Abstract
We developed a competitive index assay for murine listeriosis that tests the virulence of Listeria monocytogenes strains in different organs and at various times postinoculation. Studies presented here demonstrate the reproducibility of this assay during primary and secondary infection of inbred and outbred mice. We verified the validity of this assay by performing competitive index analysis of a well-characterized strain of L. monocytogenes lacking the actA gene. In addition, we found that while L. monocytogenes strains unable to recruit vasodilator-stimulated phosphoprotein (VASP) to their surface exhibit a 10-fold virulence attenuation in the livers of naive animals, they display a 50-fold survival defect in the liver during secondary listeriosis.
Listeria monocytogenes is a facultative intracellular gram-positive pathogen of humans and animals that causes a serious, food-borne illness in immunocompromised and pregnant individuals (9). Although the oral route is the natural route of L. monocytogenes infection, highly reproducible and useful intravenous and intraperitoneal murine models have been developed that mimic characteristics of disseminated disease, specifically growth of the organism in the spleen and liver (17). Immunity to L. monocytogenes in these models is entirely cell mediated (18). Macrophages and neutrophils are essential for the initial innate resistance to L. monocytogenes (25) while cytotoxic T lymphocytes (CTLs) are central to the adaptive immune response (12).
The intracellular life cycle of L. monocytogenes has been well described in tissue culture models of infection. Once inside a host cell vacuole or phagosome, L. monocytogenes is able to escape into the cytosol and initiate replication. During intracellular growth, actin polymerizes at one pole of the bacterium and propels the bacillus through the cytoplasm and into neighboring host cells, thereby facilitating cell-to-cell spread without exposure to the extracellular environment (19, 24). ActA is an L. monocytogenes surface protein that facilitates actin nucleation at the bacterial surface (8, 14). The central region of ActA is composed of proline-rich repeats (Table 1) which bind to the eukaryotic protein vasodilator-stimulated phosphoprotein (VASP). This interaction leads to an increase in the bacterial movement rate and cell-to-cell spread (16, 20, 22). A strain of L. monocytogenes that is completely incapable of actin-based motility (ΔActA) is 1,000-fold less virulent in the murine model of listeriosis (3). Furthermore, deletion of the proline-rich repeats of ActA leads to a decreased bacterial movement rate and lower cell-to-cell spread efficiency in tissue culture cells as well as a 40-fold increase in 50% lethal dose (LD50) (22). However, glycine substitution of critical proline residues within the ActA central region ablates VASP recruitment to the surface of L. monocytogenes and results in slower movement within tissue culture cells. Nevertheless, this loss of VASP binding does not translate into any virulence attenuation detectable by the LD50 assay (22). As LD50 experiments were restricted to primary infections, it is possible that they may not have revealed all relevant aspects of L. monocytogenes pathogenesis.
TABLE 1.
Strains used in this study
| Strain No. | Strain description | Relevant ActA sequenceb | VASPc |
|---|---|---|---|
| 10403Sa | Wild type | 263:DFPPPPTDEEL-LR1-EFPPPPTDEEL-LR2-EFPPPPTEDEL-LR3-DFPPIPTEEEL:390 | ++++ |
| DP-L3903 | Reference | 263:DFPPPPTDEEL-LR1-EFPPPPTDEEL-LR2-EFPPPPTEDEL-LR3-DFPPIPTEEEL:390 | ND |
| DP-L2300 | ΔActA3 | 263:DFPPPPTDEEL-LR1-EFPPIPTEEEL:390 | ++ |
| DP-L2990 | ΔActA3-GG | 263:DFGGGGTDEEL-LR1-EFGGGGTEEEL:390 | − |
| DP-L2374 | ΔActA6 | 263:390 | − |
10403S, DP-L2300, DP-L2990, and DP-L2374 are described in reference 22.
Underlined residues indicate where prolines have been substituted with glycines. Sequences shown begin at position 263 and end at position 390. LR, long repeated sequence.
Quantification of VASP recruitment to intracellular bacteria using indirect immunofluorescence as reported in reference 22. ++++, greatest relative fluorescence; −, no fluorescence detected. ND, not determined.
We have designed a competitive index assay which allows us to compare different bacterial strains in the context of both primary and secondary listeriosis (infections of naive and immunized mice, respectively). By using this sensitive assay we show that loss of VASP binding indeed results in decreased virulence. Furthermore, we have found that strains of L. monocytogenes deficient in recruiting VASP are even less pathogenic during a secondary immune response than during a primary one.
Bacterial and mouse strains.
All L. monocytogenes strains used in this study were derived from 10403S (2). Bacteria were grown in brain heart infusion (BHI) (Becton Dickinson, Sparks, Md.) to log phase, washed once with phosphate-buffered saline, and resuspended in fresh BHI. Aliquots were frozen and stored at −80°C. Four- to six-week-old female CD-1 mice were purchased from Charles River Laboratories (Wilmington, Mass.). Four- to six-week-old female BALB/c mice and five- to seven-week-old female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine).
Selection of a L. monocytogenes reference strain.
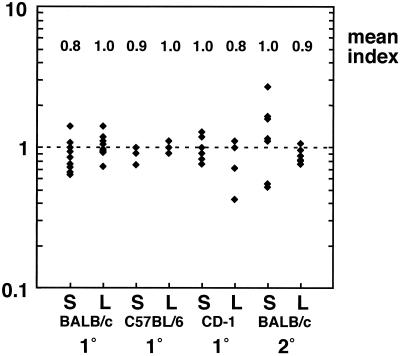
In order to establish a reliable reference strain for a competitive index assay, it was essential to ensure that the selected strain display full virulence in a murine model of infection. Accordingly, we selected for a virulent transposon-containing strain in vivo. An L. monocytogenes 10403S::Tn917-LTV3 random insertion library (5) was grown to log phase at 37°C. The culture was diluted in phosphate-buffered saline, and 104 bacteria were injected intravenously (i.v.) into a single BALB/c mouse. After 48 h, the animal was sacrificed and its liver was homogenized in 0.2% NP-40. The homogenate was pelleted, resuspended in BHI containing chloramphenicol (10 μg/ml) (to select for the transposon, which contains the erm and cat genes), and allowed to reach stationary phase. The resulting culture was diluted in BHI containing chloramphenicol (10 μg/ml) and grown to log phase at 37°C, and 104 bacteria were injected i.v. into a second BALB/c mouse. After 48 h, the animal was sacrificed and its liver was homogenized. Dilutions of the organ homogenate were plated onto Luria-Bertani agar, and the plates were incubated at 37°C. Random colonies were screened for full virulence in a primary infection competitive index assay (described below). One clone (DP-L3903) was chosen as a reference strain for our assay after characterization in three different mouse strains during primary and secondary infection (Fig. 1). DP-L3903 competed equally with 10403S in inbred (BALB/c and C57BL/6) and outbred (CD-1) mice during primary listeriosis. In addition, DP-L3903 behaved indistinguishably from the wild type during secondary infection of BALB/c mice (Fig. 1).
FIG. 1.
Competitive index analysis of the reference strain and wild-type 10403S L. monocytogenes. Groups of 3 to 10 naive or L. monocytogenes-immunized mice were infected with a 1:1 mixture of DP-L3903 and 10403S. CD-1 mice were sacrificed 48 h postinoculation, while BALB/c and C57BL/6 mice were euthanized after 60 h. A competitive index of 1 indicates that the two strains are proliferating equally in vivo. Abbreviations: S, spleen; L, liver. 1°, infection of naive mice; 2°, infection of L. monocytogenes-immunized mice; y axis, competitive index.
Competitive index analysis during primary listeriosis.
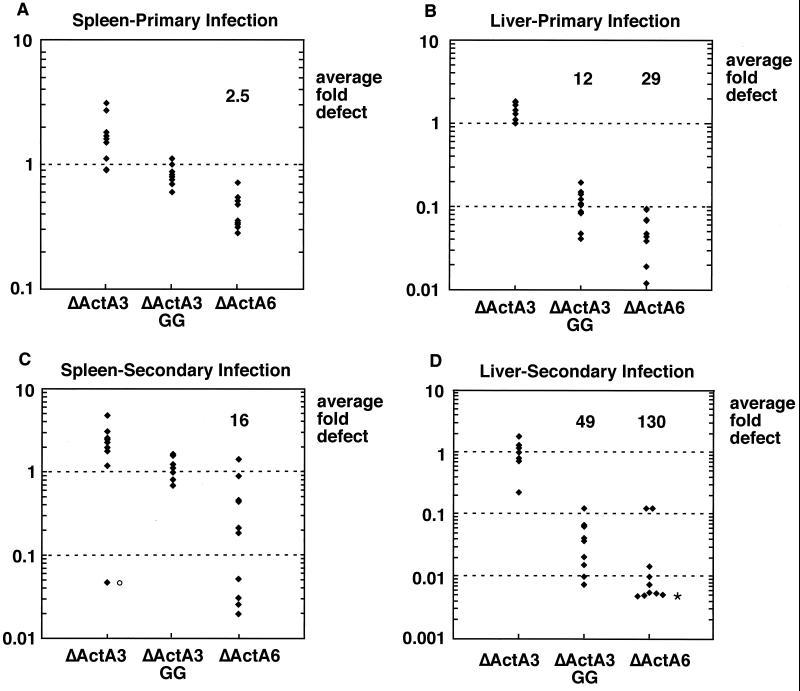
To validate the competitive index assay as an accurate measure of virulence, we compared published LD50s with our competitive indexes for several strains of L. monocytogenes. Briefly, frozen culture stocks were thawed, grown to log phase in fresh BHI for 2 h, and mixed in a 1:1 ratio. BALB/c and C57BL/6 mice received i.v. injections of 6 × 104 to 8 × 104 total bacteria (reference and test strains combined). A dose of 1 × 105 to 7 × 105 was used for CD-1 mice. Animals were sacrificed anywhere from 48 to 60 h later, and their spleens and livers were harvested. Spleens and livers were homogenized in 5 and 10 ml 0.2% NP-40, respectively, for 1 min in a tissue homogenizer (Ultra Turrax T-25 basic; IKA Works, Inc., Wilmington, N.C.), and homogenates were plated onto Luria-Bertani agar. At least 100 colonies per organ were replica plated or patched onto BHI agar containing erythromycin (2 μg/ml). Competitive indexes were calculated by dividing the number of test strain CFU (erythromycin sensitive) by the number of reference strain CFU (erythromycin resistant). The LD50 of a strain containing a large in-frame deletion within actA was reported to be 1,000-fold greater than that of its wild-type parent in BALB/c mice (3). Similarly, the mean competitive index of a ΔActA strain during primary infection demonstrated a 1,000-fold defect in the liver and 500-fold defect in the spleen of BALB/c mice 48 h postinoculation (data not shown). Likewise, the LD50 of a strain which lacks the repeat region of ActA (ΔActA6) (Table 1) was found to be 40-fold greater than that of the wild type in BALB/c mice (22). The mean competitive index of this strain in the liver during primary infection reflected approximately the same virulence attenuation 60 h postinoculation (Fig. 2B). Interestingly, the mean competitive index in the spleens of these mice was significantly higher than that in the livers (Fig. 2). We observed this difference with most L. monocytogenes actA mutant strains analyzed.
FIG. 2.
Competitive index analysis during primary infection (A and B) or secondary infection (C and D) in the spleen (A and C) or liver (B and D). Groups of four or five naive or immunized BALB/c mice were infected with a 1:1 mixture of the reference strain (DP-L3903) and ΔActA3, ΔActA3-GG, or ΔActA6. All mice were sacrificed 60 h postinoculation. Average fold defect was calculated by dividing the number of reference strain CFU by the number of test strain CFU for each organ. Primary infection competitive indexes of ΔActA6 and ΔActA3-GG were significantly lower in livers than spleens, with two-sided P values of 0.0004 for ΔActA6 and 0.0002 for ΔActA3-GG by the Mann-Whitney test as calculated by MiniTab (State College, Pa.) software. Liver competitive indexes of ΔActA6 and ΔActA3-GG were significantly lower during secondary infection than during primary infection, with two-sided P values of 0.037 for ΔActA6 and 0.0048 for ΔActA3-GG. Symbols: ○, outlying value as determined by KaleidaGraph (Synergy Software, Reading, Pa.); ∗, underestimated values (1 or 0 erythromycin-sensitive colonies out of approximately 200 screened). y axis, competitive index.
We performed competitive index analysis during primary infection of two strains of L. monocytogenes containing defined mutations within the actA gene with no reported defect in LD50 (ΔActA3 and ΔActA3-GG) (Table 1). A strain lacking two of four ActA proline-rich repeats (ΔActA3) displays a wild-type competitive index in both livers and spleens during primary infection (Fig. 2), consistent with its wild-type LD50. However, a derivative of this strain in which the prolines of the two ActA proline-rich repeats have been changed to glycines (ΔActA3-GG) exhibits a 10-fold defect in the livers of BALB/c mice (Fig. 2B). These data indicate that the competitive index assay may be more sensitive to virulence attenuation than LD50 measurements.
Competitive index analysis during secondary listeriosis.
To test whether a given bacterial strain displayed similar virulence attenuation in immunized animals compared to that in naive animals, we performed competitive index analysis during secondary infection of the three actA mutants. Mice were immunized with 2 × 103 L. monocytogenes 10403S bacteria by i.v. injection. Three weeks later, the immunized animals were challenged with 6 × 105 to 10 × 105 total bacteria (reference and test strains combined). Animals were sacrificed 60 h postinoculation and their organs were treated as described above. In order to determine the degree of resistance displayed by the immunized animals, BALB/c mice immunized as described above and naive mice of the same age were challenged with 6 × 104 L. monocytogenes 10403S bacteria by i.v. injection. After 48 h, the numbers of CFU in the spleen and liver were enumerated and the difference in bacterial load between primary and secondary infection was determined (2). Under these conditions, immunized animals had 104- to 105-fold fewer bacteria in both organs than did naive mice.
During challenge of immunized animals, ΔActA3 viability appeared unchanged compared to the reference strain during secondary infection (Fig. 2). However, the mean competitive indexes of ΔActA3-GG and ΔActA6 revealed a greater defect in the livers of immunized mice (Fig. 2D) than in those of naive mice (Fig. 2B). Indeed, ΔActA6 proliferated fivefold less well in immunized than in naive animal livers, with a two-sided P value of <0.04 as determined by the Mann-Whitney test. Similarly, ΔActA3-GG proliferated fourfold less well in livers of immunized animals than in those of naive animals (P < 0.005). In contrast, the growth of ΔActA3-GG remained unchanged compared to the reference strain in the spleens of immunized mice (Fig. 2C).
The competitive index assay has been previously used to evaluate the virulence of mutant strains of Vibrio cholerae (10, 23) and Salmonella enterica serovar Typhimurium (1, 21). For our L. monocytogenes competitive index assay, we selected for a virulent reference strain derived from the livers of infected mice based on our observations that the liver is a more restrictive environment than the spleen for L. monocytogenes. This is consistent with a previous report from our laboratory which demonstrated that the proliferation of an L. monocytogenes strain lacking phosphatidylinositol-specific phospholipase C is 30-fold lower than that of wild-type bacteria in the liver but not the spleen during infection of naive mice (6). It is not clear why ActA and phosphatidylinositol-specific phospholipase C mutants are more defective for growth in livers than in spleens. It is uncertain whether growth in the liver or spleen contributes more to death in the LD50 assay but our results suggest that the permissive nature of the spleen masks some attenuation in the liver when lethal dose alone is determined. We hypothesize that the virulence difference observed between these organs reflects the relative effectiveness of neutrophils in the spleen and liver. Neutrophils are known to be more important in the liver than the spleen for control of L. monocytogenes growth in the first 24 h after infection (7). We are currently testing whether neutrophils are involved in the organ-specific defects described here.
We found that ActA proline-rich-repeat-deficient mutants were four- to fivefold more attenuated during secondary listeriosis than in primary listeriosis. A major difference between primary and secondary infection of mice is that an established CTL response is more effective in clearing L. monocytogenes early during secondary infection (13). CTL activation and proliferation are clearly more rapid and pronounced during secondary listeriosis (4). Indeed, at 60 h postinoculation (the time point relevant to our experiments), Busch et al. report detectable levels of L. monocytogenes-specific CTLs during secondary but not primary infection. We speculate that an ongoing CTL response selects for L. monocytogenes with intact ActA proline-rich repeats. Thus, the proline rich region may contribute more to virulence of L. monocytogenes that is faced with a significant population of CTLs. Studies of L. monocytogenes infection in mice lacking CTLs will address these issues and are under way in our laboratory.
Using the competitive index assay, we showed that the ActA proline-rich repeats play a measurable role in L. monocytogenes pathogenesis. These repeats are conserved in all natural isolates of L. monocytogenes tested (26), suggesting an important role for this region. However, a previous report from our laboratory demonstrated that substituting the critical prolines for glycines resulted in no difference in LD50 (22). Here we provide the first evidence that intact ActA proline-rich repeats are necessary for full virulence during primary and secondary infection of mice. The only documented role for the ActA proline-rich repeats and VASP in the cell biology of L. monocytogenes infection is the potentiation of rapid and efficient actin-based motility (16, 20, 22), which may be required for evasion of the innate and/or acquired immune response in the host. Our data presented here suggest that the proline-rich repeats and VASP may be especially important for circumvention of acquired immunity. Interestingly, VASP is a substrate for cyclic-nucleotide-dependent kinases (11) and has been implicated as a bridge from extracellular signals to the actin cytoskeleton (15, 27). Thus, VASP may act as a signaling link between the host cell and pathogen in vivo.
In conclusion, a newly developed competitive index assay revealed that the ability of L. monocytogenes to recruit VASP is necessary for optimal growth in the liver of immune animals. Understanding the precise cell biological and immunological explanations for this observation will occupy us in the future.
Acknowledgments
We thank Mary O'Riordan for critical review of the manuscript. We thank Jonathan Hardy for his help with animal work and Jessica Lasky Su for assistance with statistical analysis.
This work was supported by U.S. Public Health Service grant AI29619. L. L. Lenz is supported by National Research Service Award AI10481.
REFERENCES
- 1.Beuzon C R, Meresse S, Unsworth K E, Ruiz-Albert J, Garvis S, Waterman S R, Ryder T A, Boucrot E, Holden D W. Salmonella maintains the integrity of its intracellular vacuole through the action of SifA. EMBO J. 2000;19:3235–3249. doi: 10.1093/emboj/19.13.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 3.Brundage R A, Smith G A, Camilli A, Theriot J A, Portnoy D A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci USA. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busch D H, Pilip I M, Vijh S, Pamer E G. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity. 1998;8:353–362. doi: 10.1016/s1074-7613(00)80540-3. [DOI] [PubMed] [Google Scholar]
- 5.Camilli A, Portnoy A, Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990;172:3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wachter M, Wuenscher M, Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. . (Erratum, 55:752.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freter R, O'Brien P C, Macsai M S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981;34:234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halbrugge M, Walter U. Analysis, purification and properties of a 50,000-dalton membrane-associated phosphoprotein from human platelets. J Chromatogr. 1990;521:335–343. doi: 10.1016/0021-9673(90)85057-3. [DOI] [PubMed] [Google Scholar]
- 12.Harty J T, Tvinnereim A R, White D W. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 13.Jensen E R, Glass A A, Clark W R, Wing E J, Miller J F, Gregory S H. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect Immun. 1998;66:4143–4150. doi: 10.1128/iai.66.9.4143-4150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 15.Krause M, Sechi A S, Konradt M, Monner D, Gertler F B, Wehland J. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J Cell Biol. 2000;149:181–194. doi: 10.1083/jcb.149.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasa I, David V, Gouin E, Marchand J B, Cossart P. The amino-terminal part of ActA is critical for the actin-based motility of Listeria monocytogenes; the central proline-rich region acts as a stimulator. Mol Microbiol. 1995;18:425–436. doi: 10.1111/j.1365-2958.1995.mmi_18030425.x. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald T T, Carter P B. Cell-mediated immunity to intestinal infection. Infect Immun. 1980;28:516–523. doi: 10.1128/iai.28.2.516-523.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–390. [PubMed] [Google Scholar]
- 19.Mounier J, Ryter A, Coquis-Rondon M, Sansonetti P J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990;58:1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl U D, Walter U, Gertler F B, Wehland J, Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997;16:5433–5444. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shea J E, Beuzon C R, Gleeson C, Mundy R, Holden D W. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun. 1999;67:213–219. doi: 10.1128/iai.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith G A, Theriot J A, Portnoy D A. The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J Cell Biol. 1996;135:647–660. doi: 10.1083/jcb.135.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite. Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unanue E R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 26.Wiedmann M, Bruce J L, Keating C, Johnson A E, McDonough P L, Batt C A. Ribotypes and virulence gene polymorphisms suggest three distinct Listeria monocytogenes lineages with differences in pathogenic potential. Infect Immun. 1997;65:2707–2716. doi: 10.1128/iai.65.7.2707-2716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wills Z, Bateman J, Korey C A, Comer A, Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–312. doi: 10.1016/s0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]