Key messages.
What is the key question?
Can longitudinal multiple breath washout examination estimate the extent and the progression rate of CF lung disease measured with chest CT?
What is the bottom line?
A low lung clearance index during childhood is associated with a lower extent and slower progression rate of structural lung damage compared with a higher lung clearance index.
Why read on?
To better understand how longitudinal lung clearance index may be used in clinical practice.
Introduction
Children with cystic fibrosis (CF) now often go through childhood with only subtle upper and lower airway-related symptoms and have well-preserved lung function assessed by spirometry.1 Despite this improvement, irreversible structural lung damages (SLD) start early in life and progress even in the absence of symptoms.2 3 An important clinical challenge in CF care is to predict the extent and foresee the progression of CF lung disease. This issue is most appropriately addressed by longitudinal studies with repeated measurements of relevant outcomes, which reflect the situation encountered in clinical practice. Multiple breath washout (MBW) has been shown to be a sensitive, non-invasive, feasible method in all ages for repeated measurements over time to track early CF lung disease.4 5 The lung clearance index (LCI) is the most commonly used outcome from MBW examinations and reflects the global ventilation inhomogeneity.4 Several studies have demonstrated that LCI responds to interventions and it is suggested that MBW may be a useful complementary tool in routine clinical care to detect and evaluate treatment responses.6 7 Chest CT has also been shown to be a sensitive marker to of early SLD in CF children, but with the disadvantage of accumulation of ionising radiation, which limits a more frequent utilisation.8 Several studies, most of them cross-sectional, have compared the sensitivity for both methods suggesting relatively similar sensitivity to detect early CF lung disease.9–12 Even though chest CT and MBW are considered complementary markers of the CF lung disease, longitudinal studies are needed to understand the potential use of LCI as a predictor of the extent and the progression of SLD.
The aims of this study were to: (A) describe the longitudinal progression of LCI in a Swedish cohort with CF between the ages 0 and 17 years, (B) investigate the association between the magnitude and progression of longitudinal LCI measurements with the levels and progression rates of SLD measured with chest CT, (C) evaluate if longitudinal LCI measurement in preschool and school-age children can predict SLD magnitude assessed by chest CT at school age. The LCI trend and the association between chest CT and longitudinal LCI measurements have partly been reported in the form of an abstract.13
Method
Study population
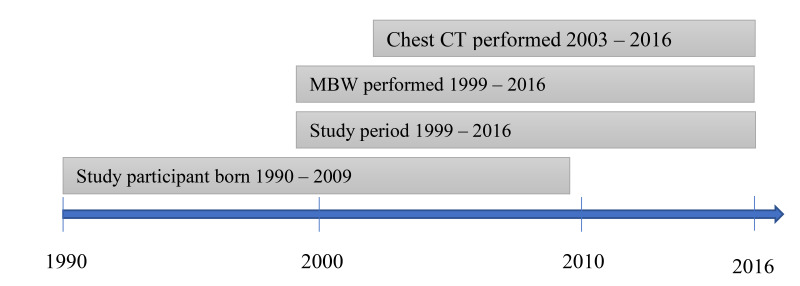
We performed a longitudinal, retrospective, observational study of Swedish children diagnosed clinically with CF. A healthy cohort of children from Sweden and the UK with cross-sectional MBW measurements served as controls. All subjects with CF included in the study were born between 1990 and 2009 and had performed at least one routine chest CT and one routine MBW examination (figure 1). All chest CT measurements with at least one MBW performed before or at the time point of chest CT were included in the analyses. No other exclusion criteria were applied. Demographic data for each subject with CF were retrieved from the Swedish CF Registry.
Figure 1.
Longitudinal overview of the cohort and the main outcomes of the study. Multiple breath washout (MBW) was performed annually between 1999–2012 and every 6 months between 2013 and 2016. Chest CT was performed from the age of 6 years and repeated every third year.
Lung function acquisition and analysis
MBW examinations were performed annually between 1999 and 2016 in clinically stable CF subjects at the paediatric Clinical Physiology laboratory at Queen Silvia Children’s, Gothenburg and at the Paediatric Department at the Central Hospital, Skovde. Additional interim MBW measurements were performed every 6 months from 2013 to 2016. A respiratory mass spectrometer (AMIS 2000, Innovision A/S, Odense, Denmark) was used to measure the expiratory gas concentrations of sulphur hexafluoride (SF6).4 14 All MBW examinations that earlier were considered clinically acceptable were reanalysed according to consensus statement by one paediatric pulmonologist (MS).15 A total of 140 MBW examinations in 140 healthy subjects aged 0–17 years with no history of pulmonary illness were also analysed to understand the relation between age and LCI in a healthy population, and to adjust LCI values in the CF cohort accordingly. The healthy cohort used the same equipment, gas mixes and procedures as in the subjects with CF. See online supplemental material 1 for further details.
thoraxjnl-2021-218178supp001.pdf (1MB, pdf)
Chest CT acquisition and analysis
High-resolution chest CTs were performed in clinical stable CF subjects every third year from 6 years of age between 2003 and 2016. The Perth-Rotterdam Annotated Grid Morphometric Analysis for Cystic Fibrosis method was used to score SLD.16 The primary outcomes for the chest CT scans were bronchiectasis (%Be) and the composite score total airways disease (%Dis). The longitudinal progression of SLD in this cohort has been presented in a previous publication and inter-rater reproducibility for both outcomes were considered excellent.17
Airway pathogens
The incidence of airway pathogens Pseudomonas aeruginosa (Pa), Staphylococcus aureus (Sa) and Aspergillus species (Asp) for the CF cohort was analysed from sputum cultures and cultures from laryngeal suctions.17 Chronic infection with Pa was defined according to the Leeds criteria.18 See OSM for more information about general antibiotic treatment strategies at Gothenburg paediatric CF-centre.
Statistical methods
For descriptive purposes, the data are presented as medians and ranges for continuous variables and as numbers (%) for categorical variables. Statistical analyses were performed using parametric statistical methods and the plausibility of model assumptions was assessed by visual inspection of residual diagnostic plots
We analysed the cross-sectional relation between LCI and age in healthy children using a piecewise linear regression model. The yearly progression of LCI in the CF cohort was estimated from longitudinal MBW data using linear mixed models with a random intercept and age slope for each subject. Robust SEs were used to account for the skewed distribution of LCI in children with CF.
To evaluate the association between longitudinal LCI measurements and SLD, we performed two different analyses. First, the correlation between the progression of SLD and progression of LCI throughout childhood was estimated by joint modelling of all available longitudinal MBW and CT data, using linear mixed effects models with correlated subject-specific random intercepts and age slopes. Similarly, we estimated the correlation between progression of SLD and mean LCI throughout childhood by omitting the age slope of LCI from the model.
To understand the association between SLD and LCI at a more detailed level, we continued with non-linear mixed effects models for SLD, with age and LCI as explanatory variables. A logit-type transformation of %Dis and %Be on the form was used to obtain approximately normally distributed and homoscedastic errors. To account for repeated measures of LCI, we estimated the best linear unbiased predictionsof LCI at chest CT and mean LCI up to chest CT, using mixed effects models of LCI on all available MBW data up to and including the time point of chest CT. The results of the logit-linear mixed effects models were subsequently used to derive SLD percentile curves at 6 and 17 years of age.
To account for natural age trends in LCI between infants, preschoolers and school-age children, all LCI values in children with CF were age-adjusted LCI values (LCIadj) according to the formula
| (1) |
as derived from the healthy reference population.
Statistical analyses were performed using SAS software, V.9.4 (SAS Institute, Cary, North Carolina). For more information about the statistical methodology, see OSM.
Results
Study population
The cohort included 75 of 75 eligible subjects with CF from Gothenburg paediatric CF centre (table 1). A total of 785 MBW examinations from the cohort were accessible and reanalysed. Eight of the 785 MBW examinations that had previously been considered acceptable were excluded following quality control according to consensus statement.15 In total, 777 MBW examinations together with 199 chest CT scans were included in the study (for more information, see OLS Figure E1). Cross-sectional data of the CF disease progression during preschool and school ages are presented in table 2. Detailed results regarding the progression of SLD in this cohort have been reported elsewhere.17
Table 1.
Demographics and descriptive statistics of the CF cohort
| Variable | n (%)/median (range) |
| Female sex | 24 (32%) |
| Pancreatic insufficiency | 67 (89%) |
| dF508/dF508, dF508/other, other/other | 38 (51%)/34 (45%)/3 (4%) |
| Age at diagnosis (years) | 0.8 (0.0–9.0) |
| Children treated with CFTR modulators | 1 (1%) |
| Children with CF-related diabetes mellitus | 2 (3%) |
| Chronically infected with Pa during study period | 22 (29%) |
| Age at onset of chronic Pa infection (years) | 13.0 (3.1–18.9) |
| Children with ABPA | 3 (4%) |
| Follow-up time* (years) | 9.0 (1.0–14.1) |
| Number of MBW examinations/child | 11 (1–18) |
| Number of chest CTs/child | 3 (1–5) |
| Chest CTs at MBW examinations (±3 days) | 146 (73%) |
*Time between the first and the last multiple breath washout or the last chest CT.
ABPA, allergic bronchopulmonary aspergillosis; CFTR, cystic fibrosis transmembrane conductance regulator; MBW, multiple breath washout; Pa, P. aeruginosa.
Table 2.
Cross-sectional overview of CF disease progression for the cohort, including the upper reference limit of normal LCI (ULN) derived from 140 individuals with no lung disease. LCI values for the CF cohort are also presented as age adjusted LCI (LCIadj)
| Variable | Age 2 years (n=27) | Age 5 years (n=41) | Age 7 years (n=44) | Age 12 years (n=44) |
| LCI | 7.7 (6.3–13.8) | 7.4 (6.2–12.2) | 7.5 (5.7–12.4) | 7.9 (5.6–11.0) |
| LCIadj | 7.2 (5.8–13.3) | 7.3 (6.1–12.1) | 7.5 (5.7–12.4) | 7.9 (5.6–11.0) |
| ULN LCI | 7.4 | 7.0 | 7.0 | 7.0 |
| FEV1 (z-score) | – | −0.1 (-2.0–2.1)* | −0.1 (-3.1–2.7) | −0.4 (-3.8–2.6) |
| Chest CT (%Dis) | – | – | 3.8 (0–17.5)† | 5.4 (0.9–32.7)‡ |
| Chest CT (%Be) | – | – | 0.8 (0–7.6)† | 2.0 (0–17.5)‡ |
| BMI (z-score) | −0.1 (−1.7–1.8) | −0.3 (−2.8–1.4) | −0.2 (−1.9–2.65) | −0.4 (−2.0–1.2) |
Data are presented as median (range).
*30 of 41 subjects performed technically acceptable spirometry.
†35 of 44 subjects had a chest CT between age 6 and 8 years.
‡30 of 44 subjects had a chest CT between age 11 and 13 years.
%Be, bronchiectasis (%); BMI, body mass index; %Dis, total airway disease (%); FEV, forced expiratory volume; LCI, lung clearance index; LCIadj, age adjusted lung clearance index; ULN, upper limit of normal.
LCI in healthy children
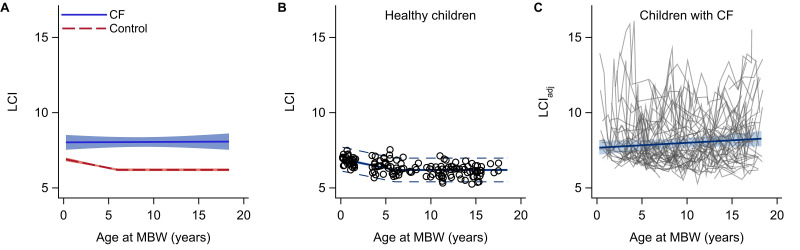
In the healthy reference population, LCI decreased by 0.12 units per year (95% CI −0.16 to −0.09, p<0.0001) up to 6 years of age, after which no further decrease was observed (p=0.21, figure 2A, B). The age-specific mean and 95% upper prediction limit (upper limit of normal) are presented in OSM Table E1.
Figure 2.
Mean LCI versus age in the CF cohort (n=75) and a healthy reference population (n=140) (A), LCI versus age in the healthy reference population only (B), and longitudinal progression of age-adjusted LCI in the CF cohort (C). Circles and grey lines are individual data. The blue lines with shaded bands show the mean trends with 95% confidence limits. Dashed lines are 95% prediction limits. CF, cystic fibrosis; LC, lung clearance index.
The progression of LCI in children with CF
Overall, there was a weak but non-significant progression of age-adjusted LCI (LCIadj) in the CF cohort (+0.03 units/year, 95% CI −0.01 to 0.08, p=0.13, figure 2C). There was a significant progression in LCIadj of 0.11 units/year in girls (95% CI 0.03 to 0.20, p=0.011) and of 0.07 units/year (0.01–0.12, p=0.025) in children born 1990–1999. No significant subgroup differences in mean LCIadj or progression in LCIadj were observed with respect to pancreatic sufficiency or age at diagnosis (online supplemental table E2).
Joint modelling of longitudinal SLD and LCI
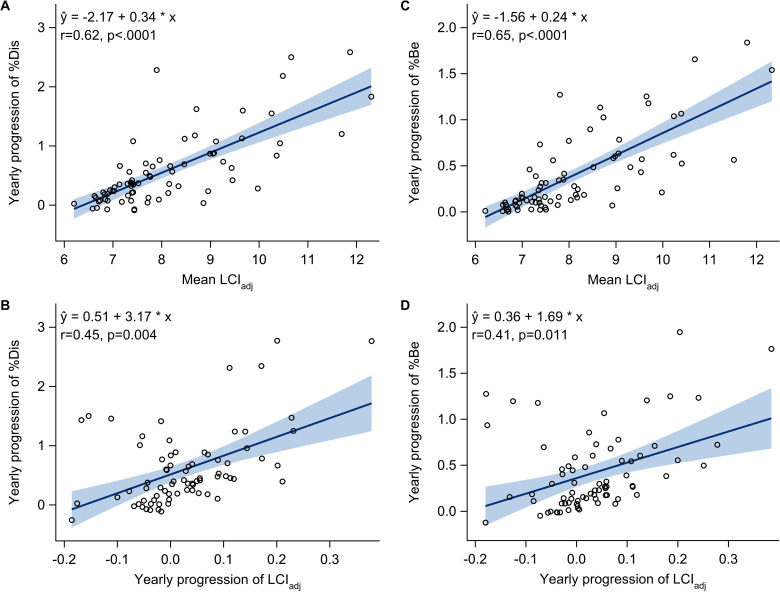
There was a positive correlation between mean LCIadj and progression rate of SLD throughout childhood (r=0.62 (95% CI 0.40 to 0.84) for %Dis, r=0.65 (95% CI 0.46 to 0.85) for %Be, both p<0.0001) (figure 3A and C). One unit increase in mean LCIadj was associated with 0.34 (95% CI 0.27 to 0.41) percentage points faster progression rate of %Dis/year and 0.24 (95% CI 0.19 to 0.29) percentage points faster progression rate of %Be/year. A slightly weaker correlation existed between progression rate of LCIadj and the progression rate of SLD (r=0.45 (95% CI 0.15 to 0.76), p=0.004 for %Dis, r=0.41 (95% CI 0.09 to 0.72), p=0.011 for %Be) (figure 3B and D). The correlations were qualitatively similar but somewhat attenuated when adjusting for age at diagnosis, sex, birth cohort and concomitant infections with Pa, Sa and Asp (online supplemental table E3).
Figure 3.
Yearly progression of total airway disease (A, B) and bronchiectasis (C, D) throughout the study period versus mean LCIadj (A, C) and progression of LCIadj (B, D) over the same period. Circles show the best linear unbiased predictions (BLUPs) of each subject’s progression and mean, estimated by joint modelling of all longitudinal chest CT and MBW data. ‘r’ is the corresponding correlation coefficient. The solid lines with shaded bands are fitted regression lines with 95% confidence limits. LC, lung clearance index; MBW, multiple breath washout.
Non-linear associations between SLD and LCI
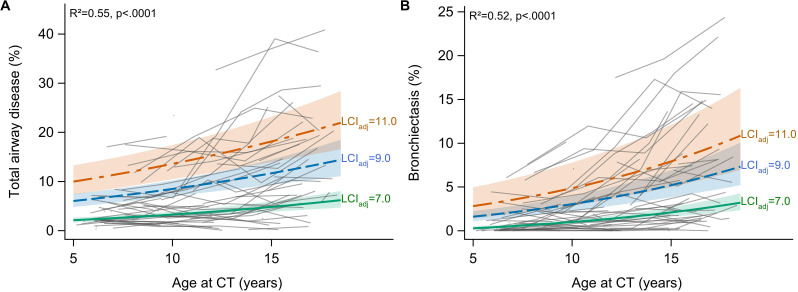
Logit-linear mixed effects models of SLD showed that LCIadj measured at CT (±3 days) together with age at CT explained 49% of the variation in %Dis (p<0.0001) and 35% of the variation in %Be (p=0.020) throughout the study period (online supplemental table E4). However, an individual LCI value beyond LCIadj=9.0 was only weakly associated with increased SLD (online supplemental figures E2, E3). In contrast, an increase in longitudinal LCI was steadily associated with more SLD and explained up to 55% of the variation in %Dis (p<0.0001) and 52% of the variation in %Be throughout the study period (p<0.0001) (figure 4, online supplemental figure E2, E3). Similar associations between LCI and SLD were observed when accounting for age at diagnosis, sex, birth cohort and concomitant infections with Pa, Sa and Asp (online supplemental table E4).
Figure 4.
Progression of total airway disease (A) and bronchiectasis (B) in the CF cohort and relation to longitudinal LCI. Grey lines are individual data. The thick lines and shaded bands represent estimated median trends with 95% confidence limits, assuming a constant LCIadj throughout childhood. Regression curves were derived from mixed effects models on logit-transformed SLD outcomes, with age at chest CT and the best linear unbiased prediction of the mean LCI at chest CT as explanatory variables. CF, cystic fibrosis; LC, lung clearance index; SLD, structural lung damage.
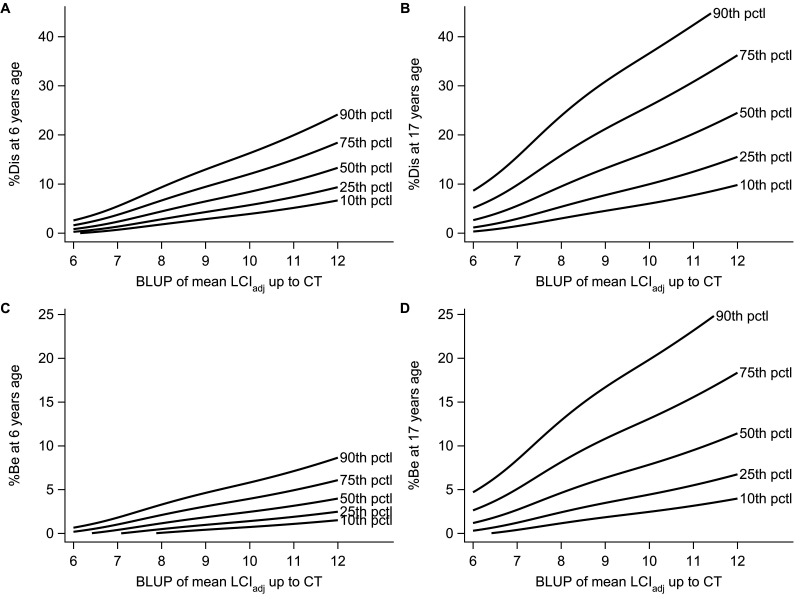
SLD percentile curves
Percentile curves of %Dis and %Be versus mean LCIadj at 6 and 17 years of age are presented in figure 5, and additionally for %Dis and %Be versus LCIadj at CT in OSM Figure E2 and E3. A non-pathological mean LCI (LCIadj=7.0) during preschool age corresponded to a median (10th–90th percentile) %Dis of 2.3% (0.7 to 5.5) and %Be of 0.4% (0.0 to 1.8) at 6 years of age. A two units higher LCI (LCIadj=9.0) corresponded to a median (10th–90th percentile) %Dis of 6.5% (2.9 to 13.0) and %Be of 1.8% (0.4 to 4.6).
Figure 5.
Estimated percentiles of total airway disease (%Dis) (A, B) and bronchiectasis (%Be) (C, D) at 6 years and 17 years of age versus longitudinal mean LCIadj at CT. The BLUP is the best linear unbiased prediction using all available MBW data up and including the time-point of chest CT. Percentile curves were derived from mixed effects models on logit-transformed SLD outcomes, using all available longitudinal MBW and chest CT data. BLUP, best linear unbiased prediction; MBW, multiple breath washout; SLD, structural lung damage.
Discussion
In this study, we have explored how longitudinal and cross-sectional LCI measurements can be used in clinical practice to estimate the extent and the progression rate of SLD in a paediatric CF population. Evaluation of LCI in a longitudinal context resulted in stronger associations with SLD measured by chest CT than only considering the most recent LCI value. A mean LCI within the normal range during childhood corresponded to a low extent and slow progression of SLD, whereas a higher mean LCI resulted in more SLD and a higher SLD progression rate. Our results support the clinical use of regular MBW examinations in children with CF with the aim of maintaining or pursuing a low LCI.
There was a weak but non-significant progression of LCI in our CF cohort. The knowledge about longitudinal changes in LCI over time in children with CF is still limited but recently a few studies have discussed this topic. A 2-year longitudinal Danish study in children with CF aged 6–18 years using Exhalyzer D and nitrogen as inert gas revealed a progression of 0.35 LCI units per years.12 Another 2-year longitudinal study in children with CF aged 5–10 years from North America using the same MBW equipment as in the Danish study showed no annual progression in LCI.19 A Swiss paediatric CF cohort, who also used Exhalyzer D over a longer period of time, revealed a slow increase in 0.1 LCI units per year in the younger school children and a more pronounced annual LCI progression rate in the adolescences.20 Results from the London CF Collaboration using the same MBW equipment as in our study revealed a deterioration of 0.18 LCI units per year in children with CF.21 In the past 10 years, we gained a lot of experience about the biological variability of LCI between LCI measurements and how pathogens and therapeutic interventions affects the course of LCI.5 7 22 23 It is reasonable to believe that this information also has changed the course of how we use LCI in clinical practice in different CF centres, which might be reflected in the longitudinal trends of the LCI progression rate. An increase in LCI in our clinic was often followed by an intervention with the aim to restore LCI to the patient’s LCI baseline, which might be one possible explanation to the absent of a significant LCI progression in our cohort. There was a significant progression in the children born between 1990 and 1999, but not in the children born after 2000. This finding most probably reflects the constant improvement in the CF healthcare over time. We also observed a significant progression in girls. A gender disparity has also been described in studies using alternative methods to track the CF lung disease.24 25 Our study further supports the hypothesis that CF lung disease progression is greater in girls but was not designed to assess this further.
In this study, we have demonstrated that both LCI at chest CT and longitudinal LCI measurements prior to and at chest CT are positively associated with SLD in a paediatric CF population. Longitudinal LCI measurements prior to and at chest CT explained 55% of the variability in %Dis, and 52% of the variability in %Be throughout the study period. Single LCI measurements (cross-sectional at chest CT) were less associated with SLD. This finding may partly be explained by the biological variability of LCI between MBW measurements in clinically stable patients with CF as well as sudden deteriorations or improvements in LCI caused by nearby pulmonary infections or interventions.6 7 20 22 23 Thus, a single LCI value is sensitive to random fluctuation and needs to be interpreted with caution and put in a clinical perspective, including when and why the MBW examination was performed. Our results indicate that longitudinal LCI measurements are more robust and reliable than single LCI measurements to track SLD. Similar findings regarding the benefit of longitudinal compared with cross-sectional measurements of risk factors have also been found in other contexts, for example, risk prediction of future cardiovascular disease.26 Nonetheless, single LCI values may be useful to capture recent or ongoing lung damages, as a sudden deterioration (increase in LCI) may indicate an early sign of progression the CF lung disease.4 In clinical practice both the present and longitudinal LCI measurements should be considered to better understand the CF lung disease.
Longitudinal LCI measurements can be used to discriminate between SLD severity in children with CF. A normal LCI (mean LCIadj=7.0) during infancy and the preschool ages corresponded to relatively low extent of SLD at the age of 6 years (median=2.3 for %Dis and 0.8 for %Be). In comparison, a two units higher mean LCIadj during early childhood resulted in more than a twofold higher SLD. This relationship between longitudinal LCI values and SLD measured with chest CT was observed throughout the entire paediatric ages. Longitudinal mean LCI also demonstrated a positive correlation with the SLD progression rate. We know from earlier studies that there is an association between the degree of airway inflammation and LCI as well as between the airway inflammation and the SLD progression rate in children with CF.16 27 28 Accordingly, a high mean LCI may reflect a high degree of airway inflammation over a longer period of time, which could explain the relationship between a high mean LCI and a faster progression rate of the CF lung disease. In clinical practice, a normal mean LCI during preschool and/or school age may be used as an indicator to perform chest CT scans less frequently. MRI of the lungs may also be an alternative, as cross-sectional MRI studies have shown a good correlation between LCI and structural airways abnormalities in younger children with CF.29 We speculate that a high stable LCI over time should be considered as a risk factor for faster SLD progression and followed by interventions with the aim to pursue a lower LCI. By keeping LCI as low as possible throughout infancy and preschool ages, we may limit the SLD progression rate as well as preserve the subject’s lung function at early school age.19 30 31
Strengths and weaknesses
This study included all available individuals at Gothenburg CF centre, with MBW and chest CT performed over a long period of time. The study also included MBW from a healthy reference cohort, all of which were performed with similar equipment and analysed with the same software and settings as used in the CF cohort. The availability of a healthy reference population enabled us to adjust LCI values of the patients with CF from MBW obtained at various ages and by different technical procedures, by removing the age trend observed in healthy preschool children. We used age-adjusted LCIadj rather than LCI z-score since age-adjusted LCIs are easier to interpret and visualise for the reader. Apart from loss of interpretability, the results would be identical if z-scores derived from the same model and population had been used.
LCI values derived from different MBW equipment or different inert gases are not considered interchangeable.15 A recent publication in 2021 revealed a systematic software error from the gas sensors of the Exhalyzer D, that is commonly used in many MBW studies.32 This error resulted in an overestimation of LCI when using nitrogen as an inert gas. This finding will affect the earlier differences seen between N2 and SF6 measurements as well as in comparison with other MBW equipment when the new software is released.15 Despite challenges in comparison between different MBW equipment and inert gases, results from our study can still provide useful information to understand the relationship between other measurements to track the CF lung disease.
This study was a retrospective, real-world study and we acknowledge several limitations. None of the data from the lung functions tests or the chest CTs were blinded for the clinician. The study stretches over many years and we do not have information about changes in medication or treatment regimens that might have affected the results. Thus, existence of unobserved confounding factors cannot be excluded due to the observational nature of the study. MBW performed in a supine position throughout all paediatric ages may further have improved the associations between LCI and SLD,33 but this information was not available during the study period. Chest CTs were analysed and blinded to patient identifiers with a fully quantitative scoring system with good reproducibility.17 A limitation is the absence of CTs during infancy or preschool ages. The pulmonary status at the time of initial CF diagnosis is variable in our cohort, as new-born screening is not yet implemented in the Swedish CF care. There were no significant differences in results regarding LCI in the cohort with early or late CF diagnoses and our results regarding the extent of SLD at early school age are similar to countries that have new-born screening, but still our results may be affected by lack of new-born screening.10
Conclusion
A low LCI during childhood was associated with less SLD and a slower SLD progression rate compared with a higher longitudinal mean LCI. This study further strengthens the clinical utility of regular MBW examinations throughout childhood, with the aim of pursuing a low LCI.
Acknowledgments
The author would like to thank all the children and caregivers that made this study possible and acknowledge the friendly co-workers at the Erasmus MC-Sophia Children’s Hospital core laboratory Lung Analysis, Rotterdam for help and support in the process of CT-scoring.
Footnotes
Twitter: @daviesgwyneth
Contributors: MS, PG, AL and HI were responsible for the design of the study. MS re-analysed all MBW examination in children with CF under supervision from PG and AL. MBW-data from healthy infants and pre-schoolers were made accessible by GD. MS performed all CT scoring supervised by HT. The statistical analyses were performed by HI. MS, AL and HI drafted the manuscript. All authors revised the manuscript, contributed with conceptual content, and approved the final manuscript. AL is responsible for content in this article.
Funding: This study was supported by grants from The Swedish CF Foundation, Erica Lederhausen Foundation, Queen Silvia’s Children Hospital Research Foundation, Freemasons Order foundation, Petter Silverskiolds Foundation and Samariten Foundation for Paediatric Research.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the local Ethics Committee (Dnr. 206–18).
References
- 1. Ratjen F, Bell SC, Rowe SM, et al. Cystic fibrosis. Nat Rev Dis Primers 2015;1:15010. 10.1038/nrdp.2015.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sly PD, Brennan S, Gangell C, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 2009;180:146–52. 10.1164/rccm.200901-0069OC [DOI] [PubMed] [Google Scholar]
- 3. Bouma NR, Janssens HM, Andrinopoulou E-R, et al. Airway disease on chest computed tomography of preschool children with cystic fibrosis is associated with school-age bronchiectasis. Pediatr Pulmonol 2020;55:141–8. 10.1002/ppul.24498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gustafsson PM, Aurora P, Lindblad A. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J 2003;22:972–9. 10.1183/09031936.03.00049502 [DOI] [PubMed] [Google Scholar]
- 5. Stanojevic S, Davis SD, Retsch-Bogart G, et al. Progression of lung disease in preschool patients with cystic fibrosis. Am J Respir Crit Care Med 2017;195:1216–25. 10.1164/rccm.201610-2158OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stahl M, Wielpütz MO, Ricklefs I, et al. Preventive inhalation of hypertonic saline in infants with cystic fibrosis (PRESIS). A randomized, double-blind, controlled study. Am J Respir Crit Care Med 2019;199:1238–48. 10.1164/rccm.201807-1203OC [DOI] [PubMed] [Google Scholar]
- 7. Rayment JH, Stanojevic S, Davis SD, et al. Lung clearance index to monitor treatment response in pulmonary exacerbations in preschool children with cystic fibrosis. Thorax 2018;73:451–8. 10.1136/thoraxjnl-2017-210979 [DOI] [PubMed] [Google Scholar]
- 8. Kuo W, Kemner-van de Corput MPC, Perez-Rovira A, et al. Multicentre chest computed tomography standardisation in children and adolescents with cystic fibrosis: the way forward. Eur Respir J 2016;47:1706–17. 10.1183/13993003.01601-2015 [DOI] [PubMed] [Google Scholar]
- 9. Gustafsson PM, De Jong PA, Tiddens HAWM, et al. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax 2008;63:129–34. 10.1136/thx.2007.077784 [DOI] [PubMed] [Google Scholar]
- 10. Ramsey KA, Rosenow T, Turkovic L, et al. Lung clearance index and structural lung disease on computed tomography in early cystic fibrosis. Am J Respir Crit Care Med 2016;193:60–7. 10.1164/rccm.201507-1409OC [DOI] [PubMed] [Google Scholar]
- 11. Owens CM, Aurora P, Stanojevic S, et al. Lung clearance index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax 2011;66:481–8. 10.1136/thx.2010.150375 [DOI] [PubMed] [Google Scholar]
- 12. Sandvik RM, Kongstad T, Green K, et al. Prospective longitudinal association between repeated multiple breath washout measurements and computed tomography scores in children with cystic fibrosis. J Cyst Fibros 2021;20:632–40. 10.1016/j.jcf.2020.09.010 [DOI] [PubMed] [Google Scholar]
- 13. Svedberg M, Imberg H, Lindblad A. Longitudinal measurements of lung clerance index and clinical application in relation to structural lung damage (Abstract). Pediatr Pulmonol 2020;55:294. [Google Scholar]
- 14. Gustafsson PM, Källman S, Ljungberg H, et al. Method for assessment of volume of trapped gas in infants during multiple-breath inert gas washout. Pediatr Pulmonol 2003;35:42–9. 10.1002/ppul.10221 [DOI] [PubMed] [Google Scholar]
- 15. Robinson PD, Latzin P, Verbanck S, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J 2013;41:507–22. 10.1183/09031936.00069712 [DOI] [PubMed] [Google Scholar]
- 16. Rosenow T, Oudraad MCJ, Murray CP, et al. PRAGMA-CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med 2015;191:1158–65. 10.1164/rccm.201501-0061OC [DOI] [PubMed] [Google Scholar]
- 17. Svedberg M, Gustafsson P, Tiddens H, et al. Risk factors for progression of structural lung disease in school-age children with cystic fibrosis. J Cyst Fibros 2020;19:910-916. 10.1016/j.jcf.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 18. Lee TWR, Brownlee KG, Conway SP, et al. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros 2003;2:29–34. 10.1016/S1569-1993(02)00141-8 [DOI] [PubMed] [Google Scholar]
- 19. Stanojevic S, Davis SD, Perrem L, et al. Determinants of lung disease progression measured by lung clearance index in children with cystic fibrosis. Eur Respir J 2021;58. 10.1183/13993003.03380-2020. [Epub ahead of print: 08 07 2021]. [DOI] [PubMed] [Google Scholar]
- 20. Frauchiger BS, Binggeli S, Yammine S, et al. Longitudinal course of clinical lung clearance index in children with cystic fibrosis. Eur Respir J 2021;58. 10.1183/13993003.02686-2020. [Epub ahead of print: 08 07 2021]. [DOI] [PubMed] [Google Scholar]
- 21. Davies G, Stanojevic S, Raywood E, et al. An observational study of the lung clearance index throughout childhood in cystic fibrosis: early years matter. Eur Respir J 2020;56. 10.1183/13993003.00006-2020. [Epub ahead of print: 01 10 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Svedberg M, Gustafsson PM, Robinson PD, et al. Variability of lung clearance index in clinically stable cystic fibrosis lung disease in school age children. J Cyst Fibros 2018;17:236–41. 10.1016/j.jcf.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 23. Oude Engberink E, Ratjen F, Davis SD, et al. Inter-test reproducibility of the lung clearance index measured by multiple breath washout. Eur Respir J 2017;50. 10.1183/13993003.00433-2017. [Epub ahead of print: 05 10 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harness-Brumley CL, Elliott AC, Rosenbluth DB, et al. Gender differences in outcomes of patients with cystic fibrosis. J Womens Health 2014;23:1012–20. 10.1089/jwh.2014.4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olesen HV, Pressler T, Hjelte L, et al. Gender differences in the Scandinavian cystic fibrosis population. Pediatr Pulmonol 2010;45:959–65. 10.1002/ppul.21265 [DOI] [PubMed] [Google Scholar]
- 26. Paige E, Barrett J, Stevens D, et al. Landmark models for optimizing the use of repeated measurements of risk factors in electronic health records to predict future disease risk. Am J Epidemiol 2018;187:1530–8. 10.1093/aje/kwy018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramsey KA, Foong RE, Grdosic J, et al. Multiple-Breath washout outcomes are sensitive to inflammation and infection in children with cystic fibrosis. Ann Am Thorac Soc 2017;14:1436–42. 10.1513/AnnalsATS.201611-935OC [DOI] [PubMed] [Google Scholar]
- 28. Belessis Y, Dixon B, Hawkins G, et al. Early cystic fibrosis lung disease detected by bronchoalveolar lavage and lung clearance index. Am J Respir Crit Care Med 2012;185:862–73. 10.1164/rccm.201109-1631OC [DOI] [PubMed] [Google Scholar]
- 29. Stahl M, Wielpütz MO, Graeber SY, et al. Comparison of lung clearance index and magnetic resonance imaging for assessment of lung disease in children with cystic fibrosis. Am J Respir Crit Care Med 2017;195:349–59. 10.1164/rccm.201604-0893OC [DOI] [PubMed] [Google Scholar]
- 30. Aurora P, Stanojevic S, Wade A, et al. Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med 2011;183:752–8. 10.1164/rccm.200911-1646OC [DOI] [PubMed] [Google Scholar]
- 31. Hardaker KM, Panda H, Hulme K, et al. Abnormal preschool lung clearance index (LCI) reflects clinical status and predicts lower spirometry later in childhood in cystic fibrosis. J Cyst Fibros 2019;18:721–7. 10.1016/j.jcf.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 32. Wyler F, Oestreich M-A, Frauchiger BS, et al. Correction of sensor crosstalk error in Exhalyzer D multiple-breath washout device significantly impacts outcomes in children with cystic fibrosis. J Appl Physiol 2021;131:1148–56. 10.1152/japplphysiol.00338.2021 [DOI] [PubMed] [Google Scholar]
- 33. Ramsey KA, McGirr C, Stick SM, et al. Effect of posture on lung ventilation distribution and associations with structure in children with cystic fibrosis. J Cyst Fibros 2017;16:713–8. 10.1016/j.jcf.2017.01.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-218178supp001.pdf (1MB, pdf)
Data Availability Statement
Data are available upon reasonable request.