Shigella causes bacillary dysentery, a disease provoking severe bloody and mucous diarrhea. When the pathogen reaches the colon, bacteria translocate through the epithelial barrier by way of the M cells that overlay the solitary lymphoid nodules (71, 72, 91). Once it reaches the underlying M cells, Shigella infects the resident macrophages and induces cell death. The infected macrophages release large amounts of interleukin-1β, which finally leads to a strong inflammatory response (101). Meanwhile, a bacterium released from the macrophages enters enterocytes via the basolateral surface by directing membrane ruffling and macropinocytosis. The bacterium is surrounded by a phagocytic vacuole; however, it immediately disrupts the membrane to escape into the cytoplasm, where it can multiply and move by inducing actin polymerization at one pole of the bacterium, allowing intracellular spread within the cytoplasm as well as into adjacent epithelial cells (3, 44). In response to bacterial infection, epithelial cells are elicited to produce proinflammatory cytokines, further promoting local inflammation in the colon. Thus, the ability of Shigella spp. to infect host cells, including the continuous intra- and intercellular spreading, is essential for leading to bacillary dysentery.
PROLOGUE TO THE DISCOVERY OF SHIGELLA MOVEMENT IN MAMMALIAN CELLS
The movement of intracellular Shigella was first reported by Ogawa et al. in 1968 (56). By exploiting phase-contrast cinemicrography, they indicated that intracellular Shigella flexneri moved conspicuously independent of the movement of the cellular organelles, and the bacterial movement had polarity. They correctly described that during bacterial movement, one bacterium always remained at the “head” end even when the direction of movement was reversed. During bacterial division in the infected cell, the newly separated ends of the daughter bacterial cells become the head. In that study, it was also demonstrated that the motility of bacteria can be blocked by adding tetracycline into the extracellular medium, suggesting that a bacterial product(s) is required for the movement. Furthermore, it was noted that motile bacteria are occasionally seen within the protrusions extending from the host cell surface. Beyond doubt, this pioneer study led to the discovery of the virG (also called icsA) gene on the large plasmid of S. flexneri required for cell-to-cell spreading (44) and actin-based motility in mammalian cells (3).
A similar intracellular actin-based movement was reported for Listeria monocytogenes by Tilney and Portnoy (87), who indicated that the pathogen is capable of lysing its phagocytic vacuole, moving intracellularly, and spreading from cell to cell. Later, spotted fever group Rickettsia and vaccinia virus were also found to have the capacity to evoke the actin polymerization required for movement in the host cell cytoplasm (9, 25, 86). Recently, molecular and cell biological approaches to the study of the linkages between the bacterial (or viral) factors and host cellular ligands have shed light on the molecular basis of actin assembly directed by intracellular pathogens. Here, we summarize and discuss the present model for the actin-based motility of Shigella in mammalian cells.
SHIGELLA VIRG (ICSA) MEDIATES ACTIN-BASED MOTILITY
Asymmetric distribution of VirG (IcsA) and its functional domains.
The actin-based motility of Shigella or enteroinvasive Escherichia coli is dependent on VirG encoded by the virG gene (3, 39, 44). VirG is a surface-exposed outer membrane protein composed of 1,102 amino acids which contains three distinctive domains, the N-terminal signal sequence (residues 1 to 52), the 706-amino acid α-domain (residues 53 to 758), and the 344-amino acid C-terminal β-core (residues 759 to 1102) (22, 39, 81). The α-domain is exposed on the surface of bacteria, while the β-core is embedded in the outer membrane, forming a membrane pore (18, 81). The α-domain is translocated through the membrane pore onto the bacterial surface, implying that VirG is a typical autotransporter protein as represented by the immunoglobulin A (IgA) protease of Neisseria gonorrhoeae (31, 61).
The asymmetric distribution of VirG along the bacterial body is a prerequisite for the polar movement of Shigella in mammalian cells, including bacterial spreading between epithelial cells (22, 64, 70, 84). Although the mechanisms are still speculative, it has recently been proposed that unipolar localization of VirG results from its direct targeting to the pole following diffusion laterally in the outer membrane (78). Several factors, including its own VirG portion, have been implicated in the establishment or maintenance of the asymmetric distribution. The N-terminal two-thirds of the VirG α-domain, which contains six glycine-rich repeats, is essential for mediating actin assembly of Shigella in host cells, since the domain serves to interact with host proteins such as vinculin and neural Wiskott-Aldrich syndrome protein (N-WASP) (see below) (82, 84). The C-terminal one-third of the α-domain is required for VirG to distribute asymmetrically, since S. flexneri expressing a VirG mutant with a deletion of this region is unable to display polar movement and is surrounded by an actin cloud in infected cells (84). Lipopolysaccharide (LPS) plays a role in either the establishment or maintenance of VirG at one pole of Shigella organisms. A number of genes involved in the biosynthesis of LPS have been shown to affect the localization of VirG (57, 58, 64, 69, 70). Indeed, removal of the O side chain results in an aberrant localization of VirG, causing a circumferential distribution over the whole bacterial body (64, 69, 70). SopA (also called IcsP), an outer membrane protease, has also been indicated to be involved in the asymmetric distribution of VirG by cleaving laterally diffused VirG protein along the bacterial body (12, 73). In addition, the absence of OmpT, another outer membrane protease encoded by the ompT gene, is crucial for VirG to be maintained on the cell surface, since OmpT specifically cleaves at Arg758-Arg759 of VirG, causing degradation of the α-domain of VirG on bacteria (18, 52). In fact, Shigella and enteroinvasive E. coli strains lack the ompT region, thus ensuring that the VirG α-domain is expressed and maintained on the bacterial surface (52).
Host cellular ligands for VirG.
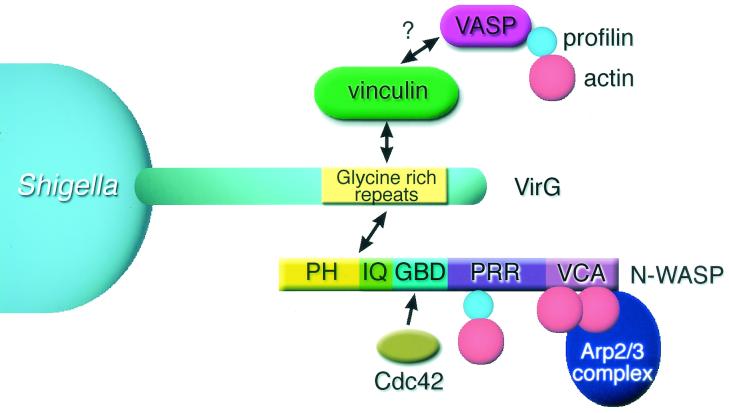
Vinculin, a protein linking focal adhesions and actin filaments, interacts directly with a portion of the VirG α-domain spanning residues 103 to 508 (Fig. 1) (84). The function of vinculin in cells is regulated by phosphatidylinositol 4,5-phosphate [PtdIns(4,5)P2]. In the inactive state, the N-terminal globular head domain interacts with the C-terminal elongated tail domain, and this interaction is disrupted by the binding of PtdIns(4,5)P2 (20, 27, 93). The exposed head and tail domains become activated to interact with other molecules. It has been indicated that vinculin, as well as the actin comet tail generated from motile bacteria in infected cells, is recruited to the Shigella surface (84). A later study revealed that the recruited vinculin is cleaved, leaving the head portion, which interacts with VirG, vasodilator-stimulating phosphoprotein (VASP), and profilin (35). Thus, the complex formed in the vicinity of the bacterium is proposed to contribute to enhancing barbed-ends growth of actin filaments. However, the role of vinculin in Shigella motility remains controversial. In a reconstituted actin tail assay, vinculin was not shown to be required for the motility of E. coli expressing VirG (41). Another group reported that Shigella is still motile with actin comet tails in a mouse embryonic carcinoma cell line, 5.51, assumed to be vinculin deficient (21). However, other investigators reported that the 5.51 cells still express adequate amounts of truncated vinculin and can support Shigella motility (76). It has also been demonstrated that microinjection of the vinculin head portion into Shigella-infected cells accelerates bacterial motility (35). In fact, the speed at which E. coli expressing VirG induces formation of the actin tail in vinculin-depleted Xenopus egg extracts is significantly decreased to less than 30% of the level in the original extracts (T. Suzuki, unpublished data). Although the reason for the controversial results is unclear, vinculin may contribute to actin assembly induced by Shigella such as through interaction with VASP (Fig. 1) (5). Alternatively, existing actin filaments bound by vinculin at the bacterial surface may facilitate actin nucleation mediated by the Arp2/3 complex interacting with the VirG–N-WASP complex (45).
FIG. 1.
Current model for VirG-induced actin polymerization on Shigella in infected mammalian cells. The surface-exposed VirG α-domain recruits vinculin and N-WASP through binding to the glycine-rich repeats of VirG. Vinculin could then interact with actin filaments and VASP which may contribute to actin polymerization (45). The activity of Cdc42 facilitates the formation of the VirG–N-WASP complex, and then the complex could achieve an activating state in which the exposed VCA domain stimulates the Arp2/3 complex to induce rapid actin polymerization. Profilin could promote the actin filament growth by interacting with both N-WASP and monomer actin. PH, plekstrin homology domain; IQ, calmodulin binding domain; GBD, GTPase binding domain that binds Cdc42; PRR, proline-rich region; V, verprolin homology domain; C, cofilin homology domain; A, acidic amino acid segment.
N-WASP is the critical cellular ligand for Shigella movement.
N-WASP is a member of the WASP family, which includes human WASP (10, 46), Saccharomyces cerevisiae WASP-like protein Las17p (also called Bee1p) (38, 40), and more distantly related WAVE (also called Scar) proteins (2, 48, 80). N-WASP and WASP possess several distinctive domains as follows: a pleckstrin homology (PH) domain that binds PtdIns(4,5)P2, a calmodulin binding IQ motif, a GTPase binding domain (GBD) that binds Cdc42, a proline-rich region (PRR), a G-actin-binding verprolin homology (V) domain, a domain (C) with homology to the actin-depolymerizing protein cofilin, and finally a C-terminal acidic (A) segment (Fig. 1) (46). The C-terminal VCA domain is indicated to mediate the interaction with the Arp2/3 complex, by which the Arp2/3 complex is activated, thus mediating actin polymerization (67). In Shigella-infected cells, N-WASP accumulates at the pole of the intracellular bacterium, assembling an actin comet tail (13, 82). Functional assays using ectopic expression of dominant-negative N-WASP in mammalian cells or immunodepletion in Xenopus egg extracts revealed that N-WASP is an essential host component for mediating the actin-based motility of intracellular Shigella (82). Importantly, none of the WASP family proteins associate with the surface of intracellular L. monocytogenes, including the actin tails (Suzuki, unpublished data). The binding of Shigella VirG to WASP family proteins is limited to only N-WASP, for which the sequence composed of the PH-IQ region of N-WASP seems to serve as the critical ligand (Suzuki, unpublished data). Consistent with this, hematopoietic cells such as J774 cells (mouse macrophages) or human monocytes, which express WASP predominantly but not N-WASP, did not support actin-based movement of intracellular S. flexneri (Suzuki, unpublished data). Also, it has been indicated that the VirG-induced formation of actin tails is not observed in the cytoplasmic extracts of human platelets (13), suggesting that these types of cells do not support the actin-based spreading of Shigella.
Activation of N-WASP by Cdc42 is required for initiating actin-based motility of intracellular Shigella
In vitro studies have indicated that activation of N-WASP in cells requires Cdc42 bound to the GBD of N-WASP (47, 62, 66, 67). The interaction of Cdc42 with GBD prevents the intramolecular interaction between the C-terminal acidic amino acids and the basic amino acids near the GBD, thus causing the unfolding of N-WASP that represents the activated form. However, when N-WASP interacts with a fragment of VirG encompassing residues 53 to 503 of VirG, the N-WASP–Arp2/3 complex-mediated actin nucleation is markedly stimulated without Cdc42 (13). The ability of VirG to activate N-WASP without Cdc42 was also reported using Clostridium difficile Tcd-10463, which inhibits Rho GTPases (51). However, actin tails were significantly shorter in the presence of the exotoxin than in infected cells without the toxin, suggesting that actin assembly by Shigella is partly affected by toxin. Recently, controversial results have been reported by our group indicating that cellular Cdc42 is certainly required for the actin-based motility of Shigella in infected cells (83). Microinjection of activated Cdc42 accelerates Shigella motility, whereas inhibiting Cdc42 activity, for example, by adding RhoGDI (a guanine nucleotide dissociation inhibitor) into cell extracts, greatly reduces bacterial motility (83). In pyrene actin polymerization assays, the VirG–N-WASP–Arp2/3 complex is insufficient to express the full activity for polymerizing actin, and rather, in the presence of activated Cdc42, the actin nucleation activity is remarkably stimulated (83). More evidence supporting our notion was the observation that Cdc42 is accumulated at one pole of Shigella in the process of initiating movement in infected cells (83). Importantly, Cdc42 is not accumulated on motile Shigella possessing an actin tail in the infected cells, implying that Cdc42 is no longer necessary after a steady speed has been reached, at which stage the VirG–N-WASP–Arp2/3 complexes would be constitutively activated. In agreement with this notion, another study has also reported that Cdc42 could not be detected on motile Shigella (50). Although the reasons for the controversial results remain unclear, the findings indicating no requirement of Cdc42 for bacterial motility may be partly due to the use of a fragment of the VirG α-domain in the in vitro actin polymerization assay (13) and the motility assay based on the analysis of steady-state bacterial motility in infected cells (51).
Current models for VirG inducing actin-based motility.
As pointed out above, VirG expressed on the bacterial surface in host cells can directly recruit N-WASP, which in turn recruits the Arp2/3 complex (Fig. 2). Consequently, at one pole of the bacterium, the VirG–N-WASP–Arp2/3 complex can be formed to mediate actin nucleation, including elongation. To initiate actin nucleation, the Arp2/3 complex is somehow activated upon physical interaction with the VCA region of N-WASP. With the aid of other host factors (see below), the VirG–N-WASP–Arp2/3 complex mediates rapid actin filament growth at the barbed-end, including cross-linking between the elongated actin filaments (Fig. 3). Thereby Shigella can gain a propulsive force in the host cytoplasm, and some motile bacteria impinge upon the host plasma membrane, leading to the extension of membranous protrusions. Through these protrusions which penetrate neighboring cells, Shigella can be transmitted into adjacent epithelial cells. In a reconstitution experiment supporting the actin-based motility of Shigella with pure proteins, host factors required for Shigella movement were confirmed to include actin, the Arp2/3 complex, and N-WASP (41). In addition, actin depolymerization factor (ADF)/cofilin, capping protein, and profilin are also indicated to be involved in the regulation of actin turnover and stabilization of the actin tail (41). Besides these, several other actin-associated proteins, such as plastin (fimbrin) (63), filamin (63), VASP (5), zyxin (15), ezrin (24), CapZ (24), Nck, and WASP-interacting protein (WIP) (50), have been identified as being localized to the actin tail or to the posterior end of intracellular bacteria. However, whether or not these host factors are functionally required for Shigella movement in infected cells awaits further study.
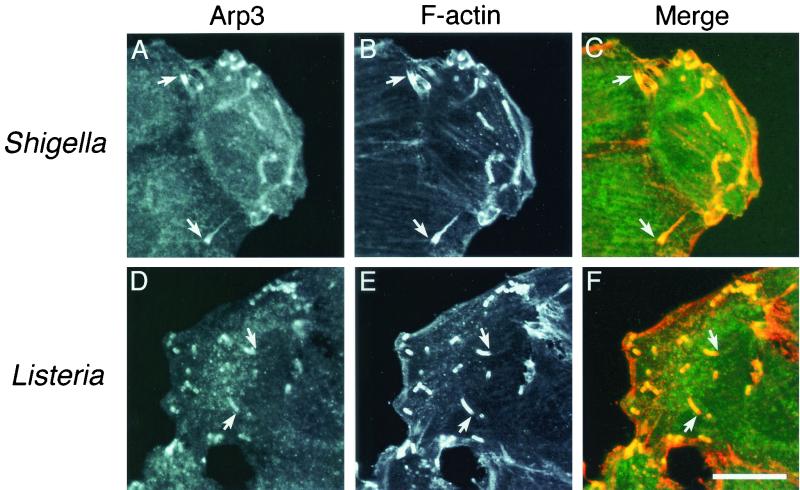
FIG. 2.
Accumulation of the Arp2/3 complex at the actin comet tail of intracellular Shigella (A, B, and C) and Listeria (D, E, and F) in infected HeLa cells. (A and D) The Arp2/3 complex was visualized with fluorescein isothiocyanate-labeled anti-Arp3 antibody. (B and E) Actin filaments were visualized with rhodamine-phalloidin. (C and F) The yellow color in the merged images indicates colocalization between the Arp2/3 complex (green) and actin filaments (red). Arrows indicate an intracellular bacterium forming an actin comet tail. Bar, 10 μm.
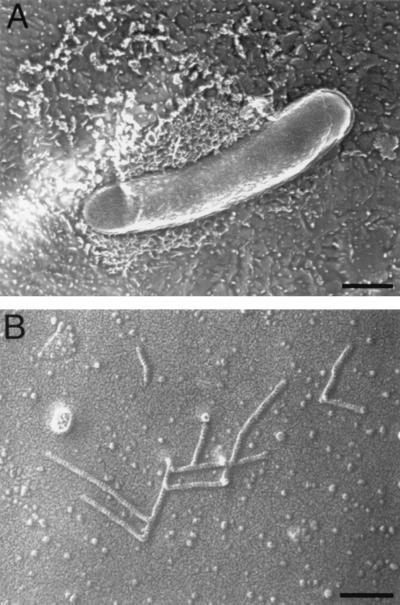
FIG. 3.
Electron micrographs of the actin assembly formed by E. coli expressing VirG in Xenopus egg extracts. Actin filaments appear as a dense cross-linked meshwork around the bacterium (A). Actin filaments form a branched network with rigid attachments and a fixed 70° angle between the filaments. The branched points have a globular mass, which would contain the Arp2/3 complex (B). Bars, 500 nm (A) and 100 nm (B).
Profilin is required for sustaining rapid movement of intracellular Shigella.
Profilin binding to actin facilitates the formation of ATP-actin monomers, the form of actin to be assembled into actin filaments. Profilin can interact with various proteins, notably proteins with a proline-rich sequence such as N-WASP, VASP, MENA, p140mDia, WAVE, and the Arp2/3 complex (19, 42, 48, 65, 79, 92). In a reconstitution assay in vitro, profilin and VASP (for Listeria) are shown to enhance bacterial motility but are not essential, suggesting that recruited profilin helps to increase the local concentration of ATP-actin (41). Profilin exists in two isoforms in mammalian cells, profilin I and II, and profilin I has a greater affinity for N-WASP (Kd = 60 nM) than does profilin II (Kd = 400 nM) (79). Hence, the role of profilin I in the actin-based motility of intracellular Shigella has recently been investigated (49). On overexpression of a profilin H133S mutant defective in interaction with the PRR of N-WASP, including poly-l-proline, Shigella motility is significantly decreased. Similarly, depletion of profilin from Xenopus egg extracts results in a decrease in bacterial motility that is rescued by adding back profilin I but not by the H133S mutant. Consistent with this, on overexpression of an N-WASP mutant lacking the PRR unable to interact with profilin, the actin tail formation of intracellular Shigella was almost completely abolished. In N-WASP-depleted extracts, the addition of wild type N-WASP but not the N-WASP mutant restores bacterial motility, indicating that profilin associated with N-WASP is an essential host factor for supporting rapid spreading of Shigella in infected cells (49). The role of the PRR of WASP family members in interaction with profilin or more generally in activation of actin assembly remains unclear. Deletion of the PRR of WAVE has a minimal effect on actin assembly (48), and profilin inhibits rather than stimulates actin polymerization in the presence of a WAVE fragment that contains PRR (43). However, in the presence of N-WASP and the Arp2/3 complex, Cdc42-stimulated nucleation of actin is enhanced by profilin (98). When the concentration of free monomeric actin is held constant, the stimulation of actin assembly by a C-terminal fragment of N-WASP is enhanced by profilin, even though the C-terminal fragment of N-WASP does not contain PRR that binds profilin (98), suggesting that a part of the enhancement that is mediated by profilin may be independent of binding to N-WASP.
IS THE ARP2/3 COMPLEX A COMMON PLAYER IN PATHOGENS FOR ACTIN-BASED MOVEMENT IN OR ATTACHMENT TO EPITHELIAL CELLS?
L. monocytogenes, spotted fever group Rickettsia, and the vaccinia virus also induce polarized actin assembly at the surface to gain propulsive force in infected cells. The Listeria surface protein ActA, which is accumulated over the posterior bacterial body during movement in host cells, is crucial for actin-based motility (11, 32, 33, 54). ActA has multiple functional domains and interacts with several host factors, the Arp2/3 complex, Drosophila Enabled (Ena)/VASP family proteins and PtdIns(4,5)P2 (5, 6, 19, 75, 77, 94). The N-terminal domain of ActA (residues 30 to 263) can not only interact with the Arp2/3 complex but can also stimulate its actin nucleation activity (60, 74, 95, 99). Thus, unlike VirG of Shigella, ActA of Listeria interacts directly with and stimulates the Arp2/3 complex and does not require N-WASP as an intermediate. The central proline-rich domain (residues 264 to 390) interacts with Ena/VASP family proteins, which in turn recruit actin filaments and profilin (55, 59, 75). Although the precise role of the central proline-rich domain remains unclear, the region contributes to the rate of movement and the percentage of moving bacteria (36, 37, 75). Ena/VASP family proteins bound to ActA are also proposed to mediate insertional actin polymerization on the surface of Listeria. However, a recent report has indicated that Ena/VASP family proteins negatively regulate the cell crawling mediated by lamellipodial membrane extension (1), suggesting that the role of Ena/VASP family proteins in Listeria motility is not necessarily the same as that in the formation of lamellipodia in locomoting cells. A recent report has indicated that ActA possesses two actin monomer-binding sites (residues 85 to 104 and 121 to 138) at the N terminus of the Arp2/3 complex-binding site (residues 144 to 170) (99). Interestingly, these motifs in the N-terminal ActA sequence share functional similarity to that of the VCA domain of N-WASP, since the VCA domain also has two actin monomer-binding verprolin homology domains and an Arp2/3 complex-interacting site (46). These tandem verprolin homology domains have been identified as the essential parts for mediating the strong activation of Arp2/3 complex-directed actin polymerization (97). Therefore, it is assumed that the two actin monomer-binding motifs of ActA sharing the function encoded by the VCA region of N-WASP serve to recruit and activate the Arp2/3 complex, thus mediating rapid actin nucleation and elongation with the aid of profilin recruited by VASP bound to the proline-rich repeats of ActA on Listeria in host cells.
Interestingly, the system underlying the intracellular movement of Rickettsia is strikingly different from that in Shigella or Listeria, since the actin comet tail of Rickettsia does not have the dendritic filamentous actin network that is generated by actin tails from motile Shigella and Listeria or during the formation of lamellipodia in mammalian cells (Fig. 3) (24, 89). In fact, neither N-WASP nor the Arp2/3 complex has been detected at Rickettsia actin tails yet (24, 89), although whether the factors would be below the limit of detection in the assay system awaits further investigation. Although the mechanism of Rickettsia movement including the bacterial factor(s) mediating actin assembly in mammalian cells is still to be characterized, a unique process for actin polymerization compared to that in Shigella or Listeria may take part in the actin-based movement of Rickettsia in mammalian cells.
The enveloped form of vaccinia virus, called intracellular enveloped virus (IEV), also induces formation of an actin comet tail in infected cells (9). The mechanism of the actin tail formation of IEV resembles that of Shigella VirG more than that of Listeria ActA with respect to the involvement of N-WASP. However, vaccinia virus movement occurs depending on protein tyrosine phosphorylation of one of the surface proteins, called A36R (16). The tyrosine-phosphorylated A36R links to N-WASP but does so indirectly via binding to adapter proteins such as Nck and WIP (50). Unlike Shigella actin-based motility, the activation of N-WASP is independent of Cdc42 (50).
Enteropathogenic E. coli (EPEC) colonizes epithelial cells in the human small intestine by provoking effacement of the microvilli and intimating attachment to the host cells, a prominent pathogenic feature called attaching and effacing (53). EPEC normally cannot invade epithelial cells and rather induces the formation of an actin pedestal structure beneath the bacterium attached to the host cell surface (23, 68, 88). To achieve an intimate attachment, EPEC delivers a set of effector proteins such as Tir, EspB, and EspD into the host cytoplasm via the type III secretion machinery (14, 26, 34, 85, 88, 90, 96). Tir has been indicated to play a major role in mediating actin polymerization in the host cells, since after its translocation Tir is tyrosine phosphorylated and subsequently inserted into the host plasma membrane to specifically interact with the bacterial surface protein intimin (29, 30). Meanwhile, the cytoplasmic domains of the inserted Tir can recruit N-WASP (or WASP) and the Arp2/3 complex, thus mediating actin polymerization and leading to the formation of dynamic actin pedestals (28). However, the link between Tir and N-WASP is indirect, and it may take place via binding to Chp, a Cdc42-like GTPase (28). In this sense, the situation of Tir would be similar to that of vaccinia virus A36R.
Our understanding of the mechanisms of actin-based movement of pathogens, including Shigella, Listeria, and vaccinia virus, has recently dramatically progressed (4, 7, 8, 17, 100). Clearly, although each pathogen exploits its own unique protein to modulate the host actin dynamics to promote the infection process, they all share mechanisms of inducing actin polymerization that are similar to each other or to those of host systems, as typically exemplified by the status of N-WASP or the Arp2/3 complex in mammalian cells (Fig. 4). Thus, our goal in the study of the actin-based motility of pathogens is not only to gain further insight into the understanding of precise mechanisms of infection but also to provide an ideal model system to uncover the complicated cellular systems for remodeling actin cytoskeletons in various cellular events.
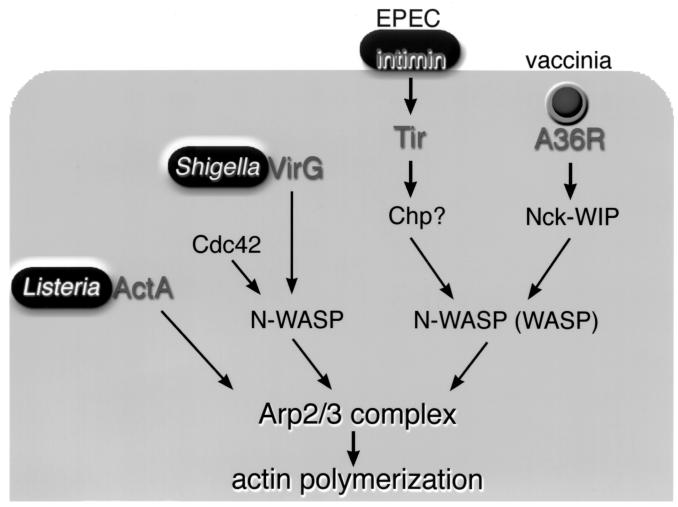
FIG. 4.
Schematic representations of the components and signaling pathways induced by pathogens for activating the Arp2/3 complex-mediated actin rearrangements. Listeria can stimulate the Arp2/3 complex by direct interaction between the bacterial ActA protein and the Arp2/3 complex. Other pathogens, such as Shigella, EPEC, and vaccinia viruses, exploit their own unique protein to modulate the signaling pathway for inducing a common actin driving system with the N-WASP–Arp2/3 complex.
ACKNOWLEDGMENTS
Work in the laboratory of C.S. was supported by the “Research for the Future” Program of the Japan Society for the Promotion of Science and a grant-in-aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
We thank Eisaku Katayama for electron microscopy for Fig. 3 and Hitomi Mimuro for aid in generating the figures used in the paper.
REFERENCES
- 1.Bear J E, Loureiro J J, Libova I, Fassler R, Wehland J, Gertler F B. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- 2.Bear J E, Rawis J F, Saxe C L., III SCAR, a WASP-related protein, isolated as a suppressor of receptor defects in late Dictyostelium development. J Cell Biol. 1998;142:1325–1335. doi: 10.1083/jcb.142.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardini M L, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron L A, Giardini P A, Soo F S, Theriot J A. Secrets of actin-based motility revealed by a bacterial pathogen. Nat Rev Mol Cell Biol. 2000;1:110–119. doi: 10.1038/35040061. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty T, Ebel F, Domann E, Niebuhr K, Gerstel B, Pistor S, Temm-Grove C J, Jockusch B M, Reinhard M, Walter U, Wehland J. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 1995;14:1314–1321. doi: 10.1002/j.1460-2075.1995.tb07117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicchetti G, Maurer P, Wagener P, Kocks C. Actin and phosphoinositide binding by the ActA protein of the bacterial pathogen Listeria monocytogenes. J Biol Chem. 1999;274:33616–33626. doi: 10.1074/jbc.274.47.33616. [DOI] [PubMed] [Google Scholar]
- 7.Cossart P. Actin-based motility of pathogens: the Arp2/3 complex is a central player. Cell Microbiol. 2000;2:195–205. doi: 10.1046/j.1462-5822.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 8.Cossart P, Bierne H. The use of host cell machinery in the pathogenesis of Listeria monocytogenes. Curr Opin Immunol. 2001;13:96–103. doi: 10.1016/s0952-7915(00)00188-6. [DOI] [PubMed] [Google Scholar]
- 9.Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 10.Derry J M J, Ochs H D, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi: 10.1016/0092-8674(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 11.Domann E, Wehland J, Rohde M, Pistor S, Hartl M, Goebel W, Leimeister-Wachter M, Wuenscher M, Chakraborty T. A novel bacterial gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992;11:1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egile C, d'Hauteville H, Parsot C, Sansonetti P J. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol Microbiol. 1997;23:1063–1073. doi: 10.1046/j.1365-2958.1997.2871652.x. [DOI] [PubMed] [Google Scholar]
- 13.Egile C, Loisel T P, Laurent V, Li R, Pantaloni D, Sansonetti P J, Carlier M-F. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol. 1999;146:1319–1332. doi: 10.1083/jcb.146.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frankel G, Phillips A D, Rosenshine I, Dougan G, Kaper J B, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol. 1998;30:911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 15.Frischknecht F, Cudmore S, Moreau V, Reckmann I, Rottger S, Way M. Tyrosine phosphorylation is required for actin-based motility of vaccinia but not Listeria or Shigella. Curr Biol. 1999;9:89–92. doi: 10.1016/s0960-9822(99)80020-7. [DOI] [PubMed] [Google Scholar]
- 16.Frischknecht F, Moreau V, Rottger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin-based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 17.Frischknecht F, Way M. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 2001;11:30–38. doi: 10.1016/s0962-8924(00)01871-7. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda I, Suzuki T, Munakata H, Hayashi N, Katayama E, Yoshikawa M, Sasakawa C. Cleavage of Shigella surface protein VirG occurs at a specific site, but the secretion is not essential for intracellular spreading. J Bacteriol. 1995;177:1719–1726. doi: 10.1128/jb.177.7.1719-1726.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gertler F B, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore A P, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4–5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg M B. Shigella actin-based motility in the absence of vinculin. Cell Motil Cytoskelet. 1997;37:44–53. doi: 10.1002/(SICI)1097-0169(1997)37:1<44::AID-CM5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg M B, Barzu O, Parsot C, Sansonetti P J. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J Bacteriol. 1993;175:2189–2196. doi: 10.1128/jb.175.8.2189-2196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goosney D L, de Grado M, Finlay B B. Putting E. coli on a pedestal: a unique system to study signal transduction and the actin cytoskeleton. Trends Cell Biol. 1999;9:11–14. doi: 10.1016/s0962-8924(98)01418-4. [DOI] [PubMed] [Google Scholar]
- 24.Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, Gounon P, Sansonetti P J, Cossart P. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci. 1999;112:1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- 25.Heinzen R A, Hayes S F, Peacock M G, Hackstadt T. Directional actin polymerization associated with spotted fever group Rickettsia infection of Vero cells. Infect Immun. 1993;61:1926–1935. doi: 10.1128/iai.61.5.1926-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis K G, Giron J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson R P, Craig S W. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- 28.Kalman D, Weiner O D, Goosney D L, Sedat J W, Finlay B B, Abo A, Bishop J M. Enteropathogenic E. coli acts through WASP and Arp2/3 complex to form actin pedestals. Nat Cell Biol. 1999;1:389–391. doi: 10.1038/14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenny B. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol Microbiol. 1999;31:1229–1241. doi: 10.1046/j.1365-2958.1999.01265.x. [DOI] [PubMed] [Google Scholar]
- 30.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 31.Klauser T, Pohlner J, Meyer T F. Extracellular transport of cholera toxin B subunit using Neisseria IgA protease β-domain: conformation-dependent outer membrane translocation. EMBO J. 1990;9:1991–1999. doi: 10.1002/j.1460-2075.1990.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. Listeria monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 33.Kocks C, Hellio R, Gounon P, Ohayon H, Cossart P. Polarized distribution of Listeria monocytogenes surface protein ActA at the site of directional actin assembly. J Cell Sci. 1993;105:699–710. doi: 10.1242/jcs.105.3.699. [DOI] [PubMed] [Google Scholar]
- 34.Kresse A U, Rohde M, Guzman C A. The EspD protein of enterohemorrhagic Escherichia coli is required for the formation of bacterial surface appendages and is incorporated in the cytoplasmic membranes of target cells. Infect Immun. 1999;67:4834–4842. doi: 10.1128/iai.67.9.4834-4842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laine R O, Zeile W, Kang F, Purich D L, Southwick F S. Vinculin proteolysis unmasks an ActA homolog for actin-based Shigella motility. J Cell Biol. 1997;138:1255–1264. doi: 10.1083/jcb.138.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasa I, David V, Gouin E, Marchand J B, Cossart P. The amino-terminal part of ActA is critical for the actin-based motility of Listeria monocytogenes; the central proline-rich region acts as a stimulator. Mol Microbiol. 1995;18:425–436. doi: 10.1111/j.1365-2958.1995.mmi_18030425.x. [DOI] [PubMed] [Google Scholar]
- 37.Laurent V, Loisel T P, Harbeck B, Wehman A, Gröbe L, Jockusch B M, Wehland J, Gertler F B, Carlier M-F. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J Cell Biol. 1999;144:1245–1258. doi: 10.1083/jcb.144.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lechler T, Li R. In vitro reconstitution of cortical actin assembly sites in budding yeast. J Cell Biol. 1997;138:95–103. doi: 10.1083/jcb.138.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lett M-C, Sasakawa C, Okada N, Sakai T, Makino S, Yamada M, Komatsu K, Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989;171:353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li R. Bee1, a yeast protein with homology to Wiskott-Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J Cell Biol. 1997;136:649–658. doi: 10.1083/jcb.136.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loisel T P, Boujemaa R, Pantaloni D, Carlier M-F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:613–616. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- 42.Machesky L M, Atkinson S J, Ampe C, Vandekerckhove J, Pollard T D. Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on Profilin-agarose. J Cell Biol. 1994;127:107–115. doi: 10.1083/jcb.127.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machesky L M, Mullins R D, Higgs H N, Kaiser D A, Blanchoin L, May R C, Hall M E, Pollard T D. Scar, a WASP-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci USA. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makino S, Sasakawa C, Kamata T, Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on a large plasmid in Shigella flexneri 2a. Cell. 1986;46:551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- 45.Marchand J B, Kaiser D A, Pollard T D, Higgs H N. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat Cell Biol. 2001;3:76–82. doi: 10.1038/35050590. [DOI] [PubMed] [Google Scholar]
- 46.Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 47.Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- 48.Miki H, Suetsugu S, Takenawa T. WAVE, a novel-WASP family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mimuro H, Suzuki T, Suetsugu S, Miki H, Takenawa T, Sasakawa C. Profilin is required for sustaining efficient intra- and intercellular spreading of Shigella flexneri. J Biol Chem. 2000;275:28893–28901. doi: 10.1074/jbc.M003882200. [DOI] [PubMed] [Google Scholar]
- 50.Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–448. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- 51.Mounier J, Laurent V, Hall A, Fort P, Carlier M-F, Sansonetti P J, Egile C. Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular motility. J Cell Sci. 1999;112:2069–2080. doi: 10.1242/jcs.112.13.2069. [DOI] [PubMed] [Google Scholar]
- 52.Nakata N, Tobe T, Fukuda I, Suzuki T, Komatsu K, Yoshikawa M, Sasakawa C. The absence of surface protease, OmpT, determines the intercellular spreading ability of Shigella: the relationship between the ompT and kcpA loci. Mol Microbiol. 1993;9:459–468. doi: 10.1111/j.1365-2958.1993.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 53.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niebuhr K, Chakraborty T, Rohde M, Gazlig T, Jansen B, Kollner P, Wehland J. Localization of the ActA polypeptide of Listeria monocytogenes in infected tissue culture cell lines: ActA is not associated with actin “comets.”. Infect Immun. 1993;61:2793–2802. doi: 10.1128/iai.61.7.2793-2802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niebuhr K, Ebel F, Frank R, Reinhard M, Domann E, Carl U D, Walter U, Gertler F B, Wehland J, Chakraborty T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997;16:5433–5444. doi: 10.1093/emboj/16.17.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogawa H, Nakamura A, Nakaya R. Cinemicrographic study of tissue cell cultures infected with Shigella flexneri. Jpn J Med Sci Biol. 1968;21:259–273. doi: 10.7883/yoken1952.21.259. [DOI] [PubMed] [Google Scholar]
- 57.Okada N, Sasakawa C, Tobe T, Talukder K A, Komatsu K, Yoshikawa M. Construction of a physical map of the chromosome of Shigella flexneri 2a and the direct assignment of nine virulence-associated loci identified by Tn5 insertions. Mol Microbiol. 1991;5:2171–2180. doi: 10.1111/j.1365-2958.1991.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 58.Okada N, Sasakawa C, Tobe T, Yamada M, Nagai S, Talukder K A, Komatsu K, Kanegasaki S, Yoshikawa M. Virulence-associated chromosomal loci of Shigella flexneri identified by random Tn5 insertion mutagenesis. Mol Microbiol. 1991;5:187–195. doi: 10.1111/j.1365-2958.1991.tb01839.x. [DOI] [PubMed] [Google Scholar]
- 59.Pistor S, Chakraborty T, Walter U, Wehland J. The bacterial actin nucleator protein ActA of Listeria monocytogenes contains multiple binding sites for host microfilament proteins. Curr Biol. 1995;5:517–525. doi: 10.1016/s0960-9822(95)00104-7. [DOI] [PubMed] [Google Scholar]
- 60.Pistor S, Gröbe L, Sechi A S, Domann E, Gerstel B, Machesky L M, Chakraborty T, Wehland J. Mutations of arginine residues within the 146-KKRRK-150 motif of the ActA protein of Listeria monocytogenes abolish intracellular motility by interfering with the recruitment of the Arp2/3 complex. J Cell Sci. 2000;113:3277–3287. doi: 10.1242/jcs.113.18.3277. [DOI] [PubMed] [Google Scholar]
- 61.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 62.Prehoda K E, Scott J A, Dyche Mullins R, Lim W A. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 63.Prévost M-C, Lesourd M, Arpin F, Vernel F, Mounier J, Hellio R, Sansonetti P J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992;60:4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rajakumar K, Jost B H, Sasakawa C, Okada N, Yoshikawa M, Adler B. Nucleotide sequence of the rhamnose biosynthetic operon of Shigella flexneri 2a and role of lipopolysaccharide in virulence. J Bacteriol. 1994;176:2362–2373. doi: 10.1128/jb.176.8.2362-2373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reinhard M, Giehl K, Abel K, Haffner C, Jarchau T, Hoppe V, Jockusch B M, Walter U. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 1995;14:1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rohatgi R, Ho H Y, Kirschner M W. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2000;150:1299–1310. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner M W. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 68.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 69.Sandlin R C, Goldberg M B, Maurelli A T. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol Microbiol. 1996;22:63–73. doi: 10.1111/j.1365-2958.1996.tb02656.x. [DOI] [PubMed] [Google Scholar]
- 70.Sandlin R C, Lampel K A, Keasler S P, Goldberg M B, Stolzer A L, Maurelli A T. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect Immun. 1995;63:229–237. doi: 10.1128/iai.63.1.229-237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sansonetti P J, Arondel J, Cantey J R, Prévost M-C, Huerre M. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun. 1996;64:2752–2764. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sansonetti P J, Arondel J, Fontaine A, d'Hauteville H, Bernardini M L. ompB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri: vaccine candidates and probes to study the pathogenesis of shigellosis. Vaccine. 1991;9:416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 73.Shere K D, Sallustio S, Manessis A, D'Aversa T G, Goldberg M B. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol. 1997;25:451–462. doi: 10.1046/j.1365-2958.1997.4681827.x. [DOI] [PubMed] [Google Scholar]
- 74.Skoble J, Portnoy D A, Welch M D. Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J Cell Biol. 2000;150:527–538. doi: 10.1083/jcb.150.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith G A, Theriot J A, Portnoy D A. The tandem repeat domain in the Listeria monocytogenes ActA protein controls the rate of actin-based motility, the percentage of moving bacteria, and the localization of vasodilator-stimulated phosphoprotein and profilin. J Cell Biol. 1996;135:647–660. doi: 10.1083/jcb.135.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Southwick F S, Adamson E D, Purich D L. Shigella actin-based motility in the presence of truncated vinculin. Cell Motil Cytoskelet. 2000;45:272–278. doi: 10.1002/(SICI)1097-0169(200004)45:4<272::AID-CM3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 77.Steffen P, Schafer D A, David V, Gouin E, Cooper J A, Cossart P. Listeria monocytogenes ActA protein interacts with phosphatidylinositol 4,5-bisphosphate in vitro. Cell Motil Cytoskelet. 2000;45:58–66. doi: 10.1002/(SICI)1097-0169(200001)45:1<58::AID-CM6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 78.Steinhauer J, Agha R, Pham T, Varga A W, Goldberg M B. The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol Microbiol. 1999;32:367–377. doi: 10.1046/j.1365-2958.1999.01356.x. [DOI] [PubMed] [Google Scholar]
- 79.Suetsugu S, Miki H, Takenawa T. The essential role of profilin in the assembly of actin for microspike formation. EMBO J. 1998;17:6516–6526. doi: 10.1093/emboj/17.22.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suetsugu S, Miki H, Takenawa T. Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex. Biochem Biophys Res Commun. 1999;260:296–302. doi: 10.1006/bbrc.1999.0894. [DOI] [PubMed] [Google Scholar]
- 81.Suzuki T, Lett M-C, Sasakawa C. Extracellular transport of VirG protein in Shigella. J Biol Chem. 1995;270:30874–30880. doi: 10.1074/jbc.270.52.30874. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki T, Miki H, Takenawa T, Sasakawa C. Neural Wiskott-Aldrich syndrome protein is implicated in actin-based motility of Shigella flexneri. EMBO J. 1998;17:2767–2776. doi: 10.1093/emboj/17.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suzuki T, Mimuro H, Miki H, Takenawa T, Sasaki T, Nakanishi H, Takai Y, Sasakawa C. Rho family GTPase Cdc42 is essential for the actin-based motility of Shigella in mammalian cells. J Exp Med. 2000;191:1905–1920. doi: 10.1084/jem.191.11.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki T, Saga S, Sasakawa C. Functional analysis of Shigella VirG domains essential for interaction with vinculin and actin-based motility. J Biol Chem. 1996;271:21878–21885. doi: 10.1074/jbc.271.36.21878. [DOI] [PubMed] [Google Scholar]
- 85.Taylor K A, O'Connell C B, Luther P W, Donnenberg M S. The EspB protein of enteropathogenic Escherichia coli is targeted to the cytoplasm of infected HeLa cells. Infect Immun. 1998;66:5501–5507. doi: 10.1128/iai.66.11.5501-5507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teysseire N, Chichi-Portiche C, Raoult D. Intracellular movements of Rickettsia conorii and R. typhi based on actin polymerization. Res Microbiol. 1992;143:821–829. doi: 10.1016/0923-2508(92)90069-z. [DOI] [PubMed] [Google Scholar]
- 87.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vallance B A, Finlay B B. Exploitation of host cells by enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2000;97:8799–8806. doi: 10.1073/pnas.97.16.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Kirk L S, Hayes S F, Heinzen R A. Ultrastructure of Rickettsia rickettsii actin tails and localization of cytoskeletal proteins. Infect Immun. 2000;68:4706–4713. doi: 10.1128/iai.68.8.4706-4713.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wachter C, Beinke C, Mattes M, Schmidt M A. Insertion of EspD into epithelial target cell membranes by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1999;31:1695–1707. doi: 10.1046/j.1365-2958.1999.01303.x. [DOI] [PubMed] [Google Scholar]
- 91.Wassef J S, Keren D F, Mailloux J L. Role of M cells in initial antigen uptake and in ulcer formation in the rabbit intestinal loop model of shigellosis. Infect Immun. 1989;57:858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch B M, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weekes J, Barry S T, Critchley D R. Acidic phospholipids inhibit the intramolecular association between the N- and C-terminal regions of vinculin, exposing actin-binding and protein kinase C phosphorylation sites. Biochem J. 1996;314:827–832. doi: 10.1042/bj3140827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Welch M D, Iwamatsu A, Mitchison T J. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- 95.Welch M D, Rosenblatt J, Skoble J, Portnoy D A, Mitchison T J. Interaction of human Arp2/3 and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 1998;281:105–108. doi: 10.1126/science.281.5373.105. [DOI] [PubMed] [Google Scholar]
- 96.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]
- 97.Yamaguchi H, Miki H, Suetsugu S, Ma L, Kirschner M W, Takenawa T. Two tandem verprolin homology domains are necessary for a strong activation of Arp2/3 complex-induced actin polymerization and induction of microspike formation by N-WASP. Proc Natl Acad Sci USA. 2000;97:12631–12636. doi: 10.1073/pnas.190351397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang C, Huang M, DeBiasio J, Pring M, Joyce M, Miki H, Takenawa T, Zigmond S H. Profilin enhances Cdc42-induced nucleation of actin polymerization. J Cell Biol. 2000;150:1001–1012. doi: 10.1083/jcb.150.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zalevsky J, Grigorova I I, Mullins R D. Activation of the Arp2/3 complex by the Listeria ActA protein: ActA binds two actin monomers and three subunits of the Arp2/3 complex. J Biol Chem. 2000;276:3468–3475. doi: 10.1074/jbc.M006407200. [DOI] [PubMed] [Google Scholar]
- 100.Zettl M, Way M. New tricks for an old dog? Nat Cell Biol. 2001;3:E74–E75. doi: 10.1038/35060152. [DOI] [PubMed] [Google Scholar]
- 101.Zychlinsky A, Fitting C, Cavaillon J M, Sansonetti P J. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Investig. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]