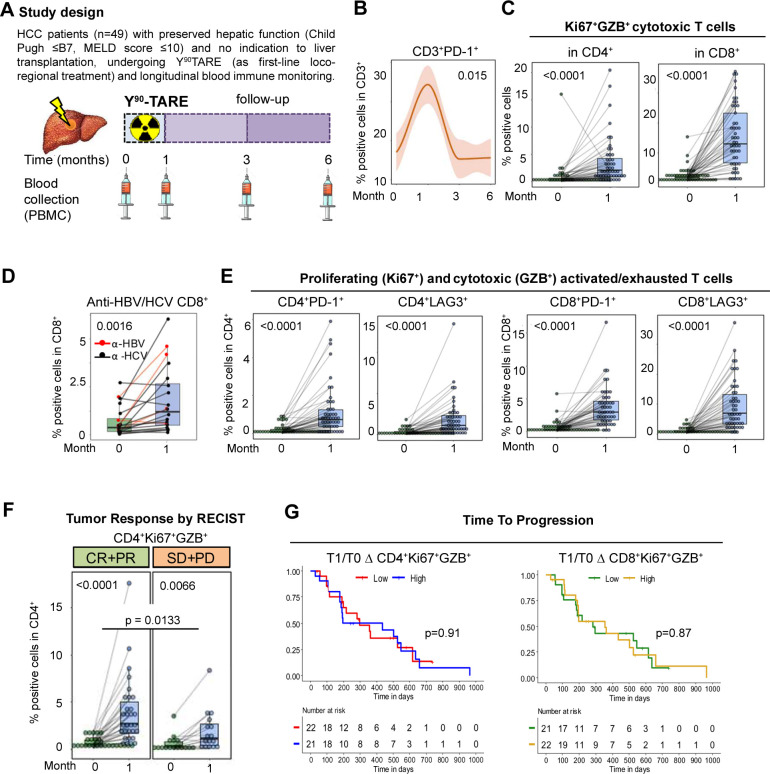
We read with interest the recent work of Chew et al 1 and we would like to share some original data that might implement the concept of immune activation as a consequence of Yttrium90 transarterial radioembolisation (Y90TARE) in hepatocellular carcinoma (HCC). In this study,1 Y90TARE was shown to mediate a significant increase in activated T and NK cells at the site of the tumour and in the peripheral blood of patients with HCC. The increase in these cell populations, particularly T cells expressing specific homing receptors (CCR5 and CXCR6), is associated with local tumour control.
In our study, we monitored immune phenotypes in the blood (figure 1A) of intermediate-advanced HCC patients (n=49) with preserved hepatic function, undergoing Y90TARE treatment during a 2-year period in our centre (table 1).
Figure 1.
Blood immune effects of Y90TARE in HCC patients. (A) Longitudinal blood immune monitoring in 49 patients undergoing Y90TARE for intermediate-advanced HCC, using flow cytometry (Gallios, Beckman Coulter). (B) CD3+PD-1+ lymphocytes, peaked one month post Y90TARE and rapidly returned to baseline levels within three months. Because of this specific kinetics, all subsequent phenotypic characterisations of immune cells were compared between baseline (time 0) and post Y90TARE (1 month), in detail. (C) Increase in cytotoxic granzyme B+ CD8+ and CD4+ T cells. (D) Increase in HBV/HCV antigen specific CD8+ cells after in vitro culture with pools of peptides restricted for the major HLA-class I alleles and derived from the HBV or HCV viral protein repertoire (ProMix Peptide Pools, PX-HCV and PX-HBV; ProImmune), tested in HCV (black dots and line) and HBV (red dots and line) infected patients, respectively. (E) Enhanced frequency of CD4+ and CD8+ T cells expressing the immune checkpoints PD-1 and LAG3, as activation or exhaustion markers. (F) Increased frequency of CD4+Ki67+GZB+LAG3+ T cells in patients experiencing tumour response (CR + PR), according to RECIST, vs progression or stable disease (SD+PD). (G) The median post vs pre Y90TARE change (delta, Δ) in the percentage of CD4+Ki67+GZB+ or CD8+Ki67+GZB+ T cells, used as a cut-off to cluster patients with high vs low activated T cells and TTP (time to progression). For statistical analyses, Friedman (B), Wilcoxon (C-F), Mann Whitney (F) and log-rank (G) tests were applied. TTP Kaplan-Meier curves were based on 32.1% recurrence among the 43 patients analysed, with a median follow-up of 578 days (95% CI 499-737) and a median TTP of 296 days (95% CI 193-525). Statistical significance was set at p<0.05. MELD, model for end stage liver disease; PBMC, peripheral blood mononuclear cells; RECIST, response evaluation criteria in solid tumour.
Table 1.
General characteristics of 49 patients undergoing radioembolisation for unresectable and untransplantable hepatocellular carcinoma
| Characteristics | Study population (n=49) |
| Age | 68 (38–87) |
| Aetiology of liver disease | |
| HCV | 22 (45) |
| HCV +other | 17 (35) |
| HBV | 4 (7) |
| NASH | 2 (4) |
| Alcohol | 3 (6) |
| Child-Pugh class | |
| A | 44 (90) |
| B≤7 | 5 (10) |
| ALBI grade | |
| 1 | 20 (41) |
| 2 | 29 (59) |
| Bilirubin (mg/dL) | 1,1 (0,4–3,3) |
| Albumin (g/dL) | 3,8 (2,8–4,6) |
| INR | 1,1 (1–1,5) |
| Platelet count (*109/L) | 118 (33–480) |
Data are expressed as the median (range) or absolute number (%) as appropriate.
ALBI, albumin-bilirubin; INR, international normalised ratio; NASH, non-alcoholic steatohepatitis.
We observed that tumour irradiation causes an altered adaptive and innate immune response, including an increased frequency of activated CD3+ T cells and CD8+ subsets, regulatory T cells (Treg) and inflammatory (PD-L1+ and HLA-DR+) monocyte populations (online supplemental figure). The immunomodulatory effect peaked 1 month after treatment and decreased significantly at 3 and 6 months, indicating the short-term nature of Y90TARE-induced immunomodulation (figure 1B).
gutjnl-2021-326869supp001.pdf (100.2KB, pdf)
The CD4+ and CD8+ T cells had higher expressions of the proliferative Ki67 and cytotoxic granzyme B markers post-treatment, suggesting an increase in activated immune effector T cells in peripheral blood (figure 1C and online supplemental figure).
Notably, CD8+ cells recognising HBV/HCV peptides were also increased, thereby indicating a rise in circulating antigens and possibly tumour-specific T lymphocytes in patients with virus-derived HCC2 after tumour irradiation (figure 1D). Nonetheless, a significant proportion of Y90TARE-induced CD4+ and CD8+ T cells expressed high levels of the inhibitory checkpoints markers PD-1 and LAG3 (figure 1E) and produced no IFN-γ despite their activation status3 (online supplemental figure). Post-Y90TARE, there were elevated levels of effector T lymphocytes and a reduced frequency of central and effector memory T cells (online supplemental figure). This depicts a dysfunctional and self-extinguishing immune response with the potential to exert immediate antitumour activity, but lacking the functional properties for long-term lasting tumour-specific T cells.4 Indeed, while the CD4+Ki67+GZB+LAG3+T cell subset was significantly higher in patients reaching tumour response compared with stable or progressing patients (figure 1F), no subset was associated with long-term disease control, as measured by time-to-progression (figure 1G). This suggests that the potent immune boosting effect caused by tumour irradiation does not affect long-term clinical outcomes.
It is still unclear as to whether the dysfunctional phenotype of Y90TARE-induced T cells is due to suboptimal immune priming by locoregional radiation or to the immune status of patients with virus-related HCC. It is tempting to speculate that these T-cell defects may instead result from the ‘molecular scar of chronicity’, which characterises the exhausted T-cell repertoire of patients with chronic viral disease and HCC.5 6 Nevertheless, the timely delivery of immune checkpoint inhibitors (ICIs) may restore the antitumour immune responses of immune checkpoint-expressing T cells in the blood of Y90TARE-treated patients with HCC.
Furthermore, as Y90TARE-induced T cells are short lived, possibly due to PD-1 and LAG3 expression, the provision of multiple immune ICIs could enhance long-term antitumour immunological memory.7 8 On the other hand, the peak of T cells expressing immune checkpoints detected 1 month after Y90TARE could be the potential period for ICIs to be administered to enhance both the immunological and clinical efficacy of irradiation treatment.
In conclusion, in the expanding debate on combination strategies for a personalised HCC treatment,9 10 our results suggest that Y90TARE treatment, followed by administration of PD-1 and LAG3 inhibitors after 1 month, represents a promising combination to induce optimal immune-mediated disease control in patients with HCC.
Acknowledgments
English language editing was provided by Editage (www.editage.com).
Footnotes
Contributors: LR and VM contributed to study design, results interpretation and manuscript preparation. SB and DC contributed to data retrieval and interpretation. LB, CCam and CCas performed immunological analyses. PF and LL performed statistical analyses.
Funding: This research received nonprofit grants from Associazione Italiana per la Ricerca sul Cancro (number CC IG-15192) and Ministero della Salute (number 52/RF-2010-2312620).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Comitato Etico Istituto Nazionale Tumori Milano Study number INT 110/13. Approval date 28 October 2014. Participants gave informed consent to participate in the study before taking part.
References
- 1. Chew V, Lee YH, Pan L, et al. Immune activation underlies a sustained clinical response to yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019;68:335–46. 10.1136/gutjnl-2017-315485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng Y, Gunasegaran B, Singh HD, et al. Non-terminally exhausted tumor-resident memory HBV-specific T cell responses correlate with relapse-free survival in hepatocellular carcinoma. Immunity 2021;54:1825–40. 10.1016/j.immuni.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 3. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. 10.1172/JCI91190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer 2021;21:345–59. 10.1038/s41568-021-00347-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hofmann M, Tauber C, Hensel N, et al. CD8+ T Cell Responses during HCV Infection and HCC. J Clin Med 2021;10:991. 10.3390/jcm10050991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hensel N, Gu Z, Sagar G, et al. Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat Immunol 2021;22:229–39. 10.1038/s41590-020-00817-w [DOI] [PubMed] [Google Scholar]
- 7. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:293–313. 10.1038/s41575-020-00395-0 [DOI] [PubMed] [Google Scholar]
- 8. Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol 2018;29:71–83. 10.1093/annonc/mdx686 [DOI] [PubMed] [Google Scholar]
- 9. De Toni EN. Immune checkpoint inhibitors: use them early, combined and instead of TACE? Gut 2020;69:1887–8. 10.1136/gutjnl-2019-319658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerbes A, Zoulim F, Tilg H, et al. Gut roundtable meeting paper: selected recent advances in hepatocellular carcinoma. Gut 2018;67:380–8. 10.1136/gutjnl-2017-315068 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2021-326869supp001.pdf (100.2KB, pdf)