Abstract
Objective
To investigate prevalence, incidence and medication of interstitial lung disease (ILD) among German individuals with rheumatoid arthritis (RA).
Methods
Nationwide BARMER claims data from 2007 to 2020 were used. RA-ILD was identified by diagnosis codes, prescription of disease-modifying antirheumatic drugs (DMARDs) and lung diagnostics. ILD was assigned as incident or prevalent relative to the year of the first diagnosis. We identified prescriptions of glucocorticoids, conventional synthetic (cs), biological (b) and targeted synthetic (ts)DMARDs, antifibrotics and rheumatology and/or pulmonology care.
Results
Among all persons with RA (40 686 in 2007 to 85 175 in 2020), 1.7%–2.2%/year had ILD with a slight decline since 2013. Incident ILD was 0.13%–0.21% per year and remained stable over time. ILD was more common in seropositive RA, in men and in the elderly (mean age 72 years in 2020). Glucocorticoids (84% to 68%), csDMARD (83% to 55%) and non-steroidal anti-inflammatory drug use (62% to 38%) declined, while bDMARDs (16% to 24%) rose. In 2020, 7% received tsDMARDs, 3% antifibrotics, 44% analgesics and 30% opioids. DMARD therapy was more common if a rheumatologist was involved and antifibrotics if a pulmonologist was involved. Opioid use was highest if no specialist was involved (39%) but also common in rheumatology care (32%) and less frequent in pulmonology care (21%).
Conclusions
RA-ILD is rare and mainly affects elderly persons. No trend in incidence was observed but treatment strategies have enlarged. Specialist care is necessary to provide disease-specific therapies. The continuing high analgesic and opioid demand shows unmet needs in these patients.
Keywords: Epidemiology; Arthritis, Rheumatoid; Health services research
WHAT IS ALREADY KNOWN ON THIS TOPIC
Interstitial lung disease (ILD) increases morbidity and mortality in patients with rheumatoid arthritis (RA).
WHAT THIS STUDY ADDS
Between 2010 and 2020, ILD was present in around 2% of persons with RA per year.
Patients with RA and ILD have received less glucocorticoids, non-steroidal anti-inflammatory drugs and conventional synthetic disease-modifying antirheumatic drugs (DMARDs) and more biological/targeted synthetic DMARDs in recent years.
Opioids and analgesics are frequently prescribed to persons with RA-ILD.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The high prescription rate of analgesics including opioids needs to be addressed.
Further evidence for effective treatment strategies in RA-ILD is needed.
Introduction
Interstitial lung disease (ILD) is a serious extra-articular manifestation in rheumatoid arthritis (RA) with a significant morbidity and increased mortality.1 2 Persons with RA have a threefold to fourfold increased risk of ILD compared with the general population,3 and if radiological criteria were applied, considerably more patients would be detected.4 Depending on age, disease duration and duration of follow-up of the examined populations with RA, ILD is found in 4%–8% of the studied RA collectives.5–7 As patients with RA are getting older, absolute numbers of patients with RA-ILD increase, while reported incidence rates have not relevantly changed, as shown by data from Denmark and the USA.3 8
There is still no evidence-based therapy for RA-ILD and only limited evidence supports the efficacy of immunosuppressive therapies for autoimmune-related ILD.9 Glucocorticoids are used for the acute management of RA-ILD.4 Despite previous fears, evidence emerges that methotrexate (MTX) may be beneficial as part of a treatment strategy in RA-ILD.4 10 Among the biological disease-modifying antirheumatic drugs (bDMARDs) used in patients with RA, preliminary evidence indicates abatacept, rituximab and tocilizumab as possible treatment options for RA-ILD.4 10–12
RA-ILD has a variable course of clinical progression and not every ILD develops into progressive fibrosis. If fibrotic progressive ILD is present, nintedanib or pirfenidone are followed as a new treatment approach13 as these drugs are known to slow disease progression in patients with idiopathic pulmonary fibrosis.9 In Germany, nintedanib has been approved since April 2020 for adults with progressive fibrosing ILD of other causes (including RA).
Little is known about the prevalence and incidence of RA-ILD in Germany and about medical provision by rheumatology, pulmonology or general care. Due to the rarity of the disease, there are few patients followed in the observational registry studies14 as these only include patients in rheumatology care. In case of predominant ILD, patients may be more likely referred to pulmonology care. For this reason, we used data from a large nationwide health insurance fund that includes all insured persons, irrespective of specialised care. The aim of this study was to examine the occurrence of ILD in persons with RA, to provide data on specialist and drug care and on developments in incidence and treatments over the last 13 years.
Methods
Data source
We used claims data from the BARMER statutory health insurance fund. The BARMER statutory health insurance fund is one of the largest health insurance companies in Germany and covers around 8.8 million people, corresponding to 12% of all inhabitants with a statutory health insurance. Around 73 million people (90%) of the German population are members of a statutory health insurance. For the analysis, data from the years 2005–2020 were available.
Inclusion criteria
Persons ≥18 years with ≥2 German modification of the International Statistical Classification of Diseases (ICD-10-GM) diagnoses of RA (M05: seropositive RA, M06: seronegative RA according to ICD-10-GM) in the referring year were included. Persons with additional diagnosis of systemic sclerosis (M34) or sarcoidosis (D86) were excluded, assuming that ILD diagnosis is not primarily related to RA in these persons.
Definition of RA-ILD
ILD was considered if the ICD-10-GM diagnoses (J84.1: other interstitial pulmonary diseases with fibrosis, J84.8: other specified interstitial pulmonary diseases, J84.9: interstitial pulmonary disease, unspecified or M05.1+J99.0: rheumatoid lung disease) were present once in case of an inpatient diagnosis or at least two times in an interval of ≥2 quarters within 12 months in case of an outpatient diagnosis. Drug-induced interstitial lung disorders (J70.2–4) were not included.
To increase the specificity of the RA diagnosis, we additionally required a DMARD therapy, which could have been prescribed at any time point prior to or during the index year. Our previous validation of the RA ICD-10 diagnosis in the BARMER claims data revealed that the requirement of a prescribed DMARD significantly increases the specificity.15 To increase the specificity of ILD diagnosis, we additionally required the performance of a lung diagnostic test (function tests, chest radiographs, high-resolution CT (HRCT) scans, bronchoscopy or bronchoalveolar lavage).
Prevalence and incidence of ILD
Prevalent ILD was considered if the ILD codes were present in the respective year and ≥1 diagnostic pulmonary procedure code had been performed in the present or prior to the index year. Incident ILD was considered if no ILD diagnosis was present in the 24 months prior to the index year. Inclusion requirements are illustrated in online supplemental figure 1.
rmdopen-2022-002777supp001.pdf (257.6KB, pdf)
Our algorithm approach to identify prevalent and incident ILD in RA is in line with the validated approach of Meehan et al,16 requiring a long period without ILD diagnosis for incident ILD.
Data report
Patient characteristics including additional comorbidities, namely hypertension, coronary heart disease, pulmonary hypertension, gastrointestinal reflux, chronic obstructive pulmonary disease (COPD), diabetes mellitus, bronchial asthma and lung diagnostics are reported for each of the years 2007–2020 and refer to all persons with prevalent ILD. The proportion of persons with specialist care (rheumatology/pulmonology) is reported starting 2008 since that is the first year specialists can be reliably identified in the data.
Incidence and prevalence data are illustrated for each year, stratified by sex (male/female), age groups (31–50, 51–70, >70 years) and seropositivity (M05: seropositive/M06: seronegative RA). Prevalence data in 2019 and 2020 are also provided for persons with b/targeted synthetic (ts)DMARD therapy.
Antirheumatic and antifibrotic therapies are reported for each year and for the year 2020 stratified by specialised care (rheumatology/pulmonology/both/none). Specific treatments were identified via the anatomical therapeutic chemical classification (ATC) and include non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, conventional synthetic (cs)/b/tsDMARDs, analgesics, opioids, nintedanib and pirfenidone. All ICD-10, ATC and procedure codes for lung diagnostics as well as identification codes for specialist care are reported in online supplemental table 1. To estimate the amount of glucocorticoids taken, the Defined Daily Doses (DDDs) dispensed are reported, providing information on the assumed average maintenance dose per day for a drug used for its main indication.17 To compare the amount of drug prescriptions in patients with ILD with patients with standard RA therapy, persons with RA and ever DMARD prescription but without ILD were selected as comparator group.
Patient and public involvement
Within the framework of the TARISMA research project, patient partners have accompanied our research from application to implementation.
The research paper was written in accordance with the REporting of studies Conducted using Observational Routinely-collected Data guideline.18
Results
Included persons
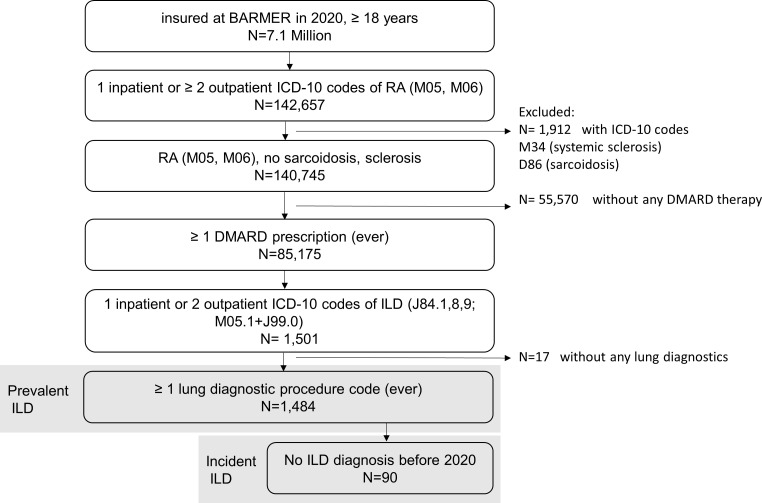
From 2007 to 2020, ≈7 million people per year, aged ≥18 years, were insured at the BARMER. Of these, 98 435 (2007) to 142 657 (2020) had an RA diagnosis (M05 or M06) and 40 686 (2007) to 85 175 (2020) persons had ever been prescribed a DMARD. A total of 257 (2007) to 1484 (2020) persons had prevalent ILD and 18 (2007) to 90 (2020) persons had incident ILD (table 1). For 2020, the individual inclusion steps are shown in figure 1.
Table 1.
Included persons, 2007–2020
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| >18 years, in million | 8.1 | 7.0 | 7.1 | 7.1 | 7.1 | 7.3 | 7.4 | 7.5 | 7.4 | 7.3 | 7.3 | 7.2 | 7.1 | 7.1 |
| RA diagnosis, in 1000 | 98 | 93 | 96 | 102 | 103 | 106 | 112 | 117 | 120 | 138 | 141 | 143 | 144 | 143 |
| RA+DMARD, in 1000 | 41 | 41 | 44 | 48 | 52 | 55 | 59 | 63 | 66 | 68 | 78 | 81 | 83 | 85 |
| Prevalent ILD | 257 | 632 | 840 | 1033 | 1130 | 1186 | 1285 | 1351 | 1406 | 1463 | 1553 | 1548 | 1541 | 1484 |
| Incident ILD | 18 | 63 | 82 | 88 | 94 | 70 | 125 | 120 | 114 | 125 | 157 | 141 | 145 | 90 |
Required for inclusion: RA: 1 inpatient or ≥2 outpatient ICD-10 codes M05, M06 in the respective year AND a prescription of a DMARD (ever). Prevalent ILD in RA: 1 inpatient or ≥2 outpatient ICD-10 codes of J84.1, 8, 9; M05.1+J99.0 in the respective year+≥1 lung diagnostic procedure code (ever). Incident ILD in RA: no ILD diagnosis in all years prior to the index year.
DMARD, disease-modifying antirheumatic drug; ICD-10, International Statistical Classification of Diseases; ILD, interstitial lung disease; RA, rheumatoid arthritis.
Figure 1.
Flow chart for 2020. DMARD, disease-modifying antirheumatic drug; ICD-10, International Statistical Classification of Diseases; ILD, interstitial lung disease; RA, rheumatoid arthritis.
Characteristics of persons with RA and prevalent ILD
The mean age was 66±10 years in 2007 and increased by 6 years over time. In 2020, the proportion of people over 70 years of age was 59%. The proportion of women was ≈68%, and seropositive RA (ICD-10 M05) was coded in ≈44% in each of the years.
Comorbidity was present in the majority of persons, with hypertension being the most common additional disease (70%), followed by COPD (28%), diabetes (26%) and coronary heart disease (26%). In line with the age increase, the proportion of comorbidities increased over time.
Two-thirds of the persons had visited a rheumatologist during the selected time periods and 46% a pulmonologist. Outpatient X-rays were taken in 95% and outpatient CT in 75%. Half of the patients underwent bronchoscopy, slightly less in recent years, and 20% underwent biopsy. All data are reported in table 2.
Table 2.
Characteristics of persons with rheumatoid arthritis (RA) and prevalent interstitial lung disease from 2007 to 2020
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| N | 257 | 632 | 840 | 1033 | 1130 | 1186 | 1285 | 1351 | 1406 | 1463 | 1553 | 1548 | 1541 | 1484 |
| Age, mean, years (SD) | 66 (10) | 66 (10) | 67 (10) | 67 (10) | 67 (10) | 68 (10) | 69 (10) | 69 (10) | 70 (10) | 70 (10) | 71 (10) | 71 (10) | 72 (10) | 72 (10) |
| 31–50 years | 10 | 8 | 6 | 7 | 6 | 6 | 4 | 3 | 4 | 3 | 3 | 3 | 3 | 2 |
| 51–70 years | 55 | 57 | 57 | 53 | 50 | 48 | 45 | 44 | 42 | 42 | 41 | 41 | 40 | 39 |
| >70 years | 35 | 35 | 36 | 39 | 43 | 47 | 51 | 53 | 54 | 55 | 55 | 56 | 57 | 59 |
| Female | 67 | 69 | 68 | 68 | 70 | 69 | 68 | 69 | 68 | 67 | 68 | 68 | 69 | 69 |
| M05: seropositive RA | 46 | 42 | 43 | 42 | 42 | 43 | 43 | 42 | 43 | 45 | 46 | 47 | 48 | 45 |
| Comorbidities | ||||||||||||||
| Hypertension | 58 | 60 | 62 | 62 | 63 | 64 | 66 | 67 | 68 | 68 | 69 | 69 | 70 | 70 |
| Coronary heart disease | 19 | 20 | 21 | 19 | 20 | 20 | 22 | 23 | 24 | 25 | 26 | 26 | 26 | 26 |
| Pulmonary hypertension | 6 | 4 | 4 | 3 | 3 | 4 | 5 | 5 | 5 | 5 | 6 | 6 | 6 | 5 |
| Gastrointestinal reflux | 11 | 15 | 16 | 16 | 17 | 17 | 18 | 19 | 20 | 20 | 21 | 22 | 23 | 24 |
| COPD | 26 | 23 | 20 | 22 | 21 | 22 | 24 | 25 | 27 | 27 | 28 | 29 | 28 | 28 |
| Diabetes mellitus | 21 | 22 | 21 | 22 | 23 | 25 | 25 | 25 | 26 | 26 | 27 | 27 | 27 | 26 |
| Asthma | 13 | 12 | 12 | 13 | 13 | 13 | 14 | 15 | 16 | 17 | 18 | 20 | 19 | 19 |
| Specialised care | ||||||||||||||
| Rheumatology care | n.a. | 66 | 68 | 69 | 67 | 68 | 68 | 67 | 66 | 65 | 65 | 65 | 65 | 62 |
| Pulmonology care | n.a. | 39 | 46 | 42 | 40 | 40 | 43 | 44 | 46 | 47 | 50 | 49 | 53 | 53 |
| Outpatient lung diagnostics* | ||||||||||||||
| CT | 59 | 63 | 65 | 67 | 68 | 71 | 71 | 72 | 74 | 74 | 74 | 75 | 75 | 75 |
| Bronchoalveolar lavage | 6 | 5 | 6 | 6 | 6 | 6 | 6 | 5 | 5 | 6 | 5 | 5 | 5 | 5 |
| X-ray | 90 | 93 | 94 | 95 | 95 | 96 | 96 | 96 | 96 | 96 | 95 | 95 | 95 | 95 |
| Functional tests | 63 | 62 | 60 | 60 | 59 | 59 | 59 | 59 | 60 | 59 | 59 | 59 | 58 | 57 |
| Biopsy | 21 | 21 | 22 | 22 | 22 | 23 | 22 | 23 | 23 | 21 | 21 | 21 | 20 | 20 |
| Spirometry | 39 | 47 | 50 | 52 | 51 | 52 | 52 | 53 | 53 | 53 | 53 | 52 | 52 | 52 |
| Bronchoscopy | 61 | 53 | 53 | 53 | 53 | 52 | 52 | 53 | 52 | 51 | 50 | 49 | 48 | 48 |
Numbers are percentages unless otherwise indicated.
*Anytime in 2005–2020.
COPD, chronic obstructive pulmonary disease; n.a., not applicable.
Prevalence and incidence of ILD in persons with RA
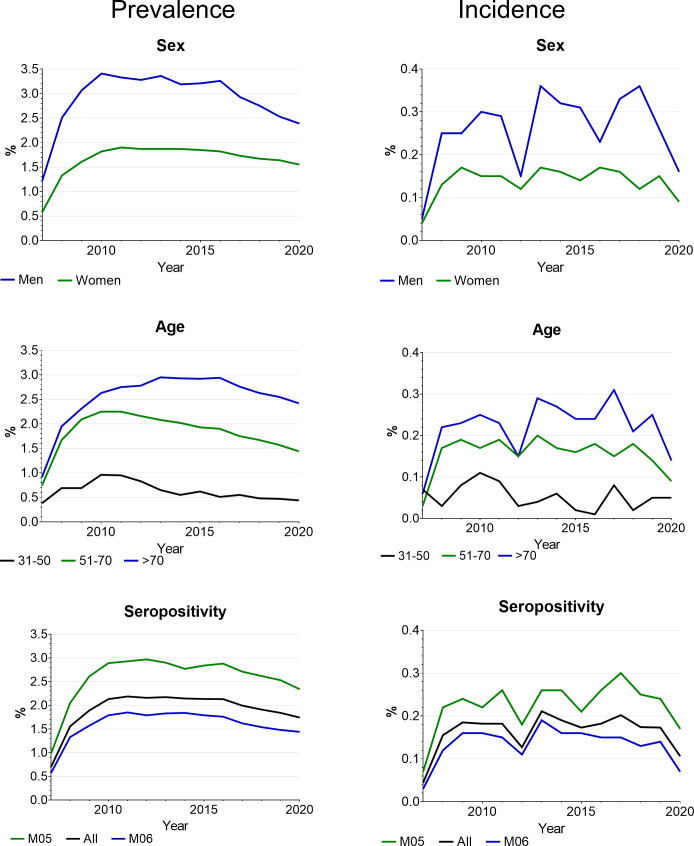
Prevalent ILD in persons with RA was between 1.6% in 2008 and 1.7% in 2020, with the highest proportion of 2.2% in 2011–2013 and a continuous slight decline until 2020 (figure 2). Low numbers in 2007 are due to the study design. RA-ILD was more frequent in seropositive RA (2.1%–3.0%) than in seronegative RA (1.3%–1.9%), in men (2.5%–3.4%) than in women (1.3%–1.9%) and in persons aged above 70 years (1.4%–2.3%) than in the younger persons. Prevalence was also higher in persons with b/tsDMARD therapy (2.5% in 2019 and 2.4% in 2020) than in persons without (1.7% in 2019 and 1.6% in 2020).
Figure 2.
Prevalence and incidence of RA-ILD by sex, age and seropositivity. ILD, interstitial lung disease; RA, rheumatoid arthritis.
Incident ILD was between 0.13% and 0.21% per year without a clear trend over time. It was also more frequent in persons with seropositive RA (in 2020: 0.17% in seropositive RA, 0.07% in seronegative RA), men (in 2020: 0.16% in men, 0.09% in women) and in the older age groups (in 2020: 0.14% in >70 years old, 0.09% in 51–70 years old). All data on incidence and prevalence are reported in online supplemental table 2.
Drug prescriptions
The majority of persons with RA-ILD received glucocorticoids with a decline over time (84% in 2007 to 68% in 2020). Among patients with glucocorticoid prescriptions, the DDDs declined over the years from 282 in 2007 to 196 in 2020 (table 3). The prescription of csDMARDs also decreased (83% to 55%) with MTX (46% to 33%) as the most common followed by leflunomide (22% to 10%) and hydroxychloroquine (12% to 8%). Mycophenolate and sulfasalazine were rarely used. The bDMARD prescriptions increased from 16% in 2007 to 24% in 2020 with tumour necrosis factor (TNF) inhibitors (15% to 11%) and abatacept (6%) as the most common. tsDMARDs (7% in 2020) and antifibrotics (3%) have emerged in the last years. Eleven per cent were prescribed a csDMARD and a bDMARD.
Table 3.
Proportion of persons (%) with drug treatment, 2007–2020
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| N | 257 | 632 | 840 | 1033 | 1130 | 1186 | 1285 | 1351 | 1406 | 1463 | 1553 | 1548 | 1541 | 1484 |
| Glucocorticoids | 84 | 79 | 79 | 77 | 79 | 76 | 80 | 78 | 77 | 76 | 76 | 74 | 71 | 68 |
| DDDs of persons with a glucocorticoid prescription, mean (SD) | 282 (244) | 254 (197) | 233 (167) | 237 (191) | 226 (182) | 211 (159) | 210 (153) | 220 (170) | 217 (166) | 211 (155) | 203 (148) | 204 (156) | 200 (155) | 196 (150) |
| Any csDMARD | 83 | 79 | 77 | 76 | 73 | 70 | 70 | 68 | 67 | 66 | 63 | 59 | 59 | 55 |
| Methotrexate | 39 | 46 | 45 | 44 | 43 | 40 | 40 | 40 | 38 | 37 | 36 | 35 | 35 | 33 |
| Leflunomide | 22 | 23 | 21 | 18 | 18 | 18 | 16 | 16 | 15 | 15 | 14 | 13 | 12 | 10 |
| HCQ | 12 | 10 | 10 | 10 | 10 | 11 | 11 | 11 | 11 | 11 | 11 | 10 | 8.6 | 8.2 |
| Mycophenolate | 3.5 | 1.6 | 1.7 | 1.0 | 0.8 | 0.6 | 0.8 | 0.9 | 1.0 | 1.0 | 0.6 | 1.0 | 1.4 | 2.1 |
| Sulfasalazine | 0.8 | 1.3 | 0.8 | 0.8 | 0.6 | 0.4 | 0.4 | 0.4 | 0.2 | 0.2 | 0.1 | 0.0 | 0.4 | 0.5 |
| Any bDMARD | 16 | 16 | 17 | 18 | 19 | 19 | 19 | 19 | 20 | 22 | 23 | 23 | 24 | 24 |
| TNF inhibitor | 15 | 14 | 15 | 14 | 15 | 15 | 13 | 12 | 13 | 13 | 13 | 12 | 11 | 11 |
| Abatacept | 0.3 | 0.8 | 1.1 | 0.8 | 1.2 | 2.3 | 2.1 | 1.9 | 3.0 | 3.9 | 4.5 | 5.5 | 5.5 | |
| Rituximab | 1.2 | 1.3 | 1.5 | 2.2 | 2.1 | 2.4 | 3.0 | 2.5 | 2.6 | 3.4 | 2.9 | 3.2 | 2.3 | 2.7 |
| Tocilizumab | 0.6 | 1.3 | 2.0 | 2.6 | 2.3 | 3.5 | 3.7 | 3.7 | 3.4 | 3.6 | 4.2 | 3.8 | ||
| JAK inhibitor | 2.1 | 4.0 | 5.6 | 6.5 | ||||||||||
| csDMARD+bDMARD | 11 | 10 | 10 | 12 | 12 | 11 | 10 | 10 | 11 | 11 | 11 | 10 | 11 | 11 |
| NSAIDs | 62 | 61 | 59 | 58 | 57 | 56 | 55 | 52 | 51 | 49 | 46 | 44 | 43 | 38 |
| Analgesics | 35 | 29 | 32 | 31 | 31 | 31 | 36 | 34 | 39 | 40 | 42 | 44 | 43 | 44 |
| Opioids | 35 | 30 | 29 | 29 | 29 | 30 | 31 | 28 | 28 | 30 | 32 | 31 | 30 | 30 |
| Nintedanib | 0.1 | 0.3 | 0.2 | 0.5 | 0.5 | 2.2 | ||||||||
| Pirfenidone | 0.1 | 0.3 | 0.3 | 0.4 | 0.4 | 0.2 | 0.3 | 0.2 | 0.3 | 0.4 |
cs/bDMARD, conventional synthetic/biological disease-modifying antirheumatic drug; DDDs, defined daily doses; HCQ, hydroxychloroquine; JAK, Janus kinase; NSAIDs, non-steroidal anti-inflammatory drugs; TNF, tumour necrosis factor.
There was a decline in the use of NSAIDs (62% to 38%) accompanied by an increase in other analgesics (35% to 44%), mostly metamizole. In all years, ≈30% of the persons received opioids. All data on medication are reported in table 3.
Compared with persons with RA without ILD, patients with ILD are less frequently prescribed MTX, more frequently glucocorticoids and bDMARDs, especially abatacept, rituximab, tocilizumab and also Janus kinase (JAK) inhibitors but not TNF inhibitors. Analgesics and opioids were also more frequently described in RA+ILD compared with persons with RA only (online supplemental table 3).
Specialist care
In 2020, of the 1484 people with RA and ILD, 379 (26%) visited a rheumatologist, 244 (16%) visited a pulmonologist, 540 (36%) visited both and 321 (22%) visited none of the specialists. Persons in general care were on average 3 years older (75 years) and less often female (68%) than persons in rheumatology care (72 years, 74% female). Persons in rheumatology care received csDMARDs (64% vs 40%), bDMARDs (31% vs 13%) and tsDMARDs (8% vs 3%) more often compared with persons without rheumatology care. Opioid use was most frequent in general care (39%), followed by rheumatology (32%) and pulmonology care (21%). Antifibrotics were rarely and almost only prescribed if a pulmonologist was involved in care (table 4).
Table 4.
Percentage of persons with prescribed drugs in 2020 by specialist care*
| Total | None | Rheumatology | Pulmonology | Both | |
| N | 1484 | 321 | 379 | 244 | 540 |
| Age, mean, years (SD) | 72 (10) | 75 (10) | 72 (10) | 71 (10) | 71 (10) |
| 31–50 (%) | 2.4 | 0.9 | 1.6 | 2.5 | 3.9 |
| 51–70 (%) | 39 | 33 | 37 | 45 | 40 |
| >70 (%) | 59 | 66 | 62 | 53 | 56 |
| Female (%) | 69 | 68 | 74 | 65 | 67 |
| Glucocorticoids | 68 | 58 | 70 | 66 | 74 |
| Any csDMARD | 55 | 40 | 64 | 43 | 64 |
| Methotrexate | 33 | 25 | 39 | 23 | 37 |
| Leflunomide | 10 | 5.0 | 14 | 7.8 | 12 |
| HCQ | 8.2 | 5.6 | 7.7 | 4.9 | 12 |
| Mycophenolate | 2.1 | 1.9 | 1.6 | 3.7 | 1.9 |
| Sulfasalazine | 0.5 | 0.0 | 1.3 | 0.4 | 0.2 |
| Any bDMARD | 24 | 13 | 31 | 18 | 29 |
| TNF inhibitor | 11 | 7.8 | 12 | 9.4 | 13 |
| Abatacept | 6 | 2.2 | 6.3 | 3.3 | 8.0 |
| Rituximab | 2.7 | 0.0 | 3.4 | 0.8 | 4.6 |
| Tocilizumab | 3.8 | 1.9 | 6.3 | 3.3 | 3.5 |
| JAK inhibitor | 6.5 | 3.4 | 8.4 | 2.9 | 8.7 |
| NSAIDs | 38 | 34 | 39 | 39 | 39 |
| Analgesics | 44 | 46 | 48 | 41 | 42 |
| Opioids | 30 | 39 | 32 | 21 | 26 |
| Nintedanib | 2.2 | 0.3 | 0.3 | 4.0 | 4.0 |
| Pirfenidone | 0.4 | 0.3 | 0.8 | 0.6 |
*At least one contact to the corresponding specialist in 2020.
cs/bDMARD, conventional synthetic/biological disease-modifying antirheumatic drug; HCQ, hydroxychloroquine; JAK, Janus kinase; NSAIDs, non-steroidal anti-inflammatory drugs; TNF, tumour necrosis factor.
Discussion
This study provides information on the occurrence and treatment of clinically significant RA-ILD in Germany over the last 13 years. While the yearly prevalence and incidence have not changed markedly, the range of therapies prescribed has enlarged in the more recent years. A high amount of prescribed pain medication and opioids indicates unmet needs in these mostly elderly patients.
With an observed prevalence of ~2% and incidence of ~0.2% per year, ILD remains a rare diagnosis in persons with RA. Similar to the Danish data,3 however, absolute numbers have increased as considerably more persons are diagnosed with RA today. ILD was more common in elderly people, especially over 70 years of age, as well as in men and in seropositive RA, which is in line with known risk factors for RA-ILD.2 19 Other comorbidities, especially hypertension, are common and related to the high age of the cohort. The frequent coexistence of COPD is reported from other studies6 20 and is discussed as an additional risk factor for ILD.20 However, misdiagnosis related to ILD or vice versa cannot be excluded without clinical validation.
Prevalence and incidence data appear to be relatively stable over the other years, and a slight decline in prevalence can be observed since 2013. The incidence and prevalence in 2007 are probably lower because of our inclusion criteria. One of the criteria is patients ever had a diagnostic measure or ever had a DMARD prescription. Those diagnosed in 2007 had the smallest amount of time to fulfil that criterion. As newer therapies with b/tsDMARDs can effectively suppress the disease activity of RA in the long term, there is some hope that ILD will develop less frequently. Whether this effect really occurs is still unclear. The relative number of patients with ILD within the RA cohort is not increasing, although there are more elderly people with RA today than 13 years ago. Increased prevalence rates over time as reported by Raimundo et al21 refer to the proportion of RA-ILD per 100 000 people and not to ILD within a population with RA. Data from the US Rochester cohort show unchanged incidence for the past six decades (until 2014) but improved survival of RA-ILD.8
In terms of therapy, more pronounced changes can be observed. Glucocorticoids, NSAIDs and csDMARDs have been prescribed less frequently over time, while b/tsDMARDs are used more often. The overall decrease in the use of glucocorticoids is encouraging. Glucocorticoids have been identified as a risk factor for ILD after accounting for disease activity.22 Both the number of patients with glucocorticoid and also the average dose in patients with glucocorticoids have decreased. Among the bDMARDs, all approved drugs are used. TNF inhibitors are prescribed slightly less frequently in the more recent years which may be due to their unclear risk–benefit ratio concerning ILD.10 Therapy with abatacept is slowly increasing. Current evidence indicates abatacept as a feasible treatment option for stabilising ILD in many and improving it in some patients11 23 24 with contradictory results on MTX co-medication.11 25 The comparison of the medication with patients with RA without ILD reflects quite well the tendency to increasingly prescribe abatacept, rituximab, tocilizumab or even JAK inhibitors instead of TNF inhibitors when ILD is present. Several cohort studies provide comparable encouraging results with rituximab26–28 as with abatacept. In a comparative analysis including all bDMARDs (TNF inhibitors, abatacept, rituximab and tocilizumab) as treatments in patients with RA-ILD, no differences were found with regard to hospitalisation rates in relation to the individual drug groups.29 Little data exist on tocilizumab12 and on JAK inhibitors in RA-ILD therapy30 31; thus, it is a little bit surprising that more patients are prescribed JAK inhibitors than rituximab in our data. One possible explanation could be the good manageability of JAK inhibitors due to their short half-life.
Since with nintedanib, antifibrotic therapy has just been approved for RA-ILD, the meaningfulness of the data is still limited.
Concerning pain management, NSAID use has decreased while analgesics and opioids are frequently prescribed to persons with RA-ILD. Their proportions exceed the use of opioids and analgesics in RA overall while NSAID use in patients with RA-ILD is equally frequent as in patients with RA only.
Considering specialist care, DMARDs are prescribed more often with rheumatological care while opioid use is highest in persons without specialist care. The higher proportion of elderly patients in general care points to the difficulty of specialist care with increasing age, and the high proportion of pain medication including opioids is a concern. Nevertheless, also one-third of patients in rheumatological care receive opioids. The proportion of patients on DMARD therapy and also on the individual DMARDs is comparable with the prevalent RA-ILD cases from US Medicare claims data, among whom as many as 63% have opioids.6 There is a high need for pain management in these patients, which, together with the continuing high, although declining, proportion of glucocorticoids, points to the need for more effective disease-modifying therapy.
The algorithm to identify ILD in RA is decisive for the output. Cho et al analysed US Medicare data showing that an algorithm with ≥2 ILD diagnosis codes by specialists provides the best positive predictive values (PPVs).32 If we require specialist contact for inclusion, many cases are lost that are relevant to the reality of care in the absence of specialist care. We have therefore decided to require a DMARD therapy for inclusion as RA-ILD case and indicated the specialist contact separately. Meehan et al showed that outpatient diagnosis with a CT diagnostic provides best PPVs.16 We decided to require a lung diagnostic, but not to limit it to a CT scan, because a CT scan may have been performed some time ago or may have been performed in an inpatient setting, which is not displayed in the data.
Limitations and strengths
Using national healthcare database to evaluate RA-ILD only allows us to evaluate clinically significant ILD and may not provide information about infraclinical ILD. The population-based claims data are valuable for the estimation of the frequency of the rare clinically significant RA-ILD and for its drug treatment as a large number of persons with RA are available. All diagnoses and prescriptions are included, irrespective of general or specialist care. We lack this information in our cohort studies. The use of painkillers and comorbidity are underestimated when only considering data from rheumatology care.33
Limitations are not clinically validated diagnoses; therefore, algorithms are necessary to increase the accuracy of diagnoses through medication or diagnostics. Specialist contact may be underestimated as specialists working in general practitioners’ offices or in outpatient settings within university hospitals are not recorded as such. Inpatient diagnostic procedures are not recorded as well. Seropositive RA may also be underestimated as ICD-10 coding in Germany is often unspecific.15 As women are over-represented in the BARMER statutory health insurance, but ILD is more common in men, this might lead to an underestimation of the number of persons with RA-ILD in this analysis.
It is not possible to identify all rheumatologists in the claims data. Rheumatologists who work in general practices, medical care facilities or even in university outpatient clinics are sometimes not billed under the rheumatology physician number. Therefore, we must assume under-reporting, so that the proportion of patients with RA-ILD without specialist care is probably smaller than reported. The same applies to the proportion of persons with an HRCT. Inpatient HRCT diagnostics and diagnostics prior to a change in the insurance are not included.
In summary, we identified ILD diagnosis in around 2% of persons with RA per year in the data of the BARMER statutory insurance. Our results show changes in the supply with medication with a shift from conventional glucocorticoid, NSAID and csDMARD therapy to modern treatment strategies including bDMARDs, tsDMARDs and also initial antifibrotic therapy. Our results show the need for specialist care to provide specific therapy and they point to the unmet needs regarding pain management.
Acknowledgments
The authors thank the BARMER for providing access to data via their data warehouse for this study. We thank the patient partners in the TARISMA Project for dedicating their time to add the patient view to this project.
Footnotes
Twitter: @callhoffj
Contributors: JC is responsible for the overall content as guarantor. KA, AS and JC conceived the idea for the article. UM and JC were involved in data acquisition. KA, AS and JC designed the study, planned analyses and interpreted the results. JC extracted data and performed analyses. KA wrote the first draft of the manuscript. All authors critically reviewed the manuscript and agreed with the submission. JC had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The study was supported by the Federal Ministry of Education and Research within the network TARISMA (01EC1902A).
Competing interests: JC received speaker fees from Janssen-Cilag. AS received speaker fees from lectures for AbbVie, Celltrion, MSD, Roche, BMS, Lilly and Pfizer, all unrelated to this manuscript. KA has no conflicts of interest. UM is an employee of the BARMER. There were no financial and personal relationships with other people or organisations that could inappropriately influence (bias) this work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study protocol was approved by the ethics committee of the Charité-Universitätsmedizin Berlin (EA2/233/22).
References
- 1.Hyldgaard C, Ellingsen T, Hilberg O, et al. Rheumatoid arthritis-associated interstitial lung disease: clinical characteristics and predictors of mortality. Respiration 2019;98:455–60. 10.1159/000502551 [DOI] [PubMed] [Google Scholar]
- 2.Spagnolo P, Lee JS, Sverzellati N, et al. The lung in rheumatoid arthritis: focus on interstitial lung disease. Arthritis Rheumatol 2018;70:1544–54. 10.1002/art.40574 [DOI] [PubMed] [Google Scholar]
- 3.Hyldgaard C, Hilberg O, Pedersen AB, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 2017;76:1700–6. 10.1136/annrheumdis-2017-211138 [DOI] [PubMed] [Google Scholar]
- 4.Fragoulis GE, Nikiphorou E, Larsen J, et al. Methotrexate-associated pneumonitis and rheumatoid arthritis-interstitial lung disease: current concepts for the diagnosis and treatment. Front Med (Lausanne) 2019;6:238. 10.3389/fmed.2019.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiely P, Busby AD, Nikiphorou E, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERas and ERAN inception cohorts. BMJ Open 2019;9:e028466. 10.1136/bmjopen-2018-028466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparks JA, Jin Y, Cho S-K, et al. Prevalence, incidence and cause-specific mortality of rheumatoid arthritis-associated interstitial lung disease among older rheumatoid arthritis patients. Rheumatology (Oxford) 2021;60:3689–98. 10.1093/rheumatology/keaa836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62:1583–91. 10.1002/art.27405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samhouri BF, Vassallo R, Achenbach SJ, et al. Incidence, risk factors, and mortality of clinical and subclinical rheumatoid arthritis-associated interstitial lung disease: a population-based cohort. Arthritis Care Res (Hoboken) 2022;74:2042–9. 10.1002/acr.24856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer A, Distler J. Progressive fibrosing interstitial lung disease associated with systemic autoimmune diseases. Clin Rheumatol 2019;38:2673–81. 10.1007/s10067-019-04720-0 [DOI] [PubMed] [Google Scholar]
- 10.Krüger K. Interstitial lung disease (ILD) -when and how to treat. Z Rheumatol 2020;79:780–1. 10.1007/s00393-020-00829-9 [DOI] [PubMed] [Google Scholar]
- 11.Tardella M, Di Carlo M, Carotti M, et al. Abatacept in rheumatoid arthritis-associated interstitial lung disease: short-term outcomes and predictors of progression. Clin Rheumatol 2021;40:4861–7. 10.1007/s10067-021-05854-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manfredi A, Cassone G, Furini F, et al. Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: a multicentre retrospective study. Intern Med J 2020;50:1085–90. 10.1111/imj.14670 [DOI] [PubMed] [Google Scholar]
- 13.Bendstrup E, Møller J, Kronborg-White S, et al. Interstitial lung disease in rheumatoid arthritis remains a challenge for clinicians. J Clin Med 2019;8:2038. 10.3390/jcm8122038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramien R, Rudi T, Schneider M, et al. OP0306 IMPACT of inflammation on interstitial lung disease in patients with rheumatoid arthritis - an analysis of the german biologics register rabbit. Ann Rheum Dis 2022;81:203. 10.1136/annrheumdis-2022-eular.1539 [DOI] [Google Scholar]
- 15.Callhoff J, Albrecht K, Marschall U, et al. Identification of rheumatoid arthritis in german claims data using different algorithms: validation by cross-sectional patient-reported survey data. Pharmacoepidemiol Drug Saf 2022; 10.1002/pds.5562 [DOI] [PubMed] [Google Scholar]
- 16.Meehan M, Shah A, Lobo J, et al. Validation of an algorithm to identify incident interstitial lung disease in patients with rheumatoid arthritis. Arthritis Res Ther 2022;24:2. 10.1186/s13075-021-02655-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organisation W . ATC-DDD toolkit. defined daily dose (DDD). 2022. Available: https://www.who.int/tools/atc-ddd-toolkit/about-ddd [Accessed 27 Sep 2022].
- 18.Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (record) statement. PLoS Med 2015;12:e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamora-Legoff JA, Krause ML, Crowson CS, et al. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology (Oxford) 2017;56:344–50. 10.1093/rheumatology/kew391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng B, Soares de Moura C, Machado M, et al. Association between chronic obstructive pulmonary disease, smoking, and interstitial lung disease onset in rheumatoid arthritis. Clin Exp Rheumatol 2022;40:1280–4. 10.55563/clinexprheumatol/i9au1r [DOI] [PubMed] [Google Scholar]
- 21.Raimundo K, Solomon JJ, Olson AL, et al. Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol 2019;46:360–9. 10.3899/jrheum.171315 [DOI] [PubMed] [Google Scholar]
- 22.Kronzer VL, Huang W, Dellaripa PF, et al. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J Rheumatol 2021;48:656–63. 10.3899/jrheum.200863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Díaz C, Castañeda S, Melero-González RB, et al. Abatacept in interstitial lung disease associated with rheumatoid arthritis: national multicenter study of 263 patients. Rheumatology (Oxford) 2020;59:3906–16. 10.1093/rheumatology/keaa621 [DOI] [PubMed] [Google Scholar]
- 24.Vicente-Rabaneda EF, Atienza-Mateo B, Blanco R, et al. Efficacy and safety of abatacept in interstitial lung disease of rheumatoid arthritis: a systematic literature review. Autoimmun Rev 2021;20:102830. 10.1016/j.autrev.2021.102830 [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Díaz C, Atienza-Mateo B, Castañeda S, et al. Abatacept in monotherapy vs combined in interstitial lung disease of rheumatoid arthritis-multicentre study of 263 caucasian patients. Rheumatology (Oxford) 2021;61:299–308. 10.1093/rheumatology/keab317 [DOI] [PubMed] [Google Scholar]
- 26.Md Yusof MY, Kabia A, Darby M, et al. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology (Oxford) 2017;56:1348–57. 10.1093/rheumatology/kex072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology (Oxford) 2019;58:2031–8. 10.1093/rheumatology/kez177 [DOI] [PubMed] [Google Scholar]
- 28.Vadillo C, Nieto MA, Romero-Bueno F, et al. Efficacy of rituximab in slowing down progression of rheumatoid arthritis-related interstitial lung disease: data from the NEREA registry. Rheumatology (Oxford) 2020;59:2099–108. 10.1093/rheumatology/kez673 [DOI] [PubMed] [Google Scholar]
- 29.Curtis JR, Sarsour K, Napalkov P, et al. Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor α agents, a retrospective cohort study. Arthritis Res Ther 2015;17:319. 10.1186/s13075-015-0835-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronin O, McKnight O, Keir L, et al. A retrospective comparison of respiratory events with JAK inhibitors or rituximab for rheumatoid arthritis in patients with pulmonary disease. Rheumatol Int 2021;41:921–8. 10.1007/s00296-021-04835-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tardella M, Di Carlo M, Carotti M, et al. A retrospective study of the efficacy of JAK inhibitors or abatacept on rheumatoid arthritis-interstitial lung disease. Inflammopharmacology 2022;30:705–12. 10.1007/s10787-022-00936-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho S-K, Doyle TJ, Lee H, et al. Validation of claims-based algorithms to identify interstitial lung disease in patients with rheumatoid arthritis. Semin Arthritis Rheum 2020;50:592–7. 10.1016/j.semarthrit.2020.04.006 [DOI] [PubMed] [Google Scholar]
- 33.Albrecht K, Marschall U, Callhoff J. Prescription of analgesics in patients with rheumatic diseases in germany: a claims data analysis. Z Rheumatol 2021;80:68–75. 10.1007/s00393-021-00971-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002777supp001.pdf (257.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.