INTRODUCTION
According to the U.S. Centers for Disease Control and Prevention (CDC), 100,306 drug overdose deaths occurred in the U.S. during a 12-month period ending in April 2021.1 This is a total increase of 28.5% from 78,056 deaths during the same period the year before. Opioids were involved in 75% of these related deaths; 82% being synthetic.
Fentanyl, an analgesic compound, is 50 times more potent than heroin and 100 times more potent than morphine.2 The latest progression of the opioid epidemic is due to the introduction of non-pharmaceutical fentanyl (NPF), including fentanyl analogs and other novel synthetic opioids. The illicit drug industry is plagued with widespread fentanyl adulteration, which increases overdose risk for persons who use non-prescribed opioids.3 Fentanyl testing strips have grown in popularity to help individuals with safer drug use practices, but they are considered illegal to use in outpatient clinics per the Drug Enforcement Administration (DEA); advanced and legal technology to detect NPF and analogs more easily and accurately is required.4
Literature Review
A literature search was performed using three databases (MEDLINE, PubMed, and Google Scholar) to identify English-language articles. Search terms were fentanyl, buprenorphine, opioid use disorder, opioid dependence, pharmacokinetics, and non-pharmaceutical fentanyl. Studies were selected based on novel information related to NPF, its pertinent analogs, and differences in pharmacokinetics. Surveys were included because NPF is not used clinically and population sizes for trials were limited.
History of Fentanyl
Fentanyl was synthesized initially by Paul Jensen in 1960 in Belgium for pain management.4 By 1972, it was approved by the U.S. Food and Drug Administration in an intravenous form as Sublimaze® for high-dose analgesia in the operating room.2 Transdermal fentanyl was utilized mostly in the 1990s for palliative use for chronic pain in cancer patients who needed consistent, sustained blood levels of a potent opioid.4 However, this came with increased reports of overdoses caused by the non-medical use of transdermal patches in the early 2000s. By 2010, many people who previously used pharmaceutical opioids had transitioned to drugs such as heroin (later fentanyl), which had become cheaper and easier to obtain.5 Since its creation in 1960, many fentanyl analogs, including sufentanil, alfentanil, remifentanil, and carfentanil, have been produced for medical use, however, these have limited reports of non-medical use.2 Various non-pharmaceutical analogs have been identified (α-methylfentanyl, 3-methylfentanyl, acetylfentanyl, furanyl fentanyl) and subsequently added as Schedule I drugs. Starting in 2010, NPF in various forms such as tablets/powder, as well as fentanyl-laced heroin, cocaine, and benzodiazepines, began to emerge. Government organizations such as the DEA and CDC have attempted to introduce legislation to regulate these compounds but have been unable to keep up with the readily available analogs produced abroad.4
Current State of Non-Pharmaceutical Fentanyl
There are key differences between NPF and alternatives such as heroin or pharmaceutical grade fentanyl. As shown in Figure 1, a recent study in Arizona found a dramatic increase in the availability of NPF attempting to mimic 30 mg oxycodone, popularly known as “dirty thirties” or “M-30 tabs”.6 The similarities between these tablets and commonly used pharmaceutical drugs likely reduced the risk perception for people who use drugs, especially compared to heroin. Additionally, NPF is significantly easier to manufacture and distribute, leading to reduced prices in comparison to heroin and pharmaceutical opioids.
Figure 1.
Authentic oxycodone M30 tablets (top) vs. fake oxycodone M30 tablets containing fentanyl (bottom). [Picture obtained from DEA website.]
The most common form of heroin is black tar, which when available, had an increasing frequency of contamination with NPF.6 Other possible perceived benefits to switching to NPFs, described in some qualitative research, are route of administration and familiarity to prescription opioid pills. NPF pills allowed users to transition from the injection route of opioid administration to smoking. Some participants emphasized the switch was a “saving grace”, because smoking was viewed with less stigma and potentially a safer option. Others felt the smoking route of NPF could limit the overdose risk, however, objective data on this could not be obtained. Prior research established an increased risk of blood-borne infections such as human immunodeficiency virus (HIV) and Hepatitis B/C with injectable routes.7 For many participants, their personal experiences with NPF were based on whatever was available to purchase at the time. Abstinence from NPF also has proved to be more difficult, thought to be due to greater potency and more severe withdrawal symptoms.
Fentanyl/Analog Pharmacokinetics
Fentanyl and its analogues have varying potencies and side effects, aggravated by a short elimination half-life (219 minutes) when compared to other opioids.8 The prolonged clinical effects (miosis, constipation, and respiratory depression) may be due to sequestration in adipocytes and other tissues in chronic users.2 In humans, fentanyl is predominately (90%) metabolized by CYP3A4 in the liver into norfentanyl. The remaining 10% and various inactive metabolites (hydroxyfentanyl, hydroxynorfentanyl, and despropionylfentanyl) are metabolized by the kidneys and excreted in urine or feces.9
When analyzing chronic users, a recent study indicated that renal clearance for fentanyl in opioid use disorder was highly variable, but consistently longer than the two to four day clearance of other short-acting opioids.3 Major medical benefits in the hospital setting included minimal cardiovascular effects, relatively short-acting, and easy to administer in the nasal, buccal, or sublingual routes.4 Fentanyl is also highly lipophilic, allowing for fast diffusion, meaning plasma levels should fall within 60 minutes.8 Due to its high affinity for mu-opioid receptors, larger doses of naloxone are required to reverse the effects than are used commonly for traditional opioids.2
Although pharmaceutical fentanyl is well characterized, little is known about the metabolic pathways of new fentanyl analogs. This disparity prompted a study in 2019 which analyzed the various pathways and potencies of new analogs (Table 1).10 The activity of new analogs potentially could be predicted to assess properly for clinical relevance. A profile of fentanyl and its analogs was analyzed in Washington, D.C. through a needle-exchange program.11 Recent advances have allowed for detection of trace amounts of these analogs. Nine major compounds were identified; the four most common being fentanyl (72.29%), acetyl fentanyl (11.44%), para-fluorofentanyl (6.63%), and furanyl fentanyl (3.61%). The data illuminated a rising presence of furanyl fentanyl which was proposed to be seven times as strong as fentanyl and can be used in tandem with other isotopes. It is becoming evident that proper identification of these analogs is crucial to guide further treatment.
Table 1.
Data on metabolism and potency along with chemical structures of fentanyl and its analogs.11
| Compound | Metabolites | Relative Potency (fentanyl) | Structure |
|---|---|---|---|
| Fentanyl | hydroxyfentanyl, hydroxynorfentanyl, despropionylfentanyl | 1 |
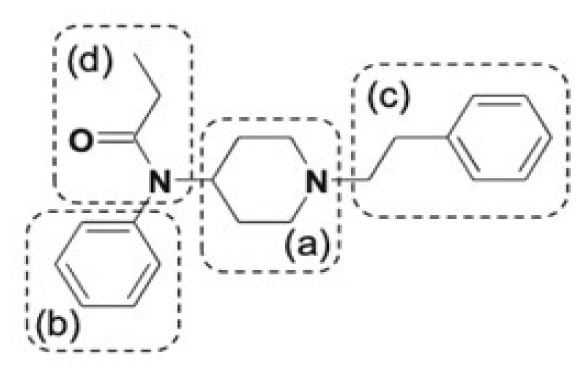
|
| Alfentanil | Noralfentanil | 0.3 |
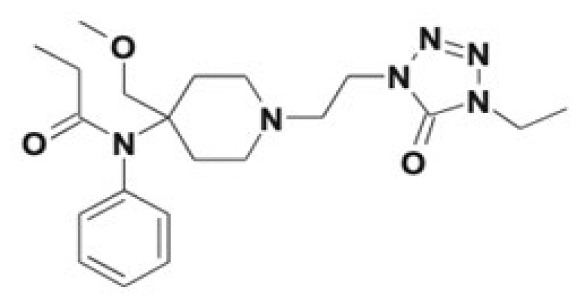
|
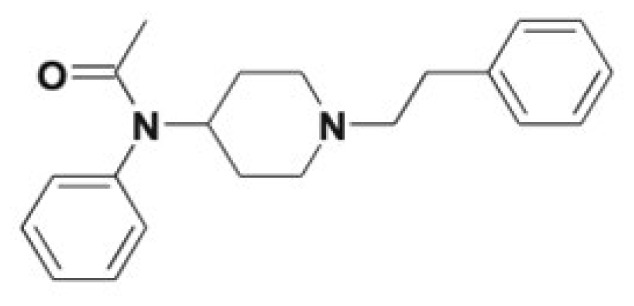
| Sufentanil | Norsufentanil, N-phenylpropanamide, demethylsufentanil | 10 |
|
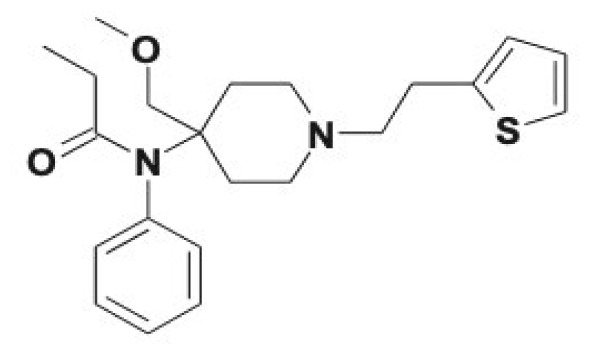
| Acetylfentanyl | Acetylnorfentanyl, 4-ANPP, β- hydroxyacetylfentanyl, 4′-hydroxy-3′-methoxy-acetylfentanyl | 0.3 |
|
| Acryloylfentanyl | Acryloylnorfentanyl, 4-ANPP, β-hydroxyacryloylfentanyl, 4′-hydroxy-3′-methoxy-acryloylfentanyl, and phase II conjugates | 0.75 |
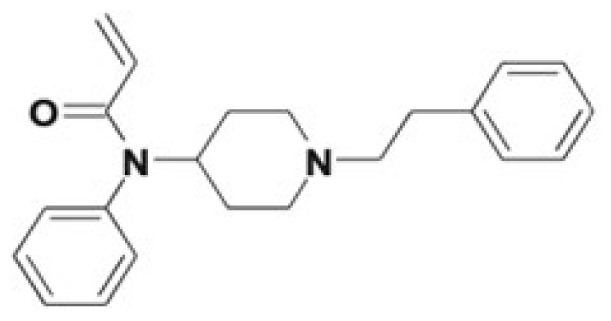
|
| α-Methylfentanyl | Norfentanyl, Despropionyl-α-methylfentanyl, alkyl/aryl hydroxy metabolites | 1 |
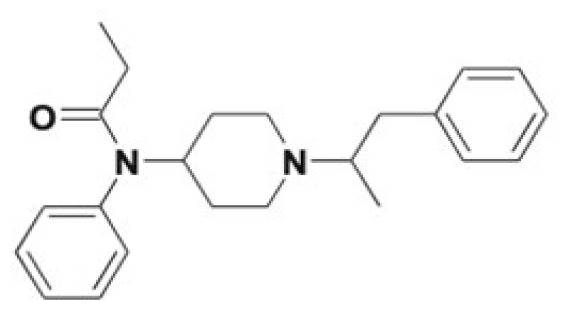
|
| Furanylfentanyl | Furano-dihydrodiol formation, 4-ANPP, norfuranylfentanyl, alkyl/aryl hydroxy metabolites, ring opening of the furanyl ring and phase II conjugates | 7 |
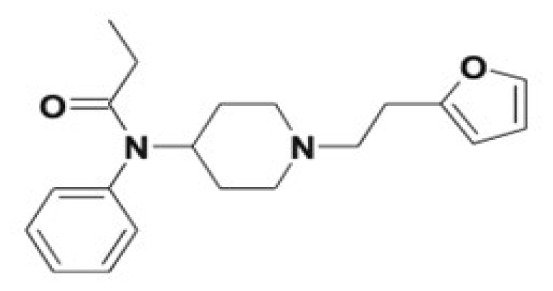
|
| Carfentanil | Norcarfentanil, alkyl/aryl hydroxy metabolites, carfentanil acid, keto-carfentanil, N-oxide formation and phase II conjugates | 30–100 |
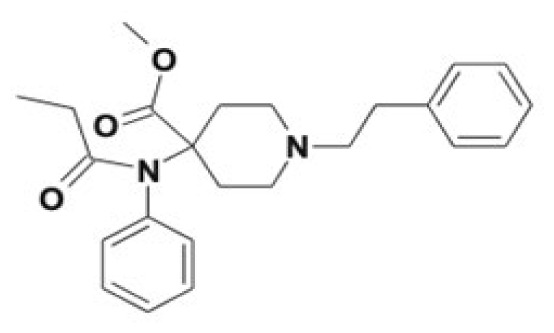
|
| Isofentanyl | Nor-3-methylfentanyl, alkyl/aryl hydroxy metabolites, carboxypropionyl-isofentanyl, 4′-hydroxy-3′-methoxyisofentanyl, N-oxide formation | NA |
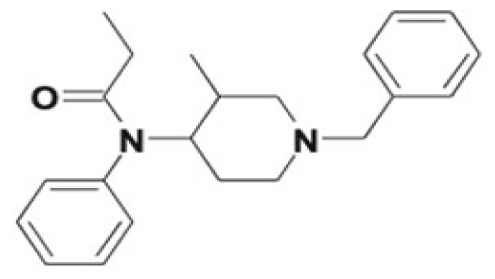
|
Buprenorphine Competition
Buprenorphine is a partial mu-agonist with high binding affinity and slow dissociation from the receptor.12 Because of this, it is suited particularly for heroin or opioid use disorder. Fentanyl, though, has a higher binding affinity compared to other mu-opioid agonists, which may limit the opioid blockade benefit of buprenorphine. It is possible that long-term buprenorphine outcomes could be affected, as those testing positive for fentanyl had lower opioid abstinence and marginally lower retention after six months of buprenorphine treatment compared to those with negative toxicology. This subtle interaction has potential to alter the overall management of opioid cessation.
CONCLUSIONS
A total of 100,306 drug overdose deaths occurred in the U.S. during a 12-month period ending in April 2021, and this number is growing yearly.1 This trend demonstrated the need for identifying these shifts in drug usage, implementation of proper policy, and novel treatments to reduce the risk of opioid overdoses.
Over the past decade, there was emerging unpredictability and accessibility of NPF and its analogs.6 There is still much to learn about the pharmaceutical properties and safety profiles of these analogs, which further exacerbates the emerging issue. Fentanyl testing strips have grown in popularity to help individuals with safer drug use practices, but they are considered illegal to use in outpatient clinics per the DEA; advanced and legal technology to detect NPF and analogs more easily and accurately is required. This will facilitate and guide proper treatment protocols.
REFERENCES
- 1.Ahmad FB, Sutton P. Provisional drug overdose death counts. 2021. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm .
- 2.Armenian P, Vo KT, Barr-Walker J, Lynch KL. Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review. Neuropharmacology. 2018;134(Pt A):121–132. doi: 10.1016/j.neuropharm.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Huhn AS, Hobelmann JG, Oyler GA, Strain EC. Protracted renal clearance of fentanyl in persons with opioid use disorder. Drug Alcohol Depend. 2020;214:108147. doi: 10.1016/j.drugalcdep.2020.108147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley TH. The fentanyl story. J Pain. 2014;15(12):1215–1226. doi: 10.1016/j.jpain.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. Jul, 2021. https://store.samhsa.gov/product/TIP-63-Medications-for-Opioid-Use-Disorder-Full-Document/PEP21-02-01-002 .
- 6.Daniulaityte R, Sweeney K, Ki S, Doebbeling BN, Mendoza N. “They say it’s fentanyl, but they honestly look like Perc 30s”: Initiation and use of counterfeit fentanyl pills”. Harm Reduct J. 2022;19(1):52. doi: 10.1186/s12954-022-00634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridge J. Route transition interventions: Potential public health gains from reducing or preventing injecting. Int J Drug Policy. 2010;21(2):125–128. doi: 10.1016/j.drugpo.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 8.McClain DA, Hug CC., Jr Intravenous fentanyl kinetics. Clin Pharmacol Ther. 1980;28(1):106–114. doi: 10.1038/clpt.1980.138. [DOI] [PubMed] [Google Scholar]
- 9.Kuip EJ, Zandvliet ML, Koolen SL, Mathijssen RH, van der Rijt CC. A review of factors explaining variability in fentanyl pharmacokinetics; focus on implications for cancer patients. Br J Clin Pharmacol. 2017;83(2):294–313. doi: 10.1111/bcp.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilde M, Pichini S, Pacifici R, et al. Metabolic pathways and potencies of new fentanyl analogs. Front Pharmacol. 2019;10:238. doi: 10.3389/fphar.2019.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giltner A, Evans A, Cicco C, Leach S, Rowe W. Fentanyl analog trends in Washington D.C. observed in needle-exchange syringes. Forensic Sci Int. 2022;338:111393. doi: 10.1016/j.forsciint.2022.111393. [DOI] [PubMed] [Google Scholar]
- 12.Wakeman SE, Chang Y, Regan S, et al. Impact of fentanyl use on buprenorphine treatment retention and opioid abstinence. J Addict Med. 2019;13(4):253–257. doi: 10.1097/ADM.0000000000000486. [DOI] [PubMed] [Google Scholar]



