatocytes costained with SYCP3 (red) and cohesin subunits RAD21 and SMC3 in leptotene and zygotene or zygotene-like spermatocytes. The scale bar is 5 µm. (C) Chromosome spread of Rbm46+/− and Rbm46−/− mice spermatocytes costained with SYCP3 (red) and γ-H2AX as indicated for DSB signal; DMC1 and RAD51 as indicated for DSB-repair foci. The scale bar is 5 µm. (D) Model of RBM46 functional mechanism in meiosis. Abnormal distribution of cohesin subunit proteins was observed in Rbm46−/− mice spermatocytes, and chromosomes showed shortened AEs and partial synapsis, resulting in failure of meiosis to progress to the zygotene stage.
Next, we observed changes in RBM46-target cohesin subunits during meiosis by costaining RAD21 and SMC3 with SYCP3 in Rbm46−/− and Rbm46+/− spermatocytes (Fig. 5B). Compared with the Rbm46+/− chromosomal uniform point distribution, RAD21 was not clearly clustered, but rather diffused around chromosomes in both Rbm46−/− leptotene and zygotene-like spermatocytes. The localization of SMC3 on chromosomes did not differ significantly between Rbm46−/− and Rbm46+/− leptotene, but the loci signal appears to be partially localized on chromosomes in zygotene-like spermatocytes of Rbm46−/− mice (Fig. 5B). These results suggest that deletion of Rbm46 disrupts the loading of cohesin subunits onto chromosomes.
Meiotic recombination is initiated by programmed DSBs (Siegel et al., 1992). We then examined the effect of Rbm46 deletion on DSB processes, as cohesin plays an important role in DSB repair. We costained PD35 Rbm46+/− and Rbm46−/− mice testes against SYCP3 and the unsynapsed chromosome region marker γ-H2AX (Fig. 5C). All of the Rbm46−/− spermatocytes were positive for γ-H2AX staining at leptotene, and the γ-H2AX signal intensity was partially reduced in the zygotene-like spermatocytes. We also stained against the DSB-repair proteins DMC1 and RAD51 and found no differences in signal intensities between Rbm46−/− zygotene-like and Rbm46+/− zygotene spermatocytes (Fig. 5C). However, without normal AEs in Rbm46−/− zygotene-like spermatocytes, accumulation of foci on axes was obviously reduced, and the DSBs were not processed normally (as the homologous partner chromosome was not available owing to incomplete synapsis of the two homologous pairs of sister chromatids). These results indicate that although DSB formation proceeds normally in Rbm46−/− testes, the formed DSBs cannot be repaired normally owing to the failure of synapsed chromosome formation.
Discussion
We generated Rbm46 knockout mice and observed their spermatocytes arrest in early meiosis. And found that RBM46 KO spermatocytes cannot synapse properly and fail to progress toward the zygotene. To investigate the mechanism(s) through which RBM46 KO causes meiotic arrest, we sorted wild-type L/Z spermatocytes by flow cytometry and used LACE-seq to profile RNA-binding targets of RBM46. Analysis of the LACE-seq-binding peaks showed that RBM46 binds to specific motifs (GCCUAU/ GUUCGA) in the 3ʹUTRs of mRNAs for cohesin subunits including Rad21, Smc3, Smc1b, and Stag3. The knockout of Rbm46 disrupts the assembly of axil elements and of synapses by reducing the protein translation of cohesin subunits.
RBPs are commonly divided into nuclear RBPs and cytoplasmic RBPs: nuclear RBPs are known to function in (e.g.) regulating nascent mRNA (pre-mRNA) and various processing events including capping, polyadenylation, and splicing; cytoplasmic RBPs often bind mature mRNA sequences as they are released from the nucleus, and have been shown to function in directing mRNA transport, regulating interactions with translation machinery (competitively or cooperatively), and regulating mRNA stability (Sutherland et al., 2015).
Numerous studies have reported that cytoplasmic RBPs can regulate mRNAs stability and translation by binding to the 3ʹUTR regions of target mRNAs. For example, NANOS2 is an RBP essential for the maintenance of SSCs through the regulation of target mRNAs stability. Using the CRAC technique, 81% of NANOS2-binding sites were identified as located in the 3ʹUTR of transcripts by a consensus motif AUKAAWU (K = G or U, W = A or U) (Codino et al., 2021). LIN28A is a positive regulator of mRNA translation in various cells (Polesskaya et al., 2007; Jin et al., 2011). HITS-CLIP experiments showed that LIN28A preferentially binds to the 3ʹUTR regions of several meiosis-associated mRNAs, and dual-luciferase assays confirmed that LIN28A binds a GGAG(A) motif in the 3ʹUTRs to regulate its target mRNAs (Wang et al., 2020a).
In cynomolgus monkey ovaries, RBM46 was observed as localized to the oocyte cytoplasm (Wang et al., 2020b). Our data for the subcellular localization of RBM46 in mouse testes indicate that RBM46 is localized in the cytoplasm of SSCs and meiotic I spermatocytes. Given this localization pattern, it is reasonable to infer that RBM46 may affect the translation of its binding target mRNA transcripts and/or may regulate their mRNA stability. In our study, two hexamer motifs (GUUCGA and GCCUAU) were identified as enriched at RBM46-binding sites, and dual-luciferase assays experimentally confirmed the specificity of RBM46 binding and demonstrated that RBM46 binding at these 3ʹUTR sites promotes the translation of bound mRNA molecules.
The functions of multiple subunits of the meiosis-specific cohesin complex have been investigated in knockout mice. Smc1b-null mice showed a phenotype of shortened chromosome axes and incomplete synapses, and also displayed aberrant telomere integrity (Revenkova et al., 2001; Adelfalk et al., 2009). Rec8-null mice also displayed shortened chromosome axes in meiotic cells, and had defects in sister SC assembly and repair of DSB (Lee et al., 2003; Xu et al., 2005). Rad21L-null mice displayed fragmented chromosome axes, nonhomologous synapsis, and impaired DSB repair (Herran et al., 2011; Lee and Hirano, 2011). Stag3-null mice displayed chromosome axis compaction, aberrant synapsis, impaired recombination, and developmental arrest (Prieto et al., 2001; Fukuda et al., 2014). Notably, Rec8 and Rad21L double-null mice exhibited a more severe phenotype than the corresponding single mutants, failing to form meiotic chromosome axes and failing to assemble AEs or SCs (Llano et al., 2012). Another study reported that double mutants of Stag3 with either Rec8 or Rad21L mice displayed defective centromeric cohesion and very short AEs, similar phenotypes to the Rec8 and Rad21L double-null mice (Ward et al., 2016).
In the present study, we observed that protein levels of cohesin subunits SMC3, STAG3, and RAD21 were decreased in PD12 Rbm46−/− testes. Chromosome spreads indicated that the localization of RAD21 and SMC3 in Rbm46−/− spermatocytes was diffuse as compared with the foci signal observed in Rbm46+/−. Knockout of RBM46 disrupts the assembly of SCs due to shortened AEs compared with normal chromosomes, which is similar to the reported phenotypes in Stag3 and Rad21L null mice (Jessberger, 2011; Fukuda et al., 2014). The knockout of Rbm46 reduces the translation of cohesin subunits and disrupts the assembly of AEs and synapses; these collectively result in the failure of meiosis to progress normally to the zygotene stage. Interestingly, unlike the other three cohesin subunits, we observed a decrease in Stag3 mRNA expression. It is known that the regulation of spermatogenesis by RBPs is complex (Legrand and Hobbs, 2018). The decreased expression of Stag3 may be due to the multiple binding sites of RBM46 on Stag3, including CDS and 3ʹUTRs regions. It is also possible that RBM46 interacts with other molecules and affects the transcription of Stag3, which needs further study.
It is clear that some tissues tend to be enriched for specialized RBPs: 90% of the 82 tissue-specific RBPs detected to date in human were identified specifically in the germline, brain, muscle, bone marrow, or liver cells. Of particular note, 57% of these (47 proteins) show enrichment in adult testis, where they have been variously shown to function in gametogenesis and fertility by regulating meiosis, stem cell maintenance, and differentiation (Kang and Han, 2011; Luteijn and Ketting, 2013; Gerstberger et al., 2014).
Characterization studies using transgenic models of reproductive system-expressed RBPs disrupted commonly present an infertile phenotype with spermatogenesis arrest. For example, the germline-specific RBPs DAZ and BOULE are required for male fertility (Reijo et al., 1995; VanGompel and Xu, 2010), while DAZL is required for both male and female fertility, and is known to influence both embryonic germ cell development and differentiation. During spermatogenesis, DAZL binds to at least 1,325 testicular mRNA transcripts at their 3ʹUTR regions (Li et al., 2019); this binding stimulates their translation by recruiting PABPs to these target mRNAs. Notably, DAZL binding had no obvious effects on the cellular levels of the mRNA transcripts per se (Li et al., 2019). Additional RBPs are also known to function in spermatogenesis, including DDX5, which exerts multiple functions in RNA splicing, export, and stability and is essential for the maintenance of spermatogenesis (Legrand et al., 2019). The RBM family protein RBM5 was identified as a male germ cell splicing factor required for spermatid differentiation, and knockout of RBM5 results in spermatogenesis arrest at the spermatid stage (O’Bryan et al., 2013). An infertility phenotype was reported for zebrafish lacking RBM46; RBM46 KO animals failed to initiate meiosis (Dai et al., 2021). In the present study, we found that both male and female Rbm46−/− mice were infertile and observed arrested meiosis in spermatocytes/oocytes at the leptotene stage.
RNA immunoprecipitation with sequencing (RIP-seq) and crosslinking immunoprecipitation coupled with high-throughput sequencing (CLIP-seq or HITS-CLIP) are two experimental approaches for identifying RBP target molecules (Licatalosi et al., 2008; Zhao et al., 2010). Several related methods have been developed seeking to reduce the required amount of input cells, such as tRIP-seq and TRIBE (McMahon et al., 2016; Masuda et al., 2020). When studying reproductive development, it is difficult to collect a large amount of cell materials at specific stages. To date, most target profiling studies of reproductive system-expressed RBPs have employed CLIP-seq. In a DAZL target profiling study, 16 testes were pooled together as one sample for identifying DAZL targets during spermatogenesis (Li et al., 2019); however, heterogeneity in the sampled developmental stages of the targeted cells (germ cells) and inclusion of other cell types (i.e., Sertoli cells, hemocytes) can reduce the accuracy of such results. Another study sought to identify MIWI targets from isolated, stage-specific spermatids using a unit gravity sedimentation method (Gou et al., 2014; Zhang et al., 2015). While this method is better than using whole testes, it requires a large number of animals.
In the present study, we used LACE-seq, a method to identify RBP-binding sites in as few as a single cell to accurately study the targeting of RBM46 in leptotene and zygotene spermatocytes. LACE-seq achieves a high repeatability and stability and found that RBM46 can bind to specific motifs (GCCUAU/GUUCGA) in the 3ʹUTRs of target RNA molecules. We believe that LACE-seq opens the door to studying the roles of RBPs in previously uncharted cell type and can expedite the discovery of RNA regulatory networks in various diseased and healthy cells.
Materials and methods
Mice generation and maintenance
Genomic DNA fragments covering exons 2–4 were deleted using a CRISPR-Cas9-mediated genome editing system (Cyagen Biosciences, USA) to generate a mouse model of Rbm46−/− in a C57BL/6 genetic background. The founders were tested by genotyping with polymerase chain reaction (PCR) and the following DNA sequencing analysis. RBM46-N-HA mice were supplied by Genome Tagging Project (GTP) Center, CEMCS, CAS, which was supported by Shanghai Municipal Commission for Science and Technology Grants (19411951800). The HA protein was coexpressed with RBM46 protein by using CRISPR/Cas9 technology without affecting the expression of RBM46 protein by inserting the HA-tag protein gene sequence in the N terminal of RBM46 protein. Mice housing and all experimental protocols were approved by the Regional Ethics Committee of Shandong University.
Tissue collection and histological analysis
Testes of mice were removed immediately after euthanasia by dissection, fixed in 4% (mass/volume) paraformaldehyde (Solarbio, Beijing, China, P1110) and embedded in paraffin after dehydration. 5 µm sections were prepared and mounted on glass slides. After degreasing the sections were stained with hematoxylin for histological analysis. The sections were also used for immunofluorescence analysis and TUNEL staining. The sections were dewaxed, rehydrated, and following treated with 10 mmol/L sodium citrate buffer (pH 6.0) for 15 min in boiling water. After cooled to room temperature, the sections were treated with phosphate-buffered saline (PBS) containing 0.1% Triton X-100, and then washed three times with PBS (pH 7.4). After blocking with 5% bovine serum albumin, primary antibodies were added to the sections and incubated overnight at 4°C, followed by incubation with Alexa Fluor 488- or 594-conjugated secondary antibodies (1:500 dilution, Abcam; ab150080, ab150120, ab150117). The primary antibodies for immunofluorescence included Anti-phospho-Histone H2A.X (Ser139) Antibody (1:1,000 dilution; Millipore, 05-636), Anti-DDX4/MVH (1:1,000 dilution; Abcam, ab13840), Anti-GCNA1 antibody (1:200 dilution; Abcam, ab82527), HA-tag (1:500 dilution; Cell Signaling Technology, 3724), and mouse anti-SYCP3 (1:500 dilution; Abcam, ab97672). After being washed with PBS three times, the slides were mounted using Mounting medium with DAPI-aqueous, fluoroshield (Abcam, ab104139). TUNEL staining was performed followed the manufacturer’s instructions (KeyGEN BioTECH, KGA7072).
Chromosome spread and immunofluorescence analysis
Spermatocyte spreads were prepared as previously described by Peters et al. (1997). Primary antibodies used for immunofluorescence were as follows: rabbit anti-SYCP3 (1:500 dilution; Abcam ab15093), mouse anti-SYCP3 (1:500 dilution; Abcam, ab97672), rabbit anti-SYCP1 (C-terminal) (1:2,000 dilution; Abcam, ab15090), Anti-phospho-Histone H2A.X (Ser139) Antibody (1:1,000 dilution; Millipore, 05-636), rabbit anti-DMC1 (1:100 dilution; Santa Cruz Biotechnology, sc-22768), rabbit anti-RAD21(1:100 dilution; Abclonal, A18850), rabbit anti-RAD51 polyclonal antibody (1:200 dilution; Thermo Fisher Scientific, PA5-27195) Primary antibody incubated overnight at four degrees in the refrigerator and were detected with Alexa Fluor 488- or 594-conjugated secondary antibodies for 1 h at room temperature. After being washed with PBS several times, the slides were mounted using Mounting medium with DAPI-aqueous, fluoroshield (Abcam, ab104139).
Western blot
Tissues were collected to prepare protein extracts from C57BL/6 mice and lysed in TAP lysis buffer containing 50 mmol/L HEPES–KOH, pH 7.5, 100 mmol/L KCl, 2 mmol/L EDTA, 10% glycerol, 0.1% NP-40, 10 mmol/L NaF, 0.25 mmol/L Na3VO4 and 50 mmol/L β-glycerolphosphate plus protease inhibitors (Roche, 04693132001). After homogenization, the cell extracts were placed on ice stand for 30 min, followed by centrifugation at 4°C at 13,000 ×g for 20 min. The supernatant was extracted for immunoblotting assays. Equal amounts of proteins were electrophoretic on a 10% BIS-TRIS protein gel (Invitrogen, NP0315) and the bands were transferred to a polyvinylidene fluoride film (Millipore). The primary antibodies for immunoblotting included RBM46 (1:500 dilution; Diaan Wuhan, customed), RAD21 (1:500 dilution; Abclonal, A18850), and GAPDH (1:5,000 dilution; Abways, AB0037).
Fluorescence-activated cell sorting of spermatocytes
Fluorescence-activated cell sorting (FACS) purification was conducted as previously described to collect leptotene/zygotene spermatocytes from PD12 HA-RBM46 mice for LACE-seq (Bastos et al., 2005; Gaysinskaya et al., 2014; Lima et al., 2017). After removing the tunica albuginea, the testes were placed in 5 mL PBS containing collagenase type I (120 U/mL) and mixed for 10 min at 35°C. The testes were digested in 5 mL of 0.25% trypsin plus 0.1 mL of deoxyribonuclease I (5 mg/mL) at 35°C for 8 min and then terminated by the addition of 0.5 mL fetal bovine serum. The suspension was passed through a 70 µm honeycomb filter. Centrifuge the cell suspension at 4°C at 500 ×g for 5 min and remove the supernatant. And then cells sediment was resuspended in 1 mL Dulbecco’s modified Eagle medium with 40 µg Hoechst 33342, 2 μL Zombie NIR™ dye and 5 μL DNase I. The cell suspension was stirred at 34°C for 20 min, then centrifuged at 500 ×g for 5 min at 4°C and resuspended in PBS at a concentration of 105 mL for sorting. Using FACS, populations of fluorescently labeled collection cells were collected according to the sorting channel into 1.5-mL LoBind microcentrifuge tubes (Eppendorf, 022431021) containing 0.5 mL PBS. The cell suspension was centrifuged and some of the supernatant was removed leaving about 10 μL PBS.
LACE-seq
Crosslinking immunoprecipitation coupled with high-throughput sequencing of FACS-purified spermatocyte populations was performed according to Su et al., (2021). The antibody used for immunoprecipitation was: HA-Tag (C29F4) Rabbit mAb (Cell Signaling Technology, 3724) with 3 µg for each test sample. And the IgG from rabbit was used for negative control.
In brief, spermatogonia cells were sorted as described above and collected in 10 μL PBS in 1.5 mL LoBind microcentrifuge tubes. Then cells were irradiated twice with UV-C light on ice at 400 mJ and stayed for 1 or 2 min between 2 radio actions. And these initial steps of LACE-seq exactly followed the options of Su et al. (2021), which are RNA immunoprecipitation and fragmentation, RNA dephosphorylation and 3ʹ linker ligation, RT on beads and first-strand cDNA capture by streptavidin beads. Next the Poly(A) tailing was replaced with a 3ʹ cDNA linker adding. The streptavidin beads with first-strand cDNA were resuspended in 20 μL ligation mixture (4.5 µL water, 2 μL 10× ligation buffer, 2 µL ATP (10 µmol/L, NEB, P0756S), 0.5 μL 3ʹ cDNA linker (1 μmol/L, /5pA/CTCGTATGCCGTCTTCTGCTTG/3NH2/, where 5pA denotes 5ʹ phosphorylated adenosine triphosphate, and 3NH2NH2 indicates 3ʹ-amino, synthesized and HPLC purified by Sangon), 1 μL T4 RNA ligase 1, truncated (NEB, M0204S), 10 μL 50% PEG8000) and transferred to a new PCR tube from 1.5-mL LoBind microcentrifuge tubes. The tubes were incubated at 25°C for 24 h in a ThermoMixer C with intermittent vortexing for 15 s at 1,000 rpm every 3 min.
After that, to generate the dsDNA template for IVT, the DNA was preamplified by first PCR with the following mixture: 12.5 μL cDNA, 0.5 μL primer A (GATCACTAATACGACTCACTATAGG, 10 μmol/L; Sangon), 0.5 μL P7 primer (CAAGCAGAAGACGGCATACGAGAT, 10 μmol/L; Sangon) and 12.5 μL 2× KAPA HiFi HotStart ReadyMix (KAPA Biosystems, KK2601). The PCR program was set as follows: 98°C for 3 min; 98°C for 15 s, 60°C for 20 s, and 72°C for 30 s (14–18 cycles); 72°C for 5 min, then hold at 12°C for long time. The PCR tube was then placed on a magnetic rack for 2 min, and the supernatant was transferred to a new LoBind tube and purified by 46.8 μL Ampure XP beads (1.8:1 ratio; Beckman Coulter, A63881) according to the manufacturer’s instructions. 13 μL Water was added to the LoBind tubes to elute PCR products and then transferred to a new PCR tube.
The subsequent IVT, RNA purification, and RT were also completely performed according to Su et al. (2021). Then PCR barcoding was performed with 20 μL cDNA, 1 μL P7 primer, 1 μL P5 index primer (AATGATACGGCGACCACCGAGATCTACAC NNNNNACACTCTTTCCCTACACGACGCTCTTCCGATCT,10 μmol/L; Sangon) and 22 μL 2× KAPA HiFi HotStart ReadyMix. And the PCR program was set as follows: 94°C for 3 min; 94°C for 15 s, 62°C for 30 s, and 72°C for 30 s (8–12 cycles); 72°C for 10 min, then hold at 12°C. PCR products between 130 and 300 bp were extracted by agarose gel electrophoresis with a 2% agarose gel and then purified using a gel extraction kit (Qiagen, 28604). The LACE-seq library was single-end sequenced using Illumina HiSeq 2500 at Novogene. LACE-seq data mapping and identification of peak, cluster, and motif are performed following Su et al. (2021).
RNA extraction, RT-qPCR, and RNA-seq
RNA was isolated from HEK 293T cells and flash frozen or fresh testes of Rbm46−/− and Rbm46+/− mice using FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme, RC112-01) following manufacturer’s instructions. The total RNA was used for subsequent RT-qPCR analysis. The sequences of the primers used are listed in Table S2. The first-strand cDNA was reverse transcribed from total RNA using HiScript III RT SuperMix for qPCR (+gDNA wiper) (Vazyme, R323-01). QPCR was conducted with the guide of SYBR Green Premix Pro Taq HS qPCR Kit (AG, 11701). Gapdh gene (LOC107788267) was used as an internal reference and three technical replicates were performed for each experiment. Finally, relative gene expression values were calculated using the 22−ΔΔCt method, which was subsequently converted to ploidy changes and plotted.
For RNA sequencing, isolated total RNA was converted into cDNA for RNA sequencing using Illumina Truseq RNA Sample Preparation Kit, followed by rRNA removal and sequenced on an Illumina HiSeq 2500 using 2 × 150 nt sequencing.
RBP immunoprecipitation
RNA-protein complex was captured for qRT-PCR by immunoprecipitation according to the manufacturer’s instructions (Geneseed, P0101). Briefly, tissues were homogenized in immunoprecipitation lysis buffer with RNasin and protease inhibitor and then divided into two samples for anti-HA-RBM46 and anti-IgG as negative controls. Protein A/G Magnetic Beads were used to capture protein at 4°C overnight. RNA of interest was immunoprecipitated with the beads and further analyzed by qPCR.
Dual-luciferase reporter assay
Dual-luciferase reporters were conducted with Secrete-Pair Dual Luminescence Assay Kit (GeneCopoeia, LF031). The RBM46-bound fragments of targets and their deletion variant, see as Fig. 5C, were inserted into the pEZX-GA02 vector at MCS2 sites. HEK 293 T cells were plated in 12-well plates. Subsequently, a mixture containing 500 ng of reporter plasmid and RBM46 overexpression plasmid was co-transfected to cells with HP DNA Transfection reagent (Roche, 06366546001). And six technical replicates were performed for each group. 72 h later, cells were collected and luciferase activities were assessed according to the manufacturer’s protocols by Microplate luminometer (Berthold, LB960).
Supplementary Material
Acknowledgments
We thank R&D Team of SDU-CUHK for help with animal and histological experiment.
Contributor Information
Yue Lv, Shandong Key Laboratory of Reproductive Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250012, China; Center for Reproductive Medicine, Shandong University, Jinan 250012, China; CUHK-SDU Joint Laboratory on Reproductive Genetics, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China.
Gang Lu, CUHK-SDU Joint Laboratory on Reproductive Genetics, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China.
Yuling Cai, Center for Reproductive Medicine, Shandong University, Jinan 250012, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China.
Ruibao Su, Key Laboratory of RNA Biology, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China; Fertility Preservation Lab, Reproductive Medicine Center, Guangdong Second Provincial General Hospital, Guangzhou 510062, China.
Liang Liang, Key Laboratory of RNA Biology, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.
Xin Wang, Key Laboratory of Systems Health Science of Zhejiang Province, School of Life Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China.
Wenyu Mu, Center for Reproductive Medicine, Shandong University, Jinan 250012, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China.
Xiuqing He, Center for Reproductive Medicine, Shandong University, Jinan 250012, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China.
Tao Huang, Center for Reproductive Medicine, Shandong University, Jinan 250012, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China.
Jinlong Ma, Center for Reproductive Medicine, Shandong University, Jinan 250012, China; CUHK-SDU Joint Laboratory on Reproductive Genetics, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China.
Yueran Zhao, Shandong Key Laboratory of Reproductive Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250012, China; Center for Reproductive Medicine, Shandong University, Jinan 250012, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China.
Zi-Jiang Chen, Shandong Key Laboratory of Reproductive Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250012, China; Center for Reproductive Medicine, Shandong University, Jinan 250012, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China; Shanghai Key Laboratory for Assisted Reproduction and Reproductive Genetics, Shanghai 200135, China; Center for Reproductive Medicine, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200135, China.
Yuanchao Xue, Key Laboratory of RNA Biology, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.
Hongbin Liu, Center for Reproductive Medicine, Shandong University, Jinan 250012, China; CUHK-SDU Joint Laboratory on Reproductive Genetics, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China; Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Jinan 250012, China.
Wai-Yee Chan, CUHK-SDU Joint Laboratory on Reproductive Genetics, School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China.
Funding
This work was supported by grants from the National Key R&D Program of China (2021YFC2700200), Academic Promotion Programme of Shandong First Medical University (2019U001), General Research Fund from Research Grants Council of Hong Kong (14103418), Basic Science Center Program of NFSC (31988101), the Shandong Provincial Key Research and Development Program (2020ZLYS02), a fund from A-Smart Group to support CUHK-SDU Joint Laboratory on Reproductive Genetics of CUHK, Major Innovation Projects in Shandong Province (2021ZDSYS16) and the Science Foundation for Distinguished Yong Scholars of Shandong (ZR2021JQ27), and Taishan Scholars Program for Young Experts of Shandong Province (tsqn202103192).
Authors’ contributions
Z.-J.C., W.-Y.C., Y.X., and H.L. designed the study and provided their valuable contributions to the whole study. Y.C., R.S., and Y.L. performed the LACE-seq experiments. W.M., X.H., and T.H. performed the animal and in vitro experiments; Y.L, L.L., and X.W. analyzed the data, J.M. and Y.Z. advised and helped data analysis. Y.L. and G.L. wrote the manuscript. All authors contributed to this work, discussed the results, critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement: The authors declare no competing interests.
Ethical approval
Mice housing and all experimental protocols were approved by the Regional Ethics Committee of Shandong University.
Informed consent
All the authors give their consent for the publication of this study.
Data availability
The generated and analyzed data are available in GEO with accession number GSE201190.
References
- Adelfalk C, Janschek J, Revenkova Eet al. Cohesin SMC1beta protects telomeres in meiocytes. J Cell Biol 2009;187:185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos H, Lassalle B, Chicheportiche Aet al. Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry A 2005;65:40–9. [DOI] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CFet al. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol 1977;74:68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MF, Philippakis AA, Qureshi AMet al. Compact, universal DNA microarrays to comprehensively determine transcription-factor binding site specificities. Nat Biotechnol 2006;24:1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas U, Hempel K, Llano Eet al. Distinct roles of meiosis-specific cohesin complexes in mammalian spermatogenesis. PLoS Genet 2016;12:e1006389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon CK, Hawley RS.. Regulating the construction and demolition of the synaptonemal complex. Nat Struct Mol Biol 2016;23:369–77. [DOI] [PubMed] [Google Scholar]
- Codino A, Turowski T, van de Lagemaat LNet al. NANOS2 is a sequence-specific mRNA-binding protein that promotes transcript degradation in spermatogonial stem cells. iScience 2021;24:102762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XY, Cheng XK, Huang JFet al. Rbm46, a novel germ cell-specific factor, modulates meiotic progression and spermatogenesis. Biol Reprod 2021;104:1139–53. [DOI] [PubMed] [Google Scholar]
- de Vries FA, de Boer E, van den Bosch Met al. Mouse Sycp1 functions in synaptonemal complex assembly, meiotic recombination, and XY body formation. Genes Dev 2005;19:1376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijpe M, Heyting C, Gross Bet al. Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci 2000;113:673–82. [DOI] [PubMed] [Google Scholar]
- Feng CW, Bowles J, Koopman P.. Control of mammalian germ cell entry into meiosis. Mol Cell Endocrinol 2014;382:488–97. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Fukuda N, Agostinho Aet al. STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J 2014;33:1243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaysinskaya V, Soh IY, van der Heijden GWet al. Optimized flow cytometry isolation of murine spermatocytes. Cytometry A 2014;85:556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstberger S, Hafner M, Tuschl T.. A census of human RNA-binding proteins. Nat Rev Genet 2014;15:829–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou LT, Dai P, Yang JHet al. Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res 2014;24:680–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering CH, Jessberger R.. Cohesin in determining chromosome architecture. Exp Cell Res 2012;318:1386–93. [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann Net al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 2010;38:576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herran Y, Gutierrez-Caballero C, Sanchez-Martin Met al. The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. EMBO J 2011;30:3091–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard B, Small C, Yang Let al. Global gene expression in the human fetal testis and ovary. Biol Reprod 2009;81:438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger R. Cohesin complexes get more complex: the novel kleisin RAD21L. Cell Cycle 2011;10:2053–4. [DOI] [PubMed] [Google Scholar]
- Jin J, Jing W, Lei XXet al. Evidence that Lin28 stimulates translation by recruiting RNA helicase A to polysomes. Nucleic Acids Res 2011;39:3724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MK, Han SJ.. Post-transcriptional and post-translational regulation during mouse oocyte maturation. BMB Rep 2011;44:147–57. [DOI] [PubMed] [Google Scholar]
- Keene JD, Komisarow JM, Friedersdorf MB.. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nat Protoc 2006;1:302–7. [DOI] [PubMed] [Google Scholar]
- Klein F, Mahr P, Galova Met al. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 1999;98:91–103. [DOI] [PubMed] [Google Scholar]
- Kramer A, Green J, Pollard J Jret al. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014;30:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hirano T.. RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol 2011;192:263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Iwai T, Yokota Tet al. Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci 2003;116:2781–90. [DOI] [PubMed] [Google Scholar]
- Legrand JMD, Chan AL, La HMet al. DDX5 plays essential transcriptional and post-transcriptional roles in the maintenance and function of spermatogonia. Nat Commun 2019;10:2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand JMD, Hobbs RM.. RNA processing in the male germline: mechanisms and implications for fertility. Semin Cell Dev Biol 2018;79:80–91. [DOI] [PubMed] [Google Scholar]
- Li ZG, Guo QY, Zhang JXet al. The RNA-binding motif protein family in cancer: friend or foe? Front Oncol 2021;11:757135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liang Z, Yang Jet al. DAZL is a master translational regulator of murine spermatogenesis. Natl Sci Rev 2019;6:455–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Mele A, Fak JJet al. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature 2008;456:464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima AC, Jung M, Rusch Jet al. A standardized approach for multispecies purification of mammalian male germ cells by mechanical tissue dissociation and flow cytometry. J Vis Exp 2017;125:55913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano E, Herran Y, Garcia-Tunon Iet al. Meiotic cohesin complexes are essential for the formation of the axial element in mice. J Cell Biol 2012;197:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luteijn MJ, Ketting RF.. PIWI-interacting RNAs: from generation to transgenerational epigenetics. Nat Rev Genet 2013;14:523–34. [DOI] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat Fet al. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 2001;27:271–6. [DOI] [PubMed] [Google Scholar]
- Masuda A, Kawachi T, Takeda JIet al. tRIP-seq reveals repression of premature polyadenylation by co-transcriptional FUS-U1 snRNP assembly. EMBO Rep 2020;21:e49890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AC, Rahman R, Jin Het al. TRIBE: hijacking an RNA-editing enzyme to identify cell-specific targets of RNA-binding proteins. Cell 2016;165:742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicoll F, Stevense M, Jessberger R.. Cohesin in gametogenesis. Curr Top Dev Biol 2013;102:1–34. [DOI] [PubMed] [Google Scholar]
- Meuwissen RL, Offenberg HH, Dietrich AJet al. A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J 1992;11:5091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryan MK, Clark BJ, McLaughlin EAet al. RBM5 is a male germ cell splicing factor and is required for spermatid differentiation and male fertility. PLoS Genet 2013;9:e1003628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, Plug AW, van Vugt MJet al. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res 1997;5:66–8. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Cuvellier S, Naguibneva Iet al. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev 2007;21:1125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi B, Bragado L, Mammi Pet al. Dengue virus targets RBM10 deregulating host cell splicing and innate immune response. Nucleic Acids Res 2020;48:6824–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I, Suja JA, Pezzi Net al. Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat Cell Biol 2001;3:761–6. [DOI] [PubMed] [Google Scholar]
- Qin H, Ni HW, Liu YCet al. RNA-binding proteins in tumor progression. J Hematol Oncol 2020;13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijo R, Lee TY, Salo Pet al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 1995;10:383–93. [DOI] [PubMed] [Google Scholar]
- Revenkova E, Eijpe M, Heyting Cet al. Novel meiosis-specific isoform of mammalian SMC1. Mol Cell Biol 2001;21:6984–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler Wet al. Integrative genomics viewer. Nat Biotechnol 2011;29:24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H, Weich S, Schwegler Het al. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol 1996;134:1109–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel K, Mesagno FP, Karus Det al. Psychosocial adjustment of children with a terminally ill parent. J Am Acad Child Adolesc Psychiatry 1992;31:327–33. [DOI] [PubMed] [Google Scholar]
- Su RB, Fan LH, Cao CCet al. Global profiling of RNA-binding protein target sites by LACE-seq. Nat Cell Biol 2021;23:664–75. [DOI] [PubMed] [Google Scholar]
- Sutherland JM, Siddall NA, Hime GRet al. RNA binding proteins in spermatogenesis: an in depth focus on the Musashi family. Asian J Androl 2015;17:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGompel MJ, Xu EY.. A novel requirement in mammalian spermatid differentiation for the DAZ-family protein Boule. Hum Mol Genet 2010;19:2360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanharanta S, Marney CB, Shu WPet al. Loss of the multifunctional RNA-binding protein RBM47 as a source of selectable metastatic traits in breast cancer. Elife 2014;3:e02734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Netto KG, Zhou Let al. Single-cell transcriptomic analysis reveals the immune landscape of lung in steroid-resistant asthma exacerbation. Proc Natl Acad Sci USA 2021;118:e2005590118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yu L, Wang Set al. LIN28A binds to meiotic gene transcripts and modulates their translation in male germ cells. J Cell Sci 2020a;133:jcs242701. [DOI] [PubMed] [Google Scholar]
- Wang S, Zheng YX, Li JYet al. Single-cell transcriptomic atlas of primate ovarian aging. Cell 2020b;180:585–600.e519. [DOI] [PubMed] [Google Scholar]
- Ward A, Hopkins J, McKay Met al. Genetic interactions between the meiosis-specific cohesin components, STAG3, REC8, and RAD21L. G3 (Bethesda) 2016;6:1713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Beasley M, Verschoor Set al. A new role for the mitotic RAD21/SCC1 cohesin in meiotic chromosome cohesion and segregation in the mouse. EMBO Rep 2004;5:378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Beasley MD, Warren WDet al. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 2005;8:949–61. [DOI] [PubMed] [Google Scholar]
- Xu XC, He S, Zhou YQet al. RNA-binding motif protein RBM47 promotes tumorigenesis in nasopharyngeal carcinoma through multiple pathways. J Genet Genomics 2021;48:595–605. [DOI] [PubMed] [Google Scholar]
- Yao YX, Yang B, Cao Het al. RBM24 stabilizes hepatitis B virus pregenomic RNA but inhibits core protein translation by targeting the terminal redundancy sequence. Emerg Microbes Infect 2018;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu JG, Zhao Jet al. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell 2000;5:73–83. [DOI] [PubMed] [Google Scholar]
- Zhang P, Kang JY, Gou LTet al. MIWI and piRNA-mediated cleavage of messenger RNAs in mouse testes. Cell Res 2015;25:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Ohsumi TK, Kung JTet al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell 2010;40:939–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The generated and analyzed data are available in GEO with accession number GSE201190.