Abstract
The most common type of idiopathic interstitial pneumonia is idiopathic pulmonary fibrosis (IPF), an irreversible, progressive disorder that has lately come into question for possible associations with COVID-19. With few geographical exceptions, IPF is a rare disease but its prevalence has been increasing markedly since before the pandemic. Environmental exposures are frequently implicated in IPF although genetic factors play a role as well. In IPF, healthy lung tissue is progressively replaced with an abnormal extracellular matrix that impedes normal alveolar function while, at the same time, natural repair mechanisms become dysregulated. While chronic viral infections are known risk factors for IPF, acute infections are not and the link to COVID-19 has not been established. Macrophagy may be a frontline defense against any number of inflammatory pulmonary diseases, and the inflammatory cascade that may occur in patients with COVID-19 may disrupt the activity of monocytes and macrophages in clearing up fibrosis and remodeling lung tissue. It is unclear if COVID-19 infection is a risk factor for IPF, but the two can occur in the same patient with complicating effects. In light of its increasing prevalence, further study of IPF and its diagnosis and treatment is warranted.
Keywords: COVID-19, Idiopathic pulmonary fibrosis, Interstitial pneumonia, Macrophagy
Key Summary Points
| The incidence and prevalence of idiopathic pulmonary fibrosis (IPF) are increasing globally. This trend predates the COVID-19 pandemic. |
| Although once considered a rare disorder, IPF may lose this distinction soon. Adjusted prevalence rates in North America are 2.40 to 2.98 per 10,000 persons. The world’s highest prevalence occurs in South Korea, 4.51 per 10,000 persons. |
| IPF is an irreversible condition in which an abnormal extracellular matrix disrupts the normal lung function and seems to involve a series of micro-injuries to the aging alveolar epithelium which triggers fibrogenic growth factors, producing myofibroblasts that build up extracellular matrices. |
| Risk favors for IPF are older age, smoking, Caucasian race, lower body mass index, exposure to particulate matter, working around livestock, and genetic predispositions. The role of COVID-19 and other chronic viral factors in current IPF epidemiology is unclear. |
| Treatments are largely supportive care as IPF is both progressive and irreversible. Lung transplant may be considered in appropriate patients. |
Introduction
The respiratory system interfaces between the internal and external environments, making it particularly vulnerable to infection. Idiopathic pulmonary fibrosis (IPF), the most common form of idiopathic interstitial pneumonia [1], is a progressive, irreversible, incurable lung disease that qualifies as a rare disease in all nations of the world except South Korea, which has the world’s highest incidence and prevalence of the disease [2]. The rarity of the disorder means that there are few physicians with extensive experience in treating these conditions and limited guidance for managing new cases [3]. The symptoms of IPF include dyspnea, coughing, reduced energy and exercise capacity, and functional deficits [2]. Left untreated, the median survival after IPF diagnosis is 2–3 years [4]. Diagnosis is made on the basis of clinical examination, high-resolution chest imaging, and—for about 21% of patients—a lung biopsy [3, 5].
Prevalence ranges from 0.33 to 4.51 per 10,000 persons with lowest rates in Asia–Pacific nations with the exception of Korea and higher rates in North America [2], but IPF may be underdiagnosed in the USA [6]. Rates of IPF have increased markedly since around 2000 [7, 8]. While it can be tempting to associate the COVID-19 pandemic and viral infection with these rising rates of IPF, the increased prevalence of IPF has long preceded the pandemic. Our objective was to better understand the epidemiology and pathogenesis of IPF and to explore the relationship of IPF to COVID-19 apart from seeing COVID-19 as its driver.
Methods
This is a narrative review. The keywords “idiopathic pulmonary fibrosis,” “pulmonary fibrosis,” and “idiopathic pulmonary fibrosis epidemiology” were searched in PubMed in June 2022. Delimited to articles published in the past year, this achieved 1373 results (without temporal restrictions, 13,804 results). We also searched Google Scholar and Cochrane library.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Increasing Incidence and Cumulative Prevalence of IPF
The incidence and prevalence of IPF are rising all over the earth and this has been attributed anecdotally to any number of potential causes. The population, particularly in developed nations, is “graying” and older individuals are more susceptible to IPF [2]. Improved diagnostic tools and awareness of IPF may increase the rate of diagnosis [2]. Greater awareness of IPF among clinicians may also contribute to increased case numbers. In addition, people who have IPF today are living longer than before, increasing prevalence statistics.
In a retrospective study based on a national cohort of US veterans, the incidence, prevalence, and geographic distribution of IPF were evaluated from 2010 through 2019 among veterans [9]. During this time period, approximately 10.7 million veterans sought care from the Veterans Health Administration (VHA) services and about 1% of them were diagnosed with IPF [9]. However, prevalence increased from 276 cases per 100,000 in 2010 to 725 cases per 100,000 in 2019. The annual incidence likewise increased from 73 per 100,000 person-years in 2010 to 210 cases per 100,000 person-years in 2019 [9]. It should be noted that there were also significant differences in incidence and prevalence by geographical regions although regionally specific environmental influences on IPF statistics have not been elucidated [9]. This increase in IPF incidence and prevalence may be particularly pronounced in the veteran population. Using an administrative claims database from Medicare beneficiaries and the years 2000 to 2011 as the range, Raghu et al. reported that the annual incidence of IPF remained constant over time with an overall estimate of 93.7 cases per 100,000 person-years with an increased cumulative prevalence from 202.2 cases per 100,000 persons in 2001 to 494.5 cases per 100,000 persons in 2011 [10]. The subjects in that database were mostly female (54%) with a mean age of 79.4 years [10]. Globally, the incidence of IPF is increasing over time when comparing data from the 1980s to twenty-first century data [11]. The reasons for this are not clear.
Epidemiology of IPF
The incidence of IPF increases with advancing age [12], and the mean age of patients at diagnosis is around 65–70 years [5, 13]. It is so rare in individuals under 50 that age itself can be a diagnostic criterion [13]. An aging respiratory system is more vulnerable to fibrosis and age is associated with stem cell depletion and aberrant intracellular communications [14]. IPF is more common in men than women [6]. Adjusted prevalence estimates range from 0.33 to 2.51 per 10,000 persons in Europe and 2.40–2.98 per 10,000 persons in North America [2]. The highest prevalence in the world was in South Korea with 4.51 per 10,000 persons, but the reasons for this have not been elucidated [2].
The incidence of IPF is increasing to the point that current cumulative prevalence threatens its status as a rare disorder [1]. Although it is tempting to associate increased rates of IPF with the COVID-19 pandemic since they share much in common, IPF rates started to increase prior to the pandemic [2]. The evidence that viral infection in general may elevate a patient’s risk for pulmonary fibrosis is mixed and inconclusive [15]. Chronic viral infection has been associated with IPF but acute viral infection has not [15]. However, pulmonary fibrosis may occur following acute respiratory distress syndrome (ARDS) which occurs in a subset of patients with COVID-19 [16]. The highly atypical inflammatory responses in COVID-19 have complicated analysis of the relationship of this viral infection to IPF [17]. While COVID-19 may not be the driver of the increasing incidence and prevalence of IPF, it is not necessarily unrelated.
Environmental exposures are often blamed for IPF. Environmental factors associated with IPF include smoking cigarettes, exposure to dust and metal particulates, dust from stone or silica, and various factors associated with agriculture, ranching, and farming [1]. Genetic factors also play a role with numerous gene variants associated with IPF [18]. At least a dozen gene loci are thought to contribute to an individual’s risk for IPF and these familial associations suggest that IPF may be on a spectrum with familial interstitial pulmonary fibrosis rather than a distinct and separate disease [19, 20].
Pathogenesis
In patients with IPF, healthy lung tissue is replaced with an abnormal extracellular matrix which disrupts normal alveolar function. That, in turn, leads to decreased respiratory compliance, a disordered gas exchange, and potentially life-threatening respiratory failure [1]. Medical experts have long struggled to understand the complex pathophysiology of IPF, which was once considered a chronic inflammatory disorder, then an immune disorder, and finally today a complex disorder caused by several interacting genetic and environmental risk factors [1].
IPF seems to involve repeated micro-injuries to the aging alveolar epithelium. These micro-injuries trigger the secretion of fibrogenic growth factors, cytokines, and coagulants, which can trigger miscommunications between the epithelium and the body’s network of fibroblasts. This, in turn, causes excess myofibroblasts to be produced, which then produce extracellular matrices that accumulate to the point that aberrant remodeling of the interstitium of the lungs occurs [1].
The damage is irreversible, because the lung’s natural repair mechanisms become dysregulated in IPF, particularly those of the type 2 alveolar epithelial cells (AEC2s). AEC2 cells are a type of stem cell in the pulmonary system that can help renew type 1 alveolar epithelial cells (AEC1s) following lung injury [21]. Patients with IPF typically exhibit both loss of AEC1 cells and abnormal AEC2 cells [1].
There is no evidence of the direct pathogenic association between fibrotic growth and the SARS-CoV-2 virus, but it is plausible, because viruses are capable of inducing fibrogenesis. Such viral infections might act by predisposing lung tissue to fibrosis or by advancing pre-existing fibrosis. Viral infections, such as the herpes virus, have been associated with IPF [15, 22, 23]. However, viruses are more often associated with asthma and chronic obstructive pulmonary disease (COPD) than IPF, which overall is considered “noninfectious” [24]. However, viral or bacterial infections have been linked to acute exacerbations of IPF [25]. The bilateral ground-glass opacities on radiographs and diffuse alveolar damage typical of COVID-19 suggest viral infection [26, 27].
Further study of the lung microbiome may aid in the elucidation of IPF and development of other respiratory diseases [28]. The microbiome has a potential role in modification of the viral infection as well as being on the front lines of an immune response [28]. The bidirectional activity of respiration makes the lung microbiome more dynamic and transient than other microbiota in the body. In healthy lungs, microbial density is low but microbial diversity is high; the opposite is true for damaged or diseased lungs [28]. In cases of pneumonia, the lung microbiota can be reflective of the cause and course of the pneumonia [29], and the lungs give evidence of an elevated microbial mass with low microbial diversity [30]. It is not determined if this occurs with IPF as well. In terms of COVID-19, the limited evidence from the study of lung microbiota in patients with COVID-19 suggests that the lung (and gut) microbiota are markedly altered in patients with COVID-19 and that such changes might be associated with disease severity [28].
The alveolar surface is most vulnerable to external pathogens and has as a defensive barrier composed of a continuous layer of pulmonary epithelial cells. This barrier is buttressed by a mucus coating, proteolytic enzymes, defensive proteins such as immunoglobulins and defensins, and lysozymes in the fluids around the alveoli as well as on its surface [31]. The epithelium of the lungs secrete multiple different types of cytokines and chemokines [31]. When the epithelium is injured, for example during infection, it can trigger the inflammatory cascade, which can culminate in lung disease [31]. Healthy lung microbiota can effectively fend off harmful pathogens by a variety of mechanisms, including limiting nutrients to them or secreting growth inhibitors [32]. This is not the case for diseased or damaged lungs.
IPF may be the result of dysfunctional changes in the alveolar epithelium triggered by age-related alterations in cellular function. Shorter-than-normal telomeres have been documented in the alveolar epithelia of patients with IPF, which are possibly related to polymorphisms affecting the telomerase enzyme [33]. Telomerase adds telomere repeats at the ends of chromosomes; telomeres shorten with each cell division until the point that they trigger a DNA “damage message” that can lead to apoptosis. Telomere shortening has been observed in various diseases associated with old age [34]. In a murine study, it appears that it is the shortest telomere length that is most crucial to cellular survival, rather than the average length of telomeres [35]. While this might explain the increased prevalence of IPF in older individuals, mutations in telomerase do not account for the majority of cases of IPF [33].
Clinical Course
Patients with IPF usually present with diffuse symptoms of dyspnea upon exercise and a dry cough; they may also complain of being tired all of the time and abnormal fatigue upon exertion [36]. While dyspnea is the predominant symptom of IPF, it is often preceded, sometimes by years, by a persistent cough [36]. In a study of 19 patients with IPF, it was found that patients had a median cough frequency per day of 9.4 (range 1.5–39.4) with coughing much more frequent in daytime than at night [37]. Few patients with IPF report sleep interrupted by coughing [37]. Chronic IPF-associated cough is likely multimechanistic in origin and can be exacerbated in the presence of gastroesophageal reflux disease [38]. Cough may be particularly distressing to patients, because it can be a marker of disease severity and progression [36]. It is thought that cough may be caused by structural changes to the lungs, an increased sensitivity of the natural cough reflex, inflammation of the pulmonary airways, and changes in mucus production [36]. However, cough may have an independent etiology, such as a comorbid disease, use of medications, or other causes [36].
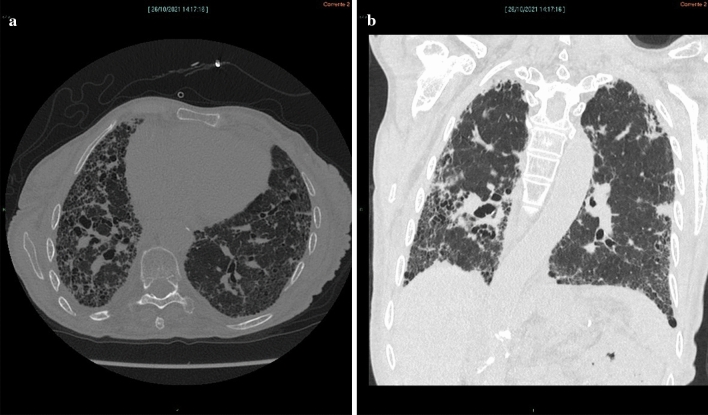
The presenting symptoms of IPF may be erroneously attributed to the aging process, being out of shape, obesity, or comorbidities, so diagnostic delays are not uncommon. In more unusual cases, patients may present with flu-like symptoms. A differential diagnosis must rule out the many forms of interstitial lung disease [1]. High-resolution computer tomography (CT) scans are needed and in some cases, a surgical lung biopsy may be advisable [1]. Histopathology will often show patterns that resemble interstitial pneumonia with interstitial fibrotic growth at intervals; there will be regions with marked fibrosis and microscopic honeycomb-like patterns (cystic spaces filled with mucin). Fibroblastic foci with a myxoid-like matrix will appear where the normal lung tissue abuts fibrotic growths [39]. See Fig. 1.
Fig. 1.
Axial computed tomography (CT) image (a) shows diffuse irregular interstitial thickening with extensive honeycombing, more evident in the subpleural region of the right lower lobe; there is evidence of bronchiectasis,irregular pleural and fissural surfaces; and reduced volume of the right lung. Coronal CT reconstruction (b) shows that fibrotic changes are more widespread in the subpleural region of the lower lobes, especially in the right lung with reduced right-lung volume
The clinical course of IPF varies widely among patients with some patients progressing far more rapidly than others. Flares or sudden exacerbations are not unusual and patients may experience acute respiratory deterioration, particularly with advanced disease [39]. Risk models have been created to account for demographic, clinical, and physiological variables that may affect prognostication. These have led to the recognition of three stages of the disease with the mortality risk 6% for stage 1, 16% for stage 2, and 39% for stage 3 [1]. The prognosis of IPF is poor and median survival after diagnosis is about 3–5 years [40]. Most patients with IPF exhibit a progressive albeit gradual deterioration of pulmonary function which typically spans years, but a subset of patients may maintain a stable condition for years, while others show a rapid and abrupt decline [39].
Risk Factors and Comorbidities
Smoking, older age, Caucasian race, lower body mass index (BMI), environmental factors such as inhalation of dust or metal particles, working around livestock, genetic predisposition, and disease-related factors such as impaired pulmonary function, greater extent of fibrosis, and the presence of severe comorbidities may worsen prognosis of IPF and shorten survival time [5, 9, 39, 41–44]. IPF is more prevalent in rural than urban areas [45]. A retrospective study using a Medicare administrative claims database reported that older age and male sex were associated with shorter survival time following IPF diagnosis [10]. While IPF is a terminal disease, survival times are increasing. People diagnosed with IPF in 2001 had a median survival of 3.3 years compared to 4.0 years in 2007 [10].
Chronic viral infections have been implicated as a risk factor for IPF, in particular the Epstein–Barr virus (EBV) and the hepatitis C virus [46–48]. The EBV protein and DNA have been found in the alveolar epithelial tissue of patients with IPF, including EBV rearrangement, which is associated with viral replication [49]. In a study of 33 patients with IPF, one or more types of herpes viruses were identified in most lungs of infected patients compared to about one-third of lungs of control patients [50]. The association with other viruses, such as SARS-CoV-2, has not been established. Moreover, these findings must be put into context. Patients with IPF are likely taking immunosuppressive drugs, which elevates their risk for viral infections and one study found the presence of EBV in the lungs of control patients to be 71% [51]. Thus, this may be correlation rather than causation. Some of the risk factors for poor outcomes with COVID infection overlap with risk factors for IPF: increasing age, male sex, and comorbidities [52]. On the other hand, while smoking is a strong risk factor for IPF, it may confer a pulmonary protective effect for acute COVID-19 infections [53]. A clinical conundrum is that diagnosis can be complicated by interstitial lung disease. In order the truncate the diagnostic process, many algorithms have been proposed [54]. Following a patient history, examination, and spirometry, if it is determined there is a probable cause for interstitial lung disease, the patient should first undergo high-resolution CT imaging and, if this is inconclusive, surgical lung biopsy or cryobiopsy may be needed. If usual interstitial pneumonia in typical or atypical presentation is detected, then IPF may be diagnosed. If tomography or biopsy is inconclusive a multidisciplinary discussion panel may be necessary [54].
Treatments
A meta-analysis of 26 studies (n = 12,956) found that antifibrotic treatment reduced all-cause mortality with a pooled risk ratio (RR) of 0.55 (95% confidence interval 0.45–0.66) [55]. This RR was consistent across subgroups, including those defined by age, bias risk, duration of follow-up, and antifibrotic subtype. Antifibrotic therapy with pirfenidone and nintedanib reduced acute exacerbations [55]. In a retrospective review of 199 records of patients with IPF, both drugs were similarly effective but pirfenidone was associated with fewer adverse events than nintedanib [56].
In the early stages of the disease, lifestyle modifications (smoking cessation, treating comorbid conditions), education, and disease-modifying antifibrotic therapy with nintedanib or pirfenidone are recommended [57–59]. Pulmonary therapy and oxygen assessment may be needed as the disease progresses. As the patient nears end of life, supplemental oxygen and palliation are required. A lung transplant can be considered, although many patients are not suitable candidates [1]. Even in appropriate candidates, the 5-year survival rate following lung transplantation in patients with IPF is approximately 50% [60].
There are no approved treatments for IPF cough and a paucity of clinical studies on the subject. In a 24-week double-blind crossover study of 98 patients with IPF, thalidomide was effective in significantly improving scores on the cough quality of life questionnaire (CQLQ) and the visual analog score (VAS) of coughing compared to placebo [61].
Since latent transforming growth factor beta (TGFβ) and its binding protein-2 (LTBP2) play a key role in the accumulation of the extracellular matrices, they might be targets for drug development [62]. Such a drug target might reduce the myofibroblast proliferation and creation of extracellular matrix. LTBP2 is a biomarker for IPF [63]. Extracellular matrix proteins include hyaluronan, fibronectin, and interstitial collagens.
COVID-19 and IPF
The increased incidence and prevalence of IPF predated the pandemic, but it is still appropriate to explore the potential links that may exist between COVID-19 and IPF, for which cases continue to increase. The presenting symptoms and clinical profile of acute COVID-19 infection are similar to those of IPF [64]. IPF has been reported in at least one study as a risk factor for COVID-19 [52]. IPF has also been reported as a COVID-19 complication as well as a symptom of long COVID [65].
Fibrosis can occur with chronic inflammation and COVID-19 is associated with inflammatory processes that in some cases were both aberrant and extreme [66]. In studies comparing COVID-19 with the other coronavirus outbreaks for which longer-term data exist, namely severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS), it was found that all of these infections could cause bilateral pneumonia with similar basic lesion patterns and the involvement of the lower lobes and the subpleural region [67].
About 5% of those infected with COVID develop ARDS, a life-threatening condition and a hallmark of the SARS and MERS epidemics as well as this most recent pandemic [68]. ARDS is characterized by bilateral opacities on radiography plus acute hypoxemia not explained by other dysfunctions and which typically occurs within a 7-day window of new-onset respiratory symptoms or a known clinical insult [69]; ARDS is not unique to COVID-19 infections. ARDS occurs in three phases. In the first phase, exudate is produced, there is diffuse alveolar damage caused by myeloid cells, there is barrier breakdown in the pulmonary system, and edema. In the next or proliferative phase, there is repair of the epithelial cells and alveolar cells and reabsorption of fluid. The third phase, involving life-threatening respiratory failure, does not occur in all patients [70]. In the case of COVID-19, ARDS paradoxically develops as the viral load diminishes, usually in the second week [71]. This has been interpreted as evidence that ARDS in patients with COVID-19 may be driven by a secondary cause, such as a dysregulated immune response [72, 73].
Macrophages play an important role in both the development and progression of inflammatory lung conditions [74]. Macrophages are most associated with phagocytosis, but in the process they can also eliminate pathogens [75]. In fact, macrophages may be considered frontline responses against pathogenic invasion and infection [76, 77]. In ARDS, the macrophages of the alveolar system differentiate into M1 or M2 macrophages and are active in the exudative phase, the rehabilitation phase, and the fibrotic phase of lung disease [76]. In the initial phase, M1 macrophages exert a pro-inflammatory response which transitions as the macrophages become M2 types after the pathogen is removed. M2 macrophages then drive the rehabilitative phase and exert an anti-inflammatory effect and inhibit pro-inflammatory mediators. At this point, M2 macrophages help repair pulmonary damage [78, 79]. Pulmonary fibrosis is to some extent shaped by the ratio of M1 to M2 macrophages. M1 macrophages are associated with matrix degradation and the inhibition of fibrotic growth [76], while M2 macrophages are associated with myofibroblast proliferation and extracellular matrix deposition [80, 81]. However there is much about this process that remains unknown, including the role of macrophages in “cytokine storm” [74]. Thus, the inflammatory cascade and hyperinflammatory state observed in certain severe cases of COVID-19 appears to be largely mediated by monocytes and macrophages, which play a role in fibrosis and remodeling [82, 83]. While the mechanisms of this pulmonary injury are not entirely elucidated, gene studies and computational analysis suggest that these COVID-19 macrophage populations are similar to the profibrotic macrophage populations of IPF, which explains why ARDS in patients with COVID-19 bears a clinical and radiological resemblance to pulmonary fibrosis [22].
Nevertheless, IPF differs from ARDS in patients with COVID-19 in important ways. First, IPF is a chronic, irreversible, and progressive and often gradual disorder, whereas the pulmonary remodeling that occurs in patients with COVID-19 is rapid and, for many patients, reversible [22]. Observational study data confirms that approximately 90% of hospitalized patients with COVID-19 experience certain sequelae of COVID-19, including lung tissue damage, diminished exercise capacity, and respiratory problems, but these resolved in about a third of all hospitalized patients within 120 days of acute infection [84].
Normally, myofibroblasts would launch the process of healing damaged lung tissue after underlying tissue has healed by remodeling the extracellular regions of the lungs to help promote replacement of parenchymal cells [85]. With COVID-19 infection, this adaptive process becomes maladaptive as the extracellular matrix accumulates, myofibroblast activity ramps up, and an environment of chronic inflammation is created, allowing for the release of pro-inflammatory cytokines, which activates fibrosis-friendly pathways, such as the TGFβ signaling pathway [85]. Aberrant activity of the lung’s myofibroblasts is evident in the differentiation between fibroblast and myofibroblast.
Pulmonary disorders have been misdiagnosed as COVID-19 infections during the pandemic, and certain conditions such as interstitial lung diseases closely mimic the viral infection; differential diagnosis may require a battery of tests [86]. Post-inflammatory pulmonary fibrosis may occur after COVID-19 infection with presenting symptoms of dyspnea upon exertion, usual interstitial pneumonia, or nonspecific interstitial pneumonia apparent on a CT scan [87]. The clinical course of this postviral illness has not been well studied.
The association between IPF and COVID-19 is intriguing but there are gaps in our current understanding. For example, patients with pre-existing IPF may be at lower risk for COVID-19 infection than those without IPF, because patients with IPF have a higher expression of AT2 cells [88]. However, fibrosis is more likely to develop in those with severe COVID-19 infections, particularly those involving inflammatory cascades [89]. Viral infections of any kind can drastically exacerbate IPF symptoms [90].
A Mendelian randomization (MR) study for IPF causality in COVID-19 was conducted using genetic variants associated with susceptibility to IPF found in early genome-wide association studies (GWAS). Using 4336 cases and 623,902 controls, investigators could make a positive genetic correlation of IPF with the severity of COVID-19 infection. When outlier data were removed, the MR results showed that a genetically increased risk for IPF had a causal effect on the severity of acute COVID-19 infection [91]. However, rs35705950 at MUC5B, an important genetic risk factor for IPF, was protective against COVID-19, whereas the combined effects of all of the other IPF risk loci increased the patient’s risk for COVID-19 [91]. In a study of the 188 differentially expressed genes (DEG) common to both IPF and COVID-19, 117 were upregulated and 71 downregulated. The upregulated genes were associated with cytokine modulation while the downregulated genes were associated with the dismantling of the extracellular matrix [85].
The commonalities between IPF and COVID-19 infection are intriguing and likely play a role in infection severity rather than susceptibility.
Conclusions
IPF is a rare lung disorder that may not be rare much longer; incidence and prevalence are increasing around the world, although the reasons for this are not entirely clear. IPF may have genetic associations but it can also be caused by environmental factors, such as inhaling dust or metal particles. COVID-19 infections can cause fibrotic damage to the lungs similar to that of IPF, but COVID-19-related pulmonary remodeling is often abrupt and reversible compared to the more gradual but irreversible and progressive changes of IPF. Associations between COVID-19 and IPF suggest that the SARS-CoV-2 infection may exacerbate IPF symptoms but does not appear to be a causative agent in triggering IPF. IPF is a devastating and severe disease and further and deeper study is warranted, both from the pathophysiologic and therapeutic points of view.
Acknowledgements
We thank the patients who allowed the use of his images for the purposes of this article and we extend our thanks to all patients who have contributed to research into this condition.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
JVP contributed to concept, editorial review, suggested references, and approved the final manuscript. JL conducted the literature search and drafted large portions of the manuscript. She approved the final manuscript, is corresponding author, and took the lead on the peer review. MV provided input to the concept, editorial review, and provided the image and caption, and he approved the final manuscript. FB provided editorial review, assisted in bibliography search, and approved the final manuscript. PM contributed to concept, wrote sections of the manuscript, and approved the final manuscript. GV contributed to the concept, outlined the first draft, provided editorial review and critiques, and approved the final manuscript.
Disclosures
The authors have no relevant disclosures. JVP has nothing to disclose. JL has nothing to disclose. MV has nothing to disclose. FB has nothing to disclose. PM has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Richeldi L, Collard H, Jones M. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 2.Maher T, Bendstrup E, Dron L, et al. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22(1):197. doi: 10.1186/s12931-021-01791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias This Joint Statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS Board of Directors, June 2001 and by The ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 4.Sharif R. Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am J Manag Care. 2017;23(11 Suppl):S176–S182. [PubMed] [Google Scholar]
- 5.Fernández-Fabrellas E, Molina-Molina M, Soriano J, et al. Demographic and clinical profile of idiopathic pulmonary fibrosis patients in Spain: the Separ National Registry. Respir Res. 2019;20(1):127. doi: 10.1186/s12931-019-1084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghu G, Weycker D, Edelsberg J, Bradford W, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174(7):810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 7.Strongman H, Kausar I, Maher T. Incidence, prevalence, and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv Ther. 2018;35(5):724–736. doi: 10.1007/s12325-018-0693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauleda J, Núñez B, Sala E, Soriano J. Idiopathic pulmonary fibrosis: epidemiology, natural history, phenotypes. Med Sci (Basel) 2018;6(4):110. doi: 10.3390/medsci6040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul B, Lee J, Zhang N, et al. Epidemiology of idiopathic pulmonary fibrosis among US veterans, 2010–2019. Ann Am Thorac Soc. 2022;19(2):196–203. doi: 10.1513/AnnalsATS.202103-295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G, Chen S, Yeh W, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014;2(7):566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson J, Fogarty A, Hubbard R, Mckeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795. doi: 10.1183/09031936.00185114. [DOI] [PubMed] [Google Scholar]
- 12.Jo H, Randhawa S, Corte T, Moodley Y. Idiopathic pulmonary fibrosis and the elderly: diagnosis and management considerations. Drugs Aging. 2016;33(5):321–334. doi: 10.1007/s40266-016-0366-1. [DOI] [PubMed] [Google Scholar]
- 13.Collard HR. The age of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(8):771–772. doi: 10.1164/rccm.201001-0049ED. [DOI] [PubMed] [Google Scholar]
- 14.Meiners S, Eickelberg O, Königshoff M. Hallmarks of the ageing lung. Eur Respir J. 2015;45(3):807–827. doi: 10.1183/09031936.00186914. [DOI] [PubMed] [Google Scholar]
- 15.Sheng G, Chen P, Wei Y, et al. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta-analysis. Chest. 2020;157(5):1175–1187. doi: 10.1016/j.chest.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mcdonald LT. Healing after COVID-19: are survivors at risk for pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2021;320(2):L257–L265. doi: 10.1152/ajplung.00238.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barros A, Oldham J, Noth I. Genetics of idiopathic pulmonary fibrosis. Am J Med Sci. 2019;357(5):379–383. doi: 10.1016/j.amjms.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kropski JA, Blackwell TS, Loyd JE. The genetic basis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(6):1717–1727. doi: 10.1183/09031936.00163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobbs B, Putman R, Araki T, et al. Overlap of genetic risk between interstitial lung abnormalities and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2019;200(11):1402–1413. doi: 10.1164/rccm.201903-0511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development renewal and cancer. Nature. 2014;507(7491):190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wendisch D, Dietrich O, Mari T, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184(26):6243–6261.E6227. doi: 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med. 2010;4(6):759–771. doi: 10.1586/ers.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore B, Moore T. Viruses in idiopathic pulmonary fibrosis. Etiology and exacerbation. Ann Am Thorac Soc. 2015;12(Suppl 2):S186–192. doi: 10.1513/AnnalsATS.201502-088AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molyneaux PL, Maher TM. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir Rev. 2013;22(129):376–381. doi: 10.1183/09059180.00000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med. 2016;194(3):265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 28.Khatiwada S, Subedi A. Lung microbiome and coronavirus disease 2019 (COVID-19): possible link and implications. Hum Microbiome J. 2020;17:100073. doi: 10.1016/j.humic.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Dai W, Qiu C, et al. Mycoplasma pneumoniae and streptococcus pneumoniae caused different microbial structure and correlation network in lung microbiota. J Thorac Dis. 2016;8(6):1316–1322. doi: 10.21037/jtd.2016.04.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinton LJ, Walkey AJ, Mizgerd JP. Integrative physiology of pneumonia. Physiol Rev. 2018;98(3):1417–1464. doi: 10.1152/physrev.00032.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Invernizzi R, Lloyd C, Molyneaux P. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology. 2020;160(2):171–182. doi: 10.1111/imm.13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin TR, Frevert CW. Innate immunity in the lungs. Proc Am Thorac Soc. 2005;2(5):403–411. doi: 10.1513/pats.200508-090JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.My A, Jjl C, Cogan Jd, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 34.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 35.Hemann M, Strong M, Hao L, Greider C. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/S0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 36.Vigeland CL, Hughes AH, Horton MR. Etiology and treatment of cough in idiopathic pulmonary fibrosis. Respir Med. 2017;123:98–104. doi: 10.1016/j.rmed.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Key AL, Holt K, Hamilton A, Smith JA, Earis JE. Objective cough frequency in idiopathic pulmonary fibrosis. Cough. 2010;6:4. doi: 10.1186/1745-9974-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakwaya Y, Ramdurai D, Swigris JJ. Managing cough in idiopathic pulmonary fibrosis. Chest. 2021;160(5):1774–1782. doi: 10.1016/j.chest.2021.05.071. [DOI] [PubMed] [Google Scholar]
- 39.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Touil I, Keskes Boudawara N, Bouchareb S, et al. Prognostic factors in idiopathic pulmonary fibrosis in a Tunisian cohort. Rev Mal Respir. 2021;38(7):681–688. doi: 10.1016/j.rmr.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Alakhras M, Decker PA, Nadrous HF, Collazo-Clavell M, Ryu JH. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest. 2007;131(5):1448–1453. doi: 10.1378/chest.06-2784. [DOI] [PubMed] [Google Scholar]
- 42.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3(4):293–298. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 43.Steele MP, Speer MC, Loyd JE, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172(9):1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Checa M, Ruiz V, Montaño M, Velázquez-Cruz R, Selman M, Pardo A. MMP-1 polymorphisms and the risk of idiopathic pulmonary fibrosis. Hum Genet. 2008;124(5):465–472. doi: 10.1007/s00439-008-0571-z. [DOI] [PubMed] [Google Scholar]
- 45.Tighe RM, Chaudhary S. Uncovering the epidemiology of idiopathic pulmonary fibrosis in the Veterans Affairs Health System. Ann Am Thorac Soc. 2022;19(2):161–162. doi: 10.1513/AnnalsATS.202108-972ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lok SS, Stewart JP, Kelly BG, Hasleton PS, Egan JJ. Epstein-Barr virus and wild P53 in idiopathic pulmonary fibrosis. Respir Med. 2001;95(10):787–791. doi: 10.1053/rmed.2001.1152. [DOI] [PubMed] [Google Scholar]
- 47.Ueda T, Ohta K, Suzuki N, et al. Idiopathic pulmonary fibrosis and high prevalence of serum antibodies to hepatitis C virus. Am Rev Respir Dis. 1992;146(1):266–268. doi: 10.1164/ajrccm/146.1.266. [DOI] [PubMed] [Google Scholar]
- 48.Meliconi R, Andreone P, Fasano L, et al. Incidence of hepatitis C virus infection in italian patients with idiopathic pulmonary fibrosis. Thorax. 1996;51(3):315–317. doi: 10.1136/thx.51.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly BG, Lok SS, Hasleton PS, Egan JJ, Stewart JP. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166(4):510–513. doi: 10.1164/rccm.2103058. [DOI] [PubMed] [Google Scholar]
- 50.Tang YW, Johnson JE, Browning PJ, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003;41(6):2633–2640. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsukamoto K, Hayakawa H, Sato A, Chida K, Nakamura H, Miura K. Involvement of Epstein-Barr virus latent membrane protein 1 in disease progression in patients with idiopathic pulmonary fibrosis. Thorax. 2000;55(11):958–961. doi: 10.1136/thorax.55.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsigaris P, Teixeira DA, Silva JA. Smoking prevalence and COVID-19 in Europe. Nicotine Tob Res. 2020;22(9):1646–1649. doi: 10.1093/ntr/ntaa121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):E3–19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 55.Petnak T, Lertjitbanjong P, Thongprayoon C, Moua T. Impact of antifibrotic therapy on mortality and acute exacerbation in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Chest. 2021;160(5):1751–1763. doi: 10.1016/j.chest.2021.06.049. [DOI] [PubMed] [Google Scholar]
- 56.Isshiki T, Sakamoto S, Yamasaki A, et al. Incidence of acute exacerbation of idiopathic pulmonary fibrosis in patients receiving antifibrotic agents: real-world experience. Respir Med. 2021;187:106551. doi: 10.1016/j.rmed.2021.106551. [DOI] [PubMed] [Google Scholar]
- 57.Richeldi L, Du-Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 58.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 59.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (capacity): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 60.Kistler KD, Nalysnyk L, Rotella P, Esser D. Lung transplantation in idiopathic pulmonary fibrosis: a systematic review of the literature. BMC Pulm Med. 2014;14(1):139. doi: 10.1186/1471-2466-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horton MR, Santopietro V, Mathew L, et al. Thalidomide for the treatment of cough in idiopathic pulmonary fibrosis: a randomized trial. Ann Intern Med. 2012;157(6):398–406. doi: 10.7326/0003-4819-157-6-201209180-00003. [DOI] [PubMed] [Google Scholar]
- 62.Zou M, Zou J, Hu X, Zheng W, Zhang M, Cheng Z. Latent transforming growth factor-Β binding protein-2 regulates lung fibroblast-to-myofibroblast differentiation in pulmonary fibrosis via Nf-Κb signaling. Front Pharmacol. 2021;12:788714. doi: 10.3389/fphar.2021.788714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enomoto Y, Matsushima S, Shibata K, et al. LTBP2 is secreted from lung myofibroblasts and is a potential biomarker for idiopathic pulmonary fibrosis. Clin Sci (Lond) 2018;132(14):1565–1580. doi: 10.1042/CS20180435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Upadhya P, Chary R, Chawla G, Vadala R, Mohanty M. New twist to an old problem: COVID-19 and idiopathic pulmonary fibrosis. Adv Respir Med. 2021;89(1):84–85. doi: 10.5603/ARM.a2020.0177. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4(9):E2127403. doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merad M, Subramanian A, Wang TT. An aberrant inflammatory response in severe COVID-19. Cell Host Microbe. 2021;29(7):1043–1047. doi: 10.1016/j.chom.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X, Zhang G, Hao SY, Bai L, Lu JJ. Similarities and differences of early pulmonary CT features of pneumonia caused by SARS-CoV-2, SARS-CoV and MERS-CoV: comparison based on a systemic review. Chin Med Sci J. 2020;35(3):254–261. doi: 10.24920/003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Osuchowski MF, Winkler MS, Skirecki T, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9(6):622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 70.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 71.Barbaro RP, Maclaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization Registry. Lancet. 2020;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from Northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D'alessio FR, Heller NM. COVID-19 and myeloid cells: complex interplay correlates with lung severity. J Clin Invest. 2020;130(12):6214–6217. doi: 10.1172/JCI143361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee JW, Chun W, Lee HJ, et al. The role of macrophages in the development of acute and chronic inflammatory lung diseases. Cells. 2021;10(4):897. doi: 10.3390/cells10040897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Wang CC. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis Int. 2014;13(2):138–152. doi: 10.1016/S1499-3872(14)60024-2. [DOI] [PubMed] [Google Scholar]
- 76.Chen X, Tang J, Shuai W, Meng J, Feng J, Han Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm Res. 2020;69(9):883–895. doi: 10.1007/s00011-020-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang X, Xiu H, Zhang S, Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018:1264913. doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 80.Braga TT, Agudelo JS, Camara NO. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol. 2015;6:602. doi: 10.3389/fimmu.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang CY, Chen CS, Yiang GT, et al. New insights into the immune molecular regulation of the pathogenesis of acute respiratory distress syndrome. Int J Mol Sci. 2018;19(2):588. doi: 10.3390/ijms19020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.E1039. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X, Shen C, Wang L, et al. Pulmonary fibrosis and its related factors in discharged patients with new corona virus pneumonia: a cohort study. Respir Res. 2021;22(1):203. doi: 10.1186/s12931-021-01798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Q, Xia S, Sui H, Shi X, Huang B, Wang T. Identification of hub genes associated with COVID-19 and idiopathic pulmonary fibrosis by integrated bioinformatics analysis. PLoS ONE. 2022;17(1):E0262737. doi: 10.1371/journal.pone.0262737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zizzo G, Caruso S, Ricchiuti E, Turato R, Stefani I, Mazzone A. Amiodarone-induced organizing pneumonia mimicking COVID-19: a case report. Eur J Med Res. 2021;26(1):62. doi: 10.1186/s40001-021-00522-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhan X, Liu B, Tong Zh. Postinflammatroy pulmonary fibrosis of COVID-19: the current status and perspective. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(9):728–732. doi: 10.3760/cma.j.cn112147-20200317-00359. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H, Zhou P, Wei Y, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21(6):746–755. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akram A. Overwhelming COVID-19 sepsis in a patient with idiopathic pulmonary fibrosis. Cureus. 2020;12(7):E9320. doi: 10.7759/cureus.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fadista J, Kraven LM, Karjalainen J, et al. Shared genetic etiology between idiopathic pulmonary fibrosis and Covid-19 severity. EBioMedicine. 2021;65:103277. doi: 10.1016/j.ebiom.2021.103277. [DOI] [PMC free article] [PubMed] [Google Scholar]