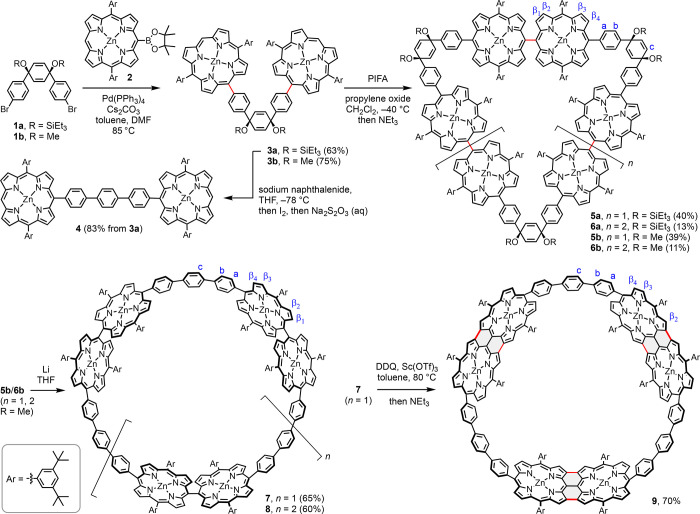
Abstract
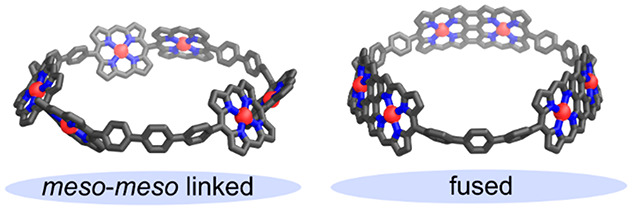
V-Shaped porphyrin dimers, with masked p-phenylene bridges, undergo efficient oxidative coupling to form meso–meso linked cyclic porphyrin oligomers. Reductive aromatization unmasks the p-phenylenes, increasing the strain. Oxidation then fuses the porphyrin dimers, providing a nanoring with curved walls. The strain in this macrocycle bends the p-phenylene and fused porphyrin dimer units (radii of curvature of 11.4 and 19.0 Å, respectively), but it does not significantly alter the electronic structure of the fused porphyrins.
π-Conjugated macrocycles2 exhibit fascinating behavior, such as exciton delocalization,3,4 global aromaticity,5 amplified two-photon absorption,6 Möbius topology,7 host–guest chemistry,8,9 enhanced semiconductivity,10 and strain-induced reactivity.11 Ring strain can favor orbital overlap by preventing neighboring subunits from twisting out of conjugation,12 while bending can also reduce the π–π* energy gap.13 Porphyrins are some of the most versatile components for constructing π-extended frameworks.14 Porphyrin nanorings mimic the ultrafast energy migration in photosynthetic light-harvesting chlorophyll arrays.4,15,16 Oxidized, reduced, and photochemically excited porphyrin nanorings are also the largest macrocycles yet to exhibit global (anti)aromatic ring currents.5 Here we present the synthesis of a strained nanoring, with three edge-fused porphyrin dimer units, via oxidative porphyrin–porphyrin coupling (Scheme 1).1
Scheme 1. Synthetic Pathway.
The blue atom labels are used to assign the 1H NMR resonances in Figure 2.
Electron-rich aromatic compounds, such as porphyrins, can often be oxidatively oligomerized.17 Porphyrins with unsubstituted meso positions readily undergo oxidative coupling to form meso–meso linked oligomers,18 and further oxidation stitches together the β positions to generate β,meso,β edge-fused porphyrin tapes.19 Here, we apply oxidative coupling of a V-shaped porphyrin dimer 3a,b, followed by reductive aromatization of the p-phenylene linker, to yield the strained p-phenylene linked porphyrin nanorings 7 and 8. Intramolecular oxidative fusion of the porphyrin units in 7 gives nanoring 9, which features three curved edge-fused porphyrin dimer tape motifs.
There have been a few reports of macrocyclization via oxidative porphyrin–porphyrin coupling.20 Several macrocycles containing a meso–meso linked porphyrin dimer motif have also been prepared by other routes,16,21 but the incorporation of a triply fused porphyrin tape into a nanoring has scarcely been explored.22 We are interested in synthesizing strained nanorings incorporating porphyrin tapes, to test how strain affects the electronic properties of the edge-fused porphyrin oligomers.
The cyclohexadienyl motif in the V-shaped porphyrin dimer 3a,b serves as a masked p-phenyl bridge. These units can be aromatized using a variety of methods, allowing strain to be added to the nanoring at a late stage in the synthesis.23−25 Previously, von Delius and co-workers used this approach to prepare cyclic porphyrin dimers and trimers,9 while Osuka and co-workers have prepared p-phenylene-linked porphyrin rings using platinum chemistry.26
V-Shaped porphyrin dimer 3a was synthesized in 63% yield by Suzuki–Miyaura coupling of triethylsilyl-protected bromide 1a and borylated porphyrin 2 (Scheme 1; see the Supporting Information for details). Porphyrins with 3,5-di-tert-butylphenyl substituents were used to provide solubility and crystallinity. The V-shaped geometry of 3a, and the syn arrangement around cyclohexadiene, were confirmed by a single-crystal X-ray diffraction analysis (Figure 1). Slow evaporation of a solution of 3a in a mixture of chloroform and methanol containing pyridine gave monoclinic crystals (space group I2/a) with one whole molecule per asymmetric unit. One of the zinc(II) ions is coordinated to methanol, and the other is coordinated to pyridine. The angle between the mean planes of the two porphyrins is 80.24(3)°.
Figure 1.
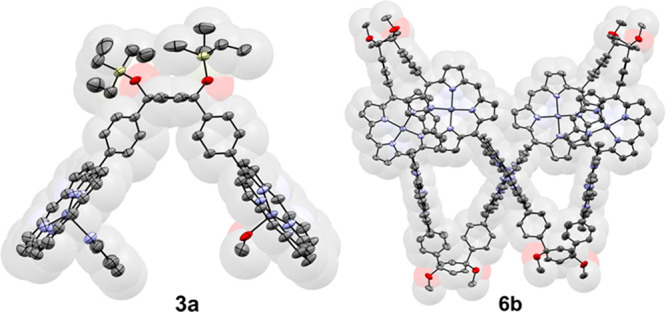
Molecular structures of 3a and 6b from X-ray crystallography (50% probability ellipsoids). Hydrogen atoms and aryl groups have been omitted from both structures for the sake of clarity, together with coordinated solvent molecules in 6b.
Oxidative cyclo-oligomerization of 3a was achieved using bis(trifluoroacetoxy)iodobenzene (PIFA) at −40 °C, in the presence of propylene oxide as an acid scavenger, generating a mixture of cyclic oligomers, which was separated by thin-layer chromatography (TLC, SiO2) to give mainly cyclic porphyrin hexamer 5a (40% yield), together with some cyclic porphyrin octamer 6a (13% yield; we did not detect any formation of the cyclic tetramer). The 1H and 13C NMR spectra reflect the high symmetry of these cyclic oligomers, and their ring sizes were determined from the MALDI mass spectra. Maintaining a low temperature (−40 °C) during this oxidative coupling reaction is important; otherwise, acid-mediated rearrangement occurs,27 manifested by the disappearance of cyclohexadienyl 1H NMR signals at 6.5–6.6 ppm and reducing the symmetry of the spectra (for details, see the Supporting Information). Use of propylene oxide as a proton scavenger improved the reproducibility of the reaction.
Next, reductive unmasking of the p-phenyl ring was tested. When porphyrin dimer 3a was deprotected (OSiEt3 → OH) with tetra-n-butylammonium fluoride (TBAF) and then treated with H2SnCl4 in THF, followed by reinsertion of zinc(II), the product gave a complex 1H NMR spectrum, and MALDI MS analysis indicated the loss of only one oxygen atom rather than two, suggesting rearrangement with migration of a phenyl moiety (see Figure S69). This behavior was reported previously during syntheses of cycloparaphenylenes (CPPs).28 Similar undesired reactivity was observed when using tin(II) chloride dihydrate without the addition of acid29 and when the same conditions were applied to macrocycles 5a and 6a. Encouraged by recent reports by von Delius and co-workers,9 we also attempted the aromatization on a nickel(II) complex instead of zinc(II), but this was similarly unsuccessful (see section 4c of the Supporting Information). Moreover, attempted cyclization of the NiII version of 3a failed because it is not sufficiently electron-rich to undergo oxidative coupling at low temperatures,30 while increasing the temperature leads to rearrangement and loss of the cyclohexadienyl signal in the 1H NMR spectrum (Figure S71).
Bearing in mind the acid sensitivity of the cyclohexadienyl derivatives, we decided to test non-acidic aromatization conditions. 3a was successfully aromatized by reaction with sodium naphthalenide at −78 °C in dry THF, before quenching with iodine and isolating product 4 in 83% yield (see Scheme 1).31 However, applying the same reaction conditions to cyclic oligomers 5a and 6a gave complex mixtures of unidentified products. After many unsuccessful attempts at the aromatization of 5a and 6a using various reducing agents (e.g., SnCl2, LiDBB, and low-valent Ti),24 we decided to change our strategy and work with methoxy derivatives (OR = OMe, rather than OSiEt3 or OH).
Porphyrin dimer 3b (the methoxy analogue of 3a) was synthesized using similar reaction conditions in even higher yield (75%). Subjecting it to our optimized PIFA-mediated coupling conditions provided cyclic oligomers 5b and 6b in 39% and 11% yields, respectively. Single crystals of 5b and 6b were grown by vapor diffusion of methanol into a solution in toluene and slow evaporation of a solution in chloroform and methanol, respectively. Porphyrin octamer 6b crystallizes in a tetragonal I41/a space group with two porphyrin moieties in the asymmetric unit, with a dihedral angle of 85.21(4)° between the 24-atom mean planes of the meso-linked porphyrins. The geometry of cyclohexadiene within each bisporphyrin unit enforces a tub-like conformation. Each zinc(II) center is coordinated to a methanol molecule (Figure 1). Trigonal (R3̅c) 5b adopts a more predictable triangularly shaped geometry with an angle between porphyrin mean planes of 89.23(4)° (Figure S79).
To our delight, after screening many conditions (see section 4 of the Supporting Information), we found that both 5b and 6b undergo reductive aromatization upon treatment with excess lithium metal in THF at 20 °C to form 7 and 8 in 65% and 60% yields, respectively. A critical factor for these reactions is the use of a glass-coated stirring bar. The reactions failed when PTFE-coated stirring bars were used, and it appears that the reduction products of PTFE interfere with aromatization.32,33 Upon conversion of 5b/6b to 7/8, the cyclohexadienyl signals in the 1H NMR spectra (5b, δ = 6.71 ppm; 6b, δ = 6.68 ppm) are replaced by p-phenyl singlets [7, δ = 8.06 ppm; 8, δ = 8.09 ppm (see Figure 2 and Figures S40 and S54)].
Figure 2.
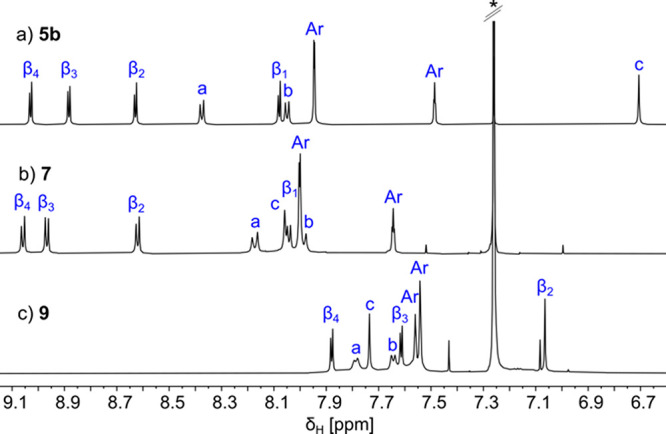
Partial 1H NMR spectra of cyclic porphyrin hexamers 5b, 7, and 9 [CDCl3, 600 MHz, 300 K (see Scheme 1 for labeling)].
Subjecting porphyrin hexamer 7 to DDQ/Sc(OTf)3 gave β,meso,β edge-fused product 9 in 70% yield. The solubility decreases drastically during the reaction, as expected from the formation of a more rigid structure with planar walls. Attempts to fuse octamer 8 were unsuccessful, perhaps due to the poor solubility of the product (Figure S78).
The UV–vis absorption spectra of cyclic porphyrin hexamers 5b, 7, and 9 are compared in Figure 3. Compounds 5b and 7 have similar absorption spectra, showing that aromatization of the p-phenylene bridges does not significantly alter the electronic structure of the porphyrins. This conclusion is confirmed by the similar redox potentials of 5b and 7 (Figure S77). Fusion of the porphyrins shifts the Q-band to the near-infrared (NIR) range at 1059 nm for 9, which is similar to the fused linear dimer 10 (1042 nm), indicating that the strain in 9 and π-conjugation through the p-phenylene bridges do not strongly affect the electronic structure of the fused porphyrin dimer units. The cyclic and square-wave voltammograms of 9 and 10 (Figure 3b) confirm that they have similar electronic structures. The absorption spectra of porphyrin octamers 6b and 8 resemble those of hexamers 5b and 7 (see the Supporting Information).
Figure 3.
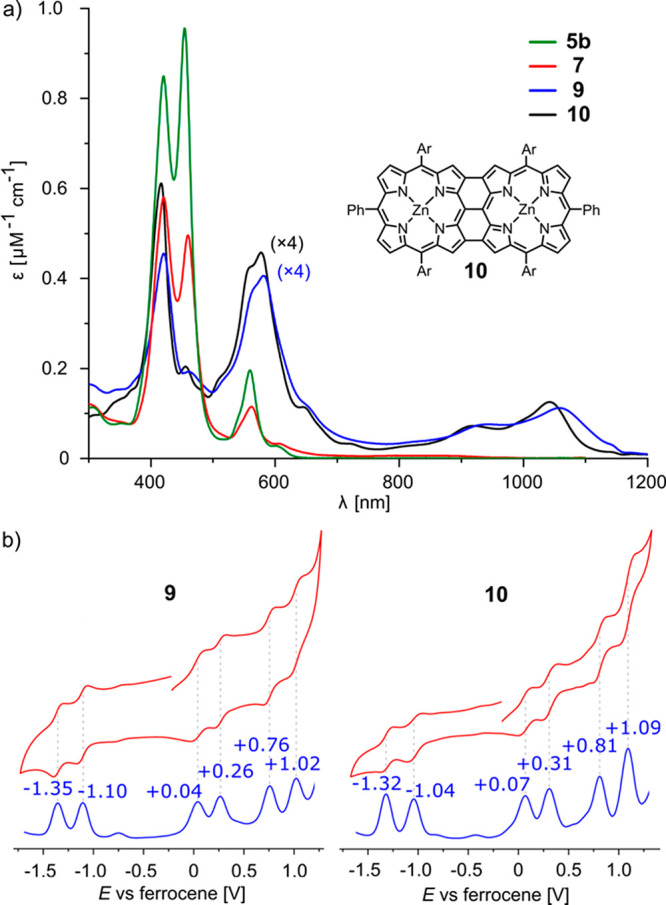
(a) UV–vis–NIR absorption spectra of cyclic porphyrin hexamers 5b, 7, and 9 and reference linear dimer 10, recorded in CH2Cl2. (b) Cyclic (red) and square-wave (blue) voltammograms of 9 and 10 recorded in CH2Cl2 containing 0.1 M Bu4NPF6. Potentials relative to internal ferrocene (Fc/Fc+ at 0 V).
The geometries and electronic structure of nanorings 7–9 were modeled using density functional theory (DFT) in the gas phase at the B3LYP 6-31G(d,p) level of theory (with the Ar solubilizing groups replaced by H to simplify the calculations). 6-Porphyrin rings 7 and 9 have diameters of 28.5 and 28.2 Å, respectively [measured from the centroid of a benzene to the centroid of the opposite meso–meso bond (Figure 4)]. 8-Porphyrin ring 8 has a diameter of 38.6 Å (measured from benzene to benzene). In 7 and 8, the meso–meso linked porphyrins are roughly orthogonal (β-meso-meso-β torsion angles of 75.7° in 7 and 81.1° in 8), as expected, and there is a substantial twist between each porphyrin and its meso-linked p-phenylene (torsion angled of 66.3° in 7 and 64.8° in 8), with a smaller twist at the biphenyl connections (torsion angles of 32.3° in 7 and 35.8° in 8). The torsion angles are similar in 9 (meso-linked p-phenylene, 59.2°; phenylene–phenylene, 37.7°), which implies that there is only weak π-conjugation around the circumference of the nanoring.
Figure 4.
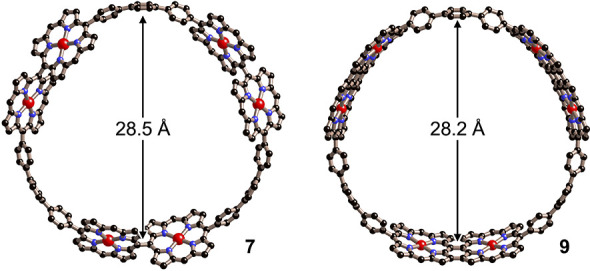
DFT-optimized structures of 7 and 9 (H atoms and meso-aryl substituents omitted solubilized).
The curvatures of the terphenylene and porphyrin dimer units in 7 and 9 were estimated by fitting the coordinates to an arc (see the Supporting Information for details). In 7, the radii of curvature of the terphenylene and porphyrin dimer units are 11.7 and 17.1 Å, respectively, which shows that the terphenylene bridges are more flexible than the porphyrins. The curvature in the p-phenylenes matches that in [17]CPP. The radii of curvature are similar in 9 (terphenylene, 11.8 Å; fused porphyrin dimer, 19.0 Å). This corresponds to the predicted curvature in a fully edge-fused 14-porphyrin nanobelt.
The strain in the nanorings was estimated by analyzing homodesmotic reactions (Table S3). As expected, 7 (154 kJ mol–1) is more stained than 8 (112 kJ mol–1) and the strain does not change significantly upon fusion of 7 to 9 (154 kJ mol–1). These six-porphyrin nanorings are more strained than a butadiyne-linked six-porphyrin nanoring (100 kJ mol–1)34 and are close to [16]CPP (149 kJ mol–1) for 7/9 and to [20]CPP (119 kJ mol–1) for 8.35 The calculated Kohn–Sham HOMO and LUMO orbitals of 9 are located on porphyrin units and have coefficients close to zero on the p-phenylene linkers (see the Supporting Information), which is consistent with the experimental observations from UV–vis–NIR spectra and voltammograms (Figure 3b and Figure S77). The electronic delocalization in the fused porphyrin dimer units in 9 is undisturbed by a substantial deviation from planarity.
In conclusion, we have demonstrated an efficient synthetic pathway to macrocyclic CPP–porphyrin hybrids using oxidative homocoupling of the porphyrins as the macrocyclization step. It is remarkable that macrocyclic products 5a and 6a are formed in good yields (40% and 13%, respectively) without using a template. The methoxy versions of these macrocycles, 5b and 6b, respectively, were synthesized in similar yields, and their crystal structures were determined. Aromatization of the terphenylene bridges and fusion of the porphyrin units in the six-porphyrin nanoring give macrocycle 9, which incorporates three curved fused porphyrin dimers, although the strain does not significantly affect the electronic structure.
Acknowledgments
The authors thank the ERC (Grant 885606, ARO-MAT) and EPSRC (Grant EP/N017188/1) for funding and Oxford Advanced Research Computing (ARC) for computational resources (10.5281/zenodo.22558).
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c04089.
General methods, synthesis, and characterization of all compounds (PDF)
Author Contributions
W.S. performed the synthesis, characterization (MS, NMR, UV–vis, and electrochemistry), and DFT calculations and wrote the initial draft of the manuscript. J.M.V.R. and C.W.P. initiated the project and performed initial experiments on the oxidative cyclization of 3a. P.N.H. and S.J.C. performed X-ray diffraction experiments on crystals grown by W.S. H.L.A. coordinated the study, secured funding, and prepared the manuscript together with W.S. All authors read and edited the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- A preprint of this manuscript was published before peer review:Stawski W.; Van Raden J. M.; Patrick C. W.; Horton P. N.; Coles J.; Anderson H. L. Strained porphyrin tape-cycloparaphenylene hybrid nanorings. chemRxiv 2022, 10.26434/chemrxiv-2022-c4dht. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Spitler E. L.; Johnson C. A. II; Haley M. M. Renaissance of annulene chemistry. Chem. Rev. 2006, 106, 5344–5386. 10.1021/cr050541c. [DOI] [PubMed] [Google Scholar]; b Iyoda M.; Yamakawa J.; Rahman M. J. Conjugated macrocycles: concepts and applications. Angew. Chem., Int. Ed. 2011, 50, 10522–10553. 10.1002/anie.201006198. [DOI] [PubMed] [Google Scholar]; c Majewski M. A.; Stępień M. Bowls, hoops, and saddles: Synthetic approaches to curved aromatic molecules. Angew. Chem., Int. Ed. 2019, 58, 86–116. 10.1002/anie.201807004. [DOI] [PubMed] [Google Scholar]
- a Aggarwal A. V.; Thiessen A.; Idelson A.; Kalle D.; Würsch D.; Stangl T.; Steiner F.; Jester S.-S.; Vogelsang J.; Höger S.; Lupton J. M. Fluctuating exciton localization in giant π-conjugated spoked-wheel macrocycles. Nat. Chem. 2013, 5, 964–970. 10.1038/nchem.1758. [DOI] [PubMed] [Google Scholar]; b Kim P.; Park K. H.; Kim W.; Tamachi T.; Iyoda M.; Kim D. Relationship between dynamic planarization processes and exciton delocalization in cyclic oligothiophenes. J. Phys. Chem. Lett. 2015, 6, 451–456. 10.1021/jz502395z. [DOI] [PubMed] [Google Scholar]
- Parkinson P.; Kondratuk D. V.; Menelaou C.; Gong J. Q.; Anderson H. L.; Herz L. M. Chromophores in molecular nanorings - When is a ring a ring?. J. Phys. Chem. Lett. 2014, 5, 4356–4361. 10.1021/jz5022153. [DOI] [PubMed] [Google Scholar]
- a Jirásek M.; Anderson H. L.; Peeks M. D. From Macrocycles to quantum rings: Does aromaticity have a size limit?. Acc. Chem. Res. 2021, 54, 3241–3251. 10.1021/acs.accounts.1c00323. [DOI] [PubMed] [Google Scholar]; b Rickhaus M.; Jirasek M.; Tejerina L.; Gotfredsen H.; Peeks M. D.; Haver R.; Jiang H.-W.; Claridge T. D. W.; Anderson H. L. Global Aromaticity at the Nanoscale. Nat. Chem. 2020, 12, 236–241. 10.1038/s41557-019-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ren L.; Gopalakrishna T. Y.; Park I.-H.; Han Y.; Wu J. Porphyrin/quinoidal-bithiophene-based macrocycles and their dications: Template-free synthesis and global aromaticity. Angew. Chem., Int. Ed. 2020, 59, 2230–2234. 10.1002/anie.201911269. [DOI] [PubMed] [Google Scholar]
- a Williams-Harry M.; Bhaskar A.; Ramakrishna G.; Goodson T. III; Imamura M.; Mawatari A.; Nakao K.; Enozawa H.; Nishinaga T.; Iyoda M. Giant thienylene-acetylene-ethylene macrocycles with large two-photon absorption cross section and semishape-persistence. J. Am. Chem. Soc. 2008, 130, 3252–3253. 10.1021/ja078246s. [DOI] [PubMed] [Google Scholar]; b Mikhaylov A.; Kondratuk D. V.; Cnossen A.; Anderson H. L.; Drobizhev M.; Rebane A. Cooperative enhancement of two-photon absorption in self-assembled zinc-porphyrin nanostructures. J. Phys. Chem. C 2016, 120, 11663–11670. 10.1021/acs.jpcc.6b01394. [DOI] [Google Scholar]
- Segawa Y.; Watanabe T.; Yamanoue K.; Kuwayama M.; Watanabe K.; Pirillo J.; Hijikata Y.; Itami K. Synthesis of a Möbius carbon nanobelt. Nat. Synth. 2022, 1, 535–541. 10.1038/s44160-022-00075-8. [DOI] [Google Scholar]
- a Kawase T.; Tanaka K.; Shiono N.; Seirai Y.; Oda M. Onion-type complexation based on carbon nanorings and a buckminsterfullerene. Angew. Chem., Int. Ed. 2004, 43, 1722–1724. 10.1002/anie.200353517. [DOI] [PubMed] [Google Scholar]; b Hogben H. J.; Sprafke J. K.; Hoffmann M.; Pawlicki M.; Anderson H. L. Stepwise effective molarities in porphyrin oligomer complexes: Preorganization results in exceptionally strong chelate cooperativity. J. Am. Chem. Soc. 2011, 133, 20962–20969. 10.1021/ja209254r. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Gsänger S.; Minameyer M. B.; Imaz I.; Maspoch D.; Shyshov O.; Schwer F.; Ribas X.; Drewello T.; Meyer B.; von Delius M. Highly strained, radially π-conjugated porphyrinylene nanohoops. J. Am. Chem. Soc. 2019, 141, 18500–18507. 10.1021/jacs.9b08584. [DOI] [PubMed] [Google Scholar]
- Ball M.; Zhang B.; Zhong Y.; Fowler B.; Xiao S.; Ng F.; Steigerwald M.; Nuckolls C. Conjugated macrocycles in organic electronics. Acc. Chem. Res. 2019, 52, 1068–1078. 10.1021/acs.accounts.9b00017. [DOI] [PubMed] [Google Scholar]
- a Miki K.; Ohe K. π-Conjugated macrocycles bearing angle-strained alkynes. Chem. - Eur. J. 2020, 26, 2529–2575. 10.1002/chem.201904114. [DOI] [PubMed] [Google Scholar]; b Kayahara E.; Hayashi T.; Takeuchi K.; Ozawa F.; Ashida K.; Ogoshi S.; Yamago S. Strain-induced double carbon–carbon bond activations of cycloparaphenylenes by a platinum complex: Application to the synthesis of cyclic diketones. Angew. Chem., Int. Ed. 2018, 57, 11418–11421. 10.1002/anie.201806591. [DOI] [PubMed] [Google Scholar]
- Darzi E. R.; Jasti R. The dynamic, size-dependent properties of [5]–[12]cycloparaphenylenes. Chem. Soc. Rev. 2015, 44, 6401–6410. 10.1039/C5CS00143A. [DOI] [PubMed] [Google Scholar]
- a Liu T.; Yang J.; Geyer F.; Conrad-Burton F. S.; Hernández Sánchez R.; Li H.; Zhu X.; Nuckolls C. P.; Steigerwald M. L.; Xiao S. Stringing the perylene diimide bow. Angew. Chem., Int. Ed. 2020, 59, 14303–14307. 10.1002/anie.202004989. [DOI] [PubMed] [Google Scholar]; b Merner B. L.; Dawe L. N.; Bodwell G. J. 1,1,8,8-Tetramethyl[8](2,11)teropyrenophane: Half of an aromatic belt and a segment of an (8,8) single-walled carbon nanotube. Angew. Chem., Int. Ed. 2009, 48, 5487–5491. 10.1002/anie.200806363. [DOI] [PubMed] [Google Scholar]
- a Wang S.-P.; Shen Y.-F.; Zhu B.-Y.; Wu J.; Li S. Recent advances in the template-directed synthesis of porphyrin nanorings. Chem. Commun. 2016, 52, 10205–10216. 10.1039/C6CC04556A. [DOI] [PubMed] [Google Scholar]; b Bols P. S.; Anderson H. L. Template-directed synthesis of molecular nanorings and cages. Acc. Chem. Res. 2018, 51, 2083–2092. 10.1021/acs.accounts.8b00313. [DOI] [PubMed] [Google Scholar]
- a Aratani N.; Kim D.; Osuka A. Discrete cyclic porphyrin arrays as artificial light-harvesting antenna. Acc. Chem. Res. 2009, 42, 1922–1934. 10.1021/ar9001697. [DOI] [PubMed] [Google Scholar]
- Gotfredsen H.; Deng J.-R.; Van Raden J.; Righetto M.; Hergenhahn J.; Clarke M.; Bellamy-Carter A.; Hart J.; O’Shea J.; Claridge T. D. W.; Duarte F.; Saywell A.; Herz L. M.; Anderson H. L. Bending a photonic wire into a ring. Nat. Chem. 2022, 14, 1436–1442. 10.1038/s41557-022-01032-w. [DOI] [PubMed] [Google Scholar]
- Grzybowski M.; Sadowski B.; Butenschön B.; Gryko D. T. Synthetic applications of oxidative aromatic coupling—from biphenols to nanographenes. Angew. Chem., Int. Ed. 2020, 59, 2998–3027. 10.1002/anie.201904934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuka A.; Shimidzu H. meso, meso-Linked porphyrin arrays. Angew. Chem., Int. Ed. Engl. 1997, 36, 135–137. 10.1002/anie.199701351. [DOI] [Google Scholar]
- a Tsuda A.; Osuka A. Fully conjugated porphyrin tapes with electronic absorption bands that reach into infrared. Science 2001, 293, 79–82. 10.1126/science.1059552. [DOI] [PubMed] [Google Scholar]; b Tanaka T.; Osuka A. Triply linked porphyrinoids. Chem. - Eur. J. 2018, 24, 17188–17200. 10.1002/chem.201802810. [DOI] [PubMed] [Google Scholar]
- a Yoshida N.; Osuka A. Control of dihedral angle of meso–meso linked diporphyrins by introducing dioxymethylene straps of various length. Org. Lett. 2000, 2, 2963–2966. 10.1021/ol006216n. [DOI] [PubMed] [Google Scholar]; b Peng X.; Aratani N.; Takagi A.; Matsumoto T.; Kawai T.; Hwang I.-W.; Ahn T. K.; Kim D.; Osuka A. A dodecameric porphyrin wheel. J. Am. Chem. Soc. 2004, 126, 4468–4469. 10.1021/ja0392972. [DOI] [PubMed] [Google Scholar]; c Nakamura Y.; Hwang I.; Aratani N.; Ahn T. K.; Ko D. M.; Takagi A.; Kawai T.; Matsumoto T.; Kim D.; Osuka A. Directly meso–meso linked porphyrin rings: Synthesis, characterization, and efficient excitation energy hopping. J. Am. Chem. Soc. 2005, 127, 236–246. 10.1021/ja045254p. [DOI] [PubMed] [Google Scholar]; d Ouyang Q.; Zhu Y.-Z.; Li Y.-C.; Wei H.-B.; Zheng J.-Y. Diastereoselective synthesis of chiral diporphyrins via intramolecular meso-meso oxidative coupling. J. Org. Chem. 2009, 74, 3164–3167. 10.1021/jo9001118. [DOI] [PubMed] [Google Scholar]
- Aratani N.; Osuka O. A meso-meso directly linked octameric porphyrin square. Chem. Commun. 2008, 4067–4069. 10.1039/b807351a. [DOI] [PubMed] [Google Scholar]
- a Sato H.; Tashiro K.; Shinmori H.; Osuka A.; Murata Y.; Komatsu K.; Aida T. Positive heterotropic cooperativity for selective guest binding via electronic communications through a fused zinc porphyrin array. J. Am. Chem. Soc. 2005, 127, 13086–13087. 10.1021/ja052993c. [DOI] [PubMed] [Google Scholar]; b Nakamura Y.; Aratani N.; Shinokubo H.; Takagi A.; Kawai T.; Matsumoto T.; Yoon Z. S.; Kim D. Y.; Ahn T. K.; Kim D.; Muranaka A.; Kobayashi N.; Osuka A. A directly fused tetrameric porphyrin sheet and its anomalous electronic properties that arise from the planar cyclooctatetraene core. J. Am. Chem. Soc. 2006, 128, 4119–4127. 10.1021/ja057812l. [DOI] [PubMed] [Google Scholar]; c Yoon M.-C.; Yoon Z. S.; Cho S.; Kim D.; Takagi A.; Matsumoto T.; Kawai T.; Hori T.; Peng X.; Aratani N.; Osuka A. A hexagonal prismatic porphyrin array: Synthesis, STM detection, and efficient energy hopping in near-infrared region. J. Phys. Chem. A 2007, 111, 9233–9239. 10.1021/jp0723923. [DOI] [PubMed] [Google Scholar]; d Kopp S. M.; Gotfredsen H.; Deng J.-R.; Claridge T. D. W.; Anderson H. L. Global aromaticity in a partially fused 8-porphyrin nanoring. J. Am. Chem. Soc. 2020, 142, 19393–19401. 10.1021/jacs.0c09973. [DOI] [PubMed] [Google Scholar]
- Jasti R.; Bhattacharjee J.; Neaton J. B.; Bertozzi C. R. Synthesis, characterization, and theory of [9]-, [12]-, and [18]cycloparaphenylene: carbon nanohoop structures. J. Am. Chem. Soc. 2008, 130, 17646–17647. 10.1021/ja807126u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. L.; Lehnherr D.; Lindner B. D.; Tykwinski R. R. Reductive Aromatization/Dearomatization and Elimination Reactions to Access Conjugated Polycyclic Hydrocarbons, Heteroacenes, and Cumulenes. ChemPlusChem. 2017, 82, 967–1001. 10.1002/cplu.201700168. [DOI] [PubMed] [Google Scholar]
- Wassy D.; Hermann M.; Wössner J. S.; Frédéric L.; Pieters G.; Esser B. Enantiopure nanohoops through racemic resolution of diketo[n]CPPs by chiral derivatization as precursors to DBP[n]CPPs. Chem. Sci. 2021, 12, 10150–10158. 10.1039/D1SC02718B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.-W.; Tanaka T.; Kim T.; Sung Y. M.; Mori H.; Kim D.; Osuka A. Synthesis of [n]cyclo-5,15-porphyrinylene-4,4’-biphenylenes displaying size-dependent excitation-energy hopping. Angew. Chem., Int. Ed. 2015, 54, 15197–15201. 10.1002/anie.201507822. [DOI] [PubMed] [Google Scholar]
- Morrow G. W.; Schwind B. Syntheses of para-terphenyl via reductive deoxygenation of quinol derivatives. Synth. Commun. 1995, 25, 269–276. 10.1080/00397919508010816. [DOI] [Google Scholar]
- Golder M. R.; Jasti R. Syntheses of the smallest carbon nanohoops and the emergence of unique physical phenomena. Acc. Chem. Res. 2015, 48, 557–566. 10.1021/ar5004253. [DOI] [PubMed] [Google Scholar]
- Lehnherr D.; Tykwinski R. R. Pentacene oligomers and polymers: functionalization of pentacene to afford mono-, di-, tri-, and polymeric materials. Org. Lett. 2007, 9, 4583–4586. 10.1021/ol702094d. [DOI] [PubMed] [Google Scholar]
- a Fuhrhop J.-H.; Mauzerall D. One-electron oxidation of metalloporphyrins. J. Am. Chem. Soc. 1969, 91, 4174–4181. 10.1021/ja01043a027. [DOI] [PubMed] [Google Scholar]; b Ouyang Q.; Zhu Y.-Z.; Zhang C.-H.; Yan K.-Q.; Li Y.-C.; Zheng J.-Y. An efficient PIFA-mediated synthesis of fused diporphyrin and triply–singly interlacedly linked porphyrin array. Org. Lett. 2009, 11, 5266–5269. 10.1021/ol902198w. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Thompson R. R.; Fronczek F. R.; Lee S. Size-selective synthesis of large cycloparaphenyleneacetylene carbon nanohoops using alkyne metathesis. Org. Lett. 2019, 21, 4680–4683. 10.1021/acs.orglett.9b01563. [DOI] [PubMed] [Google Scholar]
- Use of glass-coated magnetic stirring bars during reductive aromatization with lithium metal was reported by Itami and co-workers:Yagi A.; Segawa Y.; Itami K. Synthesis and properties of [9]cyclo-1,4-naphthylene: A π-extended carbon nanoring. J. Am. Chem. Soc. 2012, 134, 2962–2965. 10.1021/ja300001g. [DOI] [PubMed] [Google Scholar]
- a Tasker S.; Chambers R. D.; Badyal J. P. S. Surface defluorination of PTFE by sodium atoms. J. Phys. Chem. 1994, 98, 12442–12446. 10.1021/j100098a046. [DOI] [Google Scholar]; b de los Reyes C. A.; Smith McWilliams A. D.; Hernández K.; Walz-Mitra K. L.; Ergülen S.; Pasquali M.; Martí A. A. Adverse effect of PTFE stir bars on the covalent functionalization of carbon and boron nitride nanotubes using Billups–Birch reduction conditions. ACS Omega 2019, 4, 5098–5106. 10.1021/acsomega.8b03677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haver R.; Tejerina L.; Jiang H.-W.; Rickhaus M.; Jirasek M.; Grübner I.; Eggimann H. J.; Herz L. M.; Anderson H. L. Tuning the circumference of six-porphyrin nanorings. J. Am. Chem. Soc. 2019, 141, 7965–7971. 10.1021/jacs.9b02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa Y.; Omachi H.; Itami K. Theoretical studies on the structures and strain energies of cycloparaphenylenes. Org. Lett. 2010, 12, 2262–2265. 10.1021/ol1006168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.