Abstract
Objective
There has been limited systematic evaluation of outcomes and drivers of inappropriate non-vitamin K antagonist oral anticoagulants (NOACs) dosing among patients with atrial fibrillation (AF). This review identified and systematically evaluated literature on clinical and economic outcomes of inappropriate NOAC dosing and associated patient characteristics.
Methods
MEDLINE, Embase, Cochrane Library, International Pharmaceutical Abstracts, Econlit, PubMed and NHS EEDs databases were searched for English language observational studies from all geographies published between 2008 and 2020, examining outcomes of, or factors associated with, inappropriate NOAC dosing in adult patients with AF.
Results
One hundred and six studies were included in the analysis. Meta-analysis showed that compared with recommended NOAC dosing, off-label underdosing was associated with a null effect on stroke outcomes (ischaemic stroke and stroke/transient ischaemic attack (TIA), stroke/systemic embolism (SE) and stroke/SE/TIA). Meta-analysis of 15 studies examining clinical outcomes of inappropriate NOAC dosing found a null effect of underdosing on bleeding outcomes (major bleeding HR=1.04, 95% CI 0.90 to 1.19; p=0.625) but an increased risk of all-cause mortality (HR=1.28, 95% CI 1.10 to 1.49; p=0.006). Overdosing was associated with an increased risk of major bleeding (HR=1.41, 95% CI 1.07 to 1.85; p=0.013). No studies were found examining economic outcomes of inappropriate NOAC dosing. Narrative synthesis of 12 studies examining drivers of inappropriate NOAC dosing found that increased age, history of minor bleeds, hypertension, congestive heart failure and low creatine clearance (CrCl) were associated with an increased risk of underdosing. There was insufficient evidence to assess drivers of overdosing.
Conclusions
Our analysis suggests that off-label underdosing of NOACs does not reduce bleeding outcomes. Patients prescribed off-label NOAC doses are at an increased risk of all-cause mortality. These data underscore the importance of prescriber adherence to NOAC dosing guidelines to achieve optimal clinical outcomes for patients with AF.
PROSPERO registration number
CRD42020219844.
Keywords: Atrial Fibrillation, Stroke
WHAT IS ALREADY KNOWN ON THIS TOPIC
Inappropriate non-vitamin K antagonist oral anticoagulant (NOAC) dosing is a common occurrence in patients with atrial fibrillation (AF) and has been variably associated with adverse outcomes in individual studies. However, there has been limited synthesis of these studies to date.
WHAT THIS STUDY ADDS
This study provides a systematic synthesis of existing literature to highlight the importance of adhering to NOAC dosing guidelines to achieve the best clinical outcomes for patients with AF. To the best of our knowledge, this study provides the first synthesis of studies examining factors driving inappropriate NOAC dosing.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Sustained emphasis on the importance of adhering to clinical dosing guidelines for patients with AF may increase the likelihood that such patients achieve optimal clinical outcomes.
Introduction
Global guidelines recommend that non-vitamin K antagonist oral anticoagulants (NOACs) are used to prevent stroke in patients with atrial fibrillation (AF). NOACs (including apixaban, dabigatran, edoxaban and rivaroxaban) have demonstrated at least similar efficacy in preventing AF-related stroke compared with warfarin and are safer concerning serious bleeding across several large-scale clinical studies. Consequently, NOACs are recommended as first-line therapy for stroke prevention among adult patients with AF.1–5
Comorbidities (eg, chronic kidney disease and extreme body weight), as well as age and concomitant therapies, can impact the appropriate dosing of NOACs. Therefore, guidelines informed by clinical trials recommend dose adjustment to reduce the risk of thromboembolic events and bleeding complications for defined subgroups.6 7
The product information of each NOAC provides clear guidance as to which patients should be considered for dose adjustment; however, a substantial number of patients in routine clinical practice are inappropriately prescribed adjusted doses.8 9 A 2016 study conducted in the USA found that 9.4% of patients with AF were underdosed and 3.4% were overdosed.10 Prescribers may perceive that this reduces bleeding risk; however, there is currently a lack of evidence as to whether routine underdosing reduces the risk of bleeding and adequately protects patients.
Several studies have indicated that inappropriate NOAC dosing may be associated with adverse events.6 11 Underdosing has been associated with increased stroke risk,12 while overdosing may increase the risk of adverse events such as bleeding.13 Several large observational studies have been conducted in the secondary care setting (USA,12 13 South Korea14 15 and Europe16), which show an association between inappropriate NOAC dosing and adverse health outcomes. Similar results have been found in high-risk populations such as nursing home residents.17
To inform decision-makers whether the common practice of dosing outside of guidelines produces optimal patient outcomes, it is necessary to systematically evaluate the clinical and economic implications of off-label dosing and to establish what is driving decisions to dose outside of guidelines. To our knowledge, there is no published systematic review that examines the clinical and economic impacts, as well as drivers, of inappropriate NOAC dosing.
The primary objectives of this review were to identify the evidence for (1) the effect of inappropriate dosing (underdosing and overdosing) of a NOAC on clinical outcomes in adult patients with non-valvular atrial fibrillation (NVAF) and (2) the economic impact of inappropriate NOAC dosing. The secondary objective is to identify patient characteristics associated with inappropriate dosing.
Research questions
These review objectives were met by answering the following three research questions:
What is the impact of inappropriate dosing (underdosing and overdosing) of a NOAC on clinical outcomes in adult patients with AF?
What is the cost impact of inappropriate NOAC dosing?
What are the key patient characteristics associated with inappropriate NOAC dosing?
Review methods
The review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and in accordance with the Centre for Review and Dissemination (CRD) Handbook. The protocol was preregistered on the PROSPERO register of systematic reviews (CRD42020219844).
Literature search
Published literature was captured through searches of MEDLINE, Embase, Cochrane library, International Pharmaceutical Abstracts, Econlit, PubMed and NHS EEDs databases. The searches were conducted on 12 October 2020 and included material from 2008 (see online supplemental S1, Search strategy for the full search strategy).
heartjnl-2022-321114supp001.pdf (1.9MB, pdf)
Conference proceedings were date limited 2018–2020. The Conference Proceedings Citation Index- Science (Clarivate Analytics) and The Professional Society for Health Economics and Outcomes Research conference databases were searched alongside a search of Embase limited to conference proceedings. The European Society of Cardiology, the American College of Cardiology and the American Heart Association were hand searched by a researcher (CC).
Study selection and screening
Studies were screened by two researchers independently (AW and HS) based initially on study title and abstract and subsequently on full text. Disagreements over study eligibility were resolved by a third researcher (AC).
Inclusion and exclusion criteria, data extraction and quality assessment
English language studies were included if they examined outcomes of, or factors associated with, inappropriate NOAC dosing (apixaban, dabigatran, edoxaban or rivaroxaban) for adult patients with NVAF. Doses were considered inappropriate if they did not conform to label or guideline recommendations, either non-recommended high dose (overdosing) or non-recommended low dose (underdosing) (table 1).18 We applied the NOAC dosing guidelines as used in each included study.
Table 1.
Dose reduction criteria of NOACs18
| NOAC | Dose reduction criteria | Reduced dose |
| Dabigatran | Creatinine clearance: 50 mL/min | Variable doses below full 110 mg dose recommended in ESC guidelines |
| Rivaroxaban | Creatinine clearance: 50 mL/min | 15 mg once a day |
| Apixaban | 2 of 3 criteria: age ≥80 years, weight ≤60 kg, creatinine ≥1.5 mg/dL | 2.5 mg two times a day |
| Edoxaban | Creatinine clearance: ≤50 mL/min | 30 mg once a day |
NOAC, non-vitamin K antagonist oral anticoagulant.
Data were extracted by one reviewer (AW) for each included study and independently checked by a second reviewer (HS). Information extracted included study information (author, year of publication, country, study design, follow-up length, study size and NOAC studied), patient characteristics (age, sex, ethnicity, comorbidities and concomitant medications), outcomes/factors assessed, measures used and effect sizes.
Factor and outcome definitions were applied as used in the included studies.
Study quality was assessed by two researchers independently using the Newcastle-Ottawa Scale. Cut-off values of 0–3, 4–6 and 7–10 corresponding to low, moderate and high qualities, respectively, were assigned.19
Analysis
The feasibility of meta-analysis of adjusted HRs was determined based on an assessment of similarity of study design, presentation of results and factors adjusted for in regression analyses.
Fifteen studies addressing outcomes of inappropriate NOAC dosing were deemed sufficiently similar to enable a meta-analysis conducted in Stata V.16.10 12–14 17 20–29 These were all cohort studies that conducted regression analyses and reported results as HRs, and all adjusted for a minimum common set of factors (age, sex, diabetes (type unspecified), heart failure and hypertension). Statistical details from these studies were collected (online supplemental information S5, table 14). Random-effects meta-analysis was performed using aggregated data. We assume the log HR in each trial is independently normally distributed; however, studies have unequal variance and therefore regression was weighted using the inverse variance method.
Cardiovascular outcomes of underdosing assessed through meta-analysis were ‘ischaemic stroke (IS) and stroke/transient ischaemic attack (TIA)’, ‘stroke/systemic embolism (SE)’, ‘stroke/SE/TIA’ and ‘myocardial infarction (MI)’. Bleeding outcomes addressed were ‘major bleeding’, ‘gastrointestinal haemorrhage (GIB)’ and ‘intracranial haemorrhage (ICH)’. ‘Mortality’ was also assessed.
Clinical outcomes of overdosing assessed through meta-analysis were ‘IS and stroke/TIA’, ‘stroke/SE’, ‘major bleeding’ and ‘mortality’.
Due to study heterogeneity, meta-analysis was not considered appropriate for studies addressing the factors associated with inappropriate NOAC dosing. Therefore, the findings from these studies are presented as a narrative synthesis, and factors examined within a regression analysis by more than one paper were included in the narrative synthesis.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of the study.
Results
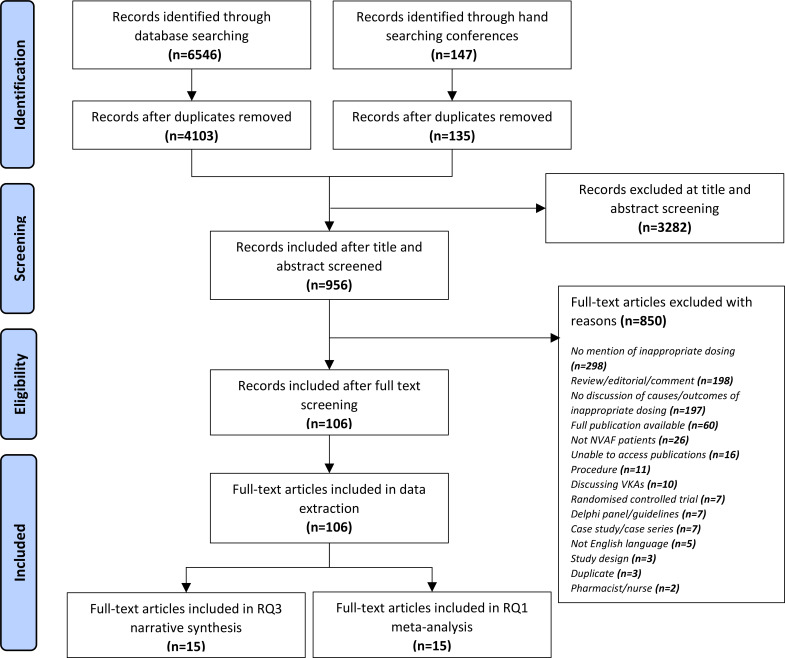
One hundred and six studies were included in this review: 34 of these examined clinical outcomes of inappropriate NOAC dosing; 41 examined drivers of inappropriate NOAC dosing; and 31 examined both outcomes and drivers of inappropriate NOAC dosing (figure 1). No studies examined economic outcomes of inappropriate NOAC dosing.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram of the study selection. NVAF, non-valvular atrial fibrillation RQ1, research question 1; RQ3, research question 3.
Sixty-three were cohort studies, and 43 were cross-sectional studies (see online supplemental S2 for an overview of study characteristics).
The following sections describe findings by outcome. Results for all subgroup analysis for each outcome can be found in online supplemental S4.
Stroke outcomes of inappropriate NOAC dosing
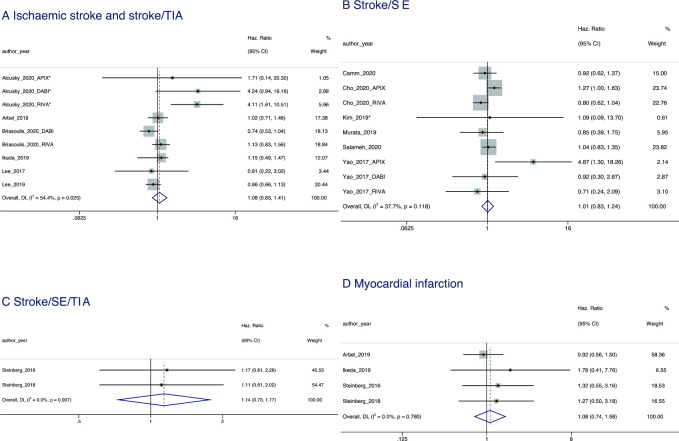
Compared with recommended NOAC dosing, underdosing was found to have a null effect on stroke outcomes. A null effect was observed for IS and stroke/TIA (HR=1.08, 95% CI 0.83 to 1.41; p=0.546), stroke/SE (HR=1.01, 95% CI 0.83 to 1.24; p=0.886) and stroke/SE/TIA (HR=1.14, 95% CI 0.73 to 1.77; p=0.569) (figure 2).
Figure 2.
Forest plot showing the HRs and 95% CIs for studies examining cardiovascular outcomes of non-vitamin K antagonist oral anticoagulant underdosing compared with recommended dosing. SE, systemic embolism; TIA, transient ischaemic attack.
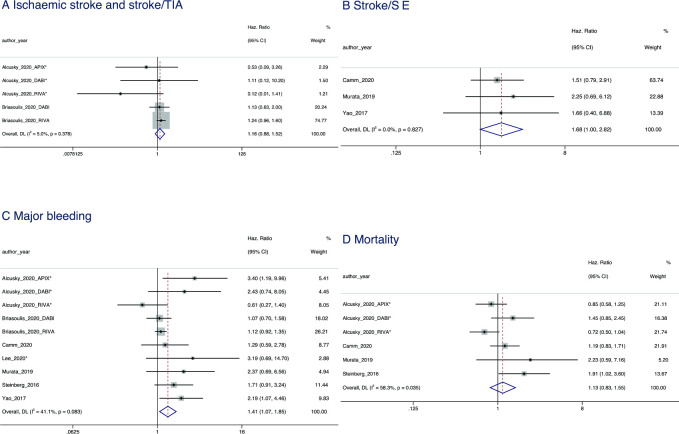
A null effect of overdosing was found for IS and stroke/TIA (HR=1.16, 95% CI 0.88 to 1.52; p=0.291) and stroke/SE (HR=1.68, 95% CI 1.00 to 2.82; p=0.052).
Cardiovascular outcomes of inappropriate NOAC dosing
Compared with recommended NOAC dosing, underdosing was associated with a null effect for MI (HR=1.08, 95% 0.74–1.58; p=0.676) (figure 2).
Bleeding outcomes of inappropriate NOAC dosing
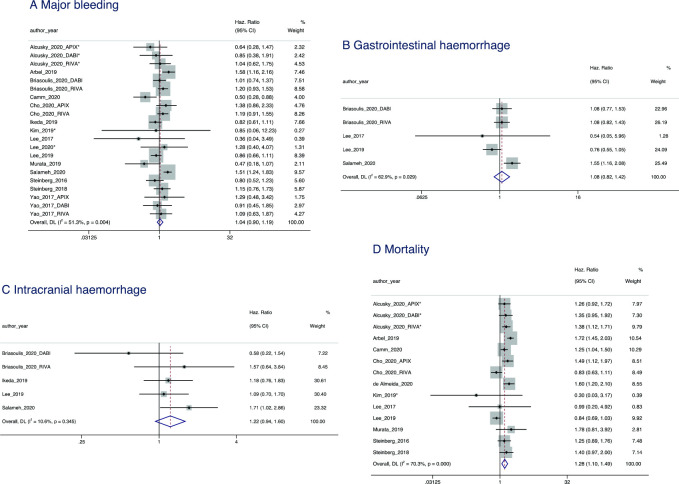
When assessed through meta-analysis, underdosing had a null effect on bleeding outcomes compared with recommended NOAC dosing. This was the case for major bleeding (HR=1.04, 95% CI 0.90 to 1.19; p=0.625), GIB (HR=1.08, 95% CI 0.82 to 1.42; p=0.590) and ICH (HR=1.22; 95% CI 0.94 to 1.60; p=0.141) (figure 3).
Figure 3.
Forest plot showing the HRs and 95% CIs for studies examining bleeding (A–C) and mortality (D) outcomes of non-vitamin K antagonist oral anticoagulant underdosing compared with recommended dosing. DL, DerSimonian and Laird approach.
Compared with recommended NOAC dosing, overdosing was associated with a greater risk of major bleeding (HR=1.41, 95% CI 1.07 to 1.85; p=0.013) (figure 3).
Mortality outcomes of inappropriate NOAC dosing
An increased risk of all-cause mortality was associated with underdosing (HR=1.28 95% CI 1.10 to 1.49; p=0.006) (figure 3). As the recorded outcome was ‘all-cause’ mortality, it was not possible to define which elements of mortality were driving this trend.
Overdosing had a null effect on all-cause mortality (HR=1.13, 95% CI 0.83 to 1.55; p=0.438) (figure 4).
Figure 4.
Forest plot showing the HRs and 95% CIs for studies examining cardiovascular (A, B), major bleeding (C) and mortality (D) outcomes of non-vitamin K antagonist oral anticoagulant overdosing compared with recommended dosing. SE, systemic embolism; TIA, transient ischaemic attack.
Economic outcomes of inappropriate NOAC dosing
No studies were identified examining economic outcomes of inappropriate NOAC dosing.
Drivers of inappropriate NOAC dosing
Fifteen studies conducted regression analyses on the drivers of inappropriate NOAC dosing, examining patient demographics (age, sex and weight), comorbidities (diabetes (type unspecified), hypertension and congestive heart failure), stroke risk factors (history of prior stroke, CHA2DS2-VASc score and CHADS2 score), renal function (CrCl and estimated glomerular filtration rate (eGFR) and bleeding risk (HAS-BLED and history of minor bleed).8 9 14 22 27 30–39
Drivers of inappropriate NOAC underdosing
A history of minor bleed (HAS-BLED <3 vs HAS-BLED ≥3) was associated with an increased risk of underdosing.8 9 14 22 27 30–39 Similarly, an increased risk of underdosing was observed with age ≥65 years and age ≥75 years (reference category: ≤65 years).8 9 14 22 27 30–39 An increased risk was also observed with CrCl levels of ≤50 mL/min. Okumura et al 9 and Yagi et al 39 found an increased risk of underdosing with CrCl levels of 50–80 and 50–63 mL/min, respectively.8 9 14 22 27 30–39 Başaran et al 8 found an increased risk of underdosing with CrCl of ≥50 mL/min.8 9 14 22 27 30–39 Hypertension (vs no hypertension) and CHF (vs no CHF) were also associated with an increased risk of underdosing.8 9 14 22 27 30–39
A null effect on underdosing was observed with age when measured as a continuous variable. Weight, sex, CHADS2 score, CHA2DS2-VASc score and HAS-BLED score also showed a null effect on underdosing (online supplemental information S3, figure 1). An inconclusive effect was observed with diabetes mellitus and history of prior stroke (online supplemental information S3, figure 2).
Drivers of inappropriate NOAC overdosing
There were variable effects between age, sex and CHA2DS2-VASc score and whether the patient had an overdose (online supplemental information S3, figure 3).
Discussion
The need for this systematic review was driven by an observation that NOACs are being inappropriately dosed in practice and a hypothesis that clinicians are prescribing lower doses to patients whom they deem to be at high risk of bleeds.9 12 24 40–42 Analysis of the drivers of inappropriate NOAC dosing supports this conjecture, identifying history of minor bleed but not major bleeding as a key driver of decisions to dose outside of guideline recommendations.
This may reflect physician perceptions about which patients have a higher risk of bleeding, reflected in the finding that age ≥65 was also associated with underdosing. Frailty might also be expected to be associated with underdosing; however, no studies quantitatively examined the impact of frailty on inappropriate NOAC dosing.
There was no difference in bleeding risk between patients with AF underdosed outside of the dosing guidelines and those dosed according to guidelines. This finding may suggest that contrary to clinicians’ assumed aim of selecting lower doses to reduce bleeding risk, in practice, no reduction in bleeding risk was found with underdosing. Underdosing conveyed no safety benefit in terms of reduced bleeding to the patient. Furthermore, differences in both stroke and cardiovascular outcomes between these patients were not found to be statistically significant, conveying no safety benefit to patients receiving reduced doses outside of guideline recommendations. While appropriate dose adjustment to avoid high levels of drug exposure have been studied in clinical trials and are necessary to avoid the unnecessary excess risk of bleeding, the fact that routinely underdosing NOACs has no safety advantage suggests that the risk of bleeding in these patients has more to do with their underlying bleeding risk than with dosing. Another explanation for this finding is that clinicians are successful in optimising NOAC doses for patients with high bleeding risk. Importantly, there is potential harm to reduce the NOAC dose in patients at presumed increased risk of bleeding, as demonstrated by the increase in all-cause mortality. Further studies are required to clarify this association due to unmeasured confounders such as frailty.
Adherence to guideline-recommend dosing is of critical importance across all the NOACs; however, different outcomes associated with individual NOAC underdosing have previously been observed.12 43 Subgroup analyses within this review echoed these findings; these results can be found in online supplemental information S4. While inappropriate low doses of dabigatran and rivaroxaban resulted in a null effect on major bleeding and all-cause mortality, inappropriate low doses of apixaban led to a paradoxically increased risk of both major bleeding and all-cause mortality. However, these associations are likely to be confounded since apixaban is more likely to be used in very high-risk patients (greater comorbidities and frail) who are also likely to be inappropriately underdosed. Therefore, it cannot be assumed that an appropriate NOAC dose would reduce all-cause mortality. Moreover, given that it is not biologically plausible that underdosing NOACs results in increased bleeding events, this emphasises that bleeding risk is more attributable to individual patient characteristics than NOAC dose.
Ashraf et al’s recently published study examining long-term clinical outcomes of underdosed NOACs similarly found that underdosing with apixaban, in particular, was associated with increased all-cause mortality.44 The current review and meta-analysis do not address this issue either, as there are currently no publications available on this issue.
While the clinical impact of inappropriate NOAC dosing is evident, there is a clear absence of evidence regarding the economic impact. This is an important area for future research.
This systematic review showed no association of underdosing with cardiovascular outcomes such as IS. However, increased stroke risk due to underdosing of NOACs has been seen in numerous randomised clinical trials. Steffel et al 45 found that half-dose edoxaban increased stroke risk among patients with NVAF.45 The study compared a lower-dose edoxaban regime comprising a standard dose of 30 mg once a day and reduced dose of 15 mg once a day to a higher-dose edoxaban regime comprising a standard and reduced dose of 60 and 30 mg once a day, respectively. Patients randomised to a lower-dose edoxaban regimen had significantly higher stroke risk than patients randomised to a higher-dose edoxaban regimen.
Similarly, the RE-LY and RELY-ABLE randomised controlled trials found higher rates of stroke and SE among patients receiving 110 mg of dabigatran two times per day than patients receiving 150 mg of dabigatran two times per day.1 46 Of note, dabigatran at a dose of 110 mg two times per day is not a dose reduction, as both dabigatran doses were approved for clinical use. Details of how underdosing was defined for dabigatran among the studies included for meta-analysis were recorded (online supplemental information S5, table 14).
The lack of a similar effect observed in this review may be linked to the inclusion of solely observational studies, creating a risk of unmeasured confounders affecting reported results. Disparity between the observational real-world data and randomised trial data illustrates the potential influence of confounders such as adherence, which was not considered or adjusted for in any of the studies included in meta-analyses. Poor adherence among standard-dosed patients could increase stroke risk among this group, obscuring any potential elevated stroke risk among underdosed patients.
Furthermore, there must be caution in clinical practice when interpreting the lack of association between underdosing and the risk of stroke based on observational studies. The impact of underdosing on AF-related clinically silent strokes and multi-infarct dementia47 was not evaluated by this review due to the paucity of data. Longitudinal studies are needed to demonstrate the lack of increased risk of these outcomes among patients underdosed with NOACs.
Low absolute rates of stroke magnify the potential influence of confounders. Calculation of absolute risk difference found that patients receiving inappropriate low doses experienced three additional IS and stroke/TIA events per 100 patients compared with those receiving a recommended dose, aligning with the conclusions drawn from the meta-analysis using relative risk (online supplemental information S3, figure 4).
A recently published systematic review by Liu et al supports the finding of null effect on bleeding and increased risk of mortality following underdosing.48 However, in contrast to this review, Liu et al also found an increased risk of stroke and SE following underdosing. This difference may be due to the broader search strings and database inclusion in this review (as demonstrated by the fact that all the 11 publications included by Liu et al were also included within the 106 selected for this review).
Strengths and limitations
To our knowledge, this is the first systematic review to assess both clinical and economic outcomes of inappropriate NOAC dosing as well as drivers of inappropriate NOAC dosing.
While observational studies are a valuable source of information for understanding NOAC dosing in clinical practice, the summary effects reported may be biased. This is due to the limitations of the primary studies in controlling for confounders both measured and unmeasured. For key variables, this review applied definitions used by the included studies. There was often a lack of clarity over definitions such as ‘history of minor bleed’, while definitions for inappropriate NOAC dosing may vary according to local guidelines. The outcome ‘all-cause mortality’ offers no further insight into specific causes of mortality driving observed trends. Understanding the causes of death in the underdosed group is an important avenue for further research to fully understand the potential detrimental impacts of inappropriate NOAC dosing.
Conclusions
This systematic review with meta-analysis indicates that off-label underdosing of NOACs does not reduce bleeding and may be associated with an increased risk of mortality. It is important to educate prescribers that this strategy will not result in less bleeding as the risk of bleeding in these patients has more to do with the patient’s underlying risk of bleeding than the dose of NOAC.
The factors identified in this review (increased age, history of minor bleeds, hypertension, congestive heart failure and low creatinine clearance) are the same risk factors that increase the overall stroke risk and may help identify vulnerable patients at high risk for routine, inappropriate underdosing.
Footnotes
Twitter: @ImMSanFer, @tseguram
Contributors: VC, JRdG, TS, CB-L, DH, SA, HW, AW, JH, AC, BV and CTR contributed to the design of the study. CC developed the study search strategy, performed the literature search and hand searching of medical society websites. AW and HS performed the study screening, selection and data extraction. AW, JH and AC planned the analysis. AW wrote the first draft of the manuscript. All authors contributed to the interpretation and subsequent edits of the manuscript. CTR acts as a guarantor for the manuscript.
Funding: Bayer AG has initiated, organised and funded this project, including payment of steering group participants and agencies involved in the development and execution of this project.
Competing interests: VC reports fees for speaking for Boehringer Ingelheim, Pfizer BMS, Bayer, Daicchi Sankyo and Mindmaze, and for participating on advisory boards for Boehringer Ingelheim, Pfizer BMS and Bayer. JRdG reports grants and personal fees from Atricure, Bayer, Daiichi Sankyo, Johnson&Johnson and Medtronic; grants from Boston Scientific, and personal fees from Biotronik, Novartis, Servier, AtrianMedical outside the submitted work. MSF reports grants and personal fees from Bayer outside the submitted work. TS reports personal fees from Bayer, Daiichi Sankyo, Novartis, AstraZeneca, Pfizer, Novo-Nordisk, Boehringer Ingelheim, Amgen, Alexion and Almirall outside the submitted work. CB-L reports personal fees from Medtronic AB, Boston Sci, Octopus, Bayer, Sanofi Aventis, BSM, Cathprint, Boeringer-Ingelheim and MSD. DH reports personal fees from Daiichi Sankyo, Bayer and Pfizer outside the submitted work. SA reports personal fees from Bayer, Pfizer/BMS, Boehringer Ingelheim and Daiichi Sankyo during the conduct of the study. HW reports personal fees from Bayer during the conduct of the study. AW, JH and AC are employees of Wickenstones and received funding from Bayer AG. BV and PF were employees of Bayer AG at the time of the study. CTR reports grants and personal fees from Anthos, Boehringer Ingelheim and Daiichi Sankyo; grants from AstraZeneca and National Institutes of Health; and personal fees from Bayer, Bristol Myers Squibb, Janssen, Pfizer outside the submitted work; and he is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from Abbott, Amgen, Anthos Therapeutics, ARCA Biopharma, AstraZeneca, Bayer HealthCare Pharmaceuticals, Daiichi-Sankyo, Eisai, Intarcia, Ionis Pharmaceuticals, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Roche, Siemens Healthcare Diagnostics, The Medicines Company and Zora Biosciences.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 4. Chen A, Stecker E, A Warden B. Direct oral anticoagulant use: a practical guide to common clinical challenges. J Am Heart Assoc 2020;9:e017559. 10.1161/JAHA.120.017559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 6. García Rodríguez LA, Martín-Pérez M, Vora P, et al. Appropriateness of initial dose of non-vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation in the UK. BMJ Open 2019;9:e031341. 10.1136/bmjopen-2019-031341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 8. Başaran Özcan, Dogan V, Beton O, et al. Suboptimal use of non-vitamin K antagonist oral anticoagulants: results from the RAMSES study. Medicine 2016;95:e4672. 10.1097/MD.0000000000004672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okumura Y, Yokoyama K, Matsumoto N, et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: findings from the SAKURA AF registry. J Arrhythm 2017;33:289–96. 10.1016/j.joa.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol 2016;68:2597–604. 10.1016/j.jacc.2016.09.966 [DOI] [PubMed] [Google Scholar]
- 11. Moudallel S, Steurbaut S, Cornu P, et al. Appropriateness of DOAC prescribing before and during hospital admission and analysis of determinants for inappropriate prescribing. Front Pharmacol 2018;9:1220. 10.3389/fphar.2018.01220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao X, Shah ND, Sangaralingham LR, et al. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol 2017;69:2779–90. 10.1016/j.jacc.2017.03.600 [DOI] [PubMed] [Google Scholar]
- 13. Steinberg BA, Shrader P, Pieper K, et al. Frequency and outcomes of reduced dose non-vitamin K antagonist anticoagulants: results from ORBIT-AF II (the outcomes registry for better informed treatment of atrial fibrillation II). J Am Heart Assoc 2018;7. 10.1161/JAHA.117.007633. [Epub ahead of print: 16 02 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee K-N, Choi J-I, Boo KY, et al. Effectiveness and safety of off-label dosing of non–vitamin K antagonist anticoagulant for atrial fibrillation in Asian patients. Sci Rep 2020;10:1801. 10.1038/s41598-020-58665-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu HT, Yang P-S, Jang E, et al. Label adherence of direct oral anticoagulants dosing and clinical outcomes in patients with atrial fibrillation. J Am Heart Assoc 2020;9:e014177. 10.1161/JAHA.119.014177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Godino C, Bodega F, Melillo F, et al. Inappropriate dose of nonvitamin-K antagonist oral anticoagulants: prevalence and impact on clinical outcome in patients with nonvalvular atrial fibrillation. J Cardiovasc Med 2020;21:751–8. 10.2459/JCM.0000000000001043 [DOI] [PubMed] [Google Scholar]
- 17. Alcusky M, Tjia J, McManus DD, et al. Comparative safety and effectiveness of direct-acting oral anticoagulants versus warfarin: a national cohort study of nursing home residents. J Gen Intern Med 2020;35:2329–37. 10.1007/s11606-020-05777-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diener H-C, Aisenberg J, Ansell J, et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: Part 2. Eur Heart J 2017;38:860–8. 10.1093/eurheartj/ehw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang J-R, Hidayat K, Chen C-L, et al. Body mass index, waist circumference, and risk of hearing loss: a meta-analysis and systematic review of observational study. Environ Health Prev Med 2020;25:25. 10.1186/s12199-020-00862-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arbel R, Sergienko R, Hammerman A, et al. Effectiveness and safety of off-label dose-reduced direct oral anticoagulants in atrial fibrillation. Am J Med 2019;132:847–55. 10.1016/j.amjmed.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 21. Briasoulis A, Gao Y, Inampudi C, et al. Characteristics and outcomes in patients with atrial fibrillation receiving direct oral anticoagulants in off-label doses. BMC Cardiovasc Disord 2020;20:42. 10.1186/s12872-020-01340-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Camm AJ, Cools F, Virdone S, et al. Mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol 2020;76:1425–36. 10.1016/j.jacc.2020.07.045 [DOI] [PubMed] [Google Scholar]
- 23. Cho MS, Yun JE, Park JJ, et al. Pattern and impact of off-label underdosing of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation who are indicated for standard dosing. Am J Cardiol 2020;125:1332–8. 10.1016/j.amjcard.2020.01.044 [DOI] [PubMed] [Google Scholar]
- 24. Ikeda T, Ogawa S, Kitazono T, et al. Outcomes associated with under-dosing of rivaroxaban for management of non-valvular atrial fibrillation in real-world Japanese clinical settings. J Thromb Thrombolysis 2019;48:653–60. 10.1007/s11239-019-01934-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HM, Choi E-K, Park CS, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in octogenarian patients with non-valvular atrial fibrillation. PLoS One 2019;14:e0211766. 10.1371/journal.pone.0211766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee KH, Park HW, Lee N, et al. Optimal dose of dabigatran for the prevention of thromboembolism with minimal bleeding risk in Korean patients with atrial fibrillation. Europace 2017;19:iv1–9. 10.1093/europace/eux247 [DOI] [PubMed] [Google Scholar]
- 27. Lee S-R, Choi E-K, Han K-D, et al. Optimal rivaroxaban dose in Asian patients with atrial fibrillation and normal or mildly impaired renal function. Stroke 2019;50:1140–8. 10.1161/STROKEAHA.118.024210 [DOI] [PubMed] [Google Scholar]
- 28. Murata N, Okumura Y, Yokoyama K, et al. Clinical outcomes of off-label dosing of direct oral anticoagulant therapy among Japanese patients with atrial fibrillation identified from the SAKURA AF registry. Circ J 2019;83:727–35. 10.1253/circj.CJ-18-0991 [DOI] [PubMed] [Google Scholar]
- 29. Salameh M, Gronich N, Stein N, et al. Stroke and bleeding risks in patients with atrial fibrillation treated with reduced apixaban dose: a real-life study. Clin Pharmacol Ther 2020;108:1265–73. 10.1002/cpt.1952 [DOI] [PubMed] [Google Scholar]
- 30. Anouassi Z, Atallah B, Alsoud LO, et al. Appropriateness of the direct oral anticoagulants dosing in the middle East Gulf region. J Cardiovasc Pharmacol 2021;77:182–8. 10.1097/FJC.0000000000000913 [DOI] [PubMed] [Google Scholar]
- 31. Atallah B, Alsolh S, El Nekidy W, et al. P281 factors influencing apixaban dosing in the middle East Gulf region. Eur Heart J 2020;41. 10.1093/ehjci/ehz872.101 [DOI] [Google Scholar]
- 32. Badreldin HA, Alreshoud L, Altoukhi R, et al. Prevalence and predictors of inappropriate apixaban dosing in patients with non-valvular atrial fibrillation at a large tertiary academic medical institution. Drugs Ther Perspect 2020;36:83–8. 10.1007/s40267-019-00696-8 [DOI] [Google Scholar]
- 33. Bosque Varela P, et al. European Stroke Organisation Conference: Abstracts. Eur Stroke J 2018;3(1_suppl:3–204. 10.1177/2396987318770127 [DOI] [Google Scholar]
- 34. Buchholz A, Ueberham L, Gorczynska K, et al. Initial apixaban dosing in patients with atrial fibrillation. Clin Cardiol 2018;41:671–6. 10.1002/clc.22949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cardoso CS, Sousa JA, Simões P, et al. Misdosing of non-vitamin K antagonist oral anticoagulants in primary care. Clin Ther 2020;42:1132–6. 10.1016/j.clinthera.2020.04.008 [DOI] [PubMed] [Google Scholar]
- 36. Ruiz Ortiz M, Muñiz J, Raña Míguez P, et al. Inappropriate doses of direct oral anticoagulants in real-world clinical practice: prevalence and associated factors. A subanalysis of the FANTASIIA registry. Europace 2018;20:1577–83. 10.1093/europace/eux316 [DOI] [PubMed] [Google Scholar]
- 37. Sabbag A, Klempfner R, Shlomo N, et al. P4020Use of novel oral anticoagulants in patients with atrial fibrillation and moderate to severe renal dysfunction: findings from a prospective national registry. Eur Heart J 2017;38. 10.1093/eurheartj/ehx504.P4020 [DOI] [Google Scholar]
- 38. Sato T, Aizawa Y, Fuse K, et al. The comparison of Inappropriate-low-doses use among 4 direct oral anticoagulants in patients with atrial fibrillation: from the database of a single-center registry. J Stroke Cerebrovasc Dis 2018;27:3280–8. 10.1016/j.jstrokecerebrovasdis.2018.07.028 [DOI] [PubMed] [Google Scholar]
- 39. Yagi N, Suzuki S, Arita T, et al. Creatinine clearance and inappropriate dose of rivaroxaban in Japanese patients with non-valvular atrial fibrillation. Heart Vessels 2020;35:110–7. 10.1007/s00380-019-01457-3 [DOI] [PubMed] [Google Scholar]
- 40. Maura G, Billionnet C, Drouin J, et al. Oral anticoagulation therapy use in patients with atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants: findings from the French healthcare databases, 2011-2016. BMJ Open 2019;9:e026645. 10.1136/bmjopen-2018-026645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brabandt v. Anticoagulants in non-valvular atrial fibrillation; 2016.
- 42. Miyazaki M, Matsuo K, Uchiyama M, et al. Inappropriate direct oral anticoagulant dosing in atrial fibrillation patients is associated with prescriptions for outpatients rather than inpatients: a single-center retrospective cohort study. J Pharm Health Care Sci 2020;6:2. 10.1186/s40780-020-0157-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nielsen PB, Skjøth F, Søgaard M, et al. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ 2017;356:j510. 10.1136/bmj.j510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ashraf H, Agasthi P, Shanbhag A, et al. Long-term clinical outcomes of underdosed direct oral anticoagulants in patients with atrial fibrillation and atrial flutter. Am J Med 2021;134:788–96. 10.1016/j.amjmed.2020.12.022 [DOI] [PubMed] [Google Scholar]
- 45. Steffel J, Ruff CT, Yin O, et al. Randomized, double-blind comparison of half-dose versus full-dose edoxaban in 14,014 patients with atrial fibrillation. J Am Coll Cardiol 2021;77:1197–207. 10.1016/j.jacc.2020.12.053 [DOI] [PubMed] [Google Scholar]
- 46. Ezekowitz MD, Eikelboom J, Oldgren J, et al. Long-term evaluation of dabigatran 150 vs. 110 Mg twice a day in patients with non-valvular atrial fibrillation. Europace 2016;18:973–8. 10.1093/europace/euv312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thacker EL, et al. Atrial fibrillation and cognitive decline. Neurology 2013;81:119–25. 10.1212/WNL.0b013e31829a33d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu X, Huang M, Ye C, et al. Effect of non-recommended doses versus recommended doses of direct oral anticoagulants in atrial fibrillation patients: a meta-analysis. Clin Cardiol 2021;44:472–80. 10.1002/clc.23586 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2022-321114supp001.pdf (1.9MB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study.