Abstract
Posttraumatic stress disorder (PTSD) is associated with neural and behavioral alterations in response to trauma exposure, including working memory impairments. Rodent models of PTSD have not fully investigated chronic or reactive working memory deficits, despite clinical relevance. The present study uses footshock to induce a posttraumatic stress state in male and female rats and evaluates the effect of footshock and trauma-paired odor cues on working memory performance in the odor span task. Results demonstrate the emergence of chronic deficits in working memory among animals exposed to footshock by 3 wk after traumatic stress. The presentation of a trauma-paired odor cue was associated with further decrement in working memory performance for male animals. Furthermore, anxiety-like behaviors associated with the PTSD-like phenotype could predict the degree of working memory impairment in response to the trauma-paired odor cue. This study enhances validation of an existing rodent model of PTSD through replication of the clinical observations of working memory deficits associated with PTSD and provides novel insight into effects in female rodents. This will facilitate work to probe underlying mechanistic dysregulation of working memory following footshock trauma exposure and future development of novel treatment strategies.
Posttraumatic stress disorder (PTSD) is a psychological condition characterized by a pervasive maladapted stress state elicited by a significant trauma. Each symptom class of PTSD is characterized by an adverse reaction to internal or external trauma reminders that demand substantial attentional and cognitive resources (Cox and Olatunji 2017). Working memory is defined as a temporary, limited-capacity memory system for goal-directed behaviors that is also dependent on attentional and cognitive resources. Given this overlap, it is not surprising that working memory is commonly impaired in PTSD patients (Honzel et al. 2014), yet working memory has been underexamined in animal models of PTSD, particularly for females, a requisite to elucidate the mechanism underlying this relationship.
The link between working memory and PTSD has yielded potential clinical interventions: Working memory training improved PTSD symptoms, suggesting intersecting mechanisms underlying working memory and impairments in PTSD (Saunders et al. 2015; Larsen et al. 2019) and underscoring the need for more preclinical studies. The medial prefrontal cortex may provide this common mechanism, as it is necessary for working memory (Yang et al. 2014; Lara and Wallis 2015; Davies et al. 2017; Bahmani et al. 2019; De Falco et al. 2019; Scott et al. 2020) and decreased medial prefrontal cortex responsivity is associated with PTSD severity (Shin et al. 2006; Koenigs and Grafman 2009). To explore brain maladaptations underlying PTSD and working memory deficits, accurate animal models must first be established.
A central feature differentiating stress and trauma lies in the long-term effects of the experience. Following stress, most individuals display short-term sensitivity but long-term resilience (Franklin et al. 2012; Dutcher and Creswell 2018). Conversely, traumas are characterized as severe stressors that exceed coping mechanisms in some individuals, thereby eliciting the chronic pathological state of PTSD after a critical period following exposure (Kilpatrick et al. 2013). While the subjective experience of an aversive event cannot be fully predicted by a selected protocol, several factors can increase the intensity of an experience, making it more likely that individuals experience the event as traumatic rather than stressful. Events that are uncontrollable and unpredictable are associated with greater trauma-like responses. Unpredictability also promotes discriminative fear conditioning in enriched contexts, increasing the likelihood of cue-associated reactivity (Lucas et al. 2014; Trow et al. 2019; Richter-Levin and Sandi 2021). The stress manipulations in the present study are designed to model long-lasting traumas rather than short-acting stressors and thus are referred to here as “trauma” (Yehuda and Antelman 1993; Richter-Levin and Sandi 2021).
Two substantial shortcomings of preclinical PTSD studies are the underexploration of sex differences and individual differences in symptom presentation. Sex differences are apparent in PTSD susceptibility, with men and women tending to experience different forms and frequency of trauma. Despite less lifetime trauma exposure, women are nearly twice as likely as men to develop PTSD (Kessler et al. 1995, 2005), and thus both sexes are included in the present study. Animal models have traditionally evaluated all animals with trauma exposure as a single, uniform group, limiting the nuanced exploration of individual differences in susceptibility (Yehuda and Antelman 1993; Richter-Levin et al. 2019). Researchers have attempted to mimic clinical diagnostic procedures by using a battery of behavioral tests selected to approximate symptom clusters to determine whether an animal exhibits a PTSD-like state (Cohen et al. 2003; Richter-Levin et al. 2019). To model this approach, the present study uses elevated zero maze, marble burying, open field, and social interaction assessments. Multivariate approaches like this accommodate individual differences in symptom presentation and may better capture sex differences in symptom profiles by embracing a quantitative spectrum of the disorder rather than a categorical approach (Ardi et al. 2016; Murphy et al. 2019).
Efforts to model trauma eliciting a PTSD-like state have largely focused on replicating physiological and reactivity phenotypes (Liberzon et al. 1997; Cohen et al. 2012). Lacking is the assessment of whether the trauma-associated memories that cause these physiological and reactivity profiles also produce generalized memory impairments, including for working memory (Nejati et al. 2018). Recent theories suggest that stress and trauma create chronic energetic and attentional demands that may contribute to working memory deficits in PTSD (Block and Liberzon 2016; Balderston et al. 2017; Peters et al. 2017). PTSD is associated with elevated attentional bias to aversive stimuli, and the persistence of trauma-related memories may serve as a low-level distractor that is omnipresent for the affected individual (Woodward et al. 2017). Clinical studies have demonstrated working memory deficits both following acute stress and in persistent states of stress such as PTSD (e.g., Scott et al. 2015; Moran 2016; Shields et al. 2016).
Rodent models have replicated acute stress effects on working memory performance, but limited preclinical studies have used stressors capable of eliciting chronic pathology (Diamond et al. 1999; Woodson et al. 2003; Davies et al. 2013). Rodent span tasks, such as the odor span task, probe working memory capacity and therefore allow for investigation of the effects of stress on working memory (Dudchenko 2004; Davies et al. 2013; Dudchenko et al. 2013). In span tasks, animals are tasked with recalling an increasing number of previously encountered stimuli to detect the stimulus not yet encountered. While a valuable point of contact with clinical working memory tasks that has been useful in schizophrenia research, span tasks may also capture cognitive features beyond working memory (April et al. 2013; Branch et al. 2014). Importantly for the present study, the odor span task allows for the direct assessment of trauma-paired environmental odors within the task.
The present study used the odor span task to assess working memory performance for male and female rats in a footshock model of PTSD. Rats underwent footshock in the presence of a trauma-paired odor (TPO), and subsequent working memory performance was evaluated in the absence and presence of that TPO. The study sought to investigate within both sexes long-term working memory deficits following footshock trauma as well as reactive deficits in working memory induced by a trauma-associated cue.
Results
Rats were assessed for the impact of footshock trauma (FST) on both anxiety-like and working memory behaviors, as illustrated in Figure 1A. To examine working memory, an odor span task was used in which rats identified novel odors in an incrementing delay non-match-to-sample (DNMS) task (Fig. 1B; see the Materials and Methods). Prior to beginning odor span training, animals performed an initial elevated zero maze test to evaluate baseline anxiety-like behavior (Fig. 1C). Anxiety-like behavior, as assessed by time spent in the open zones of the zero maze, did not differ by condition (F(1,28) = 0.0050, P = 0.94), but there was a main effect of sex (F(1,28) = 5.33, P = 0.024; Cohen's d = 0.84). Females spent more time exploring the open zones of the maze. There was no interaction between sex and condition (F(1,28) = 0.23, P = 0.64). DNMS performance, measured as proportion of correct responses, was evaluated following 2 wk of initial training to assess baseline task performance (Fig. 1D). There were no significant differences in DNMS performance between the control and FST conditions (F(1,28) = 0.27, P = 0.61) or sexes (F(1,28) = 2.07, P = 0.16) and no interaction between factors (F(1,28) = 0.11, P = 0.75). Animals were counterbalanced into control and FST groups on elevated zero maze and baseline DNMS performance, and DNMS training continued after the FST session until each animal met criterion.
Figure 1.
Experimental procedures and counterbalancing measures. (A) Experimental time line. (B) Depiction of sample odor span session. (C) Group assignments show balanced pretest elevated zero maze performance, as assessed by open zone time, although females spent greater time in the open zones of the maze than males. (*) P < 0.05, male versus female. (D) Prefootshock DNMS performance, calculated as the average proportion of correct responses on the most recent three training sessions, did not differ between the groups. (DNMS) Delayed non-match to sample, (FST) footshock trauma, (ITI) intertrial interval. N = 8 per group.
Performance in the DNMS training phase was assessed immediately following the FST session. Data are expressed as percent change in correct responses compared with the previous DNMS session (Fig. 2). There were no group differences between male and female animals (F(1,28) = 0.64, P = 0.43) or control and FST animals (F(1,28) = 0.18, P = 0.68), or interaction between sex and condition (F(1,28) = 0.043, P = 0.84).
Figure 2.

Delayed non-match to sample following acute stress. Acute stress did not significantly impact DNMS performance, expressed as a change score calculated as percent change from DNMS performance prestress. (FST) Footshock trauma. N = 8 per group.
Working memory performance was assessed during the final week of training as an average measure of baseline performance (Fig. 3A). There was a main effect of sex on odor span performance (F(1,28) = 4.87, P = 0.036; Cohen's d = 0.75), demonstrating that female working memory performance was, on average, higher than male performance. There was a main effect of condition (F(1,28) = 4.76, P = 0.038; Cohen's d = 0.73), revealing that FST animals obtained lower maximum spans, on average, than control animals. There was no interaction between sex and condition (F(1,28) = 0.19, P = 0.66). Escape omissions also were summed from the final week of training to examine whether the lowered FST spans could be explained by animals prematurely “quitting” trials through omissions (Fig. 3B). Two-way ANOVA revealed no main effect of condition (F(1,28) = 2.42, P = 0.13), supporting the fact that FST animals did not omit responses significantly more often than control animals. There was no main effect of sex (F(1,28) = 0.097, P = 0.76), suggesting that the sex difference in working memory performance was not driven by an increase in trial omissions by males. There was no interaction between the factors (F(1,28) = 0.22, P = 0.64).
Figure 3.
Footshock trauma history impacted training performance in the odor span test. (A) Maximum span, calculated as the average maximum odor span across the final 5 d of training, was significantly lower in males and FST animals. Maximum span is indicated by the dashed line. (*) P < 0.05, male versus female and FST versus control. (B) Summed trial escape omissions from the final 5 d of training did not differ between groups. (FST) Footshock trauma. N = 8 per group.
All animals received 2 d of behavioral testing, discussed below, and then entered the testing phase of the odor span task. In this phase, animals were given a normal testing day to serve as baseline and a TPO testing day. On the TPO testing day, the TPO, coriander, was presented as the target cup at span 4. Maximum span performance is presented for baseline and TPO testing days for males (Fig. 4A) and females (Fig 4B). Sexes are graphed separately for ease of visualization but were analyzed together. Two-way ANOVA revealed no main effect of sex (F(1,27) = 0.13, P = 0.72) or condition (F(1,27) = 1.71, P = 0.20) on the impact of TPO presentation on odor span. Sex and condition did significantly interact (F(1,27) = 4.36, P = 0.047; Cohen's d = 0.03), suggesting that male FST animals experienced slightly greater deficits upon exposure to the TPO compared with same-sex controls.
Figure 4.
Footshock trauma history and trauma-paired odor impacted performance in the odor span test. (A,B) Maximum span on baseline and TPO sessions. Span did not differ by sex or condition, but a significant interaction was present, suggesting male FST animals displayed greater odor span deficits in response to the TPO compared with same-sex controls. (C,D) Log-transformed latency to respond on non-TPO trials, calculated as the average latency per sampled cup on choice trials 1–3, compared with TPO trial 4. Latency was significantly greater for FST animals, regardless of sex. (*) P < 0.05, control versus FST. (E,F) Log-transformed latency to dig on the TPO trial correlated with span impairment, calculated as percent change in TPO span compared with baseline span, only for male FST animals. (*) P < 0.05, male FST. (FST) Footshock trauma, (TPO) trauma-paired odor. N = 7–8 per group.
Latency to dig is presented for non-TPO and TPO trials, calculated as the total latency to dig divided by the number of cups sampled for males (Fig. 4C) and females (Fig. 4D). There was a main effect of condition (F(1,27) = 4.67, P = 0.040, Cohen's d = 0.77), indicating that FST animals demonstrated increased latency to respond on TPO trials. There was no effect of sex (F(1,27) = 0.002, P = 0.96) or interaction between factors (F(1,27) = 1.66, P = 0.21). Deficits in span were hypothesized to result from cue reactivity, which is approximated by log-normalized latency to dig on the TPO trial. Interestingly, the latency to dig on the TPO trial was only significantly correlated with the decrement in span performance for male FST animals (Pearson's r = −0.80, P = 0.017) (Fig. 4E). All other groups demonstrated nonsignificant relationships between latency and span change (Fig. 4E, male control [r = −0.0080, P = 0.99], F, female control [r = 0.11, P = 0.81] and female FST [r = −0.37, P = 0.37]).
Susceptibility tests were run before and after the odor span testing days (Fig. 1A) to protect against the possibility that re-exposure to the trauma-paired cue substantially affected the behavior of the FST animals. Elevated zero maze behavior was measured by time spent in the open zones of the maze (Fig. 5A). Two-way ANOVA revealed no main effect of condition (F(1,28) = 0.11, P = 0.74) or sex (F(1,28) = 0.070, P = 0.79) and no interaction (F(1,28) = 0.006, P = 0.94) on open zone time. General locomotor activity was evaluated via the number of entries into the open zones during the elevated zero maze test (Fig. 5B). There was no main effect of condition (F(1,28) = 0.0049, P = 0.94) or interaction between factors (F(1,28) = 0.12, P = 0.73), but there was a main effect of sex (F(1,28) = 19.13, P = 0.0002; Cohen's d = 1.60), demonstrating that females displayed greater general locomotion during the zero maze test even though their total time in the open zones did not differ from males. Marble burying results are not analyzed because only four animals (one control and three FST) engaged in marble burying behavior. Open field behavior was scored as the total amount of time that the animal spent in the center of the arena (Fig. 5C). There were no main effects of condition (F(1,28) = 0. 025, P = 0.88) or sex (F(1,28) = 0. 48, P = 0.49) and no interaction between factors (F(1,28) = 0.030, P = 0.86) on center time. Total distance traveled, scored with ANY-Maze as number of zone crossings multiplied by the size of each zone in the maze (15 cm), was analyzed for locomotor behavior (Fig. 5D). There was no main effect of condition (F(1,28) = 0.078, P = 0.78) or interaction between factors (F(1,28) = 0.88, P = 0.36), but there was a trend toward a main effect of sex (F(1,28) = 3.99, P = 0.056), again supporting that females tend to have higher levels of general locomotion than males during anxiety-like behavior tests. Behavior in the social interaction test was evaluated by the distribution of time spent engaged in nonsocial, prosocial, and antisocial encounters (Fig. 5E). A priori hypotheses were that a PTSD-like phenotype would be associated with less total social behavior (prosocial or antisocial) and a greater tendency toward antisocial behavior. Two-way ANOVAs were performed on each of these metrics. Total time spent in social interaction did not differ by condition (F(1,28) = 0.21, P = 0.65) and there was no interaction between factors (F(1,28) = 0.13, P = 0.73), but there was a main effect of sex (F(1,28) = 8.74, P = 0.0063), demonstrating that females spent less total time engaged in social interaction. There were no main effects of condition (F(1,28) = 0.045, P = 0.83) or sex (F(1,28) = 2.43, P = 0.13) and there was no interaction between factors (F(1,28) = 1.04, P = 0.32) for time spent engaged in antisocial behavior. Data were converted to z-scores within each group (male control, male FST, female control, and female FST) to facilitate combination of male and female data by condition while accounting for baseline differences between the sexes. When normalized within condition, the change in span on TPO trials is significantly correlated with antisocial behavior for FST animals only (Pearson's r = 0.64, P = 0.007) (Fig. 5F). Control animals did not display a significant relationship between antisocial interaction and change in span (r = −0.38, P = 0.16).
Figure 5.
Behavioral profiling to assess PTSD-like phenotype. (A) Elevated zero maze open zone time did not differ between groups. (B) Open zone entries in zero maze were more numerous for females, indicating greater locomotor activity. (*) P < 0.05, male versus female. (C) Open field time in the center zone did not differ between groups. (D) Total distance traveled in the open field test did not differ between groups. (E) Time spent engaged in nonsocial, prosocial, and antisocial behaviors in the social interaction test differed by sex. (F) Correlation between normalized change in span and antisocial interaction was significant only for FST animals. Lines indicate group correlation slopes. (**) P < 0.01 FST group. (FST) Footshock trauma. N = 7–8 per group.
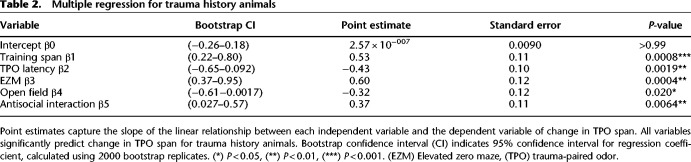
Multiple regression analysis using the PTSD-like behavioral profile significantly predicts the degree to which an animal's span was altered by presentation of the trauma-paired cue (span change) for FST animals. For this analysis, data from each predictor variable (training span, TPO latency, elevated zero maze open zone time, open field center time, and antisocial interaction time) and the outcome variable (percent change in span) were converted to z-scores to standardize data scaling across groups and collapsed across sex, since few effects of sex were present in the data. This model did not differ from the null hypothesis for control animals (F(5,9) = 1.066, P = 0.44, R2 = 0.37; no variables contributed significantly to the prediction, P > 0.05) (Table 1) but was significant for FST animals (F(5,10) = 19.61, P < 0.001, R2 = 0.91, adjusted R2 = 0.86; all variables contributed significantly to the prediction, P < 0.05) (Table 2). Due to the small sample sizes involved, bootstrapping was performed to confirm the validity of the point estimates for the regression coefficients with relaxed assumptions that may have been impacted by the sample size (Table 2). Repeated fivefold cross-validation was performed on the FST model, resulting in an average prediction error (root mean squared error) of 0.46. A covariance matrix was built for the control and FST models, which demonstrated that no multicollinearity existed between the predictor variables for either condition (Fig. 6).
Table 1.
Multiple regression for control animals

Table 2.
Multiple regression for trauma history animals
Figure 6.
Multiple linear regression covariance matrices. In accordance with assumptions for multiple linear regression, no regression parameters significantly covaried for control animals (A) or FST animals (B).
Discussion
The present study is among the first to examine working memory in an animal model of PTSD with the inclusion of female subjects. A footshock trauma procedure and the odor span task were used to evaluate the effect of footshock trauma history and trauma-paired cue reactivity on working memory performance. The data demonstrated that while FST did not acutely affect working memory performance in DNMS (Fig. 2), FST history was associated with persistent deficits in working memory performance (Fig. 3), and exposure to a trauma-paired cue further impaired working memory (Fig. 4). Additionally, multivariate analysis of PTSD phenotypic behaviors significantly predicts the degree to which an individual's working memory is impaired by the presentation of trauma-associated cues, further validating the change in span as an index that models certain features of PTSD (Table 2). Collectively, these data move the PTSD field toward an animal modeling approach that accounts for female data and allows for investigation of the mechanisms and implications for working memory deficits in PTSD.
Lack of acute effects of footshock trauma
Animals completed FST sessions one at a time and then were transported immediately to the behavioral testing room, where they performed eight DNMS trials to assess the acute impact of FST. There was no effect of acute FST on DNMS performance despite previous work demonstrating that other acute stressors impact working memory performance. Predator odor has acutely impaired DNMS performance in an object recognition working memory task (Morrow et al. 2000). Acute restraint stress impairs working memory capacity in the full odor span task, although the effect was not assessed on DNMS performance (Davies et al. 2013). Acute stress has both impaired and enhanced working memory performance in clinical working memory tasks, and the direction of effects may be partially dependent on the sex of the subject (Schoofs et al. 2013; Khayyer et al. 2021). Particularly because both cited rodent studies used only male subjects, it may not be surprising that our current results do not replicate the deficits in DNMS performance following acute stress, although the present results may also reflect nonequivalence of footshock, predator odor, and restraint stressors.
Past footshock trauma triggered chronic working memory deficits
Following FST, animals were given three additional weeks of odor span training, which also served as a stress incubation period to allow for the emergence of persistent stress phenotypes (e.g., Harvey et al. 2003). Maximum odor span was averaged across the final week of training, and FST resulted in lower overall working memory performance at 3 wk after the FST session compared with the control group. Both sexes with FST history had lower maximum spans compared with controls, although females had higher spans regardless of condition. The current study generally found higher span performance than previous studies, but this was driven by females, which have not previously been evaluated within the odor span task (Dudchenko et al. 2000; Davies et al. 2013; De Falco et al. 2019). Clinical research supports slightly better working memory performance in females than in males (Sánchez-Andrade et al. 2005; Voyer et al. 2021). Probe trial performance indicated that animals were not marking cups or selecting based on the smell of the buried food reward, but that does not rule out possible engagement in strategies like chunking promoting attainment of higher spans (Guida et al. 2012).
Trauma-paired cue reduced span performance for footshock trauma-exposed rats
Following the 3 wk of OST training and stress incubation, animals performed a baseline odor span test, which was identical to training days. On the next day, they were given a TPO test, in which the TPO was presented at span 4. Male FST animals had significantly lower span performance when the TPO was present. Female FST animals did not demonstrate a significant deficit in span in the presence of the TPO relative to same-sex controls, although this may have been driven by greater variability among FST females. Average latency to dig was significantly higher on the TPO trial for FST animals, likely indicating deliberation or hesitancy to interact with the TPO. Only male FST animals showed significant correlation between latency to dig on the TPO trial and the deficit in span performance in the presence of the TPO, suggesting that the effects of the TPO were not just trial-specific but remained as a distractor throughout the rest of the session.
The effect of the TPO on working memory demonstrates cue reactivity in the current model, which is an important aspect of the PTSD-like phenotype. Cue reactivity is associated with two of the primary PTSD symptom clusters: intrusions and hyperarousal/reactivity. The disruption of task performance, reflected in either increased latency or decreased span, in the presence of the TPO may indicate that rats were distracted by its presence. Attention is central to the working memory mechanism, and distractors generally impair working memory performance (Cowan 2008). A chronic response to trauma may allocate attentional resources to trauma-associated information (Peters et al. 2017). Therefore, working memory deficits following exposure to FST cues may be related to impaired ability to maintain attention on the task when the TPO is present. Indeed, targeting attention maintenance and working memory deficits in individuals diagnosed with PTSD reduced their symptoms (Badura-Brack et al. 2015; McDermott et al. 2016).
Span in the rodent OST can considerably exceed the working memory capacity observed in human tasks, suggesting that the OST may capture a slightly different capacity, and additional work is required to refine rodent span tasks to capture the correlate of clinical working memory more confidently (Dudchenko et al. 2013). OST may capture features of both working memory and recognition memory, which are related by their limited duration maintenance of stimulus representations but are conceptually distinguishable (Dudchenko et al. 2000; Turchi and Sarter 2000). Working memory tasks in clinical settings require the maintenance and recall of stimulus presentation order, and spans range from four to nine items, although higher capacities may be observed if stimulus order need not be maintained (Miller 1956; Cowan 2001; Hahn et al. 2021). Recognition memory relies on the simple distinction between novel and familiar stimuli, and in vivo neural recordings suggest that these broad categorization strategies are indeed used to an extent in the odor span task, a strategy that could support much higher performance (April et al. 2013; De Falco et al. 2019). Working and recognition memories are likely both dependent on attentional allocation and thus may be impacted by trauma history, as attentional deficit and bias have been associated with impairment in both forms of memory (Fosco et al. 2020; Spataro et al. 2022). Future work must expand on the current findings, particularly by examining the mechanisms underlying attentional allocation toward trauma cues and manners through which the resulting attentional bias may be corrected in animal models.
Multivariate prediction of cue reactivity
Animals were tested on elevated zero maze, marble burying, open field, and social interaction to evaluate PTSD-like phenotypes. Clinical presentation of PTSD varies from patient to patient, and being able to incorporate individual differences in symptom combinations is a critical step in rodent PTSD modeling (Richter-Levin et al. 2019). While the tasks used here are regularly used to capture anxiety-related behavior in rodents, it has been suggested that each task may approximate different clinically relevant symptoms associated with PTSD (Verbitsky et al. 2020). PTSD is a multifactorial disorder characterized by clusters of symptoms; therefore, a multiple linear regression model was built to determine whether this array of behavioral indices could predict how trauma cues impact working memory performance through the metric of change in span upon TPO presentation. Significant predictors included working memory span performance during training, latency to dig in the TPO trial, elevated zero maze open zone time, open field center time, and antisocial behavior. For animals with FST history, this combination of behaviors held significant predictive value for the degree to which trauma cue presentation would impact working memory performance. These results suggest that trauma-paired cue reactivity is associated with working memory deficits and relates to a wider profile of PTSD-like symptoms.
Previous studies have used strategies with fewer measures (generally two tests) of anxiety-like behavior to determine the extent of the PTSD-like phenotype in animals. Clinical diagnosis of PTSD requires symptoms across four primary clusters, and male and female symptoms may differ, so the utilization of multiple tests is important to capture individual variation (Carmassi et al. 2014; Gruene et al. 2015; Carragher et al. 2016). Future work will be needed to validate the use of a multiple linear or logistic regression approach to detect susceptible and resilient phenotypes in animal models of PTSD. Additionally, the current study relied on multiple behavioral endpoints, which are inherently variable. Larger sample sizes would provide additional power for detecting significant effects, particularly with respect to sex differences and susceptible phenotypes. To this end, future studies would benefit from utilization of higher-throughput models, such as an automated odor span task (Galizio et al. 2020). Additionally, because male and female rodents exhibit differing anxiety-like behavioral profiles, future work should incorporate anxiety-like tests designed to capture more female-typic behaviors (Meyerson et al. 2006; Gruene et al. 2015).
Conclusion
The current study presents a model for PTSD-like impairment of working memory performance, with different impacts in the absence versus presence of a trauma cue. Additionally, the regression model produced a quantitative assessment of the impact of the trauma-paired cue on span performance. The sample size used in the present study was quite small for use in multiple linear regression. Bootstrapping and k-fold cross-validation methods were used to provide assurance of model robustness, but the risk remains that these models built on small samples will not generalize well. This method should be validated using a larger sample size to provide further confidence about the ability of a comprehensive PTSD-like phenotype to predict reactive impairments in working memory. Alternate PTSD models must validate working memory phenotypes to identify molecular underpinnings of these persistent effects of trauma, as many of the current models have not tested this behavioral phenotype. Identification of these molecular factors may yield treatments with increased efficacy in individuals suffering PTSD-related working memory deficits.
Materials and Methods
Subjects
Three separate cohorts of adult male and female Wistar rats (initial weights male: 253–306 g; female: 146–206 g; Charles River) were obtained at 8 wk of age. Upon arrival, the rats were pair-housed with lights on a 12-h light/dark cycle (lights on at 0600) with ad libitum access to food and water. Subsequently, subjects were individually housed, mildly food-restricted to maintain 90% of free-feeding weight, and handled 5 min each day for 5 d prior to the first experimental task. All experimental procedures were conducted during the light phase of the light cycle. All procedures were approved by the Indiana University–Purdue University Indianapolis School of Science Animal Care and Use Committee.
Experimental overview
The experiment consisted of the odor span task, FST, and susceptibility tests (Fig. 1A). Five weeks is sufficient to fully train animals on the odor span task (De Falco et al. 2019). In order to remain in line with PTSD studies, which assess impact of trauma procedures 2–3 wk after trauma, the initial phases of training (shaping and DNMS, detailed below) were conducted prior to the FST session. DNMS training continued beyond the FST session for animals that had not yet met criterion at the time of the FST session. All behavioral apparatus were cleaned with water between same-sex subjects and with 70% ethanol between sexes to minimize odor distractions. Prior to each anxiety-related behavioral test, animals were given an acclimation period in the antechamber of the testing room. White noise (∼54 dB) was played during all behavioral tests, and tests were run under normal house lights unless otherwise specified. Behavioral tests were recorded via an overhead camera and scored by an observer blind to experimental condition.
Odor span task
The odor span apparatus featured a gray textured plastic platform (91.5 cm, round, 30.5-cm border, 79 cm above the ground). A round platform was selected for this task to create equal distance between center of the arena and each odor (MacQueen et al. 2011). Odors (0.5 g of dried spice; allspice, anise seed, basil, caraway, celery seed, cinnamon, cloves [0.1 g], cocoa, coffee, cumin, dill, fennel seed, garlic, ginger, lemon, marjoram, nutmeg, onion powder, orange, oregano, paprika, rosemary, sage, and thyme) were mixed in 100 g of Premium Play Sand (Quikrete Cement Products) in 3.25-oz plastic cups (5 cm tall, 7.6 cm wide). Coriander was also used in the study as a distinct trauma-paired odor. It was only present during the footshock session and during the TPO test session of OST and was otherwise never encountered during OST. Twenty-four Velcro attachment points were equally spaced along the perimeter of the platform, and cups were randomly attached to one of these points in each trial to prevent the use of spatial strategies. Animals were trained 5 d each week in either a morning group or an afternoon group, with sexes and conditions represented equally across groups. Methods for the OST followed those of Davies et al. (2013).
Initial training
Initial training involved rats learning to dig for a cereal reward (one-quarter of a Kellogg's Froot Loop) in unscented sand (3.25-oz cups containing 100 g of sand). For each trial in this phase, a single cup of unscented sand was placed in a random position on the platform. After the subject retrieved the reward or following a maximum response time of 2 min, the subject was removed from the platform and placed in a separate intertrial box for a 40-sec period. The experimenter then moved the cup to a new location before the next trial. The reward was first placed on the surface of the sand and then buried progressively further in the sand. Animals were trained until they would consistently dig for and retrieve a fully buried reward from the unscented sand. Initial training took ∼3–4 d.
Delayed non-match to sample
Rats were next trained on a DNMS task. In this phase, rats were first given a sample trial in which they were presented with a cup of scented sand randomly placed on the platform. After retrieving the reward, rats were moved to the intertrial interval box for a 40-sec delay period, during which time the experimenter moved the sample cup to a new location and randomly placed a second bowl containing a different odor and a reward on the platform. The animals were then returned to the platform for their choice trial and allowed to freely sample (sniff) the cups. A choice was determined by the animal contacting the sand with their nose or paws. An error was scored if the rats selected the sample odor rather than the novel odor. The animals were given eight DNMS choice trials each day until they selected the novel odor on six out of eight choice trials for three consecutive days. DNMS performance at the end of experiment week 2, reported as average proportion correct over the most recent 3 d, was used to counterbalance animals into control and FST groups, although animals continued training at this phase after the footshock session until they met criterion. DNMS training took ∼1.5 wk.
Odor span task
Following DNMS, animals were trained on the odor span task (Fig. 1B). These trials were conducted identically to the DNMS task, except cups containing novel odors were added following each correct choice until the rat made an incorrect selection or omitted a response, resulting in an increasing number of odors present in the arena. Span for a given trial is defined as the number of choice trials completed successfully, which is equivalent to the total number of cups present minus one. Each rat performed a maximum of three consecutive spans per day, with no trials beginning >15 min into the session. Maximum span is reported for each day. Animals that reached a stable level of high performance (span > 17 for two consecutive days) were reduced to training three times per week to reduce the likelihood of overtraining.
Following 3 wk of OST training, the effect of trauma cue presentation on working memory performance was evaluated. Animals were given a baseline testing day, which was identical to the OST training days except that following an incorrect choice, they returned to their home cage for ∼30 min between spans while other rats were tested. The following day, on span 4 (five cups present on the table), the TPO (coriander) was presented for the first time as a target odor in the OST task. Latency to interact with the coriander as well as maximum span achieved while coriander was on the platform were recorded and evaluated.
Several probe trials were conducted throughout training to confirm that subjects were using cup odor to solve the task and to ensure coriander performance could not be attributed to novelty. The first was a nonbaited trial to confirm animals were not pursuing the smell of the food reward. At span 4 (five cups on the table), a target cup was presented that did not contain a food reward. Upon contact with the correct cup, a food reward was dropped onto the surface of the sand for the animals to consume. The second probe trial was a cup change trial, in which the cups at span 1 (two cups on the table) were swapped out with clean cups of the same scents. Successful completion of this trial was indicative that the animals were not marking the cups as a strategy to identify previously sampled cups (Davies et al. 2017). Swapping out to clean cups later in the span is not feasible while maintaining a 40-sec intertrial interval. Nonbaited and cup changed trials were conducted once per week during the 3-wk odor span training period. Last, a novel odor probe trial was run with two of the three cohorts to ensure that animals could successfully perform the task when presented with a completely novel odor, confirming that any observed reactions on the TPO test day were not confounded by reactivity to task-novel odors. The novel odor trial was conducted during the final week of odor span training. Performance across all animals on the nonbaited probe was 90%, on the cup change probe was 91%, and on the novel odor probe was 96%.
Footshock trauma
Two weeks into the training procedure, animals underwent FST. They were provided 2 d of brief acclimation to the footshock chambers prior to the trauma day (<20 min). Animals were counterbalanced into FST and control groups based on DNMS training performance and initial elevated zero maze performance to maximize chances of equalizing cognitive ability and baseline anxiety-like behavior between the groups. Acclimation and footshock sessions were run in illuminated operant boxes (Med-Associates) with no additional cues preceding footshock. On the day of the FST, coriander was placed in the operant boxes, serving as a TPO cue, which subsequently was presented in the odor span test to assess the impact of trauma cues on working memory performance. The FST group had 5 min in the box before footshocks began, followed by 20 uncued shocks at 0.8 mA (1-sec duration, VI-40 schedule, resulting in ∼17-min total footshock session length). Control animals were placed in the boxes with coriander scent present but did not experience any footshock. Similar parameters elicited conditioned fear responses that were sustained 3 wk after stressor, indicating its ability to generate a PTSD-like chronic stress response (Wellman et al. 2014; Bienvenu et al. 2021). Animals were shocked one at a time and then immediately brought to the odor span room for that day's training and assessment of acute stress effects on DNMS performance. Each footshock chamber was only used for one animal per cohort.
Elevated zero maze
Elevated zero maze performance was evaluated the day following the final odor span training day. This test shows an animal's response to the conflict between exploration of a novel environment and exposure to an unsafe (open, unwalled) environment and, while frequently used to assess general anxiety-like behavior, may approximate the avoidance cluster of PTSD-like symptoms (Shepherd et al. 1994; Verbitsky et al. 2020). The maze consisted of a circular pathway (105-cm maze diameter, 10-cm track), elevated ∼50 cm above the floor, with alternating walled and open zones (two walled and two open zones) evenly dividing the circumference of the track. This circular version of the maze shows fewer sex differences than the plus-shaped version of the maze (Braun et al. 2011; Tucker and McCabe 2017). Testing was conducted in dim lighting conditions (∼0 lux in closed zones, 6 lux on open zones). Rats were placed on the maze facing the entrance of a closed zone and allowed to freely explore for 5 min. Overhead video of the session was captured and scored for time and entries into the open zone, defined by all four paws entering a zone.
Marble burying
The marble burying test is frequently used to assess obsessive-compulsive phenotypes or anxiety-related behavior but is also considered to approximate the PTSD-like symptom cluster of alterations in arousal and reactivity and was conducted on the day following elevated zero maze testing (Mikics et al. 2008; Kedia and Chattarji 2014; Verbitsky et al. 2020). Marble burying is conducted most successfully in mice, but the tendency to express neophobia through burying behaviors is common among rodent species (Himanshu et al. 2020). Following home cage acclimation to the testing room (normal light, ∼100 lux), each animal was moved into a standard shoebox cage with 5-cm packed-down bedding and allowed to freely explore for 15 min. Next, 20 marbles (2-cm diameter) were arranged in a 4 × 5 grid across one-half of the testing box (De Brouwer and Wolmarans 2018). The rats were placed on the marble-free side of the testing box and allowed 30 min to explore the testing box and interact with marbles, after which they were returned to their home cage, and the number of marbles buried was counted. A marble was considered buried if more than one-half was under the bedding.
Open field
The open field test provides an index of anxiety-like behavior, as the center of the field is an exposed and unprotected region and may capture PTSD-like symptoms of avoidance and negative alterations in mood (Katz et al. 1981; Gould et al. 2009; Verbitsky et al. 2020). This test was performed the day following TPO testing in the odor span task. The open field apparatus was a square arena surrounded by high walls (63 × 63 × 50 cm), and testing was performed under moderately dim (40-lux) illumination. The floor of the apparatus was divided into sections to quantify position relative to the walls versus center and movement via line crossings. At the beginning of the test, each rat was placed into the center of the open field arena and allowed to freely explore for 10 min. Overhead video of the session was captured and scored for time in the center of the open field and for overall locomotor activity, measured by number of zone crossings multiplied by the size of each zone.
Social interaction
This test examines whether rats show deficits in social interactions with an unknown, same-sex conspecific, and may approximate the PTSD-like symptom cluster of negative alteration in mood (File and Seth 2003; Verbitsky et al. 2020). Open field testing was performed the day prior to social interaction testing and served as the acclimation period for the test apparatus. Social interaction was tested under the same lighting conditions as the open field test (∼40 lux in the center of the arena). On the test day, rats were paired across conditions (one control and one FST) with novel partners. Social interaction most commonly involves pairing animals of identical conditions or pairing with an experimentally naïve animal. However, due to hypothesized individual differences in trauma response, animals were paired across conditions and scored individually (Hindley et al. 1985). One rat from each pair was randomly assigned to have a marked tail to allow for individual behavioral scoring during the test. At the beginning of the test, rats were placed in opposite corners of the arena and allowed to interact freely for 5 min. All behaviors initiated by the experimental rat were scored according to the following categories: nonsocial, prosocial, and antisocial behaviors toward the other rat.
One day after the social interaction test, animals were euthanized using isoflurane to induce rapid sedation, followed by decapitation.
Statistical analysis
Statistical significance was evaluated at P < 0.05, and graphed data are expressed as mean ± standard error of the mean (SEM) with individual data points overlaid, unless otherwise noted. Significant results are accompanied by effect size, reported by Cohen's d, calculated as the mean difference between groups divided by the pooled standard deviation. Change scores are used to assess effects of acute stress and presentation of the TPO while preserving baseline individual differences in the data and are calculated as difference between experimental and baseline behavior divided by baseline behavior. Two-way ANOVAs were used to compare group differences based on the factors of sex and condition (control or FST). Z-score normalization was performed within each group to preserve group differences and facilitate data collapse across sex. Pearson correlation and multiple linear regression were used to assess the significance of bivariate and multivariate linear associations. Multiple linear regression was used to determine the linear combination of the PTSD-like symptoms that correlate maximally with the TPO-induced deficits in working memory. The assumptions of linear relationships between variables, normal distribution, and no multicollinearity were met, but due to the small sample size, bootstrapping was performed to provide confidence that results were not negatively impacted by the sample size. Bootstrapping (2000 bootstrap replicates) and repeated fivefold cross-validation techniques (three replicates) were used to affirm robustness of the multiple regression model. The Shapiro–Wilk test was used to confirm normality of variables. Latency data were logarithmically transformed to achieve normal distribution. One control female was omitted from data analyses related to the trauma-paired odor test because of a time-out response omission (Fig. 4). Video recording of all behavior was captured (ANY-Maze, Stoelting Co.), and behavioral scoring was conducted in BORIS (Friard and Gamba 2016) by raters blind to condition and sex of animals. Statistical testing and graphing were performed in GraphPad Prism version 9.3.1 (GraphPad Software) and R (R Core Team 2020).
Competing interest statement
The authors declare no competing interests.
Acknowledgments
We thank Sydney T. Cook and M. Paloma Zacarias for technical assistance. This work was supported by Indiana University—Purdue University Indianapolis.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053620.122.
References
- April LB, Bruce K, Galizio M. 2013. The magic number 70 (plus or minus 20): variables determining performance in the rodent odor span task. Learn Motiv 44: 143–158. 10.1016/j.lmot.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi Z, Albrecht A, Richter-Levin A, Saha R, Richter-Levin G. 2016. Behavioral profiling as a translational approach in an animal model of posttraumatic stress disorder. Neurobiol Dis 88: 139–147. 10.1016/j.nbd.2016.01.012 [DOI] [PubMed] [Google Scholar]
- Badura-Brack AS, Naim R, Ryan TJ, Levy O, Abend R, Khanna MM, McDermott TJ, Pine DS, Bar-Haim Y. 2015. Effect of attention training on attention bias variability and PTSD symptoms: randomized controlled trials in Israeli and U.S. combat veterans. Am J Psychiatry 172: 1233–1241. 10.1176/appi.ajp.2015.14121578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahmani Z, Clark K, Merrikhi Y, Mueller A, Pettine W, Vanegas MI, Moore T, Noudoost B. 2019. Prefrontal contributions to attention and working memory. Curr Top Behav Neurosci 41: 129–153. 10.1007/7854_2018_74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderston NL, Hale E, Hsiung A, Torrisi S, Holroyd T, Carver FW, Coppola R, Ernst M, Grillon C. 2017. Threat of shock increases excitability and connectivity of the intraparietal sulcus. Elife 6: e23608. 10.7554/eLife.23608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu T, Dejean C, Jercog D, Aouizerate B, Lemoine M, Herry C. 2021. The advent of fear conditioning as an animal model of post-traumatic stress disorder: learning from the past to shape the future of PTSD research. Neuron 109: 2380–2397. 10.1016/j.neuron.2021.05.017 [DOI] [PubMed] [Google Scholar]
- Block SR, Liberzon I. 2016. Attentional processes in posttraumatic stress disorder and the associated changes in neural functioning. Exp Neurol 284: 153–167. 10.1016/j.expneurol.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Branch CL, Galizio M, Bruce K. 2014. What–where–when memory in the rodent odor span task. Learn Motiv 47: 18–29. 10.1016/j.lmot.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. 2011. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague–Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol Biochem Behav 97: 406–415. 10.1016/j.pbb.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmassi C, Akiskal HS, Bessonov D, Massimetti G, Calderani E, Stratta P, Rossi A, Dell'Osso L. 2014. . Gender differences in DSM-5 versus DSM-IV-TR PTSD prevalence and criteria comparison among 512 survivors to the L'Aquila earthquake. J Affect Disord 160: 55–61. 10.1016/j.jad.2014.02.028 [DOI] [PubMed] [Google Scholar]
- Carragher N, Sunderland M, Batterham PJ, Calear AL, Elhai JD, Chapman C, Mills K. 2016. Discriminant validity and gender differences in DSM-5 posttraumatic stress disorder symptoms. J Affect Disord 190: 56–67. 10.1016/j.jad.2015.09.071 [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar M. 2003. The relevance of differential response to trauma in an animal model of posttraumatic stress disorder. Biol Psychiatry 53: 463–473. 10.1016/S0006-3223(02)01909-1 [DOI] [PubMed] [Google Scholar]
- Cohen H, Kozlovsky N, Alona C, Matar MA, Joseph Z. 2012. Animal model for PTSD: from clinical concept to translational research. Neuropharmacology 62: 715–724. 10.1016/j.neuropharm.2011.04.023 [DOI] [PubMed] [Google Scholar]
- Cowan N. 2001. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci 24: 87–185. 10.1017/S0140525X01003922 [DOI] [PubMed] [Google Scholar]
- Cowan N. 2008. What are the differences between long-term, short-term, and working memory? Prog Brain Res 169: 323–338. 10.1016/S0079-6123(07)00020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RC, Olatunji BO. 2017. Linking attentional control and PTSD symptom severity: the role of rumination. Cogn Behav Ther 46: 421–431. 10.1080/16506073.2017.1286517 [DOI] [PubMed] [Google Scholar]
- Davies DA, Molder JJ, Greba Q, Howland JG. 2013. Inactivation of medial prefrontal cortex or acute stress impairs odor span in rats. Learn Mem 20: 665–669. 10.1101/lm.032243.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DA, Greba Q, Selk JC, Catton JK, Baillie LD, Mulligan SJ, Howland JG. 2017. Interactions between medial prefrontal cortex and dorsomedial striatum are necessary for odor span capacity in rats: role of GluN2B-containing NMDA receptors. Learn Mem 24: 524–531. 10.1101/lm.045419.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brouwer G, Wolmarans W. 2018. Back to basics: a methodological perspective on marble-burying behavior as a screening test for psychiatric illness. Behav Process 157: 590–600. 10.1016/j.beproc.2018.04.011 [DOI] [PubMed] [Google Scholar]
- De Falco E, An L, Sun N, Roebuck AJ, Greba Q, Lapish CC, Howland JG. 2019. The rat medial prefrontal cortex exhibits flexible neural activity states during the performance of an odor span task. eNeuro 6: ENEURO.0424-18.2019. 10.1523/ENEURO.0424-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Heman KL, Rose GM. 1999. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus 9: 542–552. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. 2004. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev 28: 699–709. 10.1016/j.neubiorev.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. 2000. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci 20: 2964–2977. 10.1523/JNEUROSCI.20-08-02964.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. 2013. Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev 37: 2111–2124. 10.1016/j.neubiorev.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Dutcher JM, Creswell JD. 2018. The role of brain reward pathways in stress resilience and health. Neurosci Biobehav Rev 95: 559–567. 10.1016/j.neubiorev.2018.10.014 [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. 2003. A review of 25 years of the social interaction test. Eur J Pharmacol 463: 35–53. 10.1016/S0014-2999(03)01273-1 [DOI] [PubMed] [Google Scholar]
- Fosco WD, Kofler MJ, Groves NB, Chan E, Raiker JS Jr. 2020. Which ‘working’ components of working memory aren't working in youth with ADHD? J Abnorm Child Psychol 48: 647–660. 10.1007/s10802-020-00621-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. 2012. Neural mechanisms of stress resilience and vulnerability. Neuron 75: 747–761. 10.1016/j.neuron.2012.08.016 [DOI] [PubMed] [Google Scholar]
- Friard O, Gamba M. 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7: 1325–1330. 10.1111/2041-210X.12584 [DOI] [Google Scholar]
- Galizio M, Mason MG, Bruce K. 2020. Successive incrementing non-matching-to-samples in rats: an automated version of the odor span task. J Exp Anal Behav 114: 248–265. 10.1002/jeab.619 [DOI] [PubMed] [Google Scholar]
- Gould TD, Dao DT, Kovacsics CE. 2009. The open field test BT. In Mood and anxiety related phenotypes in mice: characterization using behavioral tests (ed. Gould TD), pp. 1–20. Humana Press, New York. [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM. 2015. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4: e11352. 10.7554/eLife.11352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guida A, Gobet F, Tardieu H, Nicolas S. 2012. How chunks, long-term working memory and templates offer a cognitive explanation for neuroimaging data on expertise acquisition: a two-stage framework. Brain Cogn 79: 221–244. 10.1016/j.bandc.2012.01.010 [DOI] [PubMed] [Google Scholar]
- Hahn LA, Balakhonov D, Fongaro E, Nieder A, Rose J. 2021. Working memory capacity of crows and monkeys arises from similar neuronal computations. Elife 10: e72783. 10.7554/eLife.72783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, Stein DJ. 2003. Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res 983: 97–107. 10.1016/S0006-8993(03)03033-6 [DOI] [PubMed] [Google Scholar]
- Himanshu, Dharmila, Sarkar D, Nutan. 2020. A review of behavioral tests to evaluate different types of anxiety and anti-anxiety effects. Clin Psychopharmacol Neurosci 18: 341–351. 10.9758/cpn.2020.18.3.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley SW, Hobbs A, Paterson IA, Roberts MH. 1985. The effects of methyl β-carboline-3-carboxylate on social interaction and locomotor activity when microinjected into the nucleus raphé dorsalis of the rat. Br J Pharmacol 86: 753–761. 10.1111/j.1476-5381.1985.tb08955.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honzel N, Justus T, Swick D. 2014. Posttraumatic stress disorder is associated with limited executive resources in a working memory task. Cogn Affect Behav Neurosci 14: 792–804. 10.3758/s13415-013-0219-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. 1981. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev 5: 247–251. 10.1016/0149-7634(81)90005-1 [DOI] [PubMed] [Google Scholar]
- Kedia S, Chattarji S. 2014. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J Neurosci Methods 233: 150–154. 10.1016/j.jneumeth.2014.06.012 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048–1060. 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Khayyer Z, Saberi Azad R, Torkzadeh Arani Z, Jafari Harandi R. 2021. Examining the effect of stress induction on auditory working memory performance for emotional and non-emotional stimuli in female students. Heliyon 7: e06876. 10.1016/j.heliyon.2021.e06876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. 2013. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Traum Stress 26: 537–547. 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. 2009. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist 15: 540–548. 10.1177/1073858409333072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara AH, Wallis JD. 2015. The role of prefrontal cortex in working memory: a mini review. Front Syst Neurosci 9: 173. 10.3389/fnsys.2015.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen SE, Lotfi S, Bennett KP, Larson CL, Dean-Bernhoft C, Lee HJ. 2019. A pilot randomized trial of a dual n-back emotional working memory training program for veterans with elevated PTSD symptoms. Psychiatry Res 275: 261–268. 10.1016/j.psychres.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Krstov M, Young EA. 1997. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 22: 443–453. 10.1016/S0306-4530(97)00044-9 [DOI] [PubMed] [Google Scholar]
- Lucas M, Ilin Y, Anunu R, Kehat O, Xu L, Desmedt A, Richter-Levin G. 2014. Long-term effects of controllability or the lack of it on coping abilities and stress resilience in the rat. Stress 17: 423–430. 10.3109/10253890.2014.930430 [DOI] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. 2011. Effects of dizocilpine (MK801) on olfactory span in rats. Neurobiol Learn Mem 95: 57–63. 10.1016/j.nlm.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott TJ, Badura-Brack AS, Becker KM, Ryan TJ, Bar-Haim Y, Pine DS, Khanna MM, Heinrichs-Graham E, Wilson TW. 2016. Attention training improves aberrant neural dynamics during working memory processing in veterans with PTSD. Cogn Affect Behav Neurosci 16: 1140–1149. 10.3758/s13415-016-0459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson BJ, Augustsson H, Berg M, Roman E. 2006. The concentric square field: a multivariate test arena for analysis of explorative strategies. Behav Brain Res 168: 100–113. 10.1016/j.bbr.2005.10.020 [DOI] [PubMed] [Google Scholar]
- Mikics E, Baranyi J, Haller J. 2008. Rats exposed to traumatic stress bury unfamiliar objects–a novel measure of hyper-vigilance in PTSD models? Physiol Behav 94: 341–348. 10.1016/j.physbeh.2008.01.023 [DOI] [PubMed] [Google Scholar]
- Miller GA. 1956. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev 63: 81–97. 10.1037/h0043158 [DOI] [PubMed] [Google Scholar]
- Moran TP. 2016. Anxiety and working memory capacity: a meta-analysis and narrative review. Psychol Bull 142: 831–864. 10.1037/bul0000051 [DOI] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Elsworth JD. 2000. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bull 52: 519–523. 10.1016/S0361-9230(00)00290-2 [DOI] [PubMed] [Google Scholar]
- Murphy S, Elklit A, Chen YY, Ghazali SR, Shevlin M. 2019. Sex differences in PTSD symptoms: a differential item functioning approach. Psychol Trauma 11: 319–327. 10.1037/tra0000355 [DOI] [PubMed] [Google Scholar]
- Nejati V, Salehinejad MA, Sabayee A. 2018. Impaired working memory updating affects memory for emotional and non-emotional materials the same way: evidence from post-traumatic stress disorder (PTSD). Cogn Process 19: 53–62. 10.1007/s10339-017-0837-2 [DOI] [PubMed] [Google Scholar]
- Peters A, McEwen BS, Friston K. 2017. Uncertainty and stress: why it causes diseases and how it is mastered by the brain. Prog Neurobiol 156: 164–188. 10.1016/j.pneurobio.2017.05.004 [DOI] [PubMed] [Google Scholar]
- R Core Team. 2020. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Richter-Levin G, Sandi C. 2021. Labels matter: is it stress or is it trauma? Transl Psychiatry 11: 385. 10.1038/s41398-021-01514-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Stork O, Schmidt MV. 2019. Animal models of PTSD: a challenge to be met. Mol Psychiatry 24: 1135–1156. 10.1038/s41380-018-0272-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Andrade G, James BM, Kendrick KM. 2005. Neural encoding of olfactory recognition memory. J Reprod Dev 51: 547–558. 10.1262/jrd.17031 [DOI] [PubMed] [Google Scholar]
- Saunders N, Downham R, Turman B, Kropotov J, Clark R, Yumash R, Szatmary A. 2015. Working memory training with tDCS improves behavioral and neurophysiological symptoms in pilot group with post-traumatic stress disorder (PTSD) and with poor working memory. Neurocase 21: 271–278. 10.1080/13554794.2014.890727 [DOI] [PubMed] [Google Scholar]
- Schoofs D, Pabst S, Brand M, Wolf OT. 2013. Working memory is differentially affected by stress in men and women. Behav Brain Res 241: 144–153. 10.1016/j.bbr.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, Krystal JH, Schweinsburg BC. 2015. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull 141: 105–140. 10.1037/a0038039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GA, Liu MC, Tahir NB, Zabder NK, Song Y, Greba Q, Howland JG. 2020. Roles of the medial prefrontal cortex, mediodorsal thalamus, and their combined circuit for performance of the odor span task in rats: analysis of memory capacity and foraging behavior. Learn Mem 27: 67–77. 10.1101/lm.050195.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. 1994. Behavioural and pharmacological characterisation of the elevated ‘zero-maze’ as an animal model of anxiety. Psychopharmacology (Berl) 116: 56–64. 10.1007/BF02244871 [DOI] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, Yonelinas AP. 2016. The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci Biobehav Rev 68: 651–668. 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. 2006. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071: 67–79. 10.1196/annals.1364.007 [DOI] [PubMed] [Google Scholar]
- Spataro P, Mulligan NW, Saraulli D, Rossi-Arnaud C. 2022. The attentional boost effect facilitates the encoding of contextual details: new evidence with verbal materials and a modified recognition task. Attent Percept Psychophys 84: 1489–1500. 10.3758/s13414-022-02509-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LB, McCabe JT. 2017. Behavior of male and female C57BL/6J mice is more consistent with repeated trials in the elevated zero maze than in the elevated plus maze. Front Behav Neurosci 11: 13. 10.3389/fnbeh.2017.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trow JE, Jones AM, McDonald RJ. 2019. Comparison of the effects of repeated exposures to predictable or unpredictable stress on the behavioural expression of fear in a discriminative fear conditioning to context task. Physiol Behav 208: 112556. 10.1016/j.physbeh.2019.05.017 [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. 2000. Cortical cholinergic inputs mediate processing capacity: effects of 192 IgG-saporin-induced lesions on olfactory span performance. Eur J Neurosci 12: 4505–4514. [PubMed] [Google Scholar]
- Verbitsky A, Dopfel D, Zhang N. 2020. Rodent models of post-traumatic stress disorder: behavioral assessment. Transl Psychiatry 10: 132. 10.1038/s41398-020-0806-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D, Saint Aubin J, Altman K, Gallant G. 2021. Sex differences in verbal working memory: a systematic review and meta-analysis. Psychol Bull 147: 352–398. 10.1037/bul0000320 [DOI] [PubMed] [Google Scholar]
- Wellman LL, Fitzpatrick ME, Machida M, Sanford LD. 2014. The basolateral amygdala determines the effects of fear memory on sleep in an animal model of PTSD. Exp Brain Res 232: 1555–1565. 10.1007/s00221-014-3850-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. 2003. Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem 10: 326–336. 10.1101/lm.62903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward SH, Jamison AL, Gala S, Holmes TH. 2017. Canine companionship is associated with modification of attentional bias in posttraumatic stress disorder. PLoS One 12: e0179912. 10.1371/journal.pone.0179912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ST, Shi Y, Wang Q, Peng JY, Li BM. 2014. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol Brain 7: 61. 10.1186/s13041-014-0061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Antelman SM. 1993. Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry 33: 479–486. 10.1016/0006-3223(93)90001-T [DOI] [PubMed] [Google Scholar]