Abstract
Background:
The combination of neuromuscular impairments plus psychosocial aspects of chronic kidney disease (CKD) may predispose these patients to greater risk for experiencing increased levels of fatigability. There has been extensive clinical and scientific interest in the problem of fatigue in CKD and end-stage kidney disease (ESKD) patients, whereas less attention has been directed to understanding fatigability. Accordingly, the primary purposes of this review are to (1) discuss fatigue and fatigability and their potential interactions in patients with CKD and ESKD, (2) provide evidence for increased fatigability in CKD and ESKD patients, (3) examine how commonly experienced neuromuscular impairments in CKD and ESKD patients may contribute to the severity of performance fatigability, and (4) highlight preliminary evidence on the effects of exercise as a potential clinical treatment for targeting fatigability in this population.
Summary:
Fatigue is broadly defined as a multidimensional construct encompassing a subjective lack of physical and/or mental energy that is perceived by the individual to interfere with usual or desired activities. In contrast, fatigability is conceptualized within the context of physical activity and is quantified as the interactions between reductions in objective measures of performance (i.e., performance fatigability) and perceptual adjustments regulating activity performance (i.e., perceived fatigability). We propose herein a conceptual model to extend current understandings of fatigability by considering the interactions among fatigue, perceived fatigability, and performance fatigability. Neuromuscular impairments reported in patients with CKD and ESKD, including reductions in force capacity, skeletal muscle atrophy, mitochondrial dysfunction, abnormal skeletal muscle excitability, and neurological complications, may each contribute to the greater performance fatigability observed in these patients.
Key Messages:
Considering the interactions among fatigue, perceived fatigability, and performance fatigability provides a novel conceptual framework to advance the understanding of fatigability in CKD and ESKD patients. Measures of fatigability may provide valuable clinical insights into the overall health status of CKD and ESKD patients. Existing data suggest that CKD and ESKD patients are at greater risk of experiencing increased fatigability, partly due to neuromuscular impairments associated with reduced kidney function. Further investigations are warranted to determine the potential clinical role fatigability measures can play in monitoring the health of CKD and ESKD patients, and in identifying potential treatments targeting fatigability in this patient population.
Keywords: Fatigue, Fatigability weakness, Depression, Functional impairment, Renal failure
Introduction
Chronic kidney disease (CKD) is a debilitating condition affecting approximately 10%–15% of the global population [1]. Fatigue is one of the most common symptoms in patients with CKD not on dialysis, and in those with end-stage kidney disease (ESKD) [2–7]. Fatigue is broadly defined as a multidimensional construct encompassing a subjective lack of physical and/or mental energy that is perceived by the individual to interfere with usual or desired activities [8–10]. Patients with CKD who experience greater fatigue are at an increased risk of adverse cardiovascular events, decreased quality of life, and mortality [3, 11].
Fatigability, in contrast to fatigue, is conceptualized within the context of physical activity and is quantified as the interactions between reductions in objective measures of performance (i.e., performance fatigability) and perceptual changes regulating activity performance (i.e., perceived fatigability) [12]. While the symptom of fatigue can be chronic in nature, fatigability is reversible with rest [13, 14]. The predominant factors that affect fatigability are strongly influenced by the activity being performed [12, 15–17]. For example, during tasks isolating a specific muscle or muscle group(s), the primary mechanisms contributing to fatigability are directly related to the active muscles involved in performing the task [16, 18]. In comparison, during whole-body activities such as walking, there is an increase in the amount of active muscle mass and required support from other physiological systems to sustain muscle function [18]. This increases the potential involvement of psychobehavioral and central nervous system (CNS) factors contributing to fatigability [14, 19, 20]. In clinical populations, additional factors such as fatigue, lack of motivation, anxiety, depression, disease/injury severity, and physical inactivity may contribute more importantly to fatigability and must also be considered [12, 14, 21].
The combination of neuromuscular impairments plus psychosocial aspects of CKD may predispose these patients to greater risk for experiencing increased fatigability [22–24]. There has been extensive clinical and scientific interest in the problem of fatigue in CKD and ESKD patients, whereas less attention has been directed to understanding fatigability. Although fatigue is a major challenge to everyday life, fatigue is not synonymous with fatigability [8, 12, 13, 25–31]. Accordingly, the primary purposes of this review are to (1) discuss fatigue and fatigability and their potential interactions in patients with CKD and ESKD, (2) provide evidence for increased fatigability in CKD and ESKD patients, (3) examine how commonly experienced neuromuscular impairments in CKD and ESKD patients may contribute to the severity of performance fatigability, and (4) highlight preliminary evidence of the effects of exercise as a potential clinical treatment for targeting fatigability in this population.
Fatigue and Fatigability and Their Potential Interactions in Patients with CKD and ESKD
Fatigue is often reported as a symptom of disease reflecting a formed representation of the specific condition [10, 32]. For example, fatigue has been proposed as a state of feeling in which there is a lack of motivation to deploy resources and engage in high effort performance to cope with a situation [10]. In this context, fatigue in CKD patients may occur as an adaptive process resulting from declines in kidney function that allow for mobilization of critical resources while preserving energy necessary for the maintenance of homeostasis so as to ensure survival [33–35]. Factors associated with fatigue in non-dialysis CKD patients include unemployment, comorbidities, antidepressant medication use, and anemia [36]. In a multivariable regression analysis, Jhamb et al. [37] observed that cardiovascular disease, low serum albumin, depressive symptoms, poor subjective sleep quality, excessive daytime sleepiness, and restless leg syndrome were independently associated with greater fatigue in CKD and ESKD patients. The findings of these studies highlight the multitude of factors associated with fatigue in non-dialysis CKD and ESKD patients, and their potential differences based on the severity of disease.
Complicating the understanding of fatigue in patients with ESKD is the phenomenon of post-dialysis fatigue. Such patients often report feelings of physical and mental fatigue immediately following dialysis treatment [38]. Symptoms of physical fatigue include feeling a lack of strength, worn out, drained, or exhausted. Regarding symptoms of mental fatigue following dialysis treatment, patients reported the inability to remember conversations, name recall challenges, and forgetfulness of where they were driving in their cars [38]. The time to recover from dialysis sessions varies among individuals and ranges from minutes to days but is typically resolved within 4 h [2, 39]. Importantly, the time to recover from dialysis is significantly related to fatigue status [40]. The type and duration of kidney replacement therapy, osmotic dis-equilibrium, blood membrane interactions, isolated ultrafiltration and diffusion, and serum cytokine concentrations all influence the level of fatigue experienced after completing a dialysis session [2, 3, 41].
Psychological disorders and cognitive impairment are common in patients with CKD. Depression occurs in up to 20%, and anxiety in 10%–50%, of CKD patients [42–47]. Patients with depression and anxiety disorders are more likely to score higher on self-reported fatigue scales [41]. Cognitive impairment is associated with the severity of kidney disease and is thought to result from an accumulation of uremic neurotoxins that interact with neural progenitor cells, brain vasculature, the lymphatic system, and monoaminergic neurons [48–51]. Impaired kidney function has also been associated with reductions in cerebral gray matter volume and cortical thickness [52]. The high prevalence of depression, anxiety, and cognitive impairment is particularly salient when assessing fatigue and fatigability of CKD patients. While depression, anxiety, and fatigue constitute separate psychological states, their interrelationship has the potential to impact a person’s willingness to engage in physical activity or exert oneself (i.e., perceived fatigability) [10, 20, 53]. This may help explain the observation that fatigue and low energy levels are the most common perceived barriers to exercise in patients with CKD and ESKD [54].
From a bioenergetic perspective, dysregulation of energy use (i.e., adenosine triphosphate, ATP) is a principal mechanism responsible for performance fatigability [55]. Augmented breakdown of ATP increases circulating and tissue concentrations of metabolic byproducts (hydrogen ions and inorganic phosphate) and decreases calcium sensitivity, resulting in impaired skeletal muscle cross-bridge function during high-intensity physical activity [15, 16, 56, 57]. In comparison, fatigability experienced during moderate-intensity activity is thought to result from reductions in substrate availability and muscle activation [14, 58–62]. Sensory feedback from receptors located in skeletal muscle is sent to the CNS via myelinated (group III) and unmyelinated (group IV) nerve fibers [58, 59]. The afferent information received by the CNS from the group III/IV nerve fibers during skeletal muscle contraction exerts inhibitory influences on the central motor drive and muscle activation [58, 59]. Reductions in physiologic capacity and/or increases in energetic cost of muscle contraction with aging place greater strain on bioenergetic systems, accelerating the accumulation of metabolic by-products and the increased sense of effort required to perform a given activity [20, 56, 63, 64].
Recent efforts have been made to further clarify distinctions between fatigue and fatigability [8, 12, 25–31]. These efforts are influenced, in part, by the lack of significant associations between measures of fatigue and performance fatigability in the medical literature, suggesting potential differences in biological underpinnings [25, 65–72]. Perceived fatigablity has been shown to be significantly associated with heightened perceived effort and reduced affect, but not performance fatigability, when assessed during knee extensor contractions [73]. The transient changes in an individual’s psychophysiological state influence the decision or desire to continue with activity performance [20, 53]. In ESKD patients, this is reflected in how individuals adjust the timing and intensity of their activities to accommodate their level of fatigue [38]. Fatigability thus emerges as the interactions among fatigue, perceived fatigability, and performance fatigability[12]. Such a model also allows possible underlying determinants of fatigue, perceived fatigability, and performance fatigability to be identified, and to be used in combination to inform the clinical decision-making process. Furthermore, simultaneous monitoring of each domain may provide a more comprehensive understanding of how fatigue and fatigability respond to specific interventions [74].
Evidence of Elevated Fatigability in Patients with CKD and ESKD
Evidence from several cross-sectional studies suggests that such individuals are more susceptible to greater levels of fatigability than are their age-matched healthy counterparts (Table 1) [75–79]. For example, Johansen et al. [75] observed greater performance fatigability of the ankle dorsiflexors during maximum voluntary isometric contractions in dialysis patients versus control subjects. Similarly, performance fatigability, as determined during thirty maximal isokinetic contractions at 180°/s, was estimated to be 1.6-fold higher in kidney transplant and hemodialysis (HD) patients than in control subjects [76]. During rhythmic hand-grip exercise, HD patients exhibited greater fatigability than did transplant recipients or control subjects [79]. Macdonald et al. [77] noted that patients with CKD stages 3b and 4 reported greater ratings of perceived exertion than did control subjects when engaging in exercise intensities representative of various activities of daily living.
Table 1.
Studies examining fatigability in CKD and ESKD patients
| Author(s), year | Sample size, n | Fatigability measurement | Outcome: Performance fatigability | Outcome: Perceived fatigability |
|---|---|---|---|---|
| Johansen et al. [76], 2005 | Dialysis subjects: n = 33 Control subjects: n = 12 |
Performance fatigability = began at 10% MVC of the ankle dorsiflexors and increased by 10% every 2 min for 14 min Performance FI (%) = ([MVC preexercise − MVC postexercise]/MVC preexercise) × 100 |
All controls completed the performance fatigability protocol whereas only 18 dialysis subjects were able to complete the protocol Performance FI (%)
|
NA |
| Petersen et al. [77], 2012 | Kidney transplant subjects: n = 9 HD subjects: n = 10 Control subjects: n = 10 |
Performance fatigability = 30 maximal isokinetic knee extensor contractions at 180°s−1 Performance FI (%) = ([starting peak torque-final peak torque]/starting peak torque) × 100, where starting peak torque is the average of the highest 3 of the first 5 repetitions and final peak torque is the highest of the 3 of the last 5 repetitions |
Performance FI was significantly higher in the kidney transplant subjects (24%) and HD subjects (25%) compared to the control subjects (15%), with no differences between kidney transplant and HD subjects | NA |
| Macdonald et al. [78], 2012 | CKD subjects (stages 3b–4): n = 13 Control subjects: n = 13 |
Perceived fatigability = RPE (6–20) were recorded in the last 30 s at each intensity during submaximal cycle ergometry exercise | NA | RPE was significantly greater in CKD subjects compared to control subjects at each exercise intensity assessed (1.8 METs, 2.4 METs, 3.1 METs) |
| Petersen et al. [79], 2009 | HD subjects: n = 8 Control subjects: n = 6 |
Performance fatigability = 30 maximal isokinetic knee extensor contractions at 180°s−1 Performance FI (%) = ([starting peak torque-final peak torque]/starting peak torque) × 100, where starting peak torque is the average of the highest 3 of the first 5 repetitions and final peak torque is the highest of the 3 of the last 5 repetitions |
Performance FI data were excluded on one subject due to highly variable and unreliable results Performance FI was higher in HD subjects at baseline (27±2%, p < 0.001), pretraining (26±4%, p < 0.005), and post-training (27±9%, p < 0.001) compared to control subjects (13±3%) Performance FI was unchanged in HD subjects (baseline, 27±2%; pretraining, 26±4%; and post-training, 27±9%) following 30 min of aerobic exercise performed on a cycle ergometer 3 times per week for 6 weeks at intensities corresponding to 50–80.5% pretraining peak oxygen consumption |
NA |
| Moore et al. [80], 1993 | Chronic HD subjects: n = 11 kidney transplant recipients: n = 11 Control subjects: n = 9 |
Performance fatigability consisted of handgrip MVCs. Subjects were asked to perform 1-s isometric contractions followed by a 9-s relaxation period and instructed to attempt to equal their MVC with each repetition Performance FI was defined as the inability to maintain a percentage of the maximal voluntary contraction |
Chronic HD subjects experienced greater fatigability (71±2%) than kidney transplant recipients (78±1%) and control subjects (81±1%) | NA |
n, sample size; FI, fatigability index; RPE, rating of perceived exertion; HD, hemodialysis; CKD, chronic kidney disease; ESKD, end-stage kidney disease; MVC, maximal voluntary contraction.
To our knowledge, few longitudinal studies have been reported examining changes in measures of fatigability in patients with CKD. In one 2-year longitudinal study in patients with non-dialysis-dependent CKD, whole-body performance fatigability increased, as measured by reductions in peak oxygen consumption [80]. However, in the same study, knee extensor performance fatigability remained unchanged, despite reductions in creatinine clearance and isokinetic strength [80]. More and larger longitudinal studies are warranted to determine the extent to which fatigability levels change over time in CKD and ESKD patients [80].
Neuromuscular Impairments Influencing Fatigability in Patients with CKD and ESKD
The remainder of this review is focused on commonly observed neuromuscular impairments that are likely to exacerbate performance fatigability in CKD and ESKD patients (shown in Fig. 1). This represents just one example of how consequences associated with CKD and ESKD could exacerbate fatigability. Moreover, the influences of performance fatigability resulting from neuromuscular impairments on fatigue and perceived fatigability have yet to be thoroughly investigated. Importantly, factors other than neuromuscular impairments are likely to contribute to fatigability in CKD and ESKD patients and should also be considered [7, 81].
Fig. 1.
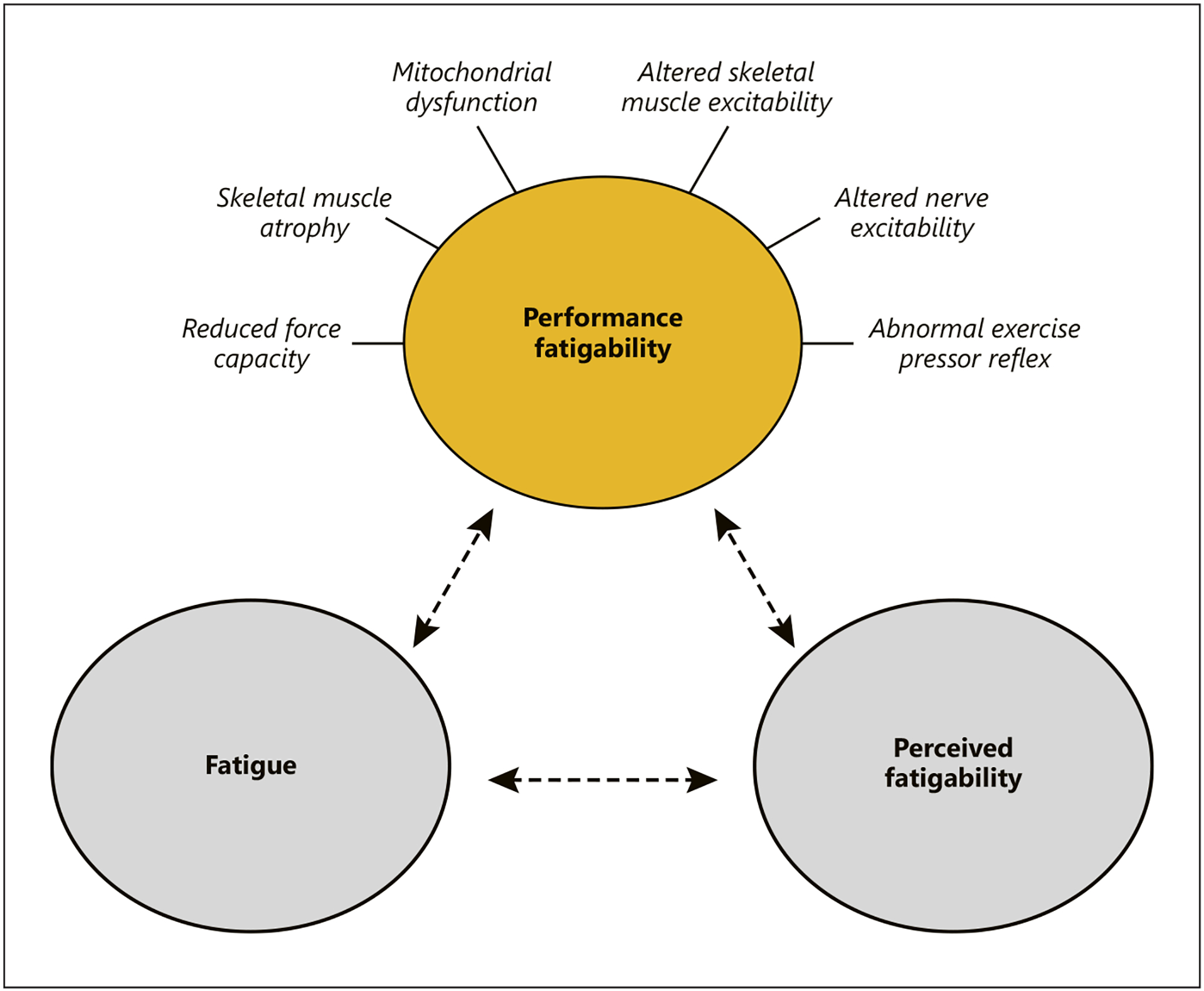
Depicts neuromuscular impairments potentially contributing to performance fatigability in patients with CKD (yellow circle). Fatigue and perceived fatigability may act to further exacerbate performance fatigability (gray circles and dashed lines). Performance fatigability is defined as declines in objective measures of performance and perceived fatigability as the perceptual adjustments regulating activity performance. Fatigue represents a self-reported symptom defined as a subjective lack of physical and/or mental energy that is perceived by the individual to interfere with usual or desired activities.
Force Capacity
Skeletal muscle weakness is commonly associated with CKD and ESKD [82–84]. Although there is some overlap between the mechanisms contributing to muscle weakness and performance fatigability, these two phenomena are not synonymous [85]. For example, after the termination of activity, performance fatigability is mitigated with rest while muscle weakness is evident even in a fully rested state [14]. The reductions in force capacity experienced with CKD may promote greater fatigability through decreases in physiologic reserve [86]. Moreover, this may have a direct impact on functional capabilities, as weaker individuals require a greater percentage of their physiologic capacity to perform a given amount of work. During exercise at lower relative intensities, more rapid depletion of phosphocreatine and increased intracellular acidosis occur in HD patients compared with transplant recipients and control subjects [79]. In comparison, isokinetic knee extensor strength is directly associated with whole-body exercise endurance in patients with CKD and ESKD, suggesting that level of strength positively influences whole-body performance fatigability [80, 87].
Skeletal Muscle Atrophy
Declines in force capacity are, in part, explained by the loss of skeletal muscle mass in individuals with CKD and ESKD [88–90]. Skeletal muscle atrophy has also been associated with declines in certain measures of physical function [88, 89, 91–93]. Individuals with compromised kidney function are predisposed to accelerated skeletal muscle loss via upregulation of protein degradation and downregulation of protein synthesis [94]. Johansen et al. [95] reported decreased amounts of contractile tissue in the anterior compartment of the lower leg in dialysis patients compared to control subjects. Using a subjective clinical assessment for skeletal muscle atrophy, Carrero et al. [96] found that 30% of patients initiating HD and 39% of prevalent HD patients exhibited signs of muscle atrophy. Skeletal muscle cross-sectional area of the mid-thigh, determined via computed tomography, declined 4.3% over a 2-year period in stage 4 CKD and HD patients [88]. Atrophy of type II muscle fibers may, in part, explain the reductions in muscle force capacity observed in this population [84]. Additional mechanisms involved in the promotion of skeletal muscle atrophy may also contribute to increased fatigability, such as alterations in mitochondrial function, which have been identified as a primary cause of skeletal muscle atrophy in aging, physical inactivity, and various diseases, including CKD [97, 98].
Mitochondrial Dysfunction
Skeletal muscle mitochondrial dysfunction is directly associated with disease severity in patients with CKD [99–104]. Mitochondrial dysfunction promotes skeletal muscle atrophy and impairs bioenergetic processes [103–106]. Thome et al. [104] reported significant impairments in oxidative phosphorylation in skeletal muscle of mice with adenine-induced CKD. In other rodent models of CKD (i.e., C57BL/6N), uremic metabolites contribute to decreased energy transfer, impaired complex III and IV enzyme activity, and elevated oxidant production within mitochondria [103]. Similarly, accumulation of the uremic metabolite indoxyl sulfate in skeletal muscle tissue upregulates glycolysis with concomitant downregulation of oxidative metabolism [107]. Indoxyl sulfate also decreases the expression of peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, a principal regulator of mitochondrial biogenesis [105]. Human vastus lateralis skeletal muscle in patients on maintenance HD exhibits reduced enzymatic activity of succinate dehydrogenase, an enzyme involved in oxidative metabolism, compared to that in control subjects [108]. Similarly, in calf muscle of HD patients, energy production via oxidative metabolism was impaired and compensated for by an increase in anaerobic glycolysis [109]. Therefore, in addition to promoting skeletal muscle loss, mitochondrial dysfunction and altered oxidative metabolism may serve as contributing factors to excess fatigability in CKD and ESKD patients.
Skeletal Muscle Excitability
Disturbances in skeletal muscle potassium (K+), sodium (Na+), chloride (Cl−), and Na+-K+ pump activity have been implicated in promoting fatigability [110–113]. In particular, elevations in skeletal muscle K+ concentrations [K+] depolarize the sarcolemma and inactivate voltage-gated Na+ channels, decreasing membrane excitability [111, 113]. During intense muscular activity, ionic shifts can exert profound effects on skeletal muscle contractile function [111]. For example, the rate of interstitial [K+] accumulation is likely to hasten the onset of activity termination by preventing calcium (Ca2+) release [111, 114]. This concept is supported by the finding that a more rapid accumulation of skeletal muscle interstitial [K+] induced by prior arm exercise was associated with a reduced time to exhaustion during subsequent leg exercise [115, 116]. However, interstitial [K+] does not seem to act independently during the fatigability process, but in combination with intracellular [K+], [Na+], and [Cl−] and Na+-K+ pump activity [111].
Abnormal K+ regulation is frequently reported in patients with CKD and may explain, in part, findings of greater fatigability in this population [76, 117–119]. Friedland & Paterson described the potential impact of elevated K+ on performance fatigability during exhaustive cycle ergometry exercise in ESKD patients on maintenance HD [120]. These authors observed arterial plasma [K]+ of about 7 mmol/L at the end of exercise and suggested that this increase in [K+] was sufficient to impair membrane excitability, thereby decreasing muscle contractility [120]. The resting transmembrane potential of myocytes in uremic patients decreases progressively with declining kidney function and assumes a linear relationship with creatinine clearance values below 6.3 mL/min per 1.73 m2 [121]. Of note, maximal Na+-K+ pump activity of the vastus lateralis is reduced by approximately 30% in HD patients and kidney transplantation recipients, and dialysis transiently normalizes sarcolemmal membrane potential, but not t-tubule function [76, 122].
Neurological Impairments
CNS contributions to the suppression of motoneuron excitability can occur via multiple processes, resulting in elevated fatigability [14, 58, 59, 123]. Neurological complications accompany CKD and become more pronounced in those with ESKD [23, 24, 124, 125]. Isaacs observed considerable dropout of motor unit activity during isometric fatigability testing of the abductor pollicis brevis in patients with CKD and clinical neuropathy [126]. Subsequent studies demonstrated that the nerves of uremic patients exhibit a chronically depolarized state before dialysis, with improvement and normalization of nerve resting membrane potential 1 h after a standard 5 h HD session [127]. The magnitude of depolarization was directly related to the serum [K]+, suggesting that depolarization due to chronic elevations in [K]+ plays an important role in the development of nerve dysfunction and performance fatigability in patients with ESKD [24, 127].
The exercise pressor reflex is a pathophysiological mechanism that elicits increased sympathetic nerve activity in the heart, blood vessels, and adrenal medulla, and decreased parasympathetic activity in the heart, so as to ensure matching the circulatory and metabolic demands of muscle contraction [128, 129]. Both mechanical (mechanoreflex) and metabolic (metaboreflex) stimuli produced by contracting skeletal muscle trigger autonomic activation during physical activity [129]. In patients with ESKD, exaggerated increases in the exercise pressor reflex may contribute to fatigability [130]. In this context, ESKD patients exhibit decreased muscle oxygenation at rest and an impaired ability of skeletal muscle to oppose sympathetically mediated vasoconstriction during exercise (functional sympatholysis) [131]. Moreover, reduced flow-mediated dilation has been significantly associated with a higher slope-of-rise in systolic blood pressure during exercise, and poorer exercise capacity, in CKD patients [132]. The aforementioned factors all contribute to greater peripheral resistance, increased myocardial work-load, and diminished blood flow.
Effects of Exercise on Fatigability in Patients with CKD and ESKD
Improvements in energy and strength are the two most desired benefits from exercise in those with ESKD [133]. Exercise interventions exert beneficial effects on fatigue, anxiety, depression, and quality of life in patients with ESKD, and both resistance exercise and aerobic exercise improve neuromuscular and functional outcomes in people with CKD and ESKD [134–139]. In comparison, the effects of exercise on fatigability in CKD and ESKD patients are not as clear, due to the relatively limited number of studies to date. In this regard, in one study, 6 weeks of cycling exercise performed for 30-min a day 3 days/week during the first hour of dialysis treatment increased whole-body performance fatigability [78]. However, in the latter study, knee extensor performance fatigability index did not change significantly despite improvements in absolute and relative peak torque [78]. Similarly, in a recent pilot study, 12 weeks of flywheel resistance exercise in adults with CKD not on dialysis elicited no change in a maximal isometric or isokinetic knee extensor performance fatigability index despite increases in torque capacity [140].
The force-fatigability relationship suggests that, in general, the degree to which fatigability is expressed is related to the amount of force produced, so that greater force elicits greater fatigability [141]. Therefore, patients with CKD and ESKD are seemingly more resistant to activity-induced fatigability following exercise, given that fatigability level was unchanged despite increased force generation [78, 140]. Further investigations are warranted to identify the most effective exercise interventions for reducing fatigability in patients with CKD and ESKD and to determine how various exercise paradigms alter neuromuscular factors implicated in fatigability.
Conclusion
Fatigability reflects activity-induced declines in performance (performance fatigability) and changes in perceptions regulating activity performance (perceived fatigability). Herein, we extend the understanding of fatigability by presenting a conceptual framework that considers fatigability as the interactions among fatigue, perceived fatigability, and performance fatigability and discuss how neuromuscular impairments reported in CKD and ESKD patients may provide a treatment target to diminish performance fatigability. Limited preliminary evidence in CKD and ESKD patients supports the notion that exercise interventions may beneficially affect elevated fatigability. It remains unclear as to whether treatment of secondary sequelae of CKD and ESKD, such as anemia, secondary hyperparathyroidism, and metabolic acidosis, will improve fatigability status. Further investigations are warranted to determine the potential clinical utility fatigability measures might play in monitoring the health of CKD and ESKD patients, and in identifying potential treatments targeting fatigability in this patient population.
Funding Sources
This work was completed as part of a Career Development Award (CDA-2; 1IK2RX003423-01A1) funded by the Rehabilitation Research & Development Service at the VA’s Office of Research & Development (J.M. Gollie). The views expressed here are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Data Availability Statement
All data used to support the findings of this study were obtained from studies included in this article’s reference list.
References
- 1.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017. Mar; 389(10075):1238–52. [DOI] [PubMed] [Google Scholar]
- 2.Bossola M, Tazza L. Postdialysis fatigue: a frequent and debilitating symptom. Semin Dial. 2016. May;29(3):222–7. [DOI] [PubMed] [Google Scholar]
- 3.Jhamb M, Weisbord SD, Steel JL, Unruh M. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis. 2008. Aug;52(2):353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almutary H, Bonner A, Douglas C. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care. 2013. Sep;39(3): 140–50. [DOI] [PubMed] [Google Scholar]
- 5.Murtagh FEM, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007. Jan;14(1):82–99. [DOI] [PubMed] [Google Scholar]
- 6.Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care. 2014. Aug;31(5):562–75. [DOI] [PubMed] [Google Scholar]
- 7.Gregg LP, Bossola M, Ostrosky-Frid M, Hedayati SS. Fatigue in CKD: epidemiology, pathophysiology, and treatment. Clin J Am Soc Nephrol. 2021. Sep;16(9):1445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010. May;2(5):406–13. [DOI] [PubMed] [Google Scholar]
- 9.Matura LA, Malone S, Jaime-Lara R, Riegel B. A systematic review of biological mechanisms of fatigue in chronic illness. Biol Res Nurs. 2018. Jul;20(4):410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends Neurosci. 2014. Jan;37(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossola M, Di Stasio E, Antocicco M, Panico L, Pepe G, Tazza L. Fatigue is associated with increased risk of mortality in patients on chronic hemodialysis. Nephron. 2015;130(2): 113–8. [DOI] [PubMed] [Google Scholar]
- 12.Enoka RM, Duchateau J. Translating fatigue to human performance: medicine & science in sports & exercise. Med Sci Sports Exerc. 2016. Nov;48(11):2228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol. 2017. Nov;13(11):662–75. [DOI] [PubMed] [Google Scholar]
- 14.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001. Jan;81(4):1725–89. [DOI] [PubMed] [Google Scholar]
- 15.Fitts RH. Cellular, molecular, and metabolic basis of muscle fatigue. In: Terjung R, editor. Comprehensive Physiology. Hoboken, NJ: John Wiley & Sons, Inc.; 2011. [Google Scholar]
- 16.Kent-Braun JA, Fitts RH, Christie A. Skeletal muscle fatigue. In: Terjung R, editor. Comprehensive Physiology. Hoboken, NJ: John Wiley & Sons, Inc.; 2012. [DOI] [PubMed] [Google Scholar]
- 17.Hunter SK. Performance fatigability: mechanisms and task specificity. Cold Spring Harb Perspect Med. 2017. May;8(7):a029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas K, Goodall S, Howatson G. Performance fatigability is not regulated to a peripheral critical threshold: exercise and sport sciences reviews. Exerc Sport Sci Rev. 2018. Oct; 46(4):240–6. [DOI] [PubMed] [Google Scholar]
- 19.McCormick A, Meijen C, Marcora S. Psychological determinants of whole-body endurance performance. Sports Med. 2015. Jul; 45(7):997–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venhorst A, Micklewright D, Noakes TD. Perceived fatigability: utility of a three-dimensional dynamical systems framework to better understand the psychophysiological regulation of goal-directed exercise behaviour. Sports Med. 2018. Nov;48(11):2479–95. [DOI] [PubMed] [Google Scholar]
- 21.Stokes MJ, Cooper RG, Edwards RH. Normal muscle strength and fatigability in patients with effort syndromes. BMJ. 1988. Oct; 297(6655):1014–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campistol JM. Uremic myopathy. Kidney Int. 2002. Nov;62(5):1901–13. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan AV, Kiernan MC. Neurological complications of chronic kidney disease. Nat Rev Neurol. 2009. Oct;5(10):542–51. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan AV, Kiernan MC. Uremic neuropathy: clinical features and new pathophysiological insights. Muscle Nerve. 2007. Mar; 35(3):273–90. [DOI] [PubMed] [Google Scholar]
- 25.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013. Jan;80(4):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kluger BM, Herlofson K, Chou KL, Lou JS, Goetz CG, Lang AE, et al. Parkinson’s disease-related fatigue: a case definition and recommendations for clinical research: parkinson’s disease-related fatigue. Mov Disord. 2016. May;31(5):625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micklewright D, St Clair Gibson AA, Gladwell V, Al Salman A. Development and validity of the rating-of-fatigue scale. Sports Med. 2017. Nov;47(11):2375–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobkin BH. Fatigue versus activity-dependent fatigability in patients with central or peripheral motor impairments. Neurorehabil Neural Repair. 2008. Mar-Apr Mar;22(2): 105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenhouse-Tucknott A, Butterworth JB, Wrightson JG, Smeeton NJ, Critchley HD, Dekerle J, et al. Toward the unity of pathological and exertional fatigue: a predictive processing model. Cogn Affect Behav Neurosci. 2021. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechstein AE, Gollie JM, Guccione AA. Fatigability and cardiorespiratory impairments in parkinson’s disease: potential non-motor barriers to activity performance. J Funct Morphol Kinesiol. 2020. Oct;5(4):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enoka RM, Almuklass AM, Alenazy M, Alvarez E, Duchateau J. Distinguishing between fatigue and fatigability in multiple sclerosis. Neurorehabil Neural Repair. 2021. Sep;35(11): 960. [DOI] [PubMed] [Google Scholar]
- 32.Quadt L, Critchley HD, Garfinkel SN. The neurobiology of interoception in health and disease: neuroscience of interoception. Ann NY Acad Sci. 2018. Sep;1428(1):112–28. [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998. Jan; 338(3):171–9. [DOI] [PubMed] [Google Scholar]
- 34.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007. Feb;21(2):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shattuck EC, Muehlenbein MP. Human sickness behavior: ultimate and proximate explanations: human sickness behavior. Am J Phys Anthropol. 2015. May;157(1):1–18. [DOI] [PubMed] [Google Scholar]
- 36.Gregg LP, Jain N, Carmody T, Minhajuddin AT, Rush AJ, Trivedi MH, et al. Fatigue in nondialysis chronic kidney disease: correlates and association with kidney outcomes. Am J Nephrol. 2019;50(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jhamb M, Liang K, Yabes J, Steel JL, Dew MA, Shah N, et al. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol. 2013; 38(6):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horigan AE, Schneider SM, Docherty S, Barroso J. The experience and self-management of fatigue in patients on hemodialysis. Nephrol Nurs J. 2013. Apr;40(2):113–23. [PMC free article] [PubMed] [Google Scholar]
- 39.Caplin B, Kumar S, Davenport A. Patients’ perspective of haemodialysis-associated symptoms. Nephrol Dial Transplant. 2011. Aug;26(8):2656–63. [DOI] [PubMed] [Google Scholar]
- 40.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R, Daily Hemodialysis Study Group London Health Sciences Centre. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol. 2006. Sep;1(5):952–9. [DOI] [PubMed] [Google Scholar]
- 41.Artom M, Moss-Morris R, Caskey F, Chilcot J. Fatigue in advanced kidney disease. Kidney Int. 2014. Sep;86(3):497–505. [DOI] [PubMed] [Google Scholar]
- 42.Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013. Jul;84(1):179–91. [DOI] [PubMed] [Google Scholar]
- 43.Hedayati SS, Finkelstein FO. Epidemiology, diagnosis, and management of depression in patients with CKD. Am J Kidney Dis. 2009. Oct;54(4):741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumanathissa M, De Silva VA, Hanwella R. Prevalence of major depressive episode among patients with pre-dialysis chronic kidney disease. Int J Psychiatry Med. 2011. Jan; 41(1):47–56. [DOI] [PubMed] [Google Scholar]
- 45.Cohen SD, Cukor D, Kimmel PL. Anxiety in patients treated with hemodialysis. Clin J Am Soc Nephrol. 2016. Dec;11(12):2250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimmel PL, Cukor D. Anxiety symptoms in patients treated with hemodialysis: measurement and meaning. Am J Kidney Dis. 2019. Aug;74(2):145–7. [DOI] [PubMed] [Google Scholar]
- 47.Schouten RW, Haverkamp GL, Loosman WL, Chandie Shaw PK, van Ittersum FJ, Smets YFC, et al. Anxiety symptoms, mortality, and hospitalization in patients receiving maintenance dialysis: a Cohort Study. Am J Kidney Dis. 2019. Aug;74(2):158–66. [DOI] [PubMed] [Google Scholar]
- 48.Etgen T, Chonchol M, Farstl H, Sander D. Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol. 2012;35(5):474–82. [DOI] [PubMed] [Google Scholar]
- 49.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease: cognitive impairment in. J Am Geriatr Soc. 2004. Nov;52(11):1863–9. [DOI] [PubMed] [Google Scholar]
- 50.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, et al. Chronic kidney disease and cognitive impairment in the elderly: the Health, Aging, and Body Composition Study. J Am Soc Nephrol. 2005. Jul;16(7):2127–33. [DOI] [PubMed] [Google Scholar]
- 51.Viggiano D, Wagner CA, Martino G, Nedergaard M, Zoccali C, Unwin R, et al. Mechanisms of cognitive dysfunction in CKD. Nat Rev Nephrol. 2020. Aug;16(8):452–69. [DOI] [PubMed] [Google Scholar]
- 52.Chang CY, Lin CC, Tsai CF, Yang WC, Wang SJ, Lin FH, et al. Cognitive impairment and hippocampal atrophy in chronic kidney disease. Acta Neurol Scand. 2017. Nov;136(5): 477–85. [DOI] [PubMed] [Google Scholar]
- 53.St Clair Gibson A, Swart J, Tucker R. The interaction of psychological and physiological homeostatic drives and role of general control principles in the regulation of physiological systems, exercise and the fatigue process – The Integrative Governor theory. Eur J Sport Sci. 2017. May;18(1):25–36. [DOI] [PubMed] [Google Scholar]
- 54.Hannan M, Bronas UG. Barriers to exercise for patients with renal disease: an integrative review. J Nephrol. 2017. Dec;30(6):729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacIntosh BR, Holash RJ, Renaud JM. Skeletal muscle fatigue: regulation of excitation-contraction coupling to avoid metabolic catastrophe. J Cell Sci. 2012. May;125(9):2105–14. [DOI] [PubMed] [Google Scholar]
- 56.Sundberg CW, Prost RW, Fitts RH, Hunter SK. Bioenergetic basis for the increased fatigability with ageing. J Physiol. 2019. Oct; 597(19):4943–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Debold EP, Fitts RH, Sundberg CW, Nosek TM. Muscle fatigue from the perspective of a single crossbridge. Med Sci Sports Exerc. 2016;48(11):2270–80. [DOI] [PubMed] [Google Scholar]
- 58.Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL. Neural contributions to muscle Fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc. 2016. Nov; 48(11):2294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amann M, Wan HY, Thurston TS, Georgescu VP, Weavil JC. On the influence of group III/IV muscle afferent feedback on endurance exercise performance. Exerc Sport Sci Rev. 2020. Oct;48(4):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Black MI, Jones AM, Blackwell JR, Bailey SJ, Wylie LJ, McDonagh STJ, et al. Muscle metabolic and neuromuscular determinants of fatigue during cycling in different exercise intensity domains. J Appl Physiol. 2017. Mar; 122(3):446–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 1991. Apr;71(2):541–85. [DOI] [PubMed] [Google Scholar]
- 62.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984. Apr; 56(4):831–8. [DOI] [PubMed] [Google Scholar]
- 63.Hortobágyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci. 2003. May;58(5):M453–60. [DOI] [PubMed] [Google Scholar]
- 64.Amann M, Venturelli M, Ives SJ, McDaniel J, Layec G, Rossman MJ, et al. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J Appl Physiol. 2013. Aug; 115(3):355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zwarts MJ, Bleijenberg G, van Engelen BG. Clinical neurophysiology of fatigue. Clin Neurophysiol. 2008. Jan;119(1):2–10. [DOI] [PubMed] [Google Scholar]
- 66.Gibson H, Carroll N, Clague JE, Edwards RH. Exercise performance and fatiguability in patients with chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1993. Sep;56(9): 993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinstein AA, Drinkard BM, Diao G, Furst G, Dale JK, Straus SE, et al. Exploratory analysis of the relationships between aerobic capacity and self-reported fatigue in patients with rheumatoid arthritis, polymyositis, and chronic fatigue syndrome. PM R. 2009. Jul; 1(7):620–8. [DOI] [PubMed] [Google Scholar]
- 68.Zijdewind I, Prak RF, Wolkorte R. Fatigue and fatigability in persons with multiple sclerosis. Exerc Sport Sci Rev. 2016. Oct;44(4): 123–8. [DOI] [PubMed] [Google Scholar]
- 69.Lou JS, Kearns G, Benice T, Oken B, Sexton G, Nutt J. Levodopa improves physical fatigue in Parkinson’s disease: a Double-Blind, Placebo-Controlled, Crossover Study. Mov Disord. 2003. Oct;18(10):1108–14. [DOI] [PubMed] [Google Scholar]
- 70.Murphy SL, Smith DM. Ecological measurement of fatigue and fatigability in older adults with osteoarthritis. J Gerontol Ser A Biol Sci Med Sci. 2010. Feb;65A(2):184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leavitt VM, DeLuca J. Central fatigue: issues related to cognition, mood and behavior, and psychiatric diagnoses. PM R. 2010. May;2(5): 332–7. [DOI] [PubMed] [Google Scholar]
- 72.Gould JR, Reineberg AE, Cleland BT, Knoblauch KE, Clinton GK, Banich MT, et al. Adjustments in torque steadiness during fatiguing contractions are inversely correlated with IQ in persons with multiple sclerosis. Front Physiol. 2018. Oct;9:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenhouse-Tucknott A, Wrightson JG, Raynsford M, Harrison NA, Dekerle J. Interactions between perceptions of fatigue, effort, and affect decrease knee extensor endurance performance following upper body motor activity, independent of changes in neuromuscular function. Psychophysiology 2020Sep; 57(9):e13602. [DOI] [PubMed] [Google Scholar]
- 74.Seamon BA, Harris-Love MO. Clinical assessment of fatigability in multiple sclerosis: a shift from perception to performance. Front Neurol. 2016. Nov;7:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johansen KL, Doyle J, Sakkas GK, Kent-Braun JA. Neural and metabolic mechanisms of excessive muscle fatigue in maintenance hemodialysis patients. Am J Physiol-Regul Integr Comp Physiol. 2005. Sep;289(3):R805–13. [DOI] [PubMed] [Google Scholar]
- 76.Petersen AC, Leikis MJ, McMahon LP, Kent AB, Murphy KT, Gong X, et al. Impaired exercise performance and muscle Na+, K+- pump activity in renal transplantation and haemodialysis patients. Nephrol Dial Transplant. 2012. May;27(5):2036–43. [DOI] [PubMed] [Google Scholar]
- 77.Macdonald JH, Fearn L, Jibani M, Marcora SM. Exertional fatigue in patients With CKD. Am J Kidney Dis. 2012. Dec;60(6):930–9. [DOI] [PubMed] [Google Scholar]
- 78.Petersen AC, Leikis MJ, McMahon LP, Kent AB, McKenna MJ. Effects of endurance training on extrarenal potassium regulation and exercise performance in patients on haemodialysis. Nephrol Dial Transplant. 2009. Sep; 24(9):2882–8. [DOI] [PubMed] [Google Scholar]
- 79.Moore GE, Bertocci LA, Painter PL. 31P-magnetic resonance spectroscopy assessment of subnormal oxidative metabolism in skeletal muscle of renal failure patients. J Clin Invest. 1993. Feb;91(2):420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leikis MJ, McKenna MJ, Petersen AC, Kent AB, Murphy KT, Leppik JA, et al. Exercise performance falls over time in patients with chronic kidney disease despite maintenance of hemoglobin concentration. Clin J Am Soc Nephrol. 2006. Apr;1(3):488–95. [DOI] [PubMed] [Google Scholar]
- 81.Kirkman DL, Bohmke N, Carbone S, Garten RS, Rodriguez-Miguelez P, Franco RL, et al. Exercise intolerance in kidney diseases: physiological contributors and therapeutic strategies. Am J Physiol Renal Physiol. 2020;320(2): F161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gollie JM, Harris-Love MO, Patel SS, Argani S. Chronic kidney disease: considerations for monitoring skeletal muscle health and prescribing resistance exercise. Clin Kidney J. 2018. Dec;11(6):822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fahal IH, Bell GM, Bone JM, Edwards RH. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrol Dial Transplant. 1997. Jan;12(1):119–27. [DOI] [PubMed] [Google Scholar]
- 84.Sawant A, Garland SJ, House AA, Overend TJ. Morphological, electrophysiological, and metabolic characteristics of skeletal muscle in people with end-stage renal disease: a critical review. Physiother Can. 2011. Jul;63(3):355–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katsiaras A, Newman AB, Kriska A, Brach J, Krishnaswami S, Feingold E, et al. Skeletal muscle fatigue, strength, and quality in the elderly: the Health ABC Study. J Appl Physiol. 2005. Jul;99(1):210–6. [DOI] [PubMed] [Google Scholar]
- 86.Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016. Apr;71(4):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diesel W, Noakes TD, Swanepoel C, Lambert M. Isokinetic muscle strength predicts maximum exercise tolerance in renal patients on chronic hemodialysis. Am J Kidney Dis. 1990. Aug;16(2):109–14. [DOI] [PubMed] [Google Scholar]
- 88.John SG, Sigrist MK, Taal MW, McIntyre CW. Natural history of skeletal muscle mass changes in chronic kidney disease stage 4 and 5 patients: an Observational Study. PLoS One. 2013. May;8(5):e65372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y, Hellberg M, Svensson P, Höglund P, Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol Dial Transplant. 2017. Mar;33(2): 342–8. [DOI] [PubMed] [Google Scholar]
- 90.Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017. May;26(3): 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Segura-Ortí E, Gordon PL, Doyle JW, Johansen KL. Correlates of physical functioning and performance across the spectrum of kidney function. Clin Nurs Res. 2018. Jun;27(5): 579–96. [DOI] [PubMed] [Google Scholar]
- 92.Johansen KL, Kaysen GA, Young BS, Hung AM, da Silva M, Chertow GM. Longitudinal Study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003. Apr;77(4):842–6. [DOI] [PubMed] [Google Scholar]
- 93.Souza VA, Oliveira D, Barbosa SR, Corrêa Jo do A, Colugnati FAB, Mansur HN, et al. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PLoS One. 2017; 12(4):e0176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol. 2014. Jul;10(9):504–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johansen KL, Shubert T, Doyle J, Soher B, Sakkas GK, Kent-Braun JA. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003. Jan;63(1):291–7. [DOI] [PubMed] [Google Scholar]
- 96.Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr. 2008. Aug;27(4):557–64. [DOI] [PubMed] [Google Scholar]
- 97.Hyatt HW, Powers SK. Mitochondrial dysfunction is a common denominator linking skeletal muscle wasting due to disease, aging, and prolonged inactivity. Antioxidants. 2021. Apr;10(4):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berru FN, Gray SE, Thome T, Kumar RA, Salyers ZR, Coleman M, et al. Chronic kidney disease exacerbates ischemic limb myopathy in mice via altered mitochondrial energetics. Sci Rep. 2019. Dec;9(1):15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roshanravan B, Kestenbaum B, Gamboa J, Jubrias SA, Ayers E, Curtin L, et al. CKD and muscle mitochondrial energetics. Am J Kidney Dis. 2016. Oct;68(4):658–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gamboa JL, Billings FT, Bojanowski MT, Gilliam LA, Yu C, Roshanravan B, et al. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol Rep. 2016. May;4(9):e12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gamboa JL, Roshanravan B, Towse T, Keller CA, Falck AM, Yu C, et al. Skeletal muscle mitochondrial dysfunction is present in patients with CKD before initiation of maintenance hemodialysis. CJASN. 2020. Jul;15(7): 926–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kestenbaum B, Gamboa J, Liu S, Ali AS, Shankland E, Jue T, et al. Impaired skeletal muscle mitochondrial bioenergetics and physical performance in chronic kidney disease. JCI Insight. 2020. Mar;5(5):e133289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thome T, Salyers ZR, Kumar RA, Hahn D, Berru FN, Ferreira LF, et al. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am J Physiol Cell Physiol. 2019. Oct;317(4):C701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thome T, Kumar RA, Burke SK, Khattri RB, Salyers ZR, Kelley RC, et al. Impaired muscle mitochondrial energetics is associated with uremic metabolite accumulation in chronic kidney disease. JCI Insight. 2021. Jan;6(1): e139826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Enoki Y, Watanabe H, Arake R, Fujimura R, Ishiodori K, Imafuku T, et al. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction: therapy for CKD-associated muscle dysfunction. J Cachexia Sarcopenia Muscle. 2017. Oct;8(5):735–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thompson CH, Kemp GJ, Taylor DJ, Ledingham JG, Radda GK, Rajagopalan B. Effect of chronic uraemia on skeletal muscle metabolism in man. Nephrol Dial Transplant. 1993;8(3):218–22. [PubMed] [Google Scholar]
- 107.Sato E, Mori T, Mishima E, Suzuki A, Sugawara S, Kurasawa N, et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep. 2016. Dec;6(1):36618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Cohen AH, et al. Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol. 2012. Jan;112(1):72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Durozard D, Pimmel P, Baretto S, Caillette A, Labeeuw M, Baverel G, et al. 31P NMR spectroscopy investigation of muscle metabolism in hemodialysis patients. Kidney Int. 1993. Apr;43(4):885–92. [DOI] [PubMed] [Google Scholar]
- 110.Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000. Jan;80(4):1411–81. [DOI] [PubMed] [Google Scholar]
- 111.McKenna MJ, Bangsbo J, Renaud J-M. Muscle K+, Na+, and Cl− disturbances and Na+ - K+ pump inactivation: implications for fatigue. J Appl Physiol. 2008. Jan;104(1):288–95. [DOI] [PubMed] [Google Scholar]
- 112.Fitts RH, Balog EM. Effect of intracellular and extracellular ion changes on E-C coupling and skeletal muscle fatigue. Acta Physiol Scand. 1996. Mar;156(3):169–81. [DOI] [PubMed] [Google Scholar]
- 113.Lindinger MI, Cairns SP. Regulation of muscle potassium: exercise performance, fatigue and health implications. Eur J Appl Physiol. 2021. Mar;121(3):721–48. [DOI] [PubMed] [Google Scholar]
- 114.Sjogaard G. Potassium and fatigue: the pros and cons. Acta Physiol Scand. 1996. Mar; 156(3):257–64. [DOI] [PubMed] [Google Scholar]
- 115.Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003. Jul;285(1):R143–8. [DOI] [PubMed] [Google Scholar]
- 116.Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996. Sep;495(Pt 2):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gilligan S, Raphael KL. Hyperkalemia and hypokalemia in CKD: prevalence, risk factors, and clinical outcomes. Adv Chronic Kidney Dis. 2017;24(5):315–8. [DOI] [PubMed] [Google Scholar]
- 118.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009. Jun; 169(12):1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sangkabutra T, Crankshaw DP, Schneider C, Fraser SF, Sostaric S, Mason K, et al. Impaired K+ regulation contributes to exercise limitation in end-stage renal failure. Kidney Int. 2003. Jan;63(1):283–90. [DOI] [PubMed] [Google Scholar]
- 120.Friedland J, Paterson D. Potassium and fatigue. Lancet. 1988. Oct;2(8617):961–2. [DOI] [PubMed] [Google Scholar]
- 121.Cotton JR, Woodard T, Carter NW, Knochel JP. Resting skeletal muscle membrane potential as an index of uremic toxicity. A proposed new method to assess adequacy of hemodialysis. J Clin Invest. 1979. Mar;63(3): 501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Z’Graggen WJ, Aregger F, Farese S, Humm AM, Baumann C, Uehlinger DE, et al. Velocity recovery cycles of human muscle action potentials in chronic renal failure. Clin Neurophysiol. 2010. Jun;121(6):874–81. [DOI] [PubMed] [Google Scholar]
- 123.Enoka RM, Baudry S, Rudroff T, Farina D, Klass M, Duchateau J. Unraveling the neurophysiology of muscle fatigue. J Electromyogr Kinesiol. 2011. Apr;21(2):208–19. [DOI] [PubMed] [Google Scholar]
- 124.Aggarwal HK, Sood S, Jain D, Kaverappa V, Yadav S. Evaluation of spectrum of peripheral neuropathy in predialysis patients with chronic kidney disease. Ren Fail. 2013. Nov; 35(10):1323–9. [DOI] [PubMed] [Google Scholar]
- 125.Arnold R, Issar T, Krishnan AV, Pussell BA. Neurological complications in chronic kidney disease. JRSM Cardiovasc Dis. 2016. Mar;5:2048004016677687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Isaacs H Electromyographic study of muscular weakness in chronic renal failure. S Afr Med J. 1969. May;43(22):683–8. [PubMed] [Google Scholar]
- 127.Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Bostock H, Kiernan MC. Altered motor nerve excitability in end-stage kidney disease. Brain. 2005. Sep;128(9): 2164–74. [DOI] [PubMed] [Google Scholar]
- 128.Mitchell JH. Neural circulatory control during exercise: early insights: neural circulatory control during exercise: early insights. Exp Physiol. 2013. Apr;98(4):867–78. [DOI] [PubMed] [Google Scholar]
- 129.Grotle AK, Macefield VG, Farquhar WB, O’Leary DS, Stone AJ. Recent advances in exercise pressor reflex function in health and disease. Auton Neurosci. 2020. Nov;228: 102698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Park J, Middlekauff HR. Abnormal neurocirculatory control during exercise in humans with chronic renal failure. Auton Neurosci. 2015. Mar;188:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sprick JD, Downey RM, Morison DL, Fonkoue IT, Li Y, DaCosta D, et al. Functional sympatholysis is impaired in end-stage renal disease. Am J Physiol Regul Integr Comp Physiol. 2019. May;316(5):R504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Downey RM, Liao P, Millson EC, Quyyumi AA, Sher S, Park J. Endothelial dysfunction correlates with exaggerated exercise pressor response during whole body maximal exercise in chronic kidney disease. Am J Physiol Renal Physiol. 2017. May;312(5):F917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moorman D, Suri R, Hiremath S, Jegatheswaran J, Kumar T, Bugeja A, et al. Benefits and barriers to and desired outcomes with exercise in patients with ESKD. Clin J Am Soc Nephrol. 2019. Feb;14(2):268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao QG, Zhang HR, Wen X, Wang Y, Chen XM, Chen N, et al. Exercise interventions on patients with end-stage renal disease: a systematic review. Clin Rehabil. 2019. Feb; 33(2):147–56. [DOI] [PubMed] [Google Scholar]
- 135.Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clin Kidney J. 2015. Dec; 8(6):753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chan D, Cheema BS. Progressive resistance training in end-stage renal disease: systematic review. Am J Nephrol. 2016;44(1):32–45. [DOI] [PubMed] [Google Scholar]
- 137.Cheema BS, Chan D, Fahey P, Atlantis E. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med. 2014. Aug;44(8):1125–38. [DOI] [PubMed] [Google Scholar]
- 138.Howden EJ, Coombes JS, Isbel NM. The role of exercise training in the management of chronic kidney disease. Curr Opin Nephrol Hypertens. 2015. Nov;24(6):480–7. [DOI] [PubMed] [Google Scholar]
- 139.Heiwe S, Jacobson SH. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014. Sep; 64(3):383–93. [DOI] [PubMed] [Google Scholar]
- 140.Gollie JM, Patel SS, Scholten JD, Harris-Love MO. Preliminary Study of the effects of eccentric-overload resistance exercise on physical function and torque capacity in chronic kidney disease. J Funct Morphol Kinesiol. 2020. Dec;5(4):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992. May; 72(5):1631–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study were obtained from studies included in this article’s reference list.


