Abstract
Background
Incidence and risk factors for seizures among women with advanced breast cancer (BC) and brain metastases are not well characterized across treatment-related or clinical subtypes. This study leveraged a large real-world dataset to describe incidence and risk factors for seizures in BRCA-associated metastatic breast cancer.
Methods
The Optum® de-identified electronic health records database was used. Females with a BC diagnoses between 2008 and 2018, with clinic visits 12 months before BC index date, evidence of BRCA mutation (BRCA+), evidence of metastasis, and no previous cancers were included. Analyses were stratified by the overall BRCA+ cohort and 4 molecular phenotypes: HER2+/HR- (human epidermal growth factor 2/hormone receptor), HER2−/HR+, HER2+/HR+, and triple negative BC (TNBC; HER2−/HR-). Seizures were identified using diagnosis codes and natural language processing. Incidence, occurrence rates, and cumulative incidence of seizures from the diagnosis of metastasis to the end of follow up were calculated. Comparisons were made between phenotypes and stratified on PARP inhibitor use, diagnosed brain metastases, history of seizures, and anticonvulsants use before BC. All comparisons included age at metastasis, number of prior lines of treatment, and metastasis location as covariates.
Results
27.8% of 7941 BRCA+ patients had ≥1 seizure over a mean follow-up time of 2.35 years. Incidence and occurrence rates were 11.83 (95% CI: 11.35–12.33) and 201.3 (95% CI: 198.05–204.50), respectively, per 100 person-years. HER2−/HR+ and TNBC patients had the lowest and highest seizure incidence rates, respectively (10.94 [95% CI: 10.23–11.71] and 16.83 [95% CI: 15.34–18.46]). With HER2−/HR+ as the reference group in a competing risk analysis, TNBC (hazard ratio, HR = 1.35; 95%CI: 1.21, 1.52; p < 0.001) and HER2+/HR- (HR = 1.29; 95%CI: 1.07, 1.56; p < 0.01) patients had a greater risk of seizures. Patients with diagnosed brain metastases or a history of seizures had higher seizure rates. Incidence trended higher with PARP inhibitor use, but patient numbers were low.
Conclusions
This study provides novel real-world evidence on seizure incidence rates in BRCA+ BC patients, even those without diagnosed brain metastases, and underscores the need to understand patients’ tumor phenotypes when assessing seizure risk. These findings may have implications for clinical practice and assessment of benefit-risk ratios of new therapeutic agents.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-10554-6.
Keywords: Metastatic breast cancer, BRCA-positive, Seizures, Predisposing factors
Introduction
Breast cancer (BC) is the leading cancer type and ranks second among the causes of cancer-related mortality among women in the United States, with estimates indicating that 287,850 new cases will be diagnosed in 2022 and that 43,250 deaths will be attributable to it during the year [1]. Although advances in treatment have raised the five-year survival rate among women with localized BC at diagnosis to 99%, lower survival rates persist for women diagnosed with regional and particularly for women with distant disease (86 and 28%, respectively) [1].
While most breast tumors arise sporadically in the general population, a small subset (10–15%) are associated with genetic mutations, of which a majority (~ 60%) are associated with the BRCA1 and BRCA2 genes (BReast CAncer susceptibility genes 1 and 2) [2–4]. Women who inherit a germline BRCA mutation are at a significantly increased risk for the development of breast cancer and ovarian cancer. Women face an approximately 72 and 69% risk of developing breast cancer associated with BRCA1 and BRCA2 mutations, respectively, by the age of 80, compared with 12% among women in the general population [3, 5]. Among BRCA mutations detected in women with breast cancer overall, approximately one-third may be somatic in origin [6, 7].
The three major molecular subtypes (or molecular phenotypes) of breast tumors based on the presence or absence of markers or overexpression of estrogen, progesterone (hormone) receptors (ER+ and/or PR+; collectively referred to as HR+), and the human epidermal growth factor 2 (HER2) are clinically meaningful to better characterize prognosis. Overall survival for women diagnosed with metastatic breast cancer also differs by molecular subtype and remains particularly low (10–13 months) for women with triple-negative tumors compared with the other molecular phenotypes (4–5 years) [8–11]. Accordingly, treatment guidelines for both non-metastatic and metastatic breast cancer are also specified by molecular subtype [8, 12]. Women diagnosed with metastatic breast cancer and HR+ phenotype receive endocrine therapy prior to the development of resistance to endocrine agents and single-agent chemotherapy thereafter, while patients with HER2+ tumors receive a combination of HER2+ targeting agents and chemotherapy in addition to endocrine therapy (the last only if the HER2+ tumors are also HR+) and patients with triple-negative tumors receive single-agent chemotherapy [8, 12]. Additional options available for the treatment of metastatic tumors harboring germline BRCA mutations include the poly [adenosine diphosphate-ribose] polymerase (PARP) inhibitors olaparib and talazoparib [13, 14]. These agents were approved in 2018 and are used in later lines of therapy for patients with HR+ and triple-negative tumors [8].
Organ sites associated with metastases from breast tumors include liver, bones, lungs, and/or brain. Diagnosed brain metastases in particular have been reported in 24% of breast cancer cases [15], with breast cancer ranking second among all causes of brain metastases [16]. The brain is the first site of metastasis in 12% of patients with breast cancer [17]. However, there is a paucity of data on rates of seizures among breast cancer patients with brain metastases and on potential risk factors associated with seizures in this patient population. Early reports on seizures in patients with brain metastases documented seizure frequencies ranging between 20 and 35% [18–20], but these studies included all cancer patients with brain metastases and not only breast cancer patients. A more recent review of 106 studies documented seizures in 12% of breast cancer patients with diagnosed brain metastases but did not explore the potential risk factors for seizures in these patients [15]. Other studies have reported agents used for cancer chemotherapy (e.g., methotrexate, fludarabine, cytarabine, vincristine, etoposide, and cisplatin) and other drugs prescribed to cancer patients (e.g., antidepressants such as tricyclics and bupropion, neuroleptic agents such as clozapine and phenothiazines, and antibiotics such as penicillin and β-lactams) as being epileptogenic [20–23]; however, no data are available on the incidence of seizures following the use of more recently approved therapeutic agents such as PARP inhibitors or on seizure rates categorized by molecular subtypes of breast cancer. This study was therefore undertaken with the objective of estimating, in a large real-world dataset, the incidence of seizures in patients with metastatic breast cancer who harbor mutations in the BRCA1 or BRCA2 genes, and examining factors associated with the development of seizures.
Methods
Data source
The Optum® de-identified electronic health records (EHR) database was used for this study [24]. The database represents an aggregation of patient-level data from more than 140,000 physicians at more than 700 hospitals and 7000 clinics that are part of 58 integrated delivery networks (IDNs) throughout the United States [25]. Extensive de-identified data on patient demographics, diagnoses, procedures, medications, laboratory results, and clinical administrative information for > 80 million patients from outpatient and inpatient settings, with > 7 million patients from each census region, are available within the database [25].
In addition to the structured data available from the EHR systems, natural language processing (NLP) was applied by Optum to the physicians’ notes to extract additional breast cancer data. The NLP step removed any identifiable information consistent with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 [26], making Institutional Review Board (IRB) review and waiver unnecessary [27]. NLP technology assists in the extraction of information on signs, diseases, and symptoms (SDS) and data on biomarkers from physicians’ notes into structured data that is subsequently used to identify disease conditions without specific medical codes (e.g., ICD9/10 or HCPCS). The SDS terms are identified along with the sentiment associated with the term. For example, to identify a patient who may have had a seizure, the SDS term “seizure” may exist in the patient’s record, but if the SDS attribute is “does not have”, it is interpreted as a physician writing in their notes that the patient does not have seizures. BRCA mutation-positive status was determined by NLP-related data fields. While the data source indicated the presence of BRCA mutations, it did not provide information on whether the mutations were germline or somatic in origin.
The Optum EHR database was selected primarily due to the number of patients available and the NLP extracted biomarker and seizure information. In addition, the Optum EHR database, is general (i.e. not oncology center specific), which allowed us to include a wider variety of treating physicians.
Inclusion criteria
Female patients with BC ICD9/10 diagnosis (ICD-9 code 174.xx or ICD-10 codes C50.01, C50.11, C50.21, C50.31, C50.41, C50.51, C50.61, C50.81, and C50.91) between January 1, 2008, and December 31, 2018, were included if they were ≥ 18 years of age on the BC index date (first BC diagnosis in the database), had evidence of EHR activity at least 12 months prior to the BC index date, had no other primary or secondary cancers 12 months prior to the BC index date, had ≥1 diagnosis or SDS term indicating metastasis more than 30 days prior to the BC index date, and had evidence of being BRCA+. The diagnosis codes used to identify patients with malignancies and the SDS terms/attributes used to identify patients with metastases but without ICD codes are listed in Additional file 1 Supplementary Table 1 and Additional file 2 Supplementary Table 2, respectively.
Analyses performed
The analyses described below were performed both for the overall BRCA+ cohort and stratified by four phenotypes: HER2+/HR+, HER2+/HR-, HER2−/HR+, and TNBC (triple negative breast cancer: HER2− and HR−). Seizures were identified using diagnosis codes (ICD-9: 345.xx [epilepsy and recurrent seizures] and 780.39 [other convulsions]; ICD-10: G40.xx [epilepsy and recurrent seizures] and R56.9 [unspecified convulsions]) and the SDS terms listed in Additional file 3 Supplementary Table 3.
The rates of occurrence and incidence of seizures in the post-metastasis period (defined as the time between the date of diagnosis of metastases and the end of follow up) were calculated for each patient and the cumulative incidence curves for drug exposures or follow up from diagnoses of brain metastases were plotted. The occurrence rate was defined as the total or aggregate number of days with a seizure divided by the aggregate duration in the post-metastasis period for patients with a seizure. The seizure incidence rate was calculated using a Poisson model where the follow-up period was defined as the date of metastasis to the date of the first seizure (for those with a seizure) or the date of metastasis to the end of follow-up (for those without a seizure). Incidence rates for the four phenotypes are presented as Forest plots.
Incidence rates expressed in units of per person-time and adjusted for age at metastasis, number of prior lines of treatment, and metastasis location were calculated, with comparisons made between patients with and without the following risk factors: use of PARP inhibitors, diagnosed brain metastases, history of seizures prior to the breast cancer diagnosis, and use of anticonvulsants prior to the diagnosis of breast cancer (see Additional file 4 Supplementary Table 4 for list of anticonvulsants and PARP inhibitors). Competing risks regression of time to seizure, with death as a competing event, and the same covariates as above were performed overall and for the four risk factors. Cumulative incidence curves of time to seizure were plotted for the overall sample and the four risk factors. These plots were stratified by the four phenotypes and a Grey’s test of equality performed to test for overall differences between the curves.
Results
Patient identification and demographics
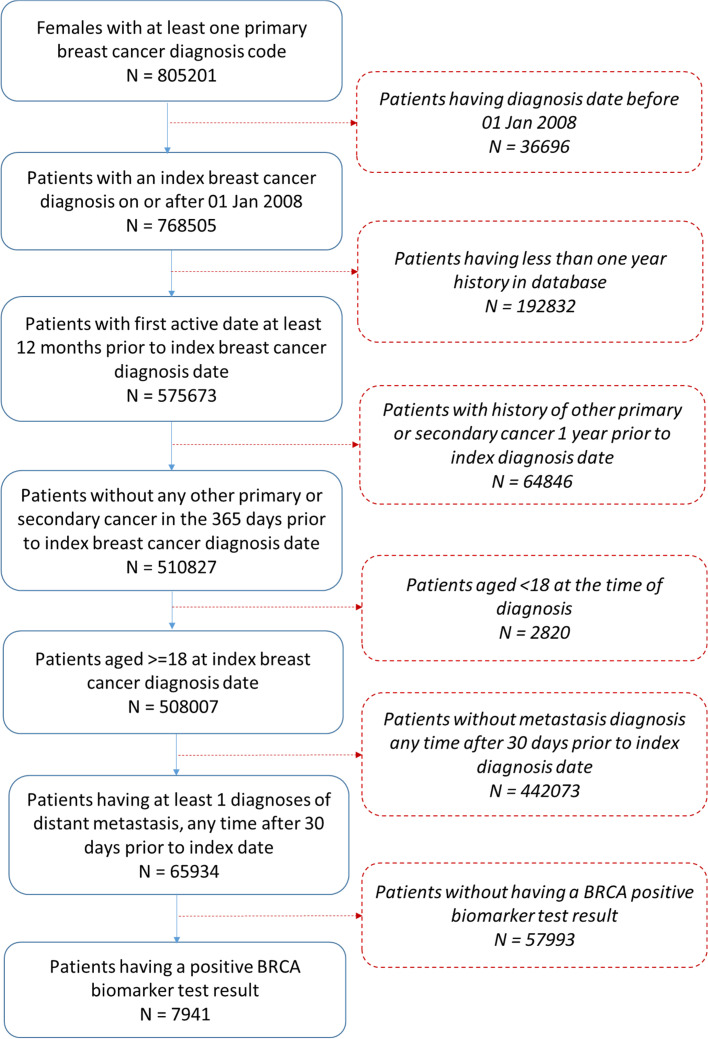
A total of 7941 BRCA+ breast cancer patients were identified in the Optum® dataset for the study period from among 65,934 (12.0%) metastatic breast cancer patients (Fig. 1).
Fig. 1.
Patient attrition
Of these, 5922 patients had known HR and HER2 status, among whom 1323 (22.3%) patients had the triple negative phenotype (ER−, PR−, and HER2−); 1039 (17.5%) patients had the HER2+/HR+ phenotype, 378 (6.4%) patients had the HER2+/HR− phenotype, and 3182 (53.7%) patients had the HER2−/HR+ phenotype (Table 1).
Table 1.
Patient demographics in BRCA+ study cohort by seizure occurrence
| Overall | With seizures | Without seizures | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| 7941 | 100% | 2207 | 28% | 5734 | 72% | |
| Race | ||||||
| Caucasian | 6454 | 81.3% | 1783 | 80.8% | 4671 | 81.5% |
| African American | 849 | 10.7% | 273 | 12.4% | 576 | 10.0% |
| Asian | 165 | 2.1% | 39 | 1.8% | 126 | 2.2% |
| Other/Unknown | 473 | 6.0% | 112 | 5.1% | 361 | 6.3% |
| Ethnicity | ||||||
| Non-Hispanic | 7292 | 91.8% | 2050 | 92.9% | 5242 | 91.4% |
| Hispanic | 308 | 3.9% | 77 | 3.5% | 231 | 4.0% |
| Unknown | 341 | 4.3% | 80 | 3.6% | 261 | 4.6% |
| Region | ||||||
| Midwest | 4065 | 51.2% | 1294 | 58.6% | 2771 | 48.3% |
| South | 1513 | 19.1% | 374 | 16.9% | 1139 | 19.9% |
| West | 888 | 11.2% | 172 | 7.8% | 716 | 12.5% |
| Northeast | 1239 | 15.6% | 285 | 12.9% | 954 | 16.6% |
| Other/Unknown | 236 | 3.0% | 82 | 3.7% | 154 | 2.7% |
| Age at diagnosis (years) | ||||||
| Mean (SD) | 53.6 (12.74) | 53.6 (12.81) | 53.6 (12.71) | |||
| Median (IQR) | 53 (44–62) | 53 (44–62) | 53 (44–62) | |||
| Min - Max | 18–89 | 19–88 | 18–89 | |||
| < 65 years of age | 6308 | 79.4% | 1753 | 79.4% | 4555 | 79.4% |
| ≥ 65 years of age | 1633 | 20.6% | 454 | 20.6% | 1179 | 20.6% |
| Hormonal Status* (n = 5922) | ||||||
| HER2+/HR+ | 1039 | 17.5% | 298 | 5.0% | 741 | 12.5% |
| HER2+/HR- | 378 | 6.4% | 124 | 2.1% | 254 | 4.3% |
| HER2−/HR+ | 3182 | 53.7% | 848 | 14.3% | 2334 | 39.4% |
| TNBC | 1323 | 22.3% | 442 | 7.5% | 881 | 14.9% |
*Of those with a known status; HER2 = human epidermal growth factor receptor 2; HR = hormone receptor; TNBC = triple negative breast cancer
The majority of the patients were Caucasians (81.3%) while geographically, most patients were based in the Midwest (51.2%). The mean age (standard deviation) of patients was 53.6 (12.7) years; this did not differ between patients with and without seizures. The majority (79.4%) of the patients were < 65 years of age; percentages of patients below and above 65 years of age did not differ between patients with and without seizures.
Incidence rates, overall and by phenotype
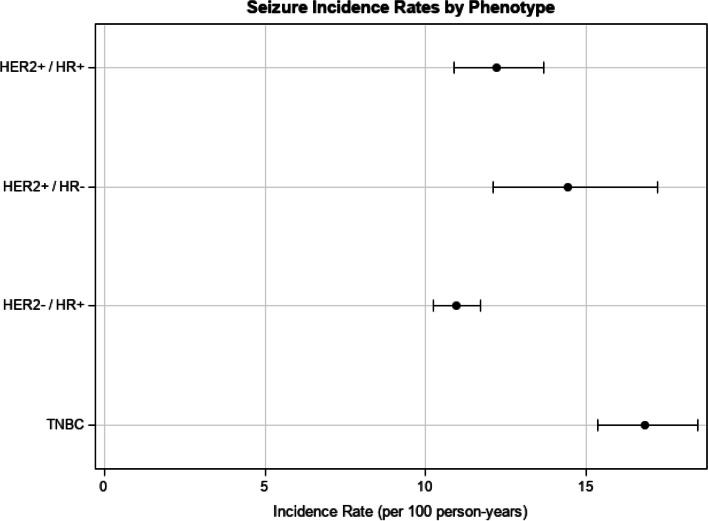
Of the 7941 patients in the overall cohort, 2207 (27.8%) had at least one seizure, with an incidence rate of 11.83 (95% CI: 11.35, 12.33) per 100 person-years and the occurrence rate of 201.3 (95% CI: 198.05, 204.50) per 100 person-years (Fig. 2 and Table 2).
Fig. 2.
Seizure incidence rates in BRCA+ patients overall and by HER2 and HR phenotype
Table 2.
Seizure incidence and occurrence rates in BRCA+ patients overall and by phenotype
| N | Number of patients with seizures | Median follow-up duration to seizure (days) | % patients with at least one seizure | Incidence person-years* | Incidence rate** (95% CI) | Total Occurrences | Occurrence frequency | Occurrence person-years† | Occurrence rate** | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 7941 | 2207 | 161 | 27.80% | 18655 | 11.83 (11.35,12.33) | 15029 | 6.80 | 7468 | 201.30 |
| HER2+/HR+ | 1039 | 298 | 193.5 | 28.68% | 2443 | 12.20 (10.89,13.66) | 2492 | 8.36 | 1051 | 237.13 |
| HER2+/HR- | 378 | 124 | 176.5 | 32.80% | 860 | 14.43 (12.10,17.20) | 1191 | 9.60 | 471 | 252.74 |
| HER2−/HR+ | 3182 | 848 | 147 | 26.65% | 7749 | 10.94 (10.23,11.71) | 5212 | 6.15 | 2917 | 178.66 |
| HER2−/HR- | 1340 | 447 | 149 | 33.36% | 2656 | 16.83 (15.34,18.46) | 3171 | 7.09 | 1312 | 241.72 |
HER2 = human epidermal growth factor receptor 2; HR = hormone receptor; TNBC = triple negative breast cancer
*Date of metastasis to first seizure date or end of follow-up for those without a seizure
**Per 100 person-years
†Date of metastasis to the end of follow-up among patients with a seizure
Among patients with seizures, there were no meaningful demographic differences between phenotype subgroups. Among phenotypes, patients in the HER2−/HR+ subgroup had the lowest seizure incidence rate of 10.94 per 100 person-years (95% CI: 10.23, 11.71), while patients with the TNBC phenotype had the highest seizure incidence rate of 16.83 per 100 person-years (95% CI: 15.34, 18.46) (Table 2).
Incidence rates by risk factors
Overall, BRCA+ patients with diagnosed brain metastases or a history of seizures had higher seizure incidence rates than those without the respective risk factors (Table 3). Prior use of anticonvulsants did not affect the seizure incidence rate. While the number of patients using PARP inhibitors is too low to draw meaningful conclusions, the incidence rates trend higher for those using PARP inhibitors versus those that do not use them.
Table 3.
Seizure incidence and occurrence rates for all BRCA+ patients (adjusted for age at metastasis, number of prior lines of treatment, and metastasis location)
| N | N patients with a seizure | Median duration to seizure (days) | % patients with at least one seizure | Incidence person-years* | Incidence rate** (95% CI) | Total Occurrences | Occurrence frequency | Occurrence person-years† | Occurrence rate** | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 7941 | 2207 | 161 | 27.8% | 18655 | 7.28 (6.01–8.83) | 15,029 | 6.81 | 7468 | 201.26 |
| Use of PARP inhibitors during evaluation period | ||||||||||
| Patients exposed to PARP inhibitors | 92 | 16 | 53.5 | 17.4% | 61 | 76.58 (7.81–751.26) | 93 | 5.81 | 20 | 458.04 |
| Patients not exposed to PARP inhibitors | 7849 | 2175 | 161 | 27.7% | 18452 | 7.20 (5.94–8.74) | 14827 | 6.82 | 7379 | 200.93 |
| Diagnosed brain metastases during evaluation period | ||||||||||
| Patients with diagnosed brain metastases | 1041 | 504 | 9 | 48.4% | 1002 | 42.55 (28.59–63.30) | 4071 | 8.08 | 951 | 428.06 |
| Patients without diagnosed brain metastases | 6900 | 1627 | 153 | 23.6% | 16874 | 5.26 (4.20–6.59) | 9994 | 6.14 | 5796 | 172.43 |
| History of seizures prior to index date | ||||||||||
| Patients with history of seizures | 788 | 465 | 22 | 59.0% | 913 | 34.76 (23.24–52.01) | 5532 | 11.90 | 1336 | 414.12 |
| Patients without history of seizures | 7153 | 1742 | 237.5 | 24.4% | 17743 | 6.35 (5.11–7.88) | 9497 | 5.45 | 6132 | 154.88 |
| Use of anticonvulsants prior to index date | ||||||||||
| Patients with history anticonvulsants | 2280 | 688 | 122.5 | 30.2% | 5057 | 7.6 (5.38–10.73) | 5956 | 8.66 | 2250 | 264.67 |
| Patients without history of anticonvulsants | 5661 | 1519 | 178 | 26.8% | 13598 | 6.93 (5.50–8.75) | 9073 | 5.97 | 5217 | 173.91 |
*Follow-up duration is from the date of metastasis to first seizure date or end of follow-up for those without a seizure. Duration is in person-years
**Per 100 person-years
†Follow-up duration is from the date of metastasis to the end of follow-up among patients with a seizure
Time to seizure
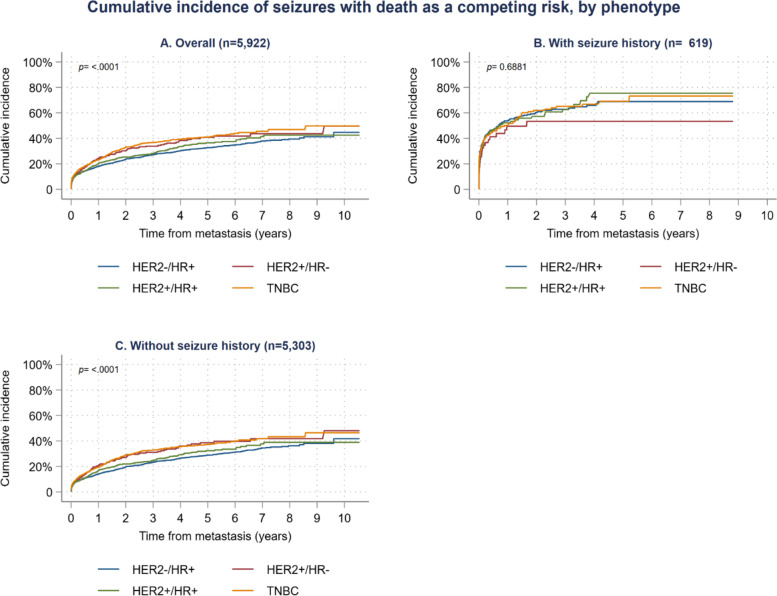
Cumulative incidence plots showed differences in the time to a seizure between the four phenotypes (p < 0.001, Fig. 3a). At 2 years, the cumulative incidence of a seizure was 33.1% for TNBC patients, 30.3% for HER2+/HR- patients, 25.4% for HER2+/HR+ patients, and 23.6% for HER2−/HR+ patients. At 8 years, these rates increased to 46.9, 43.7, 42.5, 39.5% respectively. Competing risks regression showed that overall, patients with the TNBC (HR = 1.35; 95%CI: 1.21, 1.52; p < 0.001) and HER2+/HR- (HR = 1.29; 95%CI: 1.07, 1.56; p < 0.01) phenotypes had increased risk of a seizure compared to the HER2−/HR+ phenotype (Table 4). For patients with a history of seizures, rates of seizures were higher, but not different between the phenotypes (p = 0.687, Fig. 3b). At 2 years, the cumulative incidence of a seizure was between 61.0 and 75.4% depending on their phenotype and at 8 years, it was between 53.4 and 75.4%. For patients without a history of seizures, the cumulative incidence of a seizure was 29.1% for TNBC patients, 27.1% for HER2+/HR- patients, 22.0% for HER2+/HR+ patients, and 19.6% for HER2−/HR+ patients. At 8 years, these rates increased to 43.3, 41.8, 38.9, 36.2% respectively (p < 0.001, Fig. 3c). Competing risks regression showed that in those without a history of seizures, patients with the TNBC phenotype had a significant hazard ratio of 1.40 (95%CI: 1.23, 1.60; p < 0.001) and the HER2+/HR- phenotype had significant hazard ratio of 1.38 (95%CI: 1.12, 1.71; p < 0.01) compared to the HER2−/HR+ phenotype (Table 4).
Fig. 3.
Time to seizure in BRCA+ patients by HER2/HR phenotype, overall and by prior seizure status
Table 4.
Competing risks regression of seizures by phenotype and with/without seizure history
| Phenotype | Overall (n = 5911) | With seizure history (n = 616) | Without seizure history (n = 5295) | History of anticonvulsant use (n = 1690) | History of brain metastasis (n = 818) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| HER2−/HR+ | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. | 1.00 | Ref. |
| HER2+/HR- | 1.29** | (1.07, 1.56) | 0.75 | (0.49, 1.16) | 1.38** | (1.12, 1.71) | 1.45* | (1.07, 1.96) | 1.01 | (0.79, 1.30) |
| HER2+/HR+ | 1.10 | (0.97, 1.26) | 1.00 | (0.77, 1.31) | 1.12 | (0.97, 1.30) | 1.05 | (0.82, 1.33) | 1.04 | (0.90, 1.22) |
| TNBC | 1.35*** | (1.21, 1.52) | 0.99 | (0.78, 1.26) | 1.40*** | (1.23, 1.60) | 1.05 | (0.85, 1.30) | 1.12 | (0.97, 1.29) |
Note: Adjusted for age at metastasis, number of prior lines of therapy, and site of metastasis
* p < 0.05, ** p < 0.01, *** p < 0.001
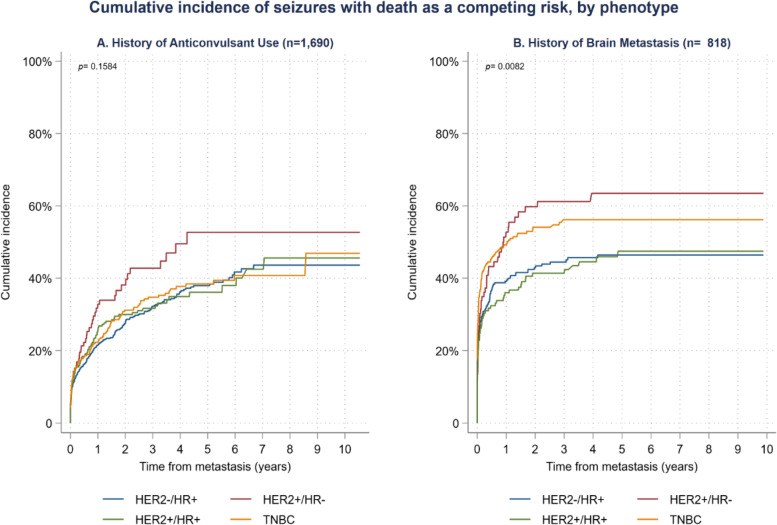
For patients with a history of anticonvulsant use, only HER2+/HR- patients showed an increased risk of seizure (HR = 1.45; 95% CI: 1.07, 1.96 l; p < 0.05; Fig. 4a). In patients with diagnosed brain metastases, both TNBC (HR = 1.39; 95%CI: 1.09, 1.76; p < 0.01) and HER2+/HR- (HR = 1.38; 95%CI: 1.02, 1.88; p < 0.05) phenotypes showed an increased risk of seizure (Fig. 4b). There was no statistically significant difference in time to seizure between the phenotypes among patients a history of using PARP inhibitors (data not shown).
Fig. 4.
Time to seizure in BRCA+ patients by phenotype and presence of anticonvulsant use or brain metastasis
Discussion
Data pertaining to the risk of seizures among women with advanced or metastatic breast cancer is limited, particularly when considering women who may harbor a mutation in the high risk BRCA1 and BRCA2 breast cancer predisposition genes. This study identified a large cohort of 7941 women with BRCA-associated metastatic breast cancer treated at centers across the United States as represented in a large EHR database and estimated the overall incidence rate of seizures to be 11.83 (95% CI: 11.35,12.33) per 100 patient-years after index diagnosis of metastatic disease.
The group of women with the highest seizure incidence rates of 16.83 events (95% CI: 15.34, 18.46) per 100 patient-years were those affected with triple-negative breast cancer. Additional risk groups were women with diagnosed brain metastases (incidence rate = 42.55 [95% CI: 28.59, 63.30] per 100 patient-years), and women with a history of seizures (incidence rate = 34.76 [95% CI: 23.24, 52.01] per 100 patient-years.
Overall, patients with brain metastasis or a history of seizures had higher seizure incidence rates, which is consistent with clinical impressions and previous studies [15]. TNBC and HER2−/HR+ patients showed a higher risk of developing seizures over the course of the study. Patients with these phenotypes were also at higher risk when metastasis was in the brain and if they had a history of anticonvulsant use. Although our study was limited in the sample size available for analyses of PARP inhibitor-treated patients, seizure incidence rates in this patient population appeared to be higher than in patients who were not treated with PARP inhibitors (76.58 per 100 person-years vs 7.20 per 100 person-years).
A notable strength of this study was the use of unstructured NLP fields in addition to the structured data (ICD9/10 diagnosis codes) to identify the seizure outcome. In a prior study, we showed how the combination of structured and unstructured data to identify adverse event outcomes is superior to using structured data alone [28].
Although BRCA mutation-positive status was discernable in the EHR data from unstructured NLP fields, a limitation of our study was the incomplete available information related to BRCA1 and BRCA2 status. Specifically, 5383 (67.8%) patients did not have details on which gene (BRCA1 versus BRCA2) was implicated. Furthermore, the Optum® EHR database did not provide differentiation on whether the BRCA mutation was a somatic or germline variant. Further, while large, we used a single EHR database for this study. This may introduce biases in terms of types of patients (e.g. the high proportion of Caucasian and Midwestern patients) and the types of treating physicians. The database does not contain information regarding healthcare access, lifestyle, or socio-economic status, which may also introduce bias into our analyses.
This study provides novel real-world evidence on the incidence rates of seizures affecting a large population of women with metastatic BRCA-associated breast cancer who received care in clinics across the U.S. The study highlights the importance of understanding patients’ molecular subtypes associated with breast cancer when assessing seizure risk. The seizure incidence rate was highest in the subgroup of women with TNBC, and significantly higher for women with diagnosed brain metastases, with a history of seizures/anti-convulsive therapy, as well as those receiving PARP inhibitor therapy. These findings have implications for clinical practice as well as for drug development when considering the benefit-risk of new oncologic therapeutic agents (such as PARP inhibitors studied here) that, once approved, are mainly introduced for treating patients with advanced disease (distant metastases) who have failed several lines of therapy. Further work may characterize seizure risk across all stages of BRCA-associated breast cancer.
Supplementary Information
Additional file 1: Supplementary Table 1. Diagnosis codes used to identify patients with malignancies.
Additional file 2: Supplementary Table 2. SDS information used to identify patients with metastases but without ICD codes in their EHRs.
Additional file 3: Supplementary Table 3. SDS terms used to identify patients with seizures.
Additional file 4: Supplementary Table 4. List of anticonvulsants and PARP inhibitors included in the analyses.
Acknowledgements
Assistance with the development of this manuscript was provided by Prasad Kulkarni, PhD, CMPP of Asclepius Medical Communications LLC, Ridgewood, New Jersey, USA and funded by AbbVie, Inc.
Abbreviations
- BC
Breast cancer
- BRCA1/BRCA2
BReast CAncer genes 1 and 2
- CI
Confidence intervals
- EHR
Electronic health record
- ER
Estrogen receptor
- HCPCS
Healthcare Common Procedural Coding System
- HER2
Human epidermal growth factor 2
- HIPAA
Health Insurance Portability and Accountability Act
- ICD
International Statistical Classification of Diseases and Related Health Problems
- IDN
Integrated delivery network
- IQR
Interquartile range
- IRB
Institutional Review Board
- NLP
Natural Language Processing
- PARP
Poly [adenosine diphosphate-ribose] polymerase
- PR
Progesterone receptor
- SD
Standard deviation
- SDS
Signs, diseases, and symptoms
- TNBC
Triple-negative breast cancer.
Authors’ contributions
JET, WS, RP, DM, RK, AL contribued to the study conception and design. JET, WS, AKRM, RK, AL helped in acquiring the data. RP, DM, AG, EP analyzed the data. JET, WS, RP, DM, EP, AL were involved in interpreting the data. JET, WS, RP, DM, EP, AL all contributed to drafting the manuscript. All authors were involved in revisions and all authors read and approved the final manuscript.
Funding
The study was funded by AbbVie, Inc., North Chicago, Illinois, USA.
Availability of data and materials
The datasets generated during and/or analysed during the current study are not publicly available due to the proprietary nature of the database from which they were derived and used under license for the current study. However, the data are available from the corresponding author on reasonable request and with permission of Optum®.
Declarations
Ethics approval and consent to participate
This was a retrospective analysis of deidentified data from a US based proprietary database. The data is a limited data set with data use agreements in place for use. It only contains de-identified health information that, as described by the HIPPA Privacy Rule, is not PHI. A limited dataset excludes specified direct identifiers of the individual or of relatives, employers, or household members of the individual. The Optum EHR database meets these criteria. Access to and use of the data available only through a license agreement. The license (obtained by AbbVie) describes the data, limitations on use, limitations on users and specifically prohibits re-identification. It also includes reference to the HIPPA Privacy Rule: 45 CFR 164.501, 164.508, 164.512(i). For these reasons, our data is not covered by the Privacy Rule and IRB approval was’t required. As this was an observational retrospective study, there was no experimental protocol, however, all analyses were approved by the sponsor (AbbVie). All procedures were performed in accordance with relevant guidelines.
Consent for publication
Individual patient data in the database from which this dataset was derived are deidentified and consent for publication is not required.
Competing interests
WS, AKRM, RK, & AL are all employees of AbbVie and own stock. At the time the study was conducted, JET was an employee of AbbVie. RP, DM, AG, EP are employed by SmartAnalyst and received funding from AbbVie to conduct the study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, Brown KL, Sharma L, Saam J, Lancaster J, Daly MB. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;123(10):1721–1730. doi: 10.1002/cncr.30498. [DOI] [PubMed] [Google Scholar]
- 3.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, van Leeuwen FE, Milne RL, Andrieu N, et al. Risks of breast, ovarian, and contralateral breast Cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 4.Peleg Hasson S, Menes T, Sonnenblick A. Comparison of patient susceptibility genes across breast Cancer: implications for prognosis and therapeutic outcomes. Pharmgenomics Pers Med. 2020;13:227–238. doi: 10.2147/PGPM.S233485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer stat facts: female breast cancer [https://seer.cancer.gov/statfacts/html/breast.html].
- 6.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter C, Nilsson MP, Olsson E, George AM, Chen Y, Kvist A, Törngren T, Vallon-Christersson J, Hegardt C, Häkkinen J, et al. Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic. Ann Oncol. 2016;27(8):1532–1538. doi: 10.1093/annonc/mdw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waks AG, Winer EP. Breast Cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 9.Bardia A, Mayer IA, Diamond JR, Moroose RL, Isakoff SJ, Starodub AN, Shah NC, O'Shaughnessy J, Kalinsky K, Guarino M, et al. Efficacy and safety of anti-Trop-2 antibody drug conjugate Sacituzumab Govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast Cancer. J Clin Oncol. 2017;35(19):2141–2148. doi: 10.1200/JCO.2016.70.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiówka M, Hewson N, Rukazenkov Y, Robertson JF. Fulvestrant 500 mg versus Anastrozole 1 mg for the FIRST-line treatment of advanced breast Cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol. 2015;33(32):3781–3787. doi: 10.1200/JCO.2015.61.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD. NCCN Guidelines Version 6.2020 Breast Cancer. PA, USA: National Comprehensive Cancer Network: Plymouth Meeting; 2020. [Google Scholar]
- 13.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, et al. Olaparib for metastatic breast Cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 14.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin M, et al. Talazoparib in patients with advanced breast Cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. J Neuro-Oncol. 2016;127(3):407–414. doi: 10.1007/s11060-016-2075-3. [DOI] [PubMed] [Google Scholar]
- 16.Yeh RH, Yu JC, Chu CH, Ho CL, Kao HW, Liao GS, Chen HW, Kao WY, Yu CP, Chao TY, et al. Distinct MR imaging features of triple-negative breast Cancer with brain metastasis. J Neuroimaging. 2015;25(3):474–481. doi: 10.1111/jon.12149. [DOI] [PubMed] [Google Scholar]
- 17.Altundag K, Bondy ML, Mirza NQ, Kau SW, Broglio K, Hortobagyi GN, Rivera E. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007;110(12):2640–2647. doi: 10.1002/cncr.23088. [DOI] [PubMed] [Google Scholar]
- 18.Villemure JG, de Tribolet N. Epilepsy in patients with central nervous system tumors. Curr Opin Neurol. 1996;9(6):424–428. doi: 10.1097/00019052-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59(9 Suppl 5):S21–S26. doi: 10.1212/WNL.59.9_suppl_5.S21. [DOI] [PubMed] [Google Scholar]
- 20.van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 21.Bromfield EB. Epilepsy in patients with brain tumors and other cancers. Rev Neurol Dis. 2004;1(Suppl 1):S27–S33. [PubMed] [Google Scholar]
- 22.Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009;63(5):761–767. doi: 10.1007/s00280-008-0876-6. [DOI] [PubMed] [Google Scholar]
- 23.Kargiotis O, Markoula S, Kyritsis AP. Epilepsy in the cancer patient. Cancer Chemother Pharmacol. 2011;67(3):489–501. doi: 10.1007/s00280-011-1569-0. [DOI] [PubMed] [Google Scholar]
- 24.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33(7):1187–1194. doi: 10.1377/hlthaff.2014.0038. [DOI] [PubMed] [Google Scholar]
- 25.Pettus JH, Zhou FL, Shepherd L, Mercaldi K, Preblick R, Hunt PR, Paranjape S, Miller KM, Edelman SV. Differences between patients with type 1 diabetes with optimal and suboptimal glycaemic control: a real-world study of more than 30 000 patients in a US electronic health record database. Diabetes Obes Metab. 2020;22(4):622–630. doi: 10.1111/dom.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edemekong PF, Annamaraju P, Haydel MJ. Health insurance portability and accountability act (HIPAA) Treasure Island (FL): StatPearls Publishing LLC; 2020. [PubMed] [Google Scholar]
- 27.Broome CM, Cunningham JM, Mullins M, Jiang X, Bylsma LC, Fryzek JP, Rosenthal A. Increased risk of thrombotic events in cold agglutinin disease: a 10-year retrospective analysis. Res Pract Thromb Haemost. 2020;4(4):628–635. doi: 10.1002/rth2.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang R, Potluri R. 35th international conference on Pharmacoepidemiology and risk management (ICPE): august 28, 2019. Philadelphia, Pennsylvania: International Society for Pharmacoepidemiology; 2019. Contribution of natural language processing in predicting risk of pleural and pericardial effusions in small cell lung Cancer by line of therapy; p. 58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Diagnosis codes used to identify patients with malignancies.
Additional file 2: Supplementary Table 2. SDS information used to identify patients with metastases but without ICD codes in their EHRs.
Additional file 3: Supplementary Table 3. SDS terms used to identify patients with seizures.
Additional file 4: Supplementary Table 4. List of anticonvulsants and PARP inhibitors included in the analyses.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to the proprietary nature of the database from which they were derived and used under license for the current study. However, the data are available from the corresponding author on reasonable request and with permission of Optum®.