Abstract
Objective
To investigate the efficacy and safety of hyperbaric oxygen therapy (HBOT) for fibromyalgia (FM).
Design
A systematic review and meta-analysis.
Data sources
PubMed, EMBASE, Cochrane Library, Web of Science, VIP (China Science and Technology Journal Database), CNKI (China National Knowledge Infrastructure) and WanFang database were searched from from inception to 22 October 2022.
Eligibility criteria
We included clinical trials (randomised controlled and non-randomised controlled trials) of HBOT for FM.
Data extraction and synthesis
Two researchers independently screened the literature, extracted data and evaluated the quality of the included studies, with disagreements resolved by a third researcher. The Cochrane Collaboration checklists and the Methodological Index for Non-randomised Studies were used to assess the risk of bias. Meta-analysis was performed by RevMan V.5.4.1 software. Random effect models were used for meta-analysis.
Results
Nine studies were included in this review, with a total of 288 patients. For pain assessment, we combined the results of the Visual Analogue Scale and Widespread Pain Index. The results showed that HBOT could relieve the pain of FM patients compared with the control intervention (standardised mean difference=−1.56, 95% CI (−2.18 to –0.93), p<0.001, I 2=51%). Most included studies reported that HBOT ameliorated tender points, fatigue, multidimensional function, patient global and sleep disturbance in FM. Adverse events occurred in 44 of 185 patients (23.8%). Twelve patients (6.5%) withdrew because of adverse reactions. No serious adverse events or complications were observed.
Conclusions
HBOT might have a positive effect in improving pain, tender points, fatigue, multidimensional function, patient global and sleep disturbance in FM, with reversible side effects. Low pressure (less than 2.0 atmospheric absolute) may be beneficial to reduce adverse events in FM. Further studies should be carried out to evaluate the optimal protocol of HBOT in FM.
PROSPERO registration number
CRD42021282920.
Keywords: Rheumatology, PAIN MANAGEMENT, Rehabilitation medicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Grading of Recommendations, Assessment, Development and Evaluations was used to assess the quality of evidence.
Rigorous methodology was used in this study, including explicit eligibility criteria, extensive database search, study selection by two reviewers working independently and risk of bias assessment.
Adverse events in hyperbaric oxygen therapy are negative outcomes that should be avoided, so it is important that we assess the risk of such effects to better understand the appropriate protocol regarding hyperbaric oxygen therapy.
The small number of randomised controlled trials included in the studies may lead to an overall risk of bias or insufficient evidence.
Introduction
Fibromyalgia (FM) is an incurable common syndrome with unclear origin.1 It is characterised by chronic pain at multiple tender points lasting for more than 3 months and is usually accompanied by clinical manifestations such as fatigue, sleep disturbance, cognitive dysfunction and depressive symptoms.2 3 It is estimated that 2%–8% of the population is affected by FM worldwide.4 FM is more frequent in females, with a female-to-male ratio of 9:1.5
The cause of FM syndrome is not yet fully understood, while the symptoms may be induced by infection, diabetes, rheumatic diseases, traumatic brain injury or mental trauma.4 6 Certain studies have reported a history of childhood sexual abuse in some patients with FM.7 8 Currently, treatment options mainly include pharmacological therapies, physical exercise, meditative exercise therapy and behavioural therapy.9–12 However, these methods only temporarily or moderately alleviate pain symptoms and often produce unbearable adverse effects that interfere with the patient’s quality of life and reduce their compliance.13 Therefore, there is a need for new and effective chronic pain treatments that can be tolerated by patients without significant adverse effects.
Accumulating evidence suggests that hyperbaric oxygen therapy (HBOT) is a non-invasive modality with lasting efficacy to treat FM.14–17 HBOT is conducted by intermittently breathing 100% oxygen in a pressure chamber above one atmospheric absolute pressure (ATA). HBOT can raise the partial pressure of oxygen in alveoli, leading to a favourable increase in dissolved oxygen in plasma.18 The increase in pressure and oxygen causes more dissolved oxygen to be delivered to the tissue through the blood, which oxygenates the ischaemic tissue.19 HBOT has shown strong anti-inflammatory potential by reducing the activation of glial cells and inflammatory mediators so that it could relieve pain under different chronic pain conditions.14 The anti-inflammatory effects of HBOT also correct associated abnormal brain activities and glial function, which may benefit FM patients.20 The increase in oxygen concentration caused by HBOT has been shown to improve the mitochondrial dysfunction of FM patients, leading to changes in brain metabolism and glial function, and may reduce the abnormal brain activities associated with FM.20 Although some studies have reported a positive effect of HBOT on FM, HBOT has not been recommended by guidelines as a complementary treatment for FM due to the lack of sufficient evidence.21 22
Mascarenhas et al 23 proposed that HBOT for the management of FM was moderate evidence in a systematic review. However, only two studies on HBOT for FM were included, and there was no meta-analysis. In addition, only two outcome measures (pain and quality of life) were investigated. To better understand the overall efficacy and safety of HBOT for FM, we conducted a systematic review and meta-analysis with more studies to investigate HBOT in the treatment of the inner Core Outcome Set of FM symptoms (pain, tenderness, fatigue, multidimensional function, patient global, sleep disturbance)24 and estimate its safety.
Methods
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.25 The protocol for this study is available online (PROSPERO trial registration number: CRD42021282920).
Search strategy
A literature search was conducted to identify all articles involving the use of hyperbaric oxygen to treat FM. The search strategy is shown in online supplemental Appendix 1. PubMed, EMBASE, Web of Science, Cochrane Library, VIP (China Science and Technology Journal Database), CNKI (China National Knowledge Infrastructure) and WanFang database were searched from from inception to 22 October 2022. The search included MeSH and free text terms such as “hyperbaric oxygen therapy”, “fibromyalgia” and synonyms.
bmjopen-2022-062322supp001.pdf (205.9KB, pdf)
Inclusion and exclusion criteria
We considered including all available information for systematic review due to the lack of data on this disease and the suspected lack of randomised controlled trials (RCTs). The criteria for inclusion were as follows: (1) study design: RCTs and non-RCTs; (2) subjects: FM patients conformed to the 2016 American College of Rheumatology (ACR) diagnostic criteria26 (ie, They met the following criteria: generalised pain for at least 3 months and a Widespread Pain Index (WPI) ≥7 and symptom severity scale (SSS) ≥5 or a WPI of 4–6 and an SSS score ≥9); (3) the intervention: patients in the experimental group received HBOT as the intervention measure, and patients in the control group received conventional treatment or nothing. The conventional treatment was any pharmacological or nonpharmacological therapy other than HBOT. The course of treatment and parameters were unlimited. (4) Outcome indicators: the inner Core Outcome Set of FM symptoms (pain, tenderness, fatigue, multidimensional function, patient global, sleep disturbance) and adverse events (AEs). The exclusion criteria were as follows: animal studies, reviews, duplicate publications, irrelevant studies, editorial materials, patients, case reports or meeting abstracts.
Literature screening and data collection
Two reviewers (JY and HM) independently assessed the eligibility of each article. Duplicate articles were eliminated. Irrelevant articles were excluded by reading the title and abstract, and then the full text was read to further screen out articles that met the inclusion criteria. Articles without full text or data were excluded after three or more attempts to email the lead author and obtain no response. The decision to include each article was made independently according to the inclusion criteria, with disagreements resolved by a third reviewer (XC). Reviewers followed PRISMA criteria for systematic evaluation.
A predesigned form was used for information extraction. The content included the article’s basic information (author, year of publication, title); research types; patient demographics (age, gender); intervention and control measures (duration, frequency, sessions, follow-up); outcome indicators; the data of results and indicators that reflected research quality. Data collection was completed independently by two researchers (JY and HM) and checked with each other. In case of disagreement, a third researcher (XC) assisted in resolving the disagreement.
Types of outcome measures
The inner Core Outcome Set of FM symptoms suggested by Mease et al 24 can be quantitatively or qualitatively analysed. The primary outcome measure was pain, and the secondary outcome measures included tenderness, fatigue, multidimensional function, patient global, sleep disturbance and AEs.
Pain and tenderness
Assessment methods included the Pain Visual Analogue Scale (VAS), number of tender points, pain threshold and WPI.
Multidimensional function
Assessment methods included the Fibromyalgia Impact Questionnaire (FIQ) and 36-Item Short Form Survey (SF-36).
Fatigue
Assessment methods of fatigue included the Fatigue Severity Scale, Functional Assessment of Chronic Illness Therapy Fatigue scale, Fatigue VAS and CR-10 Borg Scale.
Patient global
The Patient Global Impression of Change (PGIC) was used to assess this outcome measure.
Sleep disturbance
Assessment methods included the Jenkins Sleep Scale and Pittsburgh Sleep Quality Index.
Adverse events
This indicator included AEs, withdrawals due to AEs, and complications.
Risk of bias assessment
Reviewers assessed the quality of the included articles using the Cochrane Collaboration checklists27 for 3 RCTs and the Methodological Index for Non-randomised Studies (MINORS)28 for 6 non-RCTs. The Cochrane checklists assessed selection bias, implementation bias, measurement bias, attrition bias, reporting bias and other bias. In the Cochrane ROB tool, the risk of bias was classified as ‘low risk’, ‘unclear’ and ‘ high risk’. Review Manager version V.5.4.1 was used to generate the risk of bias graph of the three RCTs. The MINORS checklists included twelve items (0–24 scores) for comparative studies and eight items (0–16 scores) for non-comparative studies. The score for each item was 0 (not reported), 1 (reported but inadequate) or 2 (reported and adequate). Comparative studies scoring >19 or non-comparative studies scoring >12 were considered high quality. The quality of the included studies was assessed independently by two reviewers (JY and HM). Again, any controversy in the assessment was resolved through discussion with a third reviewer (XC).
Statistical analysis
RevMan V.5.4.1 software provided by the Cochrane Collaboration was used to conduct a meta-analysis. The standardised mean difference (SMD) and its 95% confidence intervals (CI) were used as the analysis statistics because across studies different rating tools are used to measure the same outcome.29 Forest plot tests were conducted, and meta-regression analysis was used to test heterogeneity. The χ2 test was used to analyse whether there was statistical heterogeneity among the results of each study. This study used the random effects model for meta-analysis because the random effects meta-analysis allowed for differences (treatment areas, concomitant treatments and HBOT regimen) in treatment effects among different studies.30
Grade the quality of evidence
Grading of Recommendations, Assessment, Development and Evaluations (GRADE) was used to grade the quality of the evidence.31 The risk of bias, inconsistency, indirectness, imprecision and publication bias were assessed. The quality of evidence was rated ‘high’, ‘moderate’, ‘low’ or ‘very low’.
Patient and public involvement
Patients and the public were not involved in this study.
Results
Characteristics of the included studies
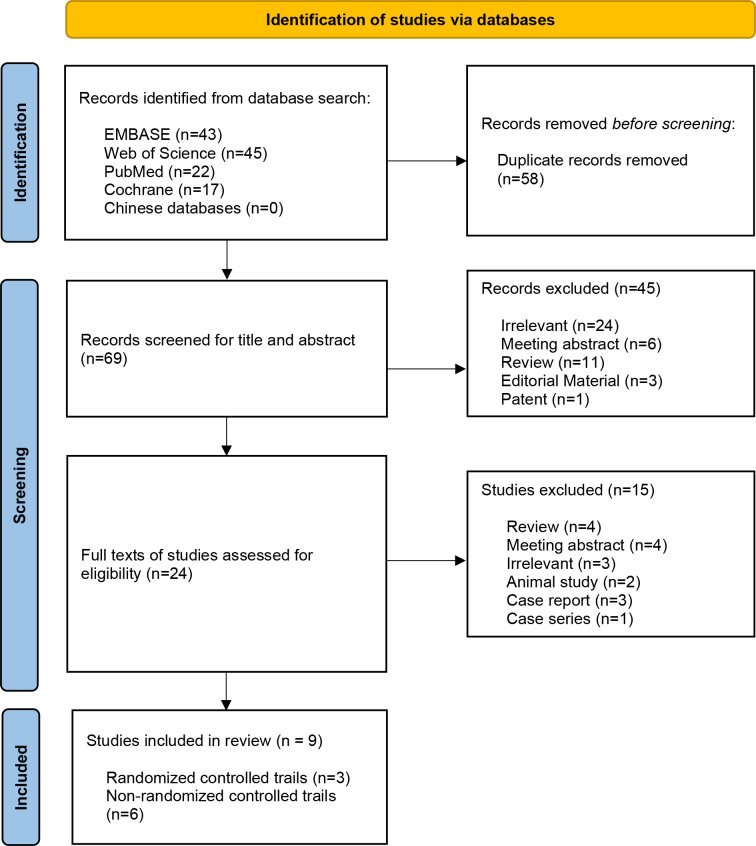
A total of 69 eligible articles were obtained by a literature search. After screening, nine studies (three RCTs and six non-RCTs) met the inclusion criteria.32–40 The flow diagram is shown in figure 1. A total of 288 patients were included in this study. Table 1 shows the characteristics of the included articles.
Figure 1.
PRISMA flow chart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of the included articles
| Author, year | Patients (N) | Intervention (HBOT) | Comparison | Outcome measures | Adverse events and the no of patients | Study design | |||
| Intervention | Control | Protocol | Sessions/ length | Adverse events | Patients (N) | ||||
| Yildiz 200440 | 26 | 24 | 90 min, 2.4ATA, 5d/w |
15/3 weeks | 90 min, 1ATA, 5d/w |
Number of tender points, Pain threshold, Pain VAS |
– | – | RCT |
| Hadanny 201838 | 15 | 15 | 90 min, 2ATA, 5d/w |
60/12 weeks | Psychotherapy | WPI, FIQ, SF-36 |
Mild barotrauma | 12 | RCT |
| Headache | 1 | ||||||||
| Izquierdo-Alventosa 202033 | 17 | 16 | 90 min, 1.45ATA, 5d/w | 40/8 weeks | Conventional therapy | VAS, Pain threshold, CR-10 Borg scale |
– | – | RCT |
| Efrati 201539 | 27 | 26 | 90 min, 2ATA, 5d/w |
40/8 weeks | No treatment | Number of tender points, Pain threshold, FIQ, SF-36 |
Mild barotrauma | 13 | NCT |
| Dizziness, claustrophobia and inability to adjust ear pressure by “ear pumping” | 5 | ||||||||
| Guggino 202034 | 22 | 14 | 90 min, 2ATA, 5d/w |
40/8 weeks | No treatment | Number of tender points, Pain VAS, Fatigue VAS, WPI, FACIT fatigue, PSQI |
– | – | NCT |
| Curtis 202132 | 9 | 8 | 90 min, 2ATA, 5d/w |
40/8 weeks | Conventional therapy | FIQR, FSS, JSS, PGIC, Fatigue VAS |
Mild middle-ear barotrauma | 3 | NCT |
| New-onset myopia | 4 | ||||||||
| Casale 20135 | 25 | – | 91 min, 2.4ATA | 20/4 weeks | – | Neuromuscular efficiency | Side effects | 2 | NCT |
| Bosco 201936 | 12 | – | 90 min, 2ATA, 5d/w |
20/4 weeks | – | WPI | – | – | NCT |
| Atzeni 201937 | 32 | – | 90 min, 2.5ATA, 3d/w |
20/4 weeks | – | Pain VAS, FACIT, PSQI, FIQR, SF-36 | Mild, reversible middle ear barotrauma | 2 | NCT |
| Dizziness | 1 | ||||||||
| Claustrophobia | 1 | ||||||||
ATA, atmospheric absolute pressure; d/w, days/week; FACIT fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue Scale; FIQ, Fibromyalgia Impact Questionnaire; FIQR, Revised FIQ; FSS, Fatigue Severity Scale; JSS, Jenkins Sleep Scale; NCT, non-randomised controlled trial; PGIC, Patient Global Impression of Change; PSQI, Pittsburgh Sleep Quality Index; RCT, randomised controlled trial; SF-36, 36-Item Short Form Survey; VAS, Visual Analogue Scale; WPI, Widespread Pain Index.
Quality assessment
Figure 2 shows the risk of bias graph of the three RCTs according to the Cochrane ROB tool. Of the RCTs included, studies by Yildiz et al 40 and Hadanny et al 38 had an unclear risk of selection bias because of the lack of specific randomisation methods and no indication of allocation concealment. All RCTs were judged to have an unclear or high risk of performance bias because researchers did not adopt blinding. All RCTs were at low risk for detection bias and attrition bias. However, the risk of reporting bias and other bias in all RCTs were unclear, mainly due to the lack of follow-up. Table 2 shows the quality assessment of the six non-RCTs. The average MINORS scores for non-comparative and comparative studies were 9.7 and 19.7, respectively. Studies by Efrati et al 39 and Curtis et al 32 were considered high quality. In non-RCTs, lack of bias assessment, study size calculation and follow-up were the most common reasons for low MINORS scores.
Figure 2.
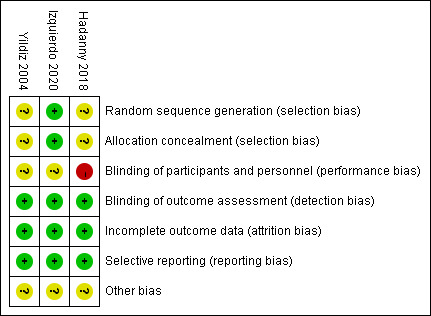
Risk of bias graph for the included randomised controlled trials across five domains. The red circle indicates a high risk of bias within that domain for a given study, the yellow circles indicate an unclear risk of bias and the green circles indicate a low risk of bias.
Table 2.
Quality assessment of the included non-randomised controlled trials using the Methodological Index for Non-randomised Studies
| Assessment | Efrati 2015 et al 39 | Guggino et al 202034 | Curtis et al 202132 | Casal et al 201935 | Bosco et al 201936 | Atzeni et al 201937 |
| 1. A clearly stated aim | 2 | 2 | 2 | 2 | 2 | 2 |
| 2. Inclusion of consecutive patients | 2 | 2 | 2 | 2 | 2 | 2 |
| 3. Prospective collection of data | 2 | 2 | 2 | 2 | 2 | 2 |
| 4. Endpoints appropriate to the aim of the study | 2 | 2 | 2 | 2 | 2 | 2 |
| 5. Unbiased assessment of the study endpoint | 2 | 0 | 1 | 1 | 0 | 0 |
| 6. Follow-up period appropriate to the aim of the study | 0 | 0 | 2 | 0 | 2 | 0 |
| 7. Lost to follow-up less than 5% | 0 | 0 | 2 | 0 | 0 | 0 |
| 8. Prospective calculation of the study size | 2 | 2 | 0 | 0 | 0 | 2 |
| 9. An adequate control group | 2 | 2 | 2 | – | – | – |
| 10. Contemporary groups | 2 | 2 | 2 | – | – | – |
| 11. Baseline equivalence of groups | 2 | 2 | 2 | – | – | – |
| 12. Adequate statistical analyses | 2 | 2 | 2 | – | – | – |
| Total score | 20 | 18 | 21 | 9 | 10 | 10 |
Efficacy of HBOT
Because of the small number of studies, insufficient data that could be pooled and heterogeneity among different study types, only pain relief from three RCTs was included in the meta-analysis, and the other outcome indicators were only analysed descriptively.
Pain relief
Seven studies (three RCTs and four non-RCTs)33 34 36–40 reported that HBOT alleviated the pain level of FM, as documented by the decrease in rating scales related to pain. We conducted a meta-analysis on pain relief of three RCTs.33 38 40 For pain assessment, we combined the results of VAS and WPI. Meta-analysis of a random effect model showed that the pain relief in the HBOT group was better than that in the control group (SMD=−1.56, 95% CI (−2.18 to –0.93), p<0.001, I 2=51%) (figure 3).
Figure 3.
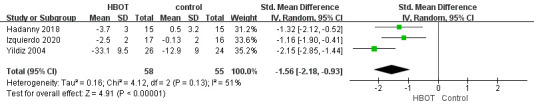
Forest plot of pain relief. HBOT, hyperbaric oxygen therapy.
Tenderness
Three studies34 39 40 reported that HBOT reduced the number of tender points in FM. Jeschonneck et al 41 found that vasoconstriction in patients with FM occurred in the skin above the tender point. This confirmed that FM syndrome was associated with local hypoxia of the skin covering the tender points. Lund et al 42 proposed that in FM with primary aetiology, muscle oxygenation was abnormal or low, at least in the muscle trigger point region, as recorded by oxygen multipoint electrodes on the muscle surface. HBOT could break the vicious cycle of pain-hypoxia because it increased the pain threshold to reduce the number of tender points in patients with FM.40
Multidimensional function
Three studies32 38 39 reported that HBOT improved FM-related functional impairment and overall symptoms, as documented by the decreased score of the FIQ or FIQ-R questionnaire. These studies may support the use of HBOT to reduce the effects of FM on global symptoms and functional activities. Studies by Hadanny et al,38 Efrati et al 39 and Atzeni et al 37 reported the SF-36, which was used to assess the quality of life. All three studies showed that HBOT could effectively improve the quality of life of FM. In addition, Hadanny et al 38 had shown that improvements in quality of life with FM were associated with improvements in brain performance parameters seen in brain function (single-photon emission computerized tomography) and structure (magnetic resonance imaging-diffusion tensor imaging). This may be because HBOT can improve brain function and microstructure by inducing neural plasticity in humans.43 44
Fatigue
Three studies33 34 37 showed that HBOT could reduce fatigue in FM patients, while Curtis et al 32 reported that HBOT had no significant effect on fatigue in FM. Studies have shown that HBOT reduced fatigue in chronic fatigue syndrome,45 which was attributed to its ability to reduce reactive oxygen species and acid-lactic acid levels, as well as muscle fatigue after exercise.46 HBOT alleviated fatigue in FM patients, possibly because HBOT increased oxygen supply to the musculoskeletal system, thereby activating cellular activity and promoting the metabolism of fatigue-related substances.47 Clinical studies have shown that increased plasma proinflammatory cytokine levels trigger symptoms such as fatigue, fever, sleep, pain and myalgia in FM patients.48 HBOT can improve FM symptoms by reducing the upregulation of proinflammatory cytokines in FM. Atzen et al 37 proposed that the fatigue of FM was only improved after 20 treatments, indicating that the number of treatments would affect the efficacy of HBOT. In Curtis et al’s study,32 the lack of an effect of HBOT on fatigue may be attributed to baseline differences in the small sample size. In addition, Casale et al 35 found that HBO did not directly increase FM muscle strength or alter muscle fibre content to alleviate fatigue but increased the ability of the central motor command to generate the same effort with fewer recruited fibres.
Patient global
Only one study32 reported PGIC, which assessed global response to treatment and has been associated with clinical symptoms in patients with FM. Curtis32 reported that patients with FM had a different degree of symptom improvement after HBOT and at a 3-month follow-up. After HBOT treatment, ‘almost the same’ was the most common impression of global symptoms in FM patients (44.4%). However, at the 3-month follow-up, ‘a great deal better’ was the most common impression of global symptoms in FM patients (41.7%). This showed that HBOT may be effective for a long time.
Sleep disturbance
Three studies reported sleep quality. Guggino et al 34 reported that HBOT did not improve the total sleep time of FM patients but improved their sleep quality. Curtis et al 32 proposed that HBOT improved sustained sleep quality in FM at a 3-month follow-up assessment. However, Atzeni et al 37 indicated that HBOT did not significantly improve the sleep quality of FM. This inconsistency may be related to the different number of HBOT sessions, which needs further study.
AEs of HBOT
Five studies reported the side effects of HBOT for FM (as shown in table 1). AEs occurred in 44 of 185 patients (23.8%). Twelve patients (6.5%) withdrew because they could not tolerate adverse reactions. Of these AEs, there were 30 cases of mild barotrauma, 4 cases of new-onset myopia, 1 case of headache, 7 cases of dizziness, claustrophobia, inability to adjust ear pressure by ‘ear pumping’ and 2 cases of side effects (not clearly reported). The predominant AE was mild barotrauma that could be resolved spontaneously and did not prevent patients from completing the treatment regimen. No serious side effects, complications or deaths were reported.
Grade analysis of the evidence
The quality of pain relief was ‘moderate’. Although there was a serious risk of bias and inconsistency, there was no serious directness or imprecision. In addition, the outcome of pain relief has a large effect. The GRADE evidence profile is shown in table 3.
Table 3.
GRADE evidence profile
| Outcome | Certainty assessment | Effect | Certainty | ||||||
| Risk of bias | Inconsistency | Directness | Imprecision | Others | Number of studies | Number of individuals | Rate (95% CI) | ||
| Pain relief | Serious* | Serious† | Not serious‡ | Not Serious§ | Large effect¶ | Three RCTs | 113 | SMD: −1.56 (−2.18 to −0.93) |
⊗⊗⊗○ Moderate |
*Most of the included studies were assessed as some concerns/high-risk bias.
†I 2 >50%.
‡Direct participants, intervention and outcomes.
§Total sample size >100.
¶SMD >0.8.
GRADE, Grading of Recommendations, Assessment, Development and Evaluations; SMD, standardised mean difference.
Discussion
In this study, we focused on the efficacy of HBOT on the inner core outcomes of FM. Pain relief was the primary outcome and could be meta-analysed (three RCTs). Tenderness, fatigue, multidimensional function, patient global, sleep disturbance and AEs were secondary outcome measures and were analysed descriptively because of the limited number of studies or limited available data that could be combined. After a systematic review, we found that HBOT could relieve the pain of FM patients compared with the control intervention (SMD=−1.56, 95% CI (−2.18 to –0.93), p<0.001, I 2=51%). In addition, most of the included studies have shown that HBOT could significantly improve tender points, fatigue, quality of life, patient global and sleep disturbance in patients with FM. However, Curtis et al 32 found that HBOT had no positive effect on fatigue reduction of FM, and Atzeni et al 37 indicated that HBOT did not significantly improve the quality of life of FM. This inconsistency might be due to baseline differences in small sample sizes or the insufficient number of HBOT sessions. Of the 185 patients with FM who received HBOT, 44 patients had adverse reactions during HBOT treatment (23.8%) and 12 patients withdrew (6.5%) because they could not tolerate the side effects. However, in one retrospective study of 1.5 million cases of treatment with HBOT, the AE rate was only 0.68%.49 We speculated that patients with FM might have a lower pain threshold and may be more sensitive to discomfort than patients with other diseases. Mild barotrauma was the most common complication of HBOT for FM. Patients may experience pressure, difficulty in ear balance, earache and discomfort during compression.50 However, mild barotrauma can be resolved spontaneously and does not prevent patients from completing the treatment, and can usually be prevented by appropriate screening.51 Oliaei et al 52 found that most complications of HBOT occurred when the pressure applied exceeded 2.0 ATA. The articles included in this study mostly used hyperbaric oxygen chambers of 2–2.5 ATA for the treatment of FM, which may lead to side effects. A randomised controlled study33 confirmed that low-pressure HBOT (1.45 ATA) was effective in the treatment of FM without AEs. Therefore, a pressure lower than 2.0 ATA may be a good choice for patients with FM to avoid side effects. Further studies are needed to explore the efficacy and safety of low-pressure HBOT for FM. In addition, contraindications for HBOT should be strictly screened before treatment, and the appropriate pressure and duration of treatment should be determined according to the patient’s tolerance.
Patients with FM in the control group received conventional treatment or nothing in the included studies. Yildiz et al,40 Efrati et al 39 and Guggino et al 34 did not give any treatment to the patients in the control group, while Hadanny et al,38 Izquierdo-Alventosa et al 33 and Curtis et al 32 performed conventional treatment for the patients in the control group. The conventional treatment that FM received included psychotherapy, medications, physical activity, nutrition therapy, massage, acupuncture, behavioural therapy, and cognitive therapy. Therefore, HBOT may be effective both as an adjunctive therapy and as an independent treatment. Most of the included studies used the same HBOT protocol, which was 100% oxygen at 2–2.5 ATA, 90 min per session, 5 days per week. Only a study by Izquierdo-Alventosa et al 33 used 1.45 ATA to avoid the side effects of HBOT. The length of treatment in the included studies ranged from three to twelve weeks, of which the study by Yildiz et al 40 lasted 3 weeks, the study by Hadanny et al 38 lasted 12 weeks, three non-comparative studies35–37 lasted 4 weeks and the rest of the studies lasted 8 weeks. A rodent study found that the anti-injury effects of HBOT were apparent immediately after treatment and lasted for up to 5 hours.19 In a rat neuropathic pain model, 2 weeks of HBOT resulted in a significant improvement in pain levels during and after treatment.53 Atzeni et al 37 proposed that 2–4 weeks of HBOT treatment significantly improved pain and anxiety symptoms in FM, while fatigue only improved after 4 weeks. In addition, sleep quality and depressive symptoms were not positively affected in FM after 4 weeks of HBOT. In this review, only Curtis et al 32 mentioned a follow-up measurement (3 months) and found that HBOT can continuously improve patient global, psychological symptoms and sleep quality in FM. Another study16 showed that HBOT for 10 days had a rapid onset, dose-dependent and long-lasting analgesic effect in patients with idiopathic trigeminal neuralgia documented a reduction in the dosage of carbamazepine analgesics and lower pain VAS. Therefore, long-term treatment with HBOT may be beneficial to improve symptoms of FM or prolong efficacy. However, the prolonged treatment window of patients is likely to cause side effects. Studies have shown that human lenses exposed to 2.0–2.5 ATA and 100% oxygen for 90 min once a day will lead to the development of myopia and cataracts after 150–850 courses of HBOT.54 However, when exposed to 2.5 ATA and 100% oxygen for 90 min once a day for 48 courses, the above side effects rarely occur.55 It is challenging to establish the effect and optimal dose‒response curves of HBOT in FM considering both safety and efficacy.
There is growing evidence that HBOT is a non-invasive way to treat chronic pain diseases with long-lasting efficacy and minor adverse effects.13 In murine models of pain, HBOT has been shown to inhibit pain sensation, which may be due to the nitric oxide-dependent release of opiate peptides and could be restrained by an antagonist, naltrexone.56 57 This effect works in the central system but also involves HBO activating µ-opioid and K-opioid receptors in the spinal cord and releasing neuronal dynorphins.58 In murine models of arthritis, HBOT has also been shown to affect inflammatory pain by reducing mechanical hypersensitivity and inflammation.59 Patients with FM often experience degenerative changes in muscle, abnormal oxygen pressure and lower muscle blood flow due to hypoxia.16 60 Local ischaemia causes mitochondria to produce higher levels of free radicals to induce apoptosis, reduce ATP synthesis and increase lactate concentration in the muscle, thus ultimately leading to muscle weakness and pain.61 62 HBOT improves muscle oxygenation in FM, which can reduce the tissue lactate concentration and help maintain ATP levels, thus possibly preventing tissue damage in ischaemic tissue.63 It raises the oxygen concentration in all tissues far above physiological levels to cause hyperoxia, which breaks the hypoxic-pain cycle in patients with FM.63 In addition, the high excitability of pain processing pathways in the brain and low activity of pain inhibition pathways may cause excessive pain in FM.64 Studies have shown that patients with FM have higher activity in the somatosensory cortex and lower activity in the frontal, medial frontal, cingulate gyrus and cerebellar cortex than healthy subjects.65 HBOT has been shown to increase neurotrophic and nitric oxide levels, reduce oxidative stress, promote cell metabolism by enhancing the mitochondrial function of neurons and glial cells, and may even promote the production of endogenous neural stem cells.66 The specific mechanism of HBOT on FM needs to be further investigated.
The quality of evidence (pain relief of HBOT for FM) assessed using the GRADE system was moderate. There are inherently ethical and logistical difficulties in handling sham control in HBOT experiments. In two RCTs,33 38 the researchers did not use the sham control/placebo in the control group, which may lower the quality of the evidence. The heterogeneity of the outcome may be caused by the population and HBOT regimen. However, the large effect (SMD >0.8) may increase the quality of the evidence. Therefore, we have a moderate degree of confidence in our estimated effect. The true value may be close to the estimated value, but there is still a chance that they could be very different.
There are some limitations in this systematic review. The main limitation is that the small number of RCTs included may lead to an overall risk of bias or insufficient evidence. Second, HBOT protocols (the length of treatment and pressure parameters) have clinical heterogeneity, which may introduce bias to the results. Third, we only retrieved data from Chinese and English databases, which may limit the data availability or cause language bias. Finally, due to the small number of included studies and heterogeneity, we did not conduct a subgroup analysis. Therefore, we cannot evaluate the efficacy of different HBOT regimens.
In conclusion, this study shows that HBOT may have a good effect in improving pain, tender points, fatigue, multidimensional function, patient global and sleep disturbance in FM, with reversible side effects. Low pressure (less than 2.0 ATA) may be beneficial to reduce AEs in patients with FM. Further high-quality and large-sample RCTs should be carried out to further evaluate its efficacy and safety.
Supplementary Material
Footnotes
XC and JY contributed equally.
Contributors: Conceptualisation: CH, XC, JY. Funding Acquisition: CH. Formal Analysis: XC. Investigation: CH. Writing-Original Draft Preparation: XC, JY, MZ, HM. Writing–Review & Editing: all the authors. Guarantor: CH. All the authors fulfill the ICMJE criteria for authorship.
Funding: Key Research and Development Project of Sichuan Provincial Science and Technology Department (No. 2018SZ0082); 1·3·5 Project for Disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2021HXFH063)
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Sueiro Blanco F, Estévez Schwarz I, Ayán C, et al. Potential benefits of non-pharmacological therapies in fibromyalgia. Open Rheumatol J 2008;2:1–6. 10.2174/1874312900802010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas EN, Blotman F. Aerobic exercise in fibromyalgia: a practical review. Rheumatol Int 2010;30:1143–50. 10.1007/s00296-010-1369-6. [DOI] [PubMed] [Google Scholar]
- 3. Alciati A, Nucera V, Masala IF, et al. One year in review 2021: fibromyalgia. Clin Exp Rheumatol 2021;39 Suppl 130:3–12. 10.55563/clinexprheumatol/gz4i3i. [DOI] [PubMed] [Google Scholar]
- 4. Siracusa R, Paola RD, Cuzzocrea S, et al. Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. Int J Mol Sci 2021;22:3891. 10.3390/ijms22083891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nat Rev Rheumatol 2011;7:518–27. 10.1038/nrrheum.2011.98. [DOI] [PubMed] [Google Scholar]
- 6. Sarzi-Puttini P, Atzeni F, Mease PJ. Chronic widespread pain: from peripheral to central evolution. Best Pract Res Clin Rheumatol 2011;25:133–9. 10.1016/j.berh.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 7. Häuser W, Galek A, Erbslöh-Möller B, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013;154:1216–23. 10.1016/j.pain.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 8. Imbierowicz K, Egle UT. Childhood adversities in patients with fibromyalgia and somatoform pain disorder. Eur J Pain 2003;7:113–9. 10.1016/S1090-3801(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 9. Elliott AM, Smith BH, Penny KI, et al. The epidemiology of chronic pain in the community. Lancet 1999;354:1248–52. 10.1016/s0140-6736(99)03057-3. [DOI] [PubMed] [Google Scholar]
- 10. Gouvinhas C, Veiga D, Mendonça L, et al. Interventional pain management in multidisciplinary chronic pain clinics: a prospective multicenter cohort study with one-year follow-up. Pain Res Treat 2017;2017:8402413. 10.1155/2017/8402413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl 2005;75:6–21. [PubMed] [Google Scholar]
- 12. Smith BH, Elliott AM, Chambers WA, et al. The impact of chronic pain in the community. Fam Pract 2001;18:292–9. 10.1093/fampra/18.3.292. [DOI] [PubMed] [Google Scholar]
- 13. Sutherland AM, Clarke HA, Katz J, et al. Hyperbaric oxygen therapy: a new treatment for chronic pain? Pain Pract 2016;16:620–8. 10.1111/papr.12312. [DOI] [PubMed] [Google Scholar]
- 14. El-Shewy KM, Kunbaz A, Gad MM, et al. Hyperbaric oxygen and aerobic exercise in the long-term treatment of fibromyalgia: a narrative review. Biomed Pharmacother 2019;109:629–38. 10.1016/j.biopha.2018.10.157. [DOI] [PubMed] [Google Scholar]
- 15. Efrati S, Hadanny A, Daphna-Tekoah S, et al. Recovery of repressed memories in fibromyalgia patients treated with hyperbaric oxygen – case series presentation and suggested Bio-Psycho-Social mechanism. Front Psychol 2018;9:848. 10.3389/fpsyg.2018.00848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu N, Niu J-Y, Liu W-T, et al. Hyperbaric oxygen therapy attenuates neuropathic hyperalgesia in rats and idiopathic trigeminal neuralgia in patients. EJP 2012;16:1094–105. 10.1002/j.1532-2149.2012.00113.x [DOI] [PubMed] [Google Scholar]
- 17. Yildiz S, Kiralp MZ, Akin A. Hyperbaric oxygen therapy reduces muscle tenderness and raises pain threshold in patients suffering from fibromyalgia. Diving Hyperb Med 2007;37:225. [Google Scholar]
- 18. J KK. Textbook of hyperbaric medicine. 3rd ed. Seattle, WA: Hogrefe and Huber Publishers, 1999. [Google Scholar]
- 19. Wilson HD, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Res 2006;1098:126–8. 10.1016/j.brainres.2006.04.088 [DOI] [PubMed] [Google Scholar]
- 20. Efrati S, Golan H, Bechor Y, et al. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome – prospective clinical trial. PLoS One 2015;10:e0127012. 10.1371/journal.pone.0127012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evcik D, Ketenci A, Sindel D. The Turkish Society of physical medicine and rehabilitation (TSPMR) guideline recommendations for the management of fibromyalgia syndrome. Turk J Phys Med Rehabil 2019;65:111–23. 10.5606/tftrd.2019.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawk C, Whalen W, Farabaugh RJ, et al. Best practices for chiropractic management of patients with chronic musculoskeletal pain: a clinical practice guideline. The Journal of Alternative and Complementary Medicine 2020;26:884–901. 10.1089/acm.2020.0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mascarenhas RO, Souza MB, Oliveira MX, et al. Association of therapies with reduced pain and improved quality of life in patients with fibromyalgia: a systematic review and meta-analysis. JAMA Intern Med 2021;181:104–12. 10.1001/jamainternmed.2020.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mease P, Arnold LM, Choy EH, et al. Fibromyalgia syndrome module at OMERACT 9: domain construct. J Rheumatol 2009;36:2318–29. 10.3899/jrheum.090367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Page MJ, McKenzie JE, Bossuyt PM. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolfe F, Clauw DJ, Fitzcharles M-A, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712–6. 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 29. Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry 2020;81:20f13681. 10.4088/JCP.20f13681. [DOI] [PubMed] [Google Scholar]
- 30. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 31. Guyatt GH, Oxman AD, Schünemann HJ, et al. Grade guidelines: a new series of articles in the Journal of clinical epidemiology. J Clin Epidemiol 2011;64:380–2. 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 32. Curtis K, Katz J, Djaiani C, et al. Evaluation of a hyperbaric oxygen therapy intervention in individuals with fibromyalgia. Pain Med 2021;22:1324–32. 10.1093/pm/pnaa416 [DOI] [PubMed] [Google Scholar]
- 33. Izquierdo-Alventosa R, Inglés M, Cortés-Amador S, et al. Comparative study of the effectiveness of a low-pressure hyperbaric oxygen treatment and physical exercise in women with fibromyalgia: randomized clinical trial. Ther Adv Musculoskelet Dis 2020;12:1759720X20930493. 10.1177/1759720X20930493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guggino G, Schinocca C, Lo Pizzo M. T helper 1 response is correlated with widespread pain, fatigue, sleeping disorders and the quality of life in patients with fibromyalgia and is modulated by hyperbaric oxygen therapy. Clin Exp Rheumatol 2020;38:1275. [PubMed] [Google Scholar]
- 35. Casale R, Boccia G, Symeonidou Z, et al. Neuromuscular efficiency in fibromyalgia is improved by hyperbaric oxygen therapy: looking inside muscles by means of surface electromyography. Clin Exp Rheumatol 2019;37 Suppl 116:75–80. [PubMed] [Google Scholar]
- 36. Bosco G, Ostardo E, Rizzato A, et al. Clinical and morphological effects of hyperbaric oxygen therapy in patients with interstitial cystitis associated with fibromyalgia. BMC Urol 2019;19:108. 10.1186/s12894-019-0545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atzeni F, Casale R, Alciati A, et al. Hyperbaric oxygen treatment of fibromyalgia: a prospective observational clinical study. Clin Exp Rheumatol 2019;37 Suppl 116:63–9. [PubMed] [Google Scholar]
- 38. Hadanny A, Bechor Y, Catalogna M, et al. Hyperbaric oxygen therapy can induce neuroplasticity and significant clinical improvement in patients suffering from fibromyalgia with a history of childhood sexual abuse-randomized controlled trial. Front Psychol 2018;9:2495. 10.3389/fpsyg.2018.02495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Efrati S, Golan H, Bechor Y, et al. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome -- prospective clinical trial. PLoS One 2015;10:e0127012. 10.1371/journal.pone.0127012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yildiz S, Kiralp MZ, Akin A, et al. A new treatment modality for fibromyalgia syndrome: hyperbaric oxygen therapy. J Int Med Res 2004;32:263–7. 10.1177/147323000403200305. [DOI] [PubMed] [Google Scholar]
- 41. Jeschonneck M, Grohmann G, Hein G, et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology 2000;39:917–21. 10.1093/rheumatology/39.8.917 [DOI] [PubMed] [Google Scholar]
- 42. Lund N, Bengtsson A, Thorborg P. Muscle tissue oxygen pressure in primary fibromyalgia. Scand J Rheumatol 1986;15:165–73. 10.3109/03009748609102084 [DOI] [PubMed] [Google Scholar]
- 43. Boussi-Gross R, Golan H, Fishlev G, et al. Hyperbaric oxygen therapy can improve post concussion syndrome years after mild traumatic brain injury - randomized prospective trial. PLoS One 2013;8:e79995. 10.1371/journal.pone.0079995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Efrati S, Fishlev G, Bechor Y, et al. Hyperbaric Oxygen Induces Late Neuroplasticity in Post Stroke Patients - Randomized, Prospective Trial. PLoS One 2013;8:e53716. 10.1371/journal.pone.0053716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Akarsu S, Tekin L, Ay H. The efficacy of hyperbaric oxygen therapy in the management of chronic fatigue syndrome. Undersea Hyperb Med 2013;40:197–200. [PubMed] [Google Scholar]
- 46. Shimoda M, Enomoto M, Horie M, et al. Effects of hyperbaric oxygen on muscle fatigue after maximal intermittent plantar flexion exercise. J Strength Cond Res 2015;29:1648–56. 10.1519/JSC.0000000000000809 [DOI] [PubMed] [Google Scholar]
- 47. Ishii Y, Deie M, Adachi N, et al. Hyperbaric oxygen as an adjuvant for athletes. Sports Med 2005;35:739–46. 10.2165/00007256-200535090-00001 [DOI] [PubMed] [Google Scholar]
- 48. Kaufmann I, Eisner C, Richter P, et al. Lymphocyte subsets and the role of Th1/Th2 balance in stressed chronic pain patients. Neuroimmunomodulation 2007;14:272–80. 10.1159/000115041 [DOI] [PubMed] [Google Scholar]
- 49. Jokinen-Gordon H, Barry RC, Watson B, et al. A retrospective analysis of adverse events in hyperbaric oxygen therapy (2012-2015): lessons learned from 1.5 million treatments. Adv Skin Wound Care 2017;30:125–9. 10.1097/01.ASW.0000508712.86959.c9 [DOI] [PubMed] [Google Scholar]
- 50. Jain KK. Textbook of hyperbaric medicine. Cham: Springer International Publishing, 2017. [Google Scholar]
- 51. Hoggan BL, Cameron AL. Systematic review of hyperbaric oxygen therapy for the treatment of non-neurological soft tissue radiation-related injuries. Support Care Cancer 2014;22:1715–26. 10.1007/s00520-014-2198-z [DOI] [PubMed] [Google Scholar]
- 52. Oliaei S, SeyedAlinaghi S, Mehrtak M, et al. The effects of hyperbaric oxygen therapy (HBOT) on coronavirus disease-2019 (COVID-19): a systematic review. Eur J Med Res 2021;26:96. 10.1186/s40001-021-00570-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thompson CD, Uhelski ML, Wilson JR, et al. Hyperbaric oxygen treatment decreases pain in two nerve injury models. Neurosci Res 2010;66:279–83. 10.1016/j.neures.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 54. Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol 1984;68:113–7. 10.1136/bjo.68.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gesell LB, Trott A. De novo cataract development following a standard course of hyperbaric oxygen therapy. Undersea Hyperb Med 2007;34:389–92. [PubMed] [Google Scholar]
- 56. Chung E, Zelinski LM, Ohgami Y, et al. Hyperbaric oxygen treatment induces a 2-phase antinociceptive response of unusually long duration in mice. J Pain 2010;11:847–53. 10.1016/j.jpain.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gibbons CR, Liu S, Zhang Y, et al. Involvement of brain opioid receptors in the anti-allodynic effect of hyperbaric oxygen in rats with sciatic nerve crush-induced neuropathic pain. Brain Res 2013;1537:111–6:S0006-8993(13)01187-6. 10.1016/j.brainres.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heeman JH, Zhang Y, Shirachi DY, et al. Involvement of spinal cord opioid mechanisms in the acute antinociceptive effect of hyperbaric oxygen in mice. Brain Res 2013;1540:42–7:S0006-8993(13)01324-3. 10.1016/j.brainres.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wilson HD, Toepfer VE, Senapati AK, et al. Hyperbaric oxygen treatment is comparable to acetylsalicylic acid treatment in an animal model of arthritis. J Pain 2007;8:924–30. 10.1016/j.jpain.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 60. Park JH, Phothimat P, Oates CT, et al. Use of P-31 magnetic resonance spectroscopy to detect metabolic abnormalities in muscles of patients with fibromyalgia. Arthritis Rheum 1998;41:406–13. . [DOI] [PubMed] [Google Scholar]
- 61. Myhill S, Booth NE, McLaren-Howard J. Chronic fatigue syndrome and mitochondrial dysfunction. Int J Clin Exp Med 2009;2:1–16. [PMC free article] [PubMed] [Google Scholar]
- 62. Behan WM, More IA, Behan PO. Mitochondrial abnormalities in the postviral fatigue syndrome. Acta Neuropathol 1991;83:61–5. 10.1007/BF00294431 [DOI] [PubMed] [Google Scholar]
- 63. Yildiz S, Uzun G, Kiralp MZ. Hyperbaric oxygen therapy in chronic pain management. Curr Pain Headache Rep 2006;10:95–100. 10.1007/s11916-006-0019-x [DOI] [PubMed] [Google Scholar]
- 64. Barilaro G, Francesco Masala I, Parracchini R, et al. The role of hyperbaric oxygen therapy in Orthopedics and Rheumatological diseases. The Israel Medical Association journal : IMAJ 2017;19:429–34. [PubMed] [Google Scholar]
- 65. Guedj E, Cammilleri S, Niboyet J, et al. Clinical correlate of brain SPECT perfusion abnormalities in fibromyalgia. J Nucl Med 2008;49:1798–803. 10.2967/jnumed.108.053264 [DOI] [PubMed] [Google Scholar]
- 66. Lin K-C, Niu K-C, Tsai K-J, et al. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J Trauma Acute Care Surg 2012;72:650–9. 10.1097/TA.0b013e31823c575f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062322supp001.pdf (205.9KB, pdf)
Data Availability Statement
Data are available upon reasonable request.