Abstract
Background:
Persistent sleep disruptions following withdrawal from abused drugs may hold keys to battle drug relapse. It is posited that there may be sleep signatures that predict relapse propensity, identifying which may open new avenues for treating substance use disorders.
Methods:
We trained male rats (~postnatal day 56) to self-administer cocaine. After long-term drug withdrawal (~postnatal day 100), we examined the correlations between the intensity of cocaine-seeking and key sleep features. To test for causal relationships, we then used behavioral, chemogenetic, or optogenetic methods to selectively increase rapid eye movement sleep (REMS), and measured behavioral and electrophysiological outcomes to probe for cellular and circuit mechanisms underlying REMS-mediated regulation of cocaine seeking.
Results:
A selective set of REMS features was preferentially associated with the intensity of cue-induced cocaine seeking after drug withdrawal. Moreover, selectively increasing REMS time and continuity by environmental warming attenuated a withdrawal time-dependent intensification of cocaine seeking – “incubation of cocaine craving”, suggesting that REMS may benefit withdrawal. Warming increased the activity of lateral hypothalamic melanin-concentrating hormone (MCH) neurons selectively during prolonged REMS episodes, and counteracted cocaine-induced synaptic accumulation of calcium-permeable AMPA receptors in the nucleus accumbens – a critical substrate for incubation. Finally, the warming effects were partly mimicked by chemogenetic or optogenetic stimulations of MCH neurons during sleep, or intra-accumbens infusions of MCH peptide during the rat’s inactive phase.
Conclusions:
REMS may encode individual vulnerability to relapse, and MCH neuron activities can be selectively targeted during REMS to reduce drug relapse.
Keywords: Long-bout REM sleep, MCH, incubation of cocaine craving, closed-loop stimulation, CP-AMPA, accumbens
Introduction
Relapse to drug use remains a major challenge in treating substance use disorders (SUDs) (1–2). Withdrawal (WD) from repeated drug use often induces persistent sleep disruptions (3–6), which, in turn, aggravate WD symptoms, increase motivational drive for drug use, and promote cue-induced drug-seeking, all precipitating drug relapse (5–15). However, it is not known whether sleep presents signature features that may predict relapse propensity, and whether those signatures may reveal neurophysiological mechanisms for potential therapy.
Sleep is broadly divided into rapid eye movement sleep (REMS) and non-REMS (NREMS). Whereas NREMS is essential for restoring physiological functions, REMS is preferentially implicated in emotion regulation (13, 16–21). Following WD from cocaine use, both NREMS and REMS are persistently disrupted by reduced total time and increased fragmentations (3, 7, 22, 23). Medications that increase NREMS or enhance slow-wave activity have been a focus in clinical practices to improve drug WD symptoms, though with limited efficacy in preventing drug relapse or associated behaviors (3, 24–26). The role of REMS in regulating WD symptoms is not clear either. While shortened REMS latency and increased REMS pressure may accompany drug or alcohol WD and predict alcohol relapse, lisuride, a medication that delays REMS onset and reduces total REMS, does not reduce drug relapse (3). By contrast, preclinical studies suggest that REMS can be beneficial in reducing cocaine seeking after WD (7). Thus, specific features of NREMS and REMS may be differentially associated with drug-relapse propensity, understanding which may further reveal the neurophysiological mechanisms.
Here, we used a cocaine self-administration (SA) model in rats to recapitulate the sleep abnormalities following long-term drug WD (7). We identified a collection of sleep/wake EEG features that were associated with future cocaine seeking, with a notable cluster around REMS. Increasing REMS time and continuity by environmental warming during sleep decreased cue-induced cocaine seeking. This was accompanied by increased activities of melanin-concentrating hormone (MCH) neurons in lateral hypothalamus (LH) preferentially during prolonged REMS episodes, and attenuation of a key form of cocaine-induced synaptic adaptation in nucleus accumbens (NAc). These results prompted us to examine MCH-NAc pathway in REMS-mediated regulation of cocaine seeking. Our findings not only depict REMS features as potential biomarkers for drug-relapse propensity, but also reveal a REMS mechanism through which cocaine seeking can be reduced.
Material and Methods
Male Sprague-Dawley rats started cocaine SA on ~postnatal day (PD) 56. Slice electrophysiology (>PD100) and sleep recordings (>PD85, most >P100) were performed in adulthood. Rats were singly housed under a 12-h reverse light/dark cycle with controlled temperature (21-22°C) and humidity (60±5%). All experiments were performed in accordance with IACUC-approved protocols. Cocaine SA training and incubation test (7, 27, 28), sleep recordings and analysis (7, 29), slice recordings (7, 27, 29), chemo- and optogenetic manipulations (29) were similar to our previously published procedures, with details in Supplementary Material. All results are presented as mean± SEM.
Results
Selective EEG features are associated with cue-induced cocaine seeking after WD
In humans, relapse to drug use is often triggered by re-exposure to environmental cues that are previously associated with drug experience (30). In rat models, cue-induced drug craving can be assessed by the drug-seeking responding upon re-exposure to drug-associated cues after WD (31). Over long-term WD, cue-induced cocaine seeking becomes progressively intensified, or “incubation of cocaine craving”, a behavioral manifestation of increased likelihood of drug relapse (32–35). We trained rats to nose-poke for cocaine SA (0.75mg/kg/infusion, 1 overnight+5d x 2h/d) followed by 45d WD (Figure 1A), a regimen that induces persistent sleep disturbances after long-term WD, consistent incubation of cocaine craving, and persistent neurophysiological changes in drug relapse studies (7, 27, 28). Cue-induced cocaine seeking was quantified by #nose pokes for cocaine (active nose-pokes, ANP; Figure 1B,C), and “incubation index” was operationally defined as #ANP on WD d45 relative to d1 in the absence of cocaine infusions. Individual linear regressions were evaluated between WD d45 behaviors and extracted EEG features from WD d41-d44 (Figure 1D,E). WD d45 ANP and incubation index showed associations with a couple of wakefulness (Wake) and NREMS features during the dark/active phase, but mostly with REMS features during the light/inactive phase, particularly during prolonged REMS episodes in the light phase (Figure 1D–I). Because of the large numbers of observations and relatively small sample sizes, the adjusted q-values after correcting for multiple comparisons were mostly not significant. Thus, we did not conclude on specific correlations, but formulated the hypothesis that light-phase REMS is preferentially associated with cue-induced cocaine seeking after WD.
Figure 1.

Correlation analysis on selective sleep/wake EEG features and cocaine seeking after long-term WD from cocaine SA. (A) Timeline. (B,C) Cocaine SA training. Among a total of 16 rats, 13 exhibited typical acquisition of cocaine SA (i.e., average #ANP ≥30, average #Infusions ≥20, and #ANP >2x #INP on the last 3 training days), and various levels of incubated cocaine seeking (B); 3 rats were low performers as they exhibited low #ANP, low #Infusions, and nonseparated ANP-INP, and they did not show incubated cocaine seeking (C). All 16 rats were included for subsequent correlation analysis. (B) Nose pokes x WD d interaction: F1, 12 = 6.82, p<0.05; #ANP, p<0.05; #INP, p=0.628; (C) F1, 2 = 0.40, p=0.591; #ANP, p=0.713; #INP, p>0.999; two-way RM ANOVA with Sidak post hoc. (D) Individual linear regressions were evaluated between WD d45 behaviors and extracted sleep/wake EEG features recorded prior to the WD d45 test. EEG data were sorted into a 3x4 table: 24-h, 12-h light phase (LT), and 12-h dark phase (DK) x Wake, NREMS, REMS, and transitional features. (E) REMS bouts were further divided into long-bout (>100 s) versus short-bout (⩽60 s) REMS. Only r values with p < 0.05 were shown. (F-I) Selective Wake, NREMS, or REMS features in relation to WD d45 behavior. p (D-I) denotes unadjusted p-value, and q denotes Benjamini-Hochberg adjusted p-value.
Warming selectively increases REMS and shifts EEG power spectrum after cocaine WD
To explore a potential causal relationship between REMS and cocaine-seeking behaviors, we manipulated REMS non-invasively by increasing the bedding temperature to near thermoneutrality (Figure 2A) (36, 37). In naïve mice, environmental warming increases REMS #bouts without changing the average bout durations (37). In rats after cocaine WD (Figure S1 A), however, bedding-warming (31.5-34.5°C) during the light phase (Zeitgeber time, ZT1-11) selectively increased the total REMS time by shortening the onset delay of REMS and prolonging the average duration of REMS bouts (Figure 2B–D), without changing #bouts (Figure 2E). Warming did not change Wake or NREMS during the light phase, nor REMS during the dark phase (Figure 2B–F). Notably, the durations of REMS bouts in the light phase did not follow a normal distribution but fell into two clusters – short ones <~60 s, and long ones >~100 s (Figure 2G). Warming reduced the cumulative frequency of short bouts and increased that of long bouts, especially those with prolonged (i.e., >200 s) durations (Figure 2H). Short versus long REMS bouts also differed in waveforms in that short bouts had higher theta, alpha, and sigma frequency power than long ones, even when transitional epochs were excluded from analyses (Figure 2I). Warming reduced the theta power in both short and long REMS bouts (Figure 2J), decreased overall theta/alpha/sigma power in averaged light-phase REMS episodes (Figure 2K), whereas NREMS (Figure 2L) and Wake (data not shown) power spectra were not affected. These results suggest that in rats after cocaine WD, warming selectively increases REMS, promotes REMS continuity, and reduces theta/alpha/sigma power for an average REMS bout.
Figure 2.

Warming selectively increases REMS and shifts EEG power spectrum in rats after long-term WD from cocaine SA. (A) Timeline and the bedding-warming apparatus. (B-E) Total times (B), onset delays (C), and bout analyses (D-E) of Wake, NREMS, or REMS over the 12-h light phase, under room temperature (RT) control conditions or following warming (WM). (B) Wake, t17=1.940, p=0.069; NREMS, t17=1.090, p=0.291; REMS, t17=5.824, p<0.001; (C) NREMS, t17=1.345, p=0.196; REMS, t17=2.655, p<0.05; NREMS+REMS, t17=1.418, p=0.174; (D) Wake, t17=0.358, p=0.725; NREMS, t17=0.971, p=0.345; REMS, t17=5.227, p<0.001; (E) Wake, t17=2.010, p=0.061; NREMS, t17=1.513, p=0.149; REMS, t17=0.586, p=0.565; unpaired t tests. (F) Time spent in Wake, NREMS, or REMS states across 12-h light and 12-h dark (shaded) phases, under RT control or WM conditions. Time x warming interaction: Wake, F11, 187=0.813, p=0.627; NREMS, F11, 187=1.086, p=0.375; REMS, F11, 187=5.015, p<0.001; two-way RM ANOVA with Sidak post hoc. (G-H) Normalized distribution frequency of light-phase REMS bouts across the bout duration range, showing short (S) and long-REMS (L) clusters. (G) p<0.001, Wilcoxon test. (H) Duration x warming interaction, F3, 51=13.35, p0.001; two-way RM ANOVA with Sidak post hoc. (I-L) 12-h light-phase power spectra of long- versus short-bout REMS episodes (I), warming effects on short- or long-bout REMS (J), and warming effects on total averaged REMS (K) or NREMS episodes (L). Transitional epochs at either ends of each REMS episodes were excluded (I-J). (I) Frequency x REMS bout duration interaction, F99, 1980=5.344, p<0.001; (J) Frequency (0.5-15 Hz) x warming interaction: Short, F29, 493=1.748, p<0.05; Long, F29, 493=1.668, p<0.05; two-way RM ANOVA with Sidak post hoc. (K) Frequency bands (δ,θ,α,σ,β,γ) x warming interaction: F5, 85=1.871, p=0.11; main effect of warming: F1, 17=4.522. p<0.05; two-way RM ANOVA. (L) Frequency bands (δ,θ,α,σ,β,γ) x warming interaction: F5, 85=0.708, p=0.62; main effect of warming: F1, 17=4.2e-006, p>0.99; two-way RM ANOVA. Data shown as mean ± SEM. * p < 0.05, *** p < 0.001; post-hoc # p < 0.05, ## p < 0.01, ### p < 0.001
REMS improvement reduces cocaine incubation and restores NAc synaptic transmission
Could warming-induced selective changes in REMS impact drug-seeking? After cocaine SA, we applied warming to the rats between WD d30-d44 during the light phase (ZT1-11; Figure 3A,B, Figure S1A). Compared to room-temperature controls, rats with sleep-warming showed a decrease in incubation index (Figure 3C–E). Moreover, there was a positive correlation between WD d1 and d45 #ANP in control rats, and warming reduced the slope of the correlation (Figure S2), consistent with the decrease in incubation.
Figure 3.

Light-phase warming after WD reduces incubation of cocaine craving and decreases NAc synaptic CP-AMPA receptors. (A) Timeline and bedding-warming apparatus. (B) Cocaine SA training. All 16 rats with optimal warming were included for (C-H), and all 12 rats with both sleep recordings and warming/sub-optimal warming were included for (I-L). (C-E) Cue-induced cocaine seeking tested on WD d1, and on WD d45 following sleep at room temperature (RT) or warming conditions (WM). (C) Treatment x WD d interaction: F1, 28 = 4.073, p=0.05; RT, p<0.01: WM, p=0.691; two-way RM ANOVA with Sidak post hoc. (E) Incubation index (#ANP WD d45/d1): t28=2.377, p<0.05; unpaired t test. (F-H) NAc MSN synaptic CP-AMPA receptor levels assessed by %Naspm (100 μM) inhibition of evoked EPSCs, shown in example traces (F), cell-based analysis (G), and animal-based analysis (H). (G) t100=5.845, p<0.001; (H) t26=5.496, p<0.001; unpaired t tests. (I) Individual linear regressions were evaluated between WD d45 behaviors and extracted EEG features recorded prior to WD d45. Only r values withp < 0.05 were shown. (J-L) Selective REMS features correlated with WD d45 behaviors, p (I-L) denotes unadjusted p-value, and q denotes Benjamini-Hochberg adjusted p-value. Data shown as mean ± SEM. * p < 0.05, *** p < 0.001; post-hoc ## p < 0.01
A key cellular mechanism mediating incubation of cocaine craving resides in NAc, a limbic-motor interface and a reward processing hub (33, 38–40), where protracted WD from cocaine SA induces gradual and persistent accumulation of calcium-permeable (CP-) AMPARs at glutamatergic synapses in principal medium spiny neurons (MSNs) (41–44). These atypical AMPARs are expressed at low levels at NAc MSN synapses in drug-naïve rats, and their accumulation after long-term WD critically contributes to the incubated cocaine seeking (41, 42, 45, 46). After WD, sleep-warming decreased CP-AMPAR levels at NAc MSN synapses, evidenced by the reduced sensitivity of MSN EPSCs to the selective CP-AMPAR antagonist Naspm (100 μM; Figure 3F–H). Thus, sleep-warming during WD restored NAc synapses to the low CP-AMPAR state, which may serve as a cellular mechanism for decreased incubation.
Correlation analysis included additional rats that underwent sub-optimal bedding-warming (~29°C), which showed intermediate changes in REMS (Figure S2). The analysis revealed clusters of REMS features (Figure 3I). For example, WD d45 ANP was inversely correlated with 24-h total REMS, and positively correlated with REMS theta, alpha, and sigma frequency components. Moreover, when REMS bouts were sorted into different duration bins, the incubation index showed a trending inverse correlation with the total amount of prolonged (>200 s) REMS bouts (Figure 3K) and positive correlation with the short ones (<= 20 s) (Figure 3L). Thus, warming increases the total time of REMS (Figure 2B) and promotes long-bout REMS (Figure 2H), the latter exhibiting lower theta, alpha, and sigma frequency power (Figure 2I), all of which are correlated with low cocaine seeking (Figure 3I–L).
Warming increases LH MCH neuron activity during prolonged REMS episodes
LH MCH neurons play an important role in the homeostatic regulation of REMS (47–49). They fire predominantly at transitions to and during REMS (50), promoting REMS initiation and/or maintenance (51–56). Environmental warming signals are relayed from the preoptic hypothalamic area through MCH neurons to diverse REMS regulatory regions (37, 57–60). Following cocaine WD, MCH neurons show decreased membrane excitability and impaired glutamatergic transmissions (29). How do they respond to warming after WD? We expressed GCaMP6f, a genetically-encoded calcium indicator (61), in LH and zona incerta (ZI), driven by MCH promoter (MCHp) (Figure 4A–C; Figure S3). After WD from cocaine SA (Figure S1B), in vivo fiberphotometry recorded Ca2+ activities from 144 out of 234 REMS episodes under warming and 155 out of 259 under room temperature control (p=0.692, paired t-test on %captured REMS episodes) during the REMS-dense period of the light phase (ZT7-11). Under both conditions, Ca2+ activity occurred mostly during REMS (Figure 4D), consistent with previous reports (50, 62). Both the normalized amplitude of Ca2+ transients and the area-under-curve (AUC) increased nonlinearly as REMS episodes lengthened (Figure 4E,F), with a half maximal effective duration (ED50) of ~60 s for the peak amplitude (Figure 4E). When separated into long-bout (>100 s) versus short-bout (≤60 s) episodes, the average amplitude was higher during long-bout REMS independent of warming (Figure 4G). Thus, although long-bout REMS represented ~20-30% of total captured REMS episodes (Figure 4H), they contributed ~85% of total Ca2+ activity in MCH neurons (Figure 4I).
Figure 4.

Warming increases MCH neuron activity during prolonged REMS episodes. (A) In vivo fiberphotometry configuration and experimental timeline. (B) Example dual-channel fluorescence images and (C) grouped data showing GCaMP expression in MCH-immunoreactive neurons. GCaMP6f was expressed in ~60% of MCH neurons with high specificity (~99%). ZI, zona incerta. Scale bars =100 μm, 50 μm. (D) Example normalized GCaMP6f signal (normalized dF = F465/Median465 – F405/Median405) over Wake (W), NREMS (N), REMS (R), and transitions to REMS (N-to-R), in rats under baseline (room temperature) or warming conditions after WD from cocaine SA. (E) GCaMP signal peak amplitude and (F) area under curve (AUC) over REMS bout durations under baseline (B) or warming (WM) conditions, normalized to baseline average per-REMS episode, and fitted with second-order linear ordinary differential equation. (E) ED50: B, 58 s; WM, 61 s. (G) Average amplitude of normalized dF in short-versus long-REMS episodes under B or WM. REMS bout duration x warming interaction: F1, 10=0.156, p=0.7015; main effect of REMS bout duration: F1, 10=37.39, p<0.001; two-way RM ANOVA with Sidak post hoc. (H-Q) Grouped data under B or WM, showing fractions of long-bout REMS bout# over total REMS bout# (H), fractions of AUC during long-bout REMS over total AUC during REMS (I), total AUC (J), average AUC per REMS episode (K), maximum peak amplitude (L), average amplitude (M), average REMS bout duration (N), average long-REMS bout duration (O), long-bout REMS total AUC (P), and short-bout REMS total AUC (Q). (H) t5=3.716, p<0.05; (I) t5=1.111, p=0.317; (J) t5=4.406, p<0.01; (K) t5=4.403, p<0.01; (L) t5=0.949, p=0.386; (M) t5=2.125, p=0.09; (N) t5=3.795, p<0.05; (O) t5=3.379, p<0.05; (P) t5=4.795, p<0.01; (Q) t5=0.982, p=0.37; paired t-tests. Data shown as mean ± SEM. * p < 0.05, ** p < 0.01; post hoc ## p < 0.01
Under warming, MCH neurons showed increased total Ca2+ AUC (Figure 4J) and average AUC per REMS episode (Figure 4K). These increases were not accompanied by changes in the maximal peak amplitude (Figure 4L), nor the average amplitude per REMS episode (Figure 4M), but were mainly attributable to the increase in the duration of REMS episodes (Figure 4N), and the further lengthening of long-bout REMS (Figure 4O). Thus, warming preferentially increases the total Ca2+ activity in MCH neurons during long-bout REMS (Figure 4P,Q).
Chemogenetic activation of MCH neurons decreases cocaine incubation and NAc CP-AMPARs
Could we mimic the warming effects by chemogenetic stimulation of MCH neurons? We expressed excitatory DREADDs (Gq) selectively in MCH neurons (Figure 5A,B; Figure S4) (29). After WD (Figure S1C), we injected the DREADDs ligand CNO (s.c., 1 mg/kg/d) on two consecutive days shortly before the onset of light phase (ZT22; Figure 5C), and measured cue-induced cocaine seeking and other behaviors in the dark phase ~16 h after 2nd CNO. This CNO/Gq-based increase in MCH neuron activities was estimated to last for ~13 h (ZT22-11; data not shown), which increased 24-h total REMS time, #bouts, and average bout durations (29).
Figure 5.

Chemogenetic activation of LH MCH neurons during the light/inactive phase decreases incubation of cocaine craving and reduces NAc CP-AMPA receptors after long-term WD. (A) AAV injection paradigm. (B) Example dual-channel fluorescence images showing DREADDs-mCherry-labeled LH neurons also exhibited MCH immunoreactivity. ZI, zona incerta. Scale bars = 100 μm. (C) Timeline. (D) Cue-induced cocaine seeking in the absence of cocaine infusions was tested on WD d1, and on WD d45 following saline or CNO injections on WD d43 and d44. Treatment x WD d interaction: F1, 31 = 4.782, p<0.05; saline, p<0.01; CNO, p=0.924: two-way RM ANOVA with Sidak post hoc. Incubation index: t31=1.914, p=0.05; unpaired t test. (E) Elevated Plus Maze test of saline or CNO-treated rats after WD. Open-arm time, t22=0.182, p=0.86; unpaired t test. #Entry, arm x CNO interaction, F1, 22=0.421, p=0.52; main effect of CNO, F1, 22=1.081, p=0.31; two-way RM ANOVA. (F) Forced-swim test of saline or CNO-treated rats after WD. Swim x CNO interaction, F2, 42=0.639, p=0.53; main effect of CNO, F1, 21=1.125, p=0.30; two-way RM ANOVA. (G-H) NAc MSN synaptic CP-AMPA receptor levels assessed by %Naspm (100 μM) inhibition of evoked EPSCs in saline or CNO-treated rats, shown in example traces (G), cell-based and animal-based analyses (H left and right) after WD. Cell-based, t39=4.945, p<0.001; animal-based, t10=3.854, p<0.01; unpaired t tests. (I) Wake bout, NREMS onset, and REMS onset features after CNO injection were inversely correlated with WD d45 ANP. (J) Post-CNO REMS time fit by second-order linear ordinary differential equations. (K) Post-CNO REMS recovery ED50 was inversely correlated with WD d45 ANP. Data shown as mean ± SEM. ** p < 0.01, *** p < 0.001; post-hoc ## p < 0.01
Compared to saline-Gq rats, CNO-Gq rats exhibited a trend decrease in incubation index (Figure 5D) and a reduction in the slope of the positive correlation between WD d1 and d45 cocaine seeking (Figure S4), suggesting reduced incubation. Another set of control rats, which received intra-LH AAV5-MCHp-GFP, showed intact incubation following CNO injections (Figure S5). Moreover, CNO-Gq rats showed similar performances compared to saline-Gq rats in elevated plus maze (EPM) test (Figure 5E) and forced swim test (FST; Figure 5F), measured ~16 h after last injections of CNO/saline, suggesting negligible effects on anxiety or depressive-like behaviors at this time. Thus, the reduction of cocaine incubation was not likely mediated by suppression of general motivation. Finally, in CNO-Gq rats, the reduction of incubation was accompanied by reduced sensitivity of NAc MSN EPSCs to Naspm (Figure 5G,H; Figure S5), suggesting NAc CP-AMPARs as a mechanism for MCH neurons to regulate cocaine incubation.
Notably, the effects of MCH neurons on promoting REMS, decreasing incubation, and reducing NAc CP-AMPARs were preferentially induced when CNO was administered preceding the onset of light phase (ZT22), but not at the reciprocal time preceding the onset of dark phase (ZT10; Figure S6)(29). CNO administration at ZT22 versus ZT10 also induced differential effects on the sleep stage-specific EEG power spectrum (Figure S7). Prolonging the dark-phase CNO treatment to 3 weeks (WD d21-d45) remained ineffective in reducing incubation or decreasing NAc CP-AMPARs (Figure S8). These diurnal-contingent properties suggest that the effectiveness of MCH neuron stimulation is circadian- and/or sleep state-dependent.
As we reported previously, CNO administration at ZT22 dose-dependently induces bi-phasic effects on sleep, promoting Wake for the first ~5 h post-injection (phase-1), followed by a large, delayed REMS rebound in the second half of light phase (phase-2)(29). WD d45 ANP was inversely correlated with the onset delays of NREMS and REMS, and the maximal durations of Wake bouts following CNO injection (Figure 5I), i.e., the extent of phase-1 effect of Gq-activation. Furthermore, the return and rebound of REMS could be fitted with second-order linear ordinary differential equations on the rising phase, with each rat exhibiting a distinct ED50 (Figure 5J). WD d45 ANP was inversely correlated with ED50 across individual rats (phase-2 effect) (Figure 5K). Such a graded correlation depicts MCH neurons as a finetuned machinery in regulating cocaine seeking.
Optogenetic activation of MCH neurons decreases cocaine incubation
To activate MCH neurons selectively during sleep, we next used a real-time optogenetic approach. We expressed AAV5-MCHp-ChR2-EYFP in LH/ZI MCH neurons (Figure 6A,B; Figure S9)(29). We used a closed-loop system to deliver laser stimulations to MCH neurons selectively during sleep (Figure 6C; <10% false negative and ~0% false positive rates (29)). In rats after WD (Figure 6D, Figure S1D), bilateral laser stimulations (1 ms x 10 Hz, 5-s-on-5-s-off) (29) selectively during light-phase sleep increased the total time of REMS (Figure 6E), decreased REMS onset delay (Figure 6F), and increased REMS #bouts and average duration (Figure 6G,H). The REMS-promoting effect sustained most of the light phase (Figure 6I), and increased REMS #bouts across different durations without changing relative distributions (Figure 6J–L). This overall increase in REMS was at the cost of reductions in light-phase NREMS time and continuity, without affecting Wake (Figure S10).
Figure 6.
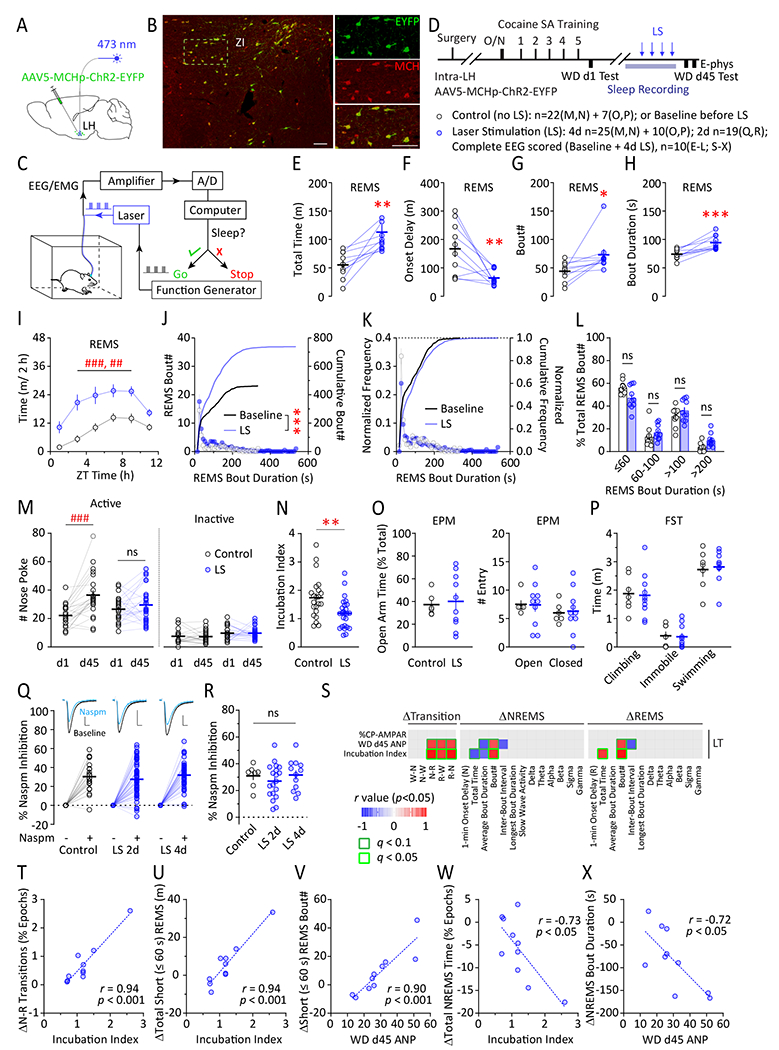
Optogenetic activation of LH MCH neurons selectively during sleep decreases incubation of cocaine craving after long-term WD, sparing NAc CP-AMPA receptors. (A) AAV injection paradigm. Example dual-channel fluorescence images showing ChR2-EYFP-labeled LH neurons also exhibited MCH immunoreactivity. ZI, zona incerta. Scale bars = 100 μm. (C) In vivo, closed-loop, optogenetic stimulation system for selective stimulation of LH MCH neurons during sleep. (D) Timeline. (E-I) Total REMS time (E), REMS onset delay (F), REMS bout# (G), REMS bout duration (H), and REMS time across the 12-h light phase (I) under baseline or ChR2 laser stimulation (LS; 473 nm, bilateral, 10 Hz, 5 s-ON-5 s-OFF, in sleep). (E) t9=3.920, p<0.01; (F) t9=3.495, p<0.01; (G) t9=2.690, p<0.05; (H) t9=5.336, p<0.001; paired t tests. (I) LS x time interaction, F5, 44=5.535, p<0.001; main effect of LS, F1, 10=14.92, p<0.01, two-way RM ANOVA with Sidak post hoc. (J) Histogram and cumulative histogram of light-phase REMS bout# across different bout durations under baseline or LS. Kolmogorov-Smirnov test. (K-L) Normalized distribution frequency of light-phase REMS bout# across different bout durations under baseline or LS. (L) Bout duration x LS interaction: F3, 54=3.263, p<0.05; p=0.11, 0.76, 0.62, 0.06 for the four duration groups (left to right), two-way RM ANOVA with Sidak post hoc. (M,N) Cue-induced cocaine seeking tested on WD d1, and on WD d45 following sham operation control or LS. (M) Treatment x WD d interaction: F1, 45 = 8.518, p<0.01; control, p<0.001; LS, p=0.461; two-way RM ANOVA with Sidak post hoc. (N) Incubation index (#ANP WD d45/d1). t45=2.978, p<0.01; unpaired t test. (O) Elevated Plus Maze test of control or LS rats after WD. Open-arm time, t14=0.278, p=0.79; unpaired t test; #Entry, arm x LS interaction, F1, 14=0.020, p=0.89; main effect of LS, F1, 14=0.013, p=0.91; two-way RM ANOVA. (P) Forced-swim test of control or LS rats after WD. Swim x LS interaction, F2, 30=0.053, p<=0.95; main effect of LS, F1, 15=0.000, p>0.999; two-way RM ANOVA. (Q-R) NAc MSN synaptic CP-AMPA receptor levels assessed by %Naspm (100 μM) inhibition of evoked EPSCs in control or LS rats, shown in example traces (Q), cell-based (Q), and animal-based analysis (R) after WD. Scale bars = 5 ms, 100 pA. Cell-based, F2, 113=0.810, p=0.45; animal-based, F2, 35=0.663, p=0.52, one-way ANOVA. (S) Individual linear regressions were evaluated between WD d45 behaviors and LS-induced changes in light-phase sleep recorded prior to WD d45 test. ΔEEG data were sorted into NREMS, REMS and transitional features. Only r values with p < 0.05 were shown. (T-V) Selective short-REMS features were correlated with incubation index (T,U) or WD d45 ANP (V). (W,X) Changes in NREMS time and average bout durations were both inversely correlated with WD d45 behaviors, albeit to a lower extent than REMS features (S-V). p (S-X) denotes unadjusted p-value, and q denotes Benjamini-Hochberg adjusted p-value. Data shown as mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001; post-hoc ## p < 0.01, ### p < 0.001
Optogenetic stimulation during light-phase sleep on 4 consecutive days after WD (d41-d44) decreased incubation of cocaine craving compared to sham control rats (Figure 6M,N, S11). The EPM and FST performances were similar between the two groups (Figure 6O,P). Notably, the reduction of incubation was not accompanied by altered sensitivity of EPSCs in NAc MSNs to Naspm (Figure 6Q,R). These results reveal that sleep-specific increase in MCH neuron activity can employ CP-AMPAR-independent mechanisms to reduce cocaine seeking.
Correlation analysis examined optogenetic stimulation-induced changes in sleep in relation to subsequent cocaine seeking (Figure 6S–X). The most prominent correlations that predict high levels of cocaine seeking were the increase in overall NREM-to-REMS transitions (Figure 6T), the increase in the total time of short-bout (≤ 60 s) REMS (Figure 6U), and the increase in #short-bout REMS episodes (Figure 6V). Additionally, related to the reciprocal decrease in NREMS time and continuity (Figure S10), WD d45 ANP and incubation index were inversely correlated with changes in total NREMS time and bout durations (Figure 6W,X), albeit to a lesser extent compared to REMS changes. Finally, optogenetic stimulations did not change the sleep EEG power spectrum (29), thus precluding correlation analysis on EEG spectrum (Figure 6S). Together, these results suggest that although increase REMS can be beneficial for reducing cocaine seeking after WD, increase in short-bout REMS serves the opposite.
Intra-NAc infusion of MCH decreases NAc CP-AMPARs and cocaine incubation
LH MCH neurons send monosynaptic projections to NAc (63). NAc expresses high levels of both MCH receptor mRNA and protein (64–68). In NAc MSNs, MCH receptor-coupled signaling regulates phosphorylation and synaptic removal of GluA1 (69), a key subunit of the cocaine-induced CP-AMPARs (41, 42, 70, 71). To test whether MCH signaling in NAc may regulate WD-induced synaptic accumulation of CP-AMPARs, we bilaterally delivered MCH peptide (1 μg/μl/side/d) or vehicle into NAc on 2 consecutive days prior to the onset of light phase after 43d WD (Figure 7A,B; Figure S1E). MCH infusions led to decreased Naspm-sensitivity in NAc MSNs after WD (Figure 7C,D), suggesting reduced synaptic CP-AMPARs. Thus, MCH-NAc projection may contribute to REMS-mediated restoration of NAc synaptic properties after cocaine WD.
Figure 7.

Intra-NAc infusions of MCH peptide recapitulate the decrease in NAc CP-AMPARs and attenuation of cocaine incubation after long-term WD. (A) Intra-NAc MCH infusion paradigm (1 μg/μl/side/d). (B) Timeline. (C-D) Example traces (C) and grouped data (D) showing NAc MSN synaptic CP-AMPA receptor levels assessed by %Naspm (100 μM) inhibition of evoked EPSCs in saline/cocaine SA x intra-NAc aCSF/MCH rats. Animal based, cocaine x MCH interaction, F1, 29=7.116, p<0.05; cocaine SA/aCSF vs. saline SA/aCSF, p<0.01: cocaine SA/MCH vs. cocaine SA/aCSF, p<0.01; saline SA/MCH vs. saline SA/aCSF, p>0.99; two-way ANOVA with Tukey post hoc. (E) Cue-induced cocaine seeking tested on WD d1, and on WD d45 following intra-NAc infusions of aCSF or MCH. Treatment x WD d interaction: F1,28=6.992, p<0.05; aCSF, p<0.01; MCH, p=0.970; two-way RM ANOVA with Sidak post hoc. (F) Incubation index following intra-NAc aCSF or MCH. t28=2.775, p<0.01, unpaired t test. (G) Food consumptions measured at 12 h post intra-NAc infusions of aCSF or MCH. t18=0.759, p=0.46; unpaired t test. (H-I) Elevated Plus Maze (H) and Forced Swim test (I) in cocaine WD rats following intra-NAc infusions of aCSF or MCH. (H) EPM open-arm time, t25=0.434, p=0.67; #entry, arm x MCH interaction, F1, 25=0.059, p=0.81; main effect of MCH, F1, 25=0.001, p=0.97; two-way RM ANOVA. (I) FST, t11=0.368, p=0.72; unpaired t test. (J-L) Bout analyses of Wake, NREMS, or REMS over the 12-h light phase following intra-NAc infusions of aCSF or MCH in rats after WD. (J)Wake, t3=0.394, p=0.72; NREMS, t3=0.020, p=0.99; REMS, t3=1.423, p=0.25; paired t tests. (K) Wake, t3=0.975, p=0.40; NREMS, t3=0.112, p=0.92; REMS, t3=1.747, p=0.18; paired t tests. (L) Wake, t3=0.349, p=0.75; NREMS, t3=0.090, p=0.93; REMS, t3=4.485, p<0.05; paired t tests. (M) %Time spent in Wake, NREMS, or REMS states across 12-h light and first 2-h dark phases following intra-NAc infusions of aCSF or MCH. Wake: MCH x time interaction, F6, 18=0.752, p=0.62; main effect of MCH, F1, 3=0.444, p=0.55; NREM: MCH x time interaction, F6, 18=1.203, p=0.35: main effect of MCH, F1, 3=0.064, p=0.82: REM: MCH x time interaction, F6, 18=0.351, p=0.90; main effect of MCH, F1, 3=5.452, p=0.10, two-way RM ANOVA. Data shown as mean ± SEM. * p < 0.05, *** p < 0.001; post-hoc # p < 0.05, ## p < 0.01
Behavioral tests were performed on WD d45 ~16 h post-infusions. Incubation of cocaine craving was abolished in MCH-treated group (Figures 7E,F,S12). Food consumption was acutely increased ~4 h post-infusions (t18=2.155, p<0.05; unpaired t test;)(69, 72–74), though diminished by 12 h post-infusions (Figure 7G), suggesting that the altered cocaine seeking was not due to changes in general motivation for reward. EPM or FST performances were not changed (Figure 7H,I), suggesting that this intra-NAc MCH manipulation was not anxiogenic or depressogenic ~16 h post-infusions.
Are the behavioral and cellular effects of intra-NAc MCH accompanied by sleep changes, considering that NAc also regulates NREMS and REMS (75–78)? Analysis of the 12-h light-phase sleep following bilateral intra-NAc infusions of MCH revealed no changes in the overall amount or bi-hourly distributions of Wake, NREMS, or REMS, nor changes in REMS bout durations (Figure 7J–M), albeit a modest decrease in REMS #bouts (Figure 7L). Thus, intra-NAc infusion of MCH peptide recapitulates the effects of warming and chemogenetic activation of MCH neurons, namely removal of NAc CP-AMPARs at MSN synapses and attenuation of cocaine incubation, without affecting the overall sleep.
Discussion
Identifying sleep/wake EEG signatures that predict future relapse propensity may open new avenues for individualized medicine in treating SUDs (79). In addition to REMS, some Wake and NREMS features also showed associations (Figure 1D–I). For example, both shorter Wake bout durations and increased NREMS time in the dark phase showed trending correlations with higher levels of cocaine seeking (Figure 1 F,H), likely reflecting increased disturbances of circadian rhythm in high seekers (8, 80). Additionally, there was an inverse correlation between cocaine incubation and EEG theta power in Wake throughout light and dark phases (Figure 1D,G). This result is reminiscent of a recent report in human methamphetamine users, in which increased drug-craving after WD is associated with reduced EEG theta power during quiet Wake (14). EEG theta power typically increases over sustained Wake, and is thought to signal sleep propensity (81, 82). Thus, reduced Wake theta power would be consistent with shortened Wake bout durations observed in high cocaine-seeking rats (Figure 1F).
Environmental warming, chemogenetic and optogenetic stimulations of MCH neurons all led to increased total REMS and long-bout REMS time (Figures 2,4–6). These changes counteract cocaine-induced REMS decrease and fragmentation (7), and all three manipulations resulted in decreased incubation after WD (Figures 3,5,6). This is consistent with the previous finding that prolonging REMS bout durations by REMS restriction-rebound reduces incubation of cocaine craving (7). Importantly, the three approaches exerted differential effects on REMS. By comparing and contrasting these effects, we found that an increase in total REMS or long-bout REMS time was associated with reduced incubation (Figure 3I,K), whereas short-bout REMS showed the opposite (Figures 3L,6U,V). Furthermore, waveform analysis revealed that a decrease in the average REMS alpha or sigma was associated with lower cocaine seeking (Figures 1D,E,I;3I,J). Under warming, this can be in part explained by a shift of REMS episodes toward longer bouts (Figure 2G,H), which contained lower alpha and sigma components compared to short-bout REMS episodes (Figure 2I).
How do long- versus short-bout REMS episodes differ, such that they may have opposing behavioral outcomes (Figures 3, 6)? The binomial distribution of the two populations (Figures 2G, 6J,K), as well as the different EEG power spectra (Figure 2I), suggest that they are two distinct states. Moreover, MCH neuron activities were preferentially engaged in long-bout REMS (Figure 4E–I). Although human studies of REMS do not typically report MCH neuron activity, it is increasingly recognized that REMS continuity is an important readout for sleep quality. For example, REMS fragmentation is a hallmark of insomnia, and insomnia increases the risk of a host of psychiatric disorders (83–90). It is not known how MCH neuronal mechanisms contribute to these and other sleep fragmentation-induced deficits in emotion regulation and memory (84, 91–95). It is also not known how MCH neuron activity is related to other microstates of REMS such as tonic versus phasic REMS states (96). Nevertheless, short- and long-bout REMS appear to be two distinct REMS states, and our results provide an MCH-based differentiation of the two.
LH MCH neurons play a double-faceted role in REMS-reward interactions. They broadly project to both REMS regulatory regions (e.g., tuberomammillary nucleus and medial septum)(51, 97–99) and reward processing centers (e.g., NAc and ventral tegmental area)(63, 100, 101). Following cocaine SA and WD, MCH neurons exhibit dampened intrinsic membrane excitability and impaired glutamatergic transmissions (29). Nevertheless, their activity may be enhanced during REMS rebound (48, 49, 102) or warming (Figure 4, (37)). Boosting their activity results in both increased REMS and reduced incubation after WD (Figures 3–6). Furthermore, we show that the “dual roles” are segregated by projection targets, such that intra-NAc infusion of MCH peptide is sufficient to reduce CP-AMPARs and attenuate cocaine incubation, with minimal effects on overall sleep (Figure 7). With such ramifying circuit properties, MCH neurons may regulate REMS versus drug-seeking in a coordinative but independent manner.
During Wake, acute activation of MCH receptors in NAc or systemically produces anxiogenic or depressive effects (65, 103, 104). However, if stimulated during sleep or inactive phase, activation of MCH neurons or NAc MCH receptors did not produce such effects in subsequent Wake period (Figures 5–7). Moreover, DREADs(Gq)-activation of MCH neurons is more effective during the sleep-enriched light phase than dark phase in improving REMS, removing NAc CP-AMPARs, and reducing cocaine incubation (Figures 5, S6–8)(29). These results emphasize the circadian phase- and/or behavioral state-sensitive consequences of MCH neuron stimulations.
Chemo- and optogenetic stimulations of MCH neurons similarly reduced incubation but differentially impacted NAc CP-AMPARs (Figures 5,6). This is likely due to the broad versus focal stimulations in addition to the activation pattern differences (29). Compared to chemogenetics that affected MCH neurons throughout LH and ZI, optogenetic stimulation was limited to a portion of ZI that was within the optical cone of the laser beam (Figures S4,S9). The robust but limited stimulations by optogenetics were sufficient to drive REMS changes and reduce incubation, yet apparently insufficient to impact MCH-to-NAc pathway. Indeed, NAc-projecting MCH neurons are sparsely dispersed along LH and ZI (63), which would be challenging for fiber-optogenetics to stimulate collectively.
Beyond NAc, there are several alternative projection targets for MCH neurons to influence relapse-like behaviors (63, 97, 98). Located adjacent to MCH neurons in LH, orexin/hypocretin (Hcrt) neurons promote cocaine seeking when activated (105), and exhibit increased number and activity following cocaine SA and WD (106). Through local inhibitory synaptic connections, MCH neurons inhibit LH Hcrt neurons (54, 107), thus providing an alternative circuit mechanism for MCH neurons to reduce cocaine seeking. Additionally, MCH-to-hippocampal projection regulates impulsivity (108) and hippocampus-dependent memory (62), and both aspects may impact relapse-associated behaviors.
Focusing on male animals alone is a limitation of current study. Both male and female rats develop incubation of drug craving (109–112), and MCH neuron activity similarly regulate REMS in both sexes (51, 53, 55). However, female rats may acquire similar or higher levels of baseline cocaine SA, and may exhibit higher WD-associated cocaine seeking depending on the estrous cycle (109–117). Thus, it will be important to determine whether the REMS-incubation association exists in females, and whether MCH neuron activities during REMS similarly regulate cocaine seeking in females. Another limitation is that, although warming is a noninvasive approach to improve human sleep (118), the temperature would have been already optimized in a thermoneutral zone aided by accessories. However, using this and other approaches in rats, our current study reveals a set of REMS features that may serve as biomarkers for cocaine relapse, and that enhanced MCH neuron activities during REMS may offer anti-relapse potential. Considering the broad spectrum of MCH projection targets and functionality in regulating emotion, motivation, and impulsivity (51, 63, 97–99, 108), the interactions between MCH neuronal network and REMS characterized in current study may find wide applications to the regulations of emotion and motivation under physiological and pathological conditions.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | Melanin Concentrating Hormone(MCH) (human,Rat, Mouse) Antibody; host: rabbit | Phoenix Pharmaceuticals,Inc. | Catalog No: H-070-47 lot No: 01629-9 | |
| Antibody | Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 488) | Abcam | AB150073 | |
| Antibody | Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 594) | Abcam | AB150076 | |
| Viral Strain | Adeno-Associated Virus serotype 5 (AAV5) | University of North Carolina Vector Core | ||
| Chemical Compound or Drug | Picrotoxin (PTX) | Tocris | #1128 | |
| Chemical Compound or Drug | 1-Naphthylacetyl Spermine Trihydrochloride (Naspm) | Toronto Research Chemicals | N377800 | |
| Chemical Compound or Drug | Clozapine N-oxide (CNO) | Tocris | #6329 | |
| Chemical Compound or Drug | Clozapine N-oxide (CNO) | NIMH Chemical Synthesis and Drug Supply Program | #C-929 | |
| Chemical Compound or Drug | (−)-Cocaine hydrochloride | NIDA Drug Supply Program | #9041-001 | |
| Commercial Assay Or Kit | ZymoPURE II plasmid maxiprep kit | Zymo Research | #D4202 | |
| Organism/Strain | Rats/ Spargue-Dawley | Envigo | ||
| Peptide, Recombinant Protein | Melanin-concentrating hormone (MCH) peptide | Tocris | #3806 | |
| Recombinant DNA | GCaMP6f | Addgene | #52925 | |
| Recombinant DNA | AAV5-MCHp-DREADDs(Gq)-mCherry | PMID: 33093653 | ||
| Recombinant DNA | AAV5-MCHp-GFP | kindly provided by Dr. Priyattam Shiromani | ||
| Recombinant DNA | AAV5-MCHp-ChR2-EYFP | PMID: 33093653 |
Acknowledgements:
We thank Dr. Oliver M. Schlüter for advice on molecular cloning; Dr. Priyattam J. Shiromani for kindly providing us with the AAV5-MCHp-GFP and AAV5-MCHp-ChR2-EYFP constructs; Myles Billard (Tucker-Davis Technologies) for fiber photometry technical support; Braden R Bubarth, Jacob Minnick, and Nicholas J Lehman for help with rat behavioral training and testing. Research reported in this publication was supported by the National Institutes of Health under Award Numbers DA043826 (YH), DA046491 (YH), AA028145 (YH), DA023206 (YD), DA040620 (YD), DA51010 (YD); DA047861 (YD), DA046346 (MG, GT, YH). Cocaine was supplied by the Drug Supply Program of NIH NIDA. Clozapine N-oxide was partly supplied by the Chemical Synthesis and Drug Supply Program of NIMH.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Giancarlo Allocca is the creator of Somnivore software, and the owner of Somnivore Pty. Ltd. Dr. Jidong Fang is the creator of SleepMaster software, and the owner of Biosoft Studio. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Hunt WA, Barnett LW, Branch LG (1971): Relapse rates in addiction programs. Journal of clinical psychology. 27:455–456. [DOI] [PubMed] [Google Scholar]
- 2.McLellan AT, Lewis DC, O’Brien CP, Kleber HD (2000): Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 284:1689–1695. [DOI] [PubMed] [Google Scholar]
- 3.Angarita GA, Emadi N, Hodges S, Morgan PT (2016): Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: a comprehensive review. Addict Sci Clin Pract. 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaehne A, Unbehaun T, Feige B, Cohrs S, Rodenbeck A, Schutz AL, et al. (2015): Sleep changes in smokers before, during and 3 months after nicotine withdrawal. Addict Biol. 20:747–755. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald MK, Moses TEH, Roehrs TA (2021): At the intersection of sleep deficiency and opioid use: mechanisms and therapeutic opportunities. Transl Res. 234:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eacret D, Veasey SC, Blendy JA (2020): Bidirectional Relationship between Opioids and Disrupted Sleep: Putative Mechanisms. Mol Pharmacol. 98:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B, Wang Y, Liu X, Liu Z, Dong Y, Huang YH (2015): Sleep Regulates Incubation of Cocaine Craving. The Journal of neuroscience : the official journal of the Society for Neuroscience. 35:13300–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logan RW, Hasler BP, Forbes EE, Franzen PL, Torregrossa MM, Huang YH, et al. (2018): Impact of Sleep and Circadian Rhythms on Addiction Vulnerability in Adolescents. Biological psychiatry. 83:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK (2007): Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 3:24–32. [PubMed] [Google Scholar]
- 10.Puhl MD, Boisvert M, Guan Z, Fang J, Grigson PS (2013): A novel model of chronic sleep restriction reveals an increase in the perceived incentive reward value of cocaine in high drug-taking rats. Pharmacology, biochemistry, and behavior. 109:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puhl MD, Fang J, Grigson PS (2009): Acute sleep deprivation increases the rate and efficiency of cocaine self-administration, but not the perceived value of cocaine reward in rats. Pharmacology, biochemistry, and behavior. 94:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roehrs T, Johanson CE, Meixner R, Turner L, Roth T (2004): Reinforcing and subjective effects of methylphenidate: dose and time in bed. Exp Clin Psychopharmacol. 12:180–189. [DOI] [PubMed] [Google Scholar]
- 13.Teplin D, Raz B, Daiter J, Varenbut M, Tyrrell M (2006): Screening for substance use patterns among patients referred for a variety of sleep complaints. The American journal of drug and alcohol abuse. 32:111–120. [DOI] [PubMed] [Google Scholar]
- 14.Zhao D, Zhang M, Tian W, Cao X, Yin L, Liu Y, et al. (2021): Neurophysiological correlate of incubation of craving in individuals with methamphetamine use disorder. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 15.Guo R, Vaughan DT, Rojo ALA, Huang YH (2022): Sleep-mediated regulation of reward circuits: implications in substance use disorders. Neuropsychopharmacology. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abel T, Havekes R, Saletin JM, Walker MP (2013): Sleep, plasticity and memory from molecules to whole-brain networks. Current biology : CB. 23:R774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baran B, Pace-Schott EF, Ericson C, Spencer RM (2012): Processing of emotional reactivity and emotional memory over sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience. 32:1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gujar N, McDonald SA, Nishida M, Walker MP (2011): A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cerebral cortex. 21:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel JM (2005): Clues to the functions of mammalian sleep. Nature. 437:1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel JM (2011): REM sleep: a biological and psychological paradox. Sleep medicine reviews. 15:139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vyazovskiy VV, Delogu A (2014): NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 20:203–219. [DOI] [PubMed] [Google Scholar]
- 22.Kowatch RA, Schnoll SS, Knisely JS, Green D, Elswick RK (1992): Electroencephalographic sleep and mood during cocaine withdrawal. Journal of addictive diseases. 11:21–45. [DOI] [PubMed] [Google Scholar]
- 23.Matuskey D, Pittman B, Forselius E, Malison RT, Morgan PT (2011): A multistudy analysis of the effects of early cocaine abstinence on sleep. Drug and alcohol dependence. 115:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan PT, Malison RT (2008): Pilot study of lorazepam and tiagabine effects on sleep, motor learning, and impulsivity in cocaine abstinence. The American journal of drug and alcohol abuse. 34:692–702. [DOI] [PubMed] [Google Scholar]
- 25.Morgan PT, Pace-Schott E, Pittman B, Stickgold R, Malison RT (2010): Normalizing effects of modafinil on sleep in chronic cocaine users. The American journal of psychiatry. 167:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan PT, Angarita GA, Canavan S, Pittman B, Oberleitner L, Malison RT, et al. (2016): Modafinil and sleep architecture in an inpatient-outpatient treatment study of cocaine dependence. Drug and alcohol dependence. 160:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. (2014): Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 83:1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma YY, Wang X, Huang Y, Marie H, Nestler EJ, Schluter OM, et al. (2016): Re-silencing of silent synapses unmasks anti-relapse effects of environmental enrichment. Proceedings of the National Academy of Sciences of the United States of America. 113:5089–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Guo R, Chen B, Rahman T, Cai L, Li Y, et al. (2020): Cocaine-induced neural adaptations in the lateral hypothalamic melanin-concentrating hormone neurons and the role in regulating rapid eye movement sleep after withdrawal. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien CP, Childress AR, Ehrman R, Robbins SJ (1998): Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 12:15–22. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y, Taylor JR, Wolf ME, Shaham Y (2017): Circuit and Synaptic Plasticity Mechanisms of Drug Relapse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 37:10867–10876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimm JW, Hope BT, Wise RA, Shaham Y (2001): Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 412:141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011): Neurobiology of the incubation of drug craving. Trends in neurosciences. 34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL (1998): Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 19:48–59. [DOI] [PubMed] [Google Scholar]
- 35.Lu L, Grimm JW, Hope BT, Shaham Y (2004): Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 47 Suppl 1:214–226. [DOI] [PubMed] [Google Scholar]
- 36.Szymusiak R, Satinoff E (1981): Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate. Physiol Behav. 26:687–690. [DOI] [PubMed] [Google Scholar]
- 37.Komagata N, Latifi B, Rusterholz T, Bassetti CLA, Adamantidis A, Schmidt MH (2019): Dynamic REM Sleep Modulation by Ambient Temperature and the Critical Role of the Melanin-Concentrating Hormone System. Current biology : CB. 29:1976–1987 e1974. [DOI] [PubMed] [Google Scholar]
- 38.Kelley AE (2004): Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and biobehavioral reviews. 27:765–776. [DOI] [PubMed] [Google Scholar]
- 39.Mogenson GJ, Jones DL, Yim CY (1980): From motivation to action: functional interface between the limbic system and the motor system. Progress in neurobiology. 14:69–97. [DOI] [PubMed] [Google Scholar]
- 40.Robbins TW, Everitt BJ (1996): Neurobehavioural mechanisms of reward and motivation. Current opinion in neurobiology. 6:228–236. [DOI] [PubMed] [Google Scholar]
- 41.Loweth JA, Tseng KY, Wolf ME (2014): Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology. 76 Pt B:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. (2008): Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 454:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M (2011): Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 31:5737–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf ME (2016): Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci. 17:351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terrier J, Luscher C, Pascoli V (2016): Cell-Type Specific Insertion of GluA2-Lacking AMPARs with Cocaine Exposure Leading to Sensitization, Cue-Induced Seeking, and Incubation of Craving. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 41:1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zinsmaier AK, Dong Y, Huang YH (2021): Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SH, Weber F (2020): Neural and Homeostatic Regulation of REM Sleep. Front Psychol. 11:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sapin E, Berod A, Leger L, Herman PA, Luppi PH, Peyron C (2010): A very large number of GABAergic neurons are activated in the tuberal hypothalamus during paradoxical (REM) sleep hypersomnia. PloS one. 5:e11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modirrousta M, Mainville L, Jones BE (2005): Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. The European journal of neuroscience. 21:2807–2816. [DOI] [PubMed] [Google Scholar]
- 50.Hassani OK, Lee MG, Jones BE (2009): Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proceedings of the National Academy of Sciences of the United States of America. 106:2418–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jego S, Glasgow SD, Herrera CG, Ekstrand M, Reed SJ, Boyce R, et al. (2013): Optogenetic identification of a rapid eye movement sleep modulatory circuit in the hypothalamus. Nat Neurosci. 16:1637–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraigne JJ, Peever JH (2013): Melanin-concentrating hormone neurons promote and stabilize sleep. Sleep. 36:1767–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konadhode RR, Pelluru D, Blanco-Centurion C, Zayachkivsky A, Liu M, Uhde T, et al. (2013): Optogenetic stimulation of MCH neurons increases sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience. 33:10257–10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konadhode RR, Pelluru D, Shiromani PJ (2014): Neurons containing orexin or melanin concentrating hormone reciprocally regulate wake and sleep. Front Syst Neurosci. 8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsunematsu T, Ueno T, Tabuchi S, Inutsuka A, Tanaka KF, Hasuwa H, et al. (2014): Optogenetic manipulation of activity and temporally controlled cell-specific ablation reveal a role for MCH neurons in sleep/wake regulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 34:6896–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kroeger D, Bandaru SS, Madara JC, Vetrivelan R (2019): Ventrolateral periaqueductal gray mediates rapid eye movement sleep regulation by melanin-concentrating hormone neurons. Neuroscience. 406:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harding EC, Yu X, Miao A, Andrews N, Ma Y, Ye Z, et al. (2018): A Neuronal Hub Binding Sleep Initiation and Body Cooling in Response to a Warm External Stimulus. Current biology : CB. 28:2263–2273 e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroeger D, Absi G, Gagliardi C, Bandaru SS, Madara JC, Ferrari LL, et al. (2018): Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun. 9:4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan CL, Cooke EK, Leib DE, Lin YC, Daly GE, Zimmerman CA, et al. (2016): Warm-Sensitive Neurons that Control Body Temperature. Cell. 167:47–59 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szymusiak R, Gvilia I, McGinty D (2007): Hypothalamic control of sleep. Sleep medicine. 8:291–301. [DOI] [PubMed] [Google Scholar]
- 61.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. (2013): Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izawa S, Chowdhury S, Miyazaki T, Mukai Y, Ono D, Inoue R, et al. (2019): REM sleep-active MCH neurons are involved in forgetting hippocampus-dependent memories. Science. 365:1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haemmerle CA, Campos AM, Bittencourt JC (2015): Melanin-concentrating hormone inputs to the nucleus accumbens originate from distinct hypothalamic sources and are apposed to GABAergic and cholinergic cells in the Long-Evans rat brain. Neuroscience. 289:392–405. [DOI] [PubMed] [Google Scholar]
- 64.Saito Y, Nothacker HP, Wang Z, Lin SH, Leslie F, Civelli O (1999): Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 400:265–269. [DOI] [PubMed] [Google Scholar]
- 65.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, et al. (2005): The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 25:2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, et al. (2000): The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. The European journal of neuroscience. 12:1194–1216. [DOI] [PubMed] [Google Scholar]
- 67.Saito Y, Cheng M, Leslie FM, Civelli O (2001): Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. The Journal of comparative neurology. 435:26–40. [DOI] [PubMed] [Google Scholar]
- 68.Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, et al. (2002): Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics. 79:785–792. [DOI] [PubMed] [Google Scholar]
- 69.Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, et al. (2010): Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. The Journal of neuroscience : the official journal of the Society for Neuroscience. 30:8263–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf ME, Tseng KY (2012): Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, et al. (2011): Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 61:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barson JR, Morganstern I, Leibowitz SF (2013): Complementary roles of orexin and melanin-concentrating hormone in feeding behavior. Int J Endocrinol. 2013:983964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dilsiz P, Aklan I, Sayar Atasoy N, Yavuz Y, Filiz G, Koksalar F, et al. (2020): MCH Neuron Activity Is Sufficient for Reward and Reinforces Feeding. Neuroendocrinology. 110:258–270. [DOI] [PubMed] [Google Scholar]
- 74.Noble EE, Hahn JD, Konanur VR, Hsu TM, Page SJ, Cortella AM, et al. (2018): Control of Feeding Behavior by Cerebral Ventricular Volume Transmission of Melanin-Concentrating Hormone. Cell metabolism. 28:55–68 e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oishi Y, Xu Q, Wang L, Zhang BJ, Takahashi K, Takata Y, et al. (2017): Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat Commun. 8:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qiu MH, Liu W, Qu WM, Urade Y, Lu J, Huang ZL (2012): The role of nucleus accumbens core/shell in sleep-wake regulation and their involvement in modafinil-induced arousal. PloS one. 7:e45471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luo YJ, Li YD, Wang L, Yang SR, Yuan XS, Wang J, et al. (2018): Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat Commun. 9:1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCullough KM, Missig G, Robble MA, Foilb AR, Wells AM, Hartmann J, et al. (2021): Nucleus Accumbens Medium Spiny Neuron Subtypes Differentially Regulate Stress-Associated Alterations in Sleep Architecture. Biological psychiatry. 89:1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Stel J (2015): Precision in Addiction Care: Does It Make a Difference? Yale J Biol Med. 88:415–422. [PMC free article] [PubMed] [Google Scholar]
- 80.Brami-Cherrier K, Lewis RG, Cervantes M, Liu Y, Tognini P, Baldi P, et al. (2020): Cocaine-mediated circadian reprogramming in the striatum through dopamine D2R and PPARgamma activation. Nat Commun. 11:4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vyazovskiy VV, Tobler I (2005): Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain research. 1050:64–71. [DOI] [PubMed] [Google Scholar]
- 82.Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A (1995): Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 18:890–894. [DOI] [PubMed] [Google Scholar]
- 83.Baglioni C, Regen W, Teghen A, Spiegelhalder K, Feige B, Nissen C, et al. (2014): Sleep changes in the disorder of insomnia: a meta-analysis of polysomnographic studies. Sleep medicine reviews. 18:195–213. [DOI] [PubMed] [Google Scholar]
- 84.Van Someren EJW (2021): Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol Rev. 101:995–1046. [DOI] [PubMed] [Google Scholar]
- 85.Riemann D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B (2012): REM sleep instability--a new pathway for insomnia? Pharmacopsychiatry. 45:167–176. [DOI] [PubMed] [Google Scholar]
- 86.Kaplan KA, McQuaid J, Primich C, Rosenlicht N (2014): An evidence-based review of insomnia treatment in early recovery. J Addict Med. 8:389–394. [DOI] [PubMed] [Google Scholar]
- 87.Rosenblum M (2017): Substance abuse and insomnia. Minn Med. 100:38–39. [PubMed] [Google Scholar]
- 88.Fortuna LR, Cook B, Porche MV, Wang Y, Amaris AM, Alegria M (2018): Sleep disturbance as a predictor of time to drug and alcohol use treatment in primary care. Sleep medicine. 42:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brower KJ (2003): Insomnia, alcoholism and relapse. Sleep medicine reviews. 7:523–539. [DOI] [PubMed] [Google Scholar]
- 90.Brower KJ, Perron BE (2010): Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses. 74:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, et al. (2011): Optogenetic disruption of sleep continuity impairs memory consolidation. Proceedings of the National Academy of Sciences of the United States of America. 108:13305–13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee ML, Katsuyama AM, Duge LS, Sriram C, Krushelnytskyy M, Kim JJ, et al. (2016): Fragmentation of Rapid Eye Movement and Nonrapid Eye Movement Sleep without Total Sleep Loss Impairs Hippocampus-Dependent Fear Memory Consolidation. Sleep. 39:2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lipinska G, Thomas KGF (2019): Rapid eye movement fragmentation, not slow-wave sleep, predicts neutral declarative memory consolidation in posttraumatic stress disorder. J Sleep Res. 28:e12846. [DOI] [PubMed] [Google Scholar]
- 94.Lipinska G, Thomas KGF (2019): The Interaction of REM Fragmentation and Night-Time Arousal Modulates Sleep-Dependent Emotional Memory Consolidation. Front Psychol. 10:1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pesonen AK, Gradisar M, Kuula L, Short M, Merikanto I, Tark R, et al. (2019): REM sleep fragmentation associated with depressive symptoms and genetic risk for depression in a community-based sample of adolescents. J Affect Disord. 245:757–763. [DOI] [PubMed] [Google Scholar]
- 96.Simor P, van der Wijk G, Nobili L, Peigneux P (2020): The microstructure of REM sleep: Why phasic and tonic? Sleep medicine reviews. 52:101305. [DOI] [PubMed] [Google Scholar]
- 97.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, et al. (1992): The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. The Journal of comparative neurology. 319:218–245. [DOI] [PubMed] [Google Scholar]
- 98.Lagos P, Torterolo P, Jantos H, Monti JM (2011): Immunoneutralization of melanin-concentrating hormone (MCH) in the dorsal raphe nucleus: effects on sleep and wakefulness. Brain research. 1369:112–118. [DOI] [PubMed] [Google Scholar]
- 99.Torterolo P, Scorza C, Lagos P, Urbanavicius J, Benedetto L, Pascovich C, et al. (2015): Melanin-Concentrating Hormone (MCH): Role in REM Sleep and Depression. Front Neurosci. 9:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tyree SM, de Lecea L (2017): Lateral Hypothalamic Control of the Ventral Tegmental Area: Reward Evaluation and the Driving of Motivated Behavior. Front Syst Neurosci. 11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Domingos AI, Sordillo A, Dietrich MO, Liu ZW, Tellez LA, Vaynshteyn J, et al. (2013): Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. eLife. 2:e01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitka T, Adori C, Katai Z, Vas S, Molnar E, Papp RS, et al. (2011): Association between the activation of MCH and orexin immunorective neurons and REM sleep architecture during REM rebound after a three day long REM deprivation. Neurochem Int. 59:686–694. [DOI] [PubMed] [Google Scholar]
- 103.Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, et al. (2002): Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nature medicine. 8:825–830. [DOI] [PubMed] [Google Scholar]
- 104.Chung S, Parks GS, Lee C, Civelli O (2011): Recent updates on the melanin-concentrating hormone (MCH) and its receptor system: lessons from MCH1R antagonists. Journal of molecular neuroscience: MN. 43:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G (2012): Multiple roles for orexin/hypocretin in addiction. Progress in brain research. 198:79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G (2019): Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biological psychiatry. 85:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rao Y, Lu M, Ge F, Marsh DJ, Qian S, Wang AH, et al. (2008): Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 28:9101–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noble EE, Wang Z, Liu CM, Davis EA, Suarez AN, Stein LM, et al. (2019): Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat Commun. 10:4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE (2008): Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology. 198:63–75. [DOI] [PubMed] [Google Scholar]
- 110.Nicolas C, Zlebnik NE, Farokhnia M, Leggio L, Ikemoto S, Shaham Y (2022): Sex Differences in Opioid and Psychostimulant Craving and Relapse: A Critical Review. Pharmacol Rev. 74:119–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, et al. (2019): Incubation of Cocaine Craving After Intermittent-Access Self-administration: Sex Differences and Estrous Cycle. Biological psychiatry. 85:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corbett CM, Dunn E, Loweth JA (2021): Effects of Sex and Estrous Cycle on the Time Course of Incubation of Cue-Induced Craving following Extended-Access Cocaine Self-Administration. eNeuro. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, et al. (2016): Estradiol Facilitation of Cocaine Self-Administration in Female Rats Requires Activation of mGluR5. eNeuro. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jackson LR, Robinson TE, Becker JB (2006): Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 31:129–138. [DOI] [PubMed] [Google Scholar]
- 115.Lynch WJ, Taylor JR (2005): Decreased motivation following cocaine self-administration under extended access conditions: effects of sex and ovarian hormones. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 30:927–935. [DOI] [PubMed] [Google Scholar]
- 116.Ramoa CP, Doyle SE, Naim DW, Lynch WJ (2013): Estradiol as a mechanism for sex differences in the development of an addicted phenotype following extended access cocaine self-administration. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 38:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, et al. (2019): Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 44:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Harding EC, Franks NP, Wisden W (2019): The Temperature Dependence of Sleep. Front Neurosci. 13:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


