Abstract
Bartonella henselae is the causative agent of cat scratch disease (CSD), a self-limiting condition characterized by a subacute regional lymphadenopathy that may develop into disseminated bartonellosis in immunocompromised subjects. Mice experimentally infected with B. henselae display typical liver and spleen granulomas rich in T cells and macrophages. So far there are no data on the interaction between bartonellae and macrophages. In order to clarify this topic, we investigated the interaction of B. henselae with J774, a mouse macrophage cell line. Analysis of bacterial uptake by functional assays and transmission electron microscopy indicates that bartonellae can enter and survive inside J774. Entry occurred within 30 min postinfection and reached a plateau at 160 min. Infection of J774 was followed by a dose-dependent release of the proinflammatory cytokines tumor necrosis factor alpha, interleukin 1β (IL-1β), and IL-6. Bartonellae persisted intracellularly without loss of viability for at least 8 h, and their number slightly decreased 24 h postinfection. Gamma interferon (IFN-γ) treatment of J774 significantly decreased the number of recoverable bacteria at 8 and 24 h. This enhancement of macrophage bactericidal activity was associated with nitric oxide (NO) release and was prevented by the addition of the competitive inhibitor of NO synthesis NG-monomethyl l-arginine. These findings suggest that IFN-γ-mediated activation of macrophages may be important for the clearing of B. henselae infection and that anti-B. henselae microbicidal activity of IFN-γ-activated macrophages is mediated to a large extent by NO production.
In recent years the number of Bartonella species identified, as well as the spectrum of their associated clinical manifestations, has expanded dramatically (25). B. henselae, a small, fastidious gram-negative bacterium endemic among domestic cats, is considered an emerging pathogen that can be transmitted to humans occasionally, causing about 24,000 cases per year in the United States (18). In immunocompetent hosts the disease is characterized by a self-limiting subacute regional lymphadenopathy associated with low-grade fever, anorexia, and malaise, known as cat scratch disease (CSD) (12). In normal patients there is no bacteremic phase, as B. henselae is usually isolated from lymph nodes but not from blood (20). However, in patients with impaired cell-mediated immune response bacteremia may occur, and the spreading of bartonella from lymph nodes to liver, spleen, and cutaneous tissues causes bacillary peliosis hepatis and/or bacillary angiomatosis (BA), both initially observed as AIDS-related diseases. Bacillary peliosis hepatis and BA consist of nongranulomatous angioproliferative lesions, appearing as Kaposi-like nodular formations in skin and blood-filled spaces of the liver and spleen (22, 36). It has been shown that B. henselae attaches to and enters human epithelial and endothelial cells, stimulating their migration and proliferation in vitro, which may account for the angioproliferative lesions observed in vivo (7, 24, 26, 33). Granulomas rich in T cells and macrophages appear in the liver of BALB/c mice within 3 days of an intraperitoneal injection of B. henselae (34). These lesions expand in the following weeks due to the recruitment of CD4+ T cells and epithelioid cells, but their further evolution results in complete tissue healing and full recovery of the animals. Granulomas in liver and lymph nodes have been also detected in cats experimentally infected with bartonellae (17). Following systemic infection, mice develop a positive delayed-type hypersensitivity, and a T-helper-1 cytokine response has been observed upon in vitro stimulation of splenocytes (21). These findings point to a key role of CD4+ cells and macrophages in the immune response against B. henselae. Clearance of macrophage-invading microorganisms, such as mycobacteria and leishmaniae, depends on the production of gamma interferon (IFN-γ) by activated T cells, resulting in inducible nitric oxide synthase expression by macrophages and thereby the release of highly toxic reactive nitrogen intermediates (RNI) (2, 6, 29, 30). In the present study we investigated the interaction of B. henselae with the murine macrophage cell line J774 and the concomitant production of proinflammatory cytokines. Bacterial uptake was analyzed by plate counting of viable intracellular bacteria and transmission electron microscopy. Finally, in order to clarify whether cytokines may affect the survival of phagocytosed bacteria, we studied the effect of IFN-γ-treatment of macrophages on intracellular or extracellular killing and the sensitivity of B. henselae to RNI.
MATERIALS AND METHODS
Cells.
J774, a murine macrophage cell line, was maintained in Dulbecco's modified Eagle medium (Life Technologies Italia) supplemented with 10% heat-inactivated fetal calf serum (HyClone Laboratories, Logan, Utah) and 2 mM l-glutamine (Life Technologies), at 37°C in a humidified 5% CO2 atmosphere.
Bacteria.
B. henselae Houston I strain (ATCC 49882), a generous gift from Tarcisio Not (University of Trieste, Trieste Italy), was grown on 5% sheep blood Columbia agar plates (BioMerieux, Lyon, France) in anaerobic conditions (candle jar) at 37°C for 7 days. Bacteria were harvested under a laminar-flow hood by gently scraping colonies off the agar surface, suspended in SPG (sucrose, 74.4 g/liter; KH2PO4, 0.4 g/liter; K2HPO4, 1.23 g/liter; glutamic acid, 0.7 g/liter) and stored at −80°C in 1-ml aliquots. The number of CFU recoverable from a cryovial was 3 × 108. For biological assays, a stock suspension of frozen bacteria was thawed and washed three times with saline buffer, and the bacteria were added to cell culture wells at the indicated bacteria-per-cell ratio. Killed bartonellae used in the cytokine release experiments were obtained by heating thawed bacteria to 56°C for 30 min in a water bed, as described (24). We verified that this treatment achieved complete inhibition of growth.
Reagents.
Mouse recombinant IFN-γ (rIFN-γ) was purchased from Life Technologies. Monoclonal anti-mouse tumor necrosis factor alpha (TNF-α) antibody was purchased from Endogen (Woburn, Mass.). Lipopolysaccharide (LPS) (phenol-extracted and chromatographically purified from Escherichia coli serotype 0111:B4), sulfanilamide, naphthylendiamine dihydrochloride, diethylamine-NO, and sodium nitrate were purchased from Sigma Chemical Co. (St. Louis, Mo.). Phosphoric acid was from Fischer Scientific (Fair Lawn, N.J.), N-monomethil-l-arginine (L-NMMA) was from Calbiochem Co. (La Jolla, Calif.), and gentamicin was from Schering-Plough (Essex Italia).
Invasion and survival assays.
B. henselae penetration and survival inside J774 were assessed as previously described with minor modifications (39). Briefly, J774 cells were seeded into 96-well flat-bottom Falcon microtiter plates at 105 cells per well in 0.1 ml of medium and incubated overnight at 37°C. Then, the medium was replaced with 50 μl of fresh medium and cell monolayers were infected with approximately 106 bartonellae. To perform invasion assays the cultures were incubated for 30 to 240 min before gentamicin (250 μg/ml) was added to kill extracellular bacteria. After 2 h, cultures were washed twice with phosphate-buffered saline (PBS) to remove gentamicin, and cells were lysed with distilled water (200 μl/well) (27, 31) and sonicated for 1 min 30 s. The number of viable intracellular bartonellae was determined by quantitative plating of serial dilutions of the lysates on sheep blood Columbia agar. Plates were cultured in candle jars at 37°C for 10 days, after which the colonies were counted. To assess the intracellular survival, J774 cells were exposed to bartonellae for 2 h, and the number of CFU was determined at different times after gentamicin selection. Where indicated, IFN-γ (500 U/ml) and/or 500 μM L-NMMA were added to macrophage cultures for 16 h before infection and were present throughout the culture period. To assess extracellular killing by IFN-γ-treated or untreated J774 in the absence of gentamicin, extracellular bacteria were plated after a 24-h coincubation.
Nitric oxide assay.
NO production was estimated as the amount of NO2− released in the culture medium. Cells were pretreated with IFN-γ (500 U/ml) for 16 h and then exposed to B. henselae as indicated for the invasion assay. Where indicated, monoclonal antibody (MAb) anti-TNF-α or an irrelevant control was added to the cultures at a concentration of 10 μg/ml. After gentamicin selection the medium was replaced, and 50-μl aliquots of culture supernatants were collected after 8, 24, and 48 h of incubation and mixed with an equal volume of Griess reagent in a 96-well flat-bottom Falcon microplate. The A570 was monitored with a microplate reader (Behring ELISA Processor II) with background subtraction at A650, as described (1). Quantitative analysis was performed by comparison with standard solutions of NaNO2.
Cell-free bactericidal assay.
To assess the bactericidal activity of NO in a cell-free system, 106 bartonellae were incubated in the presence of different concentrations of DEA-NO. Inactive DEA-NO used as control was decomposed in phosphate buffer for 30 min before addition to bacteria as described (8). NO release from DEA-NO was measured by using the Griess reagent. Following exposure to each compound, bacteria were centrifuged and resuspended in phosphate buffer for CFU quantitation.
Electron microscopy.
Samples of Bartonella-infected J774 were centrifuged at 400 × g for 15 min. Pelleted cells were fixed for 2 h in 4% paraformaldehyde in 0.1 M PBS, washed extensively in PBS, and postfixed in 1% osmium tetroxide in cacodylate buffer. Cells were then dehydrated in acetone and embedded in Epon 812 (Fluka, Seelze, Germany). Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and observed in a Philips EM 410 electron microscope.
Measurement of cytokines in cell culture supernatants.
J774 cells were treated with different stimuli: LPS (1 μg/ml), IFN-γ (500 U/ml), LPS plus IFN-γ, and live or heat-killed bartonellae at the indicated bacteria/macrophage ratios for 24 and 48 h. Cell-free supernatants were collected and assayed for TNF-α, interleukin 1β (IL-1β), and IL-6 production by commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Amersham Pharmacia Biotech Italia).
Statistical analysis.
Comparison among treatments was performed by Student's t test or by analysis of variance, as appropriate. When a potential difference among multiple treatments was found, the Newman-Keuls multiple-comparison test was used to identify which of the means were significantly different from the others at the 0.05 significance level.
RESULTS
Kinetics of Bartonella uptake by J774.
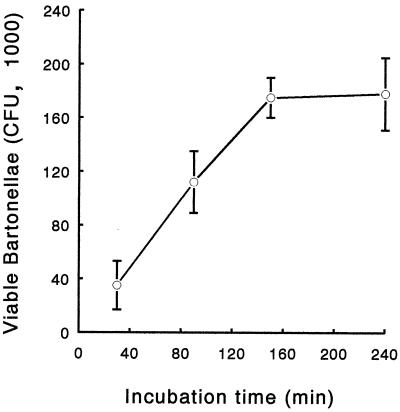
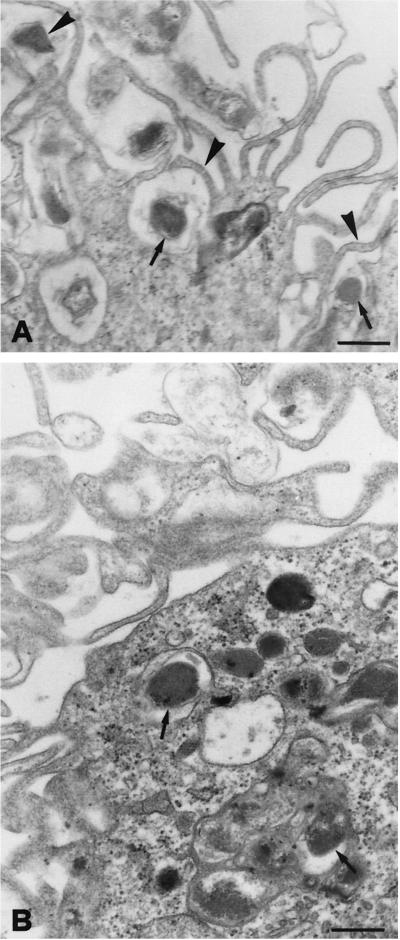
To monitor uptake, bartonellae were allowed to invade J774 cells for 30 to 240 min, and then extracellular bacteria were killed with gentamicin and the number of internalized bartonellae was determined by plating. Results are shown in Fig. 1. The number of recoverable intracellular CFU sharply increased from 30 to 160 min, whereas from 160 to 240 min the increase in CFU number was almost undetectable, indicating saturation of the uptake process around 160 min. Based on these observations, the experiments on intracellular survival were performed allowing a minimum invasion time of 2 h to get optimal internalization. The presence of bartonellae inside macrophages was confirmed by transmission electron microscopy (Fig. 2). Long microvilli can be observed in close association with the invading bacteria. Bartonellae are enclosed in membrane-bound vacuoles (arrows).
FIG. 1.
Kinetics of B. henselae entry in J774 cells. Bartonellae were allowed to invade cells for the indicated times. Gentamicin (250 μg/ml) was added to kill extracellular bacteria, cells were lysed, and the number of internalized bartonellae was determined by serial dilution and agar plating. All results are expressed as CFU recovered per microtiter well (mean ± standard deviation [error bars] obtained from triplicate cultures of a representative experiment out of three performed).
FIG. 2.
Transmission electron micrographs of J774 cells infected with B. henselae. (A) Initial contact of bartonellae with the macrophage surface. Long microvilli (arrowheads) are in close association with the invading bacteria (arrows). Scale bar, 0.4 μm. (B) Macrophage containing intracellular bacteria (arrows) within membrane-bound vacuoles 2 h postinfection. Scale bar, 0.3 μm.
B. henselae infection induces the production of proinflammatory cytokines by J774.
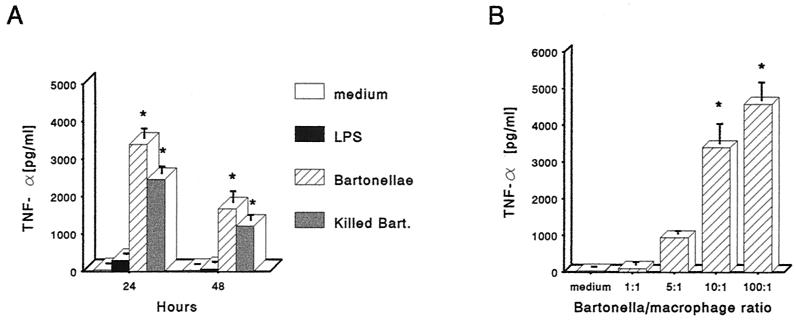
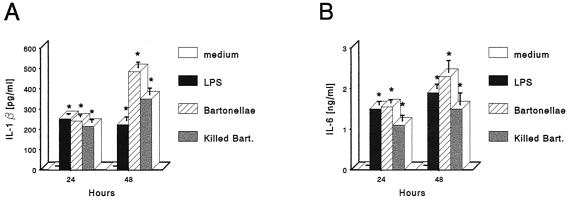
To determine whether B. henselae infection could induce the secretion of proinflammatory cytokines, J774 cells were incubated with live or heat-killed B. henselae at a 10:1 bacteria/macrophage ratio, and supernatants from 24- and 48-h cultures were tested by ELISA for TNF-α. Cell culture medium and LPS (1 μg/ml) were used as negative and positive controls, respectively. Very little TNF-α was present in supernatants from uninfected cells. J774 treated with live or heat-killed B. henselae produced significantly greater amounts of TNF-α compared with uninfected or LPS-treated cells. TNF-α concentration in supernatants from live or heat-killed B. henselae-treated cultures peaked at 24 h and decreased upon further incubation through 48 h (Fig. 3A). To determine whether TNF-α production correlated with the concentration of bartonellae, macrophages were exposed to the bacteria at the bacteria/macrophage ratios of 1:1, 5:1, 10:1, and 100:1, and supernatants from 24-h cultures were tested. This experiment showed a dose-dependent response (Fig. 3B). J774 supernatants were also assayed for IL-1β and IL-6 production. Untreated J774 did not produce detectable levels of IL-1β and IL-6. Treatment with live or heat-killed B. henselae markedly increased both IL-1β and IL-6 protein levels, with a progressive accumulation peaking at 48 h (Fig. 4). The amount of IL-1β and IL-6 induced by live or heat-killed B. henselae in J774 was not significantly different from that obtained with LPS (1 μg/ml).
FIG. 3.
Induction of TNF-α by B. henselae. (A) TNF-α production by J774 treated with viable or heat-killed B. henselae at the 10:1 bacteria/macrophage ratio and cultured for the indicated times. Cell culture medium was used as a negative control and, LPS (1 μg/ml) was used as a positive control. (B) Dose-response of TNF-α secretion in culture supernatants of J774 infected with viable B. henselae at different bacteria/macrophage ratios. Cultures were incubated for 24 h, and supernatants were tested for TNF-α. The data represent the means ± standard errors/error bars) from three independent experiments. Asterisks indicate significant increase of cytokine production compared to unstimulated cells (P < 0.05).
FIG. 4.
IL-1β (A) and IL-6 (B) production by J774 cells treated with viable or heat-killed B. henselae at a 10:1 bacteria/macrophage ratio and cultured for the indicated times. Culture medium was used as a negative control, and LPS (1 μg/ml) was used as a positive control. The data represent the means ± standard errors (error bars) from three independent experiments. Asterisks indicate significant increase of cytokine production compared to unstimulated cells (P < 0.05).
Intracellular survival of bartonellae in nonactivated and IFN-γ-activated J774.
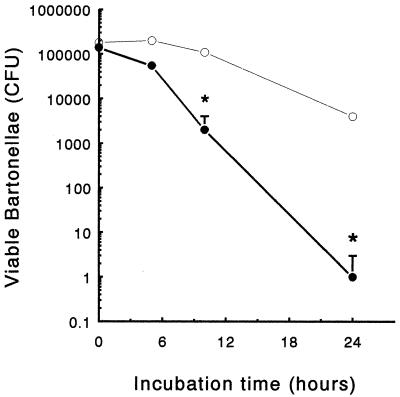
To monitor the intracellular survival of B. henselae, J774 cells were exposed to bacteria for 2 h and lysed at different time points after gentamicin selection. Results show that up to 8 h postinfection, the number of viable bacteria inside nonactivated J774 cells remained almost constant (Fig. 5). A slight decrease was observed between 8 and 24 h. In order to test the effect of IFN-γ on bartonella survival, J774 were incubated with IFN-γ (500 U/ml) for 16 h before the challenge. Immediately after infection the same number of bacteria could be found inside treated and untreated cells, suggesting that IFN-γ did not affect the infection rate. Beginning at 8 h postinfection, significantly lower numbers were recovered from IFN-γ-treated cells (Fig. 5).
FIG. 5.
Intracellular survival of B. henselae in J774. A total of 105 cells were incubated with (closed circles) or without (open circles) IFN-γ (500 U/ml) for 16 h and then infected with approximately 106 B. henselae cells. The number of CFU was determined at different times after gentamicin selection. Each data point represents the mean ± standard deviation (error bars) obtained from triplicate cultures of a representative experiment out of three performed. Asterisks indicate significant decrease of CFU number compared to unstimulated cells (P < 0.05).
Production of NO by J774 after infection with B. henselae.
As NO has been implicated in the IFN-γ-induced antimicrobial action of macrophages (30), we assessed NO production by IFN-γ-treated, bartonella-infected J774. Cells were pretreated with IFN-γ (500 U/ml) for 16 h and then exposed to B. henselae. At 8, 24, and 48 h after gentamicin selection, supernatants were collected and the amount of nitric oxide was measured as NO2− production. As shown in Table 1, NO2− release was not detectable in the supernatants of untreated J774. IFN-γ alone did not enhance NO2− production, whereas a significant increase was observed in B. henselae-infected cultures. However, the maximal NO2− levels were produced by IFN-γ-activated J774 infected with B. henselae. L-NMMA (2 mM), an arginine analog which inhibits NO production, markedly reduced the production of nitrite by IFN-γ-activated J774 infected with B. henselae. In our hands, 2 mM L-NMMA was the minimum concentration able to diminish NO2− accumulation (data not shown). Given the reported role of TNF-α as a cofactor in NO production by macrophages, we examined the effect of the addition of anti-TNF-α MAb. As reported in Table 1, anti-TNF-α MAb induced a partial but significant reduction of NO production.
TABLE 1.
NO2− release by J774 macrophages infected with B. henselaea
| Treatment | NO2− release (μM)
|
||
|---|---|---|---|
| 8 h | 24 h | 48 h | |
| Medium | 0.57 (0.2) | 1.14 (2.4) | 3.4 (2.1) |
| IFN-γ (500 U/ml) | 2.5 (1.5) | 1.5 (0.9) | 3.4 (3) |
| B. henselae (10:1) | 2 (0.9) | 5.8 (1.8) | 13 (4.9) |
| B. henselae (10:1) + IFN-γ | 16 (2.2)c | 52 (3.6)c | 73 (6.2)c |
| B. henselae (10:1) + IFN-γ + L-NMMA (2 mM) | 1.1 (1)d | 3 (4.2)d | 12 (2.8)d |
| B. henselae (10:1) + IFN-γ + anti-TNF-α MAbb | NDe | 24 (3.2)d | ND |
| B. henselae (10:1) + IFN-γ + rat IgGb | ND | 58 (4) | ND |
NO2− release was analyzed after culture of J774 macrophages for 8, 24, and 48 h performed in triplicate; numbers in parentheses are standard deviations. The experiment shown is representative of five performed.
Anti-TNF-α MAb and rat immunoglobulin G (IgG) were used at 10 μg/ml.
Significant NO2− release compared to medium (P < 0.05).
Significant inhibition of NO2− release in comparison to macrophages treated with B. henselae and IFN-γ (P < 0.05).
ND, not determined.
Bactericidal activity of NO towards B. henselae.
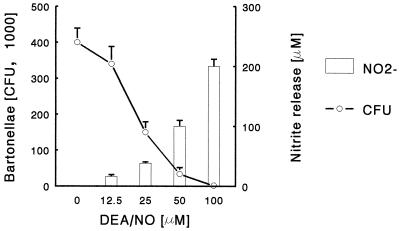
NO is one of the primary mediators of the host cell defense against many intracellular and extracellular bacteria, parasites, and fungi (23). We investigated the effect of exogenous NO on B. henselae in order to examine its susceptibility to RNI-mediated bactericidal mechanisms. Bacteria were exposed to the NO-donating compound DEA-NO for 2 h in a macrophage-free system, and bacterial survival was measured by plating. As shown in Fig. 6 the number of CFU was markedly and dose-dependently reduced in the presence of DEA-NO. Inactive DEA-NO did not affect bacteria viability (data not shown). These results suggest that B. henselae is very susceptible to the bactericidal effects of RNI.
FIG. 6.
Susceptibility of B. henselae to NO. Bartonellae (106) were incubated in medium in the absence or in the presence of different concentrations of DEA-NO. After 2 h the number of CFU (left y axis) and the concentration of nitrite released by DEA-NO in the supernatants (right y axis) were determined. The data represent the mean ± standard deviation (error bars) obtained from triplicate cultures of a representative experiment out of three performed.
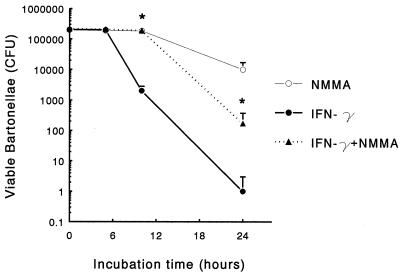
To examine whether NO mediated the decreased intracellular survival of B. henselae observed in IFN-γ-activated J774, we tested the effects of 2 mM L-NMMA on the killing of intracellular bartonellae by IFN-γ-activated J774. IFN-γ-activated J774 were incubated with 2 mM L-NMMA or plain medium and infected 16 h later with bartonellae at 10:1 multiplicity. As shown in Fig. 7, the reduction in the number of live bacteria inside macrophages observed following IFN-γ treatment was markedly inhibited by the addition of 2 mM L-NMMA. To assess the effect of IFN-γ on the extracellular killing we determined the number of CFU following 24-h incubation of bartonellae on IFN-γ-treated or untreated J774 monolayers in the absence of gentamicin. In IFN-γ-treated cocultures we observed a 35% reduction of CFU [(2.7 ± 0.32) × 106 versus (4.1 ± 0.2) × 106; P < 0.05].
FIG. 7.
Effect of L-NMMA on the intracellular survival of B. henselae. J774 were treated with IFN-γ (500 U/ml) for 16 h in the presence or absence of 2 mM L-NMMA and then incubated with B. henselae. The number of viable bacteria in macrophage lysates was determined by agar plating. The data represent the mean ± standard deviation (error bars) obtained from triplicate cultures of a representative experiment out of three performed. Asterisks indicate significant difference in CFU number compared to IFN-γ-treated cells (P < 0.05).
DISCUSSION
We report that B. henselae is rapidly internalized in vitro, in the absence of opsonins, by the murine macrophage cell line J774. Phagocytosis of B. henselae by unstimulated murine macrophages reached complete saturation within 4 h; the process is characterized by the formation of endocytic vacuoles, as documented by functional phagocytosis assays and electron microscopy. B. henselae uptake by macrophages is paralleled by the secretion of large amounts of proinflammatory cytokines: we observed that viable and heat-killed bartonellae induced essentially the same cytokine response. TNF-α production peaked at 24 h, whereas IL-6 and IL-1 peaked at 48 h. The decrease of TNF-α levels at 48 h has been observed by other authors (5, 16). The transient accumulation of TNF-α could be explained by consumption due to an autocrine process. The secretion by phagocytizing cells of proinflammatory cytokines accounts for CSD-characteristic granuloma formation. Cytokine concentrations were comparable to those detected after macrophage stimulation with other live microorganisms or LPS. TNF-α produced by macrophages following exposure to gram-negative bacteria is considered the main factor responsible for septic shock observed in animal models of bacterial proliferation in the bloodstream (37). Together with IL-1, TNF-α could account for the acute-phase response symptoms such as fever and lethargy observed in cats experimentally infected with B. henselae (17). In humans, a similar clinical response characterized by fever and asthenia has been observed a week after infection in children with CSD (40). These symptoms are usually of low severity, which is in agreement with the experimental finding that intravenous injection of B. henselae does not induce lethal septic shock in mice, probably because of the slow proliferation rate of these bacteria and of their rapid clearance from the bloodstream (21, 34). Immunocompetent CSD patients do not typically develop a bacteremic phase, and most isolates derive from lymph node biopsies of infected individuals (20). The investigation of intracellular bacterial survival in the first 48 h after J774 infection demonstrated that a significant number of viable microorganisms was detectable within 24 h, but their number steadily decreased after this time point. These results suggest that in the first 48 h the killing rate is higher than the replication rate, in contrast to what has been reported for other intracellular pathogens such as brucella, listeria, or salmonella (4, 9, 13). This is not surprising, as bartonellae are known for growing very slowly in vitro. However, as far as we know, there are no data on their intracellular replication rate, and follow-up for more prolonged periods of time is required to establish the percentage of long-term intracellular survival.
The kinetics of phagocytosis and killing of B. henselae by macrophages observed in vitro could reflect the fate of B. henselae in vivo. A murine model of B. henselae infection showed that following an intraperitoneal challenge, bacteria were detectable by PCR in liver and mesenteric lymph nodes as early as 6 h postinfection (21, 34). Our results provide further support to the theory that the rapid relocation of bacteria in lymph nodes and liver after intraperitoneal inoculation may depend on the capacity of tissue-resident macrophages to phagocytize injected microorganisms. Bacteria may persist intracellularly in tissues since bartonellae have been cultured from organ homogenates within a 24-h harvest period, while no viable organisms were recovered by 48 h, as reported by Karem et al. (21). Similarly, Regnath et al. did not detect any B. henselae survival 72 h after challenge (34). Therefore, B. henselae seems incapable of maintaining viability in mice for extended time and the recovery of viable bacteria at 24 to 72 h might be due to residual organisms from the inoculum rather than to in vivo growth. Absence of bacteremia and rapid clearance of culturable bacteria seem to indicate resistance of immunocompetent mice to B. henselae, which in the light of our results could be mediated by activated macrophages. Despite the absence of viable organisms in the tissues, microbial DNA can be detected by PCR up to 3 months after infection (21). It is uncertain whether the detection of bacterial DNA in tissues for prolonged periods of time reflects microorganism persistence in the host. It can be hypothesized that a low number of viable microorganisms persisting inside infected cells without replicating can account for relapses in susceptible animals. Persistent infection of liver and spleen by B. henselae may occasionally be observed in some immunocompetent CSD patients, mostly children, and may require up to 6 months for complete recovery (11, 40). Cats, which are the natural host of B. henselae, may harbor the microorganism for at least 18 months, suggesting that some bacteria probably survive inside macrophage or endothelial cells. Difference in virulence among strains may also account for different outcomes of bartonella infections: experimental infection of cats with the strain LSU16 of B. henselae resulted in a more severe clinical syndrome characterized by prolonged bacteremia (32, 35). The mechanism of bacterial survival for such prolonged periods of time is unclear, but it is likely that the microorganism escape the immune response by persisting inside macrophages or in other cell types. Recent findings by Munana et al. (28) indicate that B. henselae can survive for at least 4 weeks inside cat microglia without replicating. Infected macrophages might serve as a vehicle for transmission to other organs and tissues, including the central nervous system, while endothelial and microglial cells might constitute the bacterial reservoir.
It is likely that the complete clearance of intracellular survivors is performed by activated macrophage-mediated killing following the engagement of the specific immune response. Interaction of macrophages with gram-negative bacteria results in the production of proinflammatory cytokines, such as IL-1 and TNF-α, that enhance phagocytosis and microbial killing. However, macrophages may not be capable of eliminating microorganisms without further stimulation by cytokines released by antigen-activated CD4+ lymphocytes. It has been shown that IFN-γ, the most-powerful enhancer of macrophage antimicrobial activities mainly secreted by Th1 cells, is produced upon specific stimulation by splenocytes of bartonella-infected mice and is involved, together with CD4+ cells, in mediating a delayed-type hypersensitivity response after reexposure of animals to bartonella antigens (21). Our observation that IFN-γ treatment of J774 results in a significant enhancement of B. henselae killing suggests that this cytokine may contribute to the development of protective cell-mediated immunity against this microorganism.
We show that in bartonella-infected J774 cells IFN-γ induced the release of large amounts of NO. This finding is in agreement with inducible nitric oxide synthase protein detection in granulomatous tissues from patients with CSD (14). The inhibitory effect of anti-TNF-α MAb indicates that endogenous TNF-α is involved in NO release by infected J774. We also demonstrate that bartonellae are sensitive to NO generated in cell-free conditions, being killed within 30 min of exposure to DEA-NO. Similarly, Turco et al. observed that Rickettsia prowazekii is very sensitive to NO, and NO-mediated damage to rickettsiae is responsible for the inhibition of infection in L929 and RAW cells (38). The contribution of NO to microbicidal activity is not uniform for all pathogens: Staphylococcus aureus or Burkholderia pseudomallei survive a 2-h exposure to NO but are killed by a 24-h exposure (19, 27). Some bacteria such as E. coli or Salmonella enterica serovar Typhimurium are much more sensitive to peroxynitrite, a product of the reaction of NO with superoxide, and are killed by peroxynitrite-generating compounds but not by NO-generating compounds (3, 10). Recent data on the intracellular killing of Rickettsia conori by human cells indicate that RNI mediate killing by hepatocytes and endothelial cells (15).
Synthesis of NO by IFN-γ-activated J774 may be relevant not only for the extracellular killing of bartonellae; a specific role of NO in intracellular killing of phagocytized bacteria in IFN-γ-activated macrophages can be postulated on the basis of the effect of the competitive inhibitor of the l-arginine-dependent production of NO radicals, L-NMMA. We present evidence that L-NMMA treatment is effective in decreasing both NO production and intracellular killing of bartonellae, suggesting that induction of NO synthase could represent an important mechanism of elimination of intracellular microorganisms by IFN-γ-activated murine macrophages. The role of IFN-γ in macrophage activation is well known, and this cytokine is considered one of the main mediators of healing from intracellular pathogen infections. It has been shown that it is mainly produced by NK cells during primary infections and by antigen-specific CD4+ T cells in secondary infections (31). In patients with impaired CD4+ T-cell functions, secondary production does not occur, and unrestrained intracellular replication of bacteria causes lesions typical of chronic infections (peliosus hepatis and BA) with the concurrency of bacterial angiogenic factors. This hypothesis is supported by the evidence that B. henselae produces some soluble factor(s) which induce human umbilical vein endothelial cell proliferation (24).
Taken together, our results suggest that in a murine experimental system IFN-γ-mediated activation of macrophages is important for the resolution of B. henselae infection and that anti-B. henselae microbicidal activity of IFN-γ-activated macrophages is mediated to a large extent by NO production. Since the most quantitatively relevant source of IFN-γ is constituted by antigen-activated T cells, it can be hypothesized that conditions characterized by a reduced production of IFN-γ or a low number of CD4+ cells, as observed in immunodeficient subjects, may result in increased susceptibility to B. henselae. In these cases, therapeutic use of the cytokine might become an additional tool for the management of these patients.
ACKNOWLEDGMENTS
We thank Enza Ferrero for careful review of the manuscript.
This work was partly supported by the Italian Ministry of University and Scientific Research Cofin MURST 2000 grant to A.N.P. and Cofin MURST 1999 to the Department of Pediatrics, Brescia University (R.B.), and Torino University Local Research ex 60% grant to T.M.
REFERENCES
- 1.Bekker L G, Freeman S, Murray P J, Ryffel B, Kaplan G. TNF-α controls intracellular mycobacterial growth by both inducible nitric oxide synthase-dependent and inducible nitric oxide synthase-independent pathways. J Immunol. 2001;166:6728–6734. doi: 10.4049/jimmunol.166.11.6728. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan C, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev 2000. 2001;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 3.Brunelli L, Crow J P, Beckman J S. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch Biochem Biophys. 1995;316:327–334. doi: 10.1006/abbi.1995.1044. [DOI] [PubMed] [Google Scholar]
- 4.Buchmeier N, Bossie S, Chen C, Fang F C, Guiney D G, Libby S J. SlyA, a trascriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect Immun. 1997;65:3725–3730. doi: 10.1128/iai.65.9.3725-3730.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caron E, Peyrard T, Kolhler S, Cabane S, Liautard J P, Dornand J. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U 937-derived phagocytes. Infect Immun. 1994;62:5267–5274. doi: 10.1128/iai.62.12.5267-5274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conley T, Slater L, Hamilton K. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J Lab Clin Med. 1994;124:521–528. [PubMed] [Google Scholar]
- 8.Darrah P A, Hondalus M K, Chen Q, Ischiropoulos H, Mosser D M. Cooperation between reactive oxigen and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages. Infect Immun. 2000;68:3587–3593. doi: 10.1128/iai.68.6.3587-3593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994;62:543–553. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Groote M A, Granger D, Xu Y, Campbell G, Prince R. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc Natl Acad Sci USA. 1995;92:6399–6403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Prete R, Fumarola D, Ungari S, Fumarola L, Miragliotta G. Polymerase chain reaction detection of Bartonella henselae bacteraemia in an immunocompetent child with cat-scratch disease. Eur J Pediatr. 2000;159:356–9. doi: 10.1007/s004310051286. [DOI] [PubMed] [Google Scholar]
- 12.Dolan M J, Wong M T, Regnery R L, Jorgensen J H, Garcia M, Peters J, Drehner D. Syndrome of Rochalimaea henselae adenitis suggesting cat scratch disease. Ann Intern Med. 1993;118:331–336. doi: 10.7326/0003-4819-118-5-199303010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Eze M O, Yuan L, Crawford R M, Paranavitana C M, Hadfield T L, Bhattacharjee A K, Warren R L, Hoover D L. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect Immun. 2000;68:257–263. doi: 10.1128/iai.68.1.257-263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facchetti F, Vermi W, Fiorentini S, Chilosi M, Caruso A, Duse M, Notarangelo L D, Badolato R. Expression of inducible nitric oxide synthase in human granulomas and histiocytic reactions. Am J Pathol. 1999;154:145–152. doi: 10.1016/S0002-9440(10)65261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng H M, Walker D H. Mechanisms of intracellular killing of rickettsia conorii in infected human endothelial cells, hepatocytes, and macrophages. Infect Immun. 2000;68:6729–6736. doi: 10.1128/iai.68.12.6729-6736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodrum K J, Dierksheide J, Yoder B J. Tumor necrosis factor alpha acts as an autocrine second signal with gamma interferon to induce nitric oxide in group B Streptococcus-treated macrophages. Infect Immun. 1995;63:3715–3717. doi: 10.1128/iai.63.9.3715-3717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guptill L, Slater L, Wu C C, Lin T L, Glickman L T, Welch D F, HogenEsch H. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 18.Jackson L A, Perkins B A, Wenger J D. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707–1711. doi: 10.2105/ajph.83.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan S S, Lancaster J R J, Basford R E, Simmons R L. Effect of nitric oxide on staphylococcal killing and interactive effect with superoxide. Infect Immun. 1996;64:69–76. doi: 10.1128/iai.64.1.69-76.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karem K L. Immune aspects of Bartonella. Crit Rev Microbiol. 2000;26:133–145. doi: 10.1080/10408410008984173. [DOI] [PubMed] [Google Scholar]
- 21.Karem K L, Dubois K A, McGill S L, Regnery R L. Characterization of Bartonella henselae-specific immunity in BALB/c mice. Immunology. 1999;97:352–358. doi: 10.1046/j.1365-2567.1999.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knobler E H, Silvers D N, Fine K C, Lefkowitch J H, Grossman M E. Unique vascular skin lesions associated with human immunodeficiency virus. JAMA. 1988;260:524–527. [PubMed] [Google Scholar]
- 23.MacMicking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 24.Maeno N, Oda H, Yoshiie K, Wahid M R, Fujimura T, Matayoshi S. Live Bartonella henselae enhances endothelial cell proliferation without direct contact. Microb Pathog. 1999;27:419–427. doi: 10.1006/mpat.1999.0315. [DOI] [PubMed] [Google Scholar]
- 25.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 26.Minnick M F, Mitchell S J, McAllister S J. Cell entry and the pathogenesis of Bartonella infections. Trends Microbiol. 1996;4:343–347. doi: 10.1016/0966-842x(96)10055-x. [DOI] [PubMed] [Google Scholar]
- 27.Miyagi K, Kawakami K, Saito A. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect Immun. 1997;65:4108–4113. doi: 10.1128/iai.65.10.4108-4113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munana K R, Vitek S M, Hegarty B C, Kordick D L, Breitschwerdt E B. Infection of fetal feline brain cells in culture with Bartonella henselae. Infect Immun. 2001;69:564–569. doi: 10.1128/IAI.69.1.564-569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray H W, Nathan C F. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J Exp Med. 1999;189:741–746. doi: 10.1084/jem.189.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan C, Shiloh M U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohya S, Tanabe Y, Makino M, Nomura T, Xiong H, Arakawa M, Mitsuyama M. The contributions of reactive oxygen intermediates and reactive nitrogen intermediates to listericidal mechanisms differ in macrophages activated pre- and postinfection. Infect Immun. 1998;66:4043–4049. doi: 10.1128/iai.66.9.4043-4049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Reilly K L, Bauer R W, Freeland R L, Foil L D, Hughes K J, Rohde K R, Roy A F, Stout R W, Triche P C. Acute clinical disease in cats following infection with a pathogenic strain of Bartonella henselae (LSU16) Infect Immun. 1999;67:3066–3072. doi: 10.1128/iai.67.6.3066-3072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmari J, Teysseire N, Dussert C, Raoult D. Image cytometry and topographical analysis of proliferation of endothelial cells in vitro during Bartonella (Rochalimaea) infection. Anal Cell Pathol. 1996;11:13–30. [PubMed] [Google Scholar]
- 34.Regnath T, Mielke M E A, Arvand M, Hahn H. Murine model of Bartonella henselae infection in the immunocompetent host. Infect Immun. 1998;66:5534–5536. doi: 10.1128/iai.66.11.5534-5536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Relman D A. Editorial response: are all Bartonella henselae strains created equal? Clin Infect Dis. 1998;26:1300–1301. doi: 10.1086/516347. [DOI] [PubMed] [Google Scholar]
- 36.Slater L N, Welch D F, Min K W. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatis. Arch Intern Med. 1992;152:602–606. [PubMed] [Google Scholar]
- 37.Tracey K J, Cerami A. Metabolic responses to cachectin/TNF. A brief review. Ann N Y Acad Sci. 1990;587:325–331. doi: 10.1111/j.1749-6632.1990.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 38.Turco J, Liu H, Gottlieb S F, Winkler H H. Nitric oxide-mediated inhibition of the ability of Rickettsia prowazekii to infect mouse fibroblasts and mouse macrophagelike cells. Infect Immun. 1998;66:558–566. doi: 10.1128/iai.66.2.558-566.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valentin-Weigand P, Benkel P, Rohde M, Chhatwal G. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun. 1996;64:2467–2473. doi: 10.1128/iai.64.7.2467-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ventura A, Massei F, Not T, Massimetti M, Bussani R, Maggiore G. Systemic Bartonella henselae infection with hepatosplenic involvement. J Pediatr Gastroenterol Nutr. 1999;29:52–56. doi: 10.1097/00005176-199907000-00014. [DOI] [PubMed] [Google Scholar]