Abstract
Immune checkpoint inhibitors tremendously improve cancer prognosis; however, severe-grade immune-related adverse events may cause premature death. Current recommendations for checkpoint inhibitor-related pneumonitis (CIP) treatment are mainly about immunosuppressive therapy, and anti-fibrotic agents are also needed, especially for patients with poor response to corticosteroids and a longer pneumonitis course. This is because fibrotic changes play an important role in the pathological evolution of CIP. Here, we report a case demonstrating that nintedanib is a promising candidate drug for CIP management or prevention, as it has potent anti-fibrotic efficacy and a safety profile. Moreover, nintedanib could partially inhibit tumor growth in patients with non-small-cell lung cancer, and its efficacy can be improved in combination with other anti-tumor therapies.
Keywords: checkpoint inhibitor-related pneumonitis, nintedanib, pulmonary fibrosis, non-small-cell lung cancer, immunotherapy
Highlights:
Steroid-refractory CIP in patients with non-small-cell lung cancer (NSCLC) may be particularly lethal owing to progressive respiratory failure and compromised immune system during immunosuppressive treatment of CIP.
Nintedanib showed great efficacy with good tolerance in treating sustained interstitial fibrosis and reducing pulmonary function decline in the long course of CIP.
Nintedanib is an anti-tumor-supporting agent in patients with NSCLC, which can positively control tumor growth in combination with chemotherapy and might boost immunotherapy and prevent CIP when used with immune checkpoint inhibitors.
1 Introduction
Immune checkpoint inhibitors (ICIs), including programmed cell death 1 (PD-1) or its ligand programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) inhibitors, have made major breakthroughs in improving the progression-free survival (PFS) and overall survival (OS) rates of lung cancer patients (1). The US Food and Drug Administration has moved quickly to incorporate ICIs as a first-line treatment for advanced non-small-cell lung cancer (NSCLC) (2). However, toxicities from ICIs that lead to immune-related adverse events (irAEs) and even fatal adverse events (FAEs) pose a great challenge to immunotherapy (3, 4). Approximately 46.2% of FAEs involves the respiratory system (5, 6). Checkpoint inhibitor-related pneumonitis (CIP) ranges in severity from mild and self-limiting (grade 1–2) to fulminant and life threatening (grade 3–4) and often necessitates immunomodulatory treatments (5, 7, 8). The incidence of any grade CIP in patients with NSCLC ranges from 2% to 39.3% and that for grade ≥3 CIP is approximately 0.6%–4% (9, 10). The mean time from ICI initiation to CIP onset is approximately 10 weeks (2.5 months) (9). The overall fatality rate of any CIP grade ranges from 10% to 27% (5, 10, 11).
Patients with NSCLC are at risk of a higher incidence and severity of CIP (11) owing to increased susceptibility to frequent tobacco exposure (12) and/or underlying chronic respiratory diseases [chronic obstructive pulmonary disease (13), pulmonary fibrosis (14) and tumoral involvement (15)]. Previous real-world studies revealed that patients with NSCLC with CIP had a higher overall response rate but a significantly shorter overall survival after ICI initiation than those without CIP (16–18). Thus, this highlights the importance of better management of CIP to achieve the best outcome of immunotherapy.
Corticosteroids are the core treatment for any grade of CIP according to the current guidelines (19–21). Some patients might be steroid refractory (22) and require other immunosuppressive agents to control the rapid progressive symptoms (especially for those in severe grades) (20), leading to a higher mortality during CIP treatment (12). Some researchers have proposed classifying the clinical phenotype of CIP as acute, subacute, and chronic phases and have divided the pathological process of CIP into inflammatory, profibrotic, and fibrotic stages (23, 24). These concepts provide a new direction for CIP treatment besides immunosuppression therapy, which is anti-fibrotic treatment.
Nintedanib is an oral triple angiokinase inhibitor (25) and has been approved for the anti-fibrotic treatment of patients with idiopathic pulmonary fibrosis (IPF) and chronic interstitial lung diseases (ILDs) (26), even for those with a progressive phenotype (27). Nintedanib is also currently used in patients with coronavirus disease 2019 (COVID-19) to significantly reduce the duration of ventilator use and improve imaging performance (28). In addition, nintedanib has shown sufficient antitumor efficacy in patients with NSCLC (29) and has been approved in the European Union for the treatment of patients with lung adenocarcinoma following first-line chemotherapy (30). Thus, we hypothesized that nintedanib has dual favorable anti-fibrotic and anti-tumor efficacy in patients with NSCLC-CIP and shows good tolerance.
Herein, we report a case of severe steroid-refractory CIP in an elderly patient with NSCLC treated with a PD-1 inhibitor. The patient’s condition was successfully improved by immunosuppression therapy (high-dose corticosteroids and infliximab), medical intensive care unit (MICU) support, and nintedanib administration. We also present a literature review of the contradictory clinical outcomes of CIP in patients with NSCLC and the potential pathogenic mechanisms of CIP. Our findings suggest that nintedanib is a potential anti-fibrotic agent that could act as an important supportive drug in alleviating chronic fibrotic development of CIP and could exert certain antitumor efficacy under critical conditions of severe CIP with good tolerance.
2 Case presentation
2.1 The basic condition and diagnosis
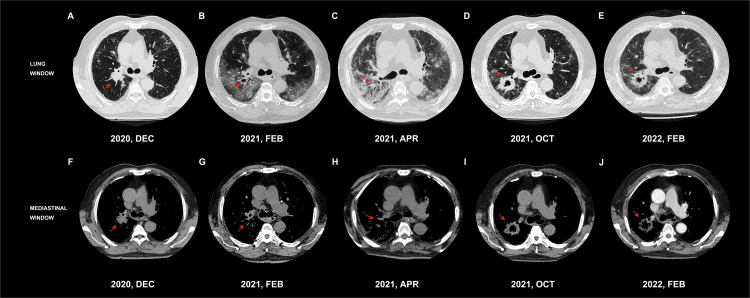
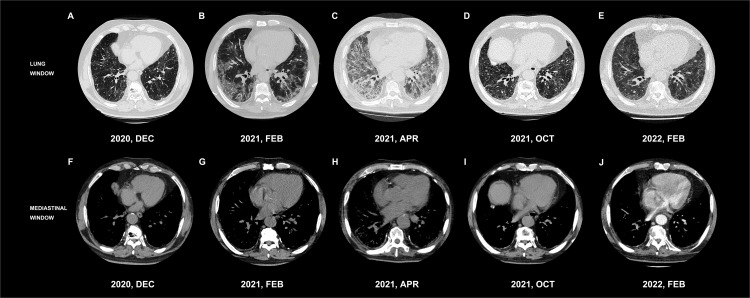
The patient was a 72-year-old male heavy smoker (50 pack-years). During a routine examination in December 2020, a space-occupying lesion (2.9 cm × 2.6 cm) near the lung hilum (Figures 1A, F) and mild fibrotic changes of unclear origin were detected (Figures 2A, F). He underwent EBUS-TBNA pathological examination and PET-CT to confirm the final diagnosis of squamous cell lung carcinoma (SqCLC; T1N1MO, stage IIb). Genomic sequencing test showed no driver mutation for targeted therapy (ALK(−), BRAF(−), BRCA1(−), BRCA2(−), EGFR(−), ERBB2(HER2)(−), FGFR2(−), FGFR3(−), KIT(−), KRAS(−), MET(−), NRAS(−), NTRK1(−), NTRK2 (–), NTRK3(−), PDGFRA(−), RET(−), ROS1(−), and IDH2(+)); however, the patient tested positive for PD-L1 (TPS=25%, IPS<1%, tested using a Ventana SP263 assay), with tumor mutation burden of 7.26 Muts/Mb (tested using a next-generation sequencing (NGS) panel and paired with peripheral blood sample sequencing). Other genetic mutations related to immunotherapy were also tested as follows: CD274(−), PDCD1LG2(−), MLH1(−), MSH2(−), MSH6(−), PMS2(−), POLD1(−), POLE(−), TP53(+), ATM(−), ATR(−), BRIP1(−), CHEK2(−), FANCA(−), RAD50(−), PALB2(−), CHEK1(−), MRE11(−), PBRM1(−), MDM2(−), MDM4(−), DNMT3A(−), JAK1(−), JAK2(+), PTEN(−), STK11(−), CCND1(−), FGF19(−), FGF3(−), FGF4(−). The patient had no history of cardiopulmonary or connective tissue disease, and the Eastern Cooperative Oncology Group (ECOG) performance status was rated as 2 points.
Figure 1.
Chest CT of tracheal carina level in the lung and mediastinum windows. dynamic changes of the space-occupying lesions. (A)/(F) A space-occupying lesion (2.9 × 2.6 cm) in the upper lobe of the right lung. (B)/(G) The primary foci shrank significantly and was surrounded by GGOs and consolidation shadow. (C)/(H) Multiple GGOs, reticulation, grid lesions, and traction bronchiectasis in the dominant lobe around the primary foci with patchy lesions in the opposite lobe. (D)/(I) A recurred lobulated mass (5.0×2.6 cm) in the upper lobe of the right lung. (E)/(J) Mildly reduced lesion (4.2×4.3 cm).
Figure 2.
Chest CT of inferior lungs in the lung and mediastinum windows. Dynamic changes in the interstitial lesions in the bilateral inferior lobes of the lung. (A)/(F) Mild basic interstitial changes. (B)/(G) Significant peribronchovascular and subpleural GGOs, reticulation, and consolidation. (C)/(H) Significant fibrotic with GGOs, reticulation, consolidation, honeycomb shadows, and thickened interlobular septa. (D)/(I) Significantly improved interstitial changes and better transparency. (E)/(J) Stable mild interstitial changes.
2.2 Two cycles of PD-1 inhibitor + nab-paclitaxel → progressive respiratory failure
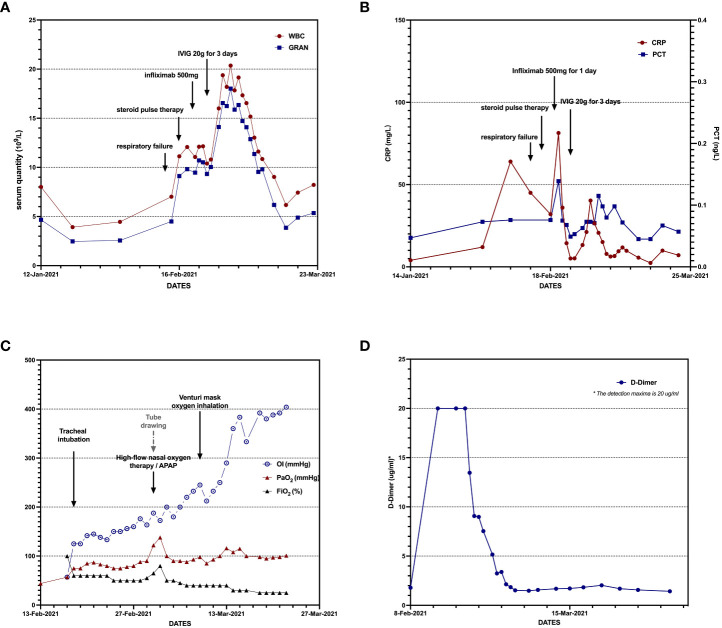
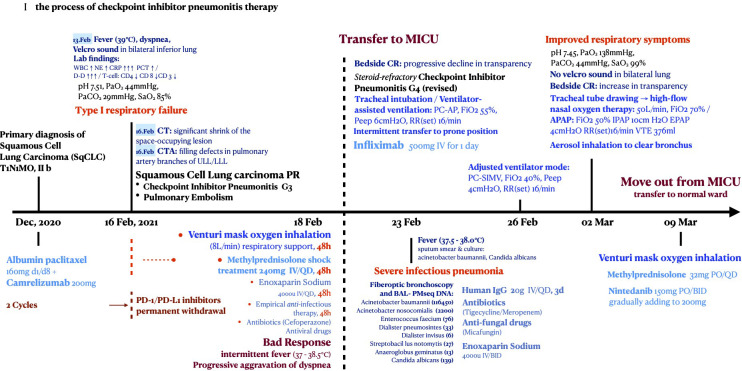
Owing to the patient’s age and tumor location, systemic chemotherapy combined with immunotherapy was administered: intravenous infusion of albumin-paclitaxel 160 mg (D1, D8) combined with camrelizumab 200 mg (D1). After two cycles of the above treatment, the patient developed fever after fatigue and colds on 13 February, 2021, with a maximum temperature of 39°C and shortness of breath. The patient was admitted immediately the next day. Bilateral bronchial breathing sounds and Velcro rales were detected. Laboratory examination revealed an inflammatory blood reaction (Figures 3A, B), decreased oxygen partial pressure (Figure 3C), decreased oxygen saturation (85% SaO2), and increased D-dimer levels (≥20 μg/ml) (Figure 3D). Images showed that the primary cancer foci shrank significantly and were surrounded by patchy ground-glass opacities (GGOs) and consolidation shadows (Figures 1B, G). Bilateral peribronchovascular and subpleural GGOs, reticulation, and consolidation were predominant in the middle to lower lungs (Figures 2B, G). Contrast-filling defects were also observed in the pulmonary artery branches of the upper and lower lobes of the left lung. The patient was preliminarily diagnosed with type I respiratory failure and interstitial pneumonia (CIP?), pulmonary embolism, and SqCLC (T1N1MO stage IIb, partial response [PR]). The patient was administered with mask oxygen inhalation therapy (8 L/min), systemic corticosteroid pulse therapy (methylprednisolone sodium succinate, 240 mg Q.D. for 2 days), and anticoagulation treatment (enoxaparin sodium, 4,000 U Q.D.) on February 16. Considering that infection could not be ruled out, empirical anti-inflammatory and antiviral treatments were administered, along with anti-asthmatic, gastric-protecting, calcium-supplementing, and other symptomatic support treatments. After 48 h, respiratory failure progressively aggravated, and the oxygenation index (PaO2/FiO2) was calculated as 125 mmHg. The patient was then transferred to the MICU for further treatment on February 18.
Figure 3.
Dynamic changes in laboratory examination: inflammatory reaction, hypoxia with hypercoagulable state (A–D).
2.3 In MICU: Steroid-refractory CIP grade 4 + compromised immune system
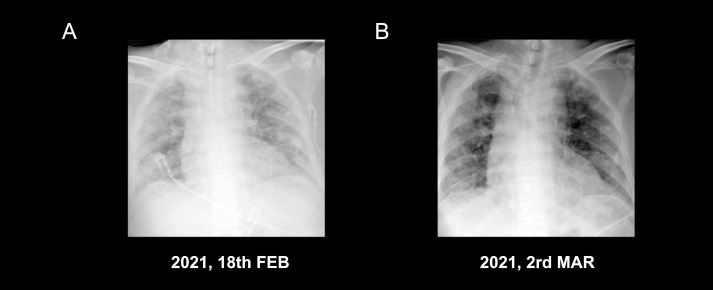
The patient was intubated for mechanical ventilation. Bedside chest radiography revealed poor lung transparency (Figure 4A). As a diagnosis of exclusion, we considered that the pulmonary symptoms and signs were caused by ICI toxicity and responded inadequately to high-dose corticosteroids. The patient had no signs of other concurrent irAEs, such as dermatitis, hepatitis, nephritis, or myopericarditis. The final diagnosis was steroid-refractory CIP grade 4 (G4), defined as a life-threatening respiratory compromise. For steroid-refractory CIP G4, infliximab (500 mg for 1 day) was administered intravenously, and the ICIs were permanently discontinued. Oral corticosteroids were slowly tempered. Two days after immunomodulatory treatment, the inflammation indicators increased again (Figures 3A, B), and plenty of sticky sputum was collected from the patient. We performed sputum culture and sputum smear and found G+ cocci, G− bacillus, Acinetobacter baumannii, and Candida albicans. We then examined the bronchoalveolar lavage fluid (BALF) using NGS and found Acinetobacter baumannii, C. albicans, and Enterocooccusum faecium infection (Table 1).
Figure 4.
Bedside chest radiograph: both lung translucency and consolidation shadow decreased after treatment (A, B).
Table 1.
Detected bacteria and fungi in BALF by NGS.
| Typea | Species | Genus | ||
|---|---|---|---|---|
| Name | Number of detected sequencesb | Name | Number of detected sequencesb | |
| G− | Acinetobacter | 221,142 |
Acinetobacter baumannii
Acinetobacter nosocoomialis |
116,450 2,200 |
| G+ | Enterococcus | 86 | Enterocooccusum faecium | 33 |
| G− | Dialister | 39 |
Dialister pneumosintes
Dialister invisus |
33 6 |
| G− | Streptobacillus | 27 | Streptobacillus notomytis | 27 |
| G− | Anaeroglobus | 13 | Anaeroglobus geminatus | 13 |
| Fungi | Candida | 139 | Candida albicans | 128 |
Type a: G+ → Gram-positive bacteria/G− → Gram-negative bacteria.
number of detected sequences b: refers to the number of strictly matched sequences of the microorganism detected at the genus/species level.
Considering severe comorbid infectious pneumonia due to compromised immune system, the patient was administered with tigecycline combined with meropenem as anti-inflammatory therapy, micafungin as antifungal therapy, and intravenous immunoglobulin pulse therapy for 3 days to enhance the self-immunological barrier. Enoxaparin sodium anticoagulant therapy was continued for pulmonary embolism. After 3 days, the temperature and laboratory indicators improved (Figure 3). Bedside chest radiography showed improved bilateral lung transmittance (Figure 4B). On March 2, endotracheal intubation was removed, and the patient was administered with nasal high-flow oxygen (45 L/min, oxygen concentration 50%).
Figure 5 shows the management axis of CIP G4 and the critical complications in this patient. After sufficient medical care in the MICU, the patient’s condition stabilized, and he was transferred to the general ward on March 9. To manage pulmonary interstitial fibrotic changes and potentially inhibit tumor growth, nintedanib 150 mg was prescribed orally twice daily during the subacute phase of CIP. With good tolerance, the dosage was increased to 200 mg orally twice daily 1 week later and has been maintained to date.
Figure 5.
The management axis of acute severe steroid-refractory CIP.
2.4 SqCLC maintenance therapy: nab-paclitaxel + nintedanib + afatinib
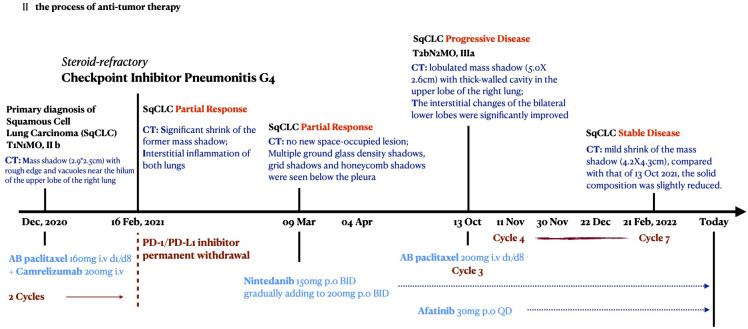
On 2 April 2021, the patient’s ECOG score was rated 4 before discharge; lung CT reexamination showed no new space-occupying lesions, and the original occupation was significantly smaller than the previous one in December 2020 (Figures 1C, H). The therapeutic efficacy of SqCLC was evaluated as PR. For CIP, pulmonary injury had evolved into an organizing and fibrotic stage. Multiple GGOs, reticulation, grid lesions, and traction bronchiectasis in a cryptogenic organizing pneumonia (OP) pattern were found in the dominant lobe around the primary tumor site, with patchy lesions in the opposite lobe (Figures 1C, H). The bilateral lower lungs were predominantly fibrotic with GGOs, reticulation, consolidation, honeycomb shadows, and thickened interlobular septa (Figures 2C, H).
Owing to the patient’s deteriorating physical status and COVID-19 public control policy, the patient had recuperated at home for 6 months and maintained oral nintedanib therapy (200 mg twice daily), with slow tapering of oral corticosteroid therapy. The patient visited the hospital for a review on 13 October 2021. The performance status improved, with an ECOG score of 2. The CT scan showed a lobulated mass with a size of 5.0 cm × 2.6 cm in the upper lobe of the right lung, surrounded by fine burrs and thick-walled cavities (Figures 1D, I). The interstitial changes in the bilateral lower lobes were significantly improved compared to the previous films (Figures 2D, I). There were swollen lymph nodes in the mediastinum, and the largest one was approximately 2.6 cm × 2.0 cm in size. Moreover, the levels of serum tumor markers increased (U/L): CEA, 16.6; AFP, 2.55; CA125, 102; CA153, 36.1; CYFRA, 9.43; NSE, 20.00; and SCCA, 13.6.
The therapeutic efficacy of lung cancer was evaluated as progressive disease (PD), with T2bN2M0 stage IIIa. After consultation with the patient’s family, the third chemotherapeutic cycle, comprising albumin–paclitaxel, was started at 200 mg (D1, D8/q3w), and oral nintedanib treatment was continued. In addition, afatinib 30 mg orally once daily was administered as second-line cancer therapy. The patient received four to seven cycles of albumin-paclitaxel treatment on 11 and 30 November 2021, 22 December 2021, and 21 February 2022.
Lung CT examination showed that the former shadow was 4.2 cm × 4.3 cm in size (Figures 1E, J) and stable mild interstitial changes (Figures 2E, J). The largest lymph node in the mediastinum was approximately 2.4 cm × 1.8 cm in size. The remaining abdominal CT, brain MRI, and whole-body bone ECT scans showed no obvious abnormalities or metastasis. A response of stable disease was achieved. The patient showed mild adverse reactions: slightly increased levels of liver transaminase (72 U/L AST (15–40) and 69 U/L GGT (10–60). To date, the patient has maintained the current lung cancer treatment regimen. The process of antitumor therapy for the patient is shown in Figure 6.
Figure 6.
The time axis of anti-tumor therapy.
3 Discussion
By inhibiting PD-(L)-1 and CTLA-4, ICIs can facilitate immune surveillance and enhance immune attack in the tumor immune microenvironment (12). However, ICI-activated immune mechanisms may attack normal tissues by identifying cross-antigens between normal and tumor cells (31), leading to various types of irAEs (32). The occurrence of irAEs may indicate that immunotherapy has already activated the immune system of patients and is attributed to better tumor clearance effects. A meta-analysis including 4,971 cancer patients found a significant association between irAE occurrence and reduced risk of tumor progression after receiving ICIs (16). Multiple retrospective analyses also reported that the development of irAEs was associated with better survival outcomes in patients with NSCLC treated with PD-(L)-1 inhibitors (17, 33), and patients with greater toxicity to ICIs could attain better tumor-killing effects (18). CIP might occur more often and have a faster onset in NSCLC than in other types of cancer (32). Moreover, CIP is especially associated with better ICI efficacy than any other type of irAE in patients with NSCLC (34). In addition, the occurrence of CIP might suggest that patients with NSCLC can achieve a prolonged tumor-regression duration after ICI discontinuation (18, 35, 36).
However, among the cases of CIP with a variety of malignancies, CIP-related death was mainly observed in patients with NSCLC (15, 37). Two meta-analyses including more than 8,000 patients found that the occurrence of low-grade irAEs/CIP (grade 1–2) was significantly associated with a favorable OS and PFS, whereas severe-grade irAEs/CIP (grade 3–4) was not significantly associated with OS but was significantly associated with a favorable PFS and better overall response rate (16, 33, 36). Low-grade CIP is usually mild and easily manageable, with a shorter pneumonitis course and less impairment of the pulmonary function (11). Severe-grade (with or without steroid-refractory) CIP greatly increases the mortality risk of patients with NSCLC during ICIs treatment owing to overlapping risk factors on the respiratory system (9, 38), compromised pulmonary function (15, 39), and progressive respiratory failure (24), despite the good inhibitory effects of ICIs on the primary tumor.
This contradiction unveils the importance of a better clinical strategy for CIP management (especially severe-grade and/or steroid-refractory CIP) to obtain the best clinical efficacy from immunotherapy in patients with NSCLC. Therefore, a deeper understanding of the onset and progression of CIP and its various clinical manifestations is necessary to identify new treatment strategies. Here, we simply analyzed the pathological mechanisms in different stages of CIP disease progression and the histological and imaging manifestations of each stage. We focused on the mechanisms of the (pro)fibrotic stage in the pathological process of CIP and its possible interacting pathways with the tumor in the lung microenvironment.
Current guidelines for CIP management mainly focus on immunomodulatory therapy to relieve the onset of symptoms. In this case, we used high-dose corticosteroids and infliximab (a chimeric immunoglobulin G (IgG) monoclonal antibody directed at tumor necrosis factor alpha (TNF-α)) to control the rapid progressive respiratory failure of the patient and supported him immediately with critical care in the MICU. He was found to be infected with Acinetobacter baumannii, Candida albicans, and Enterocooccusum faecium in the lung, probably due to a compromised immune system after sufficient immunosuppression and was in a very critical condition. As experienced respiratory physicians, we supported him with flexible mechanical ventilation and prone position ventilation, with comprehensive systemic anti-infective therapy, immunomodulatory therapy, and life-support treatment. This patient improved after 1 week and showed a prominent organizing pneumonia pattern in images with GGOs, consolidation, reticulation, thickened stripes, and grids. However, there is no authoritative definition or treatment recommendation for this chronic fibrotic condition of CIP, especially for steroid-refractory patients, for whom slowly tapering oral corticosteroids cannot play a leading role in further treatment.
Based on our knowledge of the treatment of lung fibrotic disorders and lung cancers, we chose nintedanib as an important supportive drug to manage the fibrotic changes and we hoped that it could potentially inhibit tumor recurrence during recuperation. Herein, we also illustrated previous positive clinical and experimental evidence to support nintedanib as a new therapeutic consideration in CIP management, which has been approved as one of the only anti-fibrotic drugs for progressive interstitial lung diseases (ILDs) (27, 40) and idiopathic pulmonary fibrosis (IPF) (41, 42) and has been recognized as a promising anti-tumor drug for advanced patients with NSCLC (mainly adenocarcinoma) in combination with docetaxel (43, 44). Nintedanib also showed interesting performance in current studies, as it significantly enhanced immune recognition of pulmonary cancerous cells (45, 46) and significantly reduced lung consolidation when combined with immunotherapy (45).
Studies have reported that the incidence of CIP is more often associated with smoking history (47), age over 70 years (38), squamous cell carcinoma subtype(> adenocarcinoma subtype) (31, 48, 49), combination therapy (> ICI monotherapy) (50, 51), PD-1 inhibitors (> PD-L1 and CTLA-4 inhibitors) (51, 52), the presence of baseline fibrosis on chest CT scans (11, 53), and pre-existing pulmonary diseases including IPF, ILDs, chronic obstructive pulmonary disease (COPD), and asthma (14, 13, 54). As we could see, the patient in this case had been exposed to a high risk of CIP and then developed severe steroid-refractory CIP, while we could not provide any preventive treatment before CIP onset, as there were no such recommendations in the previous studies. Nintedanib might be a strong candidate to prevent CIP by applying it in combination with ICIs in patients with NSCLC with a high risk of developing CIP.
3.1 Pulmonary injury evolution stages of CIP
The most common symptoms of CIP are non-specific, such as cough, expectoration, and shortness of breath (9, 55). Radiographic features that indirectly reflect the most prominent pulmonary histopathological changes are important for the diagnosis and grading of severity in clinical settings. Despite the huge heterogeneity, some scholars have recognized the following imaging patterns in patients with CIP: acute interstitial pneumonia (AIP), acute respiratory distress syndrome (ARDS), diffuse alveolar damage (DAD), non-fibrotic or fibrotic hypersensitivity pneumonitis (HP), cryptogenic organizing pneumonia (OP), non-specific interstitial pneumonia (NSIP), and other unclassified types (31, 55–57). These patterns can be classified into different evolution phases or different toxicity grades, from the excessive inflammatory stage or the highest grade (AIP/ARDS/DAD) to the proliferative organizing (nonfibrotic HP/OP) and fibrotic stages (fibrotic HP/NSIP) in lower grades (31, 57, 58). In general, all histopathological changes develop from abnormal inflammation resulting from an ICI-activated immune system. Similar to wound healing in lung injuries, mild or severe pulmonary fibrotic changes would always accompany the exaggeration or regression of inflammation (59, 60), which means that inflammatory, organizing, and fibrotic changes could occur simultaneously within the lung tissue (61, 62). The severity and duration of inflammation and the rate of absorption of the damaging exudation determine the clinical and pathological features of CIP in the initial episode before treatment.
The current treatment strategy for CIP mainly involves negative immunomodulatory therapy based on the symptom grade from G1 to G4, including pulse and/or tapering corticosteroids, ICI discontinuation, and immunosuppressant addition (19, 20). Immunosuppressants are usually temporarily applied in steroid-refractory patients who present inadequate response after 48–72 h of corticosteroid treatment (21). Immunosuppressants should be cautiously used, considering their potent immunosuppressive efficacy and unclear negative effects on patients with NSCLC-CIP. First, excessive suppression of the immune system could lead to opportunistic infections very quickly (63–65), which would prolong the duration of critical conditions and increase the complexity of treatment. Second, durable antitumor activity might exist after discontinuation of ICI therapy (35, 66, 67), whereas immunosuppressants, such as infliximab, could weaken the ongoing antitumor immune activity initially launched by ICI treatment (22). As mentioned previously, patients with a severe-grade CIP might achieve better tumor remission, although the clinical symptoms of CIP are life threatening. However, this advantage can be counteracted by CIP treatment, leading to an extremely poor prognosis. These findings are consistent with clinical data. Previous real-world studies have reported that more than half of patients with grade 3 CIP died during ongoing corticosteroid treatment (11) or after receiving additional immunosuppressive drugs (4). In addition, infliximab can cause interstitial lung disease (ILD) (68), which increases the risk of interstitial fibrosis.
In this case, the patient developed secondary A. baumannii-induced pneumonia beyond severe-grade CIP and experienced severe interstitial fibrotic changes after receiving high-dose corticosteroids and infliximab. There are no clear recommendations for the sequential maintenance strategy of steroid-refractory CIP patients, especially for those with severe grade. Neither the optimal corticosteroid tapering doses/duration nor further treatment plan for clinical complexity after receiving immunosuppressants is uncertain. This may be attributed to the lack of clinical data caused by premature death in these patients or the lack of consensus on the follow-up pathological mechanism and trends of CIP. Here, we support the longitudinal view of pathological development (23) to divide the pulmonary injury evolution process of CIP into three phases, from inflammation to fibrosis. This separate definition of the fibrotic phase in CIP out of usual pneumonitis grades (G1–G4) typing classification is crucial for treatment: immunosuppressives for inflammation and anti-fibrotics for fibrosis.
3.1.1 Acute inflammatory stage of CIP
Acute exaggerated inflammatory reactions in lung tissues contribute to the progressive reduction in pulmonary function, the primary cause of mortality in patients (69). Histologically, the acute phase of CIP is characterized by acute inflammatory and/or fibrinous exudation in the alveolar cavities (24, 55), accompanied by interstitial inflammatory infiltration and/or fibrosis, which is called mild organization formation (2, 31, 57). Interstitial inflammatory infiltration may include elevated levels of eosinophils, poorly formed granulomas, and lymphocytes (4, 69, 70).
3.1.1.1 Cytotoxic effects of T cells on instigation of CIP
The markedly increased percentage of lymphocytes infiltrating the malignant cells shows the superior tumor immune-clearance effects of ICIs (18). However, enhanced targeted T-cell activity can attack the cross-antigens shared between the tumor and normal lung tissues, leading to off-target toxicity (2, 8, 71). Significant lymphocytosis enriched with CD4+ T cells (72) and CD8+ T cells (70, 73, 74) has been examined in the pulmonary tissues and BAL of patients with CIP, reflecting the participation of lymphocyte-mediated exaggerated immunological reactions. The CD4+ T-cell compartment showed an increase in the number of pathogenic T-helper 17.1 cells, while within the CD8+ T-cell compartment, the number of effector memory T cells were mainly increased (75). An increased proportion of activated autoimmune indicators, CD3+ T cells/HLA-DR + T cells, was associated with the severity grading of CIP (39). Malignant NSCLC tumor cells may have more cross-antigens than normal lung tissues, as they are incubated in the same pulmonary environment (76). Tumor antigens, autoantigens, and neoantigens released from cytotoxic T-lymphocyte-mediated cell lysis induced persistent amplification of immune responses through “epitope spreading” (77). T cells from tumor-infiltrating lymphocytes shared a notable overlap in receptor sequencing with the T cells infiltrating the inflammatory CIP lesions, but not with the secondary lymphoid organs or peripheral blood (78). In addition, decreased expression of PD-1 and CTLA-4 weakened the immune tolerance/anti-inflammatory function of the Treg population (79), whereas the number of central memory T cells with proinflammatory subsets increased (78), which contributes to tumor clearance and the inflammatory microenvironment.
3.1.1.2 Increased proinflammatory cytokines and autoantibodies
Laboratory plasma and BALF examinations of patients with NSCLC-CIP showed an increasing spectrum of common inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-17A, IL-35, C-reactive protein, and procalcitonin (72, 76, 80, 81). The levels of surfactant protein-A, surfactant protein-D, and Krebs von den Lungen-6 (KL-6) produced by type II alveolar epithelial cells, which reflect alveolar epithelial cell injury, were also increased (75, 76, 82, 83). These biomarkers usually would decrease to normal levels when the initial respiratory symptoms are relieved. Notably, in addition to the known autoantibodies associated with autoimmune diseases with a higher incidence of CIP, some studies reported a nearly 1.34-fold increase in anti-CD74 plasma level (84) in patients with CIP, while CD74 was related to interstitial pneumonitis (85, 86).
3.1.2 Subacute and chronic phase of CIP: profibrotic and fibrotic stages
For most CIP patients with mild to moderate grades, inflammatory exudation would be absorbed efficiently, with complete weaning off of steroids after 6–8 weeks of initial treatment, leaving mild asymptomatic lesions in the lungs (39, 55). Higher severity and longer duration of inflammation in CIP might exert a great influence on the alveolar microenvironment (72). This could excessively trigger the body’s wound-healing mechanisms (87), leading to persistent reparative attempts by the lung, which manifest as pneumocyte hyperplasia, alveolar epithelial hyperplasia, fibroblastic proliferation, fibrous tissue hyperplasia, alveolar septal thickening, interstitial fibrosis, and lymphocyte infiltration (9, 55, 58, 79). The absorption rate of the lesions is slow or unchanged over an extended period. Even when inflammation is gradually suppressed, activated, and amplified, fibrotic processes may not stop. Chronic CIP was clinically defined as the occurrence of persistent or worsened CIP after steroid taper and CIP recrudescence during steroid taper, which extended the course of immunosuppression to more than 12 weeks (23, 39, 55). During this stage, fibrosing interstitial lung diseases (such as entirely damaged normal lung structure, thickening, and occlusion of blood vessels) are dominant in histology, which might impair air–blood exchange efficiency in the lungs (2, 11, 23). The typical sequelae might be sustained pulmonary interstitial fibrosis and poor pulmonary function caused by severe CIP (39). These patients are more vulnerable to irreversible fibrosis under certain conditions that permanently deteriorate the pulmonary function. Anti-fibrotic agents are thus necessary for this condition.
3.1.2.1 Possible mechanisms in profibrotic and fibrotic stages
Multi-causal fibrotic lung injuries may have pathophysiological mechanisms that overlap with those of idiopathic pulmonary fibrosis, suggesting the potential to share common treatment approaches (59, 88, 89). Early treatment is crucial for slowing the decline in lung function and improving clinical outcomes.
Repetitive injury and reprogramming of the alveolar epithelium (59) are significant triggers of fibrosing progression, inducing an aberrant wound healing response that is characterized by proliferation of fibroblasts and myofibroblasts (61), extracellular matrix (ECM) remodeling (62), and subsequent loss of lung architecture and function (60, 88, 89). Activated fibroblasts and myofibroblasts can secrete ECM components, while myofibroblasts can produce contractile apparatus, such as α-smooth muscle actin microfilaments, which leads to the formation of fibroblastic foci and deposition of ECM (90). Fibroblastic foci in the alveolar parenchyma (91–93) and collagen expansion of the alveolar septa (94) have been detected in CIP lungs. These changes can destroy normal lung structures, causing a characteristic “honeycombing” morphology.
Profibrotic mediators, such as IL-1β (95), transforming growth factor (TGF)-β (96), sphingosine1-phosphate (S1P) (97), and WNT (98) ligands, are involved in this process. TGF-β can induce an unabated form of the fibrotic process called epithelial-to-mesenchymal transition (EMT) (99), in which alveolar epithelial type II cells (undergoing endoplasmic reticulum stress (100), mitochondrial dysfunction (101), or senescence (102) can serve as an unlimited source for the increased myofibroblast-like pool, if the primary inflammatory insult is not attenuated. The microvascular pulmonary endothelium attached to the alveolar epithelium could also become another source of myofibroblasts by endothelial-to-mesenchymal transition (103), which potentially contributes to lung fibrosis and pulmonary hypertension. In addition, immune cells have been detected to infiltrate the chronic inflammatory (91, 93, 94) and fibrotic lesions of CIP. Mast cells (MCs) are innate immune cells that play a key role in the early response to tissue injury (104) and can release an array of profibrotic mediators (105–107), such as histamine, TGF-β (108), MC tryptase (109), and MC chymase (110). MCs would infiltrate immediately the surrounding fibroblast foci, forming a mutually excitatory MC–fibroblast crosstalk in fibrosis that activates the proliferation of MC and fibroblast (106). The number of MCs is positively correlated with the number of fibroblast foci, which has been linked to increased mortality.
3.1.2.2 Crosstalk between pulmonary fibrosis and lung cancer
One particularly special condition in patients with NSCLC-CIP is that both cancer development and immunotherapy adverse effects occur in the lungs. Tumor cells are not isolated but are in constant communication with other cell types within the lung tissue, which forms a particular microenvironment (111). Lung resident fibroblasts surrounding the malignancy are considered first responders to the site of insult that the tumor creates (112), while primary cancerous cells can also infiltrate the abnormal microenvironment that undergoes inflammatory and fibrotic processes of CIP, with the possibility of various cellular active substances and signaling molecules interacting with each other (113, 114).
As early as 1965, the crosstalk between lung cancer and pulmonary fibrosis has been suggested (115), which might pathologically exacerbate their progression (111, 113). The tumor stroma, fibrovascular networks, and tumor microenvironment are vital for the preservation and proliferation of the tumor and its structural integrity. Bronchiolar epithelial cells can undergo reversible EMT (116) during the early stages of carcinogenesis, cancer invasion, and metastasis. Cancer-associated fibroblasts (CAFs) are important players in lung cancer because they exhibit mesenchymal-like features and help build the structure of the tumor stroma (117) with heterogeneous phenotypes. The tumor vasculature can establish fibrovascular networks for the preparation of invasion. Endothelial-to-mesenchymal transition is thought to be the source of 40% of CAFs (118) and may play a role in tumor angiogenic sprouting into adjacent tissues. Through the action of platelet-derived growth factor-BB (PDGF-BB), vascular pericytes released by tumor microvasculature can differentiate into stromal fibroblasts (119), which significantly contributes to tumor invasion and metastasis. Profibrotic mediators, such as TGF-β, are also chronically overexpressed in lung cancer (112). Tyrosine kinase receptor ligands, such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF), are aberrantly expressed in lung cancer and IPF (114). For example, PDGF plays a critical role in stimulating the secretion of ECM components and growth factors, thereby promoting fibroblast proliferation and recruiting fibrocytes to the lungs.
3.2 Nintedanib
Nintedanib (development code: BIBF 1120) is a molecule that does not exist in nature and is a pure synthetic compound synthesized in 1998 during a study on small-molecule inhibitors of angiogenesis (25) [ATC Code: L01XE31 (120)]. Herein, we present the classic concepts and novel experimental results regarding the functional mechanisms of nintedanib to explore its potential efficacy in cancer patients with existing or secondary pulmonary fibrosis (such as CIP).
3.2.1 Mode of action
Receptor tyrosine kinases (RTKs) belong to a group of key enzymes that catalyze important cellular quality and processes, such as cell shape, cell motility, cell cycle control, functional differentiation, gene transcription, and synaptic transmission (121). Abnormal or excessive tyrosine phosphorylation is associated with various cancers (122, 123), inflammatory diseases (124, 125), and pulmonary fibrotic diseases, such as IPF and ILD (126). Therefore, drugs that antagonize related protein tyrosine kinases and phosphatases are expected to control these diseases.
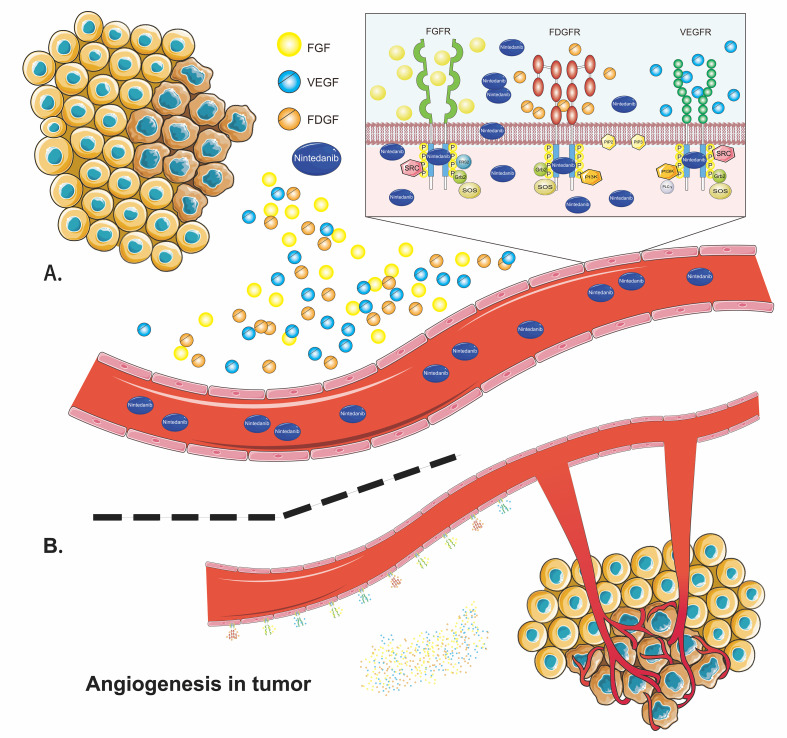
Nintedanib is a small-molecule tyrosine kinase inhibitor that competitively binds to the ATP-binding pocket of kinase to block intracellular signaling cascades (Figure 7) (127). It potently inhibits the RTKs implicated in the pathogenesis of various diseases, including vascular endothelial growth factor receptors (VEGFRs), fibroblast growth factor receptors (FGFRs), and platelet-derived growth factor receptors (PDGFRs) (128, 129). Nintedanib also inhibits the receptor tyrosine kinase FLT-3 and non-receptor tyrosine kinases Lck, Lyn, and Src (128), although the contribution of inhibition of these kinases to the therapeutic activity of nintedanib has been less reported (130). In addition to competitively binding to ATP receptor pockets, nintedanib blocks protein kinase activity by allosterically modulating (131) the conformation of ATP-binding sites to inhibit phosphorylation (132). Therefore, nintedanib may exert its anti-angiogenesis, anti-fibrotic, anti-inflammatory, and anti-tumor functions by separately or simultaneously blocking these tyrosine kinases.
Figure 7.
The basic mechanism of nintedanib.
The VEGF family binds to VEGFR-1, VEGFR-2, and VEGFR-3 (133). It can promote endothelial cell proliferation and survival, increase vascular permeability, and regulate tumor-related angiogenesis, which is critical for tumor growth and metastasis (134, 135). In NSCLC, elevated VEGF/VEGFR expression is associated with poor prognosis (136). Nintedanib inhibits the proliferation of three types of cells involved in angiogenesis by blocking VEGFRs: endothelial cells, pericytes, and smooth muscle cells (128, 137). There are four types of FGF receptors: FGFR-1, FGFR-2, FGFR-3, and FGFR-4 (138). FGFs play an important role in tissue repair, angiogenesis, inflammation, and tumor initiation and progression (139, 140). Studies have shown that blocking FGFRs can reduce alveolar interstitial fibrosis and inhibit proliferation, migration, and differentiation of fibroblasts to myofibroblasts (141, 142). FGFRs might be anti-tumor therapeutic targets, as FGFR-1 amplification occurs in approximately 20% of patients with SqCLC and is associated with a poor prognosis (143). The PDGF family interacts with homodimers or heterodimers of PDGFR-α/β (144). The PDGF/PDGFR pathway promotes the proliferation, survival, and migration primarily of cells of mesenchymal origin (142). It is associated with fibrosis, cancer proliferation, metastasis, invasion, and angiogenesis (145, 146). Together with FGFRs, PDGFRs regulate the migration and adhesion of pericytes and the transformation of smooth muscle cells to endothelial cells, providing support and stability to vascular walls (144). Nintedanib could reduce the number of blood platelets, block the differentiation of fibroblasts to myofibroblasts, inhibit EMT, and suppress inflammation and angiogenesis by inhibiting these receptors (133, 147).
Pulmonary interstitial inflammatory infiltration in fibrotic lesions may include elevated levels of eosinophils, poorly formed granulomas, and lymphocytes (4). Nintedanib significantly reduced the infiltration of immune cells (including mast cells [MCs] and eosinophils) and neutrophils (147), but not that of macrophages, inhibited granuloma formation (148), and decreased the levels of other proinflammatory cytokines, including IL-4, IL-5, IL-6, and IL-13 (149), during lung fibrosis. Notably, the significant reduction in lymphocyte count by nintedanib was dependent on the early initiation of treatment (150). As mentioned in Section 3.1.2, the number of MCs was increased in the region immediately surrounding the fibroblast foci, activating crosstalk with fibroblasts in fibrosis (106). Nintedanib could inhibit recombinant stem cell factor-induced MC survival by directly blocking the phosphorylation of stem cell factor-stimulated c-kit (a type of tyrosine kinase receptor also called stem cell factor receptor) and could reduce the infiltration of MCs (147). Nintedanib also significantly reduced the secretion of TIMP-1, IL-1β, and TGF-β, factors that play key roles in the proinflammatory and profibrotic pathways of pulmonary fibrotic changes (40). Nintedanib reduced pulmonary fibrosis by inhibiting growth-factor-induced proliferation (151) and motility (150) of lung fibroblasts, TGF-β-induced transformation of fibroblasts to myofibroblasts, EMT with increased E-cadherin levels (152), and ECM collagen secretion and ECM deposition (40), and reducing fibrotic gene expression (including collagen 1a1 and fibronectin) (153). Repetitive injury and reprogramming of the lung epithelium are considered critical drivers of fibrosis progression. Nintedanib increased the expression of the transcription factor Nkx2.1 in isolated ATII cells and stabilized the expression of distal lung epithelial cell markers, especially SP-C, to restore normal alveolar epithelial cell function that contributes to its anti-fibrotic effects (153).
Only a few studies have explored the antitumor mechanism of nintedanib. Nintedanib notably increased the ratio of α-smooth muscle actin+/CD31+, a sign of tumor vessel normalization, and reduced distorted vessel density in tumors (45). It also suppressed tumor proliferation by inhibiting CAFs (46, 154) and may inhibit tumor metastasis by blocking EMT (147). Moreover, recent evidence suggests that nintedanib can boost the efficacy of immunotherapy by upregulating MHC-I and PD-L1 expression and increasing the infiltration of CD8+ T cells and DC cell maturation to enhance tumor sensitivity to immunotherapy in both in vivo and in vitro experiments (45, 46), which warrants further clinical evaluation.
3.2.2 Therapeutic efficacy and safety
As a triple angiokinase inhibitor, nintedanib has been clinically studied for pulmonary fibrosis and solid tumors. It is one of the only two drugs currently available to treat IPF (155) and is the only drug approved for use in patients with other progressive fibrosing ILDs and SSc-ILD (156). Nintedanib, in combination with docetaxel, has also been approved by the European Union (30) for the treatment of patients with advanced adenocarcinoma NSCLC. Herein, we summarize previous clinical and experimental evidence of the anti-fibrotic and anti-tumor therapeutic efficacy of nintedanib.
3.2.2.1 In pulmonary fibrosis: potent anti-fibrotic efficacy
Anti-fibrotic drugs, although not curative, can slow ILD progression and have facilitated a paradigm shift in the treatment of IPF over the last decade (150). Nintedanib has been shown to have anti-fibrotic and anti-inflammatory effects in animal models (148) and has shown potent anti-fibrotic effects in well-designed clinical trials of IPF and ILDs (27). It significantly reduced forced vital capacity decline and prolonged the time to first acute exacerbation in patients with IPF (157), irrespective of existing physiological impairment, persistent positive anti-fibrotic effects to slow down IPF progression over more than 4 years of treatment in the INPULSIS trial (42), and improved OS and PFS in the EMPIRE registry (158). Nintedanib has achieved similar effects in reducing annual rates of forced vital capacity decline in patients with chronic fibrosing pulmonary interstitial diseases with a progressive phenotype (such as chronic hypersensitivity pneumonitis and autoimmune ILDs) in the INBUILD (40, 159) and SENSCIS trials (26). Nintedanib has also been shown to reduce lung function deterioration in patients with other fibrotic patterns to reduce lung function deterioration (28, 27, 160).
3.2.2.2 In cancer: Positive anti-tumor efficacy
Nintedanib has shown positive anti-tumor effects in clinical trials on esophageal (161), colon (162), breast (163), prostate (164), and lung cancers, thus attracting increased attention from researchers. Modest disease stabilization was observed in patients undergoing metastatic esophagogastric treatment with nintedanib (161). Nintedanib modulates tumor blood flow and permeability in patients with advanced refractory colorectal cancer (162). In combination with standard chemotherapy, a full dose of nintedanib achieved a 50% pathological complete response in early HER-2-negative breast cancer (163). Nintedanib in combination with afatinib (BIBW 2992), an ErbB family blocker, has shown limited antitumor activity in patients with advanced castration-resistant prostate cancer (164). Combined nintedanib plus bevacizumab treatment achieved a disease control rate of 72.2% in patients with solid tumors (lung, colon, and cervical), even in those with bevacizumab resistance (165).
According to clinical trials of patients with lung cancer, nintedanib monotherapy exhibited relatively inferior efficacy compared to combination therapy (Table 2). In 2011, Reck et al. studied the efficacy of nintedanib monotherapy for patients with advanced NSCLC (172). They reported an mPFS of 6.9 weeks, mOS of 21.9 weeks, and disease control rate (DCR) of 49.3% (172). Nintedanib monotherapy has also shown limited activity in relapsed SCLC (173). In later trials, the combination of nintedanib plus pemetrexed achieved a better DCR ranging from 60.9% to 66.7% in patients with advanced recurrent NSCLC (44, 166, 167). Doebele et al. tested nintedanib, in combination with paclitaxel plus carboplatin, in chemotherapy-naive patients with advanced NSCLC and observed a DCR of 84.6% (168). For patients with advanced SqCLC, the LUME-Lung 3 study reported a DCR of 81.3% under first-line combination therapy of nintedanib plus cisplatin plus gemcitabine, with an mPFS of 4.2 months and an mOS of 6.7 months (169). In lung adenocarcinoma, combined nintedanib and docetaxel therapy has been approved as first-line chemotherapy in the European Union (30). The DCR in trials of this combination after first-line chemotherapy ranged from 54.7% to 72.7%, with the longest mPFS and mOS of 5.7 and 12.6 months, respectively (43, 170, 171, 174–176). Three non-interventional studies reported the efficacy of nintedanib and docetaxel after chemo-immunotherapy with the DCR of 78.2%–86% (29, 177, 178). Nintedfanib has shown potent antitumor efficacy combined with chemotherapeutics in patients with NSCLC after first-line chemotherapy or chemo-immunotherapy. As most of these trials were conducted in European countries, more clinical evidence from other regions is warranted.
Table 2.
From phase I to phase III clinical trials on nintedanib for lung cancers.
| Clinical trial (phase) | Reference (details in the annotation) | Year | Patient characteristics | After chemo or chemoimmunotherapy | Patients(n) | Drug combination | Comparator | N dose/frequency | Response n(%) | Disease control | mPFS (mo) | mOS (mo) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete response | Partial response | Stable disease | ||||||||||||
| I | Ellis et al. (166) | 2010 | Recurrent NSCLC | previously treated with one first-line platinum-based chemotherapy regimen | 26 | Pemetrexed + BIBF 1120 (Former Nintedanib) |
– | 100 or 150 or 200 or 250 mg/bid | 1(0.04%) | 4(15.4%) | 13(50%) | 17(65.3%) | 5.4 | NA |
| I | Daga et al. (167) | 2015 | Stage IIIB/IV or recurrent NSCLC | after or failure of prior first-line chemotherapy | 18 | Pemetrexed + Nintedanib | – | 100 or 150 or 200 mg/bid | 0 | 2(11.1%) | 10(55.6%) | 12(66.7%) | NA | NA |
| I | Doebele et al. (168) | 2012 | Chemotherapy-naive advanced NSCLC | as first-line treatment | 26 | Paclitaxel + carboplatin + Nintedanib | – | 50 mg/bid | 0 | 7(26.9%) | 15(57.7%) | 22(84.6%) | NA | NA |
| I | LUME-Lung 3 study (169) | 2018 | Advanced sqNSCLC | as first-line treatment | 4+12 | Cisplatin + Gemcitabine + Nintedanib (150mg bid) | Cisplatin + Gemcitabine + Nintedanib (200mg bid) | 150 or 200 mg/bid | 0 | 5(31.3%) | 8(50%) | 13(81.3%) | 4.2 | 6.7 |
| I | Okamoto et al. (170) | 2015 | Stage IIIB/IV or recurrent NSCLC | had received one platinum-based chemotherapy regimen (not containing docetaxel) | 38 | Docetaxel + Nintedanib | – | 100 or 150 or 200 mg/bid | 0 | 10(26.3%) | 18(47.3%) | 28(73.7%) | 5.7 | NA |
| Ib | Yamamoto et al. (171) | 2018 | Lung adenocarcinoma | after the failure of first-line platinum- based chemotherapy | 10 | Docetaxel + Nintedanib | – | 200 mg/bid | 0 | 4(40%) | 3(30%) | 7(70%) | NA | NA |
| II | Reck et al. (172) | 2011 | Stage IIIB/IV or recurrent NSCLC | after chemotherapy (including one platinum-based chemotherapy) | 73 | BIBF 1120 (Former Nintedanib) |
– | 150 or 250 mg/bid | 0 | 1(1.4%) | 35(47.9%) | 36(49.3%) | 6.9w | 21.9w |
| II | Youn Han et al. (173) | 2016 | relapsed/refractory SCLC | during or after treatment with at least one platinum-based chemotherapy | 22 | Nintedanib | – | 200 mg/bid | 0 | 1(0.05%) | 7(31.8%) | 8(36%) | 1 | 9.8 |
| II | REFRACT GFPC 02-15 study (174) | 2021 | Advanced NsqNSCLC | documented progression during first-line chemotherapy based on a platinum-doublet and third- generation drug for ≤ 4 cycles | 53 | Docetaxel + Nintedanib | – | 200 mg/bid | 0 | 10(18.9%) | 19(35.8%) | 29(54.7%) | 2.7 | 6.9 |
| IIb | SENECA trial (175) | 2019 | Recurrent NsqNSCLC | received one previous chemotherapy regimen | 85+85 | Docetaxel (33mg/mq) + Nintedanib | Docetaxel (75mg/mq) + Nintedanib | 200 mg/bid | NA | NA | NA | 107(63.0%) vs. 124(72.7%) | 4.79 vs. 4.82 | 8.49 vs. 9.62 |
| III | LUME-Lung 1 study (43) | 2014 | Stage IIIB/IV or recurrent NSCLC | after first-line chemotherapy | 655+659 | Docetaxel + Nintedanib | Docetaxel + Placebo | 200 mg/bid | 0 vs. 1 (0.2%) | 29(4.4%) vs. 21(3.2%) | 325(49.6%) vs. 250(37.9%) | 354(54.0%) vs. 272(41.3%) | 3.4 vs. 2.7 | 10.1 vs. 9.1 |
| III | LUME-Lung 1 study (43) | 2014 | Stage IIIB/IV or recurrent lung adenocarcinoma | after first-line chemotherapy | 332+336 | Docetaxel + Nintedanib | Docetaxel + Placebo | 200 mg/bid | 0 vs. 0 | 15(4.7%) vs. 12(3.6%) | 179(55.6%) vs. 136(40.5%) | 194(60.2%) vs. 148(44.0%) | 4.2 vs. 1.5 | 12.6 vs. 10.3 |
| III | LUME-Lung 2 study (44) | 2016 | Stage IIIB/IV or recurrent NsqNSCLC | had received one prior chemotherapy | 353+360 | Pemetrexed + Nintedanib | Pemetrexed + Placebo | 200 mg/bid | 0 | 32(9.1%) vs. 30(8.3%) | 183(51.8%) vs. 162(45.0%) | 215(60.9%) vs. 192(53.3%) | 4.4 vs. 3.6 | 12.0 vs. 12.7 |
| III | LUME-Lung 2 study (44) | 2016 | Stage IIIB/IV or recurrent lung adenocarcinoma | had received one prior chemotherapy | 335+335 | Pemetrexed + Nintedanib | Pemetrexed + Placebo | 200 mg/bid | 0 | 32(9.1%) vs. 30(8.3%) | 175(52.2%) vs. 153(45.7%) | 207(61.8%) vs. 183(54.6%) | 4.5 vs. 3.9 | 12.3 vs. 13.1 |
| III | Cid JR et al. (176) | 2016 | Advanced lung adenocarcinoma | NA | 99 | Docetaxel + Nintedanib | – | 200 mg/bid | 0 | 27(27.2%) | 52(52.5%) | 79(79.6%) | NA | NA |
| *Non-interventional study | Corral et al. (29) | 2019 | Advanced lung adenocarcinoma after chemotherapy and immunotherapy | After chemo-immunotherapy | 11 | Docetaxel + Nintedanib | – | 200mg/bid | 0 | 4(36%) | 5(46%) | 9(82%) | 3.2 | NA |
| *Non-interventional study | VARGADO (177) | 2022 | Advanced lung adenocarcinoma after chemotherapy and immunotherapy | After chemo-immunotherapy | 80 | Docetaxel + Nintedanib | – | 200mg/bid | 1(1.6%) | 31(48.4%) | 23(35.9%) | 55(86%)(total=64) | 6.4 | 12.1 |
| *Non-interventional study | LUME-BioNIS study (178) | 2020 | Advanced lung adenocarcinoma after chemotherapy and immunotherapy | After chemo-immunotherapy | 55 | Docetaxel + Nintedanib | – | 200mg/bid | 0 | 10(18.2%) | 33(60%) | 43(78.2%) | 4.6 | 8.8 |
3.2.2.3 In patients with NSCLC-CIP: Potential of dual anti-fibrotic and anti-tumor efficacy
Whereas baseline pulmonary fibrosis diseases, such as IPF and ILD, are considered to limit ICI application and severely impair the survival outcomes of patients with NSCLC, nintedanib therapy is a valuable strategy for patients with cancer, complicated with preexisting pulmonary fibrosis. Fukunaga et al. reported that nintedanib inhibited the growth of a newly discovered nodule for 9 months during AE-IPF treatment; the tumor started to increase in size 4 months after the cessation of nintedanib, and the patient was diagnosed with squamous cell carcinoma (41). Nintedanib can exert dual anti-fibrotic and anti-tumor effects in patients with NSCLC with baseline pulmonary fibrosis. Shiratori et al. recently reported that nintedanib monotherapy successfully relieved pulmonary fibrosis and achieved remission of the primary tumor and pleural disseminations in an elderly patient with NSCLC complicated by IPF when he could not tolerate cytotoxic chemotherapy due to IPF progression (179).
According to immunotherapy, a significantly higher incidence rate (10) of any grade of CIP has been reported in patients with NSCLC with preexisting ILD. Unspecified baseline fibrosis was the strongest independent predictor of CIP in patients with NSCLC (11), compared with pertinent demographics and PD-L1 expression. Risk factors for chronic pulmonary fibrosis diseases (180) considerably overlap with those for lung cancer, which may account for the increased burden of CIP in lung cancer patients with preexisting pulmonary fibrosis. However, some of these overlapping risk markers, such as smoking history, are associated with a better clinical outcome in patients with NSCLC treated with ICIs (181); this may explain the favorable clinical overall response rate of immunotherapy observed in patients with NSCLC with preexisting ILD (182–186), as most had a history of smoking. Another meta-analysis that included 10 East Asian studies pooled a significantly higher overall response rate and DCR after PD-(L)-1 inhibitor treatment in patients with preexisting ILD than in those without ILD (10). The reported favorable efficacy might sway the common belief that preexisting pulmonary fibrosis is a contraindication for immunotherapy. It might be an appropriate choice to combine nintedanib with immunotherapy for patients with preexisting pulmonary fibrosis.
In addition, the occurrence of CIP many be strongly correlated with a better tumor remission outcome, as mentioned previously at the beginning of Section 3. Beyond baseline fibrosis, patients with NSCLC with a smoking history, older age, squamous cell carcinoma subtype, and pre-existing pulmonary diseases, and those using PD-1 inhibitor were more likely to be at risk of CIP. Thus, patients with a higher risk of developing CIP, especially those with preexisting pulmonary fibrosis, should be closely monitored during ICI therapy or after ICI termination and should be given early diagnosis and intervention under a multidisciplinary approach to obtain the best clinical efficacy from ICIs. Notably, CIP may develop months after ICIs discontinuation (187). Nintedanib could slow down the decline rate of forced vital capacity (40) and potentially strengthen the prevention of CIP (188) when used in combination with ICIs or after ICIs in cancer patients.
Beyond its potential to alleviate pulmonary fibrosis and overcome ICI side effects, nintedanib can increase the efficacy of immunotherapy and overcome ICI resistance (46, 45). The synergistic effect of nintedanib and anti-PD-1 therapy on lung cancers was revealed in in vitro and in vivo experiments (45); significantly increased ICI therapy responses, relieved aggravated lung injuries, inhibited tumor metastasis, and activated tumor immune microenvironment were observed (46). Nintedanib upregulated the expression of PD-L1 and MHC-I in tumor cells, boosted the immunological recognition and immune clearance of tumor cells, and increased the infiltration and activation of immune cells in tumor tissues, thus improving the efficacy of immunotherapy and overcoming partial ICI resistance (45). In addition, it significantly reduced pulmonary fibrosis in the nintedanib+anti-PD-L1 group, whereas evident pulmonary consolidation was observed in the control and anti-PD-L1 groups (45). These results suggest the dual potential of nintedanib to boost the tumor clearance effects of immunotherapy and prevent or alleviate the development of CIP when applied in combination with ICIs.
A current monocentric phase Ib cohort that studied the safety and efficacy of nintedanib in combination with pembrolizumab in patients with advanced solid tumors reported no case of CIP after combined treatment (189), and nintedanib increased Treg recruitment and circulation of helper memory T cells in the tumor microenvironment to enhance the tumoral immune response (189). These findings confirm the observed antitumor efficacy of nintedanib independent of angiokinase activity (130), which warrants further investigations to explore the antitumor usage of nintedanib in combination with immunotherapy.
Pharmacokinetic studies have shown that nintedanib is rapidly absorbed after oral administration. The pharmacokinetics of nintedanib are time independent and dose linear (172). In patients with advanced cancer, twice-daily administration could achieve a stable state for up to 7 days (127, 190), with a terminal half-life of 7–19 h (129). The cumulative effect of repeated administration was negligible (190). However, current studies have demonstrated that nintedanib can act against a wide range of diseases; however, its low bioavailability is a future challenge for maximizing its effects (191).
In clinical trials, nintedanib has a controlled safety profile, in combination with docetaxel (192), pemetrexed (166), paclitaxel/carboplatin (168), and afatinib (193), with a maximum tolerated dose of 200 mg B.I.D. Blockade of VEGF leads to decreased platelet activity and decreased leukocyte adhesion (194). It has anticoagulant effects and increases the risk of bleeding and thrombosis (165). In addition, blocking PDGF-α and PDGF-β can lead to thrombocytopenia by affecting platelet production (161). Adverse events associated with anti-angiogenic agents in cancer therapy include thromboembolic events, gastrointestinal perforation, bleeding, hypertension, and proteinuria (194). In SqCLCs, bevacizumab (a monoclonal antibody that targets the VEGFR ligand) is not recommended because of the increased risk of severe or even fatal pulmonary hemorrhage (195). Other anti-VEGF small-molecule TKIs, sorafenib and sunitinib, can induce skin-associated adverse events (135), and sorafenib, in combination with carboplatin and paclitaxel, increases the risk of death (196).
Nintedanib is an antiangiogenic agent with an acceptable safety profile and good tolerance in patients with cancer (174). In clinical trials, gastrointestinal reactions, such as mild nausea or vomiting, were the most common adverse events associated with nintedanib (189, 192). Although nintedanib blocks VEGF and PDGF receptors, resulting in a potentially increased risk of bleeding, there is no increased incidence of cardiovascular or bleeding complications observed in large clinical trials involving nintedanib (43, 157, 175). It should be noted that clinical trials have excluded patients with a bleeding tendency during enrollment, which might have resulted in biased results. Moreover, a post-marketing data review showed that <5% of 6,758 patients treated with nintedanib experienced adverse bleeding events, and <1% of patients experienced major bleeding events for approximately 1 year (30). Bleeding events most often involve the digestive, respiratory, and central nervous systems (42). Mild epistaxis was the most common bleeding event (177).
The most common drug-related adverse reactions in patients with advanced NSCLC treated with nintedanib monotherapy were nausea (57.5%), diarrhea (47.9%), vomiting (42.5%), anorexia (28.8%), abdominal pain (13.7%), and reversible elevation of alanine aminotransferase (13.7%) and aspartate aminotransferase (9.6%) levels (172). In the LUME-BioNIS study, the most common on-treatment ADRs/adverse events of nintedanib plus docetaxel were diarrhea (32.3%), malignant neoplasm progression (29.2%), and nausea (15.4%) (178). A phase II study of nintedanib therapy in relapsing small cell lung cancer identified elevated AST/ALT levels and neutropenia as major causes of treatment delay and subsequent dose adjustment (173).
In the present case, the elderly patient showed good tolerance to nintedanib monotherapy (200 mg B.I.D) for long-term treatment and had no adverse hemorrhagic reactions, except for slight dizziness and nausea after nintedanib initiation, which improved later.
3.2.3 Potential of nintedanib in patients with NSCLC with G3-G4 CIP
3.2.3.1 Dual management advantages in critical stage of CIP with good tolerance
Patients who experienced deteriorated or maintained CIP were significantly more likely to have a poor prognosis than those who experienced improved or resolved CIP (9, 11). Those who experienced G3–G4 CIP had to quit ICI therapy to avoid lethal respiratory failure (20), and their physicians tended to hesitate or delay commencement or continuation of aggressive anti-tumor treatment owing to deteriorating physical status, abrasive pulmonary symptoms, and prolonged immunosuppressive management for CIP. Moreover, some immunosuppressive agents, such as infliximab, might weaken the ongoing antitumor immune activity initially launched by ICI treatment (22) during this critical stage.
Nintedanib, with its good safety profile, may be a potent candidate to fill this gap by inhibiting tumor growth under critical conditions and alleviating potential pulmonary fibrosis caused by CIP pathogenesis. However, its efficacy and safety in CIP treatment have only been reported in one case. Xie et al. reported a case of CIP caused by pembrolizumab in a patient with NSCLC (adenocarcinoma) who was successfully treated with nintedanib. However, the patient experienced rapid tumor progression after 2 weeks of nintedanib monotherapy and died 2 months later (83). The patient in this study also showed significant tumor progression after 25 weeks of monotherapy with nintedanib, which must be controlled with other combination therapies.
3.2.3.2 Combination with other anti-tumor therapies in NSCLC
Long-term nintedanib monotherapy was not sufficiently effective to inhibit tumor progression in patients with NSCLC-CIP; however, it significantly ameliorated pulmonary fibrosis, reduced pulmonary function decline, and restored physical activity levels. Thus, physicians should combine other antitumor therapies with nintedanib after physical strength recovery. Here, we chose afatinib as second-line therapy, in conjunction with albumin–paclitaxel chemotherapy, leading to stabilized tumor progression with good tolerance of this case.
Afatinib is a broad-spectrum irreversible blocker of the ErbB receptor family that inhibits the EGFR (ErbB-1/HER1) signaling pathway, ErbB-2/neu/HER2, ErbB-3/HER3, and ErbB-4/HER4 (197). Afatinib has a broader inhibition spectrum than first-generation reversible EGFR-specific drugs, such as erlotinib and gefitinib (198). Although mutations in EGFR/ErbB-1/HER1 have rarely been identified, this pathway has been reported to play a role in the pathophysiology of SqCLC (199). Afatinib can be used as first-line treatment for patients with EGFR mutation-positive NSCLC and has shown considerable efficacy (200). However, given the scarcity of EGFR mutations (<4%) in SqCLCs (201, 202), most patients with advanced SqCLCs do not receive EGFR-TKIs, resulting in paucity of relevant clinical data. Compared with those of first-generation EGFR-TKIs (erlotinib/gefitinib), preclinical data of afatinib showed a lower IC50 value, greater potency capacity against wild-type EGFR antibodies, and the ability to inhibit ErbB-2/neu/HER2 (203–205).
EGFR/ErbB-1/HER1 overexpression occurs in >50% of NSCLCs, especially in >80% of SqCLC tissues (206, 207). The gene copy number of EGFR increases in >25% of patients with SqCLC (199). In addition to EGFR, other ErbB-2/neu/HER2 and ErbB-3/HER3 proteins are overexpressed in more than 20% of SqCLC patients (208, 209). These indicate the potential efficacy of afatinib in mutation-negative patients with SqCLC. The LUX-Lung 8 study reported that the efficacy of afatinib in squamous NSCLC is independent of EGFR mutations (210). Two other phase II studies also showed that afatinib was effective in patients with advanced NSCLC with wild-type EGFR (211).
4 Conclusion
Severe-grade (with or without steroid-refractory) CIP is particularly worrisome and is potentially lethal in patients with NSCLC. Sufficient immunomodulator therapy to suppress progressive respiratory symptoms could trigger secondary infectious pneumonia and further deteriorate pulmonary function during CIP treatment. The MICU successfully rescued the case patient from danger. Pulmonary fibrosis, a key stage in the pathological evolution of CIP, warrants the use of anti-fibrotic agents beyond immunosuppressive agents for CIP. Nintedanib, a triple tyrosine kinase inhibitor mostly applied in ILDs, IPF, and NSCLC-adenocarcinoma, has shown potent anti-fibrotic efficacy in the case patient with severe steroid-refractory CIP; however, it has insufficient long-term anti-tumor efficacy by monotherapy and thus requires combination with other anti-tumor therapies to boost efficacy. The dual anti-fibrotic and anti-tumor effects of nintedanib on patients with NSCLC with CIP are worthy of further investigation with clinical trials. It might be a good supportive choice for patients with NSCLC and severe CIP to inhibit tumor and fibrosis progression when they cannot tolerate further toxic anti-tumor therapy.
Ethics statement
Written informed consent was obtained from the patient for publication of this case report and any accompanying data and images.
Author contributions
LP and FM: conceptualization, methodology, writing-editing, data curation, and writing—original draft preparation, visualization. WW: supervision and writing—reviewing. X-hW, HS, and PB: visualization, Investigation. JK: supervision, validation. D-lK: supervision and writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the Respiratory and Critical Care Medicine Department of the First Hospital of China Medical University for the great healthcare services and warm supports for this paper especially in the crisis of the COVID-19.
Glossary
| NSCLC | non-small cell lung cancer |
| CIP | checkpoint inhibitor-related pneumonitis |
| irAEs | immune-related adverse events |
| PD-1 | programmed cell death-1 |
| PD-L1 | programmed cell death ligand-1 |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| PFS | progression-free survival |
| OS | overall survival |
| FAEs | fatal adverse events |
| MICU | medical intensive care unit |
| IPF | idiopathic pulmonary fibrosis |
| ILDs | interstitial lung diseases |
| SqCLC | squamous cell lung carcinoma |
| NGS | next-generation sequencing |
| ECOG | Eastern Cooperative Oncology Group |
| GGOs | ground-glass opacities |
| PR | partial response |
| G1–4 | grades 1–4 |
| BALF | bronchoalveolar lavage fluid |
| IVIG | intravenous immunoglobulin |
| OP | organizing pneumonia |
| PD | progressive disease |
| TIME | tumor immune microenvironment |
| COPD | chronic obstructive pulmonary disease |
| AIP | acute interstitial pneumonia |
| ARDS | acute respiratory distress syndrome |
| DAD | diffuse alveolar damage |
| HP | hypersensitivity pneumonitis |
| NSIP | nonspecific interstitial pneumonia |
| CRP | C-reactive protein |
| PCT | procalcitonin |
| SP-A | surface protein-A |
| ECM | extracellular matrix |
| α-SMA | α-smooth muscle actin |
| TGF | transforming growth factor |
| S1P | sphingosine1-phosphate |
| EMT | pithelial-to-mesenchymal transition |
| AT | alveolar epithelial type |
| EnMT | endothelial-to-mesenchymal transition |
| MCs | mast cells |
| CAFs | cancer-associated fibroblasts |
| PDGF | platelet-derived growth factor |
| VEGF | vascular endothelial growth factor |
| FGF | fibroblast growth factor |
| RTKs | receptor tyrosine kinases |
| VEGFRs | vascular endothelial growth factor receptors |
| FGFRs | fibroblast growth factor receptors |
| PDGFRs | platelet-derived growth factor receptors |
| SCF | stem cell factor |
| FVC | forced vital capacity |
| MTD | maximum tolerated dose |
| AE | adverse events |
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/Annurev-Pathol-042020-042741 [DOI] [PubMed] [Google Scholar]
- 2. Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo H, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non-small cell lung cancer patients. Cancer Biol Med (2020) 17(3):599–611. doi: 10.20892/J.Issn.2095-3941.2020.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang PF, Chen Y, et al. Immune-related adverse events associated with anti-Pd-1/Pd-L1 treatment for malignancies: A meta-analysis. Front Pharmacol (2017) 8:730. doi: 10.3389/Fphar.2017.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/Programmed death ligand 1 therapy. J Clin Oncol (2017) 35(7):709–17. doi: 10.1200/Jco.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol (2018) 4(12):1721–8. doi: 10.1001/Jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat Rev Clin Oncol (2019) 16(9):563–80. doi: 10.1038/S41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 7. Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann Oncol (2017) 28(10):2377–85. doi: 10.1093/Annonc/Mdx286 [DOI] [PubMed] [Google Scholar]
- 8. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med (2018) 378(2):158–68. doi: 10.1056/Nejmra1703481 [DOI] [PubMed] [Google Scholar]
- 9. Zhang Q, Tang L, Zhou Y, He W, Li W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: Current understanding in characteristics, diagnosis, and management. Front Immunol (2021) 12:663986. doi: 10.3389/Fimmu.2021.663986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang M, Fan Y, Nie L, Wang G, Sun K, Cheng Y. Clinical outcomes of immune checkpoint inhibitor therapy in patients with advanced non-small cell lung cancer and preexisting interstitial lung diseases: A systematic review and meta-analysis. Chest (2022) 161(6):1675–86. doi: 10.1016/J.Chest.2021.12.656 [DOI] [PubMed] [Google Scholar]
- 11. Atchley WT, Alvarez C, Saxena-Beem S, Schwartz TA, Ishizawar RC, Patel KP, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: Real-world incidence, risk factors, and management practices across six health care centers in north Carolina. Chest (2021) 160(2):731–42. doi: 10.1016/J.Chest.2021.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shannon VR. Pneumonitis associated with immune checkpoint inhibitors among patients with non-small cell lung cancer. Curr Opin Pulm Med (2020) 26(4):326–40. doi: 10.1097/Mcp.0000000000000689 [DOI] [PubMed] [Google Scholar]
- 13. Mark NM, Kargl J, Busch SE, Yang GHY, Metz HE, Zhang H, et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Am J Respir Crit Care Med (2018) 197(3):325–36. doi: 10.1164/Rccm.201704-0795oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shibaki R, Murakami S, Matsumoto Y, Yoshida T, Goto Y, Kanda S, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother (2020) 69(1):15–22. doi: 10.1007/S00262-019-02431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, et al. Impact of checkpoint inhibitor pneumonitis on survival in nsclc patients receiving immune checkpoint immunotherapy. J Thorac Oncol (2019) 14(3):494–502. doi: 10.1016/J.Jtho.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 16. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? a systematic review and meta-analysis. BMC Med (2020) 18(1):87. doi: 10.1186/S12916-020-01549-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol (2020) 6(12):1952–6. doi: 10.1001/Jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ono K, Ono H, Toi Y, Sugisaka J, Aso M, Saito R, et al. Association of immune-related pneumonitis with clinical benefit of anti-programmed cell death-1 monotherapy in advanced non-small cell lung cancer. Cancer Med (2021) 10(14):4796–804. doi: 10.1002/Cam4.4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/Jco.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020. J Natl Compr Canc Netw (2020) 18(3):230–41. doi: 10.6004/Jnccn.2020.0012 [DOI] [PubMed] [Google Scholar]
- 21. Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28(Suppl_4):Iv119–42. doi: 10.1093/Annonc/Mdx225 [DOI] [PubMed] [Google Scholar]
- 22. Andruska N, Mahapatra L, Hebbard C, Patel P, Paul V. Severe pneumonitis refractory to steroids following anti-PD-1 immunotherapy. BMJ Case Rep (2018) 2018:225937. doi: 10.1136/Bcr-2018-225937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou C, Yang Y, Lin X, Fang N, Chen L, Jiang J, et al. Proposed clinical phases for the improvement of personalized treatment of checkpoint inhibitor-related pneumonitis. Front Immunol (2022) 13:935779. doi: 10.3389/Fimmu.2022.935779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imran S, Golden A, Feinstein M, Plodkowski A, Bodd F, Rekhtman N, et al. Immune check-point inhibitor-related pneumonitis: Acute lung injury with rapid progression and organising pneumonia with less severe clinical disease. Histopathology (2022) 81(6):724–31. doi: 10.1111/His.14704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roth GJ, Binder R, Colbatzky F, Dallinger C, Schlenker-Herceg R, Hilberg F, et al. Nintedanib: From discovery to the clinic. J Med Chem (2014) 58(3):1053–63. doi: 10.1021/Jm501562a [DOI] [PubMed] [Google Scholar]
- 26. Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med (2019) 380(26):2518–28. doi: 10.1056/Nejmoa1903076 [DOI] [PubMed] [Google Scholar]
- 27. Lamb YN. Nintedanib: A review in fibrotic interstitial lung diseases. Drugs (2021) 81(5):575–86. doi: 10.1007/S40265-021-01487-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Umemura Y, Mitsuyama Y, Minami K, Nishida T, Watanabe A, Okada N, et al. Efficacy and safety of nintedanib for pulmonary fibrosis in severe pneumonia induced by covid-19: An interventional study. Int J Infect Dis (2021) 108:454–60. doi: 10.1016/J.Ijid.2021.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corral J, Majem M, Rodríguez-Abreu D, Carcereny E, Cortes ÁA, Llorente M, et al. Efficacy of nintedanib and docetaxel in patients with advanced lung adenocarcinoma treated with first-line chemotherapy and second-line immunotherapy in the nintedanib NPU program. Clin Transl Oncol (2019) 21(9):1270–9. doi: 10.1007/S12094-019-02053-7 [DOI] [PubMed] [Google Scholar]
- 30. European Medicines Agency . (2019). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/vargatef (Accessed December 15, 2022). Nintedanib(Vargatef®).
- 31. Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: Benefits and pulmonary toxicities. Chest (2018) 154(6):1416–23. doi: 10.1016/J.Chest.2018.08.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-related adverse events (Iraes): Diagnosis, management, and clinical pearls. Curr Oncol Rep (2020) 22(4):39. doi: 10.1007/S11912-020-0897-9 [DOI] [PubMed] [Google Scholar]
- 33. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - a systematic review and meta-analysis. Cancer Treat Rev (2021) 92:102134. doi: 10.1016/J.Ctrv.2020.102134 [DOI] [PubMed] [Google Scholar]
- 34. Sugano T, Seike M, Saito Y, Kashiwada T, Terasaki Y, Takano N, et al. Immune checkpoint inhibitor-associated interstitial lung diseases correlate with better prognosis in patients with advanced non-Small-Cell lung cancer. Thorac Cancer (2020) 11(4):1052–60. doi: 10.1111/1759-7714.13364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, pd-L1-Positive, advanced non-Small-Cell lung cancer (Keynote-010): A randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 36. Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage iii melanoma randomized to receive pembrolizumab or placebo: A secondary analysis of a randomized clinical trial. JAMA Oncol (2020) 6(4):519–27. doi: 10.1001/Jamaoncol.2019.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol (2016) 2(12):1607–16. doi: 10.1001/Jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 38. Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer (2018) 125:150–6. doi: 10.1016/J.Lungcan.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 39. Naidoo J, Cottrell TR, Lipson EJ, Forde PM, Illei PB, Yarmus LB, et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Cancer (2020) 8(1):E000840. doi: 10.1136/Jitc-2020-000840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fabre A, Nicholson AG. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med (2020) 382(8):780. doi: 10.1056/Nejmc1917224 [DOI] [PubMed] [Google Scholar]
- 41. Fukunaga K, Yokoe S, Kawashima S, Uchida Y, Nakagawa H, Nakano Y. Nintedanib prevented fibrosis progression and lung cancer growth in idiopathic pulmonary fibrosis. Respirol Case Rep (2018) 6(8):E00363. doi: 10.1002/Rcr2.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crestani B, Huggins JT, Kaye M, Costabel U, Glaspole I, Ogura T, et al. Long-term safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis: Results from the open-label extension study, INPULSIS-ON. Lancet Respir Med (2019) 7(1):60–8. doi: 10.1016/S2213-2600(18)30339-4 [DOI] [PubMed] [Google Scholar]
- 43. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-Small-Cell lung cancer (Lume-lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol (2014) 15(2):143–55. doi: 10.1016/S1470-2045(13)70586-2 [DOI] [PubMed] [Google Scholar]
- 44. Hanna NH, Kaiser R, Sullivan RN, Aren OR, Ahn MJ, Tiangco B, et al. Nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with relapsed or refractory, advanced non-small cell lung cancer (LUME-lung 2): A randomized, double-blind, phase III trial. Lung Cancer (2016) 102:65–73. doi: 10.1016/J.Lungcan.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 45. Tu J, Xu H, Ma L, Li C, Qin W, Chen X, et al. Nintedanib enhances the efficacy of PD-L1 blockade by upregulating MHC-I and PD-L1 expression in tumor cells. Theranostics (2022) 12(2):747–66. doi: 10.7150/Thno.65828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kato R, Haratani K, Hayashi H, Sakai K, Sakai H, Kawakami H, et al. Nintedanib promotes antitumour immunity and shows antitumour activity in combination with PD-1 blockade in mice: Potential role of cancer-associated fibroblasts. Br J Cancer (2021) 124(5):914–24. doi: 10.1038/S41416-020-01201-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Voong KR, Hazell SZ, Fu W, Hu C, Lin CT, Ding K, et al. Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-Small-Cell lung cancer. Clin Lung Cancer (2019) 20(4):E470–9. doi: 10.1016/J.Cllc.2019.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non- small-cell lung cancer. New Engl J Med (2015) 373(2):123–35. doi: 10.1056/Nejmoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nishino M, Chambers ES, Chong CR, Ramaiya NH, Gray SW, Marcoux JP, et al. Anti-PD-1 inhibitor-related pneumonitis in non-small cell lung cancer. Cancer Immunol Res (2016) 4(4):289–93. doi: 10.1158/2326-6066.Cir-15-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/Nejmoa1810865 [DOI] [PubMed] [Google Scholar]
- 51. Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: A systematic review and meta-analysis of trials. Chest (2017) 152(2):271–81. doi: 10.1016/J.Chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 52. Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: A systematic review and meta-analysis. Front Immunol (2019) 10:108. doi: 10.3389/Fimmu.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yamaguchi T SJ, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-Pd-1-Related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung Cancer (2018) 125:212–7. doi: 10.1016/J.Lungcan.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 54. Galant-Swafford J, Troesch A, Tran L, Weaver A, Doherty TA, Patel SP. Landscape of immune-related pneumonitis in cancer patients with asthma being treated with immune checkpoint blockade. Oncology (2020) 98(2):123–30. doi: 10.1159/000503566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lin X, Deng H, Chen L, Wu D, Chen X, Yang Y, et al. Clinical types of checkpoint inhibitor-related pneumonitis in lung cancer patients: A multicenter experience. Transl Lung Cancer Res (2021) 10(1):415–29. doi: 10.21037/Tlcr-20-1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun Y, Shao C, Li S, Xu Y, Xu K, Zhang Y, et al. Programmed cell death 1(PD-1)/PD-Ligand 1(PD-L1) inhibitors-related pneumonitis in patients with advanced non-small cell lung cancer. Asia Pac J Clin Oncol (2020) 16(6):299–304. doi: 10.1111/Ajco.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naidoo J, Nishino M, Patel SP, Shankar B, Rekhtman N, Illei P, et al. Immune-related pneumonitis after chemoradiotherapy and subsequent immune checkpoint blockade in unresectable stage III non-Small-Cell lung cancer. Clin Lung Cancer (2020) 21(5):E435–44. doi: 10.1016/J.Cllc.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 58. Larsen BT, Chae JM, Dixit AS, Hartman TE, Peikert T, Roden AC. Clinical and histopathologic features of immune checkpoint inhibitor-related pneumonitis. Am J Surg Pathol (2019) 43(10):1331–40. doi: 10.1097/Pas.0000000000001298 [DOI] [PubMed] [Google Scholar]
- 59. Jenkins G, Blanchard A, Borok Z, Bradding P, Ehrhardt C, Fisher A, et al. In search of the fibrotic epithelial cell: Opportunities for a collaborative network. Thorax (2012) 67(2):179–82. doi: 10.1136/Thoraxjnl-2011-200195 [DOI] [PubMed] [Google Scholar]
- 60. Parimon T, Hohmann MS, Yao C. Cellular senescence: Pathogenic mechanisms in lung fibrosis. Int J Mol Sci (2021) 22(12):6214. doi: 10.3390/Ijms22126214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peyser R, MacDonnell S, Gao Y, Cheng L, Kim Y, Kaplan T, et al. Defining the activated fibroblast population in lung fibrosis using single-cell sequencing. Am J Respir Cell Mol Biol (2019) 61(1):74–85. doi: 10.1165/Rcmb.2018-0313oc [DOI] [PubMed] [Google Scholar]
- 62. Deng Z, Fear MW, Suk Choi Y, Wood FM, Allahham A, Mutsaers SE, et al. The extracellular matrix and mechanotransduction in pulmonary fibrosis. Int J Biochem Cell Biol (2020) 126:105802. doi: 10.1016/J.Biocel.2020.105802 [DOI] [PubMed] [Google Scholar]
- 63. Rashdan S, Minna JD, Gerber DE. Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir Med (2018) 6(6):472–8. doi: 10.1016/S2213-2600(18)30172-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matsumoto T, Fujita M, Hirano R, Sasaki T, Watanabe K. Risk factors for pneumocystis pneumonia onset in hiv-negative patients treated with high-dose systemic corticosteroids. Infect Dis (Lond) (2019) 51(4):305–7. doi: 10.1080/23744235.2018.1558368 [DOI] [PubMed] [Google Scholar]
- 65. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med (2001) 345(15):1098–104. doi: 10.1056/Nejmoa011110 [DOI] [PubMed] [Google Scholar]
- 66. Gauci ML, Lanoy E, Champiat S, Caramella C, Ammari S, Aspeslagh S, et al. Long-term survival in patients responding to anti-Pd-1/Pd-L1 therapy and disease outcome upon treatment discontinuation. Clin Cancer Res (2019) 25(3):946–56. doi: 10.1158/1078-0432.Ccr-18-0793 [DOI] [PubMed] [Google Scholar]
- 67. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol (2018) 36(17):1668–74. doi: 10.1200/Jco.2017.75.6270 [DOI] [PubMed] [Google Scholar]
- 68. Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C, Pego-Reigosa JM, Retamozo S, Bove A, et al. Interstitial lung disease induced or exacerbated by tnf- targeted therapies: Analysis of 122 cases. Semin Arthritis Rheumatol (2011) 41(2):256–64. doi: 10.1016/J.Semarthrit.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 69. Lin X, Deng J, Deng H, Yang Y, Sun N, Zhou M, et al. Comprehensive analysis of the immune microenvironment in checkpoint inhibitor pneumonitis. Front Immunol (2022) 12:818492. doi: 10.3389/Fimmu.2021.818492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Strippoli S, Fucci L, Negri A, Putignano D, Cisternino ML, Napoli G, et al. Cellular analysis of bronchoalveolar lavage fluid to narrow differential diagnosis of checkpoint inhibitor-related pneumonitis in metastatic melanoma. J Transl Med (2020) 18(1):473. doi: 10.1186/S12967-020-02650-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liang J, Wang H, Ding W, Huang J, Zhou X, Wang H, et al. Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity. Sci Adv (2020) 6(35):Eabc3646. doi: 10.1126/Sciadv.Abc3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest (2019) 129(10):4305–15. doi: 10.1172/Jci128654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang GX, Kurra V, Gainor JF, Sullivan RJ, Flaherty KT, et al. Immune checkpoint inhibitor cancer therapy: Spectrum of imaging findings. Radiographics (2017) 37(7):2132–44. doi: 10.1148/Rg.2017170085 [DOI] [PubMed] [Google Scholar]
- 74. Shea M, Rangachari D, Hallowell RW, Hollie NI, Costa DB, VanderLaan PA. Radiologic and autopsy findings in a case of fatal immune checkpoint inhibitor-associated pneumonitis. Cancer Treat Res Commun (2018) 15:17–20. doi: 10.1016/J.Ctarc.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 75. Franken A, Van Mol P, Vanmassenhove S, Donders E, Schepers R, Van Brussel T, et al. Single-cell transcriptomics identifies pathogenic T-helper 17.1 cells and pro-inflammatory monocytes in immune checkpoint inhibitor-related pneumonitis. J Immunother Cancer (2022) 10(9):E005323. doi: 10.1136/Jitc-2022-005323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li X, Lv F, Wang Y, Du Z. Establishment and validation of nomogram for predicting immuno checkpoint inhibitor related pneumonia. BMC Pulm Med (2022) 22(1):331. doi: 10.1186/S12890-022-02127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fedorov VD, Themeli M, Sadelain M. PD-1- and ctla-4-Based inhibitory chimeric antigen receptors (Icars) divert off-target immunotherapy responses. Sci Trans Med (2013) 5(215):215ra172. doi: 10.1126/Scitranslmed.3006597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Läubli H, Koelzer VH, Matter MS, Herzig P, Dolder Schlienger B, Wiese MN, et al. The T cell repertoire in tumors overlaps with pulmonary inflammatory lesions in patients treated with checkpoint inhibitors. Oncoimmunology (2018) 7(2):E1386362. doi: 10.1080/2162402x.2017.1386362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rapoport BL, Shannon VR, Cooksley T, Johnson DB, Anderson L, Blidner AG, et al. Pulmonary toxicities associated with the use of immune checkpoint inhibitors: An update from the immuno-oncology subgroup of the neutropenia, infection & myelosuppression study group of the multinational association for supportive care in cancer. Front Pharmacol (2021) 12:743582. doi: 10.3389/Fphar.2021.743582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, Walker PR. Interleukin-6 as one of the potential mediators of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint blockade: Evidence from a case report. Acta Oncol (2018) 57(5):705–8. doi: 10.1080/0284186x.2017.1406668 [DOI] [PubMed] [Google Scholar]
- 81. Wang YN, Lou DF, Li DY, Jiang W, Dong JY, Gao W, et al. Elevated levels of il-17a and il-35 in plasma and bronchoalveolar lavage fluid are associated with checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer. Oncol Lett (2020) 20(1):611–22. doi: 10.3892/Ol.2020.11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Utsumi H, Araya J, Okuda K, Watanabe J, Takekoshi D, Fujita Y, et al. Successful treatment of steroid-refractory immune checkpoint inhibitor- related pneumonitis with triple combination therapy: A case report. Cancer Immunol Immunother (2020) 69(10):2033–9. doi: 10.1007/S00262-020-02600-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xie XH, Deng HY, Lin XQ, Wu JH, Liu M, Xie ZH, et al. Case report: Nintedanib for pembrolizumab-related pneumonitis in a patient with non-small cell lung cancer. Front Oncol (2021) 11:673877. doi: 10.3389/Fonc.2021.673877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. de Souza Costa VH, Jr, Baurakiades E, Viola Azevedo ML, Traiano G, Kowal Rosales J, Kunze Larsen KS, et al. Immunohistochemistry analysis of pulmonary infiltrates in necropsy samples of children with non-pandemic lethal respiratory infections (Rsv; adv; Piv1; Piv2; Piv3; flu a; flu b). J Clin Virol (2014) 61(2):211–5. doi: 10.1016/J.Jcv.2014.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Su H, Na N, Zhang X, Zhao Y. The biological function and significance of Cd74 in immune diseases. Inflammation Res (2017) 66(3):209–16. doi: 10.1007/S00011-016-0995-1 [DOI] [PubMed] [Google Scholar]
- 86. Majdoul S, Compton AA. Lessons in self-defence: Inhibition of virus entry by intrinsic immunity. Nat Rev Immunol (2022) 22(6):339–52. doi: 10.1038/S41577-021-00626-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wilkinson HN, Hardman MJ. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol (2020) 10(9):200223. doi: 10.1098/Rsob.200223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Collins BF, Raghu G. Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. Eur Respir Rev (2019) 28(153):190022. doi: 10.1183/16000617.0022-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cottin V, Wollin L, Fischer A, Quaresma M, Stowasser S, Harari S. Fibrosing interstitial lung diseases: Knowns and unknowns. Eur Respir Rev (2019) 28(151):180100. doi: 10.1183/16000617.0100-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cui H, Xie N, Banerjee S, Ge J, Jiang D, Dey T, et al. Lung myofibroblasts promote macrophage profibrotic activity through lactate-induced histone lactylation. Am J Respir Cell Mol Biol (2021) 64(1):115–25. doi: 10.1165/Rcmb.2020-0360oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Foukas PG, Tsiodras S, Economopoulou P, Spathis A, Mademli M, Leventakos K, et al. Concomitant human herpes virus 6 and nivolumab-related pneumonitis: Potential pathogenetic insights. Idcases (2018) 11:101–3. doi: 10.1016/J.Idcr.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kanai O, Kim YH, Fujita K, Okamura M, Mio T. Concurrence of nivolumab-induced interstitial lung disease and cancer invasion. Respirol Case Rep (2017) 5(6):E00257. doi: 10.1002/Rcr2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shibaki R, Akamatsu H, Fujimoto M, Koh Y, Yamamoto N. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol (2017) 28(6):1404–5. doi: 10.1093/Annonc/Mdx115 [DOI] [PubMed] [Google Scholar]
- 94. Li H, Ma W, Yoneda KY, Moore EH, Zhang Y, Pu LL, et al. Severe nivolumab-induced pneumonitis preceding durable clinical remission in a patient with refractory, metastatic lung squamous cell cancer: A case report. J Hematol Oncol (2017) 10(1):64. doi: 10.1186/S13045-017-0433-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aumiller V, Balsara N, Wilhelm J, Günther A, Königshoff M. Wnt/Beta-catenin signaling induces il-1beta expression by alveolar epithelial cells in pulmonary fibrosis. Am J Respir Cell Mol Biol (2013) 49(1):96–104. doi: 10.1165/Rcmb.2012-0524oc [DOI] [PubMed] [Google Scholar]
- 96. Saito A, Horie M, Nagase T. Tgf-β signaling in lung health and disease. Int J Mol Sci (2018) 19(8):2460. doi: 10.3390/Ijms19082460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol (2010) 43(6):662–73. doi: 10.1165/Rcmb.2009-0345oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest (2009) 119(4):772–87. doi: 10.1172/Jci33950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-Beta1: Potential role in idiopathic pulmonary fibrosis. Am J Pathol (2005) 166(5):1321–32. doi: 10.1016/S0002-9440(10)62351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U.S.A. (2011) 108(26):10562–7. doi: 10.1073/Pnas.1107559108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yu G, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med (2018) 24:39–49. doi: 10.1038/nm.4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J (2017) 50(2):1602367. doi: 10.1183/13993003.02367-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nataraj D, Ernst A, Kalluri R. Idiopathic pulmonary fibrosis is associated with endothelial to mesenchymal transition. Am J Respir Cell Mol Biol (2010) 43(2):129–30. doi: 10.1165/Rcmb.2010-0044ed [DOI] [PubMed] [Google Scholar]
- 104. Komi DEA, Khomtchouk K, Santa Maria PL. A review of the contribution of mast cells in wound healing: Involved molecular and cellular mechanisms. Clin Rev Allergy Immunol (2020) 58(3):298–312. doi: 10.1007/S12016-019-08729-W [DOI] [PubMed] [Google Scholar]
- 105. Wulff BC, Wilgus TA. Mast cell activity in the healing wound: More than meets the eye? Exp Dermatol (2013) 22(8):507–10. doi: 10.1111/Exd.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wygrecka M, Dahal BK, Kosanovic D, Petersen F, Taborski B, von Gerlach S, et al. Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis: Role of transmembrane scf and the par-2/Pkc-α/Raf-1/P44/42 signaling pathway. Am J Pathol (2013) 182(6):2094–108. doi: 10.1016/J.Ajpath.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 107. Veerappan A, O'Connor NJ, Brazin J, Reid AC, Jung A, McGee D, et al. Mast cells: A pivotal role in pulmonary fibrosis. DNA Cell Biol (2013) 32(4):206–18. doi: 10.1089/Dna.2013.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shimbori C, Upagupta C, Bellaye PS, Ayaub EA, Sato S, Yanagihara T, et al. Mechanical stress-induced mast cell degranulation activates tgf-β1 signalling pathway in pulmonary fibrosis. Thorax (2019) 74(5):455–65. doi: 10.1136/Thoraxjnl-2018-211516 [DOI] [PubMed] [Google Scholar]
- 109. Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, et al. Mast cell tryptase stimulates human lung fibroblast proliferation Via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol (2000) 278(1):L193–201. doi: 10.1152/Ajplung.2000.278.1.L193 [DOI] [PubMed] [Google Scholar]
- 110. Lipitsä T, Siiskonen H, Naukkarinen A, Harvima IT. Mast cell chymase degrades fibrinogen and fibrin. Br J Dermatol (2019) 181(2):296–303. doi: 10.1111/bjd.17534. [DOI] [PubMed] [Google Scholar]
- 111. Samarelli AV, Masciale V, Aramini B, Coló GP, Tonelli R, Marchioni A, et al. Molecular mechanisms and cellular contribution from lung fibrosis to lung cancer development. Int J Mol Sci (2021) 22(22):12179. doi: 10.3390/Ijms222212179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cruz-Bermúdez A, Laza-Briviesca R, Vicente-Blanco RJ, García-Grande A, Coronado MJ, Laine-Menéndez S, et al. Cancer-associated fibroblasts modify lung cancer metabolism involving ROS and TGF-β signaling. Free Radic Biol Med (2019) 130:163–73. doi: 10.1016/J.Freeradbiomed.2018.10.450 [DOI] [PubMed] [Google Scholar]
- 113. Kinoshita T, Goto T. Molecular mechanisms of pulmonary fibrogenesis and its progression to lung cancer: A review. Int J Mol Sci (2019) 20(6):1461. doi: 10.3390/Ijms20061461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tzouvelekis A, Gomatou G, Bouros E, Trigidou R, Tzilas V, Bouros D. Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest (2019) 156(2):383–91. doi: 10.1016/J.Chest.2019.04.114 [DOI] [PubMed] [Google Scholar]
- 115. Meyer EC, Liebow AA. Relationship of interstitial pneumonia honeycombing and atypical epithelial proliferation to cancer of the lung. Cancer (1965) 18:322–51. doi: . [DOI] [PubMed] [Google Scholar]
- 116. Königshoff M. Lung cancer in pulmonary fibrosis: Tales of epithelial cell plasticity. Respiration (2011) 81(5):353–8. doi: 10.1159/000326299 [DOI] [PubMed] [Google Scholar]
- 117. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer (2016) 16(9):582–98. doi: 10.1038/Nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 118. Zeisberg EM, Potenta S, et al. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res (2007) 67(21):10123–8. doi: 10.1158/0008-5472.Can-07-3127 [DOI] [PubMed] [Google Scholar]
- 119. Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, et al. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci U.S.A. (2016) 113(38):E5618–27. doi: 10.1073/Pnas.1608384113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. ATC CODE . Anatomical therapeutic chemical classification system (2020) (Accessed December 15, 2022).
- 121. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell (2010) 141(7):1117–34. doi: 10.1016/j.cell.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sun C, Bernards R. Feedback and redundancy in receptor tyrosine kinase signaling: Relevance to cancer therapies. Trends Biochem Sci (2014) 39(10):465–74. doi: 10.1016/J.Tibs.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 123. Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin (2010) 59(2):111–37. doi: 10.3322/Caac.20003 [DOI] [PubMed] [Google Scholar]
- 124. Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discovery (2013) 12(7):543–59. doi: 10.1038/Nrd4025 [DOI] [PubMed] [Google Scholar]
- 125. Clark JD, Flanagan ME, Telliez JB. Discovery and development of janus kinase (Jak) inhibitors for inflammatory diseases. J Med Chem (2014) 57(12):5023–38. doi: 10.1021/Jm401490p [DOI] [PubMed] [Google Scholar]
- 126. Trenker R, Jura N. Receptor tyrosine kinase activation: From the ligand perspective. Curr Opin Cell Biol (2020) 63:174–85. doi: 10.1016/J.Ceb.2020.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wind S, Schmid U, Freiwald M, Marzin K, Lotz R, Ebner T, et al. Clinical pharmacokinetics and pharmacodynamics of nintedanib. Clin Pharmacokinet (2019) 58(9):1131–47. doi: 10.1007/S40262-019-00766-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res (2008) 68(12):4774–82. doi: 10.1158/0008-5472.Can-07-6307 [DOI] [PubMed] [Google Scholar]
- 129. Ogura T, Taniguchi H, Azuma A, Inoue Y, Kondoh Y, Hasegawa Y, et al. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J (2015) 45(5):1382–92. doi: 10.1183/09031936.00198013 [DOI] [PubMed] [Google Scholar]
- 130. Tai WT, Shiau CW, Li YS, Chang CW, Huang JW, Hsueh TT, et al. Nintedanib (Bibf-1120) inhibits hepatocellular carcinoma growth independent of angiokinase activity. J Hepatol (2014) 61(1):89–97. doi: 10.1016/J.Jhep.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 131. Wu P, Nielsen TE, Clausen MH. FDA-Approved small-molecule kinase inhibitors. Trends Pharmacol Sci (2015) 36(7):422–39. doi: 10.1016/J.Tips.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 132. Leroux AE, Biondi RM. Renaissance of allostery to disrupt protein kinase interactions. Trends Biochem Sci (2020) 45(1):27–41. doi: 10.1016/J.Tibs.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 133. Reck M. Nintedanib: examining the development and mechanism of action of a novel triple angiokinase inhibitor. Expert Rev Anticancer Ther (2015) 15(5):579–94. doi: 10.1586/14737140.2015.1031218 [DOI] [PubMed] [Google Scholar]
- 134. Shibuya M. VEGFR and type-V RTK activation and signaling. Cold Spring Harb Perspect Biol (2013) 5(10):A009092. doi: 10.1101/Cshperspect.A009092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kaufman NEM, Dhingra S, Jois SD, Vicente MDGH. Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Molecules (2021) 26(4):1076. doi: 10.3390/Molecules26041076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jantus-Lewintre E, Sanmartín E, Sirera R, Blasco A, Sanchez JJ, Tarón M, et al. Combined vegf-a and vegfr-2 concentrations in plasma: Diagnostic and prognostic implications in patients with advanced nsclc. Lung Cancer (2011) 74(2):326–31. doi: 10.1016/J.Lungcan.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 137. Jiao Q, Bi L, Ren Y, Song S, Wang Q, Wang YS. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol Cancer (2018) 17(1):36. doi: 10.1186/S12943-018-0801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ghedini GC, Ronca R, Presta M, Giacomini A. Future applications of FGF/FGFr inhibitors in cancer. Expert Rev Anticancer Ther (2018) 18(9):861–72. doi: 10.1080/14737140.2018.1491795 [DOI] [PubMed] [Google Scholar]
- 139. Itoh N, Ohta H, Konishi M. Endocrine FGFs: Evolution, physiology, pathophysiology, and pharmacotherapy. Front Endocrinol (Lausanne) (2015) 6:154. doi: 10.3389/Fendo.2015.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jing Q, Wang Y, Liu H, Deng X, Jiang L, Liu R, et al. FGFs: Crucial factors that regulate tumour initiation and progression. Cell Prolif (2016) 49(4):438–47. doi: 10.1111/Cpr.12275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int J Mol Med (2016) 38(1):3–15. doi: 10.3892/Ijmm.2016.2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Awasthi N, Schwarz RE. Profile of nintedanib in the treatment of solid tumors: the evidence to date. Onco Targets Ther (2015) 8:3691–701. doi: 10.2147/OTT.S78805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin Cancer Res (2012) 18(9):2443–51. doi: 10.1158/1078-0432.Ccr-11-2370 [DOI] [PubMed] [Google Scholar]
- 144. Heldin CH. Targeting the pdgf signaling pathway in tumor treatment. Cell Commun Signal (2013) 11:97. doi: 10.1186/1478-811x-11-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Papadopoulos N. Lennartsson J.The PDGF/PDGFR pathway as a drug target. Mol Aspects Med (2018) 62:75–88. doi: 10.1016/J.Mam.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 146. Zou X, Tang XY, Qu ZY, Sun ZW, Ji CF, Li YJ, et al. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int J Biol Macromol. Int J Biol Macromol (2022) 202:539–57. doi: 10.1016/J.Ijbiomac.2022.01.113 [DOI] [PubMed] [Google Scholar]
- 147. Overed-Sayer C, Miranda E, Dunmore R, Liarte Marin E, Beloki L, Rassl D, et al. Inhibition of mast cells: A novel mechanism by which nintedanib may elicit anti-fibrotic effects. Thorax (2020) 75(9):754–63. doi: 10.1136/Thoraxjnl-2019-214000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther (2014) 349(2):209–20. doi: 10.1124/Jpet.113.208223 [DOI] [PubMed] [Google Scholar]
- 149. Heo MJ, Lee C, Choi SY, Choi YM, An IS, Bae S, et al. Nintedanib ameliorates animal model of dermatitis. Sci Rep (2020) 10(1):4493. doi: 10.1038/S41598-020-61424-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J (2015) 45(5):1434–45. doi: 10.1183/09031936.00174914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hostettler KE, Zhong J, Papakonstantinou E, Karakiulakis G, Tamm M, Seidel P, et al. Anti-fibrotic effects of nintedanib in lung fibroblasts derived from patients with idiopathic pulmonary fibrosis. Respir Res (2014) 15(1):157. doi: 10.1186/s12931-014-0157-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Li LF, Kao KC, Liu YY, Lin CW, Chen NH, Lee CS, et al. Nintedanib reduces ventilation-augmented bleomycin-induced epithelial-mesenchymal transition and lung fibrosis through suppression of the src pathway. J Cell Mol Med (2017) 21(11):2937–49. doi: 10.1111/Jcmm.13206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lehmann M, Buhl L, Alsafadi HN, Klee S, Hermann S, Mutze K, et al. Differential effects of nintedanib and pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir Res (2018) 19(1):175. doi: 10.1186/S12931-018-0876-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yamanaka T, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Hagiwara K, et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness Via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br J Cancer (2020) 122(7):986–94. doi: 10.1038/S41416-020-0744-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wollin L, Distler JHW, Redente EF, Riches DWH, Stowasser S, Schlenker-Herceg R, et al. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur Respir J (2019) 54(3):1900161. doi: 10.1183/13993003.00161-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. USA (FDA) . FDA Approves first treatment for group of progressive interstitial lung diseases (2020) (Accessed December 15, 2022).
- 157. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med (2014) 370(22):2071–82. doi: 10.1056/Nejmoa1402584 [DOI] [PubMed] [Google Scholar]
- 158. Vancheri C, Kreuter M, Richeldi L, Ryerson CJ, Valeyre D, Grutters JC, et al. Nintedanib with Add-on Pirfenidone in Idiopathic Pulmonary Fibrosis. Results of the INJOURNEY Trial. Am J Respir Crit Care Med (2018) 197(3):356–63. doi: 10.1164/rccm.201706-1301OC. [DOI] [PubMed] [Google Scholar]
- 159. Wells AU, Flaherty KR, Brown KK, Inoue Y, Devaraj A, Richeldi L, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the inbuild trial: A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med (2020) 8(5):453–60. doi: 10.1016/S2213-2600(20)30036-9 [DOI] [PubMed] [Google Scholar]
- 160. Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med (2019) 381(18):1718–27. doi: 10.1056/Nejmoa1908681 [DOI] [PubMed] [Google Scholar]
- 161. Won E, Basunia A, Chatila WK, Hechtman JF, Chou JF, Ku GY, et al. Efficacy of combined VEGFR1-3, PDGFα/β, and FGFR1-3 blockade using nintedanib for esophagogastric cancer. Clin Cancer Res (2019) 25(13):3811–7. doi: 10.1158/1078-0432.Ccr-18-3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mross K, Büchert M, Frost A, Medinger M, Stopfer P, Studeny M, et al. Vascular effects, efficacy and safety of nintedanib in patients with advanced, refractory colorectal cancer: A prospective phase I subanalysis. BMC Cancer (2014) 14:510. doi: 10.1186/1471-2407-14-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Quintela-Fandino M, Urruticoechea A, Guerra J, Gil M, Gonzalez-Martin A, Marquez R, et al. Phase I clinical trial of nintedanib plus paclitaxel in early her-2-Negative breast cancer (Cnio-Br-01-2010/Geicam-2010-10 study). Br J Cancer (2014) 111(6):1060–4. doi: 10.1038/bjc.2014.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Molife LR, Omlin A, Jones RJ, Karavasilis V, Bloomfield D, Lumsden G, et al. Randomized phase ii trial of nintedanib, afatinib and sequential combination in castration-resistant prostate cancer. Future Oncol (2014) 10(2):219–31. doi: 10.2217/Fon.13.250 [DOI] [PubMed] [Google Scholar]
- 165. Paluri R, Madan A, Li P, Jones B, Saleh M, Jerome M, et al. Phase 1b trial of nintedanib in combination with bevacizumab in patients with advanced solid tumors. Cancer Chemother Pharmacol (2019) 83(3):551–9. doi: 10.1007/S00280-018-3761-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ellis PM, Kaiser R, Zhao Y, Stopfer P, Gyorffy S, Hanna N. Phase I open-label study of continuous treatment with BIBF1120, a triple angiokinase inhibitor, and pemetrexed in pretreated non-small cell lung cancer patients. Clin Cancer Res (2010) 16(10):2881–9. doi: 10.1158/1078-0432.Ccr-09-2944 [DOI] [PubMed] [Google Scholar]
- 167. Daga H, Takeda K, Okada H, Miyazaki M, Ueda S, Kaneda H, et al. Phase I study of nintedanib in combination with pemetrexed as second-line treatment of Japanese patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol (2015) 76(6):1225–33. doi: 10.1007/S00280-015-2896-3 [DOI] [PubMed] [Google Scholar]
- 168. Doebele RC, Conkling P, A Phase I. Open-label dose-escalation study of continuous treatment with bibf 1120 in combination with paclitaxel and carboplatin as first-line treatment in patients with advanced non-Small-Cell lung cancer. Ann Oncol (2012) 23(8):2094–102. doi: 10.1093/Annonc/Mdr596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Orster M, Hackshaw A, De Pas T, Cobo M, Garrido P, Summers Y, et al. A phase I study of nintedanib combined with Cisplatin/Gemcitabine as first-line therapy for advanced squamous non-small cell lung cancer (LUME-lung 3). Lung Cancer (2018) 120:27–33. doi: 10.1016/J.Lungcan.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 170. Okamoto I, Miyazaki M, Takeda M, Terashima M, Azuma K, Hayashi H, et al. Tolerability of nintedanib (BIBF 1120) in combination with docetaxel: A phase 1 study in Japanese patients with previously treated non-Small-Cell lung cancer. J Thorac Oncol (2015) 10(2):346–52. doi: 10.1097/Jto.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 171. Yamamoto N, Kenmotsu H, Goto K, Takeda K, Kato T, Takeda M, et al. An open-label feasibility study of nintedanib combined with docetaxel in Japanese patients with locally advanced or metastatic lung adenocarcinoma after failure of first-line chemotherapy. Cancer Chemother Pharmacol (2018) 82(4):685–94. doi: 10.1007/s00280-018-3649-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Reck M, Kaiser R, Eschbach C, Stefanic M, Love J, Gatzemeier U, et al. A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-Small-Cell lung cancer. Ann Oncol (2011) 22(6):1374–81. doi: 10.1093/Annonc/Mdq618 [DOI] [PubMed] [Google Scholar]
- 173. Han JY, Kim HY, Lim KY, Hwangbo B, Lee JS. A phase II study of nintedanib in patients with relapsed small cell lung cancer. Lung Cancer (2016) 96:108–12. doi: 10.1016/J.Lungcan.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 174. Auliac JB, Monnet I, Bizieux A, Greillier L, Geier M, Falchero L, et al. Multicenter phase II trial of nintedanib plus docetaxel in second-line treatment in advanced non-squamous non-small cell lung cancer patients refractory to first-line platin-based chemotherapy (Refract gfpc 02-15 study). Lung Cancer (2021) 161:122–7. doi: 10.1016/J.Lungcan.2021.09.007 [DOI] [PubMed] [Google Scholar]
- 175. Capelletto E, Migliorino MR, Morabito A, Chiari R, Grossi F, Tiseo M, et al. Final results of the SENECA (Second line nintedanib in non-small cell lung cancer) trial. Lung Cancer (2019) 134:210–7. doi: 10.1016/J.Lungcan.2019.06.028 [DOI] [PubMed] [Google Scholar]
- 176. Cid JR, Montes VG. P3.02a-032 multicenter trial of nintedanib in combination with docetaxel in metastatic lung adenocarcinoma: Expertise in the real-life setting. J Thorac Oncol (2017) 12(1):S1181–2. [Google Scholar]
- 177. Grohé C, Blau W, Gleiber W, Haas S, Hammerschmidt S, Krüger S, et al. Real-world efficacy of nintedanib plus docetaxel after progression on immune checkpoint inhibitors: Results from the ongoing, non-interventional VARGADO study. Clin Oncol (R Coll Radiol) (2022) 34(7):459–68. doi: 10.1016/J.Clon.2021.12.010 [DOI] [PubMed] [Google Scholar]
- 178. Reck M, Syrigos K, Miliauskas S, Zöchbauer-Müller S, Fischer JR, Buchner H, et al. Non-interventional lume-bionis study of nintedanib plus docetaxel after chemotherapy in adenocarcinoma non-small cell lung cancer: A subgroup analysis in patients with prior immunotherapy. Lung Cancer (2020) 148:159–65. doi: 10.1016/J.Lungcan.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 179. Shiratori T, Tanaka H, Tabe C, Tsuchiya J, Ishioka Y, Itoga M, et al. Effect of nintedanib on non-small cell lung cancer in a patient with idiopathic pulmonary fibrosis: A case report and literature review. Thorac Cancer (2020) 11(6):1720–3. doi: 10.1111/1759-7714.13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: A meta-analysis. Front Pharmacol (2018) 9:1430. doi: 10.3389/fphar.2018.01430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/Nejmoa1606774 [DOI] [PubMed] [Google Scholar]
- 182. Ikeda S, Kato T, Kenmotsu H, Ogura T, Iwasawa S, Sato Y, et al. A phase 2 study of atezolizumab for pretreated NSCLC with idiopathic interstitial pneumonitis. J Thorac Oncol (2020) 15(12):1935–42. doi: 10.1016/J.Jtho.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Fujimoto D, Morimoto T, Ito J, Sato Y, Ito M, Teraoka S, et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer (2017) 111:1–5. doi: 10.1016/J.Lungcan.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 184. Fujita T, Kuroki T, Hayama N, Shiraishi Y, Amano H, Nakamura M, et al. Pembrolizumab for previously untreated patients with advanced non-Small-Cell lung cancer and preexisting interstitial lung disease. Intern Med (2020) 59(16):1939–45. doi: 10.2169/Internalmedicine.4552-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Byeon S, Cho JH, Jung HA, Sun JM, Lee SH, Ahn JS, et al. Pd-1 inhibitors for non-small cell lung cancer patients with special issues: Real-world evidence. Cancer Med (2020) 9(7):2352–62. doi: 10.1002/Cam4.2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Yamaguchi O, Kaira K, Shinomiya S, Mouri A, Hashimoto K, Shiono A, et al. Pre-existing interstitial lung disease does not affect prognosis in non-small cell lung cancer patients with pd-L1 expression ≥50% on first-line pembrolizumab. Thorac Cancer (2021) 12(3):304–13. doi: 10.1111/1759-7714.13725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Mandalà M, Merelli B, Indriolo A, Tondini C. Late-occurring toxicity induced by an immune checkpoint blockade in adjuvant treatment of a stage III melanoma patient. Eur J Cancer (2018) 95:130–2. doi: 10.1016/J.Ejca.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 188. Reguera-Nuñez E, Xu P, Chow A, Man S, Hilberg F, Kerbel RS. Therapeutic impact of nintedanib with paclitaxel and/or a pd-L1 antibody in preclinical models of orthotopic primary or metastatic triple negative breast cancer. J Exp Clin Cancer Res (2019) 38(1):16. doi: 10.1186/S13046-018-0999-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Baldini C, Danlos FX, Varga A, Texier M, Halse H, Mouraud S, et al. Safety, recommended dose, efficacy and immune correlates for nintedanib in combination with pembrolizumab in patients with advanced cancers. J Exp Clin Cancer Res (2022) 41(1):217. doi: 10.1186/S13046-022-02423-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Luedtke D, Marzin K, Jungnik A, von Wangenheim U, Dallinger C. Effects of ketoconazole and rifampicin on the pharmacokinetics of nintedanib in healthy subjects. Eur J Drug Metab Pharmacokinet (2018) 43(5):533–41. doi: 10.1007/s13318-018-0467-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Velagacherla V, Suresh A, Mehta CH, Nayak UY. Advances and challenges in nintedanib drug delivery. Expert Opin Drug Delivery (2021) 18(11):1687–706. doi: 10.1080/17425247.2021.1985460 [DOI] [PubMed] [Google Scholar]
- 192. Reck M, Mellemgaard A, von Pawel J, Gottfried M, Bondarenko I, Cheng Y, et al. Anti-angiogenic-specific adverse events in patients with non-small cell lung cancer treated with nintedanib and docetaxel. Lung Cancer (2015) 90(2):267–73. doi: 10.1016/j.lungcan.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 193. Bahleda R, Hollebecque A, Varga A, Gazzah A, Massard C, Deutsch E, et al. Phase I study of afatinib combined with nintedanib in patients with advanced solid tumours. Br J Cancer (2015) 113(10):1413–20. doi: 10.1038/bjc.2015.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Frezzetti D, Gallo M, Maiello MR, D'Alessio A, Esposito C, Chicchinelli N, et al. Vegf as a potential target in lung cancer. Expert Opin Ther Targets (2017) 21(10):959–66. doi: 10.1080/14728222.2017.1371137 [DOI] [PubMed] [Google Scholar]
- 195. Proto C, Ferrara R, Signorelli D, Lo Russo G, Galli G, Imbimbo M, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (Nsclc): What to add and what to leave out. Cancer Treat Rev (2019) 75:39–51. doi: 10.1016/J.Ctrv.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 196. Flaherty KT, Lee SJ, Zhao F, Schuchter LM, Flaherty L, Kefford R, et al. Phase iii trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol (2013) 31(3):373–9. doi: 10.1200/Jco.2012.42.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (Bibw 2992), an irreversible erbb family blocker. J Pharmacol Exp Ther (2012) 343(2):342–50. doi: 10.1124/Jpet.112.197756 [DOI] [PubMed] [Google Scholar]
- 198. Kohsaka S, Nagano M, Ueno T, Suehara Y, Hayashi T, Shimada N, et al. A method of high-throughput functional evaluation of egfr gene variants of unknown significance in cancer. Sci Trans Med (2017) 9(416):eaan6566. doi: 10.1126/scitranslmed.aan6566 [DOI] [PubMed] [Google Scholar]
- 199. Lee Y, Shim HS, Park MS, Kim JH, Ha SJ, Kim SH, et al. High egfr gene copy number and skin rash as predictive markers for egfr tyrosine kinase inhibitors in patients with advanced squamous cell lung carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res (2012) 18(6):1760–8. doi: 10.1158/1078-0432.CCR-11-2582 [DOI] [PubMed] [Google Scholar]
- 200. European Medicines Agency . (2019). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/giotrif (Accessed December 15, 2022). Afatinib Tablets (Gilotrif™).
- 201. Zhang Q, Zhu L, Zhang J. Epidermal growth factor receptor gene mutation status in pure squamous-cell lung cancer in Chinese patients. BMC Cancer (2015) 15:88. doi: 10.1186/S12885-015-1056-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Liam CK, Leow HR, Pang YK. EGFR mutation testing for squamous cell lung carcinoma. J Thorac Oncol (2013) 8(12):E114. doi: 10.1097/Jto.0b013e3182a4e111 [DOI] [PubMed] [Google Scholar]
- 203. Felip E, Hirsh V, Popat S, Cobo M, Fülöp A, Dayen C, et al. Symptom and quality of life improvement in LUX-lung 8, an open-label phase III study of second-line afatinib versus erlotinib in patients with advanced squamous cell carcinoma of the lung after first-line platinum-based chemotherapy. Clin Lung Cancer (2018) 19(1):74–83.e11. doi: 10.1016/j.cllc.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 204. Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol (2016) 17(5):577–89. doi: 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 205. Hirsh V. New developments in the treatment of advanced squamous cell lung cancer: focus on afatinib. Onco Targets Ther (2017) 10:2513–26. doi: 10.2147/OTT.S104177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Joshi A, Zanwar S, Noronha V, Patil VM, Chougule A, Kumar R, et al. EGFR mutation in squamous cell carcinoma of the lung: does it carry the same connotation as in adenocarcinomas? Onco Targets Ther (2017) 10:1859–63. doi: 10.2147/OTT.S125397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Hirsch FR, Varella-Garcia M, Bunn PA, Jr, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol (2003) 21(20):3798–807. doi: 10.1200/JCO.2003.11.069 [DOI] [PubMed] [Google Scholar]
- 208. Ugocsai K, Mándoky L, Tiszlavicz L, Molnár J. Investigation of HER2 overexpression in non-small cell lung cancer. Anticancer Res (2005) 25(4):3061–6. [PubMed] [Google Scholar]
- 209. Yi ES, Harclerode D, Gondo M, Stephenson M, Brown RW, Younes M, et al. High c-Erbb-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mod Pathol (1997) 10(2):142–8. [PubMed] [Google Scholar]
- 210. Soria JC, Felip E, Cobo M, Lu S, Syrigos K, Lee KH, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol (2015) 16(8):897–907. doi: 10.1016/S1470-2045(15)00006-6 [DOI] [PubMed] [Google Scholar]
- 211. Xu Y, Ding VW, Zhang H, Zhang X, Jablons D, He B. Spotlight on afatinib and its potential in the treatment of squamous cell lung cancer: The evidence so far. Ther Clin Risk Manag (2016) 12:807–16. doi: 10.2147/Tcrm.S92996 [DOI] [PMC free article] [PubMed] [Google Scholar]