Abstract
Two genes homologous to lpxL and lpxM from Escherichia coli and other gram-negative bacteria, which are involved in lipid A acyloxyacylation, were identified in Neisseria meningitidis strain H44/76 and insertionally inactivated. Analysis by tandem mass spectrometry showed that one of the resulting mutants, termed lpxL1, makes lipopolysaccharide (LPS) with penta- instead of hexa-acylated lipid A, in which the secondary lauroyl chain is specifically missing from the nonreducing end of the GlcN disaccharide. Insertional inactivation of the other (lpxL2) gene was not possible in wild-type strain H44/76 expressing full-length immunotype L3 lipopolysaccharide (LPS) but could be readily achieved in a galE mutant expressing a truncated oligosaccharide chain. Structural analysis of lpxL2 mutant lipid A showed a major tetra-acylated species lacking both secondary lauroyl chains and a minor penta-acylated species. The lpxL1 mutant LPS has retained adjuvant activity similar to wild-type meningococcal LPS when used for immunization of mice in combination with LPS-deficient outer membrane complexes from N. meningitidis but has reduced toxicity as measured in a tumor necrosis factor alpha induction assay with whole bacteria. In contrast, both adjuvant activity and toxicity of the lpxL2 mutant LPS are strongly reduced. As the combination of reduced toxicity and retained adjuvant activity has not been reported before for either lpxL or lpxM mutants from other bacterial species, our results demonstrate that modification of meningococcal lipid A biosynthesis can lead to novel LPS species more suitable for inclusion in human vaccines.
Neisseria meningitidis is a human pathogen for which no fully effective vaccine is available. As do almost all gram-negative bacteria, it contains lipopolysaccharide (LPS) as a major component of the outer membrane. Novel vaccines based on outer membrane vesicles of this organism also contain LPS, which due to its endotoxin activity can have both positive and negative effects (20, 39). On the one hand, it can function as a natural adjuvant, increasing the antibody response against outer membrane proteins (OMPs) (27); on the other hand, its toxicity can result in significant reactogenicity which might limit acceptance of LPS-containing vaccines, as has been the case with whole-cell Bordetella pertussis vaccines. Lipid A, the part anchoring LPS in the outer membrane, is primarily responsible for its endotoxin activity. Biosynthetic modification of lipid A might be a way to find novel LPS species more suitable for inclusion in vaccines based on products directly derived from pathogenic gram-negative bacteria such as N. meningitidis. Meningococci seem to be particularly amenable for such studies, as we have recently found that in contrast to Escherichia coli and many other gram-negative bacteria, they can grow without LPS after inactivation of lpxA, which encodes the UDP-GlcNAc acyltransferase required for the first step of lipid A biosynthesis (26).
Two late-functioning acyltransferases of lipid A biosynthesis in E. coli were identified as the products of the htrB and msbB genes (3, 4); the htrB gene was previously described as required for growth on rich media above 33°C (11), and the msbB gene was described as a multicopy suppressor of htrB (12). These genes are also known as waaM or lpxL and waaN or lpxM, respectively (2, 22). In the optimal reaction, LpxL transfers laurate to (2-keto-3-deoxyoctulosonic acid)2-lipid IVA [(KDO)2-lipid IVA], after which LpxM can add myristate to complete lipid A acylation. The predominant products formed by lpxL and lpxM mutants are tetra- and penta-acyl species, respectively (3, 4). The genes display 27.5% identity; a third gene belonging to this family named lpxP is also present in the E. coli genome and encodes a palmitoleoyl transferase replacing lpxL at lower temperature (2). Similar mutants lacking secondary fatty acyl chains from lipid A have been described for Haemophilus influenzae lpxL (16) and Salmonella enterica serovar Typhimurium lpxL (28) and lpxM (13). Interestingly, in all cases such mutants make LPS with altered biological activity. In particular, a strong reduction in the ability to stimulate tumor necrosis factor alpha (TNF-α) production by monocytes has been reported for lpxL and/or lpxM LPS mutants in E. coli, S. enterica serovar Typhimurium, and H. influenzae (7, 10, 13, 18, 25).
N. meningitidis lipid A has a different structure compared to the above-mentioned bacteria, in that it has a symmetrical distribution of the acyloxyacyl chains; in the major species both the N-linked 3-OH myristoyl chains at the 2 and 2′ positions carry a secondary lauroyl chain (15). Also, the O-linked fatty acyl chains are 3-OH laurate instead of 3-OH myristate. It can thus be expected that the meningococcal acyloxyacyl transferases will function somewhat differently from their E. coli counterparts. In the present study, we have identified and inactivated two lpxL and lpxM homologues in N. meningitidis and analyzed lipid A structure and biological activity from the corresponding mutant LPS species.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. coli strains NM522 and INVαF′ were grown on Luria-Bertani medium at 37°C. The N. meningitidis strain H44/76 and its derivatives with inactivated galE (9), lpxA (26), and lpxL1 and lpxL2 genes (this study) were grown at 37°C on GC medium base (Difco) supplemented with IsoVitaleX (Becton Dickinson) in a humid atmosphere containing 5% CO2 or in liquid medium as described (32). Bacterial suspensions were heat inactivated for 30 min at 56°C. For selection of meningococcal transformants (34) kanamycin was used at a concentration of 100 μg/ml. With E. coli, the following antibiotics were used at the indicated concentrations: ampicillin, 100 μg/ml; kanamycin, 100 μg/ml. For cloning of PCR fragments, the TA cloning kit with the vector pCRII (Invitrogen) was used.
Antibiotic susceptibility determination.
Meningococcal strains were grown overnight on GC agar plates, three colonies of each were resuspended in 200 μl of medium, 175 μl of this was spread on fresh GC plates and filter paper disks containing different antibiotics were placed on the agar surface. The filter paper disks (Oxoid) contained rifampin (5 μg), bacitracin (10 U), tetracycline (10 μg), or novobiocin (30 μg). After overnight incubation at 37°C, the halo of growth inhibition around each disk was measured.
Recombinant DNA techniques.
Most recombinant DNA techniques were carried out as described by Sambrook et al. (24). Plasmid DNA was isolated using the pLASmix kit (Talent). The PCR was performed on a Perkin-Elmer GeneAmp PCR system 9700 with Taq polymerase. Sequence analysis was performed with an Applied Biosystems automatic sequencer on double-stranded plasmid DNA templates (isolated with Qiagen columns) and with a cycle sequencing protocol. The oligonucleotides that were used for amplification of the lpxL1 and lpxL2 genes were pr670-1 (5′-ATCCTTCGGGGATGCAGGTC-3′), pr447-2 (5′-CGGCCTTTCAAAATCTGTTC-3′), pr481-1 (5′-AAACAGATACTGCGTCGGAA-3′), pr481-2 (5′-CCCTTTGCGAACCGCCAT-3′), and pr753-1 (5′-CTTCCCTTTTTCAGACGGCA-3′).
Characterization of outer membrane composition.
Binding of monoclonal antibodies (MAbs) specific for the major OMPs PorA (MN5C11G), PorB (MN15A14H6), and RmpM (MN2D6D) and for the oligosaccharide part of immunotype L3 LPS (MN4A8B2) and its galE derivative (MN31B11.18) was tested in a whole-cell enzyme-linked immunosorbent assay (ELISA) (33, 34). Isolation of outer membrane complexes (OMCs) by sarcosyl extraction and their analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were done as described previously (32).
LPS structural analysis.
Tricine-SDS-PAGE was performed in 4% stacking and 16% separating gels as described by Lesse et al. (17). Proteinase K-treated, boiled bacterial cells were used as samples. The gels were run for 17 h at a constant current of 20 mA and silver stained by the method of Tsai and Frasch (30). For fatty acid analysis by gas chromatography-mass spectrometry (GC-MS), OMC samples were acetylated for 3 h at 90°C in pyridine and acetic acid anhydride in order to completely dissolve the LPS. The samples were subsequently heated for 3 h at 65°C in tetrahydrofuran in the presence of LiAlH4 to reduce the O-linked fatty acids to the free alcohols. These were derivatized to trimethylsilane ethers for 1 h at 60°C with N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA)–1% trimethylchlorosilane (TMCS) in pyridine and analyzed by GC-MS on an Autospec (Micromass, Manchester, United Kingdom) in the electron impact mode. The amount of 3-OH C12 in the samples was quantified using 2-OH C12 as an internal standard. LPS was isolated by the hot phenol-water extraction method (35). For isolation of lipid A, LPS was subjected to mild acid hydrolysis (1% acetic acid; 2.5 h at 100°C), followed by precipitation and final fractionation in chloroform-methanol-water. Structural analysis of purified lipid A was performed by nanoelectrospray tandem MS on a Finnigan LCQ in the positive ion mode (36). Rhodobacter sphaeroides LPS was obtained from List Biological Laboratories.
Biological activity of LPS.
The chromogenic Limulus amebocyte lysate (LAL) assay for endotoxin activity was performed using the QCL-1000 kit from BioWhittaker Inc. (Walkersville, Md.) according to the instructions of the manufacturer. Overnight cultures were diluted in meningococcal medium to an optical density at 620 nm (OD620) of 0.1, and serial dilutions of these stocks were used as samples in the LAL assay. TNF-α induction by heat-inactivated bacterial suspensions was tested with the human macrophage cell line MM6 (38). MM6 cells were seeded in microtiter plates (105/well) in 100 μl of IMDM (Gibco BRL) supplemented with 10% fetal calf serum (Gibco BRL) and penicillin-streptomycin (Gibco BRL) and stimulated with 100 μl of serial dilutions of a bacterial stock solution with an OD620 of 0.1, for 16 to 18 h at 37°C in a humid atmosphere containing 5% CO2. TNF-α in the culture supernatants was quantitated using a bioassay with the TNF-α-sensitive cell line WEHI 164 as described by Espevik and Nissen (5). Human recombinant TNF-α (Roche) was used as a standard.
Immunization of mice.
Six- to eight-week-old BALB/c mice, five animals in each group, were immunized subcutaneously on day 0 with 20 μg of LPS-deficient H44/76 OMC protein supplemented with adjuvant (5 μg of LPS) and dissolved in 0.5 ml of phosphate-buffered saline. At day 14 and day 28 immunization was repeated, and mice were bled at day 42. Sera were collected and stored at 4°C. Antibody titers were determined for each individual serum against H44/76 whole cells in ELISA as described previously (27). A four-parameter curve fit was made for the optical density values obtained with serial dilutions of the sera, and the antibody titers were calculated as reciprocal dilutions that gave 50% of the maximum absorbance. The serum bactericidal activity was assayed against H44/76 as described in Hoogerhout et al. (8), using a final concentration of 20% rabbit complement. Sera were heat inactivated for 30 min at 56°C prior to use. Serum samples and bacteria were incubated for 10 to 15 min at room temperature before the addition of complement. The serum bactericidal titer is expressed as the reciprocal serum dilution showing killing of more than 90% of the number of bacteria used. Results of antibody and bactericidal titers are expressed as the mean log10 titers of five separate sera. Analysis of variance was used for evaluation of statistical data. The significance of the differences between the mean values was determined by the least-significant difference test at a confidence level of 95%.
RESULTS
Construction of an N. meningitidis lpxL1 mutant with altered lipid A.
Using the lpxL and lpxM gene sequences from E. coli and H. influenzae, we performed BLAST searches on the N. gonorrhoeae genome sequences made available on the Internet by the University of Oklahoma. Several contigs with significant homology were identified, and PCR primers were designed based on these gonococcal sequences. Using meningococcal chromosomal DNA as the template, primers pr447-2 and pr670-1 gave a 0.5-kb PCR product which upon cloning in vector pCRII and sequencing was found to be homologous to the N-terminal half of lpxL and lpxM sequences from several bacterial species. This fragment was used as a probe for isolation of a larger chromosomal fragment containing the complete gene, which shows 31 and 30% amino acid sequence identity with E. coli lpxL and lpxM, respectively, and will be referred to here as lpxL1. Immediately upstream an open reading frame with homology to the ruvC gene from E. coli was found, which presumably is involved in DNA repair and recombination and not LPS biosynthesis. A kanamycin resistance cassette was inserted into the BglI site located within the cloned lpxL1 PCR product, and the resulting construct (plasmid pBSNK6, containing also the neisserial uptake sequence) was used to transform meningococcal strain H44/76 to kanamycin resistance. PCR with primers pr447-2 and pr670-1 was used to verify that correct allelic exchange with the chromosomal lpxL1 gene had occurred, as the 0.5-kb PCR product was replaced by a 1.8-kb fragment resulting from insertion of the kanamycin resistance cassette. All transformants thus obtained showed a slightly increased mobility of their LPS when analyzed by Tricine-SDS-PAGE followed by silver staining (Fig. 1). However, binding of MAbs specific for the oligosaccharide part of meningococcal LPS was not affected by the mutation, suggesting that only the lipid A part must have been altered (results not shown).
FIG. 1.
Tricine-SDS-PAGE analysis of LPS from H44/76 wild type (lane 1) and its lpxL1 derivative (lane 2) and from H44/76 galE (lane 3) and its lpxL2 derivative (lane 4).
Construction of an N. meningitidis lpxL2 mutant with altered lipid A.
Another gene with a slightly lower homology to lpxL and lpxM from E. coli (29 and 24% amino acid sequence identity, respectively) was similarly identified and insertionally inactivated; it will be referred to here as lpxL2. Using gonococcal chromosomal DNA as the template, primers pr447-2 and pr753-1 gave a 0.95-kb PCR product which was cloned in the vector pCRII and sequenced to confirm its identity. A kanamycin resistance cassette was inserted into the cloned lpxL2 PCR product, and the resulting construct (plasmid pBSK481b, containing also the neisserial uptake sequence) was used for transformation of meningococcal strain H44/76. Transformants containing the kanamycin resistance cassette were found only very rarely and in all cases seemed to carry secondary mutations, resulting in LPS with either a truncated oligosaccharide chain or a strongly reduced level of its expression (results not shown). As it has been reported for both E. coli and H. influenzae that lpxL mutants can show temperature-sensitive growth (11, 16), we also tried the transformation at 30°C instead of 37°C, with the same result. However, when the transformation was done with a galE mutant derivative of strain H44/76, lpxL2 knockout mutants were readily isolated in large numbers. PCR with primers pr481-1 and pr481-2 was used to verify that correct allelic exchange with the chromosomal lpxL2 gene had occurred, as the 0.85-kb PCR product was replaced by a 2.1-kb fragment resulting from insertion of the kanamycin resistance cassette. Transformants were analyzed by Tricine-SDS-PAGE followed by silver staining, which showed increased mobility of their LPS compared to the galE parent strain (Fig. 1). In all cases a second, minor band at a slightly higher position (but still below that of galE LPS) could also be distinguished. Their MAb binding pattern was the same as that of the galE parent strain, again suggesting that only the lipid A part is altered (results not shown). Neither the lpxL1 nor the lpxL2 mutation resulted in temperature-sensitive growth or altered colony morphology.
Structural analysis of lpxL1 and lpxL2 mutant lipid A.
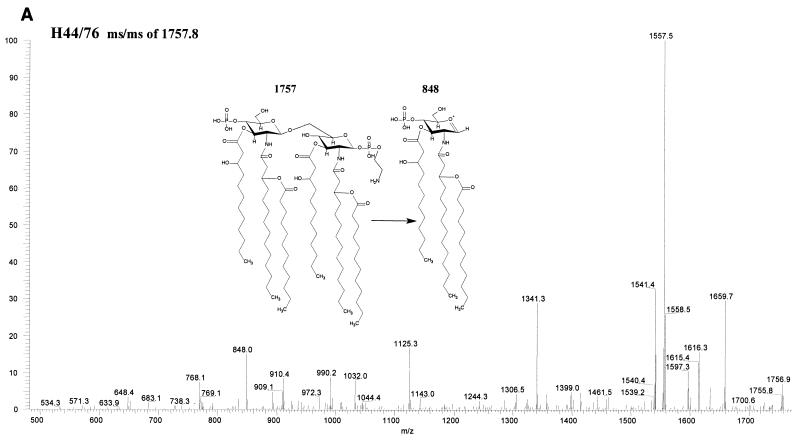
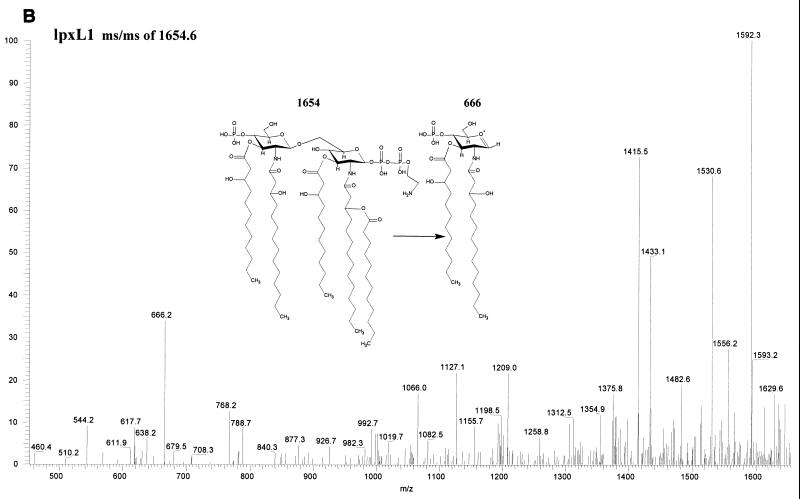
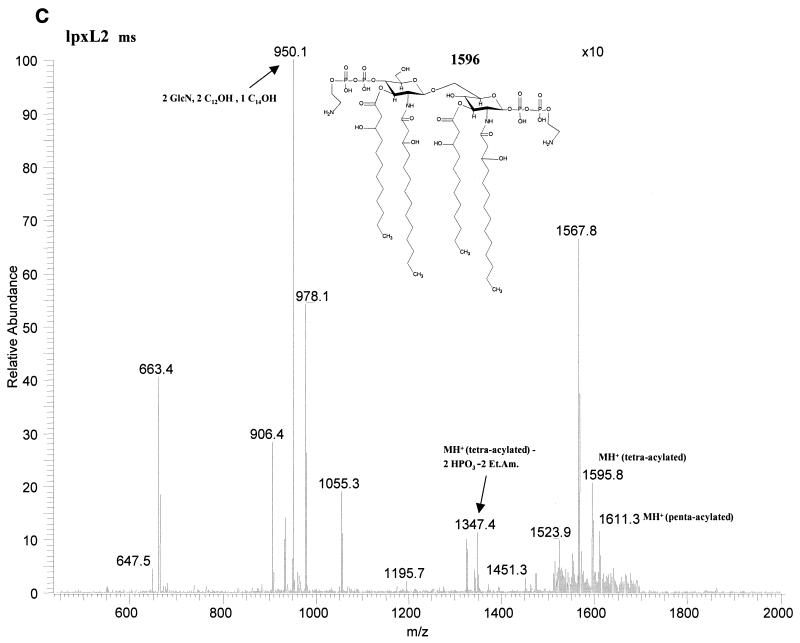
Fatty acid analysis by GC-MS of whole cells and OMCs showed a reduced ratio of C12 to 3-OH C12 in the lpxL1 mutant compared to the wild-type parent strain, indicating a (partial) loss of the secondary C12 acyl chain(s). LPS from this mutant was purified through hot phenol-water extraction, and the lipid A fraction was obtained after acid hydrolysis and chloroform-methanol extraction. Its structure was subsequently investigated using tandem MS, which revealed a major penta-acyl species in which the C12 acyloxyacyl chain was missing from the nonreducing end of the molecule (Fig. 2). An additional difference from the parent strain was found in the phosphorylation pattern at the reducing end of the disaccharide, where an additional phosphate group was present. For the lpxL2 mutant, a major tetra-acyl species was found which lacks both secondary C12 acyl chains (Fig. 2); in addition, a minor penta-acyl species was present, but the position of the remaining C12 chain could not be unequivocally established. For both species, heterogeneity in the phosphorylation pattern was observed, although the major component of the tetra-acyl species is fully substituted with P-P-ethanolamine at both the 1 and 4′ positions. The presence of a major tetra-acyl and a minor penta-acyl species agrees with the two bands seen on Tricine-SDS-PAGE (Fig. 1).
FIG. 2.
Structural analysis by MS of lipid A from wild-type H44/76 and its lpxL1 and lpxL2 mutants. In positive ion mass spectra of lipid A, oxonium ions have been shown to be formed (6). These oxonium ions are considered to be the distal ion due to charge transfer to the left sugar ring and can therefore be used to discriminate between substitutions at the two GlcN residues. (A) In the positive-ion ms/ms spectrum of the parent ion 1757 of H44/76 wild type, the oxonium ion is 848 atomic mass units. (B) In the positive-ion ms/ms spectrum of the parent ion 1654 of the lpxL1 mutant, the oxonium ion is 666 atomic mass units, showing that the C12 acyloxyacyl chain is missing from the left sugar ring. (C) In the positive-ion mass spectrum of the lpxL2 mutant, a major tetra-acylated lipid A species corresponding to a mass of 1596 and a minor penta-acylated lipid A species corresponding to a mass of 1611 were found. In the positive-ion ms/ms spectrum of the 1611 ion no oxonium ion could be found, so the position of the remaining C12 chain in this minor species could not be established (results not shown).
Antibiotic susceptibility of lpxL1 and lpxL2 mutants.
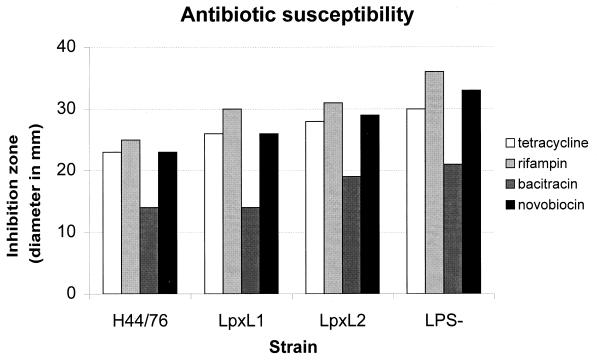
In order to determine the effect of the lpxL1 and lpxL2 mutations on the barrier function of the outer membrane, the susceptibilities of these mutants to four hydrophobic antibiotics were determined. As shown in Fig. 3, the inhibition zone displayed by the lpxL1 and lpxL2 mutants was generally intermediate in size between wild-type H44/76 and the completely LPS-deficient lpxA mutant, with the mainly tetra-acylated lpxL2 mutant showing higher sensitivity than the penta-acylated lpxL1 mutant.
FIG. 3.
Antibiotic susceptibility of strain H44/76, its lpxL1 and lpxL2 mutants, and the LPS-deficient lpxA mutant. Shown are the diameters of the inhibition zones around filter paper disks containing each of four different hydrophobic antibiotics.
Biological activity of lpxL1 and lpxL2 mutant LPS.
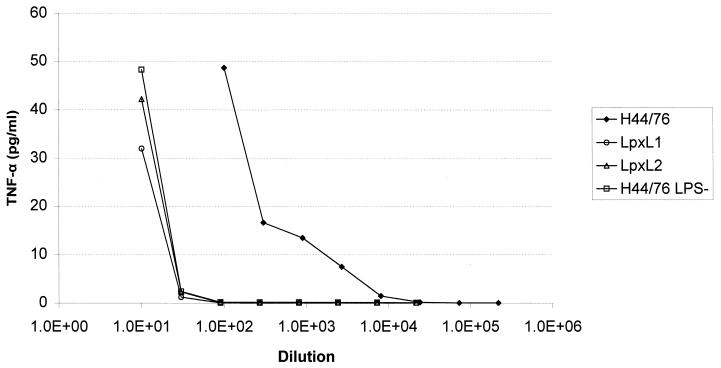
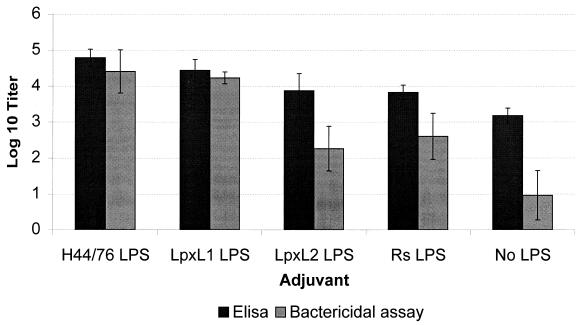
The lpxL1 and lpxL2 mutant strains were tested for their LPS-associated biological activity. In an LAL assay, whole-cell suspensions with an OD620 of 0.1 gave 20 × 104 endotoxin units (EU)/ml for the wild type, 3 × 104 EU/ml for the lpxL1 mutant, and 0.3 EU/ml for the LPS-deficient lpxA mutant. With TNF-α induction in cells of the human macrophage cell line MM6, both lpxL1 and lpxL2 whole bacterial cells showed approximately a 100-fold reduction in activity compared to the wild type, similar to the reduction found for whole cells of a completely LPS-deficient mutant (Fig. 4). Immunization of mice with OMCs isolated from the LPS-deficient lpxA meningococcal mutant was used to compare the adjuvant activities of various LPS preparations. Antibody responses were measured by whole-cell ELISA and a bactericidal assay against parent strain H44/76. We have previously shown that the bactericidal antibodies induced in this way are mainly PorA-specific and not directed against LPS itself which therefore mainly functions as adjuvant and not as immunogen (27). Immunogenicity of the major OMPs could be restored to normal levels by addition of either wild-type or lpxL1 mutant LPS, but less so by lpxL2 mutant LPS and the atoxic LPS from R. sphaeroides (Fig. 5). Specifically, the bactericidal titers obtained with lpxL1 mutant LPS were 100-fold higher than those obtained with lpxL2. These results are in marked contrast to those of the TNF-α induction assay, where the lpxL1 and lpxL2 mutants displayed the same reduced activity. Thus, only the lpxL1 mutant LPS has retained adjuvant activity in spite of decreased toxicity.
FIG. 4.
TNF-α induction in MM6 cells by whole bacteria of strain H44/76, its lpxL1 and lpxL2 mutants, and the LPS-deficient lpxA mutant. The horizontal axis gives the dilutions made from a bacterial suspension with an OD620 of 1.0. The data shown represent one of several experiments with similar results.
FIG. 5.
Comparison of adjuvant activity of LPS preparations from strain H44/76 and its lpxL1 and lpxL2 mutants and from R. sphaeroides (Rs) when used for immunization of mice together with LPS-deficient OMCs. Shown are antibody titers measured by whole-cell ELISA and bactericidal assay against wild-type strain H44/76. The data represent the average of five mice in each group. Error bars, standard deviations.
DISCUSSION
The specific acylation pattern of the lipid A moiety of LPS largely determines its biological activity (23). Biosynthetic modification of lipid A biosynthesis thus offers the potential to create novel LPS species more suitable for inclusion in those human vaccines which by their nature always include LPS, such as outer membrane vesicle- or whole-cell based vaccines. For this reason, we have studied the meningococcal homologues of the lpxL1 and lpxL2 genes, which have been shown to encode late-functioning acyltransferases of lipid A biosynthesis in E. coli (3, 4). These enzymes work in a preferred order, with LpxL first adding laurate to the 3-OH C14 at the 2′ position in (KDO)2-lipid IVA, followed by myristate addition by LpxM to the 3-OH C14 at the 3′ position. The corresponding biosynthetic steps can be expected to be somewhat different in N. meningitidis, as the secondary fatty acids are here bound to the 3-OH C14 acyl chains at the 2 and 2′ positions, i.e., at both GlcN residues instead of only GlcN II (15). We identified two lpxL and lpxM homologues in the gonococcal and meningococcal genome sequences; both genes displayed a slightly higher homology with lpxL than lpxM, which would agree with the observed absence of acylation at the LpxM position. By construction of knockout mutants for both genes and structural analysis of their lipid A, we have identified their most likely role in its biosynthetic pathway (Fig. 6). The observed penta-acylated lipid A made by the lpxL1 mutant strongly suggests that this enzyme adds the C12 to the N-linked 3-OH C14 at the 2′ position of GlcN II. As the major lipid A species found in the lpxL2 mutant is tetra-acylated, this enzyme must add the other C12, i.e., to the N-linked 3-OH C14 at the 2 position of GlcN I. The presence of a minor penta-acylated species in the lpxL2 mutant suggests that this step should precede the other one, but this preference is not absolute as some LpxL1-mediated acylation can still occur, resulting in a component with a secondary C12 presumably only at GlcN II. However, as the position of the remaining secondary acyl chain in this minor component could not be identified unequivocally, other interpretations remain possible. The observed differences in the phosphorylation pattern are most likely caused by an indirect effect of incomplete acylation on the activity of enzymes required for addition of the phosphate and ethanolamine groups. Other indirect effects on the structure of the oligosaccharide part of LPS cannot be completely excluded but seem unlikely, as no difference was found in the binding pattern of several MAbs whose epitopes have been previously characterized using a set of defined mutants with truncated oligosaccharide chains (34).
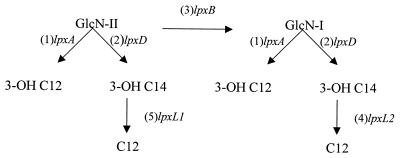
FIG. 6.
Schematic representation of the meningococcal lipid A biosynthesis pathway. After acylation of UDP-GlcN by LpxA and LpxD, dimerization by LpxB takes place. Secondary acylation by LpxL2 and LpxL1 (in that order) follows later, presumably after addition of KDO to GlcN II.
For both E. coli and H. influenzae, lpxL mutants with predominantly tetra-acylated lipid A show temperature-sensitive growth; indeed, this phenotype was the basis for the identification of lpxL in the first place (11). We did not find this to be the case in N. meningitidis, as growth at 37 or 42°C was not different for the mutants compared to the wild type; instead, the lpxL2 mutant displayed a different conditional phenotype, i.e., the requirement for a truncated galactose-deficient oligosaccharide chain. Conceivably, a proper balance between the size of the hydrophobic and hydrophilic parts of LPS is required for maintenance of outer membrane stability. In both lpxL1 and lpxL2 mutants, the major OMPs PorA, PorB, and RmpM were observed in normal amounts, suggesting no major changes in outer membrane structure or composition (results not shown). The same lack of effect on expression of these OMPs was previously observed for a meningococcal lpxA mutant completely deficient in LPS due to a block in the first step of lipid A biosynthesis (26). However, an increase in susceptibility to several hydrophobic antibiotics was observed in both lpxL1 and lpxL2 mutants, indicating that the barrier function of their outer membranes is compromised. This could be a direct effect of altered packing of the underacylated lipid A molecules or due to secondary changes in, e.g., phospholipid distribution as reported for an E. coli lpxL mutant (37). These findings differ somewhat from those reported by Vaara and Nurminen (31) who concluded on the basis of similar antibiotic susceptibility experiments with lpxL and lpxM mutants of E. coli that hexa-acylated lipid A is not a prerequisite for the normal function of the outer membrane permeability barrier. This may reflect differences in LPS-LPS and LPS-OMP interactions in these organisms, which is also apparent from the fact that LPS-deficient lpxA knockout mutants are viable in N. meningitidis but not in E. coli.
The lpxL1 mutant LPS displayed a particularly interesting pattern of biological activity. Its ability to induce TNF-α synthesis in MM6 cells was strongly reduced, but in contrast to the also nontoxic lpxL2 and R. sphaeroides LPS its adjuvant activity is the same as that of wild-type LPS when used in combination with OMCs from the LPS-deficient lpxA meningococcal mutant. The lpxL1 mutant lipid A molecule has a unique structure not found in any of the mutants described previously for other gram-negative bacteria, as the remaining acyloxyacyl chain is present at the reducing end of the molecule instead of the nonreducing end as is the case for lpxM mutants of E. coli with penta-acylated lipid A (4, 25) and for the also penta-acylated R. sphaeroides lipid A (21). The combination of reduced toxicity and retained adjuvant activity has not been reported before for either lpxL or lpxM mutants from other bacterial species and may thus be critically dependent on this particular acylation pattern found only in the meningococcal lpxL1 mutant LPS.
We have previously shown that the antibody response against the major OMPs was strongly reduced when mice were immunized with OMCs of the LPS-deficient lpxA mutant compared to wild-type OMCs (27). In our view, this is not due to a requirement for LPS to maintain the right conformation of the major OMPs for an optimal immune response, since other non-LPS-derived adjuvants could effectively replace LPS. Also, it would be difficult to envisage how lpxL1 and lpxL2 LPS could differ so strongly in stabilizing the conformation of OMPs, as both apparently allow normal OMP assembly. More likely, they differentially activate the mammalian LPS response system. Recent studies have demonstrated the essential role of members of the Toll-like receptor family for induction of proinflammatory cytokines (1, 14). In mice, TLR4 transduces the LPS signal while the structurally related TLR2 appears to play a role in recognition of peptidoglycan (19, 29). Several other as-yet-uncharacterized members of this family exist in both mice and humans, which may be involved in recognition of other classes of microbial components. Alternatively, some members might recognize similar signals but be expressed in different cell types. Investigating the interaction of mutant lipid A molecules with various members of the Toll-like receptor family should thus lead to a better understanding of the complex range of biological activities displayed by LPS. Whatever the molecular basis for the different biological activities displayed by the lpxL1 and lpxL2 mutants will turn out to be, the improved ratio between toxicity and adjuvant activity found here for lpxL1 LPS makes it a promising candidate for inclusion in novel outer membrane vesicle vaccines against meningococcal disease.
REFERENCES
- 1.Beutler B. Endotoxin, Toll-like receptor 4, and the afferent limb of innate immunity. Curr Opin Immunol. 2000;3:23–28. doi: 10.1016/s1369-5274(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 2.Carty S M, Kodangattil K R, Raetz C R H. Effect of cold shock on lipid A biosynthesis in Escherichia coli. Induction at 12°C of an acyltransferase specific for palmitoleoyl-acyl carrier protein. J Biol Chem. 1999;274:9677–9685. doi: 10.1074/jbc.274.14.9677. [DOI] [PubMed] [Google Scholar]
- 3.Clementz T, Bednarski J J, Raetz C R H. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid A. J Biol Chem. 1996;271:12095–12102. doi: 10.1074/jbc.271.20.12095. [DOI] [PubMed] [Google Scholar]
- 4.Clementz T, Zhou Z, Raetz C R H. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. J Biol Chem. 1997;272:10353–10360. doi: 10.1074/jbc.272.16.10353. [DOI] [PubMed] [Google Scholar]
- 5.Espevik T, Nissen M J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 6.Harrata A K, Domelsmith L N, Cole R B. Electrospray mass spectrometry for characterization of lipid A from Enterobacter agglomerans. Biol Mass Spectr. 1993;22:59–67. doi: 10.1002/bms.1200220108. [DOI] [PubMed] [Google Scholar]
- 7.Hone D M, Powell J, Crowley R W, Maneval D, Lewis G K. Lipopolysaccharide from an Escherichia coli htrB msbB mutant induces high levels of MIP-1α secretion without inducing TNF-α and IL-1β. J Hum Virol. 1998;1:251–256. [PubMed] [Google Scholar]
- 8.Hoogerhout P, Donders E M L M, van Gaans-van den Brink J A M, Kuipers B, Brugghe H F, van Unen L M A, Timmermans H A M, ten Hove G J, de Jong A P J M, Peeters C C A M, Wiertz E J H J, Poolman J T. Conjugates of synthetic cyclic peptides elicit bactericidal antibodies against a conformational epitope on a class 1 outer membrane protein of Neisseria meningitidis. Infect Immun. 1995;63:3473–3478. doi: 10.1128/iai.63.9.3473-3478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennings M P, van der Ley P, Wilks K E, Maskell D J, Poolman J T, Moxon E R. Cloning and molecular analysis of the galE gene of Neisseria meningitidis and its role in lipopolysaccharide biosynthesis. Mol Microbiol. 1993;10:361–369. [PubMed] [Google Scholar]
- 10.Jones B D, Nichols W A, Gibson B W, Sunshine M G, Apicella M A. Study of the role of the htrB gene in Salmonella typhimurium virulence. Infect Immun. 1997;65:4778–4783. doi: 10.1128/iai.65.11.4778-4783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karow M, Fayet O, Cegielska A, Ziegelhoffer T, Georgopoulos C. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33°C in rich media. J Bacteriol. 1991;173:741–750. doi: 10.1128/jb.173.2.741-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karow M, Georgopoulos C. Isolation and characterization of the Escherichia coli msbB gene, a multicopy suppressor of null mutations in the high-temperature requirement gene htrB. J Bacteriol. 1992;174:702–710. doi: 10.1128/jb.174.3.702-710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan S A, Everest P, Servos S, Foxwell N, Zähringer U, Brade H, Rietschel E T, Dougan G, Charles I G, Maskell D. A lethal role for lipid A in Salmonella infections. Mol Microbiol. 1998;29:571–579. doi: 10.1046/j.1365-2958.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- 14.Kopp E B, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 15.Kulshin V A, Zähringer U, Lindner B, Frasch C E, Tsai C, Dimitriev A, Rietschel E T. Structural characterization of the lipid A component of pathogenic Neisseria meningitidis. J Bacteriol. 1992;174:1793–1800. doi: 10.1128/jb.174.6.1793-1800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N-G, Sunshine M G, Engstrom J J, Gibson B W, Apicella M A. Mutation of the htrB locus of Haemophilus influenzae nontypable strain 2019 is associated with modifications of lipid A and phosphorylation of the lipo-oligosaccharide. J Biol Chem. 1995;270:27151–27159. [PubMed] [Google Scholar]
- 17.Lesse A J, Campagnari A A, Bittner W E, Apicella M A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilising tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 18.Nichols W A, Raetz C R H, Clementz T, Smith A L, Hanson J A, Ketterer M R, Sunshine M, Apicella M A. htrB of Haemophilus influenzae: determination of biochemical activity and effects on virulence and lipooligosaccharide toxicity. J Endotoxin Res. 1997;4:163–172. [Google Scholar]
- 19.Poltorak A, He X, Smirnova I, Liu M-Y, van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 2000;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 20.Poolman J T. Development of a meningococcal vaccine. Infect Agents Dis. 1995;4:13–28. [PubMed] [Google Scholar]
- 21.Qureshi N, Honovich J P, Hara H, Cotter R J, Takayama K. Location of fatty acids in lipid A obtained from lipopolysaccharide of Rhodopseudomonas sphaeroides ATCC 17023. J Biol Chem. 1988;263:5502–5504. [PubMed] [Google Scholar]
- 22.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R H, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 23.Rietschel E Th, Kirikae T, Schade F U, Ulmer A J, Holst O, Brade H, Schmidt G, Mamat U, Grimmecke H-D, Kusumoto S, Zähringer U. The chemical structure of bacterial endotoxin in relation to bioactivity. Immunobiology. 1993;187:169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Somerville J E, Cassiano L, Bainbridge B, Cunningham M D, Darveau R P. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J Clin Investig. 1996;97:359–365. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steeghs L, den Hartog R, den Boer A, Zomer B, van der Ley P. Meningitis bacterium is viable without endotoxin. Nature. 1998;392:449–450. doi: 10.1038/33046. [DOI] [PubMed] [Google Scholar]
- 27.Steeghs L, Kuipers B, Hamstra H J, Kersten G, van Alphen L, van der Ley P. Immunogenicity of outer membrane proteins in an LPS-deficient mutant of Neisseria meningitidis: influence of adjuvants on the immune response. Infect Immun. 1999;67:4988–4993. doi: 10.1128/iai.67.10.4988-4993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunshine M G, Gibson B W, Engstrom J J, Nichols W A, Jones B D, Apicella M A. Mutation of the htrB gene in a virulent Salmonella typhimurium strain by intergeneric transduction: strain construction and phenotypic characterization. J Bacteriol. 1997;179:5521–5533. doi: 10.1128/jb.179.17.5521-5533.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 30.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 31.Vaara M, Nurminen M. Outer membrane permeability barrier in Escherichia coli mutants that are defective in the late acyltransferases of lipid A biosynthesis. Antimicrob Agents Chemother. 1999;43:1459–1462. doi: 10.1128/aac.43.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Ley P, van der Biezen J, Hohenstein P, Peeters C, Poolman J T. Use of transformation to construct antigenic hybrids of the class 1 outer membrane protein in Neisseria meningitidis. Infect Immun. 1993;61:4217–4224. doi: 10.1128/iai.61.10.4217-4224.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Ley P, van der Biezen J, Poolman J T. Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine. 1995;13:401–407. doi: 10.1016/0264-410x(95)98264-b. [DOI] [PubMed] [Google Scholar]
- 34.van der Ley P, Kramer M, Steeghs L, Kuipers B, Andersen S R, Jennings M P, Moxon E R, Poolman J T. Identification of a locus involved in meningococcal lipopolysaccharide biosynthesis by deletion mutagenesis. Mol Microbiol. 1996;19:1117–1125. doi: 10.1046/j.1365-2958.1996.464992.x. [DOI] [PubMed] [Google Scholar]
- 35.Westphal O, Jann J K. Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 36.Wilm M, Mann M. Analytical properties of the nanoelectrospray ion source. Anal Chem. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Z, White K A, Polissi A, Georgopoulos C, Raetz C R. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J Biol Chem. 1998;273:12466–12475. doi: 10.1074/jbc.273.20.12466. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler-Heitbrock H W L, Thiel E, Fütterer A, Herzog V, Wirtz A, Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988;41:456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]
- 39.Zollinger W D. New and improved vaccines against meningococcal disease. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1997. pp. 469–488. [Google Scholar]