Abstract
Objective
To explore the association between ovulation induction drugs and ovarian cancer.
Design
Systematic review and meta-analysis.
Setting
Not applicable.
Patient(s)
Women without ovarian cancer who ever or never underwent ovarian induction.
Intervention(s)
An extensive electronic search of the following databases was performed: PubMed, EMBASE, MEDLINE, Google Scholar, Cochrane Library and CNKI, from inception until January 2022. A total of 34 studies fulfilled our inclusion criteria and were included in the final meta-analysis. The odds ratio (OR) and random-effects model were used to estimate the pooled effects. The Newcastle-Ottawa Scale was used to assess the quality of included studies. Funnel plots and Egger tests were used to assess publication bias.
Main outcomes
New diagnosed borderline ovarian tumor (BOT) and invasive ovarian cancer (IOC) between ovulation induction (OI) group and control (CT) group considering fertility outcome, OI cycles and specific OI drugs.
Results
Primarily, there was no significant difference in the incidence of IOC and BOT between the OI and CT groups. Secondly, OI treatment did not increase the risk of IOC and BOT in the multiparous women, nor did it increase the risk of IOC in the nulliparous women. However, the risk of BOT appeared to be higher in nulliparous women treated with OI treatment. Thirdly, among women exposed to OI, the risk of IOC and BOT was higher in nulliparous women than in multiparous women. Fourthly, the risk of IOC did not increase with increasing OI cycles. Lastly, exposure to specific OI drugs also did not contribute to the risk of IOC and BOT.
Conclusion
Overall, OI treatment did not increase the risk of IOC and BOT in most women, regardless of OI drug type and OI cycle. However, nulliparous women treated with OI showed a higher risk of ovarian cancer, necessitating their rigorous monitoring and ongoing follow-up.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-022-01084-z.
Keywords: Clomiphene, Gonadotrophin, Gonadotropin-releasing hormone analogues, Ovulation induction, Ovarian stimulation, Borderline ovarian tumor, Invasive ovarian cancer, Meta-analysis
Introduction
Infertility affects more than 48.5 million couples worldwide [1–3]. It is emerging as a public health problem, driving the demand for assisted reproductive treatment [4]. Ovulation induction (OI) is a process in which the ovaries are drugged to stimulate the production of many follicles containing eggs, which usually begins early in the menstrual cycle. OI treatment is highly desirable, especially for isolated anovulatory infertility [5]. OI treatment is associated with ovarian hyper-stimulation and multiple follicular ovulations. As we know, ovulation is a common injurious process associated with an inflammatory response and destruction of ovarian epithelial cells [6, 7]. According to the incessant ovulation and gonadotropin hypothesis, high levels of gonadotropin and excessive ovulation may engage patients into repeated cycles of injury, inducing inflammation and regeneration, which could potentially increase the risk of ovarian cancer by inducing somatic cell mutations [8–10]. Previous studies have debated whether OI could increase the risk of invasive ovarian cancer (IOC) and borderline ovarian tumors (BOT) [11, 12]. Although most studies have concluded that OI does not contribute to the risk of IOC and BOT, some scholars still proposed that OI may be associated with them. Therefore, we performed this updated systematic review and meta-analysis to find out whether exposure to OI treatment significantly increases the risk of IOC and BOT.
Materials and methods
Search strategy
The PRISMA guidelines were used for this study. A systematic literature search was then conducted in PubMed, EMBASE, MEDLINE, Google Scholar, Cochrane Library and CNKI, which included records up to January 2022. The main keywords included the following domains of Medical Subject Heading terms: “ ovulation induction “ and “ ovarian cancer “. The retrieval strategy adopted the combination of subject terms and free words. These terms were then combined with “AND” or “OR”. Also, to broaden the search, review articles were used to ensure that all relevant citations were identified and imported.
Study screening
Two independent researchers (YL and WQQ) simultaneously screened the titles, abstracts and full text of the literature according to the inclusion and exclusion criteria. Any disagreements were discussed and solved by consensus or third-party arbitration (ZS). The inclusion criteria were as follows: (1) Cohort studies and case-control studies with adequate samples; (2) Exposure to ovulation induction drugs such as clomiphene citrate (CC), gonadotrophin (GDT) and gonadotropin-releasing hormone analogs (GnRH-a); (3) Follow-up in the cohort study was sufficiently long to demonstrate treatment differences; (4) The study had a clear description of the exposure to OI drugs and essential information about enrolled patients;(5) The type of cancer included borderline ovarian tumor (BOT) or invasive ovarian cancer (IOC). The exclusion criteria were as follows: (1) Non-English or Non-Chinese literature; (2) Non-human studies; (3) Literature with incomplete data; (4) Duplicate and inaccessible literature.
Data extraction
Two independent researchers (YL and SJF) performed the data extraction after viewing the complete manuscripts of the eligible literature. Relevant data was input into separate spreadsheets and then cross-checked by each researcher to maintain the quality of the data. The data of bibliography (year and author), study design (sample size, study type, study duration and study location), outcome measures (cancer type and incidence of individual ovarian cancers in group) and other endpoint evaluation (fertility outcome, OI drug type and OI cycles) were extracted from each study. If necessary, discussions with the third-party arbitration (XW) would solve all disputes.
Quality evaluation
Two researchers (YL and YWN) independently assessed the quality of the literature by using the NOS scale (Newcastle-Ottawa Scale). The main components of the NOS scale included: patient selection, intergroup comparability and outcome measurement [13]. Disagreements were solved by consensus or third-party arbitration (WXL) when they appeared. A total score of more than 6 was considered to be of satisfactory quality [14].
Statistical analysis
Data aggregation and basic meta-analysis
The meta-analysis was performed by using STATA 12.0. Binary variables were evaluated by odds ratio (OR) and its 95% confidence interval (95% CI). P < 0.05 was regarded as statistically significant.
Depending on heterogeneity, the appropriate model (random or fixed) was then selected to merge the outcome indicators [15]. The I2 value less than 50% were deemed to be low heterogeneity, 51–75% were deemed to be moderate heterogeneity, and greater than 75% were deemed to be high heterogeneity [16]. If the I2 value exceeded 50%, the random-effect model was chosen. Otherwise, if the I2 value was less than 50%, both the random effects and fixed effects models were acceptable [17].
Assessment of publication bias
In principle, funnel plot analyses were performed to accompany meta-analyses involving more 10 studies and to judge the publication bias [18]. If there was no significant publication bias, the funnel plot was supposed to be symmetrical. A complementary approach for funnel plots was to perform Egger’s test to objectively measure bias [19].
Details of ethical approval
This meta-analysis was based on the data from published articles and independent of any patient participation. As such, institutional review board (IRB) approval was not required.
Results
Study characteristics and quality evaluation
A flowchart detailing the process of identification and inclusion for the target literature was shown in Supplemental Material Fig. 1. Three hundred seven articles were included in the initial screening phase. Of these articles, 42 articles met the criteria for full-text review. Finally, a total of 34 articles were included in the meta-analysis, 14 of which were case-control studies and 20 of which were cohort studies. The final meta-analysis included a total of 3,643,303 participants. All the included literature was of adequate quality. The quality evaluation of the included literature was presented in Supplemental Material Table S1.
Part I: the risk of ovarian cancer between OI and CT group
Of the 34 studies, 12 reported BOT [12, 20–30] and 30 reported IOC [11, 12, 20, 21, 23–26, 29, 31–51]. Basic information of the included studies was given in Supplemental Material Table S2. For further study, we conducted subgroup analyses to assess the risk of IOC and BOT between groups according to study type.
The cancer risk between groups in case-control study
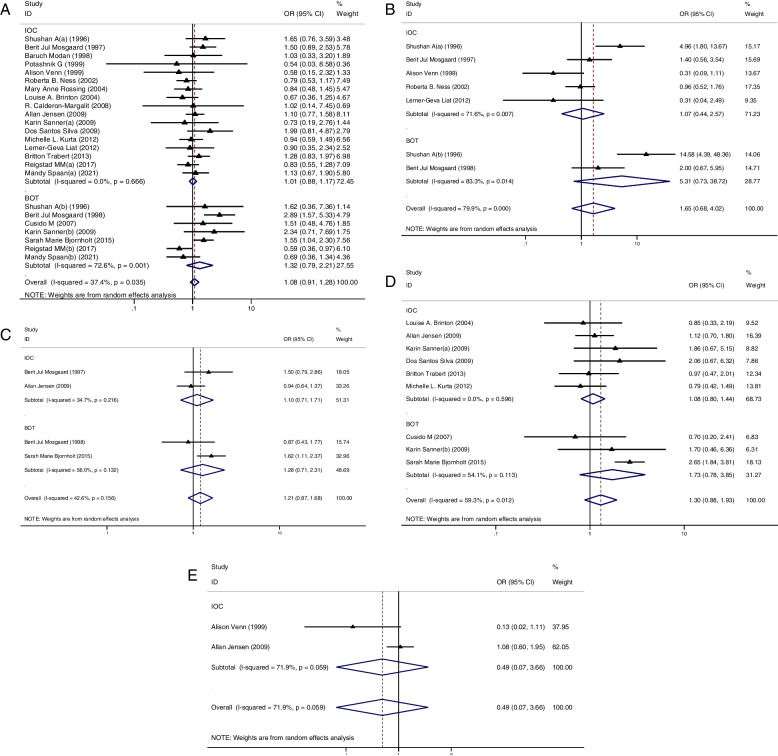
In the subgroup analysis of case-control studies, 12 studies reported IOC [11, 23, 29, 32, 36–43] and 5 studies reported BOT [23, 27–30]. Among these studies, only 1 study showed a significantly higher risk of IOC in the OI group than in the CT group [11] and 3 studies showed a higher risk of BOT in the OI group than in the CT group [28–30]. Pooled result indicated that the risk of IOC (OR = 1.09, 95%CI: 0.88–1.35, I2 = 54.9%, Table 1, Fig. 1A) and BOT (OR = 1.90, 95%CI: 0.89–4.09, I2 = 73.4%, Table 1, Fig. 1B) did not show significant difference between groups.
Table 1.
Odd ratios (with confidence intervals) and heterogeneity for each of the cancer risks analysed
| Outcome | OR | 95% CI | I2 | Degree of heterogeneity |
|---|---|---|---|---|
| The risk of IOC between OI and CT group (based on case-control study) | 1.09 | 0.88-1.35 | 54.9% | Moderate |
| The risk of BOT between OI and CT group (based on case-control study) | 1.90 | 0.89-4.09 | 73.4% | Moderate |
| The risk of IOC between OI and CT group (based on cohort study) | 1.11 | 0.91-1.35 | 21.8% | Low |
| The risk of BOT between OI and CT group (based on cohort study) | 1.34 | 0.97-1.83 | 50.5% | Moderate |
| The risk of IOC between OI and CT group (in multiparous women) | 0.83 | 0.65-1.05 | 21.3% | Low |
| The risk of BOT between OI and CT group (in nulliparous women) | 1.17 | 0.55-2.48 | 73.5% | Moderate |
| The risk of IOC between OI and CT group (in nulliparous women) | 1.55 | 0.94-2.57 | 69.5% | Moderate |
| The risk of BOT between OI and CT group (in nulliparous women) | 1.49 | 1.03-2.15 | 0% | Low |
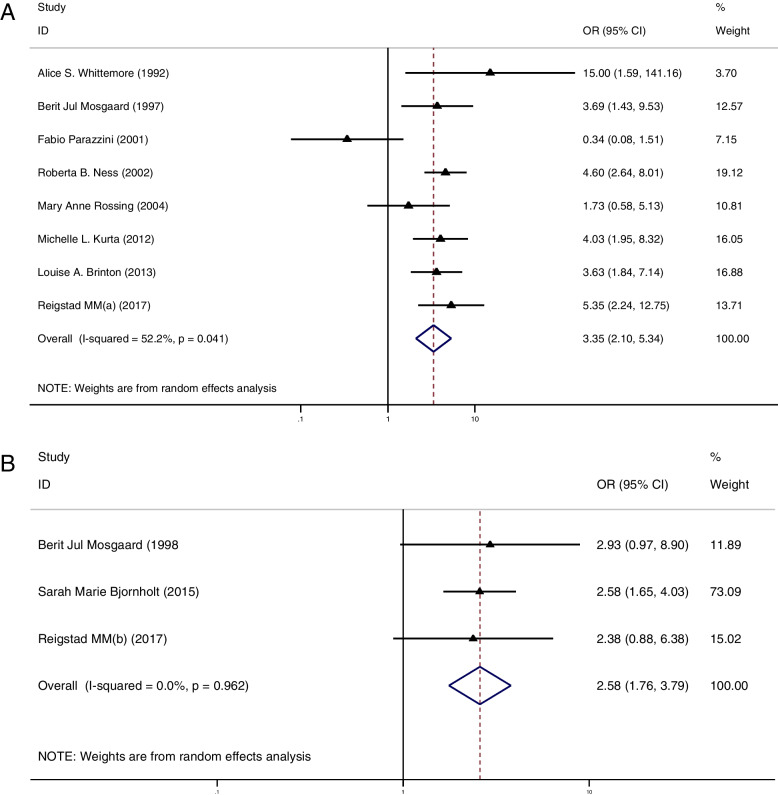
| The risk of IOC between the nulliparous and multiparous women (with ovulation induction treatment) | 3.35 | 2.10-5.34 | 52.2% | Moderate |
| The risk of BOT between the nulliparous and multiparous women (with ovulation induction treatment) | 2.58 | 1.76-3.79 | 0% | Low |
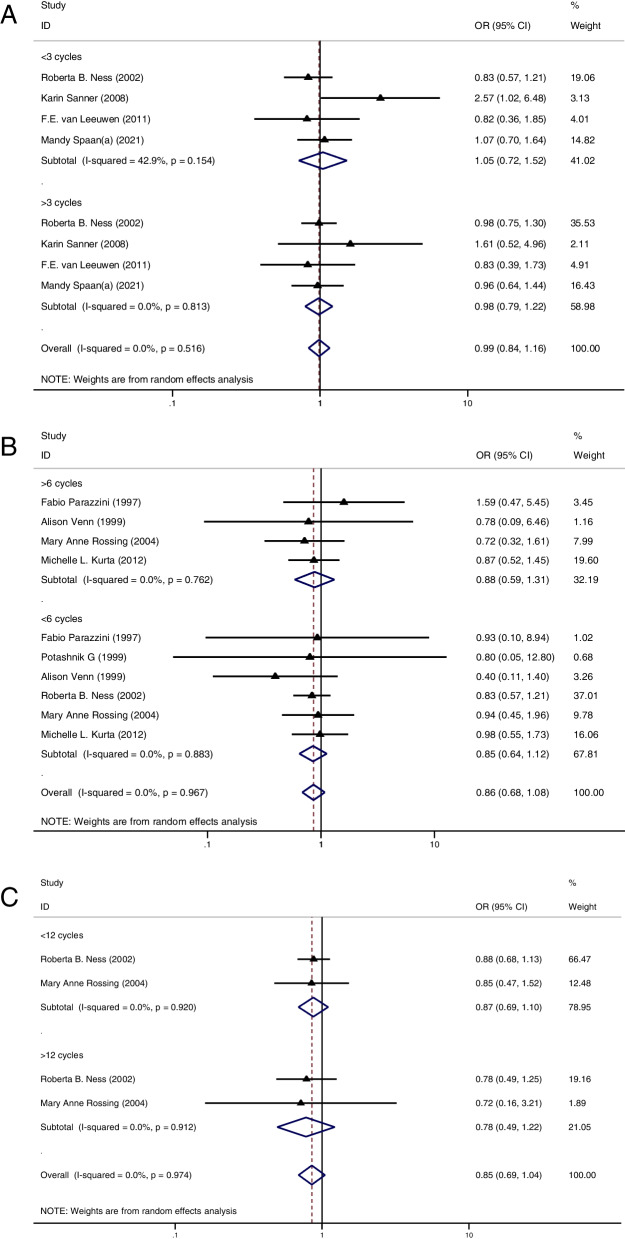
| The risk of IOC between OI and CT group (less than 3 ovulation induction cycles) | 1.05 | 0.72-1.52 | 42.9% | Low |
| The risk of IOC between OI and CT group (more than 3 ovulation induction cycles) | 0.98 | 0.79-1.22 | 0% | Low |
| The risk of IOC between OI and CT group (less than 6 ovulation induction cycles) | 0.85 | 0.64-1.12 | 0% | Low |
| The risk of IOC between OI and CT group (more than 6 ovulation induction cycles) | 0.88 | 0.59-1.31 | 0% | Low |
| The risk of IOC between OI and CT group (less than 12 ovulation induction cycles) | 0.87 | 0.69-1.10 | 0% | Low |
| The risk of IOC between OI and CT group (more than 12 ovulation induction cycles) | 0.78 | 0.49-1.22 | 0% | Low |
| The risk of IOC between CC and CT group | 1.01 | 0.88-1.17 | 0% | Low |
| The risk of BOT between CC and CT group | 1.32 | 0.79-2.21 | 72.6% | Moderate |
| The risk of IOC between GDT and CT group | 1.08 | 0.80-1.44 | 0% | Low |
| The risk of BOT between GDT and CT group | 1.73 | 0.88-1.93 | 54.1% | Moderate |
| The risk of IOC between HCG and CT group | 1.10 | 0.71-1.71 | 34.7%, | Low |
| The risk of BOT between HCG and CT group | 1.28 | 0.71-2.31 | 56% | Moderate |
| The risk of IOC between HMG and CT group | 1.07 | 0.44-2.57 | 71.6% | Moderate |
| The risk of BOT between HMG and CT group | 5.31 | 0.73-38.72 | 83.3% | High |
| The risk of IOC between GnRH-a and CT group | 0.49 | 0.07-3.66 | 71.9% | Moderate |
CC Clomiphene citrate, GDT Gonadotrophin, GnRH-a Gonadotropin-releasing hormone analogues, HCG human menopausal gonadotropin, HMG human chorionic gonadotropin, IOC invasive ovarian cancer, BOT borderline ovarian tumor, OI ovulation induction group, CT control group, OR odds ratio, 95%CI 95% confidence interval
Fig. 1.
A Forest plot of IOC risk between OI group and CT group based on case-control studies; B Forest plot of IOC risk between OI group and CT group based on cohort studies; C Forest plot of BOT risk between OI group and CT group based on case-control studies; D Forest plot of BOT risk between OI group and CT group based on cohort studies
The cancer risk between groups in cohort study
In the subgroup analysis of cohort studies, 18 studies reported IOC [12, 20, 21, 24–26, 31, 33–35, 44–51] and 7 studies reported BOT [12, 20–22, 24–26]. Of these studies, 3 studies showed a higher risk of IOC [12, 21, 31] in the OI group than in the CT group and 3 studied showed a higher risk of BOT in the OI group than in the CT group [21, 24, 25]. Again, the results showed no significant difference between groups in the incidence of IOC (OR = 1.11, 95%CI: 0.91–1.35, I2 = 21.8%, Table 1, Fig. 1C) and BOT (OR = 1.34, 95%CI: 0.97–1.83, I2 = 50.5%, Table 1, Fig. 1D).
Part II: the incidence of ovarian cancer between OI and CT group according to fertility outcome
In this section, we sought to find out whether the multiparous and nulliparous women treated with OI presented an increased risk of ovarian tumors when compared to those who had not been treated with OI. Relevant data were presented in Supplemental Material Table S3.
The cancer risk between groups in multiparous women
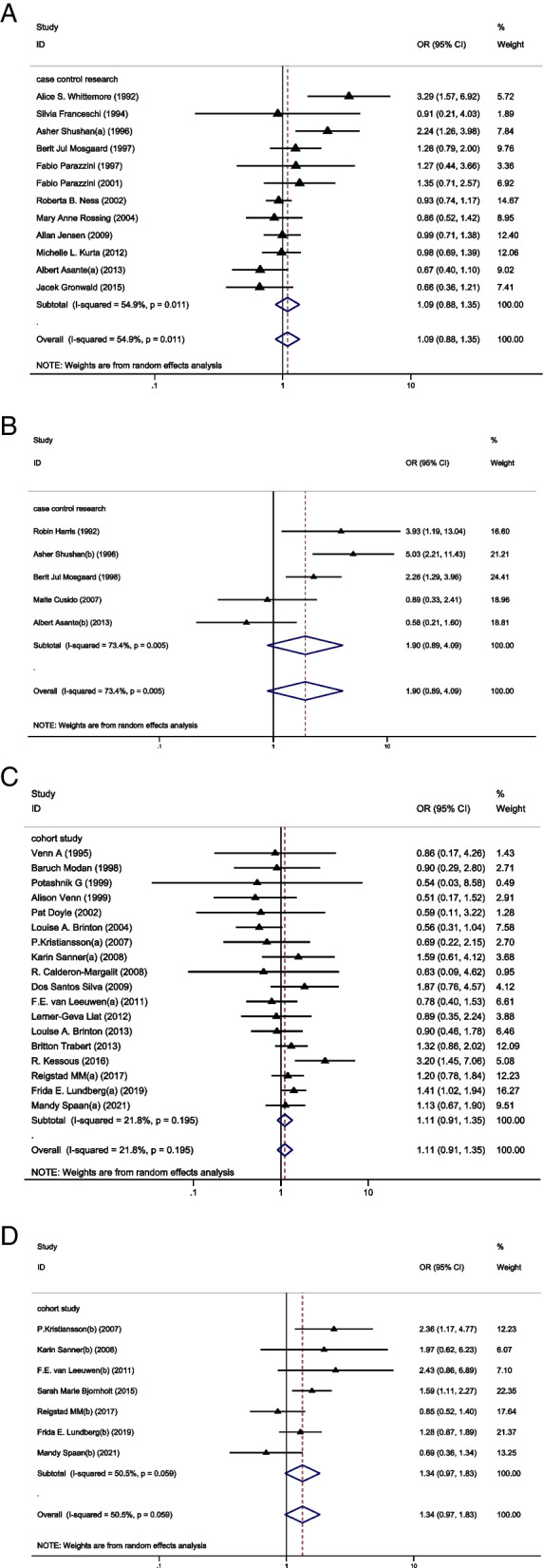
Firstly, 10 studies of IOC [11, 12, 34, 36–38, 40–42, 50] and 3 studies of BOT [12, 22, 28] analyzed the risk of ovarian cancer in multiparous women with or without OI treatment. None of these studies demonstrated a higher risk for IOC and BOT in the OI group. Pooled result remained consistent, indicating that OI treatment did not increase the risk of IOC (OR = 0.83, 95%CI: 0.65–1.05, I2 = 21.3%, Table 1, Fig. 2A) and BOT (OR = 1.17, 95%CI: 0.55–2.48, I2 = 73.5%, Table 1, Fig. 2B) in multiparous women.
Fig. 2.
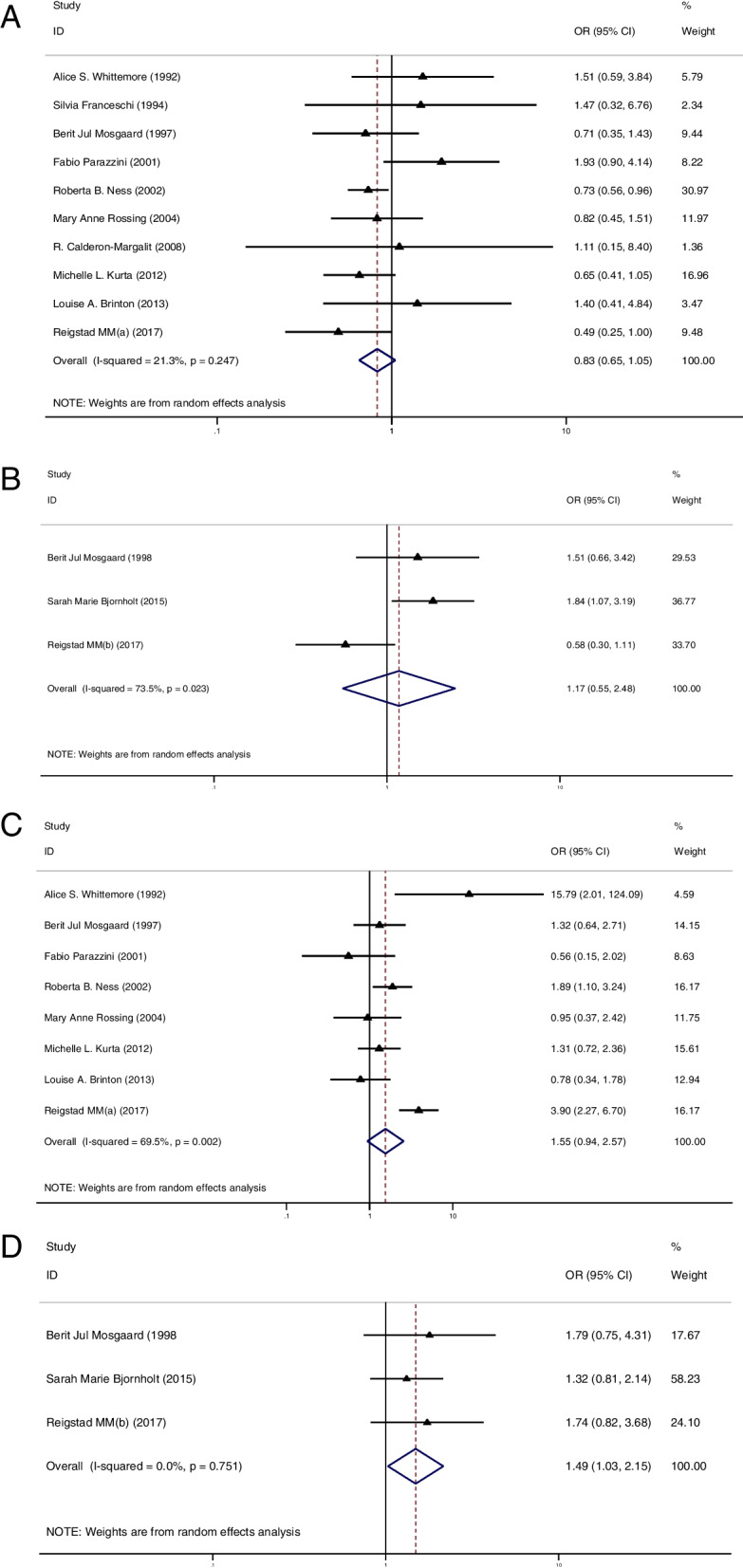
A Forest plot of IOC risk between OI group and CT group in multiparous women; B Forest plot of BOT risk between OI group and CT group in multiparous women; C Forest plot of IOC risk between OI group and CT group in nulliparous women; D Forest plot of BOT risk between OI group and CT group in nulliparous women
The cancer risk between groups in nulliparous women
In the second part, 8 studies of IOC [11, 12, 34, 36, 38, 40–42] and 3 studies of BOT [12, 22, 28] reported the risk of ovarian cancer in nulliparous women with or without OI treatment. Of these studies, only 1 study showed a significantly higher risk of IOC in the OI group than in the CT group [11]. The summarized result for IOC showed no difference in cancer risk between groups (OR = 1.55, 95%CI: 0.94–2.57, I2 = 69.5%, Table 1, Fig. 2C). Additionally, none of these studies reported a higher risk of BOT in the OI group. However, after pooled analysis, the risk of BOT appeared to be higher in nulliparous women treated with OI than in those nulliparous women who had not been treated with OI (OR = 1.49, 95%CI: 1.03–2.15, I2 = 0%, Table 1, Fig. 2D).
Part III: the risk of ovarian cancer between the multiparous and nulliparous women in OI group
In this chapter, we attempted to figure out the differences in cancer risk between the multiparous and nulliparous woman in the OI group. Relevant data were presented in Supplemental Material Table S4. In total, 8 studies of IOC [11, 12, 34, 36, 38, 40–42] and 3 studies of BOT [12, 22, 28] reported on the risk of ovarian cancer in the nulliparous and multiparous women treated with OI. The summarized results showed a significantly higher risk of IOC (OR = 3.35, 95%CI: 2.10–5.34, I2 = 52.2%, Table 1, Fig. 3A) and BOT (OR = 2.58, 95%CI: 1.76–3.79, I2 = 0%, Table 1, Fig. 3B) in the nulliparous women treated with OI than in those multiparous women treated with OI.
Fig. 3.
A Forest plot of IOC risk between nulliparous and multiparous women with OI treatment; B Forest plot of BOT risk between nulliparous and multiparous women with OI treatment
Part IV: the relationship between number of OI cycles and cancer risk
Then, we tried to find out whether cancer risk increased with more OI cycles. Totally, 8 studies provided relevant data for IOC [20, 24, 25, 36, 39, 41, 42, 46, 47]. Regrettably, data for BOT were not available for meta-analysis. Relevant data were presented in Supplemental Material Table S5.
Using a cut-off of 3 cycles, we did not find a higher cancer risk in those women who received less than 3 cycles when compared to the CT group (OR = 1.05, 95%CI: 0.72–1.52, I2 = 42.9%, Table 1, Fig. 4A). Meanwhile, we found a similar result in those women who received more than 3 cycles (OR = 0.98, 95%CI: 0.79–1.22, I2 = 0%, Table 1, Fig. 4A). Using 6 cycles as a cut-off, those women who received less than 6 cycles did not present an increased cancer risk when compared to the CT group (OR = 0.85, 95%CI: 0.64–1.12, I2 = 0%, Table 1, Fig. 4B) and a similar result was found in those women who received more than 6 OI cycles (OR = 0.88, 95%CI: 0.59–1.31, I2 = 0%, Table 1, Fig. 4B). Lastly, using 12 cycles as a cut-off, we did not find a significantly increased cancer risk in those women who received less than 12 cycles when compared to the CT group (OR = 0.87, 95%CI: 0.69–1.10, I2 = 0%, Table 1, Fig. 4C). Also, a similar result was found in those women who received more than 12 OI cycles (OR = 0.78, 95%CI: 0.49–1.22, I2 = 0%, Table 1, Fig. 4C).
Fig. 4.
A Forest plot of IOC risk between OI group and CT group based on a cut-off value of 3 cycles; B Forest plot of IOC risk between OI group and CT group based on a cut-off value of 6 cycles; A Forest plot of IOC risk between OI group and CT group based on a cut-off value of 12 cycles
Part V: the relationship between specific OI treatment and cancer risk
At last, we wished to find out whether specific OI drugs were associated with an increased cancer risk. For further study, we divided the subjects into three groups according to the type of OI drug. These were the clomiphene citrate group (CC), the gonadotrophin group (GDT) and the gonadotropin-releasing hormone analog group (GnRH-a). Relevant data were provided in Supplemental Material Table S6.
The relationship between CC and cancer risk
We firstly analyzed the relationship between CC and cancer risk. This part of analysis included 17 studies of IOC [25, 29, 35, 36, 38, 41–43, 45–47, 50, 51] and 7 studies of BOT [12, 20, 22, 25, 27–29]. Only 1 study reported a higher cancer risk in the CC group than in the CT group [12]. However, pooled results showed that the risk of IOC (OR = 1.01, 95%CI: 0.88–1.17, I2 = 0%, Table 1, Fig. 5A) and BOT (OR = 1.32, 95%CI: 0.79–2.21, I2 = 72.6%, Table 1, Fig. 5A) were not significantly higher in the CC group when compared to the CT group.
Fig. 5.
A Forest plot of IOC and BOT risk between CC group and CT group; B Forest plot of IOC and BOT risk between HMG group and CT group; C Forest plot of IOC and BOT risk between HCG group and CT group; D Forest plot of IOC and BOT risk between GDT group and CT group; E Forest plot of IOC risk between GnRH group and CT group
The relationship between GDT and cancer risk
Secondarily, we focused our attention on GDT and performed a subgroup analysis in this section. GDTs mainly consisted of human menopausal gonadotropin (HMG) and human chorionic gonadotropin (HCG). However, some studies did not further categorized GDT. For IOC, there were 5 studies of HMG [29, 35, 38, 41, 47], 2 studies of HCG [38, 43] and 6 studies of unclassified GDT [25, 33, 36, 43, 49, 51]. Only 1 study reported a higher cancer risk in HMG group than in the CT group [29]. Nevertheless, pooled results indicated that HMG (OR = 1.07, 95%CI: 0.44–2.57, I2 = 71.6%, Table 1, Fig. 5B), HCG (OR = 1.10, 95%CI: 0.71–1.71, I2 = 34.7%, Table 1, Fig. 5C) and unclassified GDT (OR = 1.08, 95%CI: 0.80–1.44, I2 = 0%, Table 1, Fig. 5D) did not increase the cancer risk.
While for BOT, 2 studies of HMG [28, 29], 2 studies of HCG [22] and 3 studies of GDT [22, 25, 27] were included in this part of analysis. Consistently, we found similar results that HMG (OR = 5.31, 95%CI: 0.73–38.72, I2 = 83.3%, Table 1, Fig. 5B), HCG (OR = 1.28, 95%CI: 0.71–2.31, I2 = 56%, Table 1, Fig. 5C) and unclassified GDT (OR = 1.73, 95%CI: 0.88–1.93, I2 = 54.1%, Table 1, Fig. 5D) did not increase tumor risk.
The relationship between GnRH-a and cancer risk
Thirdly, we only found 2 studies which provided analyzable data on the relationship between the risk of IOC and GnRH-a [43, 47]. Along the same lines, we did not find an increased risk of IOC (OR = 0.49, 95%CI: 0.07–3.66, I2 = 71.9%, Table 1, Fig. 5E) in the GnRH-a group when compared to the CT group. However, it was regrettable that another meta-analysis focusing on the relationship between the risk of BOT and GnRH-a could not be performed due to lack of data.
Publication bias
In our analysis, funnel plot analysis and Egger regression analysis were performed to judge the publication bias of the included studies. Neither the funnel plot (Supplemental Material Fig. 2A) nor the Egger test showed evidence of publication bias in our analysis (Supplemental Material Fig. 2B).
Discussion
The following points are currently discussed regarding the possible induction of ovarian cancer with the use of OI drugs: (1) the incessant ovulation hypothesis stated that ovarian epithelium could be destroyed and repaired during uninterrupted ovulation. When a sufficient amount of damage is caused, malignant transformation of ovarian epithelial cells will be triggered [8]. Furthermore, cancer risk had been found to decrease with increasing numbers of pregnancies and live births, longer duration of breastfeeding and use of oral contraceptives [52–57]. The effects of these anovulation factors confirmed the above observations. Thus, it is thought that the number of ovulatory cycles during the lifetime was associated with ovarian cancer risk, this finding has been observed in several animal models and epidemiological studies [58–62]. (2) The gonadotropin hypothesis suggested that excess gonadotropins could hyper-stimulate the ovaries and induce estrogen production. The amount of estrogen secreted in one gonadotropin-stimulated cycle was equivalent to the total production of natural cycles over a two-year period [63]. Meanwhile, there appeared to be growing evidence that estrogen conferred increased ovarian cancer risk [64–66]. Thus, gonadotropin-induced elevated estrogen levels might promote the malignant transformation of normal ovarian epithelium [63, 67]. The above hypotheses had been tested in hen models but not in humans [68, 69]. Therefore, speculation regarding the relationship between the use of ovulation induction drug use and ovarian cancer development continues.
Research in this area was based on cohort studies, case series and case-control studies. And, there were still no randomized controlled trials regarding the relationship between ovulation inducing drugs and ovarian cancer due to ethical issues, the relatively low incidence of ovarian cancer and recall bias after ovulation induction [70]. This updated systematic review and meta-analysis was based on cohort studies and case-control studies. Some of included studies provided supportive evidence that OI treatment might increase the risk of IOC. However, according to subgroup analysis based on study type, we found no convincing evidence that OI treatment could induce an increased risk of IOC. Compared with previous systematic reviews [71, 72], we expanded our search to include more recent studies in our analysis and obtained consistent results.
BOTs are morphologically similar to IOCs and follow a similar pathogenesis [73, 74]. As the etiology of BOT was still unknown, it was difficult to explain the possible causal relationship between infertility and OI drugs. In our analysis, no significant increased risk of BOT was found following OI treatment, which appeared to contradict the increasing risk of BOT reported by Barcroft et.al (OR = 1.69, 95%CI: 1.27–2.25). With more studies included in our pooled analysis, we used further subgroup analyses based on study type to circumvent the heterogeneity issues caused by retrospective studies. Ultimately, the results of subgroup analysis were highly consistent in that OI treatment did not increase the risk of BOT.
In addition, during the review of the literature, we found several studies supporting that OI treatment could induce an increased risk of ovarian cancer in the nulliparous women [11, 12]. A cumulative analysis conducted by Whittemore et.al indicated an increased risk of ovarian cancer (OR = 27.0, 95%CI: 2.3–315.6) in the nulliparous women who ever received OI treatment. Reigstad et.al also noted a greater increased risk of ovarian cancer (HR 2.49, 95% CI 1.30 to 4.78) in the nulliparous women treated with OI. As we know, parity was known as an established protective factor for ovarian cancer [75]. Previous studies have shown that the greatest reduction in ovarian cancer risk was associated with the first pregnancy and each subsequent pregnancy could also reduce the risk of ovarian cancer [11, 75, 76]. This protective mechanism has been attributed to anovulation, reduced gonadotropin production and increased progesterone levels [77]. Hence, whether OI treatment would increase the ovarian cancer risk in nulliparous and multiparous women remained controversial. Therefore, we conducted supplementary analyses based on fertility outcomes in light of the above questions. Among the multiparous women, we did not find a higher risk of IOC and BOT in the OI group than in the CT group. Similarly, among the nulliparous women, OI treatment also did not increase the risk of IOC. However, an increased risk of BOT was found in the nulliparous women treated with OI when compared to those nulliparous women who had not been treated with OI. Nonetheless, none of these included studies initially reported a higher risk of BOT in nulliparous women treated with OI. Based on a review of the included studies, this finding might be due to a lack of ovulatory pause caused by pregnancy and exposure to ovarian hyper-stimulation [8–10, 12]. Notably, the BOTs were generally seen in younger women [78–81]. Hence, the above association might also be due to a diagnostic bias occurring in young nulliparous women who might pursue medical attention and undergo intensive monitoring [41].
Rodriguez et.al previously found that infertility itself might increase ovarian cancer risk without concomitant exposure to OI drugs [82]. A current meta-analysis based on nine prospective cohort studies also suggested that infertility in women was associated with an increased risk of ovarian cancer [83]. Moreover, a number of diseases that cause infertility, including polycystic ovary syndrome (PCOS) and endometriosis, had been found to be associated with ovarian cancer development. Previous studies had indicated that the genetic and epigenetic profile of patients with PCOS was similar to that of ovarian cancer [84]. Further, the risk of ovarian cancer, particularly serous borderline ovarian tumor, was shown to be increased in patients with PCOS [85–87]. We also found that ovarian clear cell carcinoma and endometrioid carcinoma were most often associated with ovarian endometriosis in previous studies [88, 89]. Thus, infertility itself might be an independent risk factor for ovarian cancer [90]. In parallel, whether there existed a difference in cancer risk between nulliparous and multiparous women treated with OI was under discussion, as it was difficult to separate OI treatment from infertility as a risk factor for ovarian cancer. In our analysis, we used OI exposure as a control variable to evaluate the relationship between infertility and ovarian cancer and found that the nulliparous women treated with OI showed a higher risk of IOC and BOT than those multiparous women treated with OI. Nieto et.al performed a retrospective study of ovarian cancer in first-degree relatives of infertile patients and showed an increased risk of ovarian cancer in infertile patients who failed to conceive despite receiving OI treatment, which supported our findings [91]. Rizzuto et.al also noted that the risk of BOT was slightly higher in nulliparous women treated with OI than in multiparous women [72]. In summary, we believed that there was a necessity to conduct a rigorous medical follow-up in those nulliparous patients treated with OI. Consistent with previous studies, the vast majority of patients were found within 5 years after ovulation induction [92–98]. In our analysis, the included cohort studies had a follow-up period of more than 5 years, which in our opinion is sufficient to detect ovarian cancer. Therefore, follow-up periods longer than 5 years should be considered.
Indeed, it would be arbitrary to diagnose the relationship between OI and ovarian cancer solely based on the history of OI exposure. According to incessant ovulation hypothesis, more ovulatory cycles appeared to be associated with a higher risk of developing ovarian cancer [75, 99, 100]. Whether such a cumulative effect exists remained controversial. After reviewing previous studies, we found no meta-analysis reported an association between OI cycles and the risk of ovarian cancer. Thereby, we performed a further subgroup analysis based on OI cycles, which was the focal point of our analysis. In our analysis, we used 3, 6 and 12 OI cycles as cut-off points, respectively. Compared to the control population, we found no correlation between increasing OI cycles and increased cancer risk. Unfortunately, the data for BOT in this aspect were not available for meta-analysis.
In accession, several studies had reported the risk of individual ovarian cancers due to specific OI drug exposure [12, 29, 101]. Consequently, for further study, we performed subgroup analyses according to the type of OI drug to assess whether specific OI drugs would increase the risk of ovarian cancer. CC was the most common drug to induce ovulation, especially in patients with ovulatory disturbances [102]. Reigstad et.al reported an increased risk of cancer in nulliparous women exposed to CC (HR = 2.5, 95%CI: 1.3–4.8). Rossing et.al also reported an increased ovarian tumor risk in women exposed to CC (SIR = 2.5, 95%CI: 1.3–4.5). A current meta-analysis conducted by Barcroft et.al supported the view mentioned above, which concluded that the exposure to CC was associated with a significant increased cancer risk (OR = 1.40, 95%CI: 1.10–1.77). However, in our meta- analysis, we included additional studies but did not find an increased cancer risk in those women exposed to CC. GDTs were also commonly used in women with proven hypopituitarism and in women who were not sensitive to CC [103, 104]. Shan et.al reported a slight increased ovarian cancer risk in women exposed to HMG (OR = 3.95, 95%CI: 1.3–12.2). While in our study, we found that GDTs were not associated with an increased risk of IOC and BOT. GnRH-a was introduced in anovulatory women, which could reproduce spontaneous menstrual cycle and induce ovulation [105, 106]. Our findings indicated that GnRH-a did not increase the risk of IOC. Due to the lack of the data on BOT risk in women exposed to GnRH-a, further meta-analysis could not be performed. In summary, CC, GDT and GnRH-a were proven to be safe for OI treatment without increasing ovarian tumor risk.
Most of our findings were generally consistent with previous studies on this topic [71, 72, 107, 108]. A new study was included in this latest update of the systematic review and meta-analysis compared to previous studies in this area. This study provides new data on the risk of BOT and IOC to CC exposure. To assess the impact of the latest studies on the outcome of this update, an additional sensitivity analysis was conducted. The sensitivity analysis without the latest study did not change the results that exposure to CC did not increase the risk of IOC (OR = 1.05, 95%CI: 0.89–1.21) and BOT (OR = 1.73, 95%CI: 0.96–2.50). And this latest study made the results more reliable. The results of the sensitivity analysis were shown in Supplemental Material Table S7. To summarize, OI treatment was relatively safe and cancer risk was not increased more cycles of OI and specific OI drugs. However, for those nulliparous women treated with OI, they appeared to have a higher tumor risk. Therefore, rigorous monitoring and sufficiently long follow-up were necessary for these women.
Strengths and limitations of the study
This study included 34 studies from around the world and provided an up-to-date meta-analysis to explore the potential impact of OI treatment on ovarian cancer risk. The inclusion and exclusion criteria for this systematic review and meta-analysis had been made more rigorous. In addition, the included studies were updated and the process of meta-analysis was made more rigorous. In our analysis, lessons learned from previous studies were incorporated and further subgroup analyses were conducted based on study type, tumor type, parity, OI cycle and specific OI drugs. Of note, this meta-analysis was the first study to evaluate the relationship between the OI cycles and ovarian cancer.
However, our study still had some objective shortcomings. Firstly, further work should focus more attention on patient demographics and specific data including drug combinations, cycles of use, use dosage and administration methods. Secondly, loss of follow-up existed in included studies in our analysis and retrospective studies were always considered to be lower quality evidence due to the presence of recall bias. We needed more large and long-term prospective cohort studies with careful follow-up. Thus, follow-up process needed to be improved. Last but not least, the formation of symbiotic relationships between cancer registries and fertility services should be encouraged to link fertility data with cancer information. Communication and collaboration between fertility services should also be encouraged in order to collect adequate data. We believe that further exploration in this area will facilitate the further development of reproductive science.
Conclusions
OI treatment did not increase risk of ovarian cancer, regardless of treatment regimen and treatment cycle. However, nulliparous women treated with OI might have an increased risk of BOT compared to the nulliparous women not treated with OI. Meanwhile, nulliparous women treated with OI appeared to have a higher risk of IOC and BOT than multiparous women treated with OI. In view of the above, OI treatment was relatively safe but those nulliparous women treated with OI must be followed up rigorously.
Supplementary Information
Additional file 1: Supplemental Material Fig. 1. The flowchart of systematic search and screening process.
Additional file 2: Supplemental Material Fig. 2. (A) Funnel plot of all the included studies; (B) Egger’s regression test of all the included studies.
Additional file 3: Supplementary Table S1a. Quality evaluation of case-control study assessed by the Newcastle-Ottawa Scale. Supplementary Table S1b. Quality evaluation of cohort study assessed by the Newcastle-Ottawa Scale.
Additional file 4: Supplementary Table S2. ovarian cancer study characteristics.
Additional file 5: Supplementary Table S3a. Ovarian tumor in the nulliparous women. Supplementary Table S3b. Ovarian tumor in the multiparous women.
Additional file 6: Supplementary Table S4. Ovarian tumor between the nulliparous and multiparous group.
Additional file 7: Supplementary Table S5a. Ovarian tumor and stimulation cycles(< 3 cycles). Supplementary Table S5b. Ovarian tumor and stimulation cycles(≥3 cycles). Supplementary Table S5c. Ovarian tumor and stimulation cycles(< 6 cycles). Supplementary Table S5d. Ovarian tumor and stimulation cycles(≥6 cycles). Supplementary Table S5e. Ovarian tumor and stimulation cycles(< 12 cycles). Supplementary Table S5f. Ovarian tumor and stimulation cycles(≥12 cycles).
Additional file 8: Supplementary Table S6a. Ovarian tumor in women who ever received CC. Supplementary Table S6b. Ovarian tumor in women who ever received HMG. Supplementary Table S6c. Ovarian tumor in women who ever received HCG. Supplementary Table S6d. Ovarian tumor in women who ever received GDT. Supplementary Table S6e. Ovarian tumor in women who ever received GnRH-a.
Additional file 9: Supplementary Table S7. Sensitivity analyses for the novel study during this update.
Abbreviations
- OI
Ovulation induction
- OR
Odds ratio
- BOT
Borderline ovarian tumor
- IOC
Invasive ovarian cancer
- CC
Clomiphene citrate
- GDT
Gonadotrophin
- GnRH-a
Gonadotropin-releasing hormone analogues
Authors’ contributions
YL & SJF performed the database search. YL & WQQ collected the data. YL performed the analysis and wrote the manuscript. ZS & XW supplemented the database search, polished the language of the article and provided third party arbitration. WXL provided guidance on research directions. All the authors participated in this analysis and approved the final version of the manuscript.
Funding
This work was supported by Jiangsu Provincial Commission of Health and Family Planning scientific research project (H2018017), Jiangsu Provincial Women’s and Children’s Health key talent project (RC201709).
Availability of data and materials
This meta-analysis was based on the data from the published articles and independent of any patient involvement. All the data will be made available to the editors of the journal for review or query upon.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed publication of the manuscript.
Competing interests
The authors declare no financial, personal, intellectual and professional conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liang Yu, Jiafan Sun, Qiqin Wang and Wennian Yu contributed equally to this work.
References
- 1.Datta J, Palmer MJ, Tanton C, Gibson LJ, Jones KG, Macdowall W, et al. Prevalence of infertility and help seeking among 15 000 women and men. Hum Reprod. 2016;31(9):2108–2118. doi: 10.1093/humrep/dew123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Madziyire MG, Magwali TL, Chikwasha V, Mhlanga T. The causes of infertility in women presenting to gynaecology clinics in Harare, Zimbabwe; a cross sectional study. Fertil Res Practice. 2021;7(1):1. doi: 10.1186/s40738-020-00093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 Population and Fertility Collaborators. Population and fertility by age and sex for 195 countries and territories, 1950-2017: a systematic analysis for the global burden of disease study 2017. Lancet (London, England). 2018;392(10159):1995–2051. [DOI] [PMC free article] [PubMed]
- 4.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 5.Wang R, Kim BV, van Wely M, Johnson NP, Costello MF, Zhang H, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta-analysis. BMJ (Clinical research ed) 2017;356:j138. doi: 10.1136/bmj.j138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meirow D, Schenker JG. The link between female infertility and cancer: epidemiology and possible aetiologies. Hum Reprod Update. 1996;2(1):63–75. doi: 10.1093/humupd/2.1.63. [DOI] [PubMed] [Google Scholar]
- 7.Lundberg FE, Iliadou AN, Rodriguez-Wallberg K, Gemzell-Danielsson K, Johansson ALV. The risk of breast and gynecological cancer in women with a diagnosis of infertility: a nationwide population-based study. Eur J Epidemiol. 2019;34(5):499–507. doi: 10.1007/s10654-018-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet (London, England) 1971;2(7716):163. doi: 10.1016/S0140-6736(71)92335-X. [DOI] [PubMed] [Google Scholar]
- 9.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71(4):717–721. [PubMed] [Google Scholar]
- 10.Mertens-Walker I, Baxter RC, Marsh DJ. Gonadotropin signalling in epithelial ovarian cancer. Cancer Lett. 2012;324(2):152–159. doi: 10.1016/j.canlet.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136(10):1184–1203. doi: 10.1093/oxfordjournals.aje.a116427. [DOI] [PubMed] [Google Scholar]
- 12.Reigstad MM, Storeng R, Myklebust T, Oldereid NB, Omland AK, Robsahm TE, et al. Cancer risk in women treated with fertility drugs according to parity status-a registry-based cohort study. Cancer Epidemiol Biomarkers Prev. 2017;26(6):953–962. doi: 10.1158/1055-9965.EPI-16-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Yamada T, Shojima N, Yamauchi T, Kadowaki T. J-curve relation between daytime nap duration and type 2 diabetes or metabolic syndrome: a dose-response meta-analysis. Sci Rep. 2016;6:38075. doi: 10.1038/srep38075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddaway AP, Wood AM, Hedges LV. How to do a systematic review: a best practice guide for conducting and reporting narrative reviews, meta-analyses, and meta-syntheses. Annu Rev Psychol. 2019;70:747–770. doi: 10.1146/annurev-psych-010418-102803. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Ren T, Li H, Zhu L, Tang Q. Effectiveness and safety of acupuncture for anxiety disorder of coronavirus disease 2019: a protocol of systematic review and meta-analysis. Medicine. 2020;99(38):e22177. doi: 10.1097/MD.0000000000022177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song F, Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ (Clinical research ed) 1998;316(7129):471. [PMC free article] [PubMed] [Google Scholar]
- 20.Spaan M, van den Belt-Dusebout AW, Lambalk CB, van Boven HH, Schats R, Kortman M, et al. Long-term risk of ovarian cancer and borderline tumors after assisted reproductive technology. J Natl Cancer Inst. 2021;113(6):699–709. doi: 10.1093/jnci/djaa163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg FE, Johansson ALV, Rodriguez-Wallberg K, Gemzell-Danielsson K, Iliadou AN. Assisted reproductive technology and risk of ovarian cancer and borderline tumors in parous women: a population-based cohort study. Eur J Epidemiol. 2019;34(11):1093–1101. doi: 10.1007/s10654-019-00540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjørnholt SM, Kjaer SK, Nielsen TS, Jensen A. Risk for borderline ovarian tumours after exposure to fertility drugs: results of a population-based cohort study. Hum Reprod. 2015;30(1):222–231. doi: 10.1093/humrep/deu297. [DOI] [PubMed] [Google Scholar]
- 23.Asante A, Leonard PH, Weaver AL, Goode EL, Jensen JR, Stewart EA, et al. Fertility drug use and the risk of ovarian tumors in infertile women: a case-control study. Fertil Steril. 2013;99(7):2031–2036. doi: 10.1016/j.fertnstert.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Leeuwen FE, Klip H, Mooij TM, van de Swaluw AM, Lambalk CB, Kortman M, et al. Risk of borderline and invasive ovarian tumours after ovarian stimulation for in vitro fertilization in a large Dutch cohort. Hum Reprod. 2011;26(12):3456–3465. doi: 10.1093/humrep/der322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanner K, Conner P, Bergfeldt K, Dickman P, Sundfeldt K, Bergh T, et al. Ovarian epithelial neoplasia after hormonal infertility treatment: long-term follow-up of a historical cohort in Sweden. Fertil Steril. 2009;91(4):1152–1158. doi: 10.1016/j.fertnstert.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 26.Kristiansson P, Björ O, Wramsby H. Tumour incidence in Swedish women who gave birth following IVF treatment. Hum Reprod. 2007;22(2):421–426. doi: 10.1093/humrep/del411. [DOI] [PubMed] [Google Scholar]
- 27.Cusidó M, Fábregas R, Pere BS, Escayola C, Barri PN. Ovulation induction treatment and risk of borderline ovarian tumors. Gynecol Endocrinol. 2007;23(7):373–376. doi: 10.1080/09513590701350341. [DOI] [PubMed] [Google Scholar]
- 28.Mosgaard BJ, Lidegaard O, Kjaer SK, Schou G, Andersen AN. Ovarian stimulation and borderline ovarian tumors: a case-control study. Fertil Steril. 1998;70(6):1049–1055. doi: 10.1016/S0015-0282(98)00337-9. [DOI] [PubMed] [Google Scholar]
- 29.Shushan A, Paltiel O, Iscovich J, Elchalal U, Peretz T, Schenker JG. Human menopausal gonadotropin and the risk of epithelial ovarian cancer. Fertil Steril. 1996;65(1):13–18. doi: 10.1016/S0015-0282(16)58020-0. [DOI] [PubMed] [Google Scholar]
- 30.Harris R, Whittemore AS, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. III. Epithelial tumors of low malignant potential in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136(10):1204–1211. doi: 10.1093/oxfordjournals.aje.a116428. [DOI] [PubMed] [Google Scholar]
- 31.Kessous R, Davidson E, Meirovitz M, Sergienko R, Sheiner E. The risk of female malignancies after fertility treatments: a cohort study with 25-year follow-up. J Cancer Res Clin Oncol. 2016;142(1):287–293. doi: 10.1007/s00432-015-2035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronwald J, Glass K, Rosen B, Karlan B, Tung N, Neuhausen SL, et al. Treatment of infertility does not increase the risk of ovarian cancer among women with a BRCA1 or BRCA2 mutation. Fertil Steril. 2016;105(3):781–785. doi: 10.1016/j.fertnstert.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 33.Trabert B, Lamb EJ, Scoccia B, Moghissi KS, Westhoff CL, Niwa S, et al. Ovulation-inducing drugs and ovarian cancer risk: results from an extended follow-up of a large United States infertility cohort. Fertil Steril. 2013;100(6):1660–1666. doi: 10.1016/j.fertnstert.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinton LA, Trabert B, Shalev V, Lunenfeld E, Sella T, Chodick G. In vitro fertilization and risk of breast and gynecologic cancers: a retrospective cohort study within the Israeli Maccabi Healthcare Services. Fertil Steril. 2013;99(5):1189–1196. doi: 10.1016/j.fertnstert.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerner-Geva L, Rabinovici J, Olmer L, Blumstein T, Mashiach S, Lunenfeld B. Are infertility treatments a potential risk factor for cancer development? Perspective of 30 years of follow-up. Gynecol Endocrinol. 2012;28(10):809–814. doi: 10.3109/09513590.2012.671391. [DOI] [PubMed] [Google Scholar]
- 36.Kurta ML, Moysich KB, Weissfeld JL, Youk AO, Bunker CH, Edwards RP, et al. Use of fertility drugs and risk of ovarian cancer: results from a U.S.-based case-control study. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1282–1292. doi: 10.1158/1055-9965.EPI-12-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franceschi S, La Vecchia C, Negri E, Guarneri S, Montella M, Conti E, et al. Fertility drugs and risk of epithelial ovarian cancer in Italy. Hum Reprod. 1994;9(9):1673–1675. doi: 10.1093/oxfordjournals.humrep.a138771. [DOI] [PubMed] [Google Scholar]
- 38.Mosgaard BJ, Lidegaard O, Kjaer SK, Schou G, Andersen AN. Infertility, fertility drugs, and invasive ovarian cancer: a case-control study. Fertil Steril. 1997;67(6):1005–1012. doi: 10.1016/S0015-0282(97)81431-8. [DOI] [PubMed] [Google Scholar]
- 39.Parazzini F, Negri E, La Vecchia C, Moroni S, Franceschi S, Crosignani PG. Treatment for infertility and risk of invasive epithelial ovarian cancer. Hum Reprod. 1997;12(10):2159–2161. doi: 10.1093/humrep/12.10.2159. [DOI] [PubMed] [Google Scholar]
- 40.Parazzini F, Pelucchi C, Negri E, Franceschi S, Talamini R, Montella M, et al. Use of fertility drugs and risk of ovarian cancer. Hum Reprod. 2001;16(7):1372–1375. doi: 10.1093/humrep/16.7.1372. [DOI] [PubMed] [Google Scholar]
- 41.Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, et al. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155(3):217–224. doi: 10.1093/aje/155.3.217. [DOI] [PubMed] [Google Scholar]
- 42.Rossing MA, Tang MT, Flagg EW, Weiss LK, Wicklund KG. A case-control study of ovarian cancer in relation to infertility and the use of ovulation-inducing drugs. Am J Epidemiol. 2004;160(11):1070–1078. doi: 10.1093/aje/kwh315. [DOI] [PubMed] [Google Scholar]
- 43.Jensen A, Sharif H, Frederiksen K, Kjaer SK. Use of fertility drugs and risk of ovarian cancer: Danish population based cohort study. BMJ (Clinical research ed) 2009;338:b249. doi: 10.1136/bmj.b249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venn A, Watson L, Lumley J, Giles G, King C, Healy D. Breast and ovarian cancer incidence after infertility and in vitro fertilisation. Lancet (London, England) 1995;346(8981):995–1000. doi: 10.1016/S0140-6736(95)91687-3. [DOI] [PubMed] [Google Scholar]
- 45.Modan B, Ron E, Lerner-Geva L, Blumstein T, Menczer J, Rabinovici J, et al. Cancer incidence in a cohort of infertile women. Am J Epidemiol. 1998;147(11):1038–1042. doi: 10.1093/oxfordjournals.aje.a009397. [DOI] [PubMed] [Google Scholar]
- 46.Potashnik G, Lerner-Geva L, Genkin L, Chetrit A, Lunenfeld E, Porath A. Fertility drugs and the risk of breast and ovarian cancers: results of a long-term follow-up study. Fertil Steril. 1999;71(5):853–859. doi: 10.1016/S0015-0282(99)00085-0. [DOI] [PubMed] [Google Scholar]
- 47.Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet (London, England) 1999;354(9190):1586–1590. doi: 10.1016/S0140-6736(99)05203-4. [DOI] [PubMed] [Google Scholar]
- 48.Doyle P, Maconochie N, Beral V, Swerdlow AJ, Tan SL. Cancer incidence following treatment for infertility at a clinic in the UK. Hum Reprod. 2002;17(8):2209–2213. doi: 10.1093/humrep/17.8.2209. [DOI] [PubMed] [Google Scholar]
- 49.Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, et al. Ovarian cancer risk after the use of ovulation-stimulating drugs. Obstet Gynecol. 2004;103(6):1194–1203. doi: 10.1097/01.AOG.0000128139.92313.74. [DOI] [PubMed] [Google Scholar]
- 50.Calderon-Margalit R, Friedlander Y, Yanetz R, Kleinhaus K, Perrin MC, Manor O, et al. Cancer risk after exposure to treatments for ovulation induction. Am J Epidemiol. 2009;169(3):365–375. doi: 10.1093/aje/kwn318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva Idos S, Wark PA, McCormack VA, Mayer D, Overton C, Little V, et al. Ovulation-stimulation drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer. 2009;100(11):1824–1831. doi: 10.1038/sj.bjc.6605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55(1):3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 53.Lee AW, Rosenzweig S, Wiensch A, Ramus SJ, Menon U, Gentry-Maharaj A, et al. Expanding our understanding of ovarian cancer risk: the role of incomplete pregnancies. J Natl Cancer Inst. 2021;113(3):301–308. doi: 10.1093/jnci/djaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Babic A, Sasamoto N, Rosner BA, Tworoger SS, Jordan SJ, Risch HA, et al. Association between breastfeeding and ovarian cancer risk. JAMA Oncol. 2020;6(6):e200421. doi: 10.1001/jamaoncol.2020.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sung HK, Ma SH, Choi JY, Hwang Y, Ahn C, Kim BG, et al. The effect of breastfeeding duration and parity on the risk of epithelial ovarian cancer: a systematic review and meta-analysis. J Prev Med Public Health = Yebang Uihakhoe Chi. 2016;49(6):349–366. doi: 10.3961/jpmph.16.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li DP, Du C, Zhang ZM, Li GX, Yu ZF, Wang X, et al. Breastfeeding and ovarian cancer risk: a systematic review and meta-analysis of 40 epidemiological studies. Asian Pac J Cancer Prev. 2014;15(12):4829–4837. doi: 10.7314/APJCP.2014.15.12.4829. [DOI] [PubMed] [Google Scholar]
- 57.Karlsson T, Johansson T, Höglund J, Ek WE, Johansson Å. Time-dependent effects of oral contraceptive use on breast, ovarian, and endometrial cancers. Cancer Res. 2021;81(4):1153–1162. doi: 10.1158/0008-5472.CAN-20-2476. [DOI] [PubMed] [Google Scholar]
- 58.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 59.Bray F, Loos AH, Tognazzo S, La Vecchia C. Ovarian cancer in Europe: cross-sectional trends in incidence and mortality in 28 countries, 1953-2000. Int J Cancer. 2005;113(6):977–990. doi: 10.1002/ijc.20649. [DOI] [PubMed] [Google Scholar]
- 60.Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet (London, England) 2008;371(9609):303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 61.Fathalla MF. Incessant ovulation and ovarian cancer - a hypothesis re-visited. Facts, Views Vision ObGyn. 2013;5(4):292–297. [PMC free article] [PubMed] [Google Scholar]
- 62.Treviño LS, Buckles EL, Johnson PA. Oral contraceptives decrease the prevalence of ovarian cancer in the hen. Cancer Prev Res (Phila) 2012;5(2):343–349. doi: 10.1158/1940-6207.CAPR-11-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fishel S, Jackson P. Follicular stimulation for high tech pregnancies: are we playing it safe? BMJ (Clinical research ed) 1989;299(6694):309–311. doi: 10.1136/bmj.299.6694.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Folsom AR, Anderson JP, Ross JA. Estrogen replacement therapy and ovarian cancer. Epidemiology. 2004;15(1):100–104. doi: 10.1097/01.ede.0000091606.31903.8e. [DOI] [PubMed] [Google Scholar]
- 65.Glud E, Kjaer SK, Thomsen BL, Høgdall C, Christensen L, Høgdall E, et al. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004;164(20):2253–2259. doi: 10.1001/archinte.164.20.2253. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Ma L, Yang X, Bie J, Li D, Sun C, et al. Menopausal hormone replacement therapy and the risk of ovarian cancer: a meta-analysis. Front Endocrinol. 2019;10:801. doi: 10.3389/fendo.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gjorgoska M, Rižner TL. Estrogens and the Schrödinger's cat in the ovarian tumor microenvironment. Cancers. 2021;13(19):5011. [DOI] [PMC free article] [PubMed]
- 68.Mocka EH, Stern RA, Fletcher OJ, Anderson KE, Petitte JN, Mozdziak PE. Chemoprevention of spontaneous ovarian cancer in the domestic hen. Poult Sci. 2017;96(6):1901–1909. doi: 10.3382/ps/pew422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pal P, Starkweather KN, Hales KH, Hales DB. A review of principal studies on the development and treatment of epithelial ovarian cancer in the laying hen Gallus gallus. Comp Med. 2021;71(4):271–284. doi: 10.30802/AALAS-CM-20-000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beltsos AN, Odem RR. Ovulation induction and ovarian malignancy. Semin Reprod Endocrinol. 1996;14(4):367–374. doi: 10.1055/s-2008-1067981. [DOI] [PubMed] [Google Scholar]
- 71.Barcroft JF, Galazis N, Jones BP, Getreu N, Bracewell-Milnes T, Grewal KJ, et al. Fertility treatment and cancers-the eternal conundrum: a systematic review and meta-analysis. Hum Reprod. 2021;36(4):1093–1107. doi: 10.1093/humrep/deaa293. [DOI] [PubMed] [Google Scholar]
- 72.Rizzuto I, Behrens RF, Smith LA. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst Rev. 2019;6(6):Cd008215. doi: 10.1002/14651858.CD008215.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Link CJ, Jr, Reed E, Sarosy G, Kohn EC. Borderline ovarian tumors. Am J Med. 1996;101(2):217–225. doi: 10.1016/S0002-9343(96)80079-9. [DOI] [PubMed] [Google Scholar]
- 74.Buis CC, van Leeuwen FE, Mooij TM, Burger CW. Increased risk for ovarian cancer and borderline ovarian tumours in subfertile women with endometriosis. Hum Reprod. 2013;28(12):3358–3369. doi: 10.1093/humrep/det340. [DOI] [PubMed] [Google Scholar]
- 75.Moorman PG, Calingaert B, Palmieri RT, Iversen ES, Bentley RC, Halabi S, et al. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am J Epidemiol. 2008;167(9):1059–1069. doi: 10.1093/aje/kwn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tung KH, Wilkens LR, Wu AH, McDuffie K, Nomura AM, Kolonel LN, et al. Effect of anovulation factors on pre- and postmenopausal ovarian cancer risk: revisiting the incessant ovulation hypothesis. Am J Epidemiol. 2005;161(4):321–329. doi: 10.1093/aje/kwi046. [DOI] [PubMed] [Google Scholar]
- 77.Merritt MA, De Pari M, Vitonis AF, Titus LJ, Cramer DW, Terry KL. Reproductive characteristics in relation to ovarian cancer risk by histologic pathways. Hum Reprod. 2013;28(5):1406–1417. doi: 10.1093/humrep/des466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kehoe S, Powell J. Long-term follow-up of women with borderline ovarian tumors. Int J Gynaecol Obstet. 1996;53(2):139–143. doi: 10.1016/0020-7292(95)02642-8. [DOI] [PubMed] [Google Scholar]
- 79.Barnhill DR, Kurman RJ, Brady MF, Omura GA, Yordan E, Given FT, et al. Preliminary analysis of the behavior of stage I ovarian serous tumors of low malignant potential: a Gynecologic Oncology Group Study. J Clin Oncol. 1995;13(11):2752–2756. doi: 10.1200/JCO.1995.13.11.2752. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Cui H, Shen DH, Zhao Y, Wei LH, Qian HN. Clinical and pathological features of borderline ovarian tumors. Zhonghua Fu Chan Ke Za Zhi. 2003;38(2):81–84. [PubMed] [Google Scholar]
- 81.Sun Y, Xu J, Jia X. The diagnosis, treatment, prognosis and molecular pathology of borderline ovarian tumors: current status and perspectives. Cancer Manag Res. 2020;12:3651–3659. doi: 10.2147/CMAR.S250394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez C, Tatham LM, Calle EE, Thun MJ, Jacobs EJ, Heath CW., Jr Infertility and risk of fatal ovarian cancer in a prospective cohort of US women. Cancer Causes Control. 1998;9(6):645–651. doi: 10.1023/A:1008845106869. [DOI] [PubMed] [Google Scholar]
- 83.Jiang YT, Gong TT, Zhang JY, Li XQ, Gao S, Zhao YH, et al. Infertility and ovarian cancer risk: evidence from nine prospective cohort studies. Int J Cancer. 2020;147(8):2121–2130. doi: 10.1002/ijc.33012. [DOI] [PubMed] [Google Scholar]
- 84.Jiao J, Sagnelli M, Shi B, Fang Y, Shen Z, Tang T, et al. Genetic and epigenetic characteristics in ovarian tissues from polycystic ovary syndrome patients with irregular menstruation resemble those of ovarian cancer. BMC Endocr Disord. 2019;19(1):30. doi: 10.1186/s12902-019-0356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin W, Falconer H, Yin L, Xu L, Ye W. Association between polycystic ovary syndrome and cancer risk. JAMA Oncol. 2019;5(1):106–107. doi: 10.1001/jamaoncol.2018.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harris HR, Titus LJ, Cramer DW, Terry KL. Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population-based case-control study. Int J Cancer. 2017;140(2):285–291. doi: 10.1002/ijc.30441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Olsen CM, Green AC, Nagle CM, Jordan SJ, Whiteman DC, Bain CJ, et al. Epithelial ovarian cancer: testing the ‘androgens hypothesis’. Endocr Relat Cancer. 2008;15(4):1061–1068. doi: 10.1677/ERC-08-0075. [DOI] [PubMed] [Google Scholar]
- 88.Vargas-Hernández VM. Endometriosis as a risk factor for ovarian cancer. Cir Cir. 2013;81(2):163–168. [PubMed] [Google Scholar]
- 89.Králíčková M, Laganà AS, Ghezzi F, Vetvicka V. Endometriosis and risk of ovarian cancer: what do we know? Arch Gynecol Obstet. 2020;301(1):1–10. doi: 10.1007/s00404-019-05358-8. [DOI] [PubMed] [Google Scholar]
- 90.Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, et al. Ovarian cancer risk associated with varying causes of infertility. Fertil Steril. 2004;82(2):405–414. doi: 10.1016/j.fertnstert.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 91.Nieto JJ, Rolfe KJ, MacLean AB, Hardiman P. Ovarian cancer and infertility: a genetic link? Lancet (London, England) 1999;354(9179):649. doi: 10.1016/S0140-6736(99)02250-3. [DOI] [PubMed] [Google Scholar]
- 92.Hull ME, Kriner M, Schneider E, Maiman M. Ovarian cancer after successful ovulation induction: a case report. J Reprod Med. 1996;41(1):52–54. [PubMed] [Google Scholar]
- 93.Abboud J, Attieh E, Atallah D, Kessrouani A, Chaoul G. Three cases of ovarian cancer after ovulation induction for infertility. Contraception, fertilite, sexualite (1992). 1997;25(1):64–5 [PubMed]
- 94.Wang Xia ZS, XiuLi W. The rapid development of bilateral ovarian krukenberg tumor after ovarian stimulation: a case report and literature review. J Int Obstet Gynecol. 2021;48(4):477–80.
- 95.Adewole IF, Babarinsa IA, Thomas JO, Ajayi AB. Ovarian cancer associated with ovulation induction: a case report. Afr J Med Med Sci. 1997;26(3–4):203–204. [PubMed] [Google Scholar]
- 96.Artini PG, Fasciani A, Cela V, Battaglia C, de Micheroux AA, D'Ambrogio G, et al. Fertility drugs and ovarian cancer. Gynecol Endocrinol. 1997;11(1):59–68. doi: 10.3109/09513599709152318. [DOI] [PubMed] [Google Scholar]
- 97.Jeremic K, Gojnic M, Milenković V, Petković S, Stojnić J, Lazović G, et al. Treatment for infertility and risk of invasive epithelial ovarian cancer--a case report. Clin Exp Obstet Gynecol. 2006;33(3):190–191. [PubMed] [Google Scholar]
- 98.Milenković V, Sparić R, Dokić M, Petković S, Atanacković J. Ovarian cancer after in vitro fertilization. Srp Arh Celok Lek. 2004;132(9–10):331–333. doi: 10.2298/SARH0410331M. [DOI] [PubMed] [Google Scholar]
- 99.Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A. 2011;108(4):1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sowamber R, Nelson O, Dodds L, DeCastro V, Paudel I, Milea A, et al. Integrative transcriptome analyses of the human fallopian tube: Fimbria and ampulla-site of origin of serous carcinoma of the ovary. Cancers. 2020;12(5):1090. [DOI] [PMC free article] [PubMed]
- 101.Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331(12):771–776. doi: 10.1056/NEJM199409223311204. [DOI] [PubMed] [Google Scholar]
- 102.Bansal S, Goyal M, Sharma C, Shekhar S. Letrozole versus clomiphene citrate for ovulation induction in anovulatory women with polycystic ovarian syndrome: a randomized controlled trial. Int J Gynaecol Obstet. 2021;152(3):345–350. doi: 10.1002/ijgo.13375. [DOI] [PubMed] [Google Scholar]
- 103.Zolton JR, Lindner PG, Terry N, DeCherney AH, Hill MJ. Gonadotropins versus oral ovarian stimulation agents for unexplained infertility: a systematic review and meta-analysis. Fertil Steril. 2020;113(2):417–25.e1. doi: 10.1016/j.fertnstert.2019.09.042. [DOI] [PubMed] [Google Scholar]
- 104.Sarhan A, Beydoun H, Jones HW, Jr, Bocca S, Oehninger S, Stadtmauer L. Gonadotrophin ovulation induction and enhancement outcomes: analysis of more than 1400 cycles. Reprod Biomed Online. 2011;23(2):220–226. doi: 10.1016/j.rbmo.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 105.Weiss JM, Ludwig M, Ortmann O, Diedrich K. GnRH antagonists in the treatment of infertility. Ann Med. 2003;35(7):512–522. doi: 10.1080/07853890310001302. [DOI] [PubMed] [Google Scholar]
- 106.Filicori M, Cognigni GE. Ovulation induction with pulsatile gonadotropin releasing hormone: missing in action. Fertil Steril. 2018;109(4):621–622. doi: 10.1016/j.fertnstert.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 107.Kashyap S, Moher D, Fung MF, Rosenwaks Z. Assisted reproductive technology and the incidence of ovarian cancer: a meta-analysis. Obstet Gynecol. 2004;103(4):785–794. doi: 10.1097/01.AOG.0000119226.39514.1d. [DOI] [PubMed] [Google Scholar]
- 108.Li LL, Zhou J, Qian XJ, Chen YD. Meta-analysis on the possible association between in vitro fertilization and cancer risk. Int J Gynecol Cancer. 2013;23(1):16–24. doi: 10.1097/IGC.0b013e318277608b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Material Fig. 1. The flowchart of systematic search and screening process.
Additional file 2: Supplemental Material Fig. 2. (A) Funnel plot of all the included studies; (B) Egger’s regression test of all the included studies.
Additional file 3: Supplementary Table S1a. Quality evaluation of case-control study assessed by the Newcastle-Ottawa Scale. Supplementary Table S1b. Quality evaluation of cohort study assessed by the Newcastle-Ottawa Scale.
Additional file 4: Supplementary Table S2. ovarian cancer study characteristics.
Additional file 5: Supplementary Table S3a. Ovarian tumor in the nulliparous women. Supplementary Table S3b. Ovarian tumor in the multiparous women.
Additional file 6: Supplementary Table S4. Ovarian tumor between the nulliparous and multiparous group.
Additional file 7: Supplementary Table S5a. Ovarian tumor and stimulation cycles(< 3 cycles). Supplementary Table S5b. Ovarian tumor and stimulation cycles(≥3 cycles). Supplementary Table S5c. Ovarian tumor and stimulation cycles(< 6 cycles). Supplementary Table S5d. Ovarian tumor and stimulation cycles(≥6 cycles). Supplementary Table S5e. Ovarian tumor and stimulation cycles(< 12 cycles). Supplementary Table S5f. Ovarian tumor and stimulation cycles(≥12 cycles).
Additional file 8: Supplementary Table S6a. Ovarian tumor in women who ever received CC. Supplementary Table S6b. Ovarian tumor in women who ever received HMG. Supplementary Table S6c. Ovarian tumor in women who ever received HCG. Supplementary Table S6d. Ovarian tumor in women who ever received GDT. Supplementary Table S6e. Ovarian tumor in women who ever received GnRH-a.
Additional file 9: Supplementary Table S7. Sensitivity analyses for the novel study during this update.
Data Availability Statement
This meta-analysis was based on the data from the published articles and independent of any patient involvement. All the data will be made available to the editors of the journal for review or query upon.