Abstract
Background
Immune effector cell-associated neurotoxicity syndrome (ICANS) is a common adverse event of CD19-directed chimeric antigen receptor (CAR) T cell therapy. Other neurological adverse events, however, have not methodically been described and studied. Furthermore, safety data on CAR-T cell therapy in patients with central nervous system (CNS) lymphoma remain limited.
Main body
We here report occurrence of a Guillain-Barré-like syndrome (GBS) and central diabetes insipidus (cDI) following tisagenlecleucel therapy for relapsed high-grade lymphoma with CNS involvement. Both complications were refractory to standard treatment of ICANS. Weakness of respiratory muscles required mechanical ventilation and tracheostomy while cDI was treated with desmopressin substitution for several weeks. Muscle-nerve biopsy and nerve conduction studies confirmed an axonal pattern of nerve damage. T cell-rich infiltrates and detection of the CAR transgene in muscle-nerve sections imply a direct or indirect role of CAR-T cell-mediated inflammation. In line with current treatment guidelines for GBS, intravenous immunoglobulin was administered and gradual but incomplete recovery was observed over the course of several months.
Conclusions
This case report highlights the risk of rare but severe neurological adverse events, such as acute GBS or cDI, in patients treated with CAR-T cells. It further underlines the importance of appropriate patient surveillance and systematic reporting of rare complications to eventually improve treatment.
Keywords: lymphoma; receptors, chimeric antigen; case reports; hematologic neoplasms; immunotherapy
Introduction
CD19-directed chimeric antigen receptor T cells (CAR-T) have induced a paradigm shift in the treatment of large B-cell lymphoma by yielding event-free survival rates of up to 41%.1 2 However, adverse events such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are relevant causes of treatment-related morbidity. ICANS typically presents as diffuse encephalopathy, hallmarked by confusion, somnolence, apraxia, aphasia, motor weakness, and seizures.3 While the underlying pathophysiology is incompletely understood, disruption of the blood–brain barrier (BBB), bystander activation of immune cells, cytokine release as well as on-target off-tumor attack of CD19-expressing pericytes of the intracranial vasculature are discussed.4 Safety data on CAR-T cell therapy in patients with central nervous system (CNS) involvement are accumulating but still remain relatively sparse.5 We observed cDI and a Guillain-Barré-like syndrome (GBS) in a patient receiving tisagenlecleucel for treatment of relapsed high-grade lymphoma. Whereas cDI has not been described in this setting, only anecdotal evidence links peripheral neuropathies to CAR-T cells.6 7 This report highlights the potential of anti-CD19 CAR-T cells to cause rare complications in both, the central and the peripheral nervous system, emphasizing the need for improved understanding of the underlying pathophysiology and development of evidence-based treatment options.
Case description
A middle-aged patient was referred to our hospital for CAR-T cell therapy due to relapsed MYC- and BCL-6 rearranged high-grade B cell (‘double-hit’) lymphoma with CNS involvement after four lines of previous therapy (online supplemental table 1). At admission, complete remission (CR) was achieved with bridging therapy (lenalidomide and rituximab) as assessed by positron emission tomography-CT, cranial MRI, and analysis of cerebrospinal fluid (CSF). Prior to lymphodepletion, the patient presented in good clinical condition (ECOG 1). Pre-existing chemotherapy-induced sensory peripheral polyneuropathy was present with hyposensitivity and paresthesia of the distal limbs. Laboratory evaluation showed mild pancytopenia and slightly elevated lactate dehydrogenase (LDH) (online supplemental figure 1). Lymphodepletion was performed with fludarabine (25 mg/m2) and cyclophosphamide (250 mg/m2) (days -4 to -2) before tisagenlecleucel infusion (3.77×106 CAR+ T cells/kg body weight).
jitc-2022-006059supp001.pdf (25.6KB, pdf)
jitc-2022-006059supp002.pdf (649.4KB, pdf)
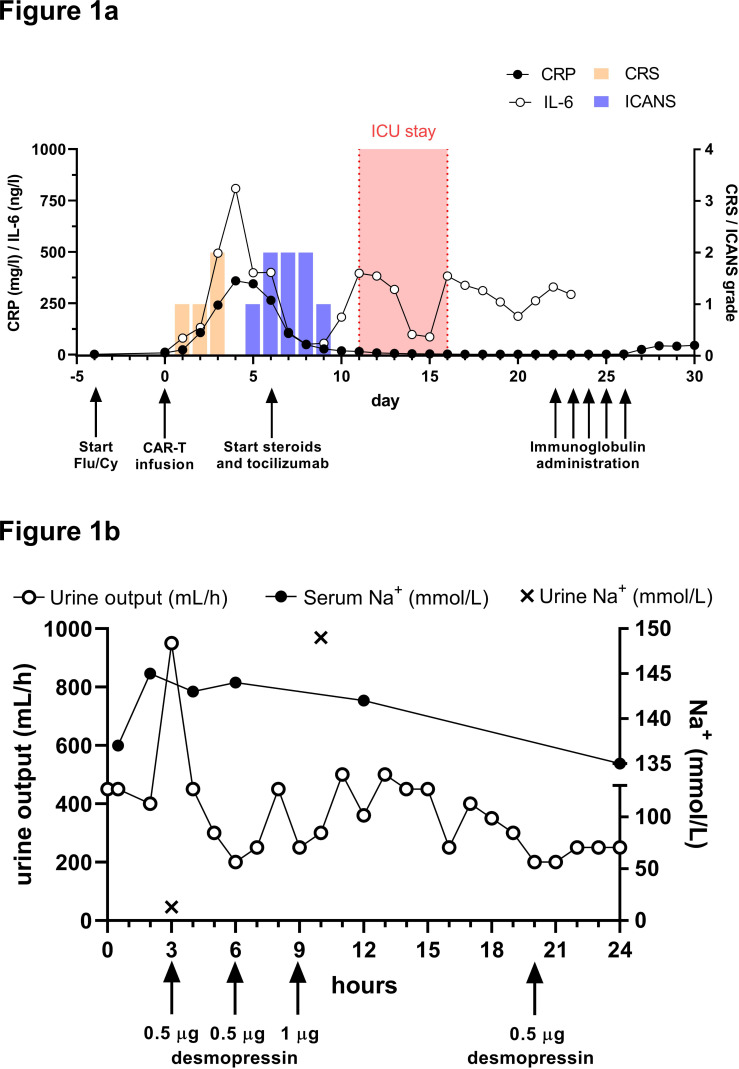
Within 24 hours after CAR-T infusion, the patient developed grade 1 CRS, which resolved under symptomatic treatment. Serum levels of C reactive protein (CRP) and interleukin-6 were elevated and peaked on day +4 (figure 1A). On day +5 and +6, peripheral facial nerve palsy (House-Brackmann grade IV) and discrete proximal motor weakness of the limbs were noticed. Simultaneously, the patient developed disorientation, consistent with grade 2 ICANS. Notably, no evidence of infection or lymphoma was present in CSF (day +14; online supplemental table 3). An MRI showed gadolinium enhancement of the right facial nerve and possibly cauda equina which is potentially suggestive of an immune-mediated etiology. Importantly, no ICANS-related imaging patterns such as signs of encephalitis, stroke or posterior reversible encephalopathy were present.8 Disorientation improved markedly under treatment with tocilizumab and dexamethasone starting from day +6. In contrast, neuromuscular weakness gradually deteriorated, resulting in incomplete quadriparesis as well as progressively impaired respiratory muscle function and upper airway reflexes. On day +11, the patient developed severe dyspnea that required emergency intubation and mechanical ventilation. Bronchoscopy did not reveal any cause of dyspnea other than a mucus plug in the airways.
Figure 1.
Clinical course and diagnosis of diabetes insipidus. (A) Timeline depicting clinical events, therapeutic interventions (black arrows), grading of CRS/ICANS and inflammatory markers from day −5 until day +30 after CAR-T cell infusion. Tocilizumab was administered once at a dose of 8 mg/kg body weight and dexamethasone initially at 8 mg every 6 hours. Intravenous immunoglobulin was administered at 2 g/kg body weight. CRS/ICANS scores were acquired according to ASTCT consensus criteria and determined under omission of GBS-related and DI-related symptoms. (B) Onset and course of central diabetes insipidus on day +11 was assessed by urine output (mL/hour) as well as urine and serum sodium concentration (mmol/L). Increased diuresis of hyponatriuric urine was reversed by repetitive administration of desmopressin (black arrows). ASTCT, American Society for Transplantation and Cellular Therapy; CAR, chimeric antigen receptor; CRP, C reactive protein; CRS, cytokine release syndrome; DI, diabetes insipidus; GBS, Guillain-Barré-like syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome.
Simultaneously, the patient developed polyuria with urine output of up to 950 mL/hour. Urine sodium concentration and osmolality were markedly decreased and, consistent with a diagnosis of cDI, the patient rapidly responded to treatment with desmopressin (figure 1B). Serum levels of thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, prolactin and human growth hormone were normal. The pituitary gland and pituitary stalk appeared normal on MRI 6 and 13 days after therapy, with no signs of inflammation or other morphological changes. Of interest, CAR-T cells were present in CSF, constituting 37% of a mild lymphocytic pleocytosis (6 cells/µL). Remarkably, the CAR-T cell fraction was >2 times higher in CSF compared with peripheral blood, suggesting disproportionate CAR-T cell expansion in the CNS. Concomitantly, mild elevation of protein (598 mg/L) and IL-6 (6.8 pg/mL) was noted in CSF.
Dilatative tracheostomy was required for weaning from mechanical ventilation before the patient was discharged from ICU with persistent peripheral muscle weakness and with continued desmopressin treatment (day +16). As GBS was suspected based on clinical presentation and nerve conduction studies (NCS), a treatment attempt with intravenous immunoglobulin (IVIG; 2 g/kg body weight) over 5 days was started (day +22). Peripheral muscle weakness recovered partially and desmopressin was successfully tapered before hospital discharge on day +56. Brain MRIs were performed 10 days before lymphodepletion as well as on days +6, +13, +19, +55 and +180 after CART cell infusion and revealed no relevant abnormalities, especially no new T2/FLAIR signal alterations in brain parenchyma or signs of demyelination. Prior to CAR-T cell therapy, remission of CNS manifestations was confirmed by absence of contrast enhancement and subtotal decrease of pre-existing leptomeningeal/pachymeningeal signal alterations. At follow-up after 9 months, the patient remained in CR with long-term persistence of CAR-T cells in the blood (0.4% CAR+ cells; absolute lymphocyte count 2.41 G/L) and motor function improved from wheelchair-bound to being able to walk without support.
Guillain-Barré-like syndrome
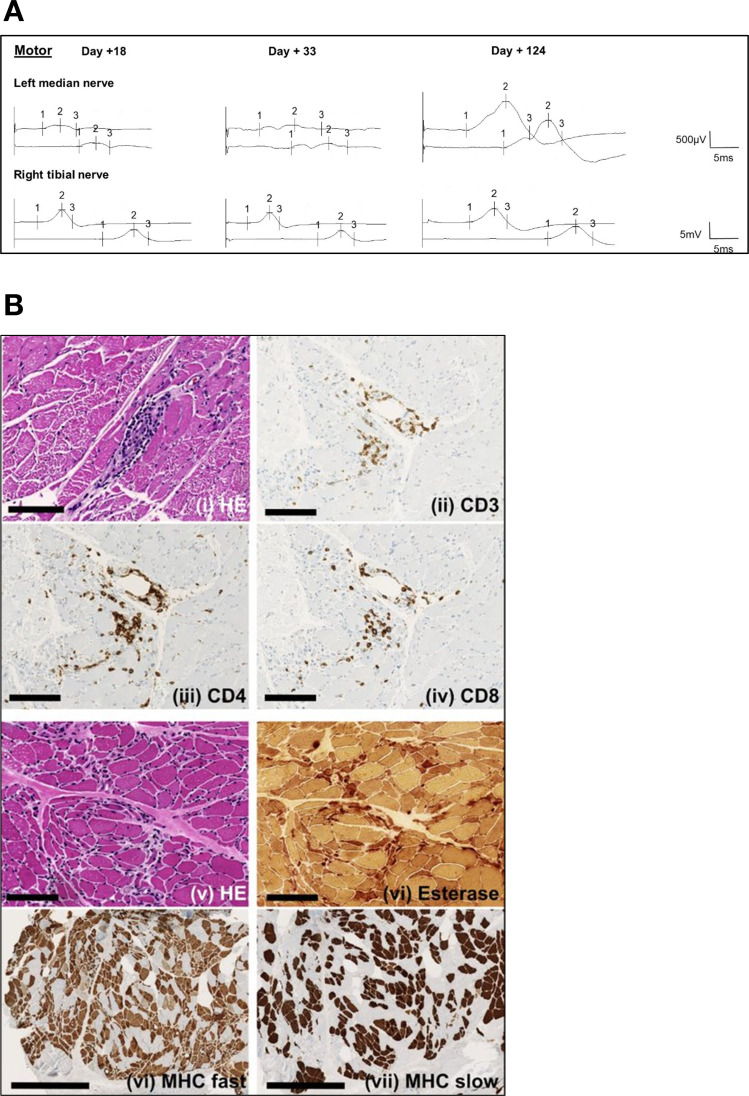
GBS comprises a group of immune-mediated acute peripheral polyradiculoneuropathies, which are classified into demyelinating and axonal disease variants.9 In this case, the clinical presentation with peripheral nerve palsy, sensory deficits/dysesthesias of the lower extremities and global hyporeflexia supported a diagnosis of a GBS-like condition. NCS demonstrated marked amplitude reductions of compound muscle action potentials while motor nerve conduction velocity and distal motor latency were only discretely reduced (figure 2A), consistent with an axonal damage-pattern. In line with clinical presentation, motor fibers were more affected than sensory fibers, confirming a diagnosis of acute motor and sensory axonal neuropathy (AMSAN). Histology of the gastrocnemius muscle (day +52) demonstrated perivascular T cell-dominant lymphocytic infiltrates and severe neurogenic damage of muscle fibers (figure 2B). The vector copy number of the CAR transgene was comparable in biopsy and peripheral blood (0.418% and 0.512%), indicating presence of CAR-T cells in muscle tissue. Anti-ganglioside and intrathecal antibody screening was negative and alternative causes for the observed neurological deficits were excluded by MRI and CSF analysis while myositis parameters were unremarkable.
Figure 2.
Hallmarks of the Guillain-Barré-like syndrome after CAR-T cell therapy. (A) Serial motor nerve conduction studies (NCS) of the left median and the right tibial nerve on day +18, +33 and +124 after CAR-T cell infusion. On day +18, drastic reduction of distal compound muscle action potentials was observed with only a modest reduction in conduction velocity, indicating an axonal damage pattern (left panel). Intravenous immunoglobulin therapy did not have immediate effects on NCS on day +33 (middle panel). After 3 months (day+ 124), signs of improvement in the upper extremities were evident (right panel). Sensory fibers were less affected in general (not shown). (B) Tissue sections of the gastrocnemius muscle were acquired on day +52 and demonstrated predominant perivascular immune cell infiltration (panels i-iv, H&E and IHC as indicated). Chronic neurogenic muscular atrophy with fiber type grouping and increase of endomysial and perimysial connective tissue was evident as a consequence of nerve damage (panels v–viii, H&E and IHC as indicated). Panels i-vi: scale bar = 100 µm. Panels vii + viii: scale bar = 500 µm. CAR, chimeric antigen receptor; IHC, immunohistochemistry; MHC, myosin heavy chain.
In summary, a severe variant of GBS, hallmarked by immune-mediated axonal nerve damage of peripheral nerve fibers, was diagnosed by clinical presentation, NCS and histopathology. While a trial of intravenous immunoglobulin failed to produce an immediate benefit, improvement of quadriparesis was observed clinically and in NCS from 2 months onwards (figure 2A).
Diabetes insipidus
cDI is caused by insufficient production of anti-diuretic hormone by hormone secreting cells in the hypothalamus, leading to excessive loss of water.10 Different pathomechanisms such as auto-immune processes or anatomical disruption of the hypothalamus–hypophysis axis can be causative, however, cDI mostly remains idiopathic. In our patient, we ruled out structural and functional causes for DI by serial MRI, electroencephalography, and unremarkable analysis of pituitary gland hormones. Therefore, a direct or indirect role of CAR-T cells is plausible. While direct attack of CAR-T cells against hypothalamic nuclei remains hypothetical, the pituitary gland is located outside of the BBB and might therefore be prone to immunological attack by CAR-T cells.
Discussion
Our patient developed an unusual pattern of deficits of the central and peripheral nervous system after tisagenlecleucel infusion, which was not compatible with ICANS and failed to resolve under established CRS/ICANS treatment.
In this report, we demonstrate that our patient’s acute-onset peripheral polyneuropathy is congruent with a GBS-like condition resembling AMSAN. In the absence of potential infectious stimuli, treatment with anti-CD19 CAR-T cells is the alleged immunostimulatory trigger. Potential differential diagnoses such as acute-onset chronic inflammatory demyelinating polyneuropathy (CIDP), critical illness polyneuropathy (CIP), critical-illness myopathy (CIM), paraneoplastic phenomena as well as the exacerbation of chemotherapy-induced polyneuropathy were considered but did not match the clinical presentation of our patient. More specifically, the clinical course with no neurological deterioration 8 weeks from onset, cranial nerve dysfunction and initial inability to walk independently contradict CIDP. The patient did not meet diagnostic criteria for CIP/CIM as he did not show systemic signs of critical illness at the time of transfer to the intensive care unit (ICU). Instead, the indication for ICU transfer was the progression of muscle weakness of peripheral and respiratory muscles, which was not related to the pre-existing chemotherapy-induced sensory polyneuropathy. Fludarabine is a known neurotoxic agent and can cause toxic leukoencephalopathy, especially at higher cumulative doses. Additionally, some cases have been reported after therapy with anti-CD19 CAR-T cells.11 This complication was ruled out in our patient by repetitive unremarkable MRI examinations. Although some case reports on peripheral neuropathy after administration of fludarabine have been published, the neurological safety profile of fludarabine in the context of adoptive engineered cell therapy is highly advantageous.12–14 Lenalidomid, an agent that is known to cause mild sensory polyneuropathy in long-term treatment, was given for a relatively short duration and at a low cumulative dose and was discontinued before lymphodepletion. In addition, development of GBS as a paraneoplastic phenomenon has been described in the context of lymphoma and after first-line chemotherapy, however, it is exceedingly rare.15 16 No other medication with high neurotoxic potential was administered (online supplemental table 3). Intrathecal chemotherapy (online supplemental table 1) was administered, however, without temporal correlation to symptom onset. Taken together, in the absence of an alternative explanation and in light of the co-occurrence of cDI and GBS at the time of peak CAR-T cell expansion, we identify GBS in our patient as complication of CD19-directed CAR-T cell therapy as previously anecdotally described.6 Importantly, GBS has already been reported as a complication after treatment with other novel immunotherapies such as checkpoint-inhibitors and TCR-engineered T cells.17–19
The pathophysiology of this complication remains to be resolved, however, direct attack of peripheral nerves, resulting from low-profile target-antigen expression or by cross-reactivity seems unlikely, since neither expression of CD19 nor cross-reactivity of the CD19 scFv to components of peripheral nerves have been reported. Therefore, an indirect damage pattern with bystander inflammation near peripheral nerves appears likely, especially given the perivascular infiltration of CD3+ lymphocytes (figure 2B). Single-cell RNA sequencing recently demonstrated CD19 expression on a subset of pericytes surrounding vessels of the BBB, hereby providing an explanation for neurotoxicity of CD19-directed immunotherapies.4 If pericytes of the blood–nerve barrier of peripheral nerves likewise express CD19 is currently unknown. A common reaction subsequent to several methodically different immunotherapies might favor an unspecific T cell-mediated immune-reaction over a specific, antigen-dependent pathomechanism. Still, a fludarabin-induced neurological side effect remains possible. In line with recommendations for GBS, we administered IVIG, which resulted in slow but clinically significant improvement.
DI has been observed as a complication of immunotherapy, for example, in the form of checkpoint-inhibitor-induced hypophysitis, but not following anti-CD19 CAR-T cell therapy.20 21 In our case, hypophysitis and locally infiltrating/compressing processes were absent. Furthermore, there were no signs of critical illness or systemic inflammation. While an etiological explanation remains hypothetical, a rare role of CAR-T cells as causative agents in the immune-mediated affection of hormone-producing cells of the hypothalamic nuclei is possible. As the changes were completely reversible, immune-mediated destruction of hormone-producing cells can be ruled out. Conceivably, structures outside the BBB, such as the pituitary gland, might be more accessible for CAR-T cells and therefore more prone to local inflammation resulting in disturbances of hormone homeostasis.
In summary, we here report the simultaneous occurrence of two rare adverse events after CAR-T cell therapy which concomitantly affected the central and peripheral nervous system. Subsuming the complications of our patient as a specific form of ICANS is not consistent with ICANS consensus definitions, especially as ICANS is characterized as a complication which exclusively affects the CNS.22 In our case, GBS and ICANS occurred simultaneously, however, they vastly differed in duration, severity and treatment response. This highlights the need for increased awareness for GBS-like conditions and cDI as potentially lethal complications of CAR-T cell therapy, especially as increasing numbers of patients with CNS lymphoma are being treated. Through the accumulation of clinical experience, we might be able to identify corresponding predisposing factors in preconditioning regimens, patients, and cell products, which eventually lead to better risk stratification and improved therapy of these complications.
Footnotes
Contributors: SU supervised treatment in the ICU, conceived the idea for the manuscript, edited and corrected the manuscript. CK, NFR,
JF and AMSM treated the patient and wrote the manuscript. TP, BS, KF and PR treated the patient and/or gave critical expert input and edited the manuscript. MGM revised the paper critically. All authors read and approved the final version of the manuscript for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AMSM received honoraria for advisory boards, consulting, and lectures/talks from Novartis, Gilead Sciences, Bristol Myers Squibb, and Janssen.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1. Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med 2022;386:640–54. 10.1056/NEJMoa2116133 [DOI] [PubMed] [Google Scholar]
- 2. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 3. Morris EC, Neelapu SS, Giavridis T, et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 2022;22:85–96. 10.1038/s41577-021-00547-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parker KR, Migliorini D, Perkey E, et al. Single-Cell analyses identify brain mural cells expressing CD19 as potential Off-Tumor targets for CAR-T immunotherapies. Cell 2020;183:126–42. 10.1016/j.cell.2020.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood 2019;134:860–6. 10.1182/blood.2019001694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuboki M, Umezawa Y, Motomura Y, et al. Severe motor weakness due to disturbance in peripheral nerves following Tisagenlecleucel treatment. In Vivo 2021;35:3407–11. 10.21873/invivo.12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov 2018;8:958–71. 10.1158/2159-8290.CD-17-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strati P, Nastoupil LJ, Westin J, et al. Clinical and radiologic correlates of neurotoxicity after axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv 2020;4:3943–51. 10.1182/bloodadvances.2020002228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol 2019;15:671–83. 10.1038/s41582-019-0250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primers 2019;5:54. 10.1038/s41572-019-0103-2 [DOI] [PubMed] [Google Scholar]
- 11. Nair R, Drillet G, Lhomme F, et al. Acute leucoencephalomyelopathy and quadriparesis after car T-cell therapy. Haematologica 2021;106:1504–6. 10.3324/haematol.2020.259952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheson BD, Vena DA, Foss FM, et al. Neurotoxicity of purine analogs: a review. J Clin Oncol 1994;12:2216–28. 10.1200/JCO.1994.12.10.2216 [DOI] [PubMed] [Google Scholar]
- 13. Johnson PW, Fearnley J, Domizio P, et al. Neurological illness following treatment with fludarabine. Br J Cancer 1994;70:966–8. 10.1038/bjc.1994.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lowe KL, Mackall CL, Norry E, et al. Fludarabine and neurotoxicity in engineered T-cell therapy. Gene Ther 2018;25:176–91. 10.1038/s41434-018-0019-6 [DOI] [PubMed] [Google Scholar]
- 15. Re D, Schwenk A, Hegener P, et al. Guillain-Barré syndrome in a patient with non-Hodgkin's lymphoma. Ann Oncol 2000;11:217–20. 10.1023/A:1008389607293 [DOI] [PubMed] [Google Scholar]
- 16. Bishay RH, Paton J, Abraham V. Variant Guillain-Barré syndrome in a patient with non-Hodgkin's lymphoma. Case Rep Hematol 2015;2015:979237. 10.1155/2015/979237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joseph J, Nathenson MJ, Trinh VA, et al. Guillain-Barre syndrome observed with adoptive transfer of lymphocytes genetically engineered with an NY-ESO-1 reactive T-cell receptor. J Immunother Cancer 2019;7:296. 10.1186/s40425-019-0759-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orcurto A, Hottinger A, Wolf B, et al. Guillain-Barré syndrome after adoptive cell therapy with tumor-infiltrating lymphocytes. J Immunother Cancer 2020;8:e001155. 10.1136/jitc-2020-001155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y, Zhang X, Zhao C. Guillain-Barré syndrome-like polyneuropathy associated with immune checkpoint inhibitors: a systematic review of 33 cases. Biomed Res Int 2021;2021:9800488. 10.1155/2021/9800488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao C, Tella SH, Del Rivero J, et al. Anti-Pd-L1 treatment induced central diabetes insipidus. J Clin Endocrinol Metab 2018;103:365–9. 10.1210/jc.2017-01905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu M, Liu L, Shi P, et al. Anti-PD-1 treatment-induced immediate central diabetes insipidus: a case report. Immunotherapy 2021;13:1255–60. 10.2217/imt-2020-0334 [DOI] [PubMed] [Google Scholar]
- 22. Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–38. 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006059supp001.pdf (25.6KB, pdf)
jitc-2022-006059supp002.pdf (649.4KB, pdf)