Abstract
Despite significant progress in clinical management, drug resistance remains a major obstacle. Recent research based on protein degradation to restrain drug resistance has attracted wide attention, and several therapeutic strategies such as inhibition of proteasome with bortezomib and proteolysis-targeting chimeric have been developed. Compared with intervention at the transcriptional level, targeting the degradation process seems to be a more rapid and direct strategy. Proteasomal proteolysis and lysosomal proteolysis are the most critical quality control systems responsible for the degradation of proteins or organelles. Although proteasomal and lysosomal inhibitors (e.g., bortezomib and chloroquine) have achieved certain improvements in some clinical application scenarios, their routine application in practice is still a long way off, which is due to the lack of precise targeting capabilities and inevitable side effects. In-depth studies on the regulatory mechanism of critical protein degradation regulators, including E3 ubiquitin ligases, deubiquitylating enzymes (DUBs), and chaperones, are expected to provide precise clues for developing targeting strategies and reducing side effects. Here, we discuss the underlying mechanisms of protein degradation in regulating drug efflux, drug metabolism, DNA repair, drug target alteration, downstream bypass signaling, sustaining of stemness, and tumor microenvironment remodeling to delineate the functional roles of protein degradation in drug resistance. We also highlight specific E3 ligases, DUBs, and chaperones, discussing possible strategies modulating protein degradation to target cancer drug resistance. A systematic summary of the molecular basis by which protein degradation regulates tumor drug resistance will help facilitate the development of appropriate clinical strategies.
Keywords: E3 ligase, DUBs, Chaperone-mediated autophagy, Protein degradation, Drug resistance
Background
For several decades, the rapid development of novel therapeutic strategies has significantly contributed to the decline in the mortality in patients with cancer [1]. However, the clinical therapeutic outcome of late-stage cancer is still far from satisfactory, where drug resistance is considered an essential molecular mechanism [2]. These molecular mechanisms of therapeutic tolerance have been studied intensively and can be categorized into pharmacokinetic and pharmacodynamic resistance pathways [3, 4]. The pharmacokinetic resistance mechanisms include increased drug efflux, reduced drug uptake, alterations in drug metabolism, and drug sequestration, which lead to decreased intracellular drug concentrations [5, 6]. Activation of DNA repair, alterations in drug targets, evasion of cellular death pathways, and remodeling of the tumor microenvironment account for pharmacodynamic resistance. Furthermore, classified according to cause, drug resistance can be divided into two categories: innate and acquired resistance [7]. Innate resistance causes chemotherapy to be ineffective before drug treatment as a result of resistance-mediating factors that preexist in the bulk of tumor cells [8]. On the other hand, acquired chemoresistance can be developed under the stress of treatment, which endows cancer cells with acquired epigenetic alterations for enhanced survival ability [9–11]. In addition to the regulation of gene transcriptional and posttranscriptional modification [12, 13], protein degradation has received increasing attention due to its direct and rapid regulation.
Protein homeostasis is essential for various cellular and organismal functions [14]. Proteins to be degraded usually contain specific recognition motifs termed “degrons,” including short amino acid sequences with (or without) specific posttranslational modifications (e.g., phosphorylation or glycosylation) [15], exposed amino acids (e.g., N-degrons and C-degrons) [16], and structural motifs [17]. After being identified, these proteins are usually conjugated with specific tags (e.g., ubiquitin) and degraded by proteolytic systems. Proteasomal proteolysis and lysosomal proteolysis are two major quality control systems responsible for the degradation of cellular proteins [18]. Generally, proteasomal proteolysis is a multi-enzyme process in which the covalent conjugation of ubiquitin to substrates induces degradation by the proteasome [19]. The most common mammalian proteasome involved in degradation is the 26S proteasome, consisting of a 20S core particle with proteolytic activity and two 19S cap subunits that recognize polyubiquitin tags on substrates [20]. With some exceptions, a small portion of proteins can interact with the 20S and be degraded through a ubiquitin-independent pathway [21]. Lysosomal proteolysis is a multistep lysosome-dependent degradation process whereby cytoplasmic constituents (e.g., protein aggregates, damaged organelles) are degraded through posttranslational modification (e.g., ubiquitin-dependent or chaperone-mediated mechanisms) [22]. The lysosomal degradation pathway can be mainly divided into autophagy and endocytosis according to the origin of the substrate from autophagosome or endosome, respectively [23]. Considered the most common lysosomal degradation pathway, autophagy can be categorized into macroautophagy (the most studied type of autophagy), microautophagy, and chaperone-mediated autophagy [24]. Substrates (e.g., specific proteins, protein aggregates, and organelles) with specific cargo signals (e.g., ubiquitin) can be recognized by autophagy receptors (e.g., p62, BNIP3), resulting in selective degradation [25]. Apart from ubiquitin-mediated selective autophagy, chaperone-mediated autophagy facilitates protein degradation by recognizing specific motifs (KFERQ-like motifs) [26]. The protein degradation process is widely adopted as a dynamic and self-regulating process for cellular quality control and a decision process for cells that discriminate between normal and malfunctioning proteins or subcellular structures [27] (Fig. 1).
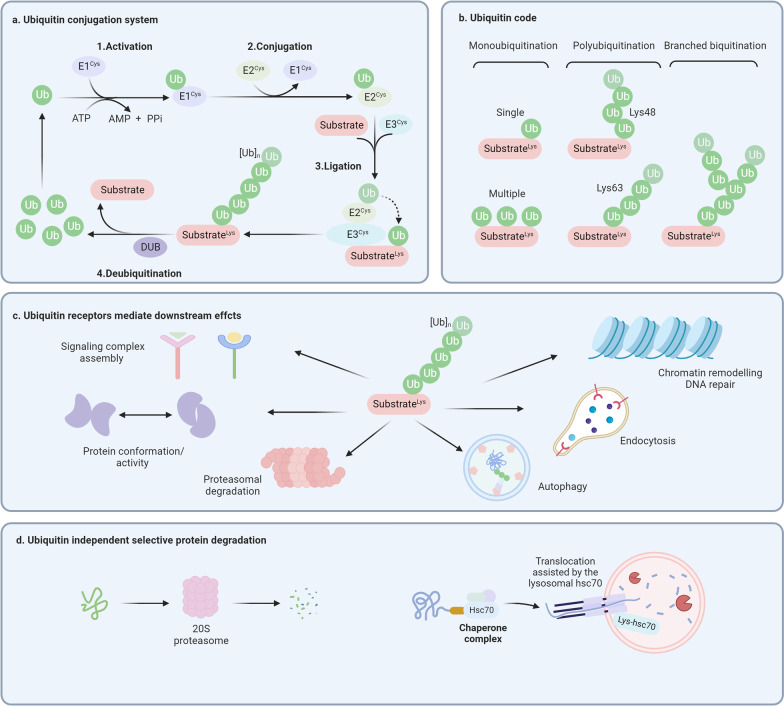
Fig. 1.
Ubiquitin-dependent and independent molecular mechanisms of protein degradation. a Ubiquitin conjugation system consists of ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3). Deubiquitylating enzymes (DUB) can remove ubiquitin chains from substrates. b Ubiquitin modification can be categorized as monoubiquitination, polyubiquitination, and branched ubiquitination, where Lys48 and Lys63 polyubiquitination chains are closely related to protein degradation. c The biological functions of substrates, including signaling transduction, protein conformation change, chromatin remodeling, proteasomal degradation, and lysosomal degradation, are further determined by the ubiquitin receptors. d Ubiquitin-independent targeted protein degradation includes 20S proteasome-mediated protein degradation and chaperone-mediated autophagy
With respect to the common recognition signal for both proteasomal and lysosomal degradation, ubiquitin (an 8.6 kD small regulatory protein) has been recognized as one of the essential posttranslational modifications governing protein degradation through both proteasomes and lysosomes [28]. Ubiquitination mainly involves three steps: ubiquitin-activating enzymes (E1s)-mediated activation, conjugating through ubiquitin-conjugating enzymes (E2s), and ligation executed by ubiquitin ligases (E3s). There exist at least 8 E1s [29], ~ 40 E2s [30], and ~ 600 E3s [31], among which E3s have the ability of substrate-specific recognition for precise regulation [32]. E3s can be categorized into three families, including homologous to the E6AP C-terminus (HECT), really interesting new gene (RING)-finger/UFD2 homology (U-box), and RING-between-RING (RBR) families [33]. In addition, ubiquitination can also be removed by deubiquitylating enzymes (DUBs), indicating that the ubiquitination system is finely regulated [34]. To date, the ~ 100 putative DUBs can be classified into two classes: cysteine proteases and metalloproteases [35]. The ubiquitination of a specific protein can be either a single ubiquitin or ubiquitin chains, where secondary ubiquitin proteins are linked to one of the lysine residues (K6, K11, K27, K29, K33, K48, K63) or N-terminal methionine (M1) [36]. The ubiquitin modification has a wide range of physiological functions, including ubiquitin-mediated degradation, ubiquitination signal transduction, chromatin remodeling, DNA repair, and protein identification [37]. Generally, proteins with K11 and K48 polyubiquitin modification tend to be degraded through the proteasome [38], and proteins with monoubiquitylation and K63 polyubiquitin modification are degraded through selective autophagy [39], which is probably due to the competitive binding of two ubiquitin-binding receptors, valosin-containing protein (VCP)/p97 and p62, to polyubiquitin chains in different degradation systems [40]. Recent studies suggest that there is a complementary and competitive relationship between the two degradation systems, and the regulatory mechanism remains to be further explored [41, 42]. In addition, the chaperone Hsc70 (also known as HSPA8) can directly bind substrates containing a KFERQ-like motif, thus mediating lysosomal degradation by interacting with lysosome-associated membrane protein type 2A (LAMP2A) [43]. Recent evidence suggests that critical regulators in selective protein degradation, including E3s, DUBs, and chaperones, are deregulated in multiple types of tumors, suggesting that studying the underlying molecular mechanisms may facilitate the identification of drug targets and the development of therapeutic regimens [44].
Compared with regulation at the transcriptional level, the protein degradation process seems to be a more rapid and direct mechanism for cancer drug resistance [45]. However, studies on endogenous degradation processes and their interplay with drug resistance are still limited due to the complex interactions between endogenous and exogenous signals [46–48]. Identifying molecular mechanisms is of significance for finding potential clinically meaningful targets and developing novel therapeutic approaches. In this review, we aim to systematically describe the molecular mechanisms between protein degradation and drug resistance, the regulatory mechanism between E3s/DUBs/chaperones and drug resistance-related proteins and their potential clinical application value. In addition, we will outline the emerging approaches targeting protein degradation in recent years and discuss their current use and application prospects.
Protein degradation-mediated aberrant drug transport and metabolism
Cancer cell pharmacokinetic resistance leads to reduced intracellular drug concentrations, including increased drug efflux, reduced drug uptake, and altered drug metabolism. Recently, multiple lines of evidence have demonstrated that protein degradation participates in the modulation of therapeutic resistance by regulating drug transport and metabolism [49].
Drug efflux modulated by protein degradation
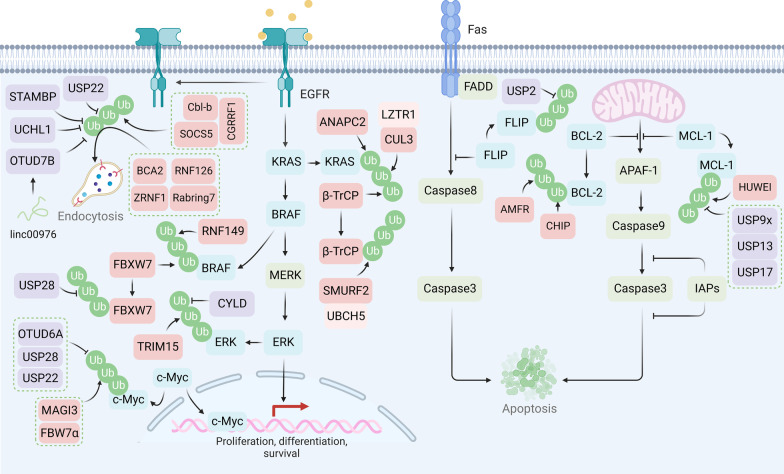
Adequate intertumoral drug exposure is a prerequisite for effective drug treatment. Once chemotherapeutic agents have reached cancer cells, passage across the plasma membrane represents the first possible impediment to effective drug delivery into cancer cells. However, when tumor cells highly express drug efflux receptors, they can sustain a low intracellular drug concentration during drug treatment, thereby promoting tumor resistance [50]. Most notably, among the 48 ATP-binding cassette (ABC) transporter family proteins, three members have been implicated as multi-drug efflux pumps capable of conferring multi-drug resistance (MDR), including multi-drug resistance protein 1 (MDR1, also known as ABCB1 and P-glycoprotein), MDR-associated protein 1 (MRP1, or ABCC1) and breast cancer resistance protein (BCRP, or ABCG2) [51]. These proteins serve as cell membrane pumps that effectively block or limit the access of drugs into cancer cells, resulting in poor therapeutic outcomes across many different cancers [52]. Interestingly, recent studies indicate that protein degradation participates in the regulation of ABC family protein expression, directly on selective degradation or indirectly via upstream signaling pathways, thus influencing the drug efflux of tumor cells (Fig. 2).
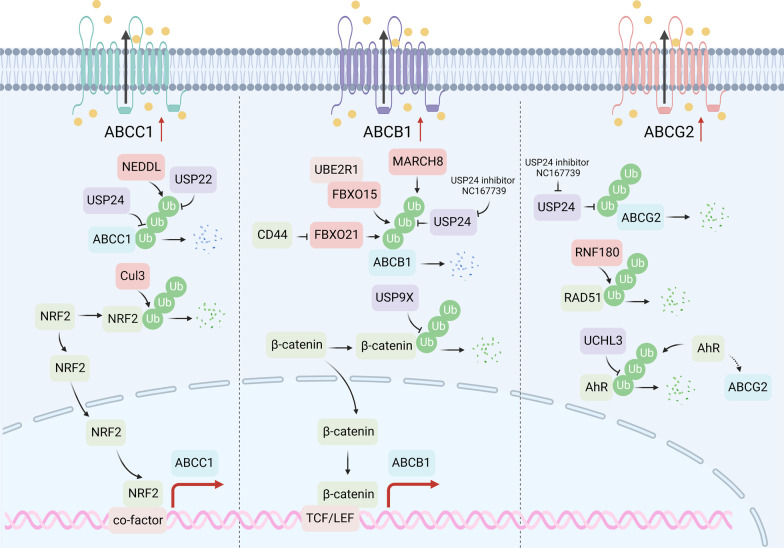
Fig. 2.
Protein degradation regulates drug efflux. ABCC1, ABCB1, and ABCG2 are the three main drug transporters. NEDDL ligases the ubiquitin chain to ABCC1, while USP22 and USP24 eliminate the ubiquitin chain. CUL3 regulates ABCC1 expression by modulating the degradation of NRF2. MARCH8, FBXO15, and FBXO21 ligase the ubiquitin chain on ABCB1, and USP24 acts as the DUB. USP24 is the DUB of ABCG2. RNF180 and UCHL3 indirectly regulate the expression of ABCG2
ABCB1
ABCB1 is the most in-depth studied ABC transporter, participating in drug resistance in multiple types of tumors [53]. Several E3s and DUBs are involved in the regulation of ABCB1 degradation through direct or indirect mechanisms. For example, evidence shows that FBXO15 can interact with ABCB1 and facilitate its ubiquitination [54]. The downregulation of ABCB1 expression by ubiquitin-conjugating enzyme E2 R1 (UBE2R1) and FBXO15-mediated ubiquitination boosted sensitivity to vincristine and doxorubicin [55]. Additionally, the F-box protein family member FBXO21 is another subunit of SCF ubiquitin E3 ligases, which function in phosphorylation-dependent ubiquitination degradation [56]. FBXO21 is involved in the proteasome-mediated degradation of ABCB1, resulting in the attenuation of MDR. However, the hyaluronic acid (HA) receptor CD44 was found to impair FBXO21-directed degradation of ABCB1, leading to the enhancement of MDR in a CD44 phosphorylation-dependent manner [57]. Therefore, targeting CD44 could represent a potential therapeutic strategy to overcome drug resistance for cancer cells overexpressing both CD44 and ABCB1. MARCH8, a member of the membrane-associated RING-CH (MARCH) protein family, is a transmembrane E3 ligase that targets glycoproteins for lysosomal destruction [58], whose downregulation modulates the drug sensitivity of breast cancer cells [59]. Mechanistically, MARCH8 can interact with ABCB1 and promote its ubiquitination and degradation, thereby antagonizing ABCB1-mediated drug efflux [60].
In addition, DUBs are also found to participate in drug resistance by regulating the ABC transporter directly or indirectly. For example, USP24 positively regulates drug resistance partially by stabilizing ABC transporters, including ABCB1, ABCG1, and ABCC1, to pump out drugs from cancer cells [61]. USP9X upregulates the expression of ABCB1 and MRP2 by stabilizing β-catenin, thus affecting cisplatin resistance in nasopharyngeal carcinoma (NPC) cells [62].
ABCC1
The first identified ABCC family protein, ABCC1, is regulated by E3s and DUBs. Neural precursor cell expressed developmentally downregulated gene 4-like (NEDD4L) is predicted as an E3 ligase to regulate the ubiquitination of ABCC1 in lung squamous cell carcinoma (LSCC) [63]. Additionally, ABCC1 is found to be indirectly upregulated by the silenced CUL3 KEAP1 E3 ligase in breast cancer, stabilizing nuclear factor erythroid 2-related factor 2 (NRF2), and subsequently increases NRF2-regulated ABCC1 transcription, which exhibits resistance to both doxorubicin and paclitaxel [64]. Likewise, inactivated ubiquitin E3 ligase Trc8 accounts for a lower ubiquitination rate of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCoAR) and higher cholesterol synthesis in the plasma membrane, which favors the activity of ABC transporters and limits the intracellular accumulation of chemotherapeutic drugs [65].
Notably, upregulation of USP22 increases ABCC1 expression and subsequently contributes to sorafenib resistance in hepatocellular carcinoma (HCC) cells, suggesting that USP22 may serve as a therapeutic target for surmounting sorafenib resistance [66]. Another study revealed that sorafenib and USP22 shRNA co-delivery system exhibits remarkedly strong antitumor efficiency by suppressing ABCC1 in the process of synergetic HCC therapy [67]. Moreover, USP22 directly interacts with sirtuin 1 (SIRT1) and positively regulates SIRT1 protein expression, leading to activation of the SIRT1/AKT/ABCC1 pathway and MDR of hepatocellular carcinoma [68]. USP22 is also found to indirectly promote the expression of MDR-related genes through upregulation of AKT and subsequently activation of the PI3K pathway in HCC [69].
ABCG2
In the case of ABCG2, a recent study revealed that RNF180 increases the sensitivity of triple-negative breast cancer cells to gefitinib by degrading RAD51 and downregulation of efflux transporters, including ABCG2, ABCC1, and ABCB1 [70]. USP24 increases the levels of ABC transporters ABCB1, ABCG2, and ezrin to enhance the pumping of taxol from cancer cells. Thus, employing NC167739, a USP24 inhibitor, can effectively block drug resistance during chemotherapy [61]. Ubiquitin C-terminal hydrolase L3 (UCHL3) stabilizes aryl hydrocarbon receptor (AhR) by its deubiquitylation and subsequently increases ABCG2 to promote stem-like properties in lung cancer [71].
Drug metabolism and protein degradation
Drug inactivation is another upstream resistance mechanism [72] that involves two distinctive pathways, including Phase I metabolism (oxidation, reduction, and hydrolysis reactions) and Phase II metabolism (binding reactions) [73]. Notably, the key metabolic enzymes responsible for Phase I metabolism are cytochrome P450 enzymes (CYP450), such as the CYP3A4, CYP1A1, CYP1A2, and CYP2 families [74]. The main enzymes involved in Phase II metabolism include UDP-sulfotransferases (SULTs), glucuronosyltransferases (UGTs), glutathione S-transferases (GSTs), N-acetyltransferases (NATs), and various methyltransferases (MTs) [75]. Recent studies indicate that targeted protein degradation participates in the modulation of drug metabolism, thereby affecting cancer drug resistance (Fig. 3).
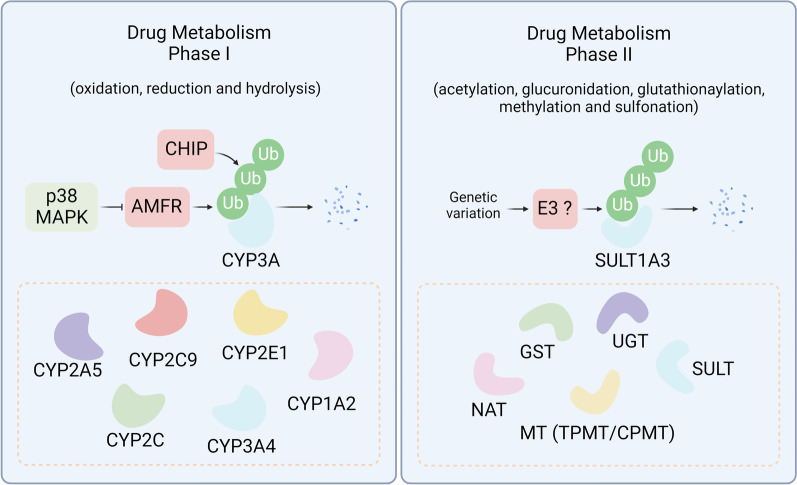
Fig. 3.
Protein degradation modulates drug metabolism. Drug metabolism can be categorized into Phase I (oxidation, reduction, and hydrolysis) and Phase II (posttranslational modification), requiring different classes of drug metabolic enzymes. In Phase I drug metabolism, CHIP and AMFR can ubiquitinate the CYP3A family for degradation. In Phase II, SULT1A3 is regulated by ubiquitin-dependent protein degradation
E3 ligase autocrine motility factor receptor (AMFR, or GP78) plays a pivotal role in metastasis, tumor progression, and recurrence [76]. Liver-specific genetic ablation of AMFR results in concurrent stabilization of several therapeutically relevant P450s by inhibiting endoplasmic reticulum-associated degradation (ERAD), including CYP2A5, CYP2C, CYP3A, and CYP2E1, which leads to corresponding enhancement of their drug-metabolizing capacities [77]. CYP3A4 is the most abundant P450 in the human liver and is responsible for the metabolism of over 50% of clinically relevant drugs [78]. Both UBC7/gp78 and UbcH5a/C terminuses of HSC70-interacting protein (CHIP) are involved in CYP3A4 ERAD, suggesting that ERAD-associated E3 ligases may influence drug metabolism by regulating the physiological CYP3A expression and its function [79, 80]. Intriguingly, endoplasmic reticulum-related protein quality control can be either proteasome or lysosome-dependent, where the regulatory mechanisms still need further elucidation [81]. Another study indicates that acetaminophen and salicylate derivatives could decrease the expression of AMFR protein through p38 MAPK activation, resulting in the inhibition of CYP3A protein degradation and a subsequent increase in enzymatic activity [82]. In addition, the detoxification-related enzyme sulfotransferase 1A3 (SULT1A3) is found to be degraded much more rapidly by a ubiquitin–proteasome system-dependent process in case of genetic variation [83].
Degradation of critical proteins in DNA damage repair-mediated drug resistance
The anticancer activity of most chemotherapy drugs relies on the induction of DNA damage in tumor cells with defective DNA repair, either directly or indirectly [84, 85]. For example, platinum-based drugs are especially used for testicular cancer, which directly form DNA adducts and lead to intrastrand and interstrand cross-links [86]. Anthracyclines induce DNA damage by inhibiting DNA topoisomerases and producing oxygen radicals indirectly [87]. Therefore, cancer cells may achieve drug resistance to DNA-damaging reagents through cell cycle arrest or DNA damage repair [88, 89]. The DNA damage response (DDR) system involves several stages, including damage sensing, signaling transduction, and damage repair [90]. Growing evidence suggests that DUB and E3 dysregulation promotes cancer cell resistance to DNA-damaging agents by mediating the ubiquitination of many proteins involved in DNA repair-associated pathways (Fig. 4).
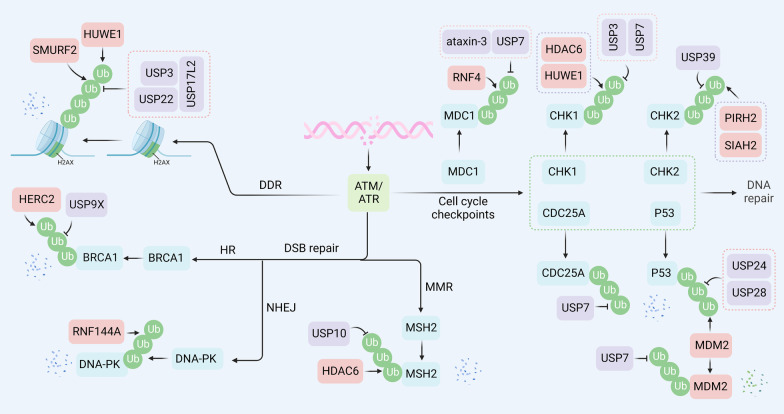
Fig. 4.
Regulatory functions of ubiquitination in DNA repair. E3 ligases usually participate in DNA damage response and DNA double-strand repair through degradation-dependent function. Specifically, during DNA damage response, H2AX can be degraded through SMURF2 and HUWEI-mediated ubiquitination, and USP3, USP22, and USP17L2 can eliminate the ubiquitin chains. During homologous recombination, BRCA1 can be degraded by HERC2 and stabilized by USP9X. In the NHEJ process, XRCC4 is ubiquitinated by FBXW7 for degradation. DNA-PK is degraded through RNF144A-mediated ubiquitination. During mismatch repair, HDAC6 ubiquitinates MSH2 for further protein degradation, which can be reversed by USP10. In the regulation of cell cycle check points, RNF4 promotes ubiquitination of MDC1, and ataxin-3 and USP7 deubiquitinate MDC1, therefore controlling the stability of MDC1. MDM2 facilitates the ubiquitinoylation of p53 for degradation, while USP4, USP24, USP7, and USP28 remove the ubiquitin chain. USP7 stabilizes CDC25A. PIRH2 and SIAH2 promote ubiquitin-dependent degradation of CHK2, and USP39 eliminates the ubiquitin chains. HDAC6 and HUWEI induce CHK1 ubiquitination, while USP3 and USP7 deubiquitinate the CHK1
DNA damage response
H2AX is a variant of histone H2A. Phosphorylation of H2AX at Ser139 (γH2AX) and subsequent ubiquitination of H2AX play a critical role in DNA damage repair, which promotes chromosome remodeling and recruitment of DNA repair enzymes [91].
The ubiquitin ligase RNF8 initiates the ubiquitination of H2AX, and RNF168 amplifies the ubiquitination response, which triggers the recruitment of p53-binding protein 1 (53BP1) and Rap80/BRCA1 to the lesion [92, 93]. RNF168 also interacts with another E3 ubiquitin ligase, SMURF2, to modulate the stability of H2AX in glioblastoma cells. Mechanistically, suppression of protein arginine methyltransferase 5 (PRMT5) attenuates the expression of RNF168 in methylthioadenosine phosphorylase (MTAP)-deficient glioblastoma cells, contributing to the destabilization of H2AX by SMURF2. These findings reveal that RNF168 and SMURF2 serve as stabilizers and destabilizers of H2AX, respectively, via the PRMT5-RNF168-SMURF2 signaling cascade [94]. In addition, RNF168 was found to mediate H2AX polyubiquitination and degradation under chronic oxidative stress, which provides new insights into the ROS-mediated regulation of H2AX turnover and is indicative of the therapeutic efficiency and survival of triple-negative breast cancer (TNBC) patients [95]. Moreover, H2AX can also be degraded via polyubiquitination mediated by HUWE1. ATM kinase, the sirtuin protein 6 (SIRT6), and the chromatin remodeler SNF2H mediate transient H2AX stabilization by blocking HUWE1 to allow γH2AX foci formation when cells are damaged [96].
Given the importance of H2AX ubiquitination in triggering the DDR, several hydrolases have been shown to regulate the ubiquitination cascade directly or indirectly. Ubiquitin-specific peptidase 22 (USP22) deubiquitinates and phosphorylates H2AX to enhance DNA damage repair and induce cisplatin resistance in lung adenocarcinoma [97]. Ubiquitin hydrolase Dub3, also known as USP17L2, counteracts the H2AX E3 ligases RNF8 and RNF168, resulting in decreases in the DNA damage-induced monoubiquitination of H2AX. Importantly, Dub3 overexpression abrogates focus formation of 53BP1 and breast cancer type 1 susceptibility protein (BRCA1) in response to genotoxic stress [98]. Similarly, USP3 removes Ub at lysine 13 and 15 of H2A and γH2AX, as well as 118 and 119 of H2AX in response to DNA damage, counteracting RNF168- and RNF8-mediated ubiquitination and impairing the accumulation of the downstream repair factors BRCA1 and 53BP1 at the damage sites [99].
Altogether, these results indicate that regulating E3s or DUBs might be a mechanism to correct DDR and to allow checkpoint recovery.
Cell cycle checkpoints
Mediator of DNA damage checkpoint protein 1 (MDC1) is a vital modular phosphoprotein in controlling proper DDR and maintaining genomic stability, which serves as a scaffold to promote the localization of various DDR components to DNA double-strand break (DSB) sites [100]. The deubiquitinating enzyme ubiquitin-specific protease 7 (USP7), also known as HAUSP, has been identified as an oncogene with essential roles in tumorigenesis and therapeutic resistance for a number of cancer types. Notably, overexpressed USP7 was positively correlated with the expression of MDC1 and worse survival for patients with cervical cancer. Significantly, USP7 physically interacted with and modulated the stabilization of MDC1, thereby sustaining the DDR and conferring cellular resistance to genotoxic insults [101]. Checkpoint kinase 1 (CHK1) and checkpoint kinase 2 (CHK2) are well-established signal transducers for checkpoint-mediated cell cycle arrest and activation of DNA repair in response to DNA damage or unreplicated DNA [102, 103]. USP7 interacted with, and stabilized CHK1 protein levels and functions through K48 deubiquitylation in acute myeloid leukemia (AML) [104]. Cell division cycle 25 A (CDC25A) is a highly conserved, dual-specificity phosphatase that regulates the cyclin-dependent kinases (CDKs) [105]. USP7 was also found to extend the half-life of CDC25A by circumventing turnover, which stabilized CDC25A and enhanced resistance to DNA-damaging agents in cervical cancer [106]. P53 is the most frequently mutated tumor suppressor in human cancer [107]. USP7 is known to stabilize the oncogenic E3 ubiquitin ligase mouse double minute 2 homolog (MDM2) that promotes the proteasomal degradation of p53 [108]. A recent study revealed that the USP7-degrading proteolysis targeting chimera (PROTAC) maintained potent cell growth inhibition in p53 mutant cancer cells, suggesting USP7-PROTAC as a promising method for potential p53 mutant cancer therapy [109]. Polo-like kinase 1 (PLK1) is a master mitotic regulator regulating DNA damage, the G2/M checkpoint, cell death pathways, and replication stress response [110]. USP7 also regulates spindle checkpoints by sustaining the protein stability of PLKI via the C233 site. Knockdown of either USP7 or PLK1 induces cell apoptosis and cell cycle G2/M arrest, resulting in reduced taxane resistance [111]. In summary, these data indicate that USP7 is of potential to be a marker and therapeutic target in overcoming resistance to treatment.
Except for USP7, which modulates the stability of p53, other types of DUBs also participate in controlling the ubiquitination of p53 and its subsequent degradation. For example, functional USP24 is required for p53 activation/stabilization upon DNA damage. Importantly, USP24 depletion inhibits p53 upregulated modulator of apoptosis (PUMA) activation and poly (ADP-ribose) polymerases (PARP) cleavage in response to ultraviolet (UV) damage, which renders cells resistant to apoptosis in a p53-dependent manner [112]. Similarly, USP28 can be cleaved and inactivated by caspase-8, preventing USP28 from deubiquitinating and stabilizing p53, thereby overriding the p53-dependent G2/M cell cycle checkpoint and eventually leading to treatment resistance [113]. Additionally, USP39 deubiquitinates and stabilizes CHK2 in lung cancer, and the knockdown of USP39 compromises G2/M checkpoint, thereby conferring decreased apoptosis and resistance to chemotherapy and radiotherapy [114]. Moreover, increased USP3 transcriptionally modulated by Smoothened (Smo) reduces Claspin polyubiquitination and proteasomal degradation, leading to activation of Claspin-dependent ATR-CHK1 signaling and radiation resistance of glioblastoma (GBM) [115].
Notably, E3s are implicated in the modulation of cell cycle checkpoints in response to DNA damage. For example, MDC1, an essential protein that triggers the DNA damage response, can be ubiquitinated and subsequent degraded by RNF4, which can be reversed by deubiquitinating enzyme ATX3 [116]. HUWE1 is a prominent CHK1 E3 ubiquitin ligase. HUWE1 knockdown results in stabilization of CHK1 and the regulation of the DDR signaling pathway [117]. PIRH2 and SIAH2 have also been shown to regulate the stability of CHK2, with significant consequences on cell cycle control involved in DDR [118, 119].
Additionally, E3 ubiquitin ligases regulate the expression levels and activities of p53 in response to exogenous and endogenous stresses. MDM2, a master regulator of p53, destabilizes p53 and causes cisplatin resistance in multiple cancers, including melanoma, ovarian, and lung cancer [120–122]. Moreover, microrchidia family CW-type zinc finger 2 (MORC2) is characterized as an oncogenic protein that facilitates DNA repair through a PARP-1-dependent mechanism [123]. Protein kinase cAMP-activated catalytic subunit alpha (PRKACA)-mediated phosphorylation of MORC2 on T582 abrogates CMA-mediated degradation of MORC2, leading to endocrine resistance of breast cancer cells [124].
Double-strand break repair
DNA double-strand breaks (DSBs) are considered one of the most lethal forms of DNA damage [125]. Homologous recombination (HR) and nonhomologous end-joining (NHEJ) are two major approaches to the repair of DSBs [126]. HR repair of DSBs requires homologous DNA as a template. In this process, the tumor suppressors BRCA1, BRCA2, BARD1, and RAD51 are indispensable for HR execution [127]. In NHEJ, DSBs are first recognized by the Ku70-Ku80 heterodimer (Ku). They then recruit other NHEJ proteins to the DNA ends, including DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and DNA ligase 4 (LIG4) [128]. NHEJ is the predominant pathway for the repair of DSBs in all mammalian cells [129]. However, the HR mechanism is either absent or impaired in BRCA1/2 mutated cells [130]. In this case, the PARP enzymes are activated and serve as a complementary approach for DNA repair. Thus, PARP inhibitors (PARPi) are effective targeting agents by competitively inhibiting the activity of PARP in cancers with BRCA1/BRCA2 mutation [131].
Remarkably, several regulators have been shown to promote drug resistance by targeting homologous recombination. For example, HERC2 has been identified as the specific E3 ligase of BRCA1 for degradation [132]. By contrast, abnormal spindle-like microcephaly-associated protein (ASPM) can promote homologous recombination by safeguarding BRCA1 stability by preventing its E3 ligase HERC2 from accessing BRCA1 under UV-induced DNA damage [133]. Correspondingly, the deubiquitinating enzyme USP9X stabilizes BRCA1 from degradation and promotes resistance to DNA-damaging agents in various types of cancer cells [134]. c-Jun N-terminal kinase 1/2 (JNK1/2)-mediates PARP-1 phosphorylation and subsequent degradation, while mitogen-activated protein kinase phosphatase-1 (MKP-1) inhibits the activity of JNK1/2 to suppress PARP-1 degradation in cisplatin-resistant ovarian cancer [135]. However, the E3 ligase of PARP-1 and the regulatory mechanism still need further investigation. Consistently, DOC-2/DAB2 interactive protein (DAB2IP) can form a complex with PARP-1 and its identified E3 ligase in renal cell carcinoma (e.g., RanBP2, TRIP12, RNF40, and VHL), leading to PARP-1 degradation. DAB2IP deficiency facilitates ionizing radiation resistance [136]. In addition to BRCA1 and PARP, several other proteins also participate in the regulation. For instance, Kelch-like ECH-associated protein 1 (KEAP1) mediates EMSY degradation, leading to enhanced homologous recombination and PARPi resistance in lung cancer [137]. In addition, exonuclease 1 (EXO1) is responsible for 3' ssDNA formation of DSB end resection, whose degradation mediated by RING-box protein 1 (RBX1) attenuates the homologous recombination pathway [138]. The RNA methyltransferase TRDMT1 has been newly identified to facilitate homologous recombination, the degradation of which by TRIM28-mediated ubiquitination results in sensitizing ovarian cancer to platinum treatment [139]. In addition to homologous combination, several E3 ligases participate in the regulation of NHEJ. RNF144A induces degradation of cytoplasmic DNA-PKcs, which is attributed to impaired NHEJ and increased sensitivity to DNA-damaging agents [140]. FBXW7 facilitates NHEJ in a degradation-independent manner via K63-linked polyubiquitination of X-ray repair cross-complementing protein 4 (XRCC4) at lysine 286, implying that inactivating FBXW7 could be a potential strategy for improving the efficacy of radiotherapy in human cancers [141].
Mismatch repair
DNA mismatch repair (MMR) is an evolutionarily conserved process that corrects base–base mismatches and insertion/deletion generated during DNA replication and recombination [142, 143]. MutS protein homolog 2 (MSH2) is the main component of the MMR system, which binds to DNA mismatches, thereby triggering cell cycle arrest or apoptosis [144]. Recent advances provide insight into the protein level of MSH2 modulated by DUBs or E3s, as well as its profound influence on MMR and treatment tolerance [145]. Previous studies have shown that HDAC6 has E3 ligase activity to promote the degradation of MSH2, leading to reduced cellular sensitivity to DNA-damaging agents [146]. In addition, DUBs have also been found to regulate MSH2. Notably, USP10 stabilizes MSH2 and reverses resistance to DNA-methylating agents and antimetabolite 6-thioguanine (6-TG) in lung cancer cells [147]. Likewise, the association and clinical implication of USP10 and MSH2 proteins are further confirmed in NSCLC tissues, indicating that USP10 stabilizes MSH2 in patients with lung cancer [148].
Taken together, a wide range of DUBs and E3s play crucial roles in regulating DNA repair by regulating DDR, cell cycle checkpoints, DSB repair, and MMR. DUB or E3 inhibitors are potential therapeutic agents for overcoming drug resistance.
Regulation of targeted therapy through modulating protein degradation
Drug efficacy of targeted therapy is largely determined by alterations of the drug targets, such as protein levels [149, 150]. Alteration of common oncogenic proteins, such as epidermal growth factor receptor (EGFR), KRAS, BRAF, and c-Myc, drives tumorigenesis across 38 cancer types [151, 152]. Multiple evidence shows that several DUBs and E3s are correlated with resistance to targeted therapy.
EGFR
EGFR (also known as ERBB1 or HER1) is a member of the ERBB family of cell surface receptor tyrosine kinases [153]. The binding of ligands triggers receptor dimerization, tyrosine phosphorylation, and activation of downstream signaling pathways, including RAS-mitogen‑activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)-AKT, and Janus kinase (JAK)-signal transducer and activator of transcription (STAT), leading to cell proliferation [154]. Following interaction with ligands, EGFR is activated and subsequently internalized into endosomes, where it can be either recycled to the membrane surface or degraded through K63-ubiquitin chain-mediated lysosomal degradation [155]. In addition, the proteasomal degradation pathway also participates in the degradation control of EGFR [156]. Despite numerous EGFR tyrosine kinase inhibitors (TKIs) exhibiting marked efficacy, resistance to these agents remains an unsolved fundamental challenge, where dysregulation of EGFR degradation may be one of the pivotal reasons [11].
Studies have revealed that E3s are vital novel players in the regulation of proteins implicated in EGFR-TKI resistance. For instance, casitas b-lineage lymphoma-b (Cbl-b) increased the sensitivity of gastric cancer cells to cetuximab at least partially by affecting EGFR expression [157]. Cbl-b overexpression partially reversed drug resistance to icotinib in EGFR-mutant NSCLC cells [158]. In human pancreatic cancer, low expression of Cbl conferred chemoresistance via stress-induced EGFR activation [159]. Furthermore, Cbl-b was expressed at a low level in MDR gastric and breast cancer cells compared with their parental cells [160]. A recent study provides a more detailed possible mechanism through which the membrane protein sarcoglycan epsilon (SGCE) stabilizes EGFR for breast cancer stem cell (BCSC) maintenance by disrupting the interaction between EGFR and its E3 ligase c-Cbl [161]. Likewise, the cell growth regulator with RING finger domain protein 1 (CGRRF1) functioned as a tumor suppressor and identified EGFR as its target in breast cancer [162]. Suppressor of cytokine signaling 5 (SOCS5), with E3 ubiquitin ligase activity, led to a marked reduction in EGFR expression levels by promoting ubiquitin-mediated EGFR degradation [163]. Furthermore, the combination of JAK and EGFR inhibitors overcomes acquired resistance to EGFR-TKIs since JAK2 inhibition uncouples the role of SOCS5 in the negative regulation of EGFR [164]. Additionally, RNF126, Rabring7, breast cancer-associated gene 2 (BCA2), and ZNRF1 are involved in the endocytosis of EGFR through their E3 ligase activity [165–167]. In-depth studies on E3-mediated alterations in EGFR may deepen our understanding of the development of potential strategies to reverse EGFR-TKI tolerance.
Apart from the downregulation of E3 ligases, DUBs also play an essential role in EGFR alteration. STAMBP (also known as AMSH) is a deubiquitinating enzyme of the Jab/MPN metalloenzyme family. High STAMBP levels predict poor survival in lung adenocarcinoma (LUAD) patients. Importantly, increased STAMBP promoted the stabilization of EGFR, suggesting STAMBP as a novel target for LUAD therapy [168]. Ubiquitin-specific protease 22 (USP22) has been shown to prevent ubiquitination-mediated EGFR degradation and activate multiple EGFR downstream signaling pathways, thus conferring resistance to EGFR-TKIs in mutant lung adenocarcinoma cells [169]. Moreover, ubiquitin carboxy terminal hydrolase-L1 (UCHL1) is involved in regulating the degradation of EGFR and promoting malignant properties in drug-resistant breast cancer, where the specific molecular mechanism needs further research [170]. Another independent study revealed that OTU domain-containing protein 7B (OTUD7B) served as a downstream gene of linc00976, deubiquitinated EGFR, and activated the MAPK signaling pathway. Thus, the linc00976/miR-137/OTUD7B/EGFR axis may act as a potential therapeutic target for pancreatic cancer [171]. Intriguingly, USP13 stabilizes mutant EGFR in a peptidase-independent manner by interacting with the EGFR-Cbl-b complex to protect EGFR from lysosomal degradation [172]. Hence, the combination of USP13 inhibitor spautin-1 with EGFR inhibitors shows a potent antitumor effect in vivo [173]. Overall, DUBs are intimately related to modulating the stability of EGFR, thus conferring resistance to EGFR-TKIs in cancer cells. Targeting these DUBs may present a promising strategy to reverse therapeutic resistance and achieve better clinical outcomes for EFGR-mutant patients.
KRAS
Kirsten rat sarcoma (KRAS) is a small membrane-bound GTPase (guanosine triphosphate hydrolase) that primarily binds to guanosine diphosphate (GDP) and is inactive [174]. Upon GDP to GTP exchange, usually in response to growth factors, KRAS cycles to an activated state, allowing multiple downstream signaling pathways to be activated, including the MAPK and PI3K pathways [175]. KRAS mutations are among the most common drivers of human cancer and are associated with tumorigenesis and poor prognosis [176]. Over the past years, despite inhibitors having shown remarkable clinical responses in KRAS-mutant cancers, resistance to KRAS inhibitors has eventually developed [177]. Thus, further understanding the mechanisms of drug resistance to KRAS-mutant inhibitors is indispensable. E3 ligase β-transducin repeat-containing protein (β-TrCP) facilitates simultaneous ubiquitination and degradation of KRAS, and intriguingly, the stability of β-TrCP is regulated by a critical E3:E2 complex composed of Smad ubiquitination regulatory factor 2 (SMURF2) and UBCH5 [178]. In addition, E3 ligases anaphase-promoting complex subunit 2 (ANAPC2) promotes KRAS degradation through the ubiquitin–proteasome pathway in colorectal cancer [179]. Leucine zipper-like transcriptional regulator 1 (LZTR1) interacts with the CUL3-based E3 ubiquitin ligase complex, which is reported to participate in KRAS ubiquitination [180]. Mechanistically, LZTR1 ubiquitinates KRAS with K48 and K63 chains, indicating that the degradation of KRAS depends on both proteasomal and lysosomal degradation, with proteasomal degradation playing a dominant role [181]. Taken together, the identification of E3s affords fascinating potential targets to reverse drug target-associated resistance owing to their biological activity and druggability.
BRAF
BRAF, a member of the RAF gene family, encodes a serine–threonine protein kinase that is an upstream factor of the MAPK pathway [182, 183]. Frequent mutations of BRAF (most commonly the V600E mutation) substantially increase kinase activity to drive the proliferation of cancer cells [184]. Therefore, several BRAF inhibitors have been developed and evaluated clinically for BRAF-mutated patients [185, 186]. Unfortunately, the emergence of acquired resistance to BRAF inhibitors (BRAFi) was inevitable and is one of the main reasons for therapy failure in BRAF-mutant cancers.
FBXW7 (also known as CDC4) is a component of the S-phase kinase-associated protein 1 (SKP1)/CUL1/F-box (SCF) E3 ubiquitin ligase complex, which functions as a tumor suppressor which is frequently altered in cancer [187, 188]. It has been reported that loss of FBXW7 in the presence of the BRAFV600E mutation is consequential and sufficient to drive tumorigenesis in mouse models [189]. Additionally, a high-throughput reversed-phase protein array revealed BRAF as a novel target of FBXW7 in adult T cell leukemia cells. Further experiments showed that mutations in FBXW7 prevent the degradation of BRAF-conferred resistance to bromodomain and extra-terminal (BET) inhibitors [190]. Intriguingly, it has been reported that loss of the deubiquitinating enzyme USP28 can destabilize its substrate FBXW7, leading to BRAF stabilization and eventually resistance to BRAF inhibitor therapy in both in vitro and in vivo models [191]. In addition, another E3 ligase, RNF149 was identified as a wild-type BRAF-interacting protein, which induced ubiquitination of wild-type BRAF and promoted its proteasomal degradation. However, RNF149 cannot bind to mutant BRAF, which may partially explain the abnormal activation of BRAF downstream pathways in BRAF-mutant patients [192].
Increasing evidence indicates that DUBs play essential roles in acquired resistance to BRAF inhibitors. USP14 participates in the resistance to BRAF inhibitors in melanoma cells. At the molecular level, targeting USP14 in melanoma increases oxidative and proteotoxic stress and subsequently triggers ROS-dependent and caspase-independent cell death that overcomes resistance to BRAF inhibitors [193]. Moreover, USP5 deprivation reversed resistance to vemurafenib and sensitized BRAF-mutant melanoma cells to apoptosis initiated by BRAF inhibitors. Thus, targeting USP5 offers a potential therapeutic strategy for BRAF inhibitor-resistant melanoma [194]. Together, unveiling the determinants of DUB and E3 functions in modulating acquired resistance to BRAFi may facilitate the development of new strategies to sensitize cancer cells to BRAFi.
c-Myc
c-Myc, a pleiotropic transcription factor, is considered one of the essential drivers in various types of tumors by controlling global gene expression including cell cycle, proliferation, differentiation, metabolism, and apoptosis [195]. Although it has excellent potential as a therapeutic target, c-Myc has long been considered untargetable due to its protein structure [196]. Recent studies revealed that E3 ligase MAGI3 promotes ubiquitination and degradation of c-Myc in colorectal cancer cells, whose expression can act as a predictor for chemotherapy response and patient survival [197]. Similarly, E3 ligase FBW7α inhibits the proliferation and progress of cholangiocarcinoma and hepatocellular carcinoma by downregulating c-Myc [198, 199]. In addition, deubiquitinating enzymes USP22 [200], USP28 [201], and OTUD6A [202] stabilize c-Myc and promote tumorigenesis in breast cancer, squamous cell lung carcinoma, and prostate cancer, respectively. Hence, regulating the expression levels of specific E3s and DUBs may provide a more convenient and effective clinical treatment method for targeting c-Myc.
Downstream adaptive responses mediated by protein degradation
When therapeutic drugs successfully bind to the effectors, pro-death signaling pathways are usually activated (chemotherapy) or pro-survival signaling pathways inhibited (targeted therapy) [203]. However, during tumor treatment, drug-resistant tumor cells are often able to evade cell death by upregulating anti-apoptotic proteins and reactivating pro-survival signaling pathways through the bypass mechanisms (Fig. 5).
Fig. 5.
Protein degradation regulates the efficacy of targeted therapy and chemotherapy. SOCS5, CGRRF1, and Cbl-b promote ubiquitination and subsequent endocytosis of the EGFR, while UPS22, STAMBP, UCHL1, and OTUD7B deubiquitinate EGFR. E3 ligases FBXW7 and RNF149 facilitate ubiquitination of BRAF. USP28 regulates the stability of BRAF by deubiquitinating FBXW7. ANAPC2, β-TrCP, and LZTR1/CUL3 complexes promote KRAS ubiquitination for degradation. E3 ligase TRIM15 and CYLD regulate the ubiquitination and deubiquitylation of ERK, respectively. E3 ligase MAGI3 and FBW7α facilitate the ubiquitination of c-Myc, and USP22, USP28, and OTUD6A stabilize c-Myc. Anti-apoptosis protein FLIP can be stabilized through USP2-mediated deubiquitylation. BCL-2 is ubiquitinated by AMFR and CHIP. MCL-1 can be ubiquitinated by HUWEI and deubiquitylated by USP9X, USP13, or USP17
Evasion of apoptosis
Apoptosis is one of the important mechanisms for first-line chemotherapeutic drugs (e.g., cisplatin, paclitaxel) in tumor therapy and is triggered by drug-mediated DNA damage, cellular stress, and immune response [204]. Selective protein degradation may promote drug resistance through the downregulation of pro-apoptotic proteins and upregulation of anti-apoptotic proteins, including B cell lymphoma 2 (BCL-2) and FLICE-inhibitory protein (FLIP). For example, USP17 stabilizes MCL-1, a BCL-2 family protein, through deubiquitylation, leading to chemoresistance in ovarian cancer [205]. Consistently, USP9x and USP13 also promote drug resistance by stabilizing MCL-1 [206, 207]. USP9x is also found to deubiquitinate and stabilize pre-B-cell leukemia homeobox-1 (PBX1) in advanced prostate cancer (PCa), thereby abrogating apoptosis and conferring chemoresistance to doxorubicin or cisplatin [208]. In addition, USP2 promotes sorafenib resistance by deubiquitinating cFLIP in hepatocellular carcinoma [209]. However, some DUBs may induce drug resistance by regulating pro-apoptotic proteins. For instance, downregulated USP15 leads to imatinib resistance by regulating the degradation of caspase-6 in myeloid leukemia cells [210], whereas blocking the DUB activity of Rpn11 activates caspase cascade and triggers apoptosis, providing the rationale for overcoming bortezomib resistance in multiple myeloma (MM) [211].
Apart from DUBs, E3 ligases also regulate the degradation of apoptosis-related proteins. The expression of the E3 ligase AMFR has been validated to be negatively correlated with the expression of the anti-apoptotic protein BCL-2 [212]. CHIP is a chaperone-dependent and U-box-containing E3 ligase that negatively correlates with breast cancer clinicopathological stages and overall survival [213]. CHIP acts as a capacitor of heterogeneous BCL-2 expression levels and prevents an increase in the anticancer drug-resistant population in breast cancer cells [214]. Moreover, cullin-RING ubiquitin ligases 4 (CRL4) E3 ligase has been reported to be upregulated and is associated with poor prognosis in ovarian cancer patients. Mechanistically, CRL4A-DDB1 stimulates STAT3 activation by degrading STAT1, leading to increased expression of Baculoviral IAP repeat containing 3 (BIRC3), one of the inhibitors of apoptosis proteins (IAPs), eventually resulting in chemoresistance against cisplatin [215]. In the intrinsic apoptotic pathway, intracellular apoptotic stimulations promote the release of cytochrome c from mitochondria, triggering activation of apoptotic protease activating factor 1 (APAF-1) and eventually activating caspase-9 apoptosome [216]. In breast cancer cells that acquired resistance to lapatinib, MDM2 ubiquitinates another ubiquitin E3 ligase, HUWE1, which indirectly inhibits apoptosome activation by preventing HUWE1 from ubiquitinating MCL-1 [217]. Furthermore, high-throughput screening revealed that RING finger containing E3 ligase SIAH2 and the signaling platform molecule SH3 domain containing RING finger 1 (SH3RF1) confer robust caspase-8 activation and suggest targeting the interaction of these two E3 ligases is a promising novel cancer therapeutic strategy [218].
Intriguingly, chaperone-mediated autophagy is reported to participate in the regulation of apoptosis. Inhibition of CMA through LAMP2A knockdown increases the expression of p53 and Bax, sensitizing the cisplatin resistance of lung cancer cells [219].
Oncogenic bypass signaling
Although targeted therapy has been developed to block specific signaling pathways in tumor progression, drug resistance still occurs due to the activation of oncogenic bypass signaling pathways (e.g., the MAPK pathway) [176, 220]. The MAPK pathway is one of the most important signaling pathways involved in the reactivation of downstream oncogenic transfactors [221]. As a USP class of cysteine proteases, cylindromatosis-associated DUB (CYLD) is identified as a tumor suppressor because its expression is inversely correlated with overall and progression-free survival. Of interest, CYLD and the E3 ligase of the extracellular signal-regulated kinase (ERK), tripartite motif-containing protein 15 (TRIM15), have been shown to participate in regulating ERK activation via the modulation of its lysine-63-linked polyubiquitination, which is important for the survival of therapeutic-resistant melanoma [222]. Cytokines activate ITCH to maintain BRAF activity and promote the tumorigenicity of melanoma cells. Mechanistically, ITCH-mediated lysine 27-linked ubiquitination of BRAF recruits protein phosphatase 2A (PP2A) to antagonize S365 phosphorylation and disrupt the inhibitory interaction with 14–3–3, leading to sustained activation of BRAF downstream MEK/ERK signaling [223]. However, whether cytokines confer resistance to BRAF inhibitors in such an ITCH-dependent manner needs further elucidation.
Recently, overexpression of yes-associated protein (YAP) was found to be associated with resistance to BRAF inhibitors in melanoma. A recent study demonstrated that ubiquitin-specific peptidase 22 (USP22) interacted with and stabilized YAP, which in turn conferred resistance to the BRAF inhibitor vemurafenib and provided new therapeutic avenues to target USP22/YAP as an option for melanoma treatment [224]. RNF44 was proposed to promote AMP-activated protein kinase α1 (AMPK-α1) degradation and consequently regulate autophagy and metabolic reprogramming in BRAFi-resistant melanoma cells [225]. Microtubule-associated serine/threonine kinase 1 (MAST1) is the main driver of drug resistance through rewiring RAF-independent MEK activation [226]. The E3 ligase CHIP can promote MAST1 degradation by ubiquitination on Lys 317 and Lys 545, which can be abrogated by HSP90B-mediated protection of ubiquitinylating sites [227]. Therefore, targeting HSP90B can predominantly sensitize cancer cells to cisplatin through MAST1 destabilization.
Cancer cell stemness and protein degradation
Cancer stem cells (CSCs) are a small subpopulation of tumor cells capable of self-renewal, enabling drug tolerance [228]. CSCs can be initialized through epithelial-to-mesenchymal transition (EMT) [229] and sustained by stemness-related pathways (e.g., the Notch pathway, Wnt pathway, and Hedgehog and Hippo pathways) [230]. Recent studies indicate that the molecular mechanisms of protein degradation regulate EMT- and stemness-related pathways (Fig. 6).
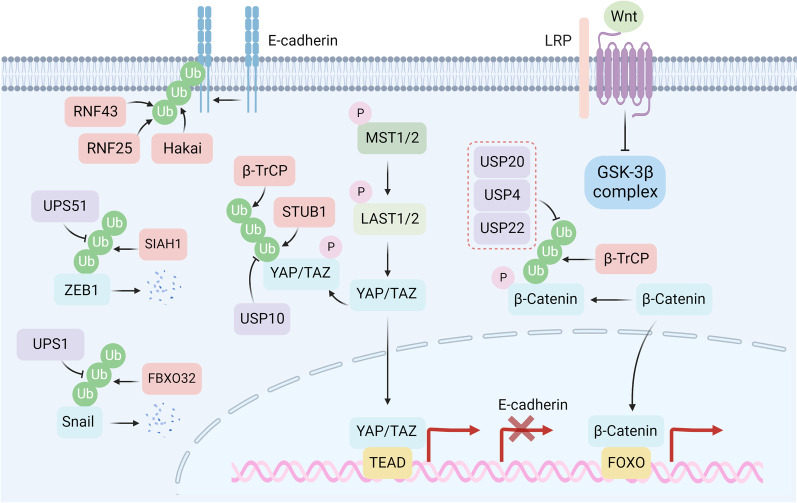
Fig. 6.
Protein degradation involved in EMT and cell stemness. Degradation of E-cadherin is regulated by E3 ligases RNF25, RNF43, and Hakai. Protein degradation of mesenchymal marker ZEB1 is mediated by E3 ligase SIAH1 and can be reversed by USP51. Snail is ubiquitinated by FBXO32 and deubiquitinated by USP1. For stemness sustaining pathways, β-TrCP participates in the ubiquitination of both YAP and β-catenin. In addition, STUB1 also facilitates the ubiquitination of YAP. USP10 facilitates deubiquitylation of YAP, and USP20, USP4, and USP22 deubiquitinate β-catenin
Cancer cells that undergo the EMT process may gain stem-like properties and a high potential for metastasis, leading to treatment refractoriness [231–233]. Loss of E-cadherin, the essential component of cell-to-cell conjugation, is considered a fundamental event in the EMT process [234, 235]. The E3 ligases Hakai and RNF43 have been reported to participate in the ubiquitin-related degradation of E-cadherin, facilitating the EMT process in tumor progression [236, 237]. Intriguingly, a recent study reveals that RNF25 plays an essential role in oxidative stress-mediated E-cadherin degradation during liver cancer metastasis [238], suggesting that changes in the tumor microenvironment may also affect the cellular protein degradation process. Apart from direct regulation of E-cadherin protein degradation, several EMT-inducing transcription factors (e.g., ZEB1, Snail, and Twist) can downregulate E-cadherin expression by repressing its transcription [239]. Downregulation of the E3 ligase SIAH1 stabilizes ZEB1 in doxorubicin-resistant HCC cells [240]. Consistently, USP51 deubiquitinates ZEB1 resulting in a poor prognosis in breast cancer patients [241]. Dysregulation of FBXO32, an E3 ligase of Snail, leads to elevated Snail expression and acquired platinum resistance in urothelial carcinoma [242]. USP1 eliminates the polyubiquitin chain on Snail, leading to resistance to platinum in ovarian cancer [243]. Conversely, RNF8 induces K63 ubiquitination of Twist to promote its translocation to the nucleus for subsequent pro-EMT and CSC functions, leading to cancer chemoresistance [244]. The context-dependent functions of ubiquitination modification suggest that the clinical application of targeted ubiquitination must consider different application scenarios.
The maintenance of CSCs is closely related to the activation of embryonic developmental pathways (e.g., the Wnt and Hippo pathways) [230, 245]. In the absence of Wnt ligand, β-catenin may be phosphorylated by the β-catenin destruction complex composed of Axin, glycogen synthase kinase 3β (GSK3β), casein kinase 1α (CK1α), and adenomatous polyposis coli (APC) and subsequently degraded by the E3 ligase β-TrCP [246]. In addition, β-TrCP can facilitate the degradation of YAP [247]. Hence, activating β-TrCP through aspirin treatment can attenuate the expression of β-catenin and YAP, resulting in the reversal of vinorelbine and docetaxel resistance in triple-negative breast cancer [248]. USP20 and USP4 induce drug resistance in breast cancer and colorectal cancer cells by stabilizing β-catenin, the key modulator of the Wnt pathway [249, 250]. Neutral red, a selective inhibitor of USP4, has shown a significant effect on suppressing colorectal cancer progression [251]. In addition, USP22 is reported to participate in colorectal cancer stemness and chemoresistance through the regulation of the Wnt/β-catenin pathway [252, 253]. However, whether this regulation is protein degradation-dependent needs further validation [254]. Apart from the Wnt pathway, the E3 ligase STIP1 homology and U-Box-containing protein 1 (STUB1) induce YAP degradation in gastric cancer cells, leading to cellular chemosensitivity [255]. Deubiquitylation of YAP by USP10 mediates the aberrant activation of the Hippo pathway and progression of hepatocellular carcinoma [256].
Tumor microenvironment remodeling by protein degradation-regulated pathways
Besides its role in intracellular molecular mechanisms of drug resistance, the deregulation of the extracellular tumor microenvironment has attracted increasing research interest [257–259]. Recent studies indicate that tumor progression needs to overcome extrinsic stress (e.g., oxidative stress, hypoxia) caused by therapy and nutrition deprivation [260–264] (Fig. 7). Redox regulates cancer drug tolerance and dormancy, which are closely related to poor prognosis [265, 266]. Depletion of the E3 ligase FBXW7 sensitizes dormant cancer cells to paclitaxel, indicating that protein degradation may participate in the regulation of dormancy [267]. Acquired drug-resistant tumor cells, on the one hand, increase reactive oxygen species (ROS) signaling (e.g., the NF-κB pathway) to maintain their active proliferative state and simultaneously activate the antioxidant defense system (e.g., NRF2, GSH, high-abundance redox proteins) to avoid oxidative stress-mediated cell death [268]. Under a normal redox state, the essential E3 ligase KEAP1 targets NRF2 for proteasome-dependent degradation. Under oxidative stress, KEAP1 may be inactivated by redox modification of Cys151, Cys273, and Cys288, resulting in the activation of NRF2-mediated transcription of antioxidant genes [269]. DUB USP17 eliminates the K48 ubiquitin chain on NRF2, resulting in camptothecin and paclitaxel resistance in colorectal cancer [270]. In addition, USP15 stabilizes KEAP1 and promotes the degradation of NRF2, leading to the sensitization of cancer cells to chemotherapy [271, 272]. Apart from KEAP1, another E3 ligase, β-TrCP also participates in the degradation of NRF2, whose downregulation is closely related to drug resistance in various types of cancer [273, 274]. Recent studies have reported that cyclophilin A (CYPA), a high-abundance redox protein, can buffer excessive ROS through self-oxidation on Cys115 and Cys161, leading to resistance to 5-fluorouracil and oxaliplatin in colorectal cancer [275]. Consistently, deubiquitylation of CYPA by USP4 promotes a poor prognosis of hepatocellular carcinoma [276]. Apart from the upregulation of the antioxidant defense system, CYLD-mediated deubiquitylation of inhibitor of NF-κB kinase (IKK) leads to the inactivation of the NF-κB pathway and reversion of drug resistance [277]. Intriguingly, KEAP1 regulates the activation of NF-κB pathway through ubiquitination and degradation of IKKβ [278]. Furthermore, β-TrCP is reported to participate in the ubiquitin-dependent degradation of IκB [279]. In addition, the secretion of pro-inflammatory or anti-inflammatory cytokines may influence the sensitivity of anticancer agents, which may be context-dependent [280]. For instance, hepatitis B virus X-interacting protein (HBXIP) inhibits CMA-mediated homeobox B13 (HOXB13) degradation by enhancing acetylation at Lys 277, leading to the expression of pro-inflammatory cytokine IL-6 and tamoxifen resistance in breast cancer [281].
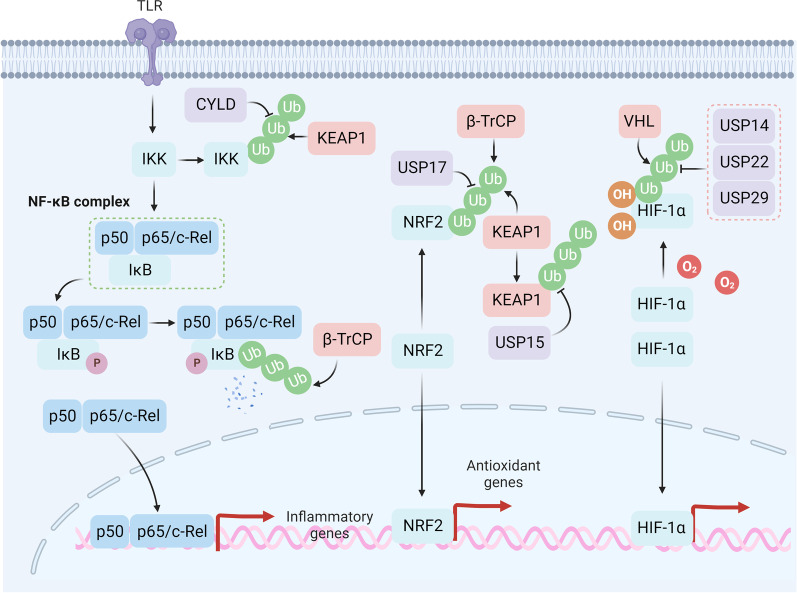
Fig. 7.
Protein degradation modulates the tumor microenvironment. IKK can be stabilized by CYLD to facilitate the phosphorylation of IκB, which can be reversed by KEAP1-mediated ubiquitylation on IKK. β-TrCP induces activation of the NF-κB pathway by ubiquitinating IκB, resulting in nucleus translocation of the NF-κB complex. β-TrCP and KEAP1 facilitate NRF2 degradation, while USP17 can deubiquitinate NRF2. KEAP1 can also be regulated by DUB USP15. Under hypoxic conditions, HIF-1α can translocate into the nucleus and facilitate transcription of hypoxic genes. While under normoxia, HIF-1α can be oxidated and further ubiquitinated by VHL for degradation. USP22, USP29, and USP14 can deubiquitylate HIF-1α
Activation of the hypoxia-inducible factor 1 alpha (HIF-1α) pathway under hypoxia is closely related to the transcription of drug resistance-related genes in multiple tumors [282–284]. Under a normal redox state, HIF-1α can be oxidized on Pro402 and Pro564 and subsequently degraded by von Hippel–Lindau protein (pVHL)-dependent degradation [285]. DUBs, including USP14, USP22, and USP29, mediate the deubiquitylation and stabilization of HIF-1α, promoting self-renewal ability and drug resistance in hepatocellular carcinoma [286–288]. Knockdown of CHIP was associated with high levels of HIF-1α and greatly enhanced growth in PCa tumor xenografts, suggesting that the use of mitotic kinase inhibitors will open new approaches for the treatment of hypoxic PCa tumors [289].
Targeting protein degradation to surmount drug resistance
Studying the regulatory mechanism of protein degradation can provide a basis for finding rapid and effective therapeutic strategies (Table 1). Several small molecules targeting E3 ligases or DUBs have entered clinical trials for treating various types of refractory tumors (Table 2). Due to the limited substrates that have been studied based on the selective regulation of E3, DUBs, and chaperones, there are quantities of proteins that are considered untargetable by modulating protein degradation [290]. Encouragingly, as a result of the in-depth study of biological mechanisms and the development of chemical synthesis technology, it has become possible to reverse the “untargetable” into a “targetable” situation [291]. At present, a variety of new technologies have been developed based on the proteasome and lysosomal pathways, which are expected to be effective in overcoming tumor drug resistance (Fig. 8).
Table 1.
Representative regulators of targeted protein degradation involved in drug resistance
| Protein degradation regulator | Category | Substrate | Therapeutic agents | Role | Tumor | Mechanism | Refs. |
|---|---|---|---|---|---|---|---|
| FBXO15 | E3 | P-gp | Vincristine and doxorubicin | Sensitive | CRC | Drug efflux | [54] |
| FBXO21 | E3 | P-gp | Valinomycin | Sensitive | Ovarian cancer | Drug efflux | [57] |
| MARCH8 | E3 | P-gp | Adriamycin, paclitaxel, and colchicine | Sensitive | Breast cancer, NSCLC | Drug efflux | [60] |
| AMFR | E3 | P450s | Enzalutamide | Resistant | – | Drug metabolism | [77] |
| FBXW7 | E3 | BRAF | BET inhibitors | Sensitive | T cell leukemia | Altering drug target | [190] |
| Cbl-b | E3 | EGFR | Adriamycin | Resistant | BC/GC | Altering drug target | [160] |
| SIAH2 | E3 | CHK2 | Genotoxic agents | Sensitive | Multiple cancer types | DNA repair | [118] |
| MDM2 | E3 | p53 | Cisplatin | Resistant | Uveal melanoma, ovarian cancer, lung cancer | DNA repair | [120–122] |
| TRIM28 | E3 | TRDMT1 | DNA-damaging agents | Sensitive | Ovarian cancer | DNA repair | [139] |
| RNF144A | E3 | DNA-PKcs | DNA-damaging agents | Sensitive | Multiple cancer types | DNA repair | [140] |
| CHIP | E3 | BCL-2 | Chemotherapy | Sensitive | Breast cancer | Apoptosis | [214] |
| CRL4 | E3 | STAT1 | Cisplatin | Resistant | Ovarian cancer | Apoptosis | [215] |
| RNF44 | E3 | AMPK-α1 | BRAFi | Resistant | Melanoma | Oncogenic bypass signaling | [225] |
| CHIP | E3 | MAST1 | Cisplatin | Sensitive | Multiple cancer types | Oncogenic bypass signaling | [227] |
| SIAH1 | E3 | ZEB1 | Doxorubicin | Sensitive | HCC | EMT | [240] |
| FBXO32 | E3 | Snail | Platinum | Sensitive | Urothelial carcinoma | EMT | [242] |
| β-TrCP | E3 | β-catenin and YAP | Vinorelbine and docetaxel | Sensitive | Triple-negative breast cancer | Stemness | [248] |
| β-TrCP | E3 | NRF2 | Temozolomide | Sensitive | Glioma | ROS signaling | [273] |
| β-TrCP | E3 | NRF2 | Chemotherapy | Sensitive | Pancreatic cancer | ROS signaling | [274] |
| USP28 | DUB | BRAF | BRAF inhibitors | Resistant | Melanoma | Altering drug target | [191] |
| USP14 | DUB | BRAF | BRAF inhibitors | Resistant | Melanoma | Altering drug target | [193] |
| USP22 | DUB | EGFR | EGFR-TKIs | Resistant | Lung adenocarcinoma | [169] | |
| USP17 | DUB | EGFR | EGFR-TKIs | Resistant | NSCLC | Altering drug target | [292] |
| UCHL1 | DUB | EGFR | EGFR-TKIs | Resistant | Breast cancer | Altering drug target | [170] |
| USP7 | DUB | MDC1 | Genotoxic insults | Resistant | Cervical cancer | DNA repair | [101] |
| USP7 | DUB | CHK1 | Cytarabine | Resistant | Acute myeloid leukemia | DNA repair | [104] |
| USP7 | DUB | CDC25A | DNA-damaging agents | Resistant | Cervical cancer | DNA repair | [106] |
| USP7 | DUB | PLK1 | Taxane | Resistant | Lung cancer | DNA repair | [111] |
| USP24 | DUB | p53 | Radiation | Sensitive | DNA repair | [112] | |
| USP28 | DUB | p53 | Genotoxic chemotherapy | Sensitive | Multiple cancer types | DNA repair | [113] |
| USP39 | DUB | CHK2 | Chemotherapy drugs and radiation treatment | Sensitive | Lung cancer | DNA repair | [114] |
| USP3 | DUB | Claspin | Radiation | Resistant | Glioblastoma | DNA repair | [115] |
| USP9x | DUB | BRCA1 | DNA-damaging agents | Resistant | Multiple cancer types | DNA repair | [134] |
| USP10 | DUB | MSH2 | N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) and 6-thioguanine (6-TG) | Sensitive | Lung cancer | DNA repair | [148] |
| USP2 | DUB | cFLIP | Sorafenib | Resistant | Hepatocellular carcinoma | Apoptosis | [209] |
| USP17 | DUB | Mcl-1 | Platinum and paclitaxel | Resistant | Ovarian cancer | Apoptosis | [292] |
| USP9x | DUB | Mcl-1 | Ionizing radiation | Resistant | Oral cancer | Apoptosis | [206] |
| USP15 | DUB | Caspase-6 | Imatinib | Sensitive | Chronic myeloid leukemia | Apoptosis | [210] |
| USP1 | DUB | Snail | Platinum | Resistant | Ovarian cancer | EMT | [243] |
| USP51 | DUB | ZEB1 | Cisplatin | Resistant | Lung cancer | EMT | [293] |
| USP20 | DUB | β-catenin | Chemotherapy | Resistant | Breast cancer | Stemness | [249] |
| USP22 | DUB | β-catenin | 5-fluorouracil | Resistant | Colorectal cancer | Stemness | [252] |
| USP10 | DUB | YAP | Chemotherapy | Resistant | Breast cancer | Stemness | [256] |
| USP17 | DUB | NRF2 | Camptothecin and paclitaxel | Resistant | Colorectal cancer | ROS signaling | [292] |
| USP15 | DUB | KEAP1 | Chemotherapy | Sensitive | Multiple cancer types | ROS signaling | [271, 272] |
| USP29 | DUB | HIF-1α | Sorafenib | Resistant | Hepatocellular carcinoma | Hypoxia | [287] |
| Hsc70 | Chaperone | HOXB13 | Tamoxifen | Sensitive | Breast cancer | Microenvironment | [281] |
| Hsc70 | Chaperone | MORC2 | Antiestrogen therapies | Sensitive | Breast cancer | DNA repair | [124] |
Table 2.
Representative small molecules targeting protein degradation under clinical evaluation
| Small molecules | Target | Application | Stage of development | Refs. |
|---|---|---|---|---|
| KSQ-4279 | USP1 | Patients with advanced solid tumors alone or in combination with an oral PARPi | Phase I | NCT05240898 |
| Perifosine | UCHL3 | Refractory tumors | Phase I or II | NCT00873457, NCT01049841, NCT00391560, NCT01097018, NCT00054145 |
| VLX1570 | UCHL and USP14 | Multiple myeloma | Phase II | NCT02372240 |
| AMG-232 | MDM2 | Treating patients with acute myeloid leukemia that has come back (recurrent), does not respond to treatment (refractory), or is newly diagnosed in combination with decitabine | Phase I | NCT03041688 |
| ALRN-6924 | MDM2/MDMX | Treatment for resistant (refractory) pediatric cancer | Phase I | NCT03654716 |
| DS-3032b | MDM2 | Treatment of relapsed and/or refractory multiple myeloma | Phase I | NCT02579824 |
| RO6839921 | MDM2 | Patients with advanced cancers | Phase I | NCT02098967, NCT01462175 |
| BI 907828 | MDM2 | Different types of advanced cancer | Phase I | NCT03449381, NCT05376800, NCT03964233 |
| NX-1607 | Cbl-b | Advanced malignancies | Phase I | NCT05107674 |
| KPG-818 | CRL4 | Hematological malignancies | Phase I | NCT04283097 |
Fig. 8.
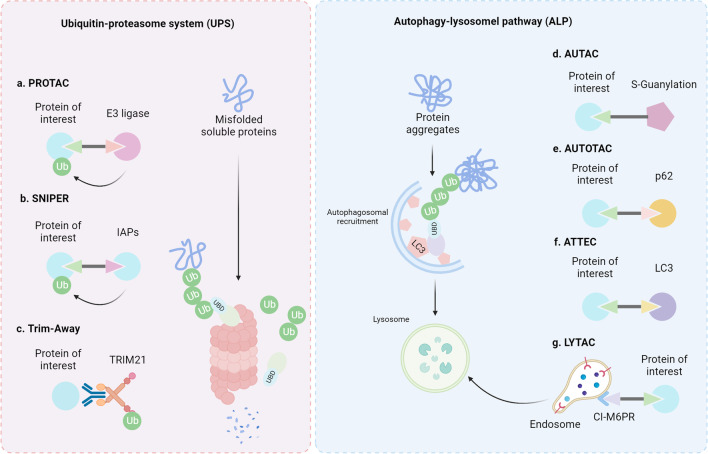
Current technologies targeting protein degradation. a PROTAC is designed to conjugate specific E3 ligases and proteins of interest for further degradation. b Based on the E3 ligase activity of IAPs, SNIPERs conjugate IAPs and proteins of interest for degradation. c TRIM21 has high affinity for antibodies. Therefore, using specific antibodies against the proteins of interest can lead to rapid degradation. d AUTAC is a heterobifunctional compound with a targeting warhead and a tag that recruits the autophagy system, which may lead to ubiquitin-dependent selective degradation through lysosomal degradation. e AUTOTAC connects the protein of interest and p62 for autophagy-induced protein degradation. f ATTEC conjugates the protein of interest and LC3 for degradation. g LYTAC interacts with membrane proteins or extracellular proteins and the CI-M6PR for endocytosis
Targeting proteasomal degradation
Small molecules
The role of the ubiquitin–proteasome system in drug resistance has long been considered closely related to cell survival and response to stress [294]. Bortezomib, a proteasome inhibitor, has been approved by the Food and Drug Administration for the treatment of multiple types of tumors since 2003 [295]. Together with the second generation of proteasome inhibitors (e.g., ixazomib, delanzomib, carfilzomib, and oprozomib), proteasome inhibitors potentiate antitumor effects against several tumors [296]. However, proteasome inhibitor resistance may occur in refractory tumors due to the lack of specific targeting ability [297]. Due to the substrate-specific selectivity of DUBs, efforts have been devoted to the development of small molecules targeting DUBs for cancer therapy [35]. Encouragingly, VLX1570, a small molecule targeting UCHL and USP14 is under clinical evaluation for treating multiple myeloma (NCT02372240) [298]. In addition, the small molecule perifosine targeting UCHL3 has been evaluated for the treatment of refractory tumors, including chronic lymphocytic leukemia (NCT00873457) [299], recurrent pediatric solid tumors (NCT01049841) [300], refractory and relapsed leukemia (NCT00391560) [301], refractory advanced colorectal cancer (NCT01097018) [302], and recurrent, refractory, locally advanced, or metastatic breast cancer (NCT00054145) [303]. Moreover, KSQ-4279 inhibits USP1 to effectively cure patients with advanced solid tumors alone or in combination with an oral PARPi, which has entered a phase I clinical trial (NCT05240898). Despite tremendous progress in the development of drugs targeting E1 enzymes [29] and E2 enzymes [30], E3 ligases have better application prospects for precise targeting due to their substrate specificity with approximately 600 members [44]. Notably, many small molecules targeting E3 ligases have been developed to surmount cancer drug resistance [304, 305]. Of note, small molecules targeting MDM2, including AMG-232 (NCT03041688) [306], ALRN-6924 (NCT03654716) [307], DS-3032b (NCT02579824) [308], RO6839921 (NCT02098967, NCT01462175) [309], BI 907,828 (NCT03449381, NCT05376800, NCT03964233) [310], are currently under clinical evaluations for treating refractory or advanced tumors. NX-1607 targeting Cbl-b is in phase I clinical trial for treating advanced malignancies (NCT05107674) [311]. In addition, KPG-818 treating hematological malignancies through targeting CRL4 is also under phase I clinical evaluation (NCT04283097) [312].
PROTAC
Despite the rapid development of studies on molecular mechanisms, some critical proteins in cancer cells are still “undruggable” due to the lack of targeted small molecule or E3 ligases [313]. In addition, there is still not a strict one-to-one correspondence between E3 and substrate, suggesting a natural defect in its targeting ability. Hence, proteolysis-targeting chimeras (PROTACs), which consist of two covalently linked protein-binding molecules targeting E3 ligase together with the protein of interest, have been developed to broaden the range of applications and improve the ability to precisely interact with E3 ligases and target proteins [314, 315]. PROTAC technology is based on a clear degradation mechanism to “hijack” the ubiquitin–proteasome system, resulting in rapid and specific degradation of indicated proteins. In 2019, two small molecules (ARV-110 (NCT03888612) and ARV-471 (NCT04072952)) that can be administered orally have entered clinical trials for the treatment of metastatic castration-resistant prostate cancer and advanced breast cancer, respectively. To date, at least 16 PROTAC molecules have entered clinical evaluation for the treatment of various tumors (Table 3).
Table 3.
Representative PROTACs under clinical evaluation
| Names | Targets | E3 ligases | Indications | Status | Refs. |
|---|---|---|---|---|---|
| AR-LDD | AR | CRBN | Prostate cancer | Phase I | NCT04428788 |
| ARV-110 | AR | CRBN | Prostate cancer | Phase II | NCT05177042, NCT03888612 |
| ARV-471 | ER | CRBN | Breast cancer | Phase II | NCT05501769, NCT05463952, NCT04072952 |
| ARV-766 | AR | VHL | Prostate cancer | Phase I | NCT05067140 |
| BGB-16673 | BTK | Undisclosed | B cell malignancies | Phase I | NCT05294731, NCT05006716 |
| CFT8634 | BRD9 | CRBN | Synovial sarcoma | Phase I/II | NCT05355753 |
| DT-2216 | BCL-XL | VHL | Liquid and solid tumors | Phase I | NCT04886622 |
| FHD-609 | BRD9 | Undisclosed | Synovial sarcoma | Phase I | NCT04965753 |
| GT20029 | AR | Undisclosed | Androgenetic alopecia and acne | Phase I | NCT05428449 |
| HSK29116 | BTK | Undisclosed | B cell malignancies | Phase I | NCT04861779 |
| KT-333 | STAT3 | Undisclosed | Liquid and solid tumors | Phase I | NCT05225584 |
| KT-413 | IRAK4 | CRBN | B cell lymphomas | Phase I | NCT05233033 |
| KT-474 | IRAK4 | Undisclosed | Atopic dermatitis and hidradenitis suppurativa | Phase I | NCT04772885 |
| LNK01002 | RAS GTPase | Undisclosed | Acute myeloid leukemia | Phase I | NCT04896112 |
| NX-2127 | BTK | CRBN | B cell malignancies | Phase I | NCT04830137 |
| NX-5948 | BTK | CRBN | B cell malignancies and autoimmune diseases | Phase I | NCT05131022 |
Other approaches targeting proteasomal degradation
Molecular glues are considered a category of small molecules that stabilize the interaction between two proteins [316]. To facilitate targeted protein degradation, molecular glues such as immunomodulatory imide drugs (IMiD, e.g., thalidomide, lenalidomide, pomalidomide) generate a novel interaction between a substrate (e.g., IKZF 1/3) [317, 318] and cereblon, a substrate receptor for CRL4 [319, 320]. Although the underlying molecular mechanisms still need further exploration, thalidomide has already been approved to treat patients with leprosy in 1975, multiple myeloma in 1998; lenalidomide has been approved for the treatment of multiple myeloma in 2006 as well as myelodysplastic syndrome, lymphoma, follicular lymphoma; pomalidomide has been approved in 2013 as a treatment for relapsed and refractory multiple myeloma [321, 322]. In addition, thalidomide (NCT00016224), lenalidomide (NCT01246557, NCT00988208) [323, 324], and pomalidomide (NCT01324947, NCT01311687) [325, 326] are currently under clinical trial for the treatment of resistant and refractory tumors.
As mentioned above, IAPs may be upregulated in refractory tumors due to their biological function of inhibiting apoptosis [327]. Similar to PROTAC, specific non-genetic IAP-based protein erasers (SNIPERs) consist of three structures, including IAP recognition structure, specific ligand for the target protein, and linker [328]. Under physiological conditions, IAP exerts its anti-apoptotic function by ubiquitinating and degrading caspase [329]. SNIPERs utilize the E3 activity of IAP to ubiquitinate and degrade the target protein by linking IAP with the target protein. The specific molecular mechanism remains to be further explored.
In addition, Trim-Away is another approach that targets protein degradation based on the high affinity of TRIM21 to antibodies [330]. TRIM21 binds to the target protein through a specific antibody to form a complex, then TRIM21 is ubiquitinated, and the entire complex is degraded through the ubiquitin–proteasome pathway [331]. Trim-Away has the advantages of a wide range of target proteins, simple and rapid technology, but due to the lack of clinical drug delivery methods, it is currently used for the mechanistic study of cell models or model organisms.
Targeting lysosomal degradation
Small molecules
Autophagy has long been considered a recycling process for coping with intracellular stress during cancer progression [332]. Much evidence indicates that autophagy is an important reason for tumor resistance [333]. Chloroquine, an autophagy‒lysosome pathway inhibitor, is currently under clinical evaluation for treating autophagy-dependent pancreatic cancer (NCT01506973) [334]. However, due to the toxicity of chloroquine and the lack of precise targeting, its clinical application is limited. In addition, autophagy plays a dual role in tumorigenesis and development, and simply inhibiting non-selective autophagy may have side effects on tumor therapy [335].
Growing approaches targeting lysosomal proteolysis
Similar to the precise targeting of proteasomal proteolysis, several technologies targeting protein degradation through lysosomal proteolysis have emerged spontaneously, including autophagy-targeting chimera (AUTAC), newly developed AUTOTAC, autophagosome-tethering compound (ATTEC), CMA-targeting motif (CTM)-directed protein degradation, and lysosome-targeting chimera (LYTAC). The design of AUTAC stems from the ubiquitin-dependent selective degradation of group A Streptococcus (GAS) by autophagy, although the specific E3 ligase has not been identified [336]. AUTOTAC is a category of small molecules connecting the protein of interest and p62, resulting in selective degradation through macroautophagy in a ubiquitin-independent way [337]. Similarly, ATTEC is a molecule that connects the protein of interest and LC3 for macroautophagy-induced degradation [338]. By designing a targeted peptide containing a CTM protein-binding domain interacting with the protein of interest, CTM-directed protein degradation can rapidly degrade endogenous proteins through chaperone-mediated autophagy [339]. LYTAC is designed to traffic secreted or membrane-associated proteins into lysosomes for degradation through the interaction between a specific antibody and a glycopolypeptide ligand for CI-M6PR [340]. At present, small molecules targeting EGFR and integrins have undergone preliminary validation and show clinical potential for the treatment of refractory tumors. [341].
Conclusions and perspectives
In this review, we have systematically summarized recent studies on protein degradation for regulating cancer drug resistance. Intriguingly, both main degradation pathways, proteasomal proteolysis and lysosomal proteolysis, participate in the regulation of drug resistance, where ubiquitin plays essential roles in the selective degradation of key regulators. Due to their strong substrate specificity, the mechanism by which E3 and DUB regulate the ubiquitination and degradation of key molecules has been deeply explored, and corresponding small molecule inhibitors are being developed to reverse tumor drug resistance. In addition, several ubiquitin-independent selective proteolysis mechanisms, such as chaperone-mediated autophagy, have also attracted significant research interest. However, due to the complexity of the molecular mechanisms and the limited substrates that have been extensively studied, some novel new small-molecule compounds based on the connection between the “undruggable” substrates and the key regulators in proteolysis have been developed and have achieved gratifying results in clinical applications.
The important impact of protein degradation regulation on drug resistance is often overlooked in the typical research process. In-depth and systematic study of the protein degradation mechanism can help improve the understanding of drug resistance and provide new targets and therapeutic strategies for drug development. In addition, traditional small molecules inhibit the enzymatic activity of the target by binding to the target protein for a long period, which remains challenging for both the structural design of small molecules and the target mutation-mediated drug resistance. By designing small molecules (e.g., PROTAC) that only require a short-term connection between the target protein and a specific key regulator of protein degradation, the target protein can be selectively degraded, which facilitates drug development and improves the killing effect on mutant tumors.
Although some research progress has been made on ubiquitination and deubiquitylation, the current research on ubiquitination is mainly focused on the proteasome pathway, while studies on the upstream ubiquitin regulation of the lysosomal degradation pathway are still limited. Among the current technologies targeting protein degradation, the molecular mechanism of PROTAC has been researched in-depth, while the others still need further study on the underlying molecular mechanisms, which may facilitate the clinical use of other technologies targeting protein degradation. In summary, continuing to further explore the regulatory mechanisms of ubiquitin-dependent and ubiquitin-independent selective degradation and clarify its regulatory relationship with tumor drug resistance will provide meaningful guidance for the clinical treatment of tumor drug resistance.
In addition to regulating genes and RNAs, targeted protein degradation has become a hot research topic due to its advantages of direct and fast action. With the development of multiomics technology, understanding the ubiquitin code that regulates key molecules of tumor resistance from a mechanistic perspective will promote the in-depth understanding and application of protein degradation mechanisms. Additionally, several ubiquitin-independent protein degradation pathways should also take into consideration for further research. Studying the interaction between proteasomal proteolysis and lysosomal proteolysis in mediating the degradation of tumor resistance-related molecules will provide a reasonable route for the development of technologies that selectively target protein degradation. Structural optimization of synthetic molecules designed for different degradation pathways will promote the specificity of degradation, reduce biotoxicity and further clinical applications. In addition, as an important part of the protein expression regulation mechanism, protein degradation should also be coordinated with research on gene expression, RNA modification, and other protein modifications, acting as a supplement to overcome the acquired drug resistance mechanism and offering new strategies to surmount cancer drug resistance.
Acknowledgements
The authors acknowledge the BioRender (www.biorender.com), as figures in this review were created with the BioRender platform.
Abbreviations
- 53BP1
P53-binding protein 1
- 6-TG
6-Thioguanine
- β-TrCP
β-Transducin repeat-containing protein
- ABC
ATP-binding cassette
- AhR
Aryl hydrocarbon receptor
- AMFR
Autocrine motility factor receptor
- AML
Acute myeloid leukemia
- AMPK-α1
AMP-activated protein kinase α1
- ANAPC2
Anaphase-promoting complex subunit 2
- APAF-1
Apoptotic protease activating factor 1
- ASPM
Abnormal spindle-like microcephaly-associated protein
- ATTEC
Autophagosome-tethering compound
- AUTAC
Autophagy-targeting chimera
- BCA2
Breast cancer-associated gene 2
- BCL-2
B cell lymphoma 2
- BCRP
Breast cancer resistance protein
- BET
Bromodomain and extra-terminal
- BIRC3
Baculoviral IAP repeat-containing 3
- BRCA1
Breast cancer type 1 susceptibility protein
- Cbl-b
Casitas b-lineage lymphoma-b
- CDC25A
Cell division cycle 25 A
- CDK
Cyclin-dependent kinase
- CGRRF1
Cell growth regulator with RING finger domain protein 1
- CHIP
C-terminus of HSC70-interacting protein
- CHK
Checkpoint kinase
- CRL4
Cullin-RING ubiquitin ligases 4
- CYP450
Cytochrome P450 enzymes
- CMA
Chaperone-mediated autophagy
- CSC
Cancer stem cell
- CTM
CMA-targeting motif
- CYLD
Cylindromatosis-associated DUB
- CYPA
Cyclophilin A
- DAB2IP
DOC-2/DAB2 interactive protein
- DDR
DNA damage response
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- DSB
DNA double-strand break
- DUB
Deubiquitylating enzyme
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-to-mesenchymal transition
- ERAD
Endoplasmic reticulum-associated degradation
- ERK
Extracellular signal-regulated kinase
- FLIP
FLICE-inhibitory protein
- GAS
Group A Streptococcus
- GBM
Glioblastoma
- GDP
Guanosine diphosphate
- GSK3β
Glycogen synthase kinase 3β
- GST
Glutathione S-transferase
- HBXIP
Hepatitis B virus X-interacting protein
- HCC
Hepatocellular carcinoma
- HECT
Homologous to the E6AP C-terminus
- HMGCoAR
3-Hydroxy-3-methylglutaryl-coenzyme A reductase
- HOXB13
Homeobox B13
- HIF-1α
Hypoxia-inducible factor 1 alpha
- HR
Homologous recombination
- IAPs
Inhibitors of apoptosis proteins
- IKK
Inhibitor of NF-κB kinase
- JAK
Janus kinase
- JNK
C-Jun N-terminal kinase
- KEAP1
Kelch-like ECH-associated protein 1
- KRAS
Kirsten rat sarcoma
- LAMP2A
Lysosome-associated membrane protein type 2A
- LSCC
Lung squamous cell carcinoma
- LUAD
Lung adenocarcinoma
- LYTAC
Lysosome-targeting chimera
- LZTR1
Leucine zipper-like transcriptional regulator 1
- MAPK
Mitogen‑activated protein kinase
- MARCH
Membrane-associated RING-CH
- MDC1
Mediator of DNA damage checkpoint protein 1
- MDM2
Mouse double minute 2 homolog
- MAST
Microtubule-associated serine/threonine kinase 1
- MDR
Multi-drug resistance
- MKP1
Mitogen-activated protein kinase phosphatase-1
- MRP
MDR-associated protein
- MORC2
Microrchidia family CW-type zinc finger 2
- MM
Multiple myeloma
- MMR
Mismatch repair
- MSH2
MutS protein homolog 2
- MT
Methyltransferase
- MTAP
Methylthioadenosine phosphorylase
- NAT
N-Acetyltransferase
- NEDD4L
Neural precursor cell expressed developmentally downregulated gene 4-like
- NHEJ
Nonhomologous end-joining
- NPC
Nasopharyngeal carcinoma
- NRF2
Nuclear factor erythroid 2-related factor 2
- NSCLC
Non-small-cell lung carcinoma
- PARP
Poly (ADP-ribose) polymerases
- PBX1
Pre-B-cell leukemia homeobox-1
- PCa
Prostate cancer
- PI3K
Phosphoinositide 3-kinase
- PLK
Polo-like kinase
- PRKACA
Protein kinase cAMP-activated catalytic subunit alpha
- PP2A
Protein phosphatase 2A
- PRMT5
Protein arginine methyltransferase 5
- PROTAC
Proteolysis targeting chimera
- PUMA
P53 upregulated modulator of apoptosis
- pVHL
Von Hippel–Lindau protein
- RBX1
DOC-2/DAB2 interactive protein 1
- RBR
RING-between-RING
- RING
Really interesting new gene
- ROS
Reactive oxygen species
- SCF
SKP1/CUL1/F-box
- SGCE
Sarcoglycan epsilon
- SH3RF1
SH3 domain-containing RING finger 1
- SIRT1
Sirtuin 1
- SKP1
S-phase kinase-associated protein 1
- SMURF2
Smad ubiquitination regulatory factor 2
- SNIPERs
Specific non-genetic IAP-based protein erasers
- SOCS5
Suppressor of cytokine signaling 5
- STAT
Signal transducer and activator of transcription
- SULT
Sulfotransferase
- STUB1
STIP1 homology and U-Box-containing protein 1
- TPD
Targeted protein degradation
- TRIM15
Tripartite motif-containing protein 15
- TKI
Tyrosine kinase inhibitor
- TNBC
Triple-negative breast cancer
- UBE2R1
Ubiquitin-conjugating enzyme E2 R1
- UCHL3
Ubiquitin C-terminal hydrolase L3
- UGT
UDP-glucuronosyltransferase
- USP
Ubiquitin-specific peptidase
- UV
Ultraviolet
- VCP
Valosin-containing protein
- XRCC4
X-ray repair cross-complementing protein 4
- YAP
Yes-associated protein
Author contributions
CH, TL, and WH designed this manuscript. HM, BL drafted the manuscript. JJ, SQ, and EN revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81821002, 82130082), National Key Research and Development Project (No. 2020YFA0509400), Guangdong Basic and Applied Basic Research Foundation (2019B030302012), 1·3·5 project for displines of excellence (ZYGD22007, ZYJC21004), and National Key Laboratory Open Fundation in 2021 (SKLKF202101).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate:
Not applicable.
Consent for publication
All authors have read and approved the final manuscript.
Competing interests
The authors declared no potential competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hui Ming, Bowen Li and Jingwen Jiang contributed equally to this work
Contributor Information
Weifeng He, Email: whe761211@hotmail.com.
Tingyuan Lang, Email: michaellang2009@163.com.
Canhua Huang, Email: hcanhua@scu.edu.cn.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 3.Guo L, Lee YT, Zhou Y, Huang Y. Targeting epigenetic regulatory machinery to overcome cancer therapy resistance. Semin Cancer Biol. 2022;83:487–502. doi: 10.1016/j.semcancer.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dallavalle S, Dobričić V, Lazzarato L, Gazzano E, Machuqueiro M, Pajeva I, Tsakovska I, Zidar N, Fruttero R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist Updat Rev Comment Antimicrob Anticancer Chemother. 2020;50:100682. doi: 10.1016/j.drup.2020.100682. [DOI] [PubMed] [Google Scholar]
- 5.Narendra G, Choudhary S, Raju B, Verma H, Silakari O. Role of genetic polymorphisms in drug-metabolizing enzyme-mediated toxicity and pharmacokinetic resistance to anti-cancer agents: a review on the pharmacogenomics aspect. Clin Pharmacokinet. 2022 doi: 10.1007/s40262-022-01174-. [DOI] [PubMed] [Google Scholar]
- 6.Brown R, Curry E, Magnani L, Wilhelm-Benartzi CS, Borley J. Poised epigenetic states and acquired drug resistance in cancer. Nat Rev Cancer. 2014;14:747–753. doi: 10.1038/nrc3819. [DOI] [PubMed] [Google Scholar]
- 7.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persi E, Wolf YI, Horn D, Ruppin E, Demichelis F, Gatenby RA, Gillies RJ, Koonin EV. Mutation-selection balance and compensatory mechanisms in tumour evolution. Nat Rev Genet. 2021;22:251–262. doi: 10.1038/s41576-020-00299-4. [DOI] [PubMed] [Google Scholar]
- 9.Iniguez AB, Alexe G, Wang EJ, Roti G, Patel S, Chen L, Kitara S, Conway A, Robichaud AL, Stolte B, et al. Resistance to epigenetic-targeted therapy engenders tumor cell vulnerabilities associated with enhancer remodeling. Cancer Cell. 2018;34:922–38.e7. doi: 10.1016/j.ccell.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspeslagh S, Morel D, Soria JC, Postel-Vinay S. Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann Oncol. 2018;29:812–824. doi: 10.1093/annonc/mdy050. [DOI] [PubMed] [Google Scholar]
- 11.Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–i19. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Jiang J, Assaraf YG, Xiao H, Chen ZS, Huang C. Surmounting cancer drug resistance: new insights from the perspective of N(6)-methyladenosine RNA modification. Drug Resist Updat. 2020;53:100720. doi: 10.1016/j.drup.2020.100720. [DOI] [PubMed] [Google Scholar]
- 13.Zhao SG, Chen WS, Li H, Foye A, Zhang M, Sjöström M, Aggarwal R, Playdle D, Liao A, Alumkal JJ, et al. The DNA methylation landscape of advanced prostate cancer. Nat Genet. 2020;52:778–789. doi: 10.1038/s41588-020-0648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savitski MM, Zinn N, Faelth-Savitski M, Poeckel D, Gade S, Becher I, Muelbaier M, Wagner AJ, Strohmer K, Werner T, et al. Multiplexed proteome dynamics profiling reveals mechanisms controlling protein homeostasis. Cell. 2018;173:260–74.e25. doi: 10.1016/j.cell.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herhaus L, Dikic I. Expanding the ubiquitin code through post-translational modification. EMBO Rep. 2015;16:1071–1083. doi: 10.15252/embr.201540891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherpa D, Chrustowicz J, Schulman BA. How the ends signal the end: regulation by E3 ubiquitin ligases recognizing protein termini. Mol Cell. 2022;82:1424–1438. doi: 10.1016/j.molcel.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng Z, Wang W, Yang Y, Chen Y, Yang X, Diehl JA, Liu X, Lei M. Structural basis of selective ubiquitination of TRF1 by SCFFbx4. Dev Cell. 2010;18:214–225. doi: 10.1016/j.devcel.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varshavsky A. The ubiquitin system, autophagy, and regulated protein degradation. Annu Rev Biochem. 2017;86:123–128. doi: 10.1146/annurev-biochem-061516-044859. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Schwesinger C. The ubiquitin-proteasome system in kidney physiology and disease. Nat Rev Nephrol. 2019;15:393–411. doi: 10.1038/s41581-019-0148-1. [DOI] [PubMed] [Google Scholar]
- 20.Dong Y, Zhang S, Wu Z, Li X, Wang WL, Zhu Y, Stoilova-McPhie S, Lu Y, Finley D, Mao Y. Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome. Nature. 2019;565:49–55. doi: 10.1038/s41586-018-0736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opoku-Nsiah KA, Gestwicki JE. Aim for the core: suitability of the ubiquitin-independent 20S proteasome as a drug target in neurodegeneration. Transl Res. 2018;198:48–57. doi: 10.1016/j.trsl.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall RS, Vierstra RD. Autophagy: the master of bulk and selective recycling. Annu Rev Plant Biol. 2018;69:173–208. doi: 10.1146/annurev-arplant-042817-040606. [DOI] [PubMed] [Google Scholar]
- 23.Birgisdottir ÅB, Johansen T. Autophagy and endocytosis - interconnections and interdependencies. J Cell Sci. 2020 doi: 10.1242/jcs.228114. [DOI] [PubMed] [Google Scholar]
- 24.Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19:12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19:365–381. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohl C, Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366:818–822. doi: 10.1126/science.aax3769. [DOI] [PubMed] [Google Scholar]
- 28.Cohen P. Ubiquitin chains as second messengers. Nat Rev Mol Cell Biol. 2018;19:212. doi: 10.1038/nrm.2018.9. [DOI] [PubMed] [Google Scholar]
- 29.Barghout SH, Schimmer AD. E1 enzymes as therapeutic targets in cancer. Pharmacol Rev. 2021;73:1–58. doi: 10.1124/pharmrev.120.000053. [DOI] [PubMed] [Google Scholar]
- 30.Osborne HC, Irving E, Forment JV, Schmidt CK. E2 enzymes in genome stability: pulling the strings behind the scenes. Trends Cell Biol. 2021;31:628–643. doi: 10.1016/j.tcb.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Sosič I, Bricelj A, Steinebach C. E3 ligase ligand chemistries: from building blocks to protein degraders. Chem Soc Rev. 2022;51:3487–3534. doi: 10.1039/D2CS00148A. [DOI] [PubMed] [Google Scholar]
- 32.Cruz Walma DA, Chen Z, Bullock AN, Yamada KM. Ubiquitin ligases: guardians of mammalian development. Nat Rev Mol Cell Biol. 2022;23:350–367. doi: 10.1038/s41580-021-00448-5. [DOI] [PubMed] [Google Scholar]
- 33.Senft D, Qi J, Ronai ZA. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer. 2018;18:69–88. doi: 10.1038/nrc.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clague MJ, Urbé S, Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat Rev Mol Cell Biol. 2019;20:338–352. doi: 10.1038/s41580-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 35.Harrigan JA, Jacq X, Martin NM, Jackson SP. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discovery. 2018;17:57–78. doi: 10.1038/nrd.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Tsai YC, Jin B, Wang B, Wang Y, Zhou H, Carpenter T, Weissman AM, Yin J. Protein engineering in the ubiquitin system: tools for discovery and beyond. Pharmacol Rev. 2020;72:380–413. doi: 10.1124/pr.118.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crunkhorn S. Cancer: targeting the ubiquitin pathway. Nat Rev Drug Discovery. 2018;17:166. doi: 10.1038/nrd.2018.33. [DOI] [PubMed] [Google Scholar]
- 38.Kolla S, Ye M, Mark KG, Rapé M. Assembly and function of branched ubiquitin chains. Trends Biochem Sci. 2022;47:759–771. doi: 10.1016/j.tibs.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Sun D, Wu R, Zheng J, Li P, Yu L. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. Cell Res. 2018;28:405–415. doi: 10.1038/s41422-018-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liebl MP, Hoppe T. It's all about talking: two-way communication between proteasomal and lysosomal degradation pathways via ubiquitin. Am J Physiol Cell Physiol. 2016;311:C166–C178. doi: 10.1152/ajpcell.00074.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun-Wang JL, Ivanova S, Zorzano A. The dialogue between the ubiquitin-proteasome system and autophagy: implications in ageing. Ageing Res Rev. 2020;64:101203. doi: 10.1016/j.arr.2020.101203. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Park S, Kim E, Lee MJ. Negative-feedback coordination between proteasomal activity and autophagic flux. Autophagy. 2019;15:726–728. doi: 10.1080/15548627.2019.1569917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pajares M, Rojo AI, Arias E, Díaz-Carretero A, Cuervo AM, Cuadrado A. Transcription factor NFE2L2/NRF2 modulates chaperone-mediated autophagy through the regulation of LAMP2A. Autophagy. 2018;14:1310–1322. doi: 10.1080/15548627.2018.1474992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dale B, Cheng M, Park KS, Kaniskan H, Xiong Y, Jin J. Advancing targeted protein degradation for cancer therapy. Nat Rev Cancer. 2021;21:638–654. doi: 10.1038/s41568-021-00365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation - lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18:401–417. doi: 10.1038/s41571-021-00479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y, Aldoss I, Qu C, Crawford JC, Gu Z, Allen EK, Zamora AE, Alexander TB, Wang J, Goto H, et al. Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL. Blood. 2021;137:471–484. doi: 10.1182/blood.2020006287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montrose DC, Saha S, Foronda M, McNally EM, Chen J, Zhou XK, Ha T, Krumsiek J, Buyukozkan M, Verma A, et al. Exogenous and endogenous sources of serine contribute to colon cancer metabolism, growth, and resistance to 5-fluorouracil. Cancer Res. 2021;81:2275–2288. doi: 10.1158/0008-5472.CAN-20-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim E, Kim JY, Smith MA, Haura EB, Anderson ARA. Cell signaling heterogeneity is modulated by both cell-intrinsic and -extrinsic mechanisms: an integrated approach to understanding targeted therapy. PLoS Biol. 2018;16:e2002930. doi: 10.1371/journal.pbio.2002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schapira M, Calabrese MF, Bullock AN, Crews CM. Targeted protein degradation: expanding the toolbox. Nat Rev Drug Discov. 2019;18:949–963. doi: 10.1038/s41573-019-0047-y. [DOI] [PubMed] [Google Scholar]
- 50.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas C, Tampé R. Structural and Mechanistic Principles of ABC Transporters. Annu Rev Biochem. 2020;89:605–636. doi: 10.1146/annurev-biochem-011520-105201. [DOI] [PubMed] [Google Scholar]
- 52.Giddings EL, Champagne DP, Wu MH, Laffin JM, Thornton TM, Valenca-Pereira F, Culp-Hill R, Fortner KA, Romero N, East J, et al. Mitochondrial ATP fuels ABC transporter-mediated drug efflux in cancer chemoresistance. Nat Commun. 2021;12:2804. doi: 10.1038/s41467-021-23071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim Y, Chen J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science. 2018;359:915–919. doi: 10.1126/science.aar7389. [DOI] [PubMed] [Google Scholar]
- 54.Katayama K, Noguchi K, Sugimoto Y. FBXO15 regulates P-glycoprotein/ABCB1 expression through the ubiquitin–proteasome pathway in cancer cells. Cancer Sci. 2013;104:694–702. doi: 10.1111/cas.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katayama K, Fujiwara C, Noguchi K, Sugimoto Y. RSK1 protects P-glycoprotein/ABCB1 against ubiquitin-proteasomal degradation by downregulating the ubiquitin-conjugating enzyme E2 R1. Sci Rep. 2016;6:36134. doi: 10.1038/srep36134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin Z, Miao J, Zhang T, He M, Wang Z, Feng X, Bai L. JUNB-FBXO21-ERK axis promotes cartilage degeneration in osteoarthritis by inhibiting autophagy. Aging Cell. 2021;20:e13306. doi: 10.1111/acel.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravindranath AK, Kaur S, Wernyj RP, Kumaran MN, Miletti-Gonzalez KE, Chan R, Lim E, Madura K, Rodriguez-Rodriguez L. CD44 promotes multi-drug resistance by protecting P-glycoprotein from FBXO21-mediated ubiquitination. Oncotarget. 2015;6:26308–26321. doi: 10.18632/oncotarget.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Xu F, Ren L, Zhao F, Huang Y, Wei L, Wang Y, Wang C, Fan Z, Mei S, et al. MARCH8 inhibits influenza A virus infection by targeting viral M2 protein for ubiquitination-dependent degradation in lysosomes. Nat Commun. 2021;12:4427. doi: 10.1038/s41467-021-24724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen W, Patel D, Jia Y, Yu Z, Liu X, Shi H, Liu H. MARCH8 suppresses tumor metastasis and mediates degradation of STAT3 and CD44 in breast cancer cells. Cancers. 2021;13:2550. doi: 10.3390/cancers13112550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou T, Zeng C, Qu J, Yan X, Lin Z. Rutaecarpine increases anticancer drug sensitivity in drug-resistant cells through MARCH8-dependent ABCB1 degradation. Biomedicines. 2021;9:1143. doi: 10.3390/biomedicines9091143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang SA, Young MJ, Wang YC, Chen SH, Liu CY, Lo YA, Jen HH, Hsu KC, Hung JJ. USP24 promotes drug resistance during cancer therapy. Cell Death Differ. 2021;28:2690–2707. doi: 10.1038/s41418-021-00778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu WX, Gan GS, Yang B. Knockdown of USP9X reverses cisplatin resistance by decreasing β-catenin expression in nasopharyngeal carcinoma cells. Neoplasma. 2021;68:810–822. doi: 10.4149/neo_2021_201227N1410. [DOI] [PubMed] [Google Scholar]
- 63.Lu M, Chen W, Zhuang W, Zhan X. Label-free quantitative identification of abnormally ubiquitinated proteins as useful biomarkers for human lung squamous cell carcinomas. Epma j. 2020;11:73–94. doi: 10.1007/s13167-019-00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loignon M, Miao W, Hu L, Bier A, Bismar TA, Scrivens PJ, Mann K, Basik M, Bouchard A, Fiset PO, et al. Cul3 overexpression depletes Nrf2 in breast cancer and is associated with sensitivity to carcinogens, to oxidative stress, and to chemotherapy. Mol Cancer Ther. 2009;8:2432–2440. doi: 10.1158/1535-7163.MCT-08-1186. [DOI] [PubMed] [Google Scholar]
- 65.Gelsomino G, Corsetto PA, Campia I, Montorfano G, Kopecka J, Castella B, Gazzano E, Ghigo D, Rizzo AM, Riganti C. Omega 3 fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol Cancer. 2013;12:137. doi: 10.1186/1476-4598-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang YS, Su CW, Chen SC, Chen YY, Liang YJ, Wu JC. Upregulation of USP22 and ABCC1 during sorafenib treatment of hepatocellular carcinoma contribute to development of resistance. Cells. 2022;11:634. doi: 10.3390/cells11040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu S, Ling S, Shan Q, Ye Q, Zhan Q, Jiang G, Zhuo J, Pan B, Wen X, Feng T, et al. Self-Activated Cascade-Responsive Sorafenib and USP22 shRNA Co-Delivery System for Synergetic Hepatocellular Carcinoma Therapy. Adv Sci (Weinh) 2021;8:2003042. doi: 10.1002/advs.202003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ling S, Li J, Shan Q, Dai H, Lu D, Wen X, Song P, Xie H, Zhou L, Liu J, et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol Oncol. 2017;11:682–695. doi: 10.1002/1878-0261.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J, Luo N, Tian Y, Li J, Yang X, Yin H, Xiao C, Sheng J, Li Y, Tang B, et al. USP22 knockdown enhanced chemosensitivity of hepatocellular carcinoma cells to 5-Fu by up-regulation of Smad4 and suppression of Akt. Oncotarget. 2017;8:24728–24740. doi: 10.18632/oncotarget.15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu P, Zhou W, Yang L, Zhang C. E3 ubiquitin ligase RNF180 reduces sensitivity of triple-negative breast cancer cells to Gefitinib by downregulating RAD51. Chem Biol Interact. 2022;354:109798. doi: 10.1016/j.cbi.2022.109798. [DOI] [PubMed] [Google Scholar]
- 71.Ouyang L, Yan B, Liu Y, Mao C, Wang M, Liu N, Wang Z, Liu S, Shi Y, Chen L, et al. The deubiquitylase UCHL3 maintains cancer stem-like properties by stabilizing the aryl hydrocarbon receptor. Signal Transduct Target Ther. 2020;5:78. doi: 10.1038/s41392-020-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kashyap A, Singh PK, Silakari O. Mechanistic investigation of resistance via drug-inactivating enzymes in Mycobacterium tuberculosis. Drug Metab Rev. 2018;50:448–465. doi: 10.1080/03602532.2018.1533966. [DOI] [PubMed] [Google Scholar]
- 73.Zeng M, Yang L, He D, Li Y, Shi M, Zhang J. Metabolic pathways and pharmacokinetics of natural medicines with low permeability. Drug Metab Rev. 2017;49:464–476. doi: 10.1080/03602532.2017.1377222. [DOI] [PubMed] [Google Scholar]
- 74.Machalz D, Pach S, Bermudez M, Bureik M, Wolber G. Structural insights into understudied human cytochrome P450 enzymes. Drug Discov Today. 2021;26:2456–2464. doi: 10.1016/j.drudis.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Oda S, Fukami T, Yokoi T, Nakajima M. A comprehensive review of UDP-glucuronosyltransferase and esterases for drug development. Drug Metab Pharmacokinet. 2015;30:30–51. doi: 10.1016/j.dmpk.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Joshi V, Upadhyay A, Kumar A, Mishra A. Gp78 E3 ubiquitin ligase: essential functions and contributions in proteostasis. Front Cell Neurosci. 2017;11:259. doi: 10.3389/fncel.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwon D, Kim SM, Jacob P, Liu Y, 3rd, Correia MA. Induction via functional protein stabilization of hepatic cytochromes P450 upon gp78/autocrine motility factor receptor (AMFR) ubiquitin E3-ligase genetic ablation in mice: therapeutic and toxicological relevance. Mol Pharmacol. 2019;96:641–654. doi: 10.1124/mol.119.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGraw J, Cherney M, Bichler K, Gerhardt A, Nauman M. The relative role of CYP3A4 and CYP3A5 in eplerenone metabolism. Toxicol Lett. 2019;315:9–13. doi: 10.1016/j.toxlet.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 79.Kim SM, Wang Y, Nabavi N, Liu Y, Correia MA. Hepatic cytochromes P450: structural degrons and barcodes, posttranslational modifications and cellular adapters in the ERAD-endgame. Drug Metab Rev. 2016;48:405–433. doi: 10.1080/03602532.2016.1195403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Kim SM, Trnka MJ, Liu Y, Burlingame AL, Correia MA. Human liver cytochrome P450 3A4 ubiquitination: molecular recognition by UBC7-gp78 autocrine motility factor receptor and UbcH5a-CHIP-Hsc70-Hsp 40 E2–E3 ubiquitin ligase complexes. J Biol Chem. 2015;290:3308–3332. doi: 10.1074/jbc.M114.611525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fregno I, Molinari M. Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways. Crit Rev Biochem Mol Biol. 2019;54:153–163. doi: 10.1080/10409238.2019.1610351. [DOI] [PubMed] [Google Scholar]
- 82.Ohtsuki Y, Sanoh S, Santoh M, Ejiri Y, Ohta S, Kotake Y. Inhibition of cytochrome P450 3A protein degradation and subsequent increase in enzymatic activity through p38 MAPK activation by acetaminophen and salicylate derivatives. Biochem Biophys Res Commun. 2019;509:287–293. doi: 10.1016/j.bbrc.2018.12.124. [DOI] [PubMed] [Google Scholar]
- 83.Kurogi K, Rasool MI, Alherz FA, El Daibani AA, Bairam AF, Abunnaja MS, Yasuda S, Wilson LJ, Hui Y, Liu MC. SULT genetic polymorphisms: physiological, pharmacological and clinical implications. Expert Opin Drug Metab Toxicol. 2021;17:767–784. doi: 10.1080/17425255.2021.1940952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Motegi A, Masutani M, Yoshioka KI, Bessho T. Aberrations in DNA repair pathways in cancer and therapeutic significances. Semin Cancer Biol. 2019;58:29–46. doi: 10.1016/j.semcancer.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Liu R, Li J, Shao J, Lee JH, Qiu X, Xiao Y, Zhang B, Hao Y, Li M, Chen Q. Innate immune response orchestrates phosphoribosyl pyrophosphate synthetases to support DNA repair. Cell Metab. 2021;33:2076–89.e9. doi: 10.1016/j.cmet.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer. 2021;21:37–50. doi: 10.1038/s41568-020-00308-y. [DOI] [PubMed] [Google Scholar]
- 87.Paukovcekova S, Krchniakova M, Chlapek P, Neradil J, Skoda J, Veselska R. Thiosemicarbazones can act synergistically with anthracyclines to downregulate CHEK1 expression and induce DNA damage in cell lines derived from pediatric solid tumors. Int J Mol Sci. 2022;23:8459. doi: 10.3390/ijms23158549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferri A, Stagni V, Barilà D. Targeting the DNA damage response to overcome cancer drug resistance in glioblastoma. Int J Mol Sci. 2020;21:4910. doi: 10.3390/ijms21144910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gobin M, Nazarov PV, Warta R, Timmer M, Reifenberger G, Felsberg J, Vallar L, Chalmers AJ, Herold-Mende CC, Goldbrunner R, et al. A DNA repair and cell-cycle gene expression signature in primary and recurrent glioblastoma: prognostic value and clinical implications. Cancer Res. 2019;79:1226–1238. doi: 10.1158/0008-5472.CAN-18-2076. [DOI] [PubMed] [Google Scholar]
- 90.Mauri G, Arena S, Siena S, Bardelli A, Sartore-Bianchi A. The DNA damage response pathway as a land of therapeutic opportunities for colorectal cancer. Ann Oncol. 2020;31:1135–1147. doi: 10.1016/j.annonc.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 91.Katsuta E, Sawant Dessai A, Ebos JM, Yan L, Ouchi T, Takabe K. H2AX mRNA expression reflects DNA repair, cell proliferation, metastasis, and worse survival in breast cancer. Am J Cancer Res. 2022;12:793–804. [PMC free article] [PubMed] [Google Scholar]
- 92.Nowsheen S, Aziz K, Aziz A, Deng M, Qin B, Luo K, Jeganathan KB, Zhang H, Liu T, Yu J, et al. L3MBTL2 orchestrates ubiquitin signalling by dictating the sequential recruitment of RNF8 and RNF168 after DNA damage. Nat Cell Biol. 2018;20:455–464. doi: 10.1038/s41556-018-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu X, Xu M, Zhu Q, Zhang J, Liu G, Bao Y, Gu L, Tian Y, Wen H, Zhu WG. RNF8-ubiquitinated KMT5A is required for RNF168-induced H2A ubiquitination in response to DNA damage. Faseb j. 2021;35:e21326. doi: 10.1096/fj.202002234R. [DOI] [PubMed] [Google Scholar]
- 94.Du C, Hansen LJ, Singh SX, Wang F, Sun R, Moure CJ, Roso K, Greer PK, Yan H, He Y. A PRMT5-RNF168-SMURF2 axis controls H2AX proteostasis. Cell Rep. 2019;28:3199–211.e5. doi: 10.1016/j.celrep.2019.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gruosso T, Mieulet V, Cardon M, Bourachot B, Kieffer Y, Devun F, Dubois T, Dutreix M, Vincent-Salomon A, Miller KM, et al. Chronic oxidative stress promotes H2AX protein degradation and enhances chemosensitivity in breast cancer patients. EMBO Mol Med. 2016;8:527–549. doi: 10.15252/emmm.201505891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Atsumi Y, Minakawa Y, Ono M, Dobashi S, Shinohe K, Shinohara A, Takeda S, Takagi M, Takamatsu N, Nakagama H, et al. ATM and SIRT6/SNF2H mediate transient H2AX stabilization when DSBs form by blocking HUWE1 to allow efficient γH2AX foci formation. Cell Rep. 2015;13:2728–2740. doi: 10.1016/j.celrep.2015.11.054. [DOI] [PubMed] [Google Scholar]
- 97.Wang A, Ning Z, Lu C, Gao W, Liang J, Yan Q, Tan G, Liu J. USP22 induces cisplatin resistance in lung adenocarcinoma by regulating γH2AX-mediated DNA damage repair and Ku70/Bax-mediated apoptosis. Front Pharmacol. 2017;8:274. doi: 10.3389/fphar.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Delgado-Díaz MR, Martín Y, Berg A, Freire R, Smits VA. Dub3 controls DNA damage signalling by direct deubiquitination of H2AX. Mol Oncol. 2014;8:884–893. doi: 10.1016/j.molonc.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sharma N, Zhu Q, Wani G, He J, Wang QE, Wani AA. USP3 counteracts RNF168 via deubiquitinating H2A and γH2AX at lysine 13 and 15. Cell Cycle. 2014;13:106–114. doi: 10.4161/cc.26814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie R, Yan Z, Jing J, Wang Y, Zhang J, Li Y, Liu X, Yu X, Wu C. Functional defects of cancer-associated MDC1 mutations in DNA damage repair. DNA Repair (Amst) 2022;114:103330. doi: 10.1016/j.dnarep.2022.103330. [DOI] [PubMed] [Google Scholar]
- 101.Su D, Ma S, Shan L, Wang Y, Wang Y, Cao C, Liu B, Yang C, Wang L, Tian S, et al. Ubiquitin-specific protease 7 sustains DNA damage response and promotes cervical carcinogenesis. J Clin Investig. 2018;128:4280–4296. doi: 10.1172/JCI120518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moiseeva TN, Yin Y, Calderon MJ, Qian C, Schamus-Haynes S, Sugitani N, Osmanbeyoglu HU, Rothenberg E, Watkins SC, Bakkenist CJ. An ATR and CHK1 kinase signaling mechanism that limits origin firing during unperturbed DNA replication. Proc Natl Acad Sci USA. 2019;116:13374–13383. doi: 10.1073/pnas.1903418116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Yang L, Wang C, Zhao W, Ju Z, Zhang W, Shen J, Peng Y, An C, Luu YT, et al. Inhibition of the ATM/Chk2 axis promotes cGAS/STING signaling in ARID1A-deficient tumors. J Clin Investig. 2020;130:5951–5966. doi: 10.1172/JCI130445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cartel M, Mouchel PL, Gotanègre M, David L, Bertoli S, Mansat-De Mas V, Besson A, Sarry JE, Manenti S, Didier C. Inhibition of ubiquitin-specific protease 7 sensitizes acute myeloid leukemia to chemotherapy. Leukemia. 2021;35:417–432. doi: 10.1038/s41375-020-0878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lara-Chica M, Correa-Sáez A, Jiménez-Izquierdo R, Garrido-Rodríguez M, Ponce FJ, Moreno R, Morrison K, Di Vona C, Arató K, Jiménez-Jiménez C, et al. A novel CDC25A/DYRK2 regulatory switch modulates cell cycle and survival. Cell Death Differ. 2022;29:105–117. doi: 10.1038/s41418-021-00845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Das S, Chandrasekaran AP, Jo KS, Ko NR, Oh SJ, Kim KS, Ramakrishna S. HAUSP stabilizes Cdc25A and protects cervical cancer cells from DNA damage response. Biochim Biophys Acta Mol Cell Res. 2020;1867:118835. doi: 10.1016/j.bbamcr.2020.118835. [DOI] [PubMed] [Google Scholar]
- 107.Giacomelli AO, Yang X, Lintner RE, McFarland JM, Duby M, Kim J, Howard TP, Takeda DY, Ly SH, Kim E, et al. Mutational processes shape the landscape of TP53 mutations in human cancer. Nat Genet. 2018;50:1381–1387. doi: 10.1038/s41588-018-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harakandi C, Nininahazwe L, Xu H, Liu B, He C, Zheng YC, Zhang H. Recent advances on the intervention sites targeting USP7-MDM2-p53 in cancer therapy. Bioorg Chem. 2021;116:105273. doi: 10.1016/j.bioorg.2021.105273. [DOI] [PubMed] [Google Scholar]
- 109.Pei Y, Fu J, Shi Y, Zhang M, Luo G, Luo X, Song N, Mi T, Yang Y, Li J, et al. Discovery of a potent and selective degrader for USP7. Angew Chem Int Ed Engl. 2022;61:e202204395. doi: 10.1002/anie.202204395. [DOI] [PubMed] [Google Scholar]
- 110.Peng B, Shi R, Bian J, Li Y, Wang P, Wang H, Liao J, Zhu WG, Xu X. PARP1 and CHK1 coordinate PLK1 enzymatic activity during the DNA damage response to promote homologous recombination-mediated repair. Nucleic Acids Res. 2021;49:7554–7570. doi: 10.1093/nar/gkab584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peng Y, Liu Y, Gao Y, Yuan B, Qi X, Fu Y, Zhu Q, Cao T, Zhang S, Yin L, et al. USP7 is a novel Deubiquitinase sustaining PLK1 protein stability and regulating chromosome alignment in mitosis. J Exp Clin Cancer Res. 2019;38:468. doi: 10.1186/s13046-019-1457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L, Nemzow L, Chen H, Lubin A, Rong X, Sun Z, Harris TK, Gong F. The deubiquitinating enzyme USP24 is a regulator of the UV damage response. Cell Rep. 2015;10:140–147. doi: 10.1016/j.celrep.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Müller I, Strozyk E, Schindler S, Beissert S, Oo HZ, Sauter T, Lucarelli P, Raeth S, Hausser A, Al Nakouzi N, et al. Cancer cells employ nuclear caspase-8 to overcome the p53-dependent G2/M checkpoint through cleavage of USP28. Mol Cell. 2020;77:970–84.e7. doi: 10.1016/j.molcel.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu J, Chen Y, Geng G, Li L, Yin P, Nowsheen S, Li Y, Wu C, Liu J, Zhao F, et al. USP39 regulates DNA damage response and chemo-radiation resistance by deubiquitinating and stabilizing CHK2. Cancer Lett. 2019;449:114–124. doi: 10.1016/j.canlet.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 115.Tu Y, Chen Z, Zhao P, Sun G, Bao Z, Chao H, Fan L, Li C, You Y, Qu Y, et al. Smoothened promotes glioblastoma radiation resistance via activating USP3-mediated claspin deubiquitination. Clin Cancer Res Off J Am Assoc Cancer Res. 2020;26:1749–1762. doi: 10.1158/1078-0432.CCR-19-1515. [DOI] [PubMed] [Google Scholar]
- 116.Pfeiffer A, Luijsterburg MS, Acs K, Wiegant WW, Helfricht A, Herzog LK, Minoia M, Böttcher C, Salomons FA, van Attikum H, et al. Ataxin-3 consolidates the MDC1-dependent DNA double-strand break response by counteracting the SUMO-targeted ubiquitin ligase RNF4. EMBO J. 2017;36:1066–1083. doi: 10.15252/embj.201695151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cassidy KB, Bang S, Kurokawa M, Gerber SA. Direct regulation of Chk1 protein stability by E3 ubiquitin ligase HUWE1. Febs j. 2020;287:1985–1999. doi: 10.1111/febs.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.García-Limones C, Lara-Chica M, Jiménez-Jiménez C, Pérez M, Moreno P, Muñoz E, Calzado MA. CHK2 stability is regulated by the E3 ubiquitin ligase SIAH2. Oncogene. 2016;35:4289–4301. doi: 10.1038/onc.2015.495. [DOI] [PubMed] [Google Scholar]
- 119.Daks A, Fedorova O, Parfenyev S, Nevzorov I, Shuvalov O, Barlev NA. The role of E3 ligase Pirh2 in disease. Cells. 2022;11:1515. doi: 10.3390/cells11091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu Q, Chen H, Li X, Wang X, Yan H. JMJD2C mediates the MDM2/p53/IL5RA axis to promote CDDP resistance in uveal melanoma. Cell Death Discov. 2022;8:227. doi: 10.1038/s41420-022-00949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu AY, Gu LY, Cang W, Cheng MX, Wang WJ, Di W, Huang L, Qiu LH. Fn14 overcomes cisplatin resistance of high-grade serous ovarian cancer by promoting Mdm2-mediated p53–R248Q ubiquitination and degradation. J Exp Clin Cancer Res. 2019;38:176. doi: 10.1186/s13046-019-1171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ning Y, Hui N, Qing B, Zhuo Y, Sun W, Du Y, Liu S, Liu K, Zhou J. ZCCHC10 suppresses lung cancer progression and cisplatin resistance by attenuating MDM2-mediated p53 ubiquitination and degradation. Cell Death Dis. 2019;10:414. doi: 10.1038/s41419-019-1635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang L, Li DQ. MORC2 regulates DNA damage response through a PARP1-dependent pathway. Nucleic Acids Res. 2019;47:8502–8520. doi: 10.1093/nar/gkz545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang F, Xie HY, Yang LF, Zhang L, Zhang FL, Liu HY, Li DQ, Shao ZM. Stabilization of MORC2 by estrogen and antiestrogens through GPER1- PRKACA-CMA pathway contributes to estrogen-induced proliferation and endocrine resistance of breast cancer cells. Autophagy. 2020;16:1061–1076. doi: 10.1080/15548627.2019.1659609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kockler ZW, Osia B, Lee R, Musmaker K, Malkova A. Repair of DNA breaks by break-induced replication. Annu Rev Biochem. 2021;90:165–191. doi: 10.1146/annurev-biochem-081420-095551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Waterman DP, Haber JE, Smolka MB. Checkpoint responses to DNA double-strand breaks. Annu Rev Biochem. 2020;89:103–133. doi: 10.1146/annurev-biochem-011520-104722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao W, Wiese C, Kwon Y, Hromas R, Sung P. The BRCA tumor suppressor network in chromosome damage repair by homologous recombination. Annu Rev Biochem. 2019;88:221–245. doi: 10.1146/annurev-biochem-013118-111058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao B, Rothenberg E, Ramsden DA, Lieber MR. The molecular basis and disease relevance of non-homologous DNA end joining. Nat Rev Mol Cell Biol. 2020;21:765–781. doi: 10.1038/s41580-020-00297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang HHY, Pannunzio NR, Adachi N, Lieber MR. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18:495–506. doi: 10.1038/nrm.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pop L, Suciu I, Ionescu O, Bacalbasa N, Ionescu P. The role of novel poly (ADP-ribose) inhibitors in the treatment of locally advanced and metastatic Her-2/neu negative breast cancer with inherited germline BRCA1/2 mutations. A review of the literature. J Med Life. 2021;14:17–20. doi: 10.25122/jml-2020-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y, Liu CF, Rao GW. A review on poly (ADP-ribose) polymerase (PARP) inhibitors and synthetic methodologies. Curr Med Chem. 2021;28:1565–1584. doi: 10.2174/0929867327666200312113011. [DOI] [PubMed] [Google Scholar]
- 132.Sala-Gaston J, Martinez-Martinez A, Pedrazza L, Lorenzo-Martín LF, Caloto R, Bustelo XR, Ventura F, Rosa JL. HERC ubiquitin ligases in cancer. Cancers. 2020;12:1653. doi: 10.3390/cancers12061653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu S, Wu X, Wang P, Cao SL, Peng B, Xu X. ASPM promotes homologous recombination-mediated DNA repair by safeguarding BRCA1 stability. iScience. 2021;24:102534. doi: 10.1016/j.isci.2021.102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu Q, Zhang FL, Lu DY, Shao ZM, Li DQ. USP9X stabilizes BRCA1 and confers resistance to DNA-damaging agents in human cancer cells. Cancer Med. 2019;8:6730–6740. doi: 10.1002/cam4.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang J, Kho DH, Zhou JY, Davis RJ, Wu GS. MKP-1 suppresses PARP-1 degradation to mediate cisplatin resistance. Oncogene. 2017;36:5939–5947. doi: 10.1038/onc.2017.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yun EJ, Lin CJ, Dang A, Hernandez E, Guo J, Chen WM, Allison J, Kim N, Kapur P, Brugarolas J, et al. Downregulation of human DAB2IP gene expression in renal cell carcinoma results in resistance to ionizing radiation. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25:4542–4551. doi: 10.1158/1078-0432.CCR-18-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marzio A, Kurz E, Sahni JM, Di Feo G, Puccini J, Jiang S, Hirsch CA, Arbini AA, Wu WL, Pass HI, et al. EMSY inhibits homologous recombination repair and the interferon response, promoting lung cancer immune evasion. Cell. 2022;185:169–83.e19. doi: 10.1016/j.cell.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xie Y, Liu YK, Guo ZP, Guan H, Liu XD, Xie DF, Jiang YG, Ma T, Zhou PK. RBX1 prompts degradation of EXO1 to limit the homologous recombination pathway of DNA double-strand break repair in G1 phase. Cell Death Differ. 2020;27:1383–1397. doi: 10.1038/s41418-019-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu X, Wang X, Yan W, Yang H, Xiang Y, Lv F, Shi Y, Li HY, Lan L. Ubiquitination-mediated degradation of TRDMT1 regulates homologous recombination and therapeutic response. NAR Cancer. 2021;3:zcab010. doi: 10.1093/narcan/zcab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ho SR, Mahanic CS, Lee YJ, Lin WC. RNF144A, an E3 ubiquitin ligase for DNA-PKcs, promotes apoptosis during DNA damage. Proc Natl Acad Sci USA. 2014;111:E2646–E2655. doi: 10.1073/pnas.1323107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang Q, Karnak D, Tan M, Lawrence TS, Morgan MA, Sun Y. FBXW7 facilitates nonhomologous end-joining via K63-linked polyubiquitylation of XRCC4. Mol Cell. 2016;61:419–433. doi: 10.1016/j.molcel.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Germano G, Amirouchene-Angelozzi N, Rospo G, Bardelli A. The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov. 2018;8:1518–1528. doi: 10.1158/2159-8290.CD-18-0150. [DOI] [PubMed] [Google Scholar]
- 143.Jin Z, Sinicrope FA. Mismatch repair-deficient colorectal cancer: building on checkpoint blockade. J Clin Oncol Off J Am Soc Clin Oncol. 2022;40:2735–2750. doi: 10.1200/JCO.21.02691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qiu W, Ding K, Liao L, Ling Y, Luo X, Wang J. Analysis of the expression and prognostic value of MSH2 in pan-cancer based on bioinformatics. Biomed Res Int. 2021;2021:9485273. doi: 10.1155/2021/9485273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: a review. Cancer Treat Rev. 2016;51:19–26. doi: 10.1016/j.ctrv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 146.Zhang M, Xiang S, Joo HY, Wang L, Williams KA, Liu W, Hu C, Tong D, Haakenson J, Wang C, et al. HDAC6 deacetylates and ubiquitinates MSH2 to maintain proper levels of MutSα. Mol Cell. 2014;55:31–46. doi: 10.1016/j.molcel.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang M, Hu C, Tong D, Xiang S, Williams K, Bai W, Li GM, Bepler G, Zhang X. Ubiquitin-specific peptidase 10 (USP10) deubiquitinates and stabilizes MutS homolog 2 (MSH2) to regulate cellular sensitivity to DNA damage. J Biol Chem. 2016;291:10783–10791. doi: 10.1074/jbc.M115.700047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zeng Z, Li D, Yu T, Huang Y, Yan H, Gu L, Yuan J. Association and clinical implication of the USP10 and MSH2 proteins in non-small cell lung cancer. Oncol Lett. 2019;17:1128–1138. doi: 10.3892/ol.2018.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Konieczkowski DJ, Johannessen CM, Garraway LA. A convergence-based framework for cancer drug resistance. Cancer Cell. 2018;33:801–815. doi: 10.1016/j.ccell.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hughes D, Andersson DI. Evolutionary consequences of drug resistance: shared principles across diverse targets and organisms. Nat Rev Genet. 2015;16:459–471. doi: 10.1038/nrg3922. [DOI] [PubMed] [Google Scholar]
- 151.Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10:1808–1825. doi: 10.1158/2159-8290.CD-20-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dentro SC, Leshchiner I, Haase K, Tarabichi M, Wintersinger J, Deshwar AG, Yu K, Rubanova Y, Macintyre G, Demeulemeester J, et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell. 2021;184:2239–54.e39. doi: 10.1016/j.cell.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol. 2022;19:499–514. doi: 10.1038/s41571-022-00639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tian X, Gu T, Lee MH, Dong Z. Challenge and countermeasures for EGFR targeted therapy in non-small cell lung cancer. Biochim Biophys Acta Rev Cancer. 2022;1877:188645. doi: 10.1016/j.bbcan.2021.188645. [DOI] [PubMed] [Google Scholar]
- 155.Surve S, Watkins SC, Sorkin A. EGFR-RAS-MAPK signaling is confined to the plasma membrane and associated endorecycling protrusions. J Cell Biol. 2021;220:e202107103. doi: 10.1083/jcb.202107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li X, Fan XX, Jiang ZB, Loo WT, Yao XJ, Leung EL, Chow LW, Liu L. Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacol Res. 2017;115:45–55. doi: 10.1016/j.phrs.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 157.Yu P, Fan Y, Qu X, Zhang J, Song N, Liu J, Liu Y. Cbl-b regulates the sensitivity of cetuximab through ubiquitin-proteasome system in human gastric cancer cells. J buon. 2016;21:867–873. [PubMed] [Google Scholar]
- 158.Zhang T, Zheng C, Hou K, Wang J, Zhang Y, Fan Y, Zhao H, Qu X, Liu Y, Kang J, et al. Suppressed expression of Cbl-b by NF-κB mediates icotinib resistance in EGFR-mutant non-small-cell lung cancer. Cell Biol Int. 2019;43:98–107. doi: 10.1002/cbin.11026. [DOI] [PubMed] [Google Scholar]
- 159.Kadera BE, Toste PA, Wu N, Li L, Nguyen AH, Dawson DW, Donahue TR. Low expression of the E3 ubiquitin ligase CBL confers chemoresistance in human pancreatic cancer and is targeted by epidermal growth factor receptor inhibition. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:157–165. doi: 10.1158/1078-0432.CCR-14-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Che X, Zhang Y, Qu X, Guo T, Ma Y, Li C, Fan Y, Hou K, Cai Y, Yu R. The E3 ubiquitin ligase Cbl-b inhibits tumor growth in multidrug-resistant gastric and breast cancer cells. Neoplasma. 2017;64:887–892. doi: 10.4149/neo_2017_610. [DOI] [PubMed] [Google Scholar]
- 161.Zhao L, Qiu T, Jiang D, Xu H, Zou L, Yang Q, Chen C, Jiao B. SGCE promotes breast cancer stem cells by stabilizing EGFR. Adv Sci. 2020;7:1903700. doi: 10.1002/advs.201903700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee YJ, Ho SR, Graves JD, Xiao Y, Huang S, Lin WC. CGRRF1, a growth suppressor, regulates EGFR ubiquitination in breast cancer. Breast Cancer Res. 2019;21:134. doi: 10.1186/s13058-019-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao X, Sun L, Mu T, Yi J, Ma C, Xie H, Liu M, Tang H. An HBV-encoded miRNA activates innate immunity to restrict HBV replication. J Mol Cell Biol. 2020;12:263–276. doi: 10.1093/jmcb/mjz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gao SP, Chang Q, Mao N, Daly LA, Vogel R, Chan T, Liu SH, Bournazou E, Schori E, Zhang H, et al. JAK2 inhibition sensitizes resistant EGFR-mutant lung adenocarcinoma to tyrosine kinase inhibitors. Sci Signal. 2016;9:ra33. doi: 10.1126/scisignal.aac8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith CJ, Berry DM, McGlade CJ. The E3 ubiquitin ligases RNF126 and Rabring7 regulate endosomal sorting of the epidermal growth factor receptor. J Cell Sci. 2013;126:1366–1380. doi: 10.1242/jcs.116129. [DOI] [PubMed] [Google Scholar]
- 166.Zhang R, Liu W, Sun J, Kong Y, Chen C. Roles of RNF126 and BCA2 E3 ubiquitin ligases in DNA damage repair signaling and targeted cancer therapy. Pharmacol Res. 2020;155:104748. doi: 10.1016/j.phrs.2020.104748. [DOI] [PubMed] [Google Scholar]
- 167.Shen CH, Chou CC, Lai TY, Hsu JE, Lin YS, Liu HY, Chen YK, Ho IL, Hsu PH, Chuang TH, et al. ZNRF1 mediates epidermal growth factor receptor ubiquitination to control receptor lysosomal trafficking and degradation. Front Cell Dev Biol. 2021;9:642625. doi: 10.3389/fcell.2021.642625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu H, Yang X, Xuan X, Wu D, Zhang J, Xu X, Zhao Y, Ma C, Li D. STAMBP promotes lung adenocarcinoma metastasis by regulating the EGFR/MAPK signaling pathway. Neoplasia. 2021;23:607–623. doi: 10.1016/j.neo.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang H, Han B, Lu H, Zhao Y, Chen X, Meng Q, Cao M, Cai L, Hu J. USP22 promotes resistance to EGFR-TKIs by preventing ubiquitination-mediated EGFR degradation in EGFR-mutant lung adenocarcinoma. Cancer Lett. 2018;433:186–198. doi: 10.1016/j.canlet.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 170.Jin Y, Zhang W, Xu J, Wang H, Zhang Z, Chu C, Liu X, Zou Q. UCH-L1 involved in regulating the degradation of EGFR and promoting malignant properties in drug-resistant breast cancer. Int J Clin Exp Pathol. 2015;8:12500–12508. [PMC free article] [PubMed] [Google Scholar]
- 171.Lei S, He Z, Chen T, Guo X, Zeng Z, Shen Y, Jiang J. Long noncoding RNA 00976 promotes pancreatic cancer progression through OTUD7B by sponging miR-137 involving EGFR/MAPK pathway. J Exp Clin Cancer Res. 2019;38:470. doi: 10.1186/s13046-019-1388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Giron P, Eggermont C, Noeparast A, Vandenplas H, Teugels E, Forsyth R, De Wever O, Aza-Blanc P, Gutierrez GJ, De Grève J. Targeting USP13-mediated drug tolerance increases the efficacy of EGFR inhibition of mutant EGFR in non-small cell lung cancer. Int J Cancer. 2020;148:2579–2593. doi: 10.1002/ijc.33404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liao Y, Guo Z, Xia X, Liu Y, Huang C, Jiang L, Wang X, Liu J, Huang H. Inhibition of EGFR signaling with Spautin-1 represents a novel therapeutics for prostate cancer. J Exp Clin Cancer Res. 2019;38:157. doi: 10.1186/s13046-019-1165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hofmann MH, Gerlach D, Misale S, Petronczki M, Kraut N. Expanding the reach of precision oncology by drugging All KRAS mutants. Cancer Discov. 2022;12:924–937. doi: 10.1158/2159-8290.CD-21-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhu C, Guan X, Zhang X, Luan X, Song Z, Cheng X, Zhang W, Qin JJ. Targeting KRAS mutant cancers: from druggable therapy to drug resistance. Mol Cancer. 2022;21:159. doi: 10.1186/s12943-022-01629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Akhave NS, Biter AB, Hong DS. Mechanisms of resistance to KRAS(G12C)-targeted therapy. Cancer Discov. 2021;11:1345–1352. doi: 10.1158/2159-8290.CD-20-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thein KZ, Biter AB, Hong DS. Therapeutics targeting mutant KRAS. Annu Rev Med. 2021;72:349–364. doi: 10.1146/annurev-med-080819-033145. [DOI] [PubMed] [Google Scholar]
- 178.Shukla S, Allam US, Ahsan A, Chen G, Krishnamurthy PM, Marsh K, Rumschlag M, Shankar S, Whitehead C, Schipper M, et al. KRAS protein stability is regulated through SMURF2: UBCH5 complex-mediated β-TrCP1 degradation. Neoplasia. 2014;16:115–128. doi: 10.1593/neo.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gong RH, Chen M, Huang C, Wong HLX, Kwan HY, Bian Z. Combination of artesunate and WNT974 induces KRAS protein degradation by upregulating E3 ligase ANACP2 and β-TrCP in the ubiquitin-proteasome pathway. Cell Commun Signal. 2022;20:34. doi: 10.1186/s12964-022-00834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bigenzahn JW, Collu GM, Kartnig F, Pieraks M, Vladimer GI, Heinz LX, Sedlyarov V, Schischlik F, Fauster A, Rebsamen M, et al. LZTR1 is a regulator of RAS ubiquitination and signaling. Science. 2018;362:1171–1177. doi: 10.1126/science.aap8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abe T, Umeki I, Kanno SI, Inoue SI, Niihori T, Aoki Y. LZTR1 facilitates polyubiquitination and degradation of RAS-GTPases. Cell Death Differ. 2020;27:1023–1035. doi: 10.1038/s41418-019-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Janku F. Advances on the BRAF front in colorectal cancer. Cancer Discov. 2018;8:389–391. doi: 10.1158/2159-8290.CD-18-0125. [DOI] [PubMed] [Google Scholar]
- 183.Ribas A, Lo RS. Trying for a BRAF slam dunk. Cancer Discov. 2020;10:640–642. doi: 10.1158/2159-8290.CD-20-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Grothey A, Fakih M, Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol. 2021;32:959–967. doi: 10.1016/j.annonc.2021.03.206. [DOI] [PubMed] [Google Scholar]
- 185.Menzer C, Menzies AM, Carlino MS, Reijers I, Groen EJ, Eigentler T, de Groot JWB, van der Veldt AAM, Johnson DB, Meiss F, et al. Targeted therapy in advanced melanoma with rare BRAF mutations. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37:3142–3151. doi: 10.1200/JCO.19.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, Keedy V, Bielle F, Hofheinz RD, Joly F, et al. BRAF inhibition in BRAF(V600)-mutant gliomas: results from the VE-BASKET study. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36:3477–3484. doi: 10.1200/JCO.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yumimoto K, Nakayama KI. Recent insight into the role of FBXW7 as a tumor suppressor. Semin Cancer Biol. 2020;67:1–15. doi: 10.1016/j.semcancer.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 188.Else M, Blakemore SJ, Strefford JC, Catovsky D. The association between deaths from infection and mutations of the BRAF, FBXW7, NRAS and XPO1 genes: a report from the LRF CLL4 trial. Leukemia. 2021;35:2563–2569. doi: 10.1038/s41375-021-01165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aydin IT, Abbate F, Rajan GS, Badal B, Aifantis I, Desman G, Celebi JT. FBXW7 inactivation in a Braf(V600E) -driven mouse model leads to melanoma development. Pigment Cell Melanoma Res. 2017;30:571–574. doi: 10.1111/pcmr.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yeh CH, Bellon M, Wang F, Zhang H, Fu L, Nicot C. Loss of FBXW7-mediated degradation of BRAF elicits resistance to BET inhibitors in adult T cell leukemia cells. Mol Cancer. 2020;19:139. doi: 10.1186/s12943-020-01254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Saei A, Palafox M, Benoukraf T, Kumari N, Jaynes PW, Iyengar PV, Muñoz-Couselo E, Nuciforo P, Cortés J, Nötzel C, et al. Loss of USP28-mediated BRAF degradation drives resistance to RAF cancer therapies. J Exp Med. 2018;215:1913–1928. doi: 10.1084/jem.20171960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hong SW, Jin DH, Shin JS, Moon JH, Na YS, Jung KA, Kim SM, Kim JC, Kim KP, Hong YS, et al. Ring finger protein 149 is an E3 ubiquitin ligase active on wild-type v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) J Biol Chem. 2012;287:24017–24025. doi: 10.1074/jbc.M111.319822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Didier R, Mallavialle A, Ben Jouira R, Domdom MA, Tichet M, Auberger P, Luciano F, Ohanna M, Tartare-Deckert S, Deckert M. Targeting the proteasome-associated deubiquitinating enzyme USP14 impairs melanoma cell survival and overcomes resistance to MAPK-targeting therapies. Mol Cancer Ther. 2018;17:1416–1429. doi: 10.1158/1535-7163.MCT-17-0919. [DOI] [PubMed] [Google Scholar]
- 194.Potu H, Peterson LF, Pal A, Verhaegen M, Cao J, Talpaz M, Donato NJ. Usp5 links suppression of p53 and FAS levels in melanoma to the BRAF pathway. Oncotarget. 2014;5:5559–5569. doi: 10.18632/oncotarget.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dhanasekaran R, Deutzmann A, Mahauad-Fernandez WD, Hansen AS, Gouw AM, Felsher DW. The MYC oncogene - the grand orchestrator of cancer growth and immune evasion. Nat Rev Clin Oncol. 2022;19:23–36. doi: 10.1038/s41571-021-00549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Llombart V, Mansour MR. Therapeutic targeting of "undruggable" MYC. EBioMedicine. 2022;75:103756. doi: 10.1016/j.ebiom.2021.103756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang H, Yang W, Qin Q, Yang X, Yang Y, Liu H, Lu W, Gu S, Cao X, Feng D, et al. E3 ubiquitin ligase MAGI3 degrades c-Myc and acts as a predictor for chemotherapy response in colorectal cancer. Mol Cancer. 2022;21:151. doi: 10.1186/s12943-022-01622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li M, Ouyang L, Zheng Z, Xiang D, Ti A, Li L, Dan Y, Yu C, Li W. E3 ubiquitin ligase FBW7α inhibits cholangiocarcinoma cell proliferation by downregulating c-Myc and cyclin E. Oncol Rep. 2017;37:1627–1636. doi: 10.3892/or.2017.5432. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Q, Li X, Cui K, Liu C, Wu M, Prochownik EV, Li Y. The MAP3K13-TRIM25-FBXW7α axis affects c-Myc protein stability and tumor development. Cell Death Differ. 2020;27:420–433. doi: 10.1038/s41418-019-0363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim D, Hong A, Park HI, Shin WH, Yoo L, Jeon SJ, Chung KC. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J Cell Physiol. 2017;232:3664–3676. doi: 10.1002/jcp.25841. [DOI] [PubMed] [Google Scholar]
- 201.Ruiz EJ, Pinto-Fernandez A, Turnbull AP, Lan L, Charlton TM, Scott HC, Damianou A, Vere G, Riising EM, Da Costa C, et al. USP28 deletion and small-molecule inhibition destabilizes c-MYC and elicits regression of squamous cell lung carcinoma. Elife. 2021;10:e71596. doi: 10.7554/eLife.71596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Peng Y, Liu J, Wang Z, Cui C, Zhang T, Zhang S, Gao P, Hou Z, Liu H, Guo J, et al. Prostate-specific oncogene OTUD6A promotes prostatic tumorigenesis via deubiquitinating and stabilizing c-Myc. Cell Death Differ. 2022;29:1730–1743. doi: 10.1038/s41418-022-00960-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nussinov R, Tsai CJ, Jang H. Anticancer drug resistance: an update and perspective. Drug Resist Updat. 2021;59:100796. doi: 10.1016/j.drup.2021.100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Carneiro B, El-Deiry W. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395–417. doi: 10.1038/s41571-020-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu X, Luo Q, Zhao P, Chang W, Wang Y, Shu T, Ding F, Li B, Liu Z. MGMT-activated DUB3 stabilizes MCL1 and drives chemoresistance in ovarian cancer. Proc Natl Acad Sci USA. 2019;116:2961–2966. doi: 10.1073/pnas.1814742116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sulkshane P, Pawar SN, Waghole R, Pawar SS, Rajput P, Uthale A, Oak S, Kalkar P, Wani H, Patil R, et al. Elevated USP9X drives early-to-late-stage oral tumorigenesis via stabilisation of anti-apoptotic MCL-1 protein and impacts outcome in oral cancers. Br J Cancer. 2021;125:547–560. doi: 10.1038/s41416-021-01421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang S, Zhang M, Jing Y, Yin X, Ma P, Zhang Z, Wang X, Di W, Zhuang G. Deubiquitinase USP13 dictates MCL1 stability and sensitivity to BH3 mimetic inhibitors. Nat Commun. 2018;9:215. doi: 10.1038/s41467-017-02693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu Y, Xu X, Lin P, He Y, Zhang Y, Cao B, Zhang Z, Sethi G, Liu J, Zhou X, et al. Inhibition of the deubiquitinase USP9x induces pre-B cell homeobox 1 (PBX1) degradation and thereby stimulates prostate cancer cell apoptosis. J Biol Chem. 2019;294:4572–4582. doi: 10.1074/jbc.RA118.006057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu D, Fan Y, Li J, Cheng B, Lin W, Li X, Du J, Ling C. Inhibition of cFLIP overcomes acquired resistance to sorafenib via reducing ER stress-related autophagy in hepatocellular carcinoma. Oncol Rep. 2018;40:2206–2214. doi: 10.3892/or.2018.6606. [DOI] [PubMed] [Google Scholar]
- 210.Nie ZY, Yao M, Yang Z, Yang L, Liu XJ, Yu J, Ma Y, Zhang N, Zhang XY, Liu MH, et al. De-regulated STAT5A/miR-202-5p/USP15/Caspase-6 regulatory axis suppresses CML cell apoptosis and contributes to Imatinib resistance. J Exp Clin Cancer Res. 2020;39:17. doi: 10.1186/s13046-019-1502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Song Y, Li S, Ray A, Das DS, Qi J, Samur MK, Tai YT, Munshi N, Carrasco RD, Chauhan D, et al. Blockade of deubiquitylating enzyme Rpn11 triggers apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Oncogene. 2017;36:5631–5638. doi: 10.1038/onc.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang Y, Ma L, Wang C, Sheng G, Feng L, Yin C. Autocrine motility factor receptor promotes the proliferation of human acute monocytic leukemia THP-1 cells. Int J Mol Med. 2015;36:627–632. doi: 10.3892/ijmm.2015.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cho Y, Kang HG, Kim SJ, Lee S, Jee S, Ahn SG, Kang MJ, Song JS, Chung JY, Yi EC, et al. Post-translational modification of OCT4 in breast cancer tumorigenesis. Cell Death Differ. 2018;25:1781–1795. doi: 10.1038/s41418-018-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tsuchiya M, Nakajima Y, Waku T, Hiyoshi H, Morishita T, Furumai R, Hayashi Y, Kishimoto H, Kimura K, Yanagisawa J. CHIP buffers heterogeneous Bcl-2 expression levels to prevent augmentation of anticancer drug-resistant cell population. Oncogene. 2015;34:4656–4663. doi: 10.1038/onc.2014.387. [DOI] [PubMed] [Google Scholar]
- 215.Hu X, Meng Y, Xu L, Qiu L, Wei M, Su D, Qi X, Wang Z, Yang S, Liu C, et al. Cul4 E3 ubiquitin ligase regulates ovarian cancer drug resistance by targeting the antiapoptotic protein BIRC3. Cell Death Dis. 2019;10:104. doi: 10.1038/s41419-018-1200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Li Y, Zhou M, Hu Q, Bai XC, Huang W, Scheres SH, Shi Y. Mechanistic insights into caspase-9 activation by the structure of the apoptosome holoenzyme. Proc Natl Acad Sci USA. 2017;114:1542–1547. doi: 10.1073/pnas.1620626114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kurokawa M, Kim J, Geradts J, Matsuura K, Liu L, Ran X, Xia W, Ribar TJ, Henao R, Dewhirst MW, et al. A network of substrates of the E3 ubiquitin ligases MDM2 and HUWE1 control apoptosis independently of p53. Sci Signal. 2013;6:ra32. doi: 10.1126/scisignal.2003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Christian PA, Fiandalo MV, Schwarze SR. Possible role of death receptor-mediated apoptosis by the E3 ubiquitin ligases Siah2 and POSH. Mol Cancer. 2011;10:57. doi: 10.1186/1476-4598-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ichikawa A, Fujita Y, Hosaka Y, Kadota T, Ito A, Yagishita S, Watanabe N, Fujimoto S, Kawamoto H, Saito N, et al. Chaperone-mediated autophagy receptor modulates tumor growth and chemoresistance in non-small cell lung cancer. Cancer Sci. 2020;111:4154–4165. doi: 10.1111/cas.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Assaraf YG, Brozovic A, Gonçalves AC, Jurkovicova D, Linē A, Machuqueiro M, Saponara S, Sarmento-Ribeiro AB, Xavier CPR, Vasconcelos MH. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist Updat. 2019;46:100645. doi: 10.1016/j.drup.2019.100645. [DOI] [PubMed] [Google Scholar]
- 221.Yaeger R, Corcoran RB. Targeting alterations in the RAF-MEK pathway. Cancer Discov. 2019;9:329–341. doi: 10.1158/2159-8290.CD-18-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhu G, Herlyn M, Yang X. TRIM15 and CYLD regulate ERK activation via lysine-63-linked polyubiquitination. Nat Cell Biol. 2021;23:978–991. doi: 10.1038/s41556-021-00732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yin Q, Han T, Fang B, Zhang G, Zhang C, Roberts ER, Izumi V, Zheng M, Jiang S, Yin X, et al. K27-linked ubiquitination of BRAF by ITCH engages cytokine response to maintain MEK-ERK signaling. Nat Commun. 2019;10:1870. doi: 10.1038/s41467-019-09844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wei Y, Jiang Z, Lu J. USP22 promotes melanoma and BRAF inhibitor resistance via YAP stabilization. Oncol Lett. 2021;21:394. doi: 10.3892/ol.2021.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li YY, Wu C, Shah SS, Chen SM, Wangpaichitr M, Kuo MT, Feun LG, Han X, Suarez M, Prince J, et al. Degradation of AMPK-α1 sensitizes BRAF inhibitor-resistant melanoma cells to arginine deprivation. Mol Oncol. 2017;11:1806–1825. doi: 10.1002/1878-0261.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jin L, Chun J, Pan C, Li D, Lin R, Alesi GN, Wang X, Kang HB, Song L, Wang D, et al. MAST1 drives cisplatin resistance in human cancers by rewiring cRaf-independent MEK activation. Cancer Cell. 2018;34:315–30.e7. doi: 10.1016/j.ccell.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pan C, Chun J, Li D, Boese AC, Li J, Kang J, Umano A, Jiang Y, Song L, Magliocca KR, et al. Hsp90B enhances MAST1-mediated cisplatin resistance by protecting MAST1 from proteosomal degradation. J Clin Investig. 2019;129:4110–4123. doi: 10.1172/JCI125963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. 2019;35:347–367. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wilson M, Weinberg R, Lees J, Guen V. Emerging mechanisms by which EMT programs control stemness. Trends Cancer. 2020 doi: 10.1016/j.trecan.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 230.Yang Y, Li X, Wang T, Guo Q, Xi T, Zheng L. Emerging agents that target signaling pathways in cancer stem cells. J Hematol Oncol. 2020;13:60. doi: 10.1186/s13045-020-00901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Soundararajan R, Paranjape AN, Maity S, Aparicio A, Mani SA. EMT, stemness and tumor plasticity in aggressive variant neuroendocrine prostate cancers. Biochim Biophys Acta Rev Cancer. 2018;1870:229–238. doi: 10.1016/j.bbcan.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lambert AW, Weinberg RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021;21:325–338. doi: 10.1038/s41568-021-00332-6. [DOI] [PubMed] [Google Scholar]
- 233.Ming H, Li B, Zhou L, Goel A, Huang C. Long non-coding RNAs and cancer metastasis: molecular basis and therapeutic implications. Biochim Biophys Acta Rev Cancer. 2021;1875:188519. doi: 10.1016/j.bbcan.2021.188519. [DOI] [PubMed] [Google Scholar]
- 234.Fang C, Kang Y. E-cadherin: context-dependent functions of a quintessential epithelial marker in metastasis. Cancer Res. 2021;81:5800–5802. doi: 10.1158/0008-5472.CAN-21-3302. [DOI] [PubMed] [Google Scholar]
- 235.Na TY, Schecterson L, Mendonsa AM, Gumbiner BM. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci USA. 2020;117:5931–5937. doi: 10.1073/pnas.1918167117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shrestha H, Ryu T, Seo YW, Park SY, He Y, Dai W, Park E, Simkhada S, Kim H, Lee K, et al. Hakai, an E3-ligase for E-cadherin, stabilizes δ-catenin through Src kinase. Cell Signal. 2017;31:135–145. doi: 10.1016/j.cellsig.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 237.Zhang Y, Sun L, Gao X, Guo A, Diao Y, Zhao Y. RNF43 ubiquitinates and degrades phosphorylated E-cadherin by c-Src to facilitate epithelial-mesenchymal transition in lung adenocarcinoma. BMC Cancer. 2019;19:670. doi: 10.1186/s12885-019-5880-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 238.Huang Z, Zhou L, Duan J, Qin S, Wang Y, Jiang J, Jin P, Li B, Luo M, He B. Oxidative stress promotes liver cancer metastasis via a PKA-activated RNF25/ECAD/YAP circuit. 2022.
- 239.Goossens S, Vandamme N, Van Vlierberghe P, Berx G. EMT transcription factors in cancer development re-evaluated: beyond EMT and MET. Biochim Biophys Acta Rev Cancer. 2017;1868:584–591. doi: 10.1016/j.bbcan.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 240.Long L, Xiang H, Liu J, Zhang Z, Sun L. ZEB1 mediates doxorubicin (Dox) resistance and mesenchymal characteristics of hepatocarcinoma cells. Exp Mol Pathol. 2019;106:116–122. doi: 10.1016/j.yexmp.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 241.Zhang Z, Li J, Ou Y, Yang G, Deng K, Wang Q, Wang Z, Wang W, Zhang Q, Wang H, et al. CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct Target Ther. 2020;5:25. doi: 10.1038/s41392-020-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tanaka N, Kosaka T, Miyazaki Y, Mikami S, Niwa N, Otsuka Y, Minamishima YA, Mizuno R, Kikuchi E, Miyajima A, et al. Acquired platinum resistance involves epithelial to mesenchymal transition through ubiquitin ligase FBXO32 dysregulation. JCI Insight. 2016;1:e83654. doi: 10.1172/jci.insight.83654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sonego M, Pellarin I, Costa A, Vinciguerra GLR, Coan M, Kraut A, D'Andrea S, Dall'Acqua A, Castillo-Tong DC, Califano D, et al. USP1 links platinum resistance to cancer cell dissemination by regulating snail stability. Sci Adv. 2019;5:eaav3235. doi: 10.1126/sciadv.aav3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lee HJ, Li CF, Ruan D, Powers S, Thompson PA, Frohman MA, Chan CH. The DNA damage transducer RNF8 facilitates cancer chemoresistance and progression through twist activation. Mol Cell. 2016;63:1021–1033. doi: 10.1016/j.molcel.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wong CC, Xu J, Bian X, Wu JL, Kang W, Qian Y, Li W, Chen H, Gou H, Liu D, et al. In colorectal cancer cells with mutant KRAS, SLC25A22-mediated glutaminolysis reduces DNA demethylation to increase WNT signaling, stemness, and drug resistance. Gastroenterology. 2020;159:2163–80.e6. doi: 10.1053/j.gastro.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 246.Ranes M, Zaleska M, Sakalas S, Knight R, Guettler S. Reconstitution of the destruction complex defines roles of AXIN polymers and APC in β-catenin capture, phosphorylation, and ubiquitylation. Mol Cell. 2021;81:3246–61.e11. doi: 10.1016/j.molcel.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Guo Q, Quan M, Dong J, Bai J, Wang J, Han R, Wang W, Cai Y, Lv YQ, Chen Q, et al. The WW domains dictate isoform-specific regulation of YAP1 stability and pancreatic cancer cell malignancy. Theranostics. 2020;10:4422–4436. doi: 10.7150/thno.42795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ma J, Fan Z, Tang Q, Xia H, Zhang T, Bi F. Aspirin attenuates YAP and β-catenin expression by promoting β-TrCP to overcome docetaxel and vinorelbine resistance in triple-negative breast cancer. Cell Death Dis. 2020;11:530. doi: 10.1038/s41419-020-2719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wu C, Luo K, Zhao F, Yin P, Song Y, Deng M, Huang J, Chen Y, Li L, Lee S, et al. USP20 positively regulates tumorigenesis and chemoresistance through β-catenin stabilization. Cell Death Differ. 2018;25:1855–1869. doi: 10.1038/s41418-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yun SI, Kim HH, Yoon JH, Park WS, Hahn MJ, Kim HC, Chung CH, Kim KK. Ubiquitin specific protease 4 positively regulates the WNT/β-catenin signaling in colorectal cancer. Mol Oncol. 2015;9:1834–1851. doi: 10.1016/j.molonc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Nguyen HH, Kim T, Nguyen T, Hahn MJ, Yun SI, Kim KK. A selective inhibitor of ubiquitin-specific protease 4 suppresses colorectal cancer progression by regulating β-catenin signaling. Cell Physiol Biochem. 2019;53:157–171. doi: 10.33594/000000127. [DOI] [PubMed] [Google Scholar]
- 252.Jiang S, Song C, Gu X, Wang M, Miao D, Lv J, Liu Y. Ubiquitin-specific peptidase 22 contributes to colorectal cancer stemness and chemoresistance via Wnt/β-catenin pathway. Cell Physiol Biochem. 2018;46:1412–1422. doi: 10.1159/000489156. [DOI] [PubMed] [Google Scholar]
- 253.Li Z, Huang X, Hu W, Lu H. Down-regulation of USP22 reduces cell stemness and enhances the sensitivity of pancreatic cancer cells to cisplatin by inactivating the Wnt/β-catenin pathway. Tissue Cell. 2022;77:101787. doi: 10.1016/j.tice.2022.101787. [DOI] [PubMed] [Google Scholar]
- 254.Ning Z, Wang A, Liang J, Xie Y, Liu J, Feng L, Yan Q, Wang Z. USP22 promotes the G1/S phase transition by upregulating FoxM1 expression via β-catenin nuclear localization and is associated with poor prognosis in stage II pancreatic ductal adenocarcinoma. Int J Oncol. 2014;45:1594–1608. doi: 10.3892/ijo.2014.2531. [DOI] [PubMed] [Google Scholar]
- 255.Tang DE, Dai Y, Lin LW, Xu Y, Liu DZ, Hong XP, Jiang HW, Xu SH. STUB1 suppresseses tumorigenesis and chemoresistance through antagonizing YAP1 signaling. Cancer Sci. 2019;110:3145–3156. doi: 10.1111/cas.14166. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 256.Zhu H, Yan F, Yuan T, Qian M, Zhou T, Dai X, Cao J, Ying M, Dong X, He Q, et al. USP10 promotes proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ. Cancer Res. 2020;80:2204–2216. doi: 10.1158/0008-5472.CAN-19-2388. [DOI] [PubMed] [Google Scholar]
- 257.Erin N, Grahovac J, Brozovic A, Efferth T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resis Updat Rev Comment Antimicrob Anticancer Chemother. 2020;53:100715. doi: 10.1016/j.drup.2020.100715. [DOI] [PubMed] [Google Scholar]
- 258.Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi: 10.1016/j.pharmthera.2020.107753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Tian H, Zhou L, Wang Y, Nice EC, Huang C, Zhang H. A targeted nanomodulator capable of manipulating tumor microenvironment against metastasis. J Control Release. 2022;348:590–600. doi: 10.1016/j.jconrel.2022.06.022. [DOI] [PubMed] [Google Scholar]
- 260.Bao MH, Wong CC. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells. 2021;10:1715. doi: 10.3390/cells10071715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta P, Wei L, Ashby CR, Jr, Yang DH, Chen ZS. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updat. 2018;41:1–25. doi: 10.1016/j.drup.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 262.Tian H, Zhang M, Jin G, Jiang Y, Luan Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J Colloid Interface Sci. 2021;587:358–366. doi: 10.1016/j.jcis.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 263.Jin P, Jiang J, Zhou L, Huang Z, Qin S, Chen HN, Peng L, Zhang Z, Li B, Luo M, et al. Disrupting metformin adaptation of liver cancer cells by targeting the TOMM34/ATP5B axis. EMBO Mol Med. 2022:e16082. [DOI] [PMC free article] [PubMed]
- 264.Jin P, Jiang J, Zhou L, Huang Z, Nice EC, Huang C, Fu L. Mitochondrial adaptation in cancer drug resistance: prevalence, mechanisms, and management. J Hematol Oncol. 2022;15:97. doi: 10.1186/s13045-022-01313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Qin S, Li B, Ming H, Nice EC, Zou B, Huang C. Harnessing redox signaling to overcome therapeutic-resistant cancer dormancy. Biochim Biophys Acta Rev Cancer. 2022;1877:188749. doi: 10.1016/j.bbcan.2022.188749. [DOI] [PubMed] [Google Scholar]
- 266.Li B, Huang Y, Ming H, Nice EC, Xuan R, Huang C. Redox control of the dormant cancer cell life cycle. Cells. 2021;10:2707. doi: 10.3390/cells10102707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shimizu H, Takeishi S, Nakatsumi H, Nakayama KI. Prevention of cancer dormancy by Fbxw7 ablation eradicates disseminated tumor cells. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed]
- 268.Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler TW, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 270.Zhang Q, Zhang ZY, Du H, Li SZ, Tu R, Jia YF, Zheng Z, Song XM, Du RL, Zhang XD. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019;26:2300–2313. doi: 10.1038/s41418-019-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Niederkorn M, et al. The deubiquitinase USP15 modulates cellular redox and is a therapeutic target in acute myeloid leukemia. Leukemia. 2022;36:438–451. doi: 10.1038/s41375-021-01394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhang L, Gao X, Qin Z, Shi X, Xu K, Wang S, Tang M, Wang W, Gao S, Zuo L, et al. USP15 participates in DBP-induced testicular oxidative stress injury through regulating the Keap1/Nrf2 signaling pathway. Sci Total Environ. 2021;783:146898. doi: 10.1016/j.scitotenv.2021.146898. [DOI] [PubMed] [Google Scholar]
- 273.Bu X, Qu X, Guo K, Meng X, Yang X, Huang Q, Dou W, Feng L, Wei X, Gao J, et al. CD147 confers temozolomide resistance of glioma cells via the regulation of β-TrCP/Nrf2 pathway. Int J Biol Sci. 2021;17:3013–3023. doi: 10.7150/ijbs.60894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yang Q, Li K, Huang X, Zhao C, Mei Y, Li X, Jiao L, Yang H. lncRNA SLC7A11-AS1 promotes chemoresistance by blocking SCF(β-TRCP)-mediated degradation of NRF2 in pancreatic cancer. Mol Ther Nucleic Acids. 2020;19:974–985. doi: 10.1016/j.omtn.2019.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Peng L, Jiang J, Chen HN, Zhou L, Huang Z, Qin S, Jin P, Luo M, Li B, Shi J, et al. Redox-sensitive cyclophilin a elicits chemoresistance through realigning cellular oxidative status in colorectal cancer. Cell Rep. 2021;37:110069. doi: 10.1016/j.celrep.2021.110069. [DOI] [PubMed] [Google Scholar]
- 276.Li T, Yan B, Ma Y, Weng J, Yang S, Zhao N, Wang X, Sun X. Ubiquitin-specific protease 4 promotes hepatocellular carcinoma progression via cyclophilin a stabilization and deubiquitination. Cell Death Dis. 2018;9:148. doi: 10.1038/s41419-017-0182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Deng M, Dai W, Yu VZ, Tao L, Lung ML. Cylindromatosis lysine 63 deubiquitinase (CYLD) regulates NF-kB signaling pathway and modulates fibroblast and endothelial cells recruitment in nasopharyngeal carcinoma. Cancers. 2020;12:1924. doi: 10.3390/cancers12071924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tian H, Zhang B, Di J, Jiang G, Chen F, Li H, Li L, Pei D, Zheng J. Keap1: one stone kills three birds Nrf 2, IKKβ and Bcl-2/Bcl-xL. Cancer Lett. 2012;325:26–34. doi: 10.1016/j.canlet.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 279.Coyaud E, Mis M, Laurent EM, Dunham WH, Couzens AL, Robitaille M, Gingras AC, Angers S, Raught B. BioID-based identification of Skp cullin F-box (SCF)β-TrCP1/2 E3 ligase substrates. Mol Cell Proteom MCP. 2015;14:1781–1795. doi: 10.1074/mcp.M114.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tan C, Hu W, He Y, Zhang Y, Zhang G, Xu Y, Tang J. Cytokine-mediated therapeutic resistance in breast cancer. Cytokine. 2018;108:151–159. doi: 10.1016/j.cyto.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 281.Liu B, Wang T, Wang H, Zhang L, Xu F, Fang R, Li L, Cai X, Wu Y, Zhang W, et al. Oncoprotein HBXIP enhances HOXB13 acetylation and co-activates HOXB13 to confer tamoxifen resistance in breast cancer. J Hematol Oncol. 2018;11:26. doi: 10.1186/s13045-018-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.McAleese CE, Choudhury C, Butcher NJ, Minchin RF. Hypoxia-mediated drug resistance in breast cancers. Cancer Lett. 2021;502:189–199. doi: 10.1016/j.canlet.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 283.Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50:1–9. doi: 10.1038/s12276-018-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Li J, Zhang T, Ren T, Liao X, Hao Y, Lim JS, Lee JH, Li M, Shao J, Liu R. Oxygen-sensitive methylation of ULK1 is required for hypoxia-induced autophagy. Nat Commun. 2022;13:1172. doi: 10.1038/s41467-022-28831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Moslehi J, Rathmell WK. The 2019 Nobel Prize honors fundamental discoveries in hypoxia response. J Clin Investig. 2020;130:4–6. doi: 10.1172/JCI134813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ling S, Shan Q, Zhan Q, Ye Q, Liu P, Xu S, He X, Ma J, Xiang J, Jiang G, et al. USP22 promotes hypoxia-induced hepatocellular carcinoma stemness by a HIF1α/USP22 positive feedback loop upon TP53 inactivation. Gut. 2020;69:1322–1334. doi: 10.1136/gutjnl-2019-319616. [DOI] [PubMed] [Google Scholar]
- 287.Gao R, Buechel D, Kalathur RKR, Morini MF, Coto-Llerena M, Ercan C, Piscuoglio S, Chen Q, Blumer T, Wang X, et al. USP29-mediated HIF1α stabilization is associated with Sorafenib resistance of hepatocellular carcinoma cells by upregulating glycolysis. Oncogenesis. 2021;10:52. doi: 10.1038/s41389-021-00338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lv C, Wang S, Lin L, Wang C, Zeng K, Meng Y, Sun G, Wei S, Liu Y, Zhao Y. USP14 maintains HIF1-α stabilization via its deubiquitination activity in hepatocellular carcinoma. Cell Death Dis. 2021;12:803. doi: 10.1038/s41419-021-04089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Biswas K, Sarkar S, Said N, Brautigan DL, Larner JM. Aurora B kinase promotes CHIP-dependent degradation of HIF1α in prostate cancer cells. Mol Cancer Ther. 2020;19:1008–1017. doi: 10.1158/1535-7163.MCT-19-0777. [DOI] [PubMed] [Google Scholar]
- 290.Simonetta KR, Taygerly J, Boyle K, Basham SE, Padovani C, Lou Y, Cummins TJ, Yung SL, von Soly SK, Kayser F, et al. Prospective discovery of small molecule enhancers of an E3 ligase-substrate interaction. Nat Commun. 2019;10:1402. doi: 10.1038/s41467-019-09358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Cowan AD, Ciulli A. Driving E3 ligase substrate specificity for targeted protein degradation: lessons from nature and the laboratory. Annu Rev Biochem. 2022;91:295–319. doi: 10.1146/annurev-biochem-032620-104421. [DOI] [PubMed] [Google Scholar]
- 292.McCann AP, Smyth P, Cogo F, McDaid WJ, Jiang L, Lin J, Evergren E, Burden RE, Van Schaeybroeck S, Scott CJ, et al. USP17 is required for trafficking and oncogenic signaling of mutant EGFR in NSCLC cells. Cell Commun Signal. 2018;16:77. doi: 10.1186/s12964-018-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhou F, Du C, Xu D, Lu J, Zhou L, Wu C, Wu B, Huang J. Knockdown of ubiquitin-specific protease 51 attenuates cisplatin resistance in lung cancer through ubiquitination of zinc-finger E-box binding homeobox 1. Mol Med Rep. 2020;22:1382–1390. doi: 10.3892/mmr.2020.11188. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 294.Narayanan S, Cai CY, Assaraf YG, Guo HQ, Cui Q, Wei L, Huang JJ, Ashby CR, Jr, Chen ZS. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resist Updat. 2020;48:100663. doi: 10.1016/j.drup.2019.100663. [DOI] [PubMed] [Google Scholar]
- 295.Liu J, Zhao R, Jiang X, Li Z, Zhang B. Progress on the application of bortezomib and bortezomib-based nanoformulations. Biomolecules. 2021;12:51. doi: 10.3390/biom12010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14:417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wallington-Beddoe CT, Sobieraj-Teague M, Kuss BJ, Pitson SM. Resistance to proteasome inhibitors and other targeted therapies in myeloma. Br J Haematol. 2018;182:11–28. doi: 10.1111/bjh.15210. [DOI] [PubMed] [Google Scholar]
- 298.Rowinsky EK, Paner A, Berdeja JG, Paba-Prada C, Venugopal P, Porkka K, Gullbo J, Linder S, Loskog A, Richardson PG, et al. Phase 1 study of the protein deubiquitinase inhibitor VLX1570 in patients with relapsed and/or refractory multiple myeloma. Invest New Drugs. 2020;38:1448–1453. doi: 10.1007/s10637-020-00915-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Friedman DR, Davis PH, Lanasa MC, Moore JO, Gockerman JP, Nelson T, Bond KM, Jiang N, Davis ED, Allgood SD. Pre-clinical and interim results of a phase II trial of perifosine in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) Blood. 2010;116:1842. doi: 10.1182/blood.V116.21.1842.1842. [DOI] [Google Scholar]
- 300.Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 2022;80:1–17. doi: 10.1016/j.semcancer.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 301.Carneiro BA, Kaplan JB, Altman JK, Giles FJ, Platanias LC. Targeting mTOR signaling pathways and related negative feedback loops for the treatment of acute myeloid leukemia. Cancer Biol Ther. 2015;16:648–656. doi: 10.1080/15384047.2015.1026510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Teo MYM, Fong JY, Lim WM, In LLA. Current advances and trends in KRAS targeted therapies for colorectal cancer. Mol Cancer Res MCR. 2022;20:30–44. doi: 10.1158/1541-7786.MCR-21-0248. [DOI] [PubMed] [Google Scholar]
- 303.McKenna M, McGarrigle S, Pidgeon GP. The next generation of PI3K-Akt-mTOR pathway inhibitors in breast cancer cohorts. Biochim Biophys Acta Rev Cancer. 2018;1870:185–197. doi: 10.1016/j.bbcan.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 304.Wei J, Meng F, Park KS, Yim H, Velez J, Kumar P, Wang L, Xie L, Chen H, Shen Y, et al. Harnessing the E3 Ligase KEAP1 for targeted protein degradation. J Am Chem Soc. 2021;143:15073–15083. doi: 10.1021/jacs.1c04841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nguyen KM, Busino L. Targeting the E3 ubiquitin ligases DCAF15 and cereblon for cancer therapy. Semin Cancer Biol. 2020;67:53–60. doi: 10.1016/j.semcancer.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 306.Sahin I, Zhang S, Navaraj A, Zhou L, Dizon D, Safran H, El-Deiry WS. AMG-232 sensitizes high MDM2-expressing tumor cells to T-cell-mediated killing. Cell Death Discov. 2020;6:57. doi: 10.1038/s41420-020-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Shulman DS, Vo KT, Fox E, Muscal JA, Walensky LD, Pikman Y, Stegmaier K, Church A, Crompton BD, Place AE. Abstract CT112: a phase I multicenter trial of the dual MDM2/MDMX inhibitor ALRN-6924 in children and young adults with relapsed/refractory pediatric cancers. Cancer Res. 2019;79:CT112-CT. doi: 10.1158/1538-7445.AM2019-CT112. [DOI] [Google Scholar]
- 308.DiNardo CD, Rosenthal J, Andreeff M, Zernovak O, Kumar P, Gajee R, Chen S, Rosen M, Song S, Kochan J. Phase 1 dose escalation study of mdm2 inhibitor ds-3032b in patients with hematological malignancies-preliminary results. Blood. 2016;128:593. doi: 10.1182/blood.V128.22.593.593. [DOI] [Google Scholar]
- 309.Chen L, Pastorino F, Berry P, Bonner J, Wood K, Veal G, Ponzoni M, Lunec J, Newell DR, Tweddle DA. Abstract LB-300: in vivo evaluation of the intravenous MDM2-p53 antagonist RO6839921 alone and in combination with temozolomide in TP53 wild-type orthotopic models of neuroblastoma. Cancer Res. 2017;77:LB-300-LB-. doi: 10.1158/1538-7445.AM2017-LB-300. [DOI] [Google Scholar]
- 310.Cornillie J, Wozniak A, Li H, Gebreyohannes YK, Wellens J, Hompes D, Debiec-Rychter M, Sciot R, Schöffski P. Anti-tumor activity of the MDM2-TP53 inhibitor BI-907828 in dedifferentiated liposarcoma patient-derived xenograft models harboring MDM2 amplification. Clin Transl Oncol. 2020;22:546–554. doi: 10.1007/s12094-019-02158-z. [DOI] [PubMed] [Google Scholar]
- 311.Wolf D, Baier G. IFNγ helps CBLB-deficient CD8+ T cells to put up resistance to tregs. Cancer Immunol Res. 2022;10:370. doi: 10.1158/2326-6066.CIR-22-0080. [DOI] [PubMed] [Google Scholar]
- 312.Ge C, Liao B, Zhang L. KPG-818, a novel cereblon modulator, inhibits hematological malignancies in preclinical models. Cancer Res. 2020;80:6367. doi: 10.1158/1538-7445.AM2020-6367. [DOI] [Google Scholar]
- 313.Lazo JS, Sharlow ER. Drugging undruggable molecular cancer targets. Annu Rev Pharmacol Toxicol. 2016;56:23–40. doi: 10.1146/annurev-pharmtox-010715-103440. [DOI] [PubMed] [Google Scholar]
- 314.Li K, Crews CM. PROTACs: past, present and future. Chem Soc Rev. 2022;51:5214–5236. doi: 10.1039/D2CS00193D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA. 2001;98:8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Domostegui A, Nieto-Barrado L, Perez-Lopez C, Mayor-Ruiz C. Chasing molecular glue degraders: screening approaches. Chem Soc Rev. 2022;51:5498–5517. doi: 10.1039/D2CS00197G. [DOI] [PubMed] [Google Scholar]
- 317.Wu W, Nelson GM, Koch R, Donovan KA, Nowak RP, Heavican-Foral TB, Nirmal AJ, Liu H, Yang L, Duffy J, et al. Overcoming IMiD resistance in T-cell lymphomas through potent degradation of ZFP91 and IKZF1. Blood. 2022;139:2024–2037. doi: 10.1182/blood.2021014701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Patil A, Manzano M, Gottwein E. CK1α and IRF4 are essential and independent effectors of immunomodulatory drugs in primary effusion lymphoma. Blood. 2018;132:577–586. doi: 10.1182/blood-2018-01-828418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Mayor-Ruiz C, Bauer S, Brand M, Kozicka Z, Siklos M, Imrichova H, Kaltheuner IH, Hahn E, Seiler K, Koren A, et al. Rational discovery of molecular glue degraders via scalable chemical profiling. Nat Chem Biol. 2020;16:1199–1207. doi: 10.1038/s41589-020-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yamamoto J, Ito T, Yamaguchi Y, Handa H. Discovery of CRBN as a target of thalidomide: a breakthrough for progress in the development of protein degraders. Chem Soc Rev. 2022;51:6234–6250. doi: 10.1039/D2CS00116K. [DOI] [PubMed] [Google Scholar]
- 321.Dong G, Ding Y, He S, Sheng C. Molecular glues for targeted protein degradation: from serendipity to rational discovery. Journal of Medicinal Chemistry. 2021;64(15):10606–10620. doi: 10.1021/acs.jmedchem.1c00895. [DOI] [PubMed] [Google Scholar]
- 322.Schreiber SL. The Rise of Molecular Glues. Cell. 2021;184(1):3–9. doi: 10.1016/j.cell.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 323.Fürstenau M, Fink AM, Schilhabel A, Weiss J, Robrecht S, Eckert R, de la Serna J, Crespo M, Coscia M, Vitale C, et al. B-cell acute lymphoblastic leukemia in patients with chronic lymphocytic leukemia treated with lenalidomide. Blood. 2021;137:2267–2271. doi: 10.1182/blood.2020008609. [DOI] [PubMed] [Google Scholar]
- 324.Vogelzang NJ, Fizazi K, Burke JM, De Wit R, Bellmunt J, Hutson TE, Crane E, Berry WR, Doner K, Hainsworth JD, et al. Circulating tumor cells in a phase 3 study of docetaxel and prednisone with or without lenalidomide in metastatic castration-resistant prostate cancer. Eur Urol. 2017;71:168–171. doi: 10.1016/j.eururo.2016.07.051. [DOI] [PubMed] [Google Scholar]
- 325.Chari A, Suvannasankha A, Fay JW, Arnulf B, Kaufman JL, Ifthikharuddin JJ, Weiss BM, Krishnan A, Lentzsch S, Comenzo R, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130:974–981. doi: 10.1182/blood-2017-05-785246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, Leleu X, Schjesvold F, Moreau P, Dimopoulos MA, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096–2107. doi: 10.1016/S0140-6736(19)32556-5. [DOI] [PubMed] [Google Scholar]
- 327.Vetma V, Guttà C, Peters N, Praetorius C, Hutt M, Seifert O, Meier F, Kontermann R, Kulms D, Rehm M. Convergence of pathway analysis and pattern recognition predicts sensitization to latest generation TRAIL therapeutics by IAP antagonism. Cell Death Differ. 2020;27:2417–2432. doi: 10.1038/s41418-020-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ma Z, Ji Y, Yu Y, Liang D. Specific non-genetic IAP-based protein erasers (SNIPERs) as a potential therapeutic strategy. Eur J Med Chem. 2021;216:113247. doi: 10.1016/j.ejmech.2021.113247. [DOI] [PubMed] [Google Scholar]
- 329.Dumétier B, Zadoroznyj A, Dubrez L. IAP-mediated protein ubiquitination in regulating cell signaling. Cells. 2020;9:1118. doi: 10.3390/cells9051118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Foss S, Watkinson R, Sandlie I, James LC, Andersen JT. TRIM21: a cytosolic Fc receptor with broad antibody isotype specificity. Immunol Rev. 2015;268:328–339. doi: 10.1111/imr.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zeng J, Santos AF, Mukadam AS, Osswald M, Jacques DA, Dickson CF, McLaughlin SH, Johnson CM, Kiss L, Luptak J, et al. Target-induced clustering activates Trim-Away of pathogens and proteins. Nat Struct Mol Biol. 2021;28:278–289. doi: 10.1038/s41594-021-00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 333.Mele L, Del Vecchio V, Liccardo D, Prisco C, Schwerdtfeger M, Robinson N, Desiderio V, Tirino V, Papaccio G, La Noce M. The role of autophagy in resistance to targeted therapies. Cancer Treat Rev. 2020;88:102043. doi: 10.1016/j.ctrv.2020.102043. [DOI] [PubMed] [Google Scholar]
- 334.Karasic TB, O'Hara MH, Loaiza-Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E, De Jesus-Acosta A, Redlinger C, Burrell JA, Laheru DA, et al. Effect of gemcitabine and nab-paclitaxel with or without hydroxychloroquine on patients with advanced pancreatic cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:993–998. doi: 10.1001/jamaoncol.2019.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605–616. doi: 10.1038/s41418-018-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Takahashi D, Arimoto H. Targeting selective autophagy by AUTAC degraders. Autophagy. 2020;16:765–766. doi: 10.1080/15548627.2020.1718362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ji CH, Kim HY, Lee MJ, Heo AJ, Park DY, Lim S, Shin S, Ganipisetti S, Yang WS, Jung CA, et al. The AUTOTAC chemical biology platform for targeted protein degradation via the autophagy-lysosome system. Nat Commun. 2022;13:904. doi: 10.1038/s41467-022-28520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Li Z, Zhu C, Ding Y, Fei Y, Lu B. ATTEC: a potential new approach to target proteinopathies. Autophagy. 2020;16:185–187. doi: 10.1080/15548627.2019.1688556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Fan X, Jin WY, Lu J, Wang J, Wang YT. Rapid and reversible knockdown of endogenous proteins by peptide-directed lysosomal degradation. Nat Neurosci. 2014;17:471–480. doi: 10.1038/nn.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ahn G, Banik SM, Miller CL, Riley NM, Cochran JR, Bertozzi CR. LYTACs that engage the asialoglycoprotein receptor for targeted protein degradation. Nat Chem Biol. 2021;17:937–946. doi: 10.1038/s41589-021-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Banik SM, Pedram K, Wisnovsky S, Ahn G, Riley NM, Bertozzi CR. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature. 2020;584:291–297. doi: 10.1038/s41586-020-2545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.