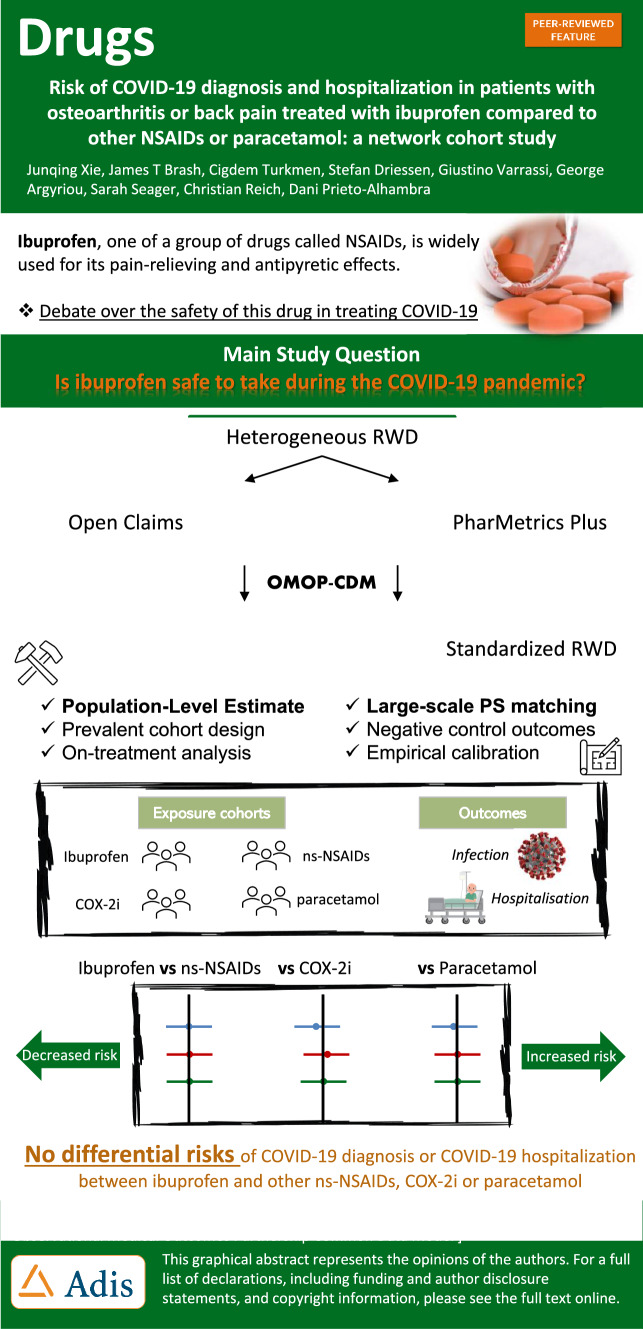
Abstract
Objective
We aimed to investigate whether ibuprofen use, compared with other non-selective non-steroidal anti-inflammatory drugs (ns-NSAIDs), cyclooxygenase-2 inhibitors (COX-2i) or paracetamol, increases the risk of coronavirus disease 2019 (COVID-19) diagnosis or hospitalisation.
Design
A prevalent user and active comparator cohort study.
Setting
Two US claims databases (Open Claims and PharMetrics Plus) mapped to the Observational Medical Outcomes Partnership Common Data Model.
Participants
Insured patients with a history of osteoarthritis or back pain and receiving ibuprofen, other ns-NSAIDs, COX-2i or paracetamol between 1 November, 2019 and 31 January, 2020 (study enrolment window 1) or between 1 February, 2020 and 31 October, 2020 (study enrolment window 2).
Main Outcome Measures
Large-scale propensity score matching and empirical calibration were used to minimise confounding. Incidence and hazard ratios of COVID-19 diagnosis and hospitalisation according to drug/s use were estimated and pooled in the same study period across data sources using a fixed-effects meta-analysis. Index treatment episode was the primary risk evaluation window, censored at the time of discontinuation.
Results
A total of 633,562 and 1,063,960 participants were included in periods 1 and 2, respectively, for the ibuprofen versus ns-NSAIDs comparison, 311,669 and 524,470 for ibuprofen versus COX-2i, and 492,002 and 878,598 for ibuprofen versus paracetamol. Meta-analyses of empirically calibrated hazard ratios revealed no significantly differential risk of COVID-19 outcomes in users of ibuprofen versus any of the other studied analgesic classes: hazard ratios were 1.13 (0.96–1.33) for the ibuprofen-ns-NSAIDs comparison, 1.03 (0.83–1.28) for the ibuprofen-COX-2i comparison and 1.13 (0.74–1.73) for ibuprofen-paracetamol comparison on COVID-19 diagnosis in the February 2020–October 2020 window. Similar hazard ratios were found on COVID-19 hospitalisation and across both study periods.
Conclusions
In patients with osteoarthritis or back pain, we found no differential risks of incident COVID-19 diagnosis or COVID-19 hospitalisation for ibuprofen users compared with other ns-NSAIDs, COX-2i or paracetamol. Our findings support regulatory recommendations that NSAIDs, including ibuprofen, should be prescribed as indicated in the same way as before the COVID-19 pandemic, especially for those who rely on ibuprofen or NSAIDs to manage chronic arthritis or musculoskeletal pain symptoms.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01822-z.
Key Points
| This study comprehensively evaluates the safety concern of ibuprofen use in the context of coronavirus disease 2019 by curating a near 10-million cohort of patients with osteoarthritis or back pain, comparing it with multiple alternative analgesics, and using state-of-the-art methods to control for residual confounding and bias. |
| Ibuprofen does not confer differential risks of coronavirus disease 2019 diagnosis or hospitalisation, compared with other non-selective non-steroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors and paracetamol. |
| Patients with arthritis or musculoskeletal pain should use ibuprofen as indicated, and clinicians should re-evaluate recommendations and advice around using these medicines with the ongoing pandemic. |
Introduction
In the early stages of the coronavirus disease 2019 (COVID-19) pandemic in 2020, a doctor in France reported four young patients with COVID-19 developing severe symptoms after using ibuprofen, a typical type of non-steroidal anti-inflammatory drug (NSAID) [1]. The French Health Minister and some UK experts immediately endorsed this case study, suggesting that patients with severe acute respiratory syndrome coronavirus 2 infection avoid using ibuprofen [2, 3]. This triggered a significant public health concern about prescribing NSAIDs in the COVID-19-naïve population, and presented a clinical dilemma for those who require NSAIDs for relieving symptoms caused by a spectrum of diseases [4]. On the one hand, there was speculation that regular use of NSAIDs might increase the expression of the angiotensin-converting enzyme 2 receptor through which severe acute respiratory syndrome coronavirus 2 enters host cells [5, 6]. On the other hand, studies found that uncontrolled inflammation due to diseases such as active arthritis was associated with an increased COVID-19 infection risk [7]. Given the absence of reliable causal evidence linking NSAID usage with COVID-19 outcomes, health regulatory agencies and clinical societies stated that NSAID therapy should not be discontinued [8, 9]. However, as a precautionary approach, they recommended that alternative analgesics, such as paracetamol, might be preferable for managing the symptoms of COVID-19 [10].
Some observational studies have since emerged showing that NSAID use does not increase the risk of severe complications or death in patients with COVID-19, yet only two studies have examined susceptibility to COVID-19 for general NSAID users in outpatient settings [11, 12]. Despite suggestive, these studies are subject to methodological limitations, particularly the indication bias, by comparing NSAID users with either non-users or with users of opioid-containing drugs. Numerous empirical studies have demonstrated that an inappropriate design of reference groups can result in entirely non-comparable participants, which is unlikely to be addressed by traditional statistical approaches based on a limited confounder adjustment.
Given the conundrum that persists for the public and medical professionals regarding the safety of ibuprofen in the context of the ongoing COVID-19 pandemic, we attempted to assess the causality between ibuprofen use and COVID-19 susceptibility and severity in the COVID-19-naive population by leveraging two large US claims databases, head-to-head comparisons and state-of-the-art statistical techniques to account for measured and unmeasured confounding. Specifically, we designed a prevalent user-active comparator cohort study, comparing ibuprofen users with other non-selective NSAID (ns-NSAID), cyclooxygenase (COX-2i) or paracetamol users in relation to developing a COVID-19 diagnosis or COVID-19 hospitalisation, and restricted to a pool of patients with osteoarthritis (OA) or back pain given that they are commonly prescribed for those analgesics. In addition, we explored the potential heterogeneity of the associations during different calendar periods considering there was a significant change in prescribing patterns for these drugs because of the pandemic.
Methods
Study Design
We conducted a prevalent user-active comparator cohort study using two US administrative claims databases that had previously been mapped to the Observational Medical Outcomes Partnership Common Data Model (v5) [13]. Specifically, we used the IQVIA US Open Claims and IQVIA PharMetrics Plus databases, which contain pre-adjudicated and adjudicated health insurance data, respectively. Additional information on these data sources is detailed in the Electronic Supplementary Material (ESM). All data partners had previous institutional review board approval or exemption for their participation.
Cohort Eligibility and Enrolment Period
We included participants registered in either data source who were aged 18 years or older, with a history of OA or back pain (Table 1 of the ESM), and received at least one eligible prescription of any study drugs between 1 November, 2019 and 31 January, 2020 (the pre-pandemic observational period), or between 1 February, 2020 and 31 October, 2020 (the pandemic period). We used the last prescription of any study drugs as the index date in the pre-pandemic period to reduce exposure misclassification due to medication switching between drugs. However, during the pandemic period, we chose the first prescription to reduce reverse causality (people taking these same drugs to treat fever and other COVID-19-related symptoms). Patients with less than 180 days observable data, previous exposure to the comparator drug within the 180 days, or with a diagnosis of COVID-19 on or before the index date were excluded.
Exposures and Follow-Up
We classified patients into the target (ibuprofen) or the active comparator groups (ns-NSAIDs, COX-2i, paracetamol,) according to the prescription received on the index date. We excluded those who had both target and comparator drugs prescribed concomitantly on the index date. Specification of these medicines with RxNorm or ATC codes are listed in Tables 2–5 of the ESM. Participants were followed from the index date to the earliest of a study outcome, death, loss or deregistration from the database, date of last data collection (last possible drug prescription start in Open Claims: August 2021, PharMetrics Plus: March 2021), record of comparator drug or end of index treatment (on-treatment [OT] analysis). We additionally performed an intention-to-treat sensitivity analysis, wherein patients were followed for 6 months following index date.
Outcomes
We assessed two outcomes for COVID-19 susceptibility: (1) COVID-19 diagnosis and (2) COVID-19 hospitalisation (hospital admission with a COVID-19 diagnosis during or up to 3 weeks before admission). The COVID-19 status for both outcomes was identified by SNOMED COVID-19 diagnostic codes. The phenotyping process of these outcomes based on claims data has been previously described and validated [14, 15]. The concept IDs relating to COVID-19 are listed in Tables 6–9 of the ESM.
Statistical Analyses
As prior knowledge was limited to estimate the minimal sample size, we instead provided a minimum detectable rate ratio that presents achieving a 5% type-I error rate and 80% statistical power for each target–comparator–outcome combination by using all patients who met the eligibility criteria from the specific data source and study window [16]. We used large-scale propensity score (PS) matching to balance target and comparison cohorts and control for measured and potential unmeasured confounding. For instance, when comparing ibuprofen with paracetamol, we first derived a PS for each individual by building a regularised logistic regression model that includes the binary treatment assignment as the dependent variable and a large set of predefined baseline patient demographics, previous conditions, drug exposures, procedures and health service use behaviours as the explanatory variables [17]. Of note, we excluded baseline features that occurred in fewer than 0.1% of patients within the target and comparator cohorts before fitting the PS model but evaluated their balance between groups after PS matching. Details of patient characteristics included in the analysis are provided in the ESM. We then created 1:1 PS-matched patient cohorts and replicated the process to assemble 12 pairs of PS-matched cohorts (1 target * 3 comparators * 2 databases * 2 study windows).
We quantified the relative risk of outcome between the target and comparator treatments by hazard ratios (HRs) derived from the Cox proportional hazards model. To account for potential residual confounding, we included up to 217 negative control outcomes (NCOs) for each comparison, i.e. outcomes for which the null hypothesis was believed to be true. Negative control outcomes were identified through a data-rich algorithm [18, 19] and reviewed by clinicians. We used the empirical null distributions to calibrate HR estimates if more than 5% of negative experiments were rejected. All NCOs used in this study are listed in Table 10 of the ESM.
We reported study diagnostics, including preference score distributions (a transformation of PS that adjusts for prevalence differences between populations), to evaluate the empirical equipoise and population generalisability, absolute standardised mean differences of patient characteristics to evaluate the cohort balance before and after propensity score matching, negative control calibration plots to assess the likelihood of residual bias and Kaplan–Meier plots to examine the proportional hazard assumption of the Cox model. For each model, we defined the HR estimate as invalidated if any absolute standardised mean difference of baseline covariates after PS matching was greater than 0.1 or the Cox model failed because of a violation of the proportionality assumption. Using a fixed-effect model, we aggregated HRs and their 95% confidence interval (CI) estimates (without correcting for multiple testing) across the data sources.
We conducted this study using the open-source OHDSI CohortMethod R package (https://ohdsi.github.io/CohortMethod/) with large-scale analytics achieved through the Cyclops R package. We developed an interactive website to promote transparency and allow for sharing and exploration of the complete results online (https://dqdashboard.iqvia.com/ibucovid/).
Patient and Public Involvement
No patients or public members were directly involved in the design or analysis of the reported data. The independent scientific advisory committee responsible for the approval of our protocol involved patients in the evaluation of our data access application.
Results
Population
Study cohorts were created from a pool of patients with OA or back pain, and were designed to enable comparisons between ibuprofen users (target cohort) and ns-NSAID users, COX-2i users or paracetamol users (comparator cohorts). The number of patients eligible for each cohort varied by data source and enrolment window (Table 1). In the pre-pandemic enrolment window (November 2019–January 2020), 1,503,207 patients were eligible for the ibuprofen versus ns-NSAID user cohorts, 3,939,853 patients were eligible for ibuprofen versus COX-2i user cohorts and 3,793,598 patients were eligible for ibuprofen versus paracetamol user cohorts. In the pandemic enrolment window (February 2020–October 2020), the corresponding figures were 6,876,630 patients, 2,370,693 patients and 5,551,200 patients, respectively. Cohorts from the Open Claims database had more patients than equivalent cohorts in the PharMetrics Plus database, consistent with Open Claims being the larger database.
Table 1.
Population size
| Comparisons | Database | Target cohort | Comparator cohort | All patients |
|---|---|---|---|---|
| Pre-pandemic enrolment (Nov 2019–Jan 2020) | ||||
| Ibuprofen vs COX-2i | PharMetrics Plus | 118,841 | 60,347 | 179,188 |
| Ibuprofen vs COX-2i | Open Claims | 930,231 | 393,788 | 1,324,019 |
| Ibuprofen vs COX-2i | Combined | 1,049,072 | 454,135 | 1,503,207 |
| Ibuprofen vs ns-NSAIDs | PharMetrics Plus | 82,742 | 508,085 | 590,827 |
| Ibuprofen vs ns-NSAIDs | Open Claims | 640,574 | 2,708,452 | 3,349,026 |
| Ibuprofen vs ns-NSAIDs | Combined | 723,316 | 3,216,537 | 3,939,853 |
| Ibuprofen vs paracetamol | PharMetrics Plus | 87,305 | 338,249 | 425,554 |
| Ibuprofen vs paracetamol | Open Claims | 611,173 | 2,756,871 | 3,368,044 |
| Ibuprofen vs paracetamol | Combined | 698,478 | 3,095,120 | 3,793,598 |
| Pandemic enrolment (Feb 2020–Oct 2020) | ||||
| Ibuprofen vs COX-2i | PharMetrics Plus | 211,088 | 98,905 | 309,993 |
| Ibuprofen vs COX-2i | Open Claims | 1,574,159 | 649,594 | 2,223,753 |
| Ibuprofen vs COX-2i | Combined | 1,785,247 | 748,499 | 2,533,746 |
| Ibuprofen vs ns-NSAIDs | PharMetrics Plus | 150,744 | 869,877 | 1,020,621 |
| Ibuprofen vs ns-NSAIDs | Open Claims | 1,105,008 | 4,391,839 | 5,496,847 |
| Ibuprofen vs ns-NSAIDs | Combined | 1,255,752 | 5,261,716 | 6,517,468 |
| Ibuprofen vs paracetamol | PharMetrics Plus | 163,801 | 536,024 | 699,825 |
| Ibuprofen vs paracetamol | Open Claims | 1,102,235 | 4,173,434 | 5,275,669 |
| Ibuprofen vs paracetamol | Combined | 1,266,036 | 4,709,458 | 5,975,494 |
Number of patients in the PharMetrics Plus and Open Claims databases that satisfied the cohort inclusion criteria of this study. Cohorts included patients with a history of osteoarthritis or back pain who are prescribed ibuprofen (target cohort) or a COX-2i, ns-NSAID or paracetamol (comparator cohorts). For each comparison pair, persons were excluded from a cohort if they had a recent record of the alternative drug. Cohorts were split into two enrolment periods; a pre-pandemic period where the index event occurred between November 2019 and January 2020, and a pandemic period where the index event occurred between February 2020 and October 2020
COX-2i cyclooxygenase-2 inhibitors, ns-NSAIDs non-selective non-steroidal anti-inflammatory drugs
Patient Characteristics and PS Matching
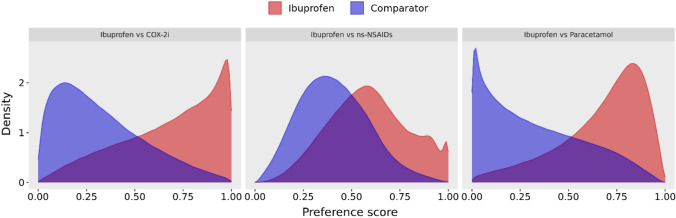
The number of baseline patient characteristics used to construct a PS model ranged from 3174 to 3789 covariates for the ibuprofen versus ns-NSAID user cohorts, 3491–4090 covariates for the ibuprofen vs COX-2i user cohorts and 3639–4227 for the ibuprofen vs paracetamol user cohorts, with variation arising between data sources and study windows. A substantial overlap in PS distribution between unmatched ibuprofen and ns-NSAID user cohorts indicated a minimal violation of the positivity assumption for causal inference [20] (Fig. 1). Indeed, unmatched ibuprofen and ns-NSAID user cohorts were similar with respect to age, sex and prevalence of clinical conditions (Table 2). In contrast, a more polarised PS distribution existed between ibuprofen user cohorts and COX-2i or paracetamol user cohorts, suggesting that these patients had less comparable baseline characteristics before PS matching (Fig. 1). The COX-2i users tended to be older than ibuprofen users, with a higher prevalence of musculoskeletal-related procedures and a lower prevalence of emergency room visits (Table 3). Paracetamol users appeared generally less healthy than ibuprofen users, as indicated by higher mean clinical index scores (Table 4). Nevertheless, following PS matching, all measured covariates were adequately balanced between analysis cohort pairs (absolute standardised mean difference < 0.1, Tables 2, 3 and 4 for covariate balance; Table 5 for PS-matched cohort counts). Further information on details of each covariate balance is provided in an interactive web application (https://dqdashboard.iqvia.com/ibucovid/).
Fig. 1.
Preference score distribution in ibuprofen versus comparator cohorts. Illustrative preference score distributions for ibuprofen versus comparator user cohorts from the February to October 2020 enrolment window, Open Claims database. Preference scores are a transformation of propensity scores, and a propensity score is the probability a patient received the target drug over the comparator drug, given the patient’s baseline covariates. ns-NSAIDs non-selective non-steroidal anti-inflammatory drugs
Table 2.
Covariate balance before and after PS matching ibuprofen-ns-NSAID cohorts
| Before PS matching | After PS matching | |||||
|---|---|---|---|---|---|---|
| Ibuprofen cohort (%) | ns-NSAID cohort (%) | ASMD | Ibuprofen cohort (%) | ns-NSAID cohort (%) | ASMD | |
| Demographics | ||||||
| Female | 63.3 | 62.7 | 0.01 | 62.1 | 62.8 | − 0.01 |
| Age, years | ||||||
| 18–19 | 1.3 | 0.5 | 0.09 | 1.1 | 0.9 | 0.01 |
| 20–24 | 3.7 | 1.5 | 0.14 | 2.8 | 2.6 | 0.01 |
| 25–29 | 4.9 | 2.1 | 0.15 | 3.7 | 3.5 | 0.01 |
| 30–34 | 6.2 | 3.1 | 0.15 | 5.1 | 4.9 | 0.01 |
| 35–39 | 7 | 4.1 | 0.12 | 6.4 | 6.5 | 0 |
| 40–44 | 7.6 | 5.3 | 0.09 | 7.5 | 7.6 | 0 |
| 45–49 | 8.7 | 6.9 | 0.07 | 8.9 | 8.8 | 0 |
| 50–54 | 10.7 | 9.5 | 0.04 | 11.2 | 11.1 | 0 |
| 55–59 | 12.7 | 12.3 | 0.01 | 13.4 | 13.8 | − 0.01 |
| 60–64 | 12.2 | 13.4 | –0.03 | 13 | 13.4 | − 0.01 |
| 65–69 | 9.9 | 13.1 | –0.1 | 10.6 | 10.5 | 0 |
| 70–74 | 6.9 | 11.4 | –0.16 | 7.5 | 7.6 | 0 |
| 75–79 | 4.1 | 7.8 | –0.16 | 4.4 | 4.5 | 0 |
| 80–84 | 4.1 | 9.1 | –0.2 | 4.4 | 4.4 | 0 |
| Conditions | ||||||
| Amenorrhea, any time prior | 6.1 | 3.4 | 0.13 | 5 | 5.2 | – 0.01 |
| Hypertension, any time prior | 52.7 | 62.3 | –0.2 | 54.8 | 55.6 | – 0.02 |
| Hip pain, any time prior | 17 | 22 | –0.13 | 17.6 | 18.1 | – 0.01 |
| Hyperlipidaemia, any time prior | 40.5 | 49.8 | –0.19 | 42.4 | 42.9 | – 0.01 |
| Nicotine dependence, any time prior | 17.8 | 13.8 | 0.11 | 16.8 | 17.8 | – 0.03 |
| Cataracts, any time prior | 13 | 20.1 | –0.19 | 13.9 | 14.1 | 0 |
| OA, any time prior | 20.1 | 28 | –0.19 | 21.3 | 22.1 | – 0.02 |
| Hip OA, any time prior | 7.9 | 12.1 | –0.14 | 8.5 | 8.6 | – 0.01 |
| Knee OA, any time prior | 27 | 38.9 | –0.26 | 28.9 | 29.8 | – 0.02 |
| Osteoporosis, any time prior | 7.9 | 12.3 | –0.15 | 8.4 | 8.5 | 0 |
| Lumbar spine stenosis, any time prior | 15.7 | 20.3 | –0.12 | 16.6 | 17.2 | – 0.01 |
| Low back pain, 6 months prior | 43.1 | 35.8 | 0.15 | 41.7 | 41.8 | 0 |
| Hip OA, 6 months prior | 3.6 | 6.1 | –0.11 | 3.9 | 4 | 0 |
| Knee OA, 6 months prior | 12.9 | 20.3 | –0.2 | 13.9 | 14.3 | – 0.01 |
| Knee OA, 1 month prior | 5.5 | 10.5 | –0.18 | 5.9 | 6.2 | – 0.01 |
| Cerebrovascular disease | 2.1 | 3 | –0.06 | 2.2 | 2.3 | – 0.01 |
| Chronic obstructive lung disease | 12.5 | 14.3 | –0.05 | 13.1 | 13.6 | – 0.02 |
| Diabetes mellitus | 0.7 | 0.7 | 0 | 0.7 | 0.8 | – 0.01 |
| Ischaemic heart disease | 0.2 | 0.3 | –0.01 | 0.2 | 0.2 | 0 |
| Obesity | 25.8 | 25.3 | 0.01 | 25.6 | 26.3 | – 0.01 |
| Malignant neoplastic disease | 0.2 | 0.2 | –0.01 | 0.2 | 0.2 | 0 |
| Procedures and visit | ||||||
| Knee arthroscopy, any time prior | 3 | 5.6 | − 0.13 | 3.2 | 3.5 | − 0.01 |
| DXA scan, any time prior | 13 | 20.3 | − 0.2 | 14 | 14.2 | − 0.01 |
| ECG, any time prior | 37.6 | 44.3 | − 0.14 | 38.9 | 39.2 | − 0.01 |
| Knee X-ray, any time prior | 15 | 20.4 | − 0.14 | 15.6 | 16.5 | − 0.02 |
| Manual/ physiotherapy , any time prior | 37 | 44.4 | − 0.15 | 38.2 | 38.6 | − 0.01 |
| Total knee replacement, any time prior | 0.6 | 1.4 | − 0.08 | 0.7 | 0.8 | − 0.01 |
| Arthrocentesis, 6 months prior | 5.3 | 8.8 | − 0.14 | 5.8 | 5.9 | 0 |
| Emergency visit, 6 months prior | 14.5 | 12.3 | 0.06 | 12 | 12.5 | − 0.02 |
| Clinical index scoresa | ||||||
| CHADS2VASc | 2.06 | 2.54 | − 0.29 | 2.12 | 2.15 | − 0.02 |
| Diabetes Comorbidity Severity Index | 1.52 | 1.95 | − 0.18 | 1.58 | 1.63 | − 0.02 |
| Charlson Index, Romano adaptation | 2.1 | 2.55 | − 0.16 | 2.17 | 2.23 | − 0.02 |
Select characteristics before and after PS matching, showing the (weighted) percentage of subjects with the characteristics in the ibuprofen versus comparator cohorts, as well as the absolute standardised mean difference. Data shown from the OpenClaims, on treatment, February to October 2020 analysis. A complete covariate balance list can be found in the accompanying supplementary files, including drug covariates that were balanced at the ingredient level
ASMD absolute standardised mean difference, DXA dual-energy X-ray absorptiometry, ECG electrocardiogram, ns-NSAIDs non-selective non-steroidal anti-inflammatory drugs, OA osteoarthritis, PS propensity score
aData for these covariates are given as a mean score for the cohort, not % of the cohort
Table 3.
Covariate balance before and after PS-matching ibuprofen-COX-2i cohorts
| Before PS matching | After PS matching | |||||
|---|---|---|---|---|---|---|
| Ibuprofen cohort (%) |
COX-2i cohort (%) |
ASMD | Ibuprofen cohort (%) |
COX-2i cohort (%) |
ASMD | |
| Demographics | ||||||
| Female | 64.5 | 63.6 | 0.02 | 63.8 | 63.1 | 0.02 |
|
Age, years 8–19 |
1.1 | 0.1 | 0.12 | 0.2 | 0.2 | 0 |
| 20–24 | 3.3 | 0.4 | 0.21 | 0.5 | 0.6 | − 0.01 |
| 25–29 | 4.5 | 0.7 | 0.24 | 0.8 | 0.9 | − 0.01 |
| 30–34 | 5.9 | 1.2 | 0.26 | 1.5 | 1.6 | − 0.01 |
| 35–39 | 6.9 | 2 | 0.24 | 2.5 | 2.7 | − 0.01 |
| 40–44 | 7.9 | 3.2 | 0.2 | 4.1 | 4.1 | 0 |
| 45–49 | 9.1 | 5 | 0.16 | 6.1 | 6.3 | − 0.01 |
| 50–54 | 11.3 | 8.1 | 0.11 | 9.7 | 9.7 | 0 |
| 55–59 | 13.3 | 11.9 | 0.04 | 13.9 | 13.4 | 0.01 |
| 60–64 | 12.4 | 14.7 | − 0.07 | 15.8 | 15.6 | 0.01 |
| 65–69 | 9.8 | 16.3 | − 0.19 | 15.5 | 15.5 | 0 |
| 70–74 | 6.7 | 14.9 | − 0.26 | 12.7 | 12.7 | 0 |
| 75–79 | 3.9 | 10.4 | − 0.25 | 8.1 | 8.3 | − 0.01 |
| 80–84 | 3.9 | 11 | − 0.27 | 8.5 | 8.4 | 0 |
| Conditions | ||||||
| Amenorrhea, any time prior | 6.3 | 2.2 | 0.2 | 2.7 | 2.6 | 0 |
| Hypertension, any time prior | 55 | 66.4 | − 0.23 | 65.5 | 64 | 0.03 |
| Hip pain, any time prior | 18.6 | 27.2 | − 0.21 | 24.7 | 23.9 | 0.02 |
| Hyperlipidaemia, any time prior | 41.9 | 54.5 | − 0.25 | 52.5 | 51.6 | 0.02 |
| Nicotine dependence, any time prior | 19.3 | 9.8 | 0.27 | 12 | 11.5 | 0.01 |
| Cataracts, any time prior | 13.5 | 24.2 | − 0.28 | 21.3 | 20.9 | 0.01 |
| OA, any time prior | 21.8 | 35 | − 0.3 | 32.3 | 31.7 | 0.01 |
| Hip OA, any time prior | 8.4 | 19.4 | − 0.32 | 15 | 14.8 | 0 |
| Knee OA, any time prior | 29.1 | 51.1 | − 0.46 | 45.7 | 44.6 | 0.02 |
| Osteoporosis, any time prior | 8.2 | 15.2 | − 0.22 | 13.7 | 13.2 | 0.01 |
| Lumbar spine stenosis, any time prior | 16.5 | 26.4 | − 0.24 | 24.6 | 23.8 | 0.02 |
| Low back pain, 6 months prior | 43.5 | 27.3 | 0.34 | 30.7 | 31 | − 0.01 |
| Hip OA, 6 months prior | 3.8 | 11.6 | − 0.29 | 7.7 | 8 | − 0.01 |
| Knee OA, 6 months prior | 14.3 | 28.6 | − 0.36 | 24 | 23.9 | 0 |
| Knee OA, 1 month prior | 5.9 | 16.3 | − 0.34 | 11.5 | 11.7 | 0 |
| Cerebrovascular disease | 2.2 | 3 | − 0.05 | 3 | 2.8 | 0.01 |
| Chronic obstructive lung disease | 13.4 | 13.8 | − 0.01 | 15.5 | 14.2 | 0.04 |
| Diabetes mellitus | 0.8 | 0.6 | 0.02 | 0.7 | 0.7 | 0 |
| Ischaemic heart disease | 0.2 | 0.3 | − 0.01 | 0.3 | 0.2 | 0.01 |
| Obesity | 27.7 | 25 | 0.06 | 26.1 | 25.2 | 0.02 |
| Malignant neoplastic disease | 0.2 | 0.3 | − 0.01 | 0.3 | 0.3 | 0 |
| Procedures and visit | ||||||
| Knee arthroscopy, any time prior | 3.1 | 11.1 | − 0.32 | 6.8 | 6.9 | 0 |
| DXA scan, any time prior | 13.5 | 25.4 | − 0.3 | 22.6 | 21.8 | 0.02 |
| ECG, any time prior | 39 | 49.2 | − 0.21 | 47.5 | 46.1 | 0.03 |
| Knee X-ray, any time prior | 16.5 | 26 | − 0.23 | 23 | 22.1 | 0.02 |
| Manual therapy/physiotherapy, any time prior | 38.6 | 53.3 | − 0.3 | 49.5 | 48.6 | 0.02 |
| Total knee replacement, any time prior | 0.6 | 4.6 | − 0.25 | 1.8 | 1.9 | − 0.01 |
| Arthrocentesis, 6 months prior | 6.1 | 11.8 | − 0.2 | 10.5 | 10.2 | 0.01 |
| Emergency visit, 6 months prior | 17.6 | 6.2 | 0.36 | 7.5 | 7.1 | 0.02 |
| Clinical index scoresa | ||||||
| CHADS2VASc | 2.12 | 2.74 | − 0.38 | 2.62 | 2.57 | 0.03 |
| Diabetes Comorbidity Severity Index | 1.62 | 1.95 | − 0.14 | 1.95 | 1.87 | 0.04 |
| Charlson Index, Romano adaptation | 2.22 | 2.57 | − 0.13 | 2.6 | 2.48 | 0.04 |
Select characteristics before and after PS matching, showing the (weighted) percentage of subjects with the characteristics in the ibuprofen vs comparator cohorts, as well as the ASMD. Data shown from the OpenClaims, on treatment, February to October 2020 analysis. A complete covariate balance list can be found in the accompanying supplementary files, including drug covariates that were balanced at the ingredient level
ASMD absolute standardised mean difference, COX-2i cyclooxygenase-2 inhibitors, DXA dual-energy X-ray absorptiometry, ECG electrocardiogram, OA osteoarthritis
aData for these covariates are given as a mean score for the cohort, not % of the cohort
Table 4.
Covariate balance before and after PS matching ibuprofen-paracetamol cohorts
| Before PS matching | After PS matching | |||||
|---|---|---|---|---|---|---|
| Ibuprofen cohort (%) | Paracetamol cohort (%) | ASMD | Ibuprofen cohort (%) | Paracetamol cohort (%) | ASMD | |
| Demographics | ||||||
| Female | 64 | 60.6 | 0.07 | 62.3 | 62.8 | − 0.01 |
| Age, years | ||||||
| 18–19 | 1.4 | 0.3 | 0.11 | 0.8 | 0.7 | 0.01 |
| 20–24 | 3.8 | 1.1 | 0.18 | 2.3 | 2.1 | 0.01 |
| 25–29 | 5 | 1.6 | 0.19 | 3.4 | 3.2 | 0.01 |
| 30–34 | 6.4 | 2.5 | 0.19 | 4.9 | 4.8 | 0 |
| 35–39 | 7.1 | 3.5 | 0.16 | 6.1 | 6.2 | 0 |
| 40–44 | 7.8 | 4.6 | 0.13 | 7.3 | 7.5 | 0 |
| 45–49 | 8.8 | 6.1 | 0.1 | 8.8 | 8.8 | 0 |
| 50–54 | 10.8 | 8.6 | 0.07 | 11.2 | 11.2 | 0 |
| 55–59 | 12.5 | 11.8 | 0.02 | 13.5 | 13.6 | 0 |
| 60–64 | 11.8 | 13.5 | − 0.05 | 13.2 | 13.4 | − 0.01 |
| 65–69 | 9.6 | 13.7 | − 0.13 | 10.9 | 11.1 | 0 |
| 70–74 | 6.9 | 12.3 | − 0.19 | 8 | 7.9 | 0 |
| 75–79 | 4.1 | 8.9 | − 0.2 | 4.8 | 4.8 | 0 |
| 80–84 | 4 | 11.5 | − 0.28 | 4.8 | 4.8 | 0 |
| Conditions | ||||||
| Amenorrhea, any time prior | 6.2 | 3 | 0.15 | 4.9 | 4.9 | 0 |
| Hypertension, any time prior | 51.4 | 68.3 | − 0.35 | 55.6 | 56.4 | − 0.02 |
| Hip pain, any time prior | 16.5 | 24.2 | − 0.19 | 17.6 | 18.3 | − 0.02 |
| Hyperlipidemia, any time prior | 39.9 | 53.7 | − 0.28 | 42.9 | 43.9 | − 0.02 |
| Nicotine dependence, any time prior | 16.6 | 16.3 | 0.01 | 16.2 | 16.9 | − 0.02 |
| Cataracts, any time prior | 13.2 | 21.5 | − 0.22 | 14.6 | 15.3 | − 0.02 |
| OA, any time prior | 19.9 | 29.1 | − 0.22 | 21.8 | 23 | − 0.03 |
| Hip OA, any time prior | 7.6 | 13.5 | − 0.19 | 8.6 | 8.8 | − 0.01 |
| Knee OA, any time prior | 27.3 | 39 | − 0.25 | 29.9 | 30.7 | − 0.02 |
| Osteoporosis, any time prior | 7.9 | 13.5 | − 0.18 | 8.9 | 9.1 | − 0.01 |
| Lumbar spine stenosis, any time prior | 13.5 | 26.9 | − 0.34 | 15.4 | 16.3 | − 0.02 |
| Low back pain, 6 months prior | 43 | 36.6 | 0.13 | 41.3 | 41.3 | 0 |
| Hip OA, 6 months prior | 3.6 | 6.5 | − 0.13 | 4.1 | 4.1 | 0 |
| Knee OA, 6 months prior | 13.9 | 18 | − 0.11 | 15.1 | 15.1 | 0 |
| Knee OA, 1 month prior | 5.9 | 9.3 | − 0.13 | 6.5 | 6.4 | 0 |
| Cerebrovascular disease | 1.9 | 4.1 | − 0.13 | 2.1 | 2.3 | − 0.01 |
| Chronic obstructive lung disease | 10.9 | 19.9 | − 0.25 | 12.4 | 13 | − 0.02 |
| Diabetes mellitus | 0.7 | 0.9 | − 0.03 | 0.7 | 0.8 | − 0.01 |
| Ischaemic heart disease | 0.2 | 0.3 | − 0.03 | 0.2 | 0.2 | 0 |
| Obesity | 25.9 | 26.1 | 0 | 25.6 | 26.1 | − 0.01 |
| Malignant neoplastic disease | 0.2 | 0.3 | − 0.03 | 0.2 | 0.2 | 0 |
| Procedures and visits | ||||||
| Knee arthroscopy, any time prior | 2.6 | 6.7 | − 0.2 | 3 | 3.2 | − 0.01 |
| DXA scan, any time prior | 13.3 | 20.5 | − 0.19 | 14.7 | 15.2 | − 0.01 |
| ECG, any time prior | 37.6 | 46.7 | − 0.18 | 39.1 | 40.1 | − 0.02 |
| Knee X-ray, any time prior | 14.9 | 21.7 | − 0.18 | 15.8 | 16.5 | − 0.02 |
| Manual/ physiotherapy, any time prior | 36.8 | 45.7 | − 0.18 | 38.1 | 39.1 | − 0.02 |
| Total knee replacement, any time prior | 0.4 | 2.1 | − 0.16 | 0.4 | 0.5 | − 0.01 |
| Arthrocentesis, 6 months prior | 5.8 | 8.2 | − 0.09 | 6.4 | 6.6 | − 0.01 |
| Emergency visit, 6 months prior | 15.4 | 13.5 | 0.05 | 12.8 | 13.4 | − 0.02 |
| Clinical index scoresa | ||||||
| CHADS2VASc | 2.02 | 2.85 | − 0.48 | 2.15 | 2.19 | − 0.03 |
| Diabetes Comorbidity Severity Index | 1.43 | 2.49 | − 0.41 | 1.57 | 1.65 | − 0.03 |
| Charlson index - Romano adaptation | 2 | 3.19 | − 0.41 | 2.14 | 2.27 | − 0.05 |
Select characteristics before and after PS matching, showing the (weighted) percentage of subjects with the characteristics in the Ibuprofen versus comparator cohorts, as well as the ASMD. Data shown from the OpenClaims, on treatment, February to October 2020 analysis. A complete covariate balance list can be found in the accompanying supplementary files, including drug covariates that were balanced at the ingredient level
ASMD absolute standardised mean difference, DXA dual-energy X-ray absorptiometry, ECG electrocardiogram, OA osteoarthritis, PS propensity score
aData for these covariates are given as a mean score for the cohort, not % of the cohort
Table 5.
Incidence of COVID-19 outcomes in PS-matched Ibu and Comp cohorts
| Comparisons | Database | Persons each cohorta | Cases | Follow-up (years) | Incidence (%) | Incidence (per 1k person-years) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ibu | Comp | Ibu | Comp | Ibu | Comp | Ibu | Comp | |||
| COVID-19 diagnosis (Nov 2019–Jan 2020) | ||||||||||
| Ibu vs COX-2i | PharMetrics Plus | 39,572 | 156 | 480 | 6931 | 14,539 | 0.39 | 1.21 | 22.5 | 33.0 |
| Ibu vs COX-2i | OpenClaims | 272,097 | 1407 | 3432 | 62,849 | 138,466 | 0.52 | 1.26 | 22.4 | 24.8 |
| Ibu vs ns-NSAIDs | PharMetrics Plus | 69,723 | 174 | 449 | 9038 | 16,081 | 0.25 | 0.64 | 19.3 | 27.9 |
| Ibu vs ns-NSAIDs | OpenClaims | 563,839 | 1885 | 4100 | 98,946 | 168,055 | 0.33 | 0.73 | 19.1 | 24.4 |
| Ibu vs paracetamol | PharMetrics Plus | 54,074 | 119 | 414 | 6830 | 10,454 | 0.22 | 0.77 | 17.4 | 39.6 |
| Ibu vs paracetamol | OpenClaims | 437,928 | 1334 | 4523 | 71,973 | 141,905 | 0.30 | 1.03 | 18.5 | 31.9 |
| COVID-19 diagnosis (Feb 2020–Oct 2020) | ||||||||||
| Ibu vs COX-2i | PharMetrics Plus | 67,190 | 532 | 1247 | 10,894 | 21,898 | 0.79 | 1.86 | 48.8 | 57.0 |
| Ibu vs COX-2i | OpenClaims | 457,280 | 3551 | 7370 | 92,004 | 193,931 | 0.78 | 1.61 | 38.6 | 38.0 |
| Ibu vs ns-NSAIDs | PharMetrics Plus | 120,954 | 689 | 1334 | 14,424 | 24,796 | 0.57 | 1.10 | 47.8 | 53.8 |
| Ibu vs ns-NSAIDs | OpenClaims | 943,006 | 5382 | 9682 | 143,309 | 241,999 | 0.57 | 1.03 | 37.6 | 40.0 |
| Ibu vs paracetamol | PharMetrics Plus | 101,635 | 575 | 834 | 11,617 | 15,820 | 0.57 | 0.82 | 49.5 | 52.7 |
| Ibu vs paracetamol | OpenClaims | 776,963 | 4325 | 8106 | 110,110 | 188,659 | 0.56 | 1.04 | 39.3 | 43.0 |
| Hospitalised with COVID-19 (Nov 2019–Jan 2020) | ||||||||||
| Ibuprofen vs COX-2i | PharMetrics Plus | 39,572 | 29 | 89 | 6963 | 14,660 | 0.07 | 0.22 | 4.2 | 6.1 |
| Ibuprofen vs COX-2i | OpenClaims | 272,097 | 446 | 961 | 63,317 | 139,848 | 0.16 | 0.35 | 7.0 | 6.9 |
| Ibuprofen vs ns-NSAIDs | PharMetrics Plus | 69,723 | 24 | 91 | 9073 | 16,183 | 0.03 | 0.13 | 2.7 | 5.6 |
| Ibuprofen vs ns-NSAIDs | OpenClaims | 563,839 | 561 | 1171 | 99,526 | 169,551 | 0.10 | 0.21 | 5.6 | 6.9 |
| Ibuprofen vs paracetamol | PharMetrics Plus | 54,074 | 18 | 82 | 6854 | 10,565 | 0.03 | 0.15 | 2.6 | 7.8 |
| Ibuprofen vs paracetamol | OpenClaims | 437,928 | 386 | 1274 | 72,392 | 143,803 | 0.09 | 0.29 | 5.3 | 8.9 |
| Hospitalised with COVID-19 (Feb 2020–Oct 2020) | ||||||||||
| Ibu vs COX-2i | PharMetrics Plus | 67,190 | 112 | 211 | 10,979 | 22,153 | 0.17 | 0.31 | 10.2 | 9.5 |
| Ibu vs COX-2i | OpenClaims | 457,280 | 1044 | 1948 | 92,847 | 196,320 | 0.23 | 0.43 | 11.2 | 9.9 |
| Ibu vs ns-NSAIDs | PharMetrics Plus | 120,954 | 128 | 262 | 14,506 | 25,020 | 0.11 | 0.22 | 8.8 | 10.5 |
| Ibu vs ns-NSAIDs | OpenClaims | 943,006 | 1456 | 2432 | 144,396 | 244,609 | 0.15 | 0.26 | 10.1 | 9.9 |
| Ibu vs paracetamol | PharMetrics Plus | 101,635 | 120 | 185 | 11,687 | 16,004 | 0.12 | 0.18 | 10.3 | 11.6 |
| Ibu vs paracetamol | OpenClaims | 776,963 | 1143 | 2228 | 110,941 | 191,379 | 0.15 | 0.29 | 10.3 | 11.6 |
On-treatment incidence rates of COVID-19 diagnosis or hospitalisation with COVID-19 in Ibu vs Comp user cohorts. For each pairwise comparison, we report the PS-matched cohort size, follow-up years, the number of events and incidences
Comp comparator, COVID-19 coronavirus disease 2019, COX-2i cyclooxygenase-2 inhibitors, Ibu ibuprofen, ns-NSAIDs non-selective non-steroidal anti-inflammatory drugs, PS propensity sore
aIbu and Comp cohorts are matched 1:1 on the PS and are therefore the same size
Incidence Rates of COVID-19 Outcomes
On-treatment incidence of COVID-19 outcomes in PS-matched cohorts are reported in Table 5 (intention-to-treat incidence rates in Tables 11–12 of the ESM). In the Open Claims database, the OT incidence rates of COVID-19 diagnosis were 37.6 versus 40.0 (ibuprofen vs ns-NSAIDs), 38.6 versus 38.0 (ibuprofen vs COX-2i) and 39.3 versus 43.0 (ibuprofen vs paracetamol) per 1000 person-years for patients enrolled during the pandemic window. Within the same database and study window, the incidence rates of COVID-19 hospitalisation were 10.1 versus 9.9 (ibuprofen vs ns-NSAIDs), 11.2 versus 9.9 (ibuprofen vs COX-2i) and 10.3 versus 11.6 (ibuprofen vs paracetamol) per 1000 person-years. In general, the incidence rates of COVID-19 diagnosis were higher in PharMetrics Plus, but the incidence rates for COVID-19 hospitalisation were similar between the two databases. Furthermore, the incidence rates of both COVID-19 outcomes were higher for cohorts enrolled during the pandemic observation period.
Empirical Calibration and HRs
The proportional hazards assumption of Cox regression was held for all comparisons, except for one paracetamol comparison (Kaplan–Meier plots available at https://dqdashboard.iqvia.com/ibucovid/). Prior to empirical calibration, all comparisons had a detectable systematic bias, defined here as > 5% significant NCOs in a comparison. After empirical calibration, most comparisons produced < 5% significant NCOs, and all comparison produced <8% NCOs (Table 13 of the ESM).
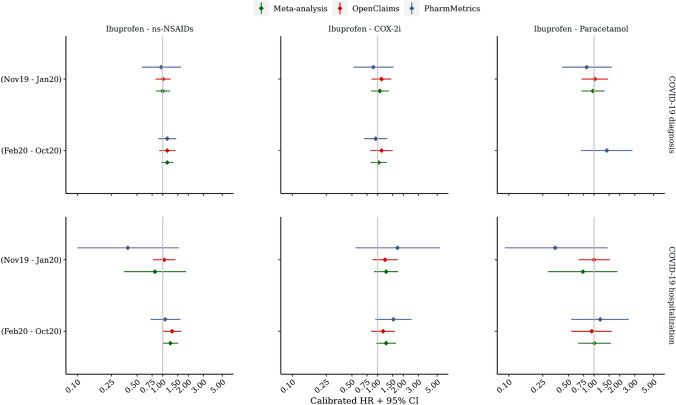
In the OT analyses, a meta-analysis of calibrated HRs revealed no significant differential risk of COVID-19 diagnosis or hospitalisation with COVID-19 in users of ibuprofen versus users of COX-2i, ns-NSAIDs or paracetamol (Table 6, Fig. 2, comparisons that violated the Cox proportional hazards assumption were excluded). For example, the aggregated HRs for COVID-19 diagnosis in the pre-pandemic cohorts were 1.00 (95% CI 0.83–1.22) for ibuprofen versus ns-NSAID users, 1.06 (95% CI 0.84–1.35) for ibuprofen versus COX-2i users and 0.97 (95% CI 0.71–1.33) for ibuprofen versus paracetamol users. Further, the aggregated HRs for hospitalisation with COVID-19 in the pandemic enrolment window were 1.23 (95% CI 0.99–1.52), 1.26 (95% CI 0.96–1.65) and 1.01 (95% CI 0.65–1.58) accordingly. Although a single significant HR was observed in the Open Claims database when comparing ibuprofen users versus ns-NSAID users for the risk of hospitalisation with COVID-19 (pandemic period), no similarly significant HRs were observed in PharMetrics Plus, nor in either database during the pre-pandemic period.
Table 6.
On-treatment calibrated HRs for COVID-19 outcomes in users of ibuprofen versus comparator analgesics
| Comparisons | Database | COVID-19 diagnosis HR (95% CI) | Hospitalisation with COVID-19 HR (95% CI) | ||
|---|---|---|---|---|---|
| Nov 2019–Jan 2020 | Feb 2020–Oct 2020 | Nov 2019–Jan 2020 | Feb 2020–Oct 2020 | ||
| On treatment | |||||
| Ibuprofen—ns-NSAIDs | Open Claims | 1.01 (0.82–1.24) | 1.13 (0.91–1.41) | 1.04 (0.77–1.41) | 1.29 (1.01–1.65) |
| Ibuprofen—ns-NSAIDs | PharMetrics Plus | 0.96 (0.57–1.64) | 1.13 (0.89–1.44) | 0.39 (0.10–1.55) | 1.07 (0.72–1.61) |
| Ibuprofen—ns-NSAIDs | Meta-analysis | 1.00 (0.83–1.22) | 1.13 (0.96–1.33) | 0.81 (0.35–1.88) | 1.23 (0.99–1.52) |
| Ibuprofen—COX-2i | Open Claims | 1.11 (0.85–1.45) | 1.11 (0.82–1.50) | 1.22 (0.87–1.72) | 1.16 (0.84–1.60) |
| Ibuprofen—COX-2i | PharMetrics Plus | 0.89 (0.52–1.54) | 0.95 (0.69–1.30) | 1.71 (0.55–5.36) | 1.53 (0.94–2.51) |
| Ibuprofen—COX-2i | Meta-analysis | 1.06 (0.84–1.35) | 1.03 (0.83–1.28) | 1.26 (0.91–1.74) | 1.26 (0.96–1.65) |
| Ibuprofen—paracetamol | Open Claims | 1.02 (0.71–1.46) | NA | 1.00 (0.66–1.53) | 0.94 (0.54–1.62) |
| Ibuprofen—paracetamol | PharMetrics Plus | 0.82 (0.42–1.62) | 1.41 (0.70–2.82) | 0.35 (0.09–1.44) | 1.18 (0.54–2.55) |
| Ibuprofen—paracetamol | Meta-analysis | 0.97 (0.71–1.33) | NA | 0.74 (0.29–1.88) | 1.01 (0.65–1.58) |
CI confidence interval, COVID-19 coronavirus disease 2019, COX-2i cyclooxygenase-2 inhibitors, HR hazard ratios, NA not available because of the failure of a negative control diagnosis, ns-NSAIDs non-selective non-steroidal anti-inflammatory drugs
Fig. 2.
On-treatment calibrated hazard ratio (HR) estimates for coronavirus disease 2019 (COVID-19) outcomes in users of ibuprofen versus comparator analgesics. CI confidence interval, COX-2i cyclooxygenase-2 inhibitors, ns-NSAIDs non-selective non-steroidal anti-inflammatory drugs
The results of the 6-month ITT sensitivity analyses were largely consistent with the OT analysis, but two comparisons (Table 14 and Fig. 1 of the ESM). Specifically, a significantly increased risk of COVID-19 diagnosis was observed in an ibuprofen-paracetamol comparison (pre-pandemic enrolment, meta-analysis HR 1.27, 95% CI 1.06–1.52), and a significantly increased risk of hospitalisation with COVID-19 was observed in an ibuprofen-COX-2i comparison (pandemic enrolment window, HR 1.25, 95% CI 1.07–1.46). Neither result was replicated in the alternative study period, nor in the OT analysis. Notably, the median length of drug use in these cohorts was substantially less than 6 months, with a large difference between median ibuprofen and COX-2i usage (e.g. Open Claims, median ibuprofen usage: 29 days, median COX-2i usage: 89 days; Tables 15–16 of the ESM).
Discussion
In this cohort study, including 6,707,247 and 10,154,597 distinct patients with OA or back pain during the two observation periods, we found no differential risks of incident COVID-19 diagnosis or COVID-19 hospitalisation among ibuprofen users compared with other ns-NSAIDs, COX-2i or paracetamol. Our findings support regulatory recommendations that NSAIDs, including ibuprofen, should be prescribed as indicated in the same way as before the COVID-19 pandemic, especially for those who rely on ibuprofen or NSAIDs to manage chronic arthritis or musculoskeletal pain symptoms.
A few laboratory-based studies have proposed possible biological mechanisms linking ibuprofen or NSAID exposure and COVID-19 complications. For example, in vitro experiments found that NSAIDs could disrupt the resolution of the inflammatory process by inhibiting prostanoid biosynthesis, which theoretically might weaken immune responses against pathogens [21, 22]. In diabetic rats, ibuprofen has the potential to upregulate the angiotensin-converting enzyme 2 receptor, to which the severe acute respiratory syndrome coronavirus 2 virus binds before entering host cells [23]. However, it remains unknown whether these results can be generalised to humans.
Based on patients hospitalised with COVID-19, several studies have consistently shown that the previous use of NSAIDs did not exacerbate the severity of COVID-19 or dying from the disease [24–27]. Nevertheless, data are limited on the susceptibility to COVID-19 associated with NSAIDs indicated for other health conditions. Among a few studies, Wong et al. found no differential risk of COVID-19-related deaths among NSAID users (536,423 users from the general community and 175,495 users from the rheumatoid arthritis/OA population) compared to non-users [12]. This study exploited clinical knowledge with an informed directed acyclic graph approach to control a potential confounding bias. However, the quantitative bias analysis in this study showed that even a moderate unmeasured confounder could fully explain the observed associations, which is likely to occur given the known systematic difference between NSAID users and non-users driven by the drug indications. Another community-based cohort study, in contrast, used an active comparator design by including 13,202 users of NSAIDs and 12,457 users of co-codamol or co-dydramol [11]. Although the observed association with COVID-19 infection was statistical no-significance [HR, 0.79 (95% CI 0.57–1.11)], it is questionable that opioid-containing drugs acted as a meaningful active comparator given that they are likely prescribed for patients with moderate or severe acute pain with different susceptibility to COVID-19.
Our study extended existing knowledge and produced more reliable causal findings in the following ways. First, for the first time, we specifically studied the effect of ibuprofen, the type of drug under the spotlight among the NSAID class, compared with other similar analgesics on COVID-19-related outcomes. It is of special importance for clinicians and patients to balance the risks associated with ibuprofen over its alternative medications because of the very large consumption of this drug in primary care and over-the-counter settings. Second, previous studies that used general practitioners’ prescription records as the source of drug exposure were likely subject to considerable medication non-compliance. Instead, we based our analyses on claim data, and, therefore, the concern of non-compliance between the drug prescription and drug dispensation can be vastly dismissed. Third, COVID-19 is a novel infectious disease, and current knowledge of its risk factors is relatively limited. The conventional approach of pre-specifying potential confounders based on clinical experience might be insufficient and defective, especially during the pandemic’s lockdown periods when doctors’ routine drug prescribing practices have been seriously disrupted. Even though we applied a large-scale propensity score approach to balance thousands of baseline covariates that maximally rules out the confounding, evidence from NCOs existed for residual confounding in our study, highlighting the importance of empirically calibrating estimates in observational healthcare database studies.
However, several limitations warrant attention in our study. First, ibuprofen, other ns-NSAIDs and paracetamol were commonly used through the over-the-counter self-medication [28], which inevitably misclassified groups of exposures and controls by only using those claim based data. Although the extect to which the misclassification could be similar in terms of ascertaning target or comparator drugs, it would likely distort associations toward the null. Second, owing to the limited availability of testing resources at the earlier pandemic stage and the fact that many infected patients are asymptomatic, under-diagnosis of people with COVID-19 infection was possible. To address this limitation inherent in identifying asymptomatic COVID-19 cases, we defined a hospital admission-based COVID-19 outcome because the claims data can capture most COVID-19 hospitalisation cases. Additionally, as the COVID-19 test capacity had been gradually improved in the US community, the underestimation of COVID-19 should be primarily mitigated in the second observational window up to 31 October, 2020. Third, when a data-driven model was applied in the context of the prevalent-user design, there was always a potential risk of inclusion of mediators in the covariate adjustment. In this specific case, all study analgesic medications were commonly prescribed for symptom relief, the pathology of underlying diseases that causes painful conditions was unlikely to be modified by these drugs. Therefore, the likelihood of over-adjustment seemed minimal. Fourth, indication bias could not be ruled out, even though the target and comparison drugs had a significant degree of overlap for their indications. Fifth, we cannot differentiate short-term or long-term users as information on indications is not readily available. By restricting to patients with OA or back pain, we expected most participants to be regular users of NSAIDs. Sixth, a proportion of people initiating any study drugs to relieve COVID-19 symptoms might be falsely included. However, this is unlikely to occur during the first enrolment window before the pandemic, showing consistent results. In summary, our findings reassured that using ibuprofen in the community, compared to other ns-NSAIDs, COX-2i or paracetamol, was not associated with an increased risk of susceptibility and severity of COVID-19.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Abbott contracted IQVIA for the conduct of this study. Prof. Prieto-Alhambra receives funding from the UK National Institute for Health Research in the form of a Senior Research Fellowship, and as part of the Oxford National Institute for Health Research Biomedical Research Centre. Prof. Giustino Varrassi had a contract with Abbott International, as a consultant for scientific projects. He is also a consultant for scientific projects with Dompé Farmaceutici and Menarini Group.
Conflicts of Interest/Competing Interests
All authors have completed the ICMJE disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare the following interests: Dani Prieto-Alhambra receives funding from the UK National Institute for Health Research in the form of a senior research fellowship and from the Oxford National Institute for Health Research Biomedical Research Centre. Junqing Xie receives the Clarendon Fund and Jardine scholarship (University of Oxford) to support her DPhil study. Dani Prieto-Alhambra’s research group has received research grants from the European Medicines Agency, the Innovative Medicines Initiative, Amgen, Chiesi and UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen, AstraZeneca and UCB Biopharma.
Ethics Approval
These assets are de-identified, commercially available data products that could be purchased and licensed by any researcher. The collection and de-identification of these data assets is a process that is commercial intellectual property and not privileged to the data licensees and the co-authors on this study. Licensees of these data have signed data use agreements with the data vendors that detail the usage protocols for running retrospective research on these databases. All analyses performed in this study were in accordance with data use agreement terms as specified by the data owners. As these data are deemed commercial assets, there is no institutional review board applicable to the usage and dissemination of these result sets or required registration of the protocol with additional ethics oversight. Compliance with data use agreement terms, which stipulate how these data can be used and for what purpose, is sufficient for the licensing commercial entities.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Patient-level data cannot be shared without approval from data custodians owing to local information governance and data protection regulations. Additional summary data, analytical code and detailed definitions of algorithms for identifying the events are available from the corresponding author on reasonable request to access the GitHub repository.
Code Availability
Not applicable.
Authors’ Contributors
JQ-X, DP-A, SS, CT and GV conceived the study and contributed to the study design. JQ-X, JB and JA conducted the statistical analyses. JQ-X and DP-A interpreted the results and wrote the manuscript. All authors contributed to writing the manuscript, approved the final version and had final responsibility for the decision to submit for publication. The lead authors (JQ-X and JB) affirm that this manuscript is an honest, accurate and transparent account of the study being reported, that no important aspects of the study have been omitted and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. DP-A is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
References
- 1.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 2.Torjesen I. Covid-19: ibuprofen can be used for symptoms, says UK agency, but reasons for change in advice are unclear. BMJ. 2020;369:m1555. doi: 10.1136/bmj.m1555. [DOI] [PubMed] [Google Scholar]
- 3.Roy S. Coronavirus: alerte sur l’ibuprofène et autres anti-inflammatoires. Le Figaro. Updated March 14, 2020. 2022. https://www.lefigaro.fr/sciences/coronavirus-alerte-sur-l-ibuprofene-et-autres-anti-inflammatoires-20200314. Accessed 28 Feb 2022.
- 4.Fernandez-Gutierrez B, Leon L, Madrid A, et al. Hospital admissions in inflammatory rheumatic diseases during the peak of COVID-19 pandemic: incidence and role of disease-modifying agents. Ther Adv Musculoskelet Dis. 2021. doi: 10.1177/1759720X20962692. [DOI] [PMC free article] [PubMed]
- 5.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency. EMA gives advice on the use of non-steroidal anti-inflammatories for COVID-19. 2020. https://www.ema.europa.eu/en/news/ema-gives-advice-use-non-steroidal-anti-inflammatories-covid-19. Accessed 5 Jul 2020 .
- 9.Monti S, Montecucco C. Non-steroidal anti-inflammatory treatment during COVID-19: friend or foe? Response to: 'Coronavirus disease 19 (Covid-19) and non-steroidal anti-inflammatory drugs (NSAID)' by Giollo et al. Ann Rheum Dis. 2021;80(2):e13.10.1136/annrheumdis-2020-217638 [DOI] [PubMed]
- 10.NICE. Key messages. COVID-19 rapid evidence summary: acute use of non-steroidal anti-inflammatory drugs (NSAIDs) for people with or at risk of COVID-19. 2020. https://www.nice.org.uk/advice/es23/chapter/Key-messages. Accessed 5 July 2020.
- 11.Chandan JS, Zemedikun DT, Thayakaran R, et al. Nonsteroidal antiinflammatory drugs and susceptibility to COVID-19. Arthritis Rheumatol. 2021;73(5):731–739. doi: 10.1002/art.41593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong AY, MacKenna B, Morton CE, et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis. 2021;80(7):943–951. doi: 10.1136/annrheumdis-2020-219517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OHDSI. OMOP Common Data Model. 2021. https://www.ohdsi.org/data-standardization/the-common-data-model/. Accessed 7 Oct 2021.
- 14.Prieto-Alhambra D, Kostka K, Duarte-Salles T, et al. Unraveling COVID-19: a large-scale characterization of 4.5 million COVID-19 cases using CHARYBDIS. Clin Epidemiol. 2022;14:369–84. doi: 10.2147/CLEP.S323292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prats-Uribe A, Sena AG, Lai LYH, et al. Use of repurposed and adjuvant drugs in hospital patients with COVID-19: multinational network cohort study. BMJ. 2021;373:n1038. doi: 10.1136/bmj.n1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodward M. Formulae for sample size, power and minimum detectable relative risk in medical studies. J R Stat Soc D. 1992;41(2):185. doi: 10.2307/2348252. [DOI] [Google Scholar]
- 17.Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47(6):2005–2014. doi: 10.1093/ije/dyy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Robust empirical calibration of p-values using observational data. Stat Med. 2016;35(22):3883–3888. doi: 10.1002/sim.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. Proc Natl Acad Sci USA. 2018;115(11):2571–2577. doi: 10.1073/pnas.1708282114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen ML, Porter KE, Gruber S, Wang Y, van der Laan MJ. Diagnosing and responding to violations in the positivity assumption. Stat Methods Med Res. 2012;21(1):31–54. doi: 10.1177/0962280210386207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 23.Qiao W, Wang C, Chen B, et al. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology. 2015;131(2):97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- 24.Abu Esba LC, Alqahtani RA, Thomas A, Shamas N, Alswaidan L, Mardawi G. Ibuprofen and NSAID use in COVID-19 infected patients is not associated with worse outcomes: a prospective cohort study. Infect Dis Ther. 2021;10(1):253–268. doi: 10.1007/s40121-020-00363-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drake TM, Fairfield CJ, Pius R, et al. Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kow CS, Hasan SS. The risk of mortality in patients with COVID-19 with pre-diagnosis use of NSAIDs: a meta-analysis. Inflammopharmacology. 2021;29(3):641–644. doi: 10.1007/s10787-021-00810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Lee S-H, You SC, Kim J, Yang K. Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19. Sci Rep. 2021;11(1):5087. doi: 10.1038/s41598-021-84539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061–1075. doi: 10.2147/TCRM.S79135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.