Abstract
The global COVID-19 coronavirus pandemic has infected over 109 million people, leading to over 2 million deaths up to date and still lacking of effective drugs for patient treatment. Here, we screened about 1.8 million small molecules against the main protease (Mpro) and papain like protease (PLpro), two major proteases in severe acute respiratory syndrome-coronavirus 2 genome, and identified 1851Mpro inhibitors and 205 PLpro inhibitors with low nmol/l activity of the best hits. Among these inhibitors, eight small molecules showed dual inhibition effects on both Mpro and PLpro, exhibiting potential as better candidates for COVID-19 treatment. The best inhibitors of each protease were tested in antiviral assay, with over 40% of Mpro inhibitors and over 20% of PLpro inhibitors showing high potency in viral inhibition with low cytotoxicity. The X-ray crystal structure of SARS-CoV-2 Mpro in complex with its potent inhibitor 4a was determined at 1.8 Å resolution. Together with docking assays, our results provide a comprehensive resource for future research on anti-SARS-CoV-2 drug development.
Keywords: high-throughput screening, SARS, CoV-2, main, papain-like, proteases
Graphical Abstract
Graphical Abstract.
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) has infected over 109 million cumulative cases with a ~2.2% case-fatality rate globally, and caused worldwide social and economic disruption (Adhikari et al. 2020; Walker et al. 2020). Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is a positive strand RNA virus that causes severe COVID-19 respiratory disease in human (Guy et al. 2020; Wu et al. 2020b). Several existing drugs that have been applied in clinic to treat COVID-19, such as Lopinavir and Ritonavir, have shown limited curative effect with relatively severe side effects (Cao et al. 2020; Grein et al. 2020b). Remdesivir, an RNA-dependent RNA polymerase (RdRp, EC:2.7.7.48) inhibitor developed for treating Ebola virus (Tchesnokov et al. 2019; Yin et al. 2020), has shown reduced time to clinical recovery, however, more data are still required to confirm its benefits on mild or moderate patients (Durante-Mangoni et al. 2020; Grein et al. 2020a). Currently, no specific anti-SARS-CoV-2 drug is available yet.
Great efforts have been made to characterize molecular targets, which are pivotal for the development of anti-coronaviral therapies. Two of the best-characterized drug targets among coronaviruses are the main protease (Mpro, also called 3CLpro, EC:3.4.22.69) and the papain-like protease (PLpro, EC:3.4.22.2), which are responsible for processing the polyproteins pp1a and pp1ab into mature non-structural proteins (Nsps) (Freitas et al. 2020; Wu et al. 2020a). Mpro is firstly auto-cleaved from poly-proteins, and then further processes downstream Nsp proteins to release Nsp4–Nsp16, including the RdRp and helicase that are essential in the life cycle of the virus (Lobo-Galo et al. 2020; Zhang et al. 2020). PLpro is responsible for the cleavages of N-terminus of the replicase polyprotein to release Nsp1, Nsp2, and Nsp3 that are required for correcting virus replication (Lin et al. 2018). In addition, PLpro was also confirmed to be significant for antagonizing the host’s immune responses (Ma-Lauer et al. 2016). Thus, both proteases are considered as drug targets for coronaviruses such as SARS-CoV-2, and many efforts had been carried out to identify their novel inhibitors. Several high affinity Mpro small molecule inhibitors have already been discovered (Dai et al. 2020b; Jin et al. 2020b), however, no effective PLpro inhibitor of SARS-CoV-2 has been reported yet.
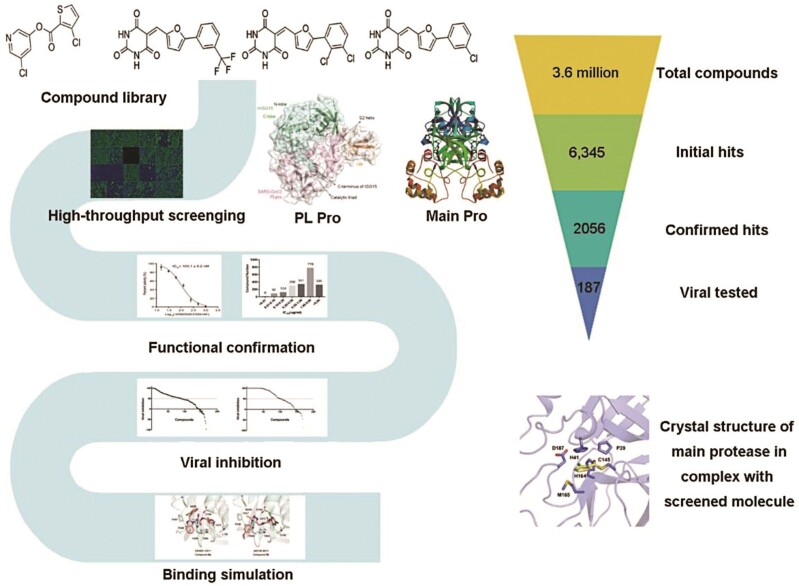
Here, we screened about 1,800,000 compounds from the Chinese National Compound Library (CNCL) for the discovery of inhibitors of Mpro and PLpro, and identified 1,851 and 205 hits targeting these two proteases, respectively, with great structural diversity. Together with molecular docking, cell-based antiviral assays, and X-ray crystallography, this work provides a systematic framework for further drug development for the treatment of COVID-19.
Results
High-throughput screening of Mpro inhibitors
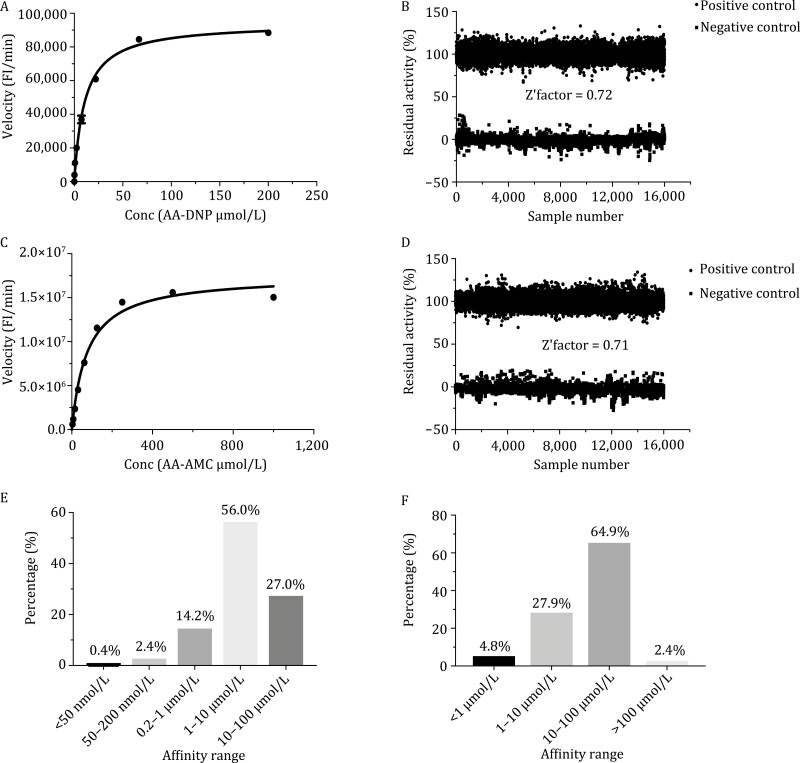
To screen inhibitors, large scale of Mpro protein sample with native N and C termini was expressed in E. coli as described and purified in a modified protocol (Fig. S1A). The protease activity was tested using the fluorescently labelled substrate MCA-AVLQSGFR-Lys(Dnp)-Lys-NH2. To better facilitate high-throughput screening, the assay system was optimized by screening the best substrate and enzyme concentrations, and the final concentrations were set at 20 µmol/L and 40 nmol/L, respectively, with Zʹ factor of 0.72 (Fig. 1A and 1B). A total 1,733,782 compounds from CNCL were initially screened with the criterion of >70% inhibition at 10 µg/mL. In total, 9,742 compounds were obtained from primary screening with the hit rate of 0.56%. These compounds were further confirmed in a second test with 1 and 10 µg/mL compound concentrations, respectively, and 2358 hits showing inhibition higher than 50% at 1 µg/mL were selected. The half maximal inhibitory concentration (IC50) of these compounds was further measured and 1,851 of them exhibit a dose dependent manner with the best IC50 of 9.0 ± 4.0 nmol/L, which is the most potent inhibitor reported to date (Dai et al. 2020b) (for detailed chemical structures and affinity data, please visit https://app.cncl.org.cn/). Among these compounds, 0.4% (8 compounds) showed an IC50 value below 50 nmol/L, 2.2% (41 compounds) fell in the IC50 range of 50–200 nmol/L, 13.7% (253 compounds) showed an IC50 value between 200 nmol/L and 1 µmol/L, 56% (1,044 compounds) showed an IC50 value of 1–10 µmol/L, and 27% (505 compounds) showed an IC50 value between 10 and 100 µmol/L (Fig. 1E; Table S1). The 1,851 compounds were analyzed using cheminformatics method and further classified into over 400 different chemical structures, showing huge diversity of chemical scaffolds and great potential in future drug development.
Figure 1.
High-throughput screening of Mpro and PLpro inhibitors. (A and B) The optimized activity assay (A) and the consistency of high-throughput screening systems (B) targeting on Mpro based on about 6000 HTS assay plates. (C and D) The optimized activity assay (C) and the consistency of high-throughput screening systems (D) targeting on PLpro based on 6000 HTS assay plates. (E and F) The IC50 distribution of Mpro (E) and PLpro (F) inhibitors.
High-throughput screening of PLpro inhibitors
Large scale of PLpro protein sample was expressed in E. coli and purified in a modified protocol (Fig. S1b). The protease activity was tested using the fluorescently labelled substrate Z-RLRGG-AMC, and the substrate and enzyme concentrations were set at 50 µmol/l and 40 nmol/L, respectively, with the Zʹ factor of 0.71 (Fig. 1C and 1D). The compounds from CNCL were initially screened with a criterion of > 50% inhibition at 8 µg/mL for 1,786,016 compounds and 20 µmol/L for 3,987 bio-active compounds including approved drugs, clinical trial drug candidates, and preclinical drug candidates. 3,987 compounds were obtained and further confirmed in a second test at concentrations of 1 and 10 µg/mL for pure compounds and 2 and 20 µmol/L for bio-active compounds. Overall 387 compounds showing simple dose dependency were selected with more than 50% inhibition against PLpro at 10 µg/mL and 40 bio-active compounds at 20 µmol/L. In total, 205 out of 387 compounds exhibited valid IC50 and the best compound showed an IC50 value of 0.18 ± 0.03 µmol/L, which is the most potent PLpro inhibitor reported to date (Baez-Santos et al. 2015) (for detailed chemical structures and affinity data, please visit https://app.cncl.org.cn/). Among these compounds, 5.3% (10 compounds) showed an IC50 value below 1 µmol/L, 29.0% (59 compounds) fell in the range of 1–10 µmol/L, 62.8% (130 compounds) showed an IC50 value between 10 and 100 µmol/L, and 2.9% (6 compounds) showed an IC50 value higher than 100 µmol/L (Fig. 1F; Table S2).
Interestingly, among the 205 PLpro inhibitors, six compounds have also shown inhibitory activity in the Mpro screening (Table S3). These compounds are classified as 4-phenyl-4,5-dihydro-1H-1,2,4-triazole derivatives. Most of these inhibitors display potency preferences to either protease. For example, compound 3a showed high inhibitory activity against Mpro (0.5 ± 0.0 µmol/L) but relatively low inhibitory activity toward PLpro (58.8 ± 15.3 µmol/L). However, another analogue, compound 3b, showed high inhibitory activity against PLpro (6.0 ± 0.0 µmol/L) but low inhibitory activity toward Mpro (21.9 ± 0.3 µmol/L). Another derivative, compound 3c, showed a weaker bias between these two proteases with roughly 2-fold difference (4.2 ± 0.6 vs. 10.8 ± 0.5 µmol/L). These results suggest that the development of inhibitors with high potencies toward both Mpro and PLpro is possible and our work thus provide attractive hints for developing better anti-SARS-CoV-2 drugs by inhibiting both of its proteases.
Structure of SARS-CoV-2 Mpro in complex with compound 4a
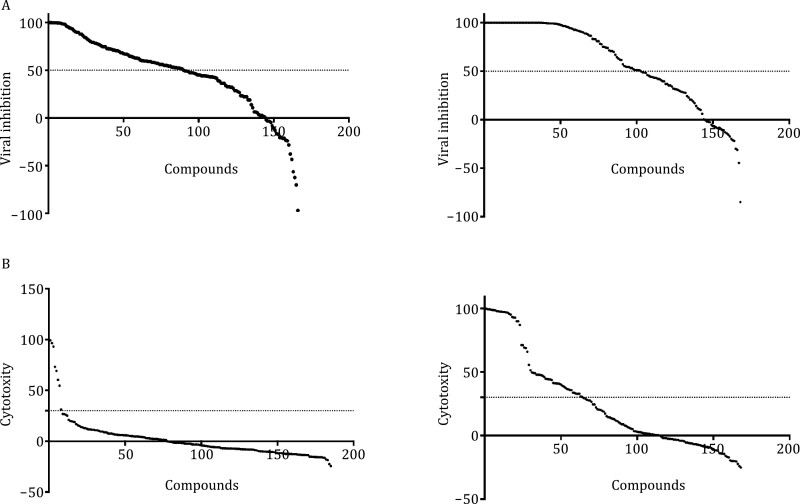
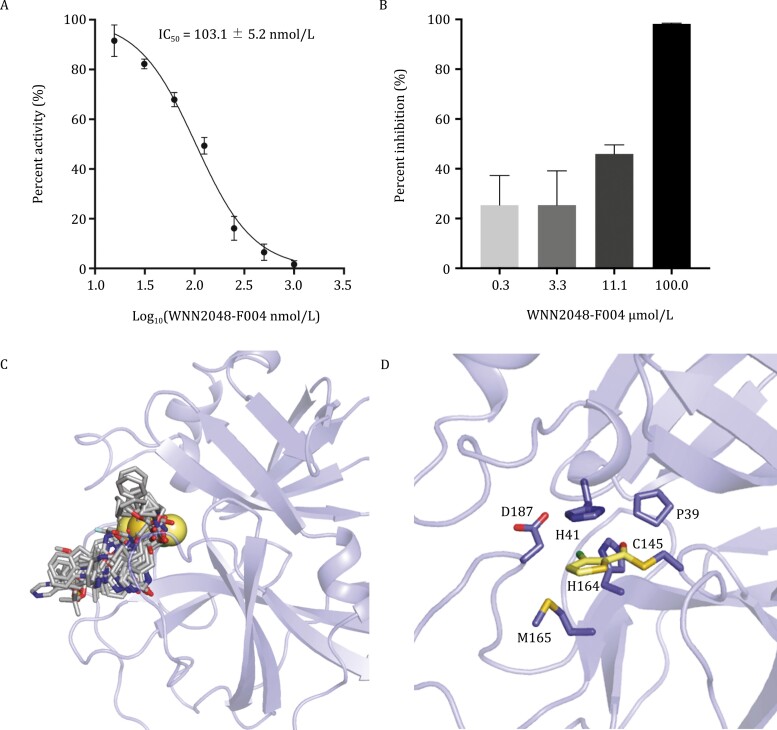
Among the Mpro inhibitors with the highest inhibitory activities, 77 compounds showed at least 50% inhibition of viral replication at the concentration of 10 µg/mL (Fig. 3A; Tables S1 and S2). An initial cytotoxicity assays using same inhibitor concentration revealed that only 4.8% (8 out of 166) of Mpro inhibitors showed over 30% of cytotoxicity, displaying good compound safety. Among these Mpro inhibitors, a compound 4a exhibited high inhibitory potency with IC50 value of 0.10 ± 0.05 µmol/L and showed good anti-SARS-CoV-2 infection activity in cell culture with IC50 value of 21.3 µmol/L without cytotoxicity effect.
Figure 3.
Distribution of virus replication inhibition and cytotoxicity. (A) The inhibition of selected Mpro inhibitors (left panel) and PLpro (right panel) on viral replication. (B) The cytotoxicity distribution of selected Mpro inhibitors (left panel) and PLpro (right panel) on viral replication. Note that the compound order between (A) and (B) is different.
We determined the crystal structure of compound 4a-bound SARS-CoV-2 Mpro to elucidate the molecular basis of the compound-induced inhibition of Mpro. The structure of SARS-CoV-2 Mpro contains three domains with the substrate-binding site located in the cleft between domains I and II (Fig. 4C; Table S4). At the active site of SARS-CoV-2 Mpro, Cys145, and His41 (Cys-His) form a catalytic dyad. The thiol of Cys145 is able to anchor inhibitors by a covalent linkage, which has been reported to be important for the inhibitors to maintain antiviral activity (Yang et al. 2005; Dai et al. 2020b; Jin et al. 2020b). The electron density map showed compound 4a covalently bind to the substrate-binding pocket of SARS-CoV-2 Mpro (Fig. 4C). The ester group of compound 4a is employed as a new warhead to form a covalent bond with the Cys145 (Fig. 4D). The thiophene group of compound 4a stacks with the imidazole ring of His41. This group is also surrounded by the side chains of Pro39, His164, Met165, and Asp187. The overall structure of the compound 4a-bound Mpro is similar to the previously reported SARS-CoV-2 Mpro complex structures (Dai et al. 2020a; Douangamath et al. 2020; Fu et al. 2020; Hoffman et al. 2020; Jin et al. 2020a; Kneller et al. 2020; Sacco et al. 2020; Su et al. 2020; Yang et al. 2020; Zhang et al. 2020; Bai et al. 2021; Lockbaum et al. 2021; Qiao et al. 2021). A major difference lies in the substrate-binding pocket, where the compound 4a has a slightly deeper insertion and induces the outward flip of the His41 to facilitate the ligand binding (Fig. 4C and 4D).
Figure 4.
Structure of SARS-CoV-2 Mpro in complex with compound 4a. (A) Biochemical inhibition of SARS-CoV-2 Mpro by WNN2048-F004. (B) Anti-SARS-CoV-2 infection activity of WNN2048-F004 at different concentrations (0.3, 3.3, 10, and 100 µmol/L). (C) The binding modes of compound 4a and known representative inhibitors with SARS-CoV-2 Mpro showed by superimposing all the crystal structures of inhibitor-bound SARS-CoV-2 Mpro (PDB codes: 5R84, 6LU7, 6LZE, 6M2N, 6XBH, 6XHM, 6XQT, 6Y2G, 7BQY, 7BRP, 7C7P, 7D1O, 7JPZ, and 7L0D). The compound 4a is shown in yellow spheres. The other inhibitors are shown in gray sticks. (D) The binding pocket of compound 4a. The key residues are shown in sticks and compound 4a is shown in yellow sticks.
Binding models of SARS-CoV-2 Mpro noncovalent inhibitors
The apparent IC50 values of selected inhibitors were measured against a series of different substrate concentrations with S/KM ratio from 1/2 to 2 (Table S5). Some inhibitors such as compound 4a showed a consistent IC50 that is not affected by substrate concentration, indicating a covalent binding manner. The IC50 of other inhibitors, such as compound 3a, were decreased as increasing of substrate concentration, suggesting these are noncovalent inhibitors of SARS-CoV-2 Mpro. To characterize the structure-activity relationships of these Mpro inhibitors, molecular docking was performed against the compound 4a bound SARS-CoV-2 Mpro and the published structures of SARS-CoV-2 Mpro (Dai et al. 2020b; Jin et al. 2020b; Zhang et al. 2020). The inhibitor-bound models were built targeting the substrate-binding site between the domains I and II of Mpro. 1,471 out of the 1,851 inhibitors could stably bind to the models of Mpro in the docking simulations, suggesting that some inhibitors are allosteric modulators or potentially recognize a different conformation of Mpro. Among the inhibitors that were docked into the Mpro models, great chemotype differences were observed and these inhibitors showed distinct binding modes to the protease. Here we present the binding models of several representative chemical scaffolds of inhibitors, which could be starting points for rational development of anti-virus drugs targeting Mpro.
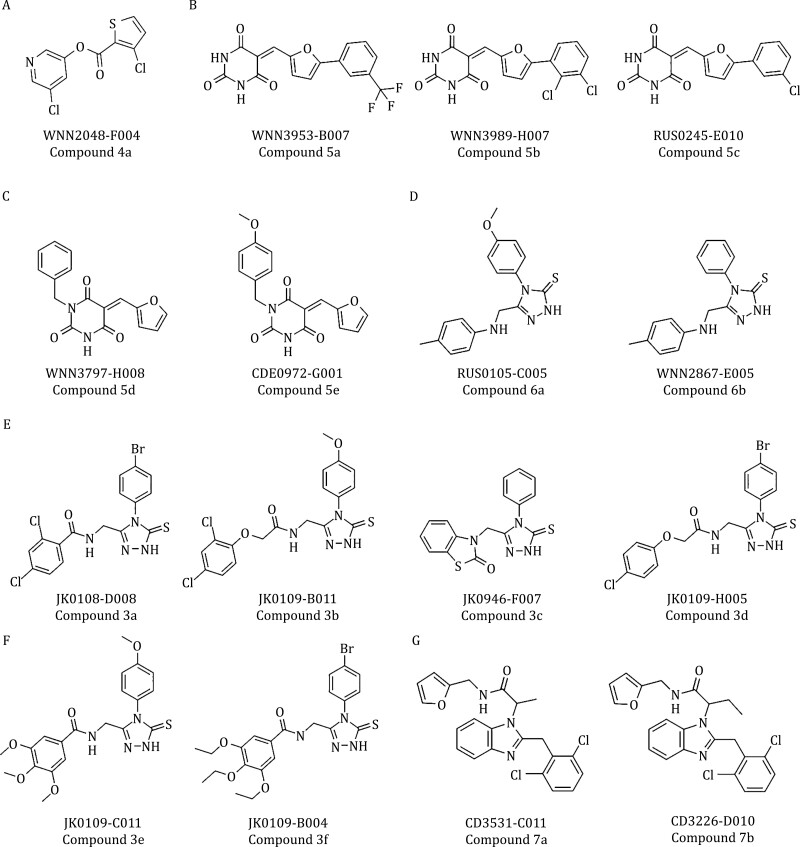
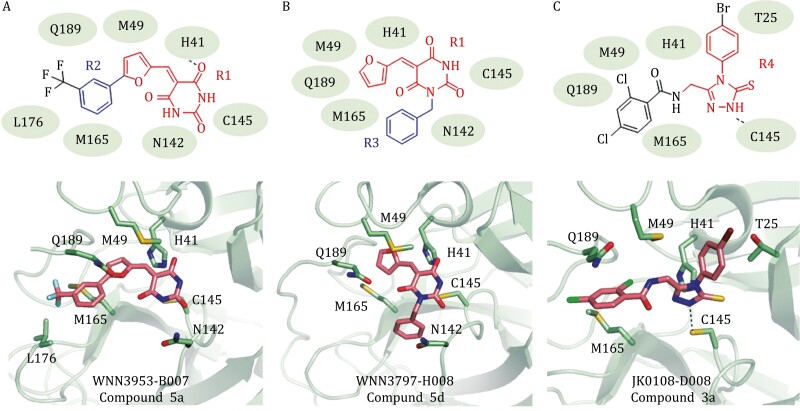
Five of the potent Mpro inhibitors (compounds 5a, 5b, 5c, 5d, and 5e) (Fig. 2B and 2C) have 5-(furan-2-ylmethylene) pyrimidine-2,4,6(1H,3H,5H)-trione group (group R1). In the docking models, this group is located in the center of the substrate-binding site and directly interacts with the Cys-His catalytic dyad (Fig. 5A and 5B). This series of compounds can be further divided into two types: a phenyl group in the furan (compound 5a, 5b, and 5c) and a phenyl group in the pyrimidinone (compounds 5d and 5e). For the furan derived compounds, e.g., compound 5a (Fig. 4A), the phenyl groups (group R2) deeply insert into the hydrophobic cavity consisting of residues Met49, Met165, and Leu176 (Fig. 5A). For the pyrimidinone derived compounds, e.g., compound 5d (Fig. 5B), their phenyl groups (group R3) attach to the edge of the substrate-binding pocket close to the residue Asn142 (Fig. 5B). The phenyl groups of these two types bind to the protein at different subsites, but both are favorable for ligand binding. Since these two types of phenyl-group substitutions are not mutually excluded, it might be possible to develop more specific potent Mpro inhibitors based on this scaffold.
Figure 2.
Chemical structures for selected hits targeting SARS-CoV-2 virus proteases. (A) A potent covalent inhibitor of Mpro. (B–F) Representative Mpro inhibitors. (G) PLpro inhibitors with the benzimidazole group.
Figure 5.
Binding modes of Mpro noncovalent inhibitors. (A) Schematic diagram and cartoon representation of the interactions between WNN3953-B007 (compound 5a) and SARS-CoV-2 Mpro (PDB code: 6LU7). (B) Schematic diagram and cartoon representation of the interactions between WNN3797-H008 (compound 5d) and SARS-CoV-2 Mpro (PDB code: 6LU7). (C) Schematic diagram and cartoon representation of the interactions between JK0108-D008 (compound 3a) and SARS-CoV-2 Mpro (PDB code: 6Y2G).
A large series of Mpro inhibitors (compounds 3a–3f, 6a, and 6b) (Fig. 2) share a same group of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (group R4). Their 1,2,4-triazole-3-thione group hydrophobically interacts with Thr25 and Met49 and electrostatically interacts with His41 and Cys145 of the catalytic site (Fig. 5C). These compounds have different substituted aromatic groups at the fifth position of the triazole ring. Such aromatic group, including benzo and phenyl, is sandwiched by the hydrophobic residues Met49 and Met165 to facilitate the ligand binding (Fig. 4C). However, some compounds such as compound 3b (Fig. 2E) have long and/or large substituting groups that cannot insert deeply into the substrate-binding pocket. As a result, these compounds showed weak effects on inhibiting Mpro (Table S1). Notably, several compounds of these series (compounds 3a–3f) also inhibit PLpro.
Binding models of SARS-CoV-2 PLpro inhibitors
A similar IC50 against a series of different substrate concentrations with S/KM ratio from 1/4 to 4 (Table S6) were also tested on selected SARS-CoV-2 PLpro inhibitors, and similar to SARS-CoV-2 Mpro, both covalent and noncovalent PLpro inhibitors were suggested. In cell-based assays, 72 out of 121 PLpro inhibitors showed at least 50% inhibition of viral replication at the concentration of 10 µg/mL (Fig. 3A; Tables S1 and S2). An initial cytotoxicity assays using same inhibitor concentration revealed that 38.8% (47 out of 121) of PLpro inhibitors exposed over 30% of cytotoxicity (Fig. 3B). This finding suggested that despite low sequence similarity, PLpro might share a similar binding site with its isozymes in host cells.
We applied molecular docking to characterize the binding modes of the potent PLpro inhibitors. The published structures of SARS-CoV-2 PLpro were used in the molecular docking of PLpro inhibitors. To include more structural information for docking analysis, we also performed molecular docking against homology models generated by using the SARS-CoV PLpro structures as templates (Ratia et al. 2006; Daczkowski et al. 2017). PLpro has an independent ubiquitin-like domain and a right-hand like architecture, including the palm, thumb, and finger domains with its catalytic triad located between the palm and thumb domains. Docking results suggest that instead of direct interactions with the catalytic triad (Ratia et al. 2008), the inhibitors bind to a cleft next to the catalytic site, inducing a loop closure that shuts down catalysis site. Compared with the docking results of Mpro, a much smaller percentage of the PLpro inhibitors bind to the docking models with high affinity, implying either more accurate models or better understanding of PLpro inhibition mechanism is required.
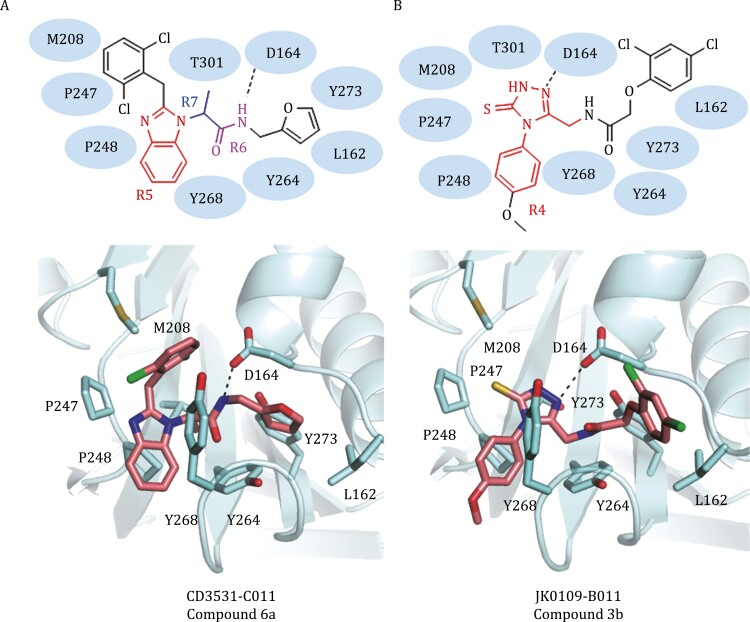
A series of PLpro inhibitors with the benzimidazole group (compounds 7a and 7b) (Fig. 2G) bind to the allosteric ligand binding site (Freitas et al. 2020). The majority of contacts between PLpro and these compounds are hydrophobic. For example, in the docking model of compound 7a, the benzimidazole group (group R5) of a compound is surrounded by residues Met208, Pro247, Pro248, Tyr268, and Thr301, and its amide group (group R6) forms a hydrogen bond with Asp164 (Fig. 5A). The methyl or ethyl group (group R7) connecting the benzimidazole and amide groups points directly into the interior of the protein between Tyr273 and Thr301. The aromatic group connected to the amide group inserts into the cleft formed by Leu162, Tyr264, and Tyr273, while the other aromatic group connected to the benzimidazole attaches to the edge of the cleft (Fig. 5A).
Several Mpro inhibitors also diminish the activity of PLpro. In the docking models of PLpro, the 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione compound series (compounds 3a–3f) had their 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione group (R4) insert into the allosteric ligand-binding site formed by Asp164, Met208, Pro247, Pro248, and Tyr268 (Fig. 6B). Asp164 is highly conserved among most coronaviral papain-like proteases and has been revealed to be important for ligand stabilization (Sulea et al. 2006; Ratia et al. 2008). For these compounds (compounds 3a–3f), the nitrogen within the triazole group electrostatically interacts with the side chain carboxyl group of the residue Asp164. The substitution at the fifth position of the triazole ring is sandwiched by three tyrosine residues Tyr264, Tyr268, and Tyr273. In general, the PLpro inhibitors contain hydrophobic aromatic rings connected by polar groups, which form hydrogen bonds with the key residue Asp164.
Figure 6.
Binding modes of PLpro inhibitors. (A) Schematic diagram and cartoon representation of the interactions between CD3531-C011 (compound 6a) and SARS-CoV-2 PLpro (template PDB code: 3MJ5). (B) Schematic diagram and cartoon representation of the interactions between JK0109-B011 (compound 3b) and SARS-CoV-2 PLpro (template PDB code: 3E9S).
Discussion
SARS-CoV-2 virus poses a continued threat and has a potential for a new global pandemic. The preparation of drug reserve with diverse compounds not only offers necessary treatments for the infected patients but also helps us quick respond to other viral outbreaks. In this work, we tested approximately 1.8 million small molecules against two major proteases in SARS-CoV-2 genome, i.e., Mpro and PLpro, and identified 1,851 Mpro inhibitors and 205 PLpro inhibitors with low nano-molar activity. 77 Mpro inhibitors and 72PLpro inhibitors showed at least 50% inhibition of viral replication at the concentration of 10 µg/mL (Fig. 3A; Tables S1 and S2). Diverse chemotypes were identified in the large number of potent inhibitors, which provide valuable information for the development of small-molecule drug reserve against SARS-CoV-2 virus.
The substrate specificity of Mpro is highly conserved among different coronavirus (Fig. S2), making it an ideal target for the development of broad-spectrum antiviral drugs (Yang et al. 2005; Pillaiyar et al. 2016). Several types of substrate-like peptidomimetic Mpro inhibitors targeting the substrate-binding site have been reported (Pillaiyar et al. 2016; Zumla et al. 2016; Dai et al. 2020b; Jin et al. 2020b). However, the classical small-molecule covalent inhibitors that are validated by complex structure determination have not emerged. Integrating enzymatic assays with X-ray protein crystallography, we identified compound 4a as the first class covalent, nonpeptidomimetic inhibitor of SARS-CoV-2 Mpro. The crystal structure reveals the binding mode of compound 4a is different from those of known Mpro inhibitors. To resemble the binding of the substrates, the previously reported peptidomimetic inhibitors bind to the substrate-binding pocket in extended delineated conformations and occupy most subsites of the pocket. As a small molecule, compound 4a only occupies one subsite of the catalytic dyad by interacting with His41 and Cys145, which are key elements for the recognition of substrates (Tan et al. 2005). It forms a covalent bond with the Cys145 and stacks with the side chain of His by the thiophene group. Even though the compound 4a only block the catalytic dyad, it is able to effectively prevent the access of substrate to the core of the active site. The unique binding mode and the high inhibitory efficiency make compound 4a a potential lead for future drug development targeting Mpro. In addition to the covalent inhibitor, we also identified a large number of noncovalent small-molecule inhibitors of Mpro. Multiple chemical series of Mpro inhibitors are identified and distinct binding modes of these inhibitors are characterized using molecular docking. Such findings provide valuable information for developing noncovalent inhibitors targeting Mpro.
PLpro is also an attractive antiviral drug target. This protease regulates the process of virus protein maturation (Baez-Santos et al. 2015), and participate in inhibiting the production of cytokines and chemokines, which are crucial in the host innate immune response against viral infection (Devaraj et al. 2007; Frieman et al. 2009; Clementz et al. 2010; Calistri et al. 2014; Mielech et al. 2014). Therefore, knowledge of PLpro inhibitors is important for the rational design of antivirus drugs. In this work, we identified 205 potent PLpro inhibitors with diverse chemical structures. 72 PLpro inhibitors showed at least 50% inhibition of viral replication at the concentration of 10 µg/mL (Fig. 3A; Tables S1 and S2), indicating PLpro is a viable target for developing antiviral drugs against SARS-CoV-2. The cytotoxicity assays revealed that 40 PLpro inhibitors exposed over 30% of cytotoxicity (Fig. 3B), suggesting PLpro may share a similar binding site with its isozymes in host cells. SARS-CoV-2 PLpro is homologous to human deubiquitinating enzymes including more than 40 cysteine proteases (Daviet and Colland 2008). The high cytotoxicity of some PLpro inhibitors might be a consequence of the off-target effects on the human deubiquitinating enzymes. In this work, we identified a number of potent PLpro inhibitors with low cytotoxicity. These inhibitors can be directly used in the development of safe drugs targeting pathogenic PLpro without inhibitor host deubiquitinating enzymes.
In short, we performed high-throughput screening of approximately 1.8 million small-molecule compounds against two major proteases, Mpro and PLpro of SARS-CoV-2. 2,050 hits were discovered, including six compounds that could inhibit both proteases. Further cell-based antiviral assays and molecular docking results identifies over 100 inhibitors with high antiviral potency as well as low cytotoxicity. A potent covalent inhibitor of Mpro was identified through these assays. The X-ray crystal structure of SARS-CoV-2 Mpro in complex with its potent inhibitor 4a was determined at 1.8 Å resolution. The compounds identified through our screening paradigm and the molecular basis of the protease-inhibitor interactions revealed in this work will greatly facilitate the future drug development targeting COVID-19 and other coronaviruses.
Materials and methods
Expression and purification of Mpro
The full-length gene encoding SARS-CoV-2Mpro protein was code optimized and synthesized in pGEX6p-1vector for E. coli expression (Genewiz). The expression plasmid was transformed into E. coli BL21 (DE3) cells and then cultured in Luria broth (LB) media containing 100 µg/mL ampicillin at 37 °C, 200 rpm. When the cells were grown to OD600 of 0.6–0.8, 0.5 mmol/L IPTG was added to the cell culture to induce the expression of the recombinant 2019-nCoV Mpro protein at 16 °C, 180 rpm, overnight, then the cells were harvested by centrifugation at 3000 ×g for 20 min. Cell pellets were resuspended in lysis buffer (20 mmol/L Tris–HCl, pH 8.0, 300 mmol/L NaCl), lysed by 4–5 rounds of high-pressure homogenization, and then centrifuged at 25,000 ×g for 40 min. The supernatant was loaded onto an Ni-NTA affinity column and binding for 2 h at 4 °C, and washed by gradient concentration of resuspension buffer containing 0–30 mmol/L imidazole. The His tagged Mpro protein was eluted by cleavage buffer (50 mmol/L Tris–HCl, pH 7.5, 150 mmol/L NaCl) containing 300 mmol/L imidazole. The sample was treated overnight with His-tagged PreScission protease to remove the C-terminal His tag. The Ni-NTA resin (Qiagen) was incubated with the sample at 4 °C for 1 h to remove the cleaved His-tag and PreScission protease. The purified Mpro protein was concentrated using a 10 kDa molecule weight cut-off concentrator (Millipore), and subjected to size exclusion chromatography Superdex 200 increase 10/300 for buffer exchange to 50 mmol/L Tris–HCl, pH 7.3, 1 mmol/L EDTA.
Expression and purification of PLpro
The PLpro was inserted into pET-22b (+) followed by a PreScission protease site and a 6× His-tag at the C terminus. The transformed E. coli BL21 (DE3) cells were cultured in LB medium containing 75 µg/mL ampicillin at 37 °C for 4 h. Protein was then induced by adding 0.5 mmol/L IPTG and incubated over-night at 16 °C. The cells were harvested by centrifugation at 6,200 ×g for 15 min at 4 °C.
The cell pellet was suspended in binding buffer (50 mmol/L Tris–HCl pH 8.0, 150 mmol/L NaCl, 2 mmol/L DTT) followed by dounce homogenization and cells were disrupted by ultra-high pressure cell disrupters (JNBIO) at 4 °C. After centrifugation at 160,000 ×g for 30 min, the supernatant was collected and incubated with Ni-NTA resin supplied with 5 mmol/L imidazole. After a 2 h-incubation at 4 °C, the resin was washed with 30 column volumes of washing buffer I (50 mmol/L Tris–HCl pH 8.0, 150 mmol/L NaCl, 10 mmol/L imidazole, 2 mmol/L DTT) followed by four column volumes of washing buffer II (50 mmol/L Tris–HCl pH 8.0, 150 mmol/L NaCl, 20 mmol/L imidazole, 2 mmol/L DTT) and three column volumes of washing buffer III (50 mmol/L Tris–HCl pH 8.0, 150 mmol/L NaCl, 30 mmol/L imidazole, 2 mmol/L DTT). Then the protein was eluted with elution buffer (50 mmol/L Tris–HCl pH 8.0, 150 mmol/L NaCl, 300 mmol/L imidazole, 2 mmol/L DTT) and further purified by gel filtration using a Superdex 75 (GE Healthcare) gel filtration column.
Enzymatic activity and inhibition assays
The enzyme activity and inhibition assays of SARS-CoV-2 Mpro have been described previously (Dai et al. 2020b; Jin et al. 2020b). Briefly, the recombinant SARS-CoV-2 Mpro (40 nmol/L at a final concentration) was mixed with each compound in 50 µL assay buffer (20 mmol/L Tris, pH7.3, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Glycerol, 0.01% Tween-20) and incubated for 10 min. The reaction was initiated by adding the fluorogenic substrate MCA-AVLQSGFRK (DNP) K (GL Biochem, Shanghai), with a final concentration of 20 µmol/L. After that, the fluorescence signal at 320 nm (excitation)/405 nm (emission) was immediately measured by continuous 8 points for 8 min with an EnVision multimode plate reader (Perkin Elmer, USA). The initial velocity was measured when the protease reaction was proceeding in a linear fashion.
The activity of SARS-CoV-2 PLpro was also measured by a continuous 8 points fluorometric assay for 8 min. Briefly, the recombinant SARS-CoV-2 PLpro (40 nmol/L at a final concentration) was mixed with each compound in 50 µL assay buffer (20 mmol/L Tris pH 8.0, 0.01% Tween20, 0.5 mmol/L DTT) and incubated for 10 min. The reaction was initiated by adding the substrate Z-RLRGG-AMC (GL Biochem, Shanghai) with a final concentration of 50 µmol/L, using wavelengths of 355 nm and 460 nm for excitation and emission, measured by an EnVision multimode plate reader (Perkin Elmer, USA).
High-throughput screen and IC50 measurement
Potential inhibitors against SARS-CoV-2 Mpro and PLpro were screened by an enzymatic inhibition assay carried outinblack 384-well plates (OptiPlateTM-384F, PerkinElmer). The Vmax of reactions added with different compounds compared to the reaction added with DMSO were calculated and used to generate inhibitory rate and IC50. The ~1,800,000 compounds of CNCL bought from ChemDiv (USA), ChemBridge (USA), Life Chemicals (Canada), Specs (Holland) and donated by NovoNordisk (Denmark) were primarily screened against Mpro and PLpro at the concentration of 10 and 8 µg/mL respectively. And 3,987 bio-active compounds containing approved drugs, clinical trial drug candidates, preclinical drug candidates bought from MCE (USA), ApeBio (USA), Selleck (USA), and TargetMol (USA) were also primarily screened against PLpro at the concentration of 20 µmol/L. Hits identified from the primary screen assayed in single well were subsequently secondary screened against SARS-CoV-2 Mpro and PLprowith two concentrations (1 and 10 µg/mL) in duplicates. For selected potential inhibitors which showed two doses dependence and more than 50% inhibition at higher concentration, IC50 values against SARS-CoV-2 Mpro and PLpro were measured at eight concentrations and three independent experiments were performed. All experimental data was analyzed using GraphPad Prism software.
Compounds clustering
The original set with 1,873 compounds was further processed by removing the molecules having poorly specified and then represented as Extended-Connectivity Fingerprints1 (ECFP) and tanimoto similarity matrix was calculated. Hierarchical clustering algorithms2 and average linking method were chosen for compounds clustering. Different similarity threshold was tried, and similarity threshold of 0.1 was chosen by clustering performance and synthetic feasibility. Finally, representative structures of 421 group were kept for further use.
Antiviral assays
Vero cells were seeded in 96-well plates at a density of 6,000 cells per well in a total volume of 100 µL per well and incubated overnight at 37 °C and 5% CO2. Cell monolayers were treated with the compounds at a final concentration of 10 µg/mL or 10 µmol/L for 1 h, and infected with SARS-CoV-2 at an MOI of 0.01. At 24 h p.i., cells were fixed and incubated with rabbit anti- NP antibody, followed by anti-rabbit Alexa488 (Abcam) and DAPI (Beyotime). The plates were imaged using Operetta (PerkinElmer) with a 10× objective. Nine images were acquired per well in both the DAPI and 488 nm channels. The percentages of infected and DAPI-positive cells were calculated using automated image analysis software (Harmony 3.5, PerkinElmer). A positive control of anti-SARS-CoV-2 compound (Chloroquine diphosphate salt, Sigma, C6628) and vehicle (DMSO) were handled in the same way as for the compound library during drug screening in a blinded fashion, with the compound identities unknown to the experimenters.
Homology modeling and molecular docking
Eight published SARS-CoV-2Mpro structures deposited in the Protein Data Bank (www.rcsb.org) were selected for molecular docking. Their PDB codes are 6LU7, 6LZE, 6M0K, 6Y2E, 6Y2F, 6Y2G, 7BQY, 7BUY. Six published SARS-CoV-2 PLpro structures deposited in the Protein Data Bank were selected for molecular docking. Their PDB codes are 6W9C, 6WRH, 6YVA, 6WX4, 6WZU, 6WUU. To include more structural information for docking analysis, we also used the ligand-bound SARS-CoV PLpro structures as templates to build the homology models of SARS-CoV-2 PLpro and performed molecular docking against these models. Their PDB codes are 3E9S, 3MJ5, 4M0W, 4OVZ, 4OW0, 5E6J, and 5Y3E. Modeller (Sali 1995) was used to perform homology modeling with the default parameters. Thirteen resulting models with the lowest RMSD from their templates were selected for further analysis. A compound of interest was docked to its receptor using Schrodinger Glide software in SP mode with default parameters (Friesner et al. 2004). The ligand was initially placed in the center of the pocket and was constrained to move within 1 nm diameter sphere, where it was allow freely moving during the docking process. The extended conformation searches were performed using Lamarckian Genetic Algorithm. The docking model with the lowest binding energy was selected for analysis. When the binding energy score of a compound to a receptor model is larger than −6.0, the resulting docking model is excluded.
Supplementary Material
Acknowledgments
The authors would like to thank J. S. Shen, H. Chen, W. B. Yang, Y. Zhou, T. F. Xu and X. J. Lu for expert advice on chemistry discussion. The High-throughput studies were performed at Chinese National Compound Library (CNCL, http://www.cncl.org.cn), Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
Glossary
Abbreviations
- 3CLpro
3C-like protease
- CNCL
the Chinese National Compound Library
- COVID-19
coronavirus disease 2019
- IC50
half maximal inhibitory concentration
- Mpro
main protease
- PLpro
papain like protease
- Nsp
non-structural protein
- HTS
high-throughput screening
- RdRp
RNA-dependent RNA polymerase
- SARS-CoV-2
Severe acute respiratory syndrome-coronavirus 2
Contributor Information
Yi Zang, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China.
Mingbo Su, School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing 210023, China.
Qingxing Wang, University of Chinese Academy of Sciences, Beijing 100049, China; State Key Laboratory of Virology, Wuhan Institute of Virology, Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan 430071, China.
Xi Cheng, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China.
Wenru Zhang, School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing 210023, China.
Yao Zhao, Shanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China.
Tong Chen, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Yingyan Jiang, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Qiang Shen, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Juan Du, School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China.
Qiuxiang Tan, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Peipei Wang, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Lixin Gao, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Zhenming Jin, Shanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China.
Mengmeng Zhang, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Cong Li, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Ya Zhu, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Bo Feng, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Bixi Tang, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Han Xie, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China.
Ming-Wei Wang, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Mingyue Zheng, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Xiaoyan Pan, State Key Laboratory of Virology, Wuhan Institute of Virology, Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan 430071, China.
Haitao Yang, Shanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China.
Yechun Xu, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Beili Wu, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China; University of Chinese Academy of Sciences, Beijing 100049, China; CAS Center for Excellence in Biomacromolecules, Chinese Academy of Sciences, Beijing 100101, China.
Leike Zhang, State Key Laboratory of Virology, Wuhan Institute of Virology, Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan 430071, China.
Zihe Rao, Shanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China.
Xiuna Yang, Shanghai Institute for Advanced Immunochemical Studies and School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China.
Hualiang Jiang, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China; University of Chinese Academy of Sciences, Beijing 100049, China.
Gengfu Xiao, State Key Laboratory of Virology, Wuhan Institute of Virology, Center for Biosafety Mega-Science, Chinese Academy of Sciences, Wuhan 430071, China.
Qiang Zhao, State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China; CAS Center for Excellence in Biomacromolecules, Chinese Academy of Sciences, Beijing 100101, China; Zhongshan Branch, the Institute of Drug Discovery and Development, Chinese Academy of Sciences, Guangdong 528400, China.
Jia Li, The National Center for Drug Screening, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; State Key Laboratory of Drug Research and CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China; School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, UCAS, Hangzhou 310024, China; School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing 210023, China; Zhongshan Branch, the Institute of Drug Discovery and Development, Chinese Academy of Sciences, Guangdong 528400, China.
Funding
This work was supported by the National Key R&D Program of China 2018YFA0507000 (B.W, Q.Z.), 2018ZX09735001 (Y.J.) and 2020YFC0844500 (J.L.), the National Science Foundation of China grants 31825010 (B.W.), 81525024 (Q.Z.), 81673489 (J.L), the Key Research Program of Frontier Sciences, CAS grants QYZDB-SSW-SMC024 (B.W.) and QYZDB-SSW-SMC054 (Q.Z.), Fund of Chinese Academy of Sciences 2020YJFK0105 (J.L.), Chinese Academy of Engineering and Jack Ma Foundation 2020-CMKYGG-05 (J.D.), the Shanghai Science and Technology Development Funds 20431900200 (J.L.) and K. C. Wong Education Foundation (J.L.), Fund of Youth Innovation Promotion Association 2018319 (X.C.), and the Hubei Science and Technology Project 2020FCA003 (G.X.). Fund of Chinese Academy of Sciences 2020YJFK0105 (J.L.)
Authors’ contributions
Yi Zang designed and performed the high-throughput studies of Mpro. Mingbo Su designed and performed the high-throughput studies of PLpro. Xi Cheng performed the molecular dockings. Wenru Zhang optimized the purification procedures and prepared the protein samples of Mpro. Tong Chen optimized the purification procedures and prepared the protein samples of PLpro. Yingyan Jiang helped in analyzing the screening data. Qiang Shen, Peipei Wang, Lixin Gao, Mengmeng Zhang, Cong Li performed the high-throughput screening of Mpro and PLpro. Bo Feng, Bixi Tang helped in the HTS performance and manuscript writing. Yao Zhao, Juan Du, Qiuxiang Tan and Ya Zhu helped in preparing the protein sample. Mingyue Zheng performed chemical structure clustering. Haitao Yang, Yechun Xu, Zihe Rao, Xiuna Yang and Beili Wu helped with purification and docking data analysis. Xiaoyan Pan, Leike Zhang designed and performed anti-viral assays. Hualiang Jiang oversaw the docking strategy and final data analyze. Gengfu Xiao oversaw anti-viral assays. Qiang Zhao oversaw the sample preparation and screening analysis and processing. Jia Li initiated the project, planned and analyzed experiments, supervised the research, and wrote the manuscript with input from all co-authors.
Conflict of interest statement: The authors declare they have no conflict of interest. Informed consent was obtained from all individual participants included in the study.
Ethical approval
This article does not contain any studies with human or animal subjects performed by the any of the authors.
References
- Adhikari SP, Meng S, Wu YJet al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 2020;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez-Santos YM, St John SE, Mesecar AD.. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res 2015;115:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Ye F, Feng Yet al. Structural basis for the inhibition of the SARS-CoV-2 main protease by the anti-HCV drug narlaprevir. Signal Transduct Target Ther 2021;6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calistri A, Munegato D, Carli Iet al. The ubiquitin-conjugating system: multiple roles in viral replication and infection. Cells 2014;3:386–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Wang Y, Wen Det al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz MA, Chen Z, Banach BSet al. Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases. J Virol 2010;84:4619–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daczkowski CM, Goodwin OY, Dzimianski JVet al. Structurally guided removal of DeISGylase biochemical activity from papain-like protease originating from middle east respiratory syndrome coronavirus. J Virol 2017;91:e01067-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Zhang B, Jiang XMet al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020a;368:1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Zhang B, Su Het al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020b;368:1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviet L, Colland F.. Targeting ubiquitin specific proteases for drug discovery. Biochimie 2008;90:270–283. [DOI] [PubMed] [Google Scholar]
- Devaraj SG, Wang N, Chen Zet al. Regulation of IRF-3-dependent innate immunity by the papain-like protease domain of the severe acute respiratory syndrome coronavirus. J Biol Chem 2007;282:32208–32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douangamath A, Fearon D, Gehrtz Pet al. Crystallographic and electrophilic fragment screening of the SARS-CoV-2 main protease. Nat Commun 2020;11:5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante-Mangoni E, Andini R, Bertolino Let al. Early experience with remdesivir in SARS-CoV-2 pneumonia. Infection 2020;48:779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas BT, Durie IA, Murray Jet al. Characterization and noncovalent inhibition of the deubiquitinase and deISGylase activity of SARS-CoV-2 papain-like protease. ACS Infect Dis. 2020;6:2099–2109. [DOI] [PubMed] [Google Scholar]
- Frieman M, Ratia K, Johnston REet al. Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-kappaB signaling. J Virol 2009;83:6689–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesner RA, Banks JL, Murphy RBet al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 2004;47:1739–1749. [DOI] [PubMed] [Google Scholar]
- Fu L, Ye F, Feng Yet al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat Commun 2020;11:4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J, Ohmagari N, Shin Det al. Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med 2020a;382:2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J, Ohmagari N, Shin Det al. Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med 2020b;382:2327–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy RK, DiPaola RS, Romanelli Fet al. Rapid repurposing of drugs for COVID-19. Science 2020;368:829–830. [DOI] [PubMed] [Google Scholar]
- Hoffman RL, Kania RS, Brothers MAet al. Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J Med Chem 2020;63:12725–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Du X, Xu Yet al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020a;582:289–293. [DOI] [PubMed] [Google Scholar]
- Jin Z, Du X, Xu Yet al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020b;582:289–293. [DOI] [PubMed] [Google Scholar]
- Kneller DW, Galanie S, Phillips Get al. Malleability of the SARS-CoV-2 3CL M(pro) active-site cavity facilitates binding of clinical antivirals. Structure 2020;28:1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Moses DC, Hsieh CHet al. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antiviral Res 2018;150:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo-Galo N, Terrazas-Lopez M, Martinez-Martinez Aet al. FDA-approved thiol-reacting drugs that potentially bind into the SARS-CoV-2 main protease, essential for viral replication. J Biomol Struct Dyn 2021;39:3419–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockbaum GJ, Reyes AC, Lee JMet al. Crystal structure of SARS-CoV-2 main protease in complex with the non-covalent inhibitor ML188. Viruses 2021;13:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Lauer Y, Carbajo-Lozoya J, Hein MYet al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc Natl Acad Sci USA 2016;113:E5192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielech AM, Kilianski A, Baez-Santos YMet al. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology 2014;45:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaiyar T, Manickam M, Namasivayam Vet al. An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J Med Chem 2016;59:6595–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Li YS, Zeng Ret al. SARS-CoV-2 M(pro) inhibitors with antiviral activity in a transgenic mouse model. Science 2021;371:1374–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratia K, Pegan S, Takayama Jet al. A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc Natl Acad Sci USA 2008;105:16119–16124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratia K, Saikatendu KS, Santarsiero BDet al. Severe acute respiratory syndrome coronavirus papain-like protease: structure of a viral deubiquitinating enzyme. Proc Natl Acad Sci USA 2006;103:5717–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco MD, Ma C, Lagarias Pet al. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M(pro) and cathepsin L. Sci Adv 2020;6:eabe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A. Comparative protein modeling by satisfaction of spatial restraints. Mol Med Today 1995;1:270–277. [DOI] [PubMed] [Google Scholar]
- Su HX, Yao S, Zhao WFet al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin 2020;41:1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulea T, Lindner HA, Purisima EOet al. Binding site-based classification of coronaviral papain-like proteases. Proteins 2006;62:760–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Verschueren KH, Anand Ket al. pH-dependent conformational flexibility of the SARS-CoV main proteinase (M(pro)) dimer: molecular dynamics simulations and multiple X-ray structure analyses. J Mol Biol 2005;354:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchesnokov EP, Feng JY, Porter DPet al. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by Remdesivir. Viruses 2019;11:326–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PGT, Whittaker C, Watson OJet al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science 2020;369:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Liu Y, Yang Yet al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B 2020a;10:766–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Zhao S, Yu Bet al. A new coronavirus associated with human respiratory disease in China. Nature 2020b;579:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KS, Ma XR, Ma Yet al. A quick route to multiple highly potent SARS-CoV-2 main protease inhibitors*. ChemMedChem 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xie W, Xue Xet al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol 2005; 3:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Mao C, Luan Xet al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by Remdesivir. Science 2020;368:1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lin D, Sun Xet al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science 2020;368:409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A, Chan JF, Azhar EIet al. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov 2016;15: 327–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.