Abstract
Background and aims
Sex differences in internet gaming disorder (IGD) remain unknown. Investigating sex-specific neural features that underlie the core risk factor (i.e., risk-taking) of IGD would help in understanding sex-specific vulnerabilities to IGD and advance sex-specific treatments and prevention for IGD.
Methods
111 participants (28 IGD males, 27 IGD females, 26 recreational game user (RGU) males, 30 RGU females) completed a probability discounting task during fMRI scanning.
Results
First, among RGUs, males showed a higher risk-taking tendency and greater neural activation associated with risk/value evaluation for reward (the ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC), left putamen) and smaller activation associated with cognitive control (the inferior frontal gyrus) than females during the contrast of risky-safe choices. Moreover, males showed a greater modulatory effect of risky choices on the connection from the vmPFC/ACC to the left putamen than females. Second, IGD males showed decreased activation in the vmPFC/ACC and left putamen compared to RGU males, whereas this decrease did not exist in IGD females.
Discussion
Males show a higher risk-taking tendency than females. Altered neural substrates associated with risky decision-making exist in IGD males but not in IGD females.
Conclusions
The present findings fill the gap in information on the behavioral and neural substrates underlying IGD among females and demonstrate that a high risk-taking tendency is a risk factor and core symptom only in IGD males but not in IGD females. It is necessary to design and adopt distinct treatments and prevention strategies for IGD in males and females.
Keywords: internet gaming disorder, sex, risk-taking, fMRI, probability discounting
Introduction
Internet gaming disorder (IGD) is characterized by a loss of control over excessive internet game-playing behaviors with various functional impairments in daily life and is considered a behavioral addiction (American Psychiatric Association, 2013). Previous studies have reported that IGD is associated with poor academic/work performance, insomnia, strained social relationships, and even a high risk of psychiatric disorders (e.g., depression and anxiety) (F. W. Paulus, Ohmann, Von Gontard, & Popow, 2018). IGD has become a serious public concern worldwide; the global prevalence of IGD ranges from 0.7% to 15.6%, with an average of 4.7% (Feng, Ramo, Chan, & Bourgeois, 2017). Given this phenomenon, the fifth version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) included IGD as a tentative disorder (American Psychiatric Association, 2013) in 2013. The eleventh version of the International Classification of Diseases (ICD-11) officially included “gaming disorder” as a disorder in 2019 (https://icd.who.int/browse11/l-m/en). Profound insights into IGD and improvements to the treatment and prevention of IGD are urgently needed.
Sex is a basic factor that mediates human neurobiological traits as well as behaviors and emotions in response to social and environmental stimuli (Cahill, 2006). Males and females show diverse susceptibility to mental diseases, illnesses and stress. Numerous studies on drug and gambling addictions have demonstrated that males and females show different characteristics in the stages of addiction (e.g., development, consequences, relapse and even treatment) and distinct neurobiological mechanisms (Becker, Perry, & Westenbroek, 2012; Fattore, Melis, Fadda, & Fratta, 2014). For example, males show a higher prevalence of dependence on several drugs (e.g., cannabis and alcohol) and gambling than females do (Blanco, Hasin, Petry, Stinson, & Grant, 2006; Wagner & Anthony, 2007). Males mostly begin using drugs and gambling due to sensation seeking, whereas negative emotions (e.g., depression, loneliness) are the major cause of drug and gambling initiation in females (Becker, McClellan, & Reed, 2017; González-Ortega, Echeburúa, de Corral, & Polo-López, 2015). Moreover, sex differences exist in the neurobiological substrates that are central to addiction, including dopamine release and delivery in the striatum and neural response in the prefrontal and subcortical regions, which might result from sex hormones. For instance, males show greater dopamine release in the nucleus accumbens (NAcc, i.e., the ventral striatum) to amphetamine than females (Munro et al., 2006). Compared to females with cocaine dependence, males with cocaine dependence showed increased corticostriatal activation (e.g., the ventral striatum and anterior cingulate cortex (ACC)) when presented with cocaine cues (Potenza et al., 2012). Please see the previous reviews (Becker et al., 2012; Fattore et al., 2014) for more information.
Many studies have revealed that IGD and drug and gambling addictions share similar clinical symptoms and behavioral and neurobiological characteristics, such as a loss of control over the strong craving for addictive drugs/events, a high level of risk-taking, increased release of dopamine in the NAcc, and altered neural features in the corticostriatal regions (Dong, Lin, Hu, Xie, & Du, 2015; Fattore et al., 2014; Fauth-Bühler & Mann, 2017; Legault, Liu, & Balodis, 2021). It is therefore reasonable to assume that sex differences exist in IGD-related behavioral and neurobiological characteristics. A few studies have demonstrated sex differences in IGD that are similar to differences in drug and gambling addictions. Males were found to have a higher prevalence rate of IGD than females (Borgonovi, 2016; L. Li, Yu, Zhang, & Jin, 2015). One questionnaire study found that male adolescents with higher reward sensitivity had a high risk of developing internet addiction (IA) (including IGD), whereas female adolescents with higher punishment sensitivity had a high risk of IA, indicating sex differences in the risk factors for developing IA (Q. Li et al., 2019). Using functional magnetic resonance imaging (fMRI), researchers found that when faced with gaming-related pictures, IGD males showed increased neural activation in the striatum compared with IGD females (Dong, Wang, Du, & Potenza, 2018). Moreover, IGD males showed decreased neural activation in the dorsolateral prefrontal cortex (DLPFC) relative to healthy control (HC) males, whereas there was no significant difference between IGD females and HC females; regardless of IGD or HC, males showed decreased DLPFC activation compared with females (Dong, Zheng et al., 2018).
Although these studies demonstrated sex differences in the risk factors and neural responses to gaming cues in IGD, the understanding of IGD females and sex differences in the behavioral and neurobiological characteristics of IGD remain unclear, such as sex differences in the typical high level of risk-taking or low level of control ability among individuals with IGD. Importantly, examining sex differences in IGD can advance sex-specific prevention and treatment for IGD. Given the many sex differences in addictions presented above, it is not surprising that males and females respond differently to the same treatments. A previous study showed that the same treatment worked differently for males and females with cocaine addiction (H. C. Fox, Morgan, & Sinha, 2014). Studies have documented many barriers for females in traditional treatments, and treatments tailored to females were likely to be more successful (Campbell & Ettorre, 2011). It is crucial to develop sex-specific prevention and treatment for addictions, including IGD. To this end, the need to investigate the behavioral and neural factors related to IGD in a sex-informed fashion is urgent.
Risk-taking is the tendency to engage in behaviors under uncertain situations despite potential negative outcomes (e.g., excessive game-playing) (Kreek, Nielsen, Butelman, & LaForge, 2005), which is crucial to the development and maintenance of addiction. A high level of risk-taking is one of the core features of addictive disorders, including IGD (Dong & Potenza, 2016; Kreek et al., 2005; Spurrier & Blaszczynski, 2014). Researchers have reported that individuals with a high level of sensation-seeking trait (i.e., greater desire for risk-taking) are more likely to develop IGD (Chiu, Lee, & Huang, 2004; Mehroof & Griffiths, 2010). During risky decision-making tasks, individuals with IGD chose more options with high risk and uncertainty than HCs, meanwhile, they showed altered neural responses in the classic reward circuit (i.e., the corticostriatal neural circuit) during risky choices (relative to safe choices), including reduced activation in the ACC, ventromedial prefrontal cortex (vmPFC)) (Dong & Potenza, 2016), inferior frontal gyrus (IFG)) (Lee, Lee, Yoon, Kee, & Jung, 2016; Lin, Zhou, Dong, & Du, 2015), and enhanced activation in the striatum (L. Wang et al., 2021), indicating maladaptive elevated risk-taking (i.e., reduced risk sensitivity) in IGD. However, these studies involved IGD samples with only males or mixed males and females. Whether reduced risk sensitivity and altered neural substrates can be extended to females with IGD or whether females and males with IGD differ in risk-taking remains unknown. This information is important for the sex-specific treatment and prevention of IGD. Thus, this study was set to investigate sex differences in risk-taking and its neural substrates among individuals with IGD.
First, we aimed to investigate sex differences in risk-taking, thus, only cisgender participants were recruited to exclude the potential effect of differences in gender identify between cisgender and transgender and other gender groups on the results. Second, for the control group, we aimed to recruit recreational game users (RGUs). RGUs refers to gamers who play internet games recreationally without developing addiction (Viriyavejakul, 2008). Their similar gaming experiences to IGD gamers can exclude the effect of different gaming experiences of IGD and control groups on the results (Dong, Li, Wang, & Potenza, 2017; L. Wang et al., 2017). Third, the probability discounting (PD) task, which is widely used when measuring individual risk-taking behavior, was employed in the present study. Probability discounting refers to the phenomenon in which the subjective value of one reward decreases as the odds against receiving it increase (B. J. Weber & Huettel, 2008). In this task, participants are asked to make decisions between a certain smaller gain (safe option) and a probabilistic larger gain (risky option), such as $10 at 100% vs. $20 at 50%. Brain regions in the corticostriatal circuit, including the striatum (the NAcc, caudate and putamen), vmPFC, ACC, IFG and DLPFC, are commonly activated during PD or risky decision-making tasks (M. X. Cohen & Ranganath, 2005; Mohr, Biele, & Heekeren, 2010; Peters & Büchel, 2009; B. J. Weber & Huettel, 2008). Using the PD task, researchers found a lower probability discounting rate (elevated risk-taking propensity) in IGD subjects than controls (L. Wang et al., 2016), however, there are studies showing no differences in probability discounting rate between IGD subjects and controls (Q. Li et al., 2016; Tian et al., 2018). Furthermore, IGD subjects showed enhanced neural response in the striatum and IFG during risky choices in the PD task (Z. Wang et al., 2019). However, there are studies showing reduced neural response in the IFG (Lin et al., 2015; L. Wang et al., 2016). Together with the findings in previous studies as stated above, the direction of group differences in neural responses and whether the groups difference (IGD vs. RGU) exists in behavioral performance cannot be concluded. Thus, we only hypothesized that compared to RGUs, participants with IGD would exhibit altered activation during risky choices relative to safe choices in these regions of the corticostriatal circuit, such as the ACC, vmPFC, IFG, DLPFC and striatal regions.
In addition, a growing number of studies have found altered resting-state functional connectivity (FC) in the corticostriatal circuit in IGD, such as, decreased FC between the ACC and putamen (Jin et al., 2016), decreased FC between the mPFC and putamen (Lee, Namkoong, Lee, & Jung, 2021) and increased FC between the putamen and middle frontal gyrus (Dong et al., 2021) in IGD subjects relative to HCs, which revealed typical neural features in IGD. Based on these findings, we wondered whether the connectivity among the corticostriatal circuit during the present risk-taking task (PD) would be altered in IGD and its sex differences. Dynamic causal modeling (DCM) is a prominent analytical framework for deducing effective connectivity that depicts the causal effect among the activities of brain regions (i.e., the modulation effect of the activity of one brain region on that of another) during experimental tasks (Friston, Harrison, & Penny, 2003). Therefore, the second aim of the present study was to further use DCM analysis to examine the group difference (IGD vs. RGU) in the effective connectivity among the corticostriatal circuit during risky choices in the PD task and its sex difference. Specifically, the regions in the corticostriatal circuit that show a significant group-by-sex interaction effect during the PD task were selected as the regions of interest (ROIs) in the DCM analysis. We hypothesized that participants with IGD would show altered connectivity among the ROIs compared with RGUs during risky choices.
With regard to sex differences in risk-taking, previous studies have demonstrated that males are more likely than females to take risks (Byrnes, Miller, & Schafer, 1999; Harris & Jenkins, 2006). Considering previous findings from questionnaire studies that high sensation seeking/reward sensitivity is the main risk factor for males to begin drug use, gambling, or playing games and develop these into addictions whereas high emotional issues and punishment sensitivity are the main risk factors for females, we hypothesized that altered neural activation and connectivity to risky choices would exist only in the male IGD group but not in the female IGD group. For the RGU population, given that there is no study explicitly examining the risk-taking and neural response under the PD task among male and female gamers, we only hypothesized that males would exhibit different risk-taking, neural activation and effective connectivity in the corticostriatal regions (e.g., the ACC, vmPFC, IFG and striatal regions) to risky choices than females. Note that the directions of the group and sex differences in behavioral performance and neural responses cannot be concluded based on previous findings, two-tailed tests were planned for all the hypotheses in the present study.
Methods
Participants
According to a previous study (Thirion et al., 2007), a sample size of >20 for each group is necessary to have sufficient and acceptable reliability for fMRI studies, and a sample size of 27 is preferable. To satisfy this requirement, 111 cisgender university students from local universities in Shanghai, China, were recruited to participate in this study, including 28 IGD males, 27 IGD females, 26 RGU males and 30 RGU females. The numbers of males and females were balanced between the IGD and RGU groups (χ 2 = 0.223, P = 0.637, φ (effect size) = 0.045). All participants were free of any psychiatric/neurological disorders and other addictive disorders, including gambling, alcohol, nicotine and illegal drug dependence, and no participants took medications that affected sex hormones. Young's Internet Addiction Test (IAT) (Young, 1998) and the DSM-5 criteria for IGD were applied in this study to diagnose IGD participants. The IAT has been proven to be reliable and valid in screening for internet addiction (Lai et al., 2013; Pawlikowski, Altstötter-Gleich, & Brand, 2013; Widyanto & Mcmurran, 2004) and has been widely used in previous studies on internet addiction and IGD (Cai et al., 2016; Dong, Liu, Zheng, Du, & Potenza, 2019; L. Wang et al., 2017; L. Wang, Zhang, et al., 2018). It includes 20 items scored on a 5-point scale (1-rarely, 5-always) and measures the degree of problems related to internet use, such as compulsive use, withdrawal symptoms, and problems related to time management, family relationships, sleep and school/work. Scores over 50 indicate frequent or occasional problems related to internet use due to undisciplined internet use. In line with previous studies on IGD, 50 was used as the cutoff score to categorize IGD and RGU participants in the present study. The DSM-5 criteria contain nine items; individuals who meet five or more criteria are diagnosed with IGD (Petry et al., 2014). In sum, gamers who met both criteria were included as the IGD group: (1) IAT scores above 50; (2) five or more DSM-5 criteria met. Gamers who did not meet these criteria for IGD were included as the RGU group (Dong et al., 2021). In the present study, the IGD and RGU groups were matched with age and gaming time. The IGD group reported significantly higher IAT and DSM-5 scores than the RGU group. There are no significant differences in gaming time, IAT and DSM scores between males and females among each of the two groups (IGD and RGU) (Table 1).
Table 1.
Demographic information of the two groups (IGD and RGU)
| IGD | RGU | F | P | Cohen's f | |||
| Male, N = 28 | Female, N = 27 | Male, N = 26 | Female, N = 30 | ||||
| Age (years) | 22.11 ± 2.47 | 21.31 ± 2.03 | 21.50 ± 2.61 | 21.00 ± 1.92 | 1.22 | 0.307 | 0.185 |
| Gaming time (months) | 22.27 ± 2.95 | 22.00 ± 5.20 | 23.19 ± 1.05 | 20.10 ± 6.11 | 2.51 | 0.063 | 0.266 |
| Gaming time (hours) | 15.07 ± 8.89 | 14.48 ± 10.57 | 11.52 ± 5.69 | 11.27 ± 6.99 | 1.60 | 0.194 | 0.212 |
| IAT scores | 67.04 ± 10.49 | 63.70 ± 7.25 | 43.58 ± 7.82 | 47.47 ± 11.35 | 41.52 | <0.001 | 1.079 |
| DSM-V scores | 6.20 ± 0.87 | 5.96 ± 0.98 | 2.47 ± 1.18 | 2.73 ± 1.14 | 101.61 | <0.001 | 1.687 |
Table values: mean ± standard deviation.
Abbreviations: IGD = internet gaming disorder; RGU = recreational gaming user; IAT = Internet Addiction Test; DSM-V = The fifth version of the Diagnostic and Statistical Manual of Mental Disorders.
Measures
The timeline of one trial in the PD task is depicted in Fig. 1A. Before the task, all participants were instructed, “Please make decisions between a fixed amount of money and a probabilistic but larger amount of money (button 1—left, button 2—right), such as 10 yuan at 100% vs. 33 yuan at 50%. After the experiment, you will be paid according to the decision in a randomly selected trial of the task. Every decision is important for the final reward.” If the subject chose the fixed money in that trial, he or she would receive the money in cash. If the subject chose the probabilistic money, they would select a card from many cards with two colors (red and black) reflecting the probability of receiving the money in that trial. In this task, first, a fixation was presented for 500 ms, and then two options with one fixed amount of money on the left and one probabilistic but larger amount of money on the right were presented. The participants needed to make decision during 4,000 ms. The position of the fixed and probabilistic options remained unchanged during the task. The amount of the probabilistic option varied randomly among 11, 12.5, 14, 17, 20, 25, 33, 50, and 100 yuan. The probability of the probabilistic option varied randomly among 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, and 100%. Once the participants make decision, the screen turned black for a (4000-reaction time (RT)) ms and 1,000–3,500 ms jitter. Then, the next trial started. The formal PD task included 81 trials. All participants completed it under fMRI scanning, and every participant was subsequently paid an extra 60 yuan.
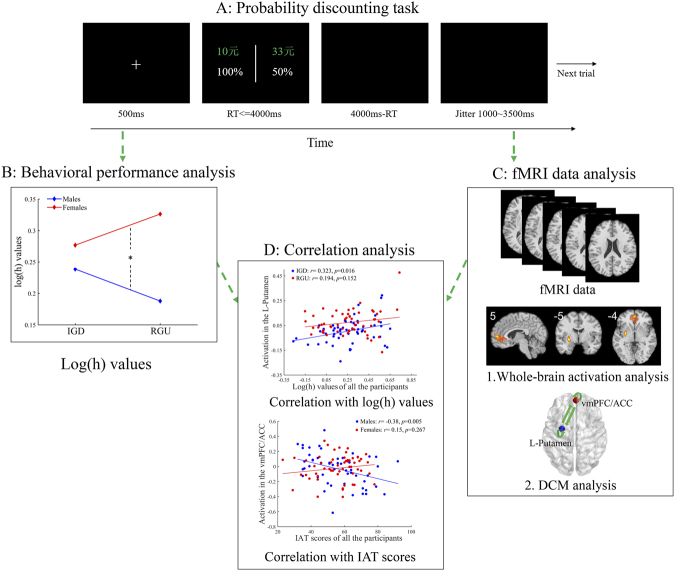
Fig. 1.
The probability discounting task and data analysis
A: The timeline of one trial in the probability discounting task. “元” represents Chinese currency. 1元 is equal to approximately $0.16. B: Behavioral performance analysis depicts the difference in log(h) values (i.e., probability discounting rates) among sexes (males and females) and groups (IGD and RGU). C: fMRI data analysis depicts the difference in whole-brain activation and effective functional connectivity between sexes (males and females) and groups (IGD and RGU). Specifically, whole-brain activation analysis depicts whole-brain activation associated with risk taking. DCM analysis depicts the modulatory effect of risky choices on the connections between and within ROIs. D: Correlation analysis depicts the correlation between brain activation and log(h) values and IAT scores. Abbreviations: RT = reaction time; IGD = internet gaming disorder; RGU = recreational game user; IAT = Internet Addiction Test; DCM = dynamic causal modeling; L = left; vmPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex.
Procedure
All participants in the present study were recruited through online advertising. First, the participants were screened face to face, which took approximately one hour for each participant and included 1) collecting the participants' demographic information (age, sex, gender identity) to identify cisgender males and females. The sex and gender identity were measured using a two-step self-reported paper-pencil questionnaire (Grant et al., 2010; Reisner et al., 2014; Sausa, Sevelius, Keatley, Iñiguez, & Reyes, 2009). First: “What sex were you assigned at birth, on your original birth certificate? (check one)” with response options “Female” and “Male”. Second: “Which best describes your current gender identity?” with response options “Female”, “Male”, “Transgender”, and “other”. Participants who were males at birth and selected “Male” on the second question were identified as cisgender males. Participants who were females at birth and selected “Female” on the second question were identified as cisgender females. Others who did not match these two types were excluded in this study; 2) confirming they were free of any current or previous psychiatric/neurological disorders (using structured psychiatric interviews) and other addictive disorders (including gambling, alcohol, nicotine and illegal drug dependence), by an experienced psychiatrist; and 3) assessing their gaming behaviors/symptoms (gaming time, IAT and DSM criteria) and classifying them into the IGD and RGU groups during the clinical interviews by an experienced psychiatrist. Second, fMRI scanning was conducted. All participants were asked not to drink tea or coffee on the day of the scanning. Before the formal fMRI scanning, participants completed a practice version with 5 trials of the PD task and the safety screening scale for fMRI scanning, which took approximately 10 min. The formal PD task took 15 min. Third, the participants were paid 0–100 yuan according to their choice in a random trial of the PD task and 60 yuan for completing the fMRI scanning.
Statistical analysis
Behavioral data analysis
The subjective value of one reward decreases as the odds against receiving it increase (i.e., the probability of receiving it decreases), which is defined as probability discounting. The discounting rate for probabilistic reward is depicted by a hyperbolic function:
| (1) |
where V represents the subjective value of an option (e.g., one monetary reward) and A represents the amount of the probabilistic reward. P represents the probability of receiving the reward, and O represents the odds against receiving the reward; h is the individual-specific discounting rate for probabilistic reward. Smaller h values reflect more risk-taking, whereas larger h values reflect less risk-taking.
The calculation of h values was based on the parameter estimation procedure (Kirby & Maraković, 1996), which has been widely used in previous studies to calculate delay/probability discounting (Amlung & MacKillop, 2014; Gilman, Curran, Calderon, Stoeckel, & Evins, 2014; Kirby, Petry, & Bickel, 1999; Liu et al., 2016). The calculation of h values is presented in detail as follows:
First, all trials in the PD task were categorized into 9 parts according to the amount of the probabilistic monetary reward (11, 12.5, 14, 17, 20, 25, 33, 50, and 100 yuan). Each part had 9 trials with different probabilities of receiving the reward (10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, and 100%), as shown in Table 2. Second, Table 2A shows the regular calculation of the h value, i.e., the participants' decisions followed a single discounting rate, switching their decisions at one trial. Table 2B shows the calculation of the h value for special situations where the participants' decisions did not perfectly follow a single discounting rate.
Table 2A.
An example for the calculation of h values
| Order | Choice | Decisions | Predicted h values | Actual h value |
| 1 | (1) 10 Yuan 100% vs. (2) 14 Yuan 90% | 2 | 3.60 | |
| 2 | (1) 10 Yuan 100% vs. (2) 14 Yuan 80% | 2 | 1.60 | |
| 3 | (1) 10 Yuan 100% vs. (2) 14 Yuan 70% | 2 | 0.93 | 0.75 |
| 4 | (1) 10 Yuan 100% vs. (2) 14 Yuan 60% | 1 | 0.60 | |
| 5 | (1) 10 Yuan 100% vs. (2) 14 Yuan 50% | 1 | 0.40 | |
| 6 | (1) 10 Yuan 100% vs. (2) 14 Yuan 40% | 1 | 0.27 | |
| 7 | (1) 10 Yuan 100% vs. (2) 14 Yuan 30% | 1 | 0.17 | |
| 8 | (1) 10 Yuan 100% vs. (2) 14 Yuan 20% | 1 | 0.10 | |
| 9 | (1) 10 Yuan 100% vs. (2) 14 Yuan 10% | 1 | 0.04 |
Table Legend: Shown are the nine trials with 14 Yuan and varied probabilities of receiving. One participant chose the probabilistic option in the top three trials and chose the certain option (i.e., 10 Yuan) from the fourth trial, thus, the h value for the current amount of probabilistic reward was 0.75, i.e., the geometric mean of the bounded ranges between 0.93 and 0.60.
Table 2B.
An example for the calculation of h values by consistency
| Order | Choice | Decisions | Predicted h values | %Consistency | Actual h value(s) |
| 44% | |||||
| 1 | (1)10 Yuan 100% vs. (2) 17 Yuan 90% | 2 | 6.30 | ||
| 56% | |||||
| 2 | (1) 10 Yuan 100% vs. (2) 17 Yuan 80% | 2 | 2.80 | ||
| 67% | |||||
| 3 | (1) 10 Yuan 100% vs. (2) 17 Yuan 70% | 2 | 1.63 | ||
| 78% | |||||
| 4 | (1) 10 Yuan 100% vs. (2) 17 Yuan 60% | 2 | 1.05 | ||
| 89% | 0.86 | ||||
| 5 | (1) 10 Yuan 100% vs. (2) 17 Yuan 50% | 1 | 0.70 | ||
| 78% | |||||
| 6 | (1) 10 Yuan 100% vs. (2) 17 Yuan 40% | 2 | 0.47 | ||
| 89% | 0.37 | ||||
| 7 | (1) 10 Yuan 100% vs. (2) 17 Yuan 30% | 1 | 0.30 | ||
| 78% | |||||
| 8 | (1) 10 Yuan 100% vs. (2) 17 Yuan 20% | 1 | 0.18 | ||
| 67% | |||||
| 9 | (1) 10 Yuan 100% vs. (2) 17 Yuan 10% | 1 | 0.08 | ||
| 56% | |||||
| Geometric mean: 0.57 | |||||
Table Legend: Shown are the nine trials with 17 Yuan and varied probabilities of receiving. Assuming that the h value was between 1.05 and 0.70, the participant should have chosen the probabilistic option in the top four and the certain option from the fifth trial. However, in the sixth trial, the participant chose the probabilistic option. Thus, the consistency for this range was (4+4)/9*100 = 89%. The consistencies of the 10 bounded ranges of h values (including unbounded) were calculated and are shown in Table 2B.
Table 2A—Regular calculation of h values: 1) for one amount of a probabilistic reward, the h value of each of the 9 trials was calculated according to Eq. (1) stated above, generating 9 predicted h values. The 9 choice trials defined 8 bounded ranges of h values. 2) The geometric mean of the bounded range between the two trials where participants switched their decisions was the h value for the current amount of the probabilistic reward. (The geometric mean was used here to avoid underweighting the smaller/larger of the two values of the bounded range. As Eq. (1) depicts, the h values follow a hyperbolic function, i.e., the h values decrease steeply at large probability and decrease gradually at small probability, which can be inferred from Table 2. Considering that the arithmetic mean is susceptible to extreme values, it is more representative to use the geometric mean rather than the arithmetic mean.)
Table 2B—Calculation of the h value for special situations: 1) the predicted h values were calculated for each trial as stated above. The 9 choice trials defined 10 bounded ranges of h values (including unbounded). 2) The consistency was calculated for each bounded range. Consistency is the proportion of decisions that are consistent with the bounded range of predicted h values (please see the legend for Table 2B for an example of the calculation of consistency). The geometric mean of the bounded range with the highest consistency among the 10 consistencies was the h value for the present amount of the probabilistic reward. In rare cases for which two or more ranges generated equally high consistency, the geometric mean of these mean h values was calculated as the final h value for the current amount of the probabilistic reward.
Consequently, 9 h values for these 9 amounts of probabilistic rewards were generated. Third, the geometric mean of these 9 h values was calculated as the final h value for the participant. (Given that the amounts of the probabilistic monetary reward (11, 12.5, 14, 17, 20, 25, 33, 50, and 100 yuan) did not increase/decrease evenly, the h values for these amounts were distributed unevenly, and using the geometric mean could be more representative of the h values than the arithmetic mean or median.)
The same process was applied to every participant to calculate the final h value, which was implemented using our custom script in MATLAB. Finally, a 2 (Group: IGD and RGU groups) × 2 (Sex: male and female) ANOVA on the h values was performed to examine the group-by-sex effect of risk-taking.
FMRI data acquisition
Functional imaging data were collected using a 3T scanner (Siemens Trio, Malvern, PA, USA) equipped for echo-planar imaging (EPI). The acquisition parameters are listed as follows: slice number = 33, slice thickness = 3 mm, interleaved sequence, repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 220 × 220 mm2 and matrix 64 × 64. Stimuli presentation and behavioral data acquisition of the PD task were completed using E-prime.
FMRI data preprocessing
Using Statistical Parametric Mapping (SPM) version 12 (https://www.fil.ion.ucl.ac.uk/spm/), functional images of all participants were first preprocessed as follows: slice-timing, realignment, normalization to Montreal Neurological Institute (MNI) standard space, and spatial smoothing (FWHM = 6 mm).
Whole-brain activation analysis
Second, using Neuroelf version 1.1 (http://neuroelf.net), a general linear model (GLM) was conducted and estimated to identify blood oxygen level-dependent (BOLD) activation associated with the task conditions of interest. In this study, trials in which participants chose probabilistic options (i.e., risky choices) and trials in which participants chose certain options (i.e., safe choices) were separately convolved with a canonical hemodynamic response function to yield the task regressors of interest. These choice regressors included the presentation duration of the choices, i.e., the response time window, given that the PD task does not differentiate between the option presentation and the response window. In addition, the six head-movement parameters estimated by the realignment step were included in the GLM to regress out their effect on brain activation. Finally, to examine the group-by-sex effect on brain activation associated with risk-taking, a 2 (Group: IGD and RGU groups) × 2 (Sex: males and females) ANOVA on the statistical contrast maps of risk-taking (regressor of risky choices-regressor of safe choices) was performed. The imaging results were corrected using familywise error (FWE) correction with voxel-level P < 0.005 and cluster extent of 50 voxels. Additionally, to examine the relationship between the participants' severity of IGD, risk-taking and brain activation, Pearson correlation analysis was performed between the IAT scores, the h values and the mean activation of clusters showing significant interaction effects and main effects.
Dynamic causal modeling (DCM) analysis
To further examine the group-by-sex effect on the relationship between those brain regions showing significant group-by-sex interaction effects on brain activation associated with risk-taking, DCM analysis was performed and implemented in SPM 12. DCM analysis includes five steps. 1) Selecting ROIs. Regions showing significant group-by-sex interaction effects on brain activation were selected and extracted as ROIs in this study. Spherical ROIs (centers: the coordinates of local peaks of significant regions; radii: 6 mm) were made for the next step. 2) Defining the GLM. The GLM was constructed to extract the time series of selected ROIs, which was constructed in Neuroelf as stated above. 3) Specifying and estimating the DCM. Referring to previous research (Dong et al., 2020; M. Wang, Dong, Zheng, Du, & Dong, 2020), for each participant, a “full” model was specified in this study, i.e., hypothesizing that the risky choices modulated all the connections (effect of task conditions), as shown in Fig. 1C. Then, hypothesized models of each participant were estimated. 4) Computing the optimal DCM model. A DCM network discovery routine was performed at the group level, which automatically searches all possible submodels of the full model and selects the optimal submodel for each participant by using post hoc model selection. 5) Group-by-sex effect on effective connectivity. The estimated parameters of each of the connections from the participants' optimal model were pooled into a 2 (Group: IGD and RGU groups) × 2 (Sex: male and female) ANOVA.
Ethics
The study procedures were carried out in conformity to the Declaration of Helsinki. The Institutional Review Board of Hangzhou Normal University approved the current study. All participants provided written informed content and the safety screening scale for MRI scan before the study.
Results
Behavioral performance
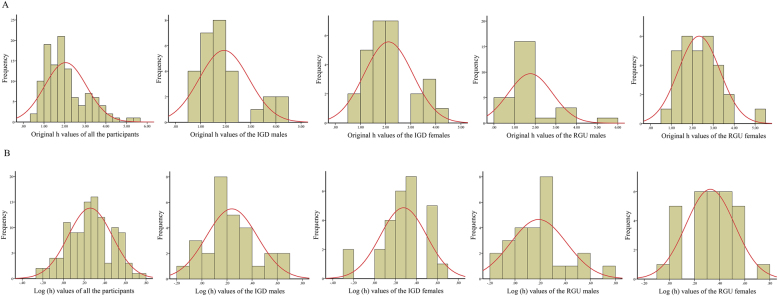
As shown in Fig. 2A, the h values did not fit the normal distribution among all the participants, the IGD males and the RGU males. Thus, a nonparametric ANOVA of Group × Sex was performed using the “scheirerRayHare” function in R v4.1.2 (https://www.r-project.org/). The results showed that the interaction effect (H = 1.16, P = 0.281, Cohen's f (effect size) = 0.107) and the main effect of group (H = 0.04, P = 0.838, Cohen's f = 0.020) on the log(h) values were not significant. The main effect of sex reached significance (H = 6.651, P = 0.010, Cohen's f = 0.255), i.e., males showed lower log(h) values than females, indicating greater risk-taking in males.
Fig. 2.
The histograms for the original h values and log(h) values
A: The histograms for the original h values of all the participants and subgroups (the IGD males, the IGD females, the RGU males, RGU females). B: The histograms for the log(h) values of all the participants and subgroups (the IGD males, the IGD females, the RGU males, RGU females). Abbreviations: IGD = internet gaming disorder; RGU = recreational game user.
Although nonparametric ANOVA can retain the features of raw data, its statistical power is lower than that of traditional parametric ANOVA. Thus, a log-10 transformation of the h values was performed to normalize the skewed distribution of these h values (Fig. 2B; the log(h) values among all the participants and subgroups fit the normal distribution). Then, a parametric ANOVA of Group × Sex was performed in IBM SPSS statistics v22.0 (https://spss.en.softonic.com/). The results showed that the interaction effect (F = 1.57, P = 0.213, Cohen's f (effect size) = 0.119) and the main effect of group (F = 0.00, P = 0.986, Cohen's f = 0.002) on the log(h) values were not significant. The main effect of sex reached significance (F = 4.86, P = 0.030, Cohen's f = 0.213), i.e., males showed lower log(h) values than females, indicating greater risk-taking in males (Fig. 1B).
Results of whole-brain activation analysis
Whole-brain activation during the contrast of risky choices-safe choices across all the participants is shown in Fig. 3 and Table 3. Regions in the corticostriatal neural circuit (the prefrontal, ACC, striatal regions and parts of parietal lobe) were activated in response to risky choices compared to safe choices, which were consistent with previous findings on the PD task (Cardinal, 2006; Peters & Büchel, 2009; Seaman et al., 2018; B. J. Weber & Huettel, 2008). The posterior cingulate cortex and precuneus showed negative activation during the task. They are primary parts of the default mode network, which is known to be deactivated during participants doing tasks (M. D. Fox et al., 2005; Fransson, 2006; Raichle, 2015).
Fig. 3.
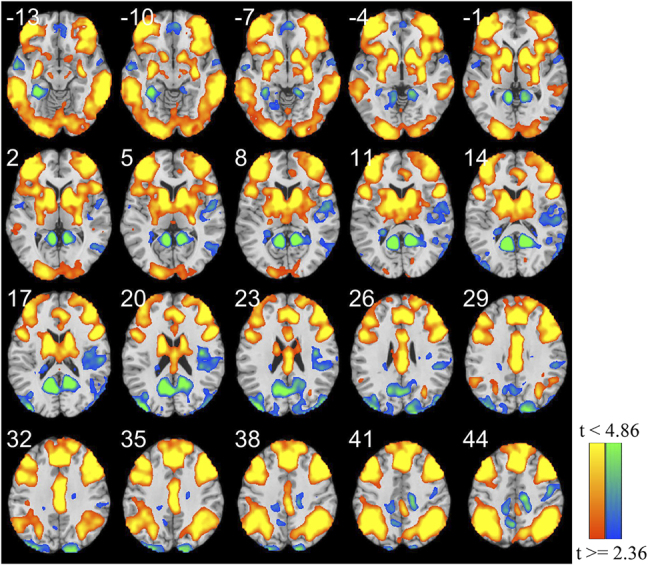
The results of whole-brain activation analysis during the contrast of risky choices-safe choices across all the participants
Clusters in red indicate the regions activated positively by the contrast of risky choices-safe choices. Clusters in blue indicate the regions activated negatively by this contrast. Statistical images were corrected using FWE with uncorrected P < 0.02 and cluster P < 0.05.
Table 3.
Brain regions showing significant activation across all the participants
| Region | x | y | z | Custer Size | t | P | Cohen's d |
| Risky-safe choices | |||||||
| Thalamus (L) | −12 | 0 | 9 | 10,421 | 7.01 | <0.001 | 0.665 |
| Caudate (L) | −12 | 12 | 0 | 164 | |||
| Caudate (R) | 12 | 3 | 9 | 378 | |||
| Putamen (L) | −24 | −6 | −6 | 115 | |||
| Putamen (L) | −27 | −12 | 9 | 111 | |||
| Putamen (R) | 30 | −15 | −9 | 134 | |||
| Medial frontal gyrus (L) | 0 | 33 | 36 | 494 | |||
| Inferior frontal gyrus (L) | −42 | 45 | 6 | 460 | |||
| Inferior frontal gyrus (R) | 54 | 15 | 18 | 338 | |||
| Anterior cingulate cortex (R) | 6 | 39 | 12 | 112 | |||
| Superior frontal gyrus (L) | −3 | 36 | 54 | 254 | |||
| Middle frontal gyrus (R) | 39 | 39 | −12 | 250 | |||
| Inferior parietal lobe (L) | −48 | −48 | 51 | 1,363 | 7.10 | <0.001 | 0.674 |
| Inferior parietal lobe (R) | 51 | −45 | 51 | 1,417 | 5.97 | <0.001 | 0.567 |
| Posterior cingulate cortex (R) | 12 | −51 | 9 | 734 | −4.70 | <0.001 | 0.446 |
| Precuneus (L) | −9 | −45 | 51 | 1,057 | −4.81 | <0.001 | 0.457 |
Table Legend: Shown are the coordinates of the local maxima in MNI space, the size of the clusters, and the t values, P values, Cohen's d (effect size) for the one-sample t-test across all the participants. Statistical images were corrected using FWE with uncorrected P < 0.005 and cluster P < 0.05. Abbreviations: L = left; R = right.
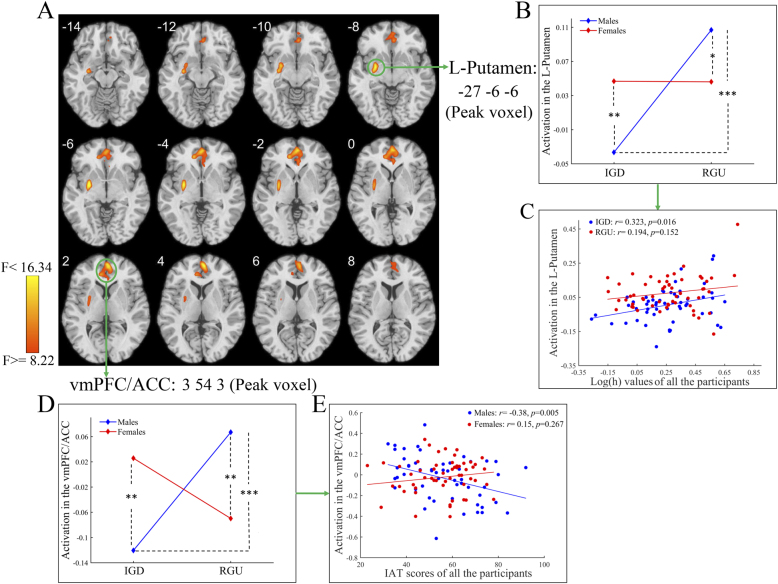
For the interaction effect of group and sex, the left putamen and ventromedial prefrontal cortex (vmPFC)/ACC were detected (Fig. 4A and Table 4). The post hoc analysis on the mean activation of the two regions showed that, IGD males exhibited lower activation than RGU males and IGD females, and RGU males exhibited higher activation than RGU females (Figures 4B and D). Moreover, the activation of the left putamen positively correlated with log(h) values among the IGD group, indicating that a lower log(h) value was associated with lower activation of the left putamen (Fig. 4C). The activation of the vmPFC/ACC negatively correlated with the IAT scores among males but not females, indicating that a higher IAT score was associated with lower activation of the vmPFC/ACC (Fig. 4E).
Fig. 4.
The results of the interaction effect for whole-brain activation analysis
A: Brain regions showing a significant interaction effect (Group × Sex) on whole-brain activation. B: The post hoc result for brain activation in the L-putamen (the coordinate indicates the peak voxel of this cluster). C: The brain activation of the L-putamen showed a significant positive correlation with log(h) values among the IGD group but not among the RGU group. D: The post hoc result for brain activation in the vmPFC/ACC (the coordinate indicates the peak voxel of this cluster). E: Brain activation of the vmPFC/ACC showed a significant negative correlation with IAT scores among males but not among females. Abbreviations: IGD = internet gaming disorder; RGU = recreational game user; IAT = Internet Addiction Test; L = left; vmPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Table 4.
Brain regions showing significant interaction effects and a main effect of sex
| Region | x | y | z | Custer Size | F | P | Cohen's f |
| Interaction effect: Group × Sex | |||||||
| Putamen (L) | −27 | −6 | −6 | 71 | 17.19 | <0.001 | 0.401 |
| vmPFC/ACC (R) | 3 | 54 | 3 | 175 | 16.15 | <0.001 | 0.388 |
| Main effect: Sex | |||||||
| Inferior frontal gyrus (R) | 42 | 15 | 30 | 52 | 16.22 | <0.001 | 0.389 |
Table Legend: Shown are the coordinates of the local maxima in MNI space, the size of the clusters, and the F values, P values, Cohen's f (effect size) for the ANOVA analysis of Group × Sex. Statistical images were corrected using FWE with uncorrected P < 0.005 and cluster P < 0.05. Abbreviations: L = left; R = right; vmPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex.
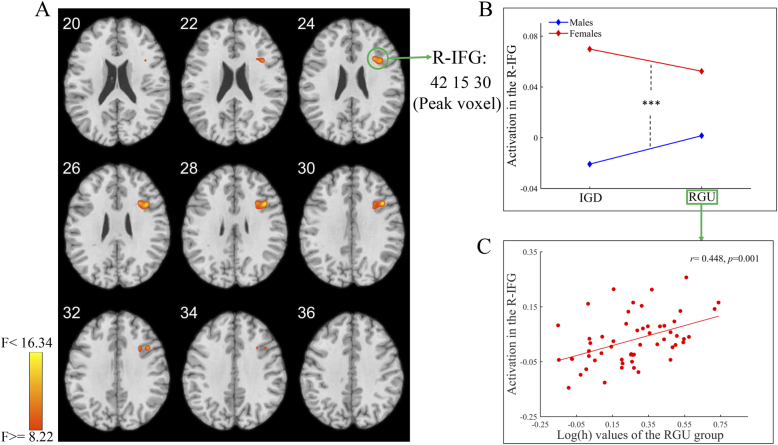
For the main effect of sex, the right IFG was detected (Fig. 5A and Table 3), i.e., males exhibited lower activation than females (Fig. 5B). The activation of the right IFG positively correlated with log(h) values among the RGU group, indicating that a lower log(h) value was associated with lower activation of the right IFG (Fig. 5C).
Fig. 5.
The results of main effect of sex for whole-brain activation analysis
A: Brain regions showing a significant main effect of sex on whole-brain activation. B: The post hoc result for brain activation in the R-IFG (the coordinate indicates the peak voxel of this cluster). C: Brain activation in the R-IFG showed a significant positive correlation with log(h) values among the RGU group. Abbreviations: IGD = internet gaming disorder; RGU = recreational game user; R-IFG = right inferior frontal gyrus; ***, P < 0.001.
Results of DCM analysis
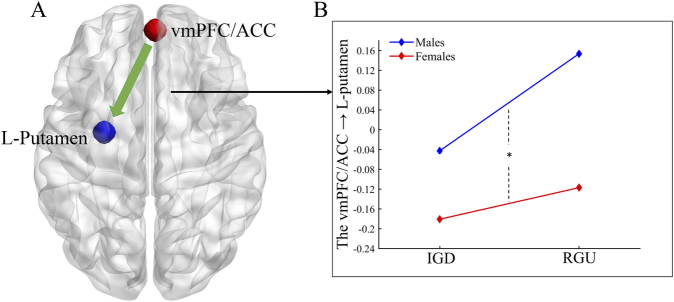
On the basis of the results of the GLM analysis, we selected two spherical ROIs as shown in Fig. 6A, i.e., the vmPFC/ACC (centers: 3 54 3; radii: 6 mm) and left putamen (centers: -27 -6 -6; radii: 6 mm). The results showed that the main effect of sex on the modulatory effect of risky choices on the connection from the vmPFC/ACC to the left putamen was significant (F = 5.13, P = 0.026, Cohen's f (effect size) = 0.220), i.e., the modulatory effect of risky choices on this connection in males was greater than that in females (Fig. 6B).
Fig. 6.
The results of main effect of sex for DCM analysis
A: Effective connectivity showing a significant main effect of sex on the modulatory effect of risky choices. B: The post hoc result for the modulatory effect of risky choices on the effective connectivity from the ACC to the L-putamen. Abbreviations: DCM = dynamic causal modeling; IGD = internet gaming disorder; RGU = recreational game user; vmPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex; L = left; *, P < 0.05.
Discussion
To the best of our knowledge, this is the first study to examine sex differences in risk-taking and the underlying neural correlates among individuals with IGD and RGUs. Our results showed that males and females differed in risk-taking, and they had distinct neural responses associated with risky decision-making in a manner that was sensitive to group (IGD and RGU). Moreover, these differences were relatively strong as reflected by the good effect sizes of the results, i.e., medium effects for the behavioral and DCM results and large effects for the whole-brain activation results (J. Cohen, 1969, 1988). Our hypotheses were supported. Detailed implications are discussed below.
Males showed a higher risk-taking tendency than females
For the probability discounting rates (h values), a significant main effect of sex was found; males showed greater risk-taking (lower h values) than females. This finding is consistent with previous studies. Males were found to have greater risk-taking across multiple domains, including health, recreational, and financial domains (Byrnes et al., 1999; Harris & Jenkins, 2006; E. U. Weber, Blais, & Betz, 2002). For instance, males were more likely to engage in drug/alcohol use, smoking, and gambling than females. Researchers have reported that males had higher sensitivity to rewarding stimuli, whereas females had higher sensitivity to punishment (Pagliaccio et al., 2016). Furthermore, males had greater expectations of enjoyment and females had a greater perceived likelihood of negative outcomes, which mediated the higher propensity toward risky behaviors in males than in females (Harris & Jenkins, 2006). Overall, the higher risk-taking tendency in males may account for the higher prevalence of addictive disorders in males than females, including IGD, substances and gambling addictions (Ha & Hwang, 2014; Moran-Santa, Flanagan, & Brady, 2014; Potenza et al., 2001).
At the neural level, among RGUs, males showed higher activation in the vmPFC/ACC and left putamen than females during risky choices compared to safe choices indexed by the result of the significant Group × Sex interaction effects; meanwhile, males showed lower activation than females in the right IFG indexed by the result of a significant main effect of sex. In addition, the DCM results showed a greater modulatory effect of risky choices on the connection from the vmPFC/ACC to the left putamen of males than to that of females. Along with the behavioral results above, these results are consistent with our hypotheses about the higher risk-taking tendency in males than females. The ACC, vmPFC, putamen and IFG belong to the classic corticostriatal circuit of reward. The ACC has been implicated in risk and uncertainty assessment during risky decision-making tasks (Critchley, Mathias, & Dolan, 2001; Knutson, Taylor, Kaufman, Peterson, & Glover, 2005; M. P. Paulus, Rogalsky, Simmons, Feinstein, & Stein, 2003). The vmPFC is widely suggested to be responsible for encoding goal-value signals and tracking subjective values of monetary rewards (Gläscher, Hampton, & O'Doherty, 2009; Hare, Camerer, & Rangel, 2009; Hare, O'doherty, Camerer, Schultz, & Rangel, 2008; Kable & Glimcher, 2007). These studies establish the crucial role of the ACC and vmPFC in risk/value evaluation and anticipation for reward.
The putamen, together with the caudate, forms the dorsal striatum and is important in habit/association learning during decision-making and habitual drug seeking (Everitt & Robbins, 2013). The dorsal striatum mediates processes linked to encoding action-outcome associations during goal-directed behaviors (caudate) and encoding stimulus-action associations (putamen) (Balleine, Delgado, & Hikosaka, 2007; Haruno & Kawato, 2006). Also, researchers have found its important role in risk/value evaluation and anticipation of reward during decision-making. For instance, using a risky decision-making task, one study revealed that the activation of the bilateral putamen correlated positively with the expected reward of choices (Kerstin, Peter, & Quartz, 2006). Another study indicated that the right putamen was significantly activated when subjects made a risky choice; moreover, increased activation of the putamen during risky choices predicted subjects' tendency to take higher risks for larger rewards (Engelmann & Tamir, 2009). Rademacher et al. found that anticipation of a monetary reward significantly activated subjects' bilateral putamen (Rademacher et al., 2010). Other studies have likewise demonstrated the involvement of the putamen in risk/value evaluation and anticipation of reward (M. X. Cohen & Ranganath, 2005; Hori, Minamimoto, & Kimura, 2009; Knutson, Fong, Adams, Varner, & Hommer, 2001; Schultz, Tremblay, & Hollerman, 2000). The IFG is known for its cognitive control function (Miller, 2000; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008). Cognitive control is involved in multiple higher-order executive functions, such as risky decision-making and problem solving (Chan, Shum, Toulopoulou, & Chen, 2008; Diamond, 2013). Prior research revealed that greater activation of reward evaluation-related regions and lower activation of control-related regions significantly predicted more risky choices in healthy populations (B. J. Weber & Huettel, 2008). The current result with a large effect showing that lower activation of the right IFG was accompanied by lower probability discounting rates (i.e., more risk-taking) in the RGU group supports this argument. Accordingly, these neural results suggest that males have greater risk/value evaluation and anticipation of reward and weaker cognitive control than females during risky decision-making, which underlies the higher risk-taking behavior of males.
Distinct neural substrates underlying IGD between males and females
The result of significant Group × Sex interaction effects at the neural level showed that among males, the IGD group showed lower neural activation in the vmPFC/ACC and left putamen than the RGU group during risky choices compared to safe choices; this difference did not exist among females. However, a nonsignificant interaction effect and a main effect of group on h values were identified in the present study. On the basis of the functions of the vmPFC/ACC and left putamen and the correlational result showing that lower activation of the left putamen was associated with greater risk-taking tendency (medium effect), the current results suggested reduced functions of risk/value evaluation and anticipation of reward in IGD males compared to RGU males during risky decision-making, demonstrating a high risk-taking tendency (reduced risk/reward sensitivity) in IGD males, which supported previous findings (Dong & Potenza, 2016; Dong et al., 2017). Interestingly, this alteration did not exist in IGD females, which might suggest that high levels of risk-taking are not a risk factor or core symptom of IGD in females. This inference could be supported by the correlation result showing that lower activation in the vmPFC/ACC was associated with higher addiction severity only among males (medium effect) but not among females.
This finding is consistent with previous findings about sex differences in drug and gambling addictions and IGD; that is, high sensation seeking/reward sensitivity is the main risk factor for males to begin drug use, gambling, or playing games that subsequently develop into addictions, whereas high emotional issues/punishment sensitivity is the main risk factor for females (as stated in the Introduction). Previous studies have revealed different neural substrates underlying IGD/gambling addiction between males and females from the perspective of drug/gaming cues (Dong, Wang, Wang, Du, & Potenza, 2019; Dong, Zheng, et al., 2018; Potenza et al., 2012; M. Wang, Dong, Wang, Zheng, & Potenza, 2018). For example, Potenza and colleagues revealed that males with cocaine dependence had a greater neural response to drug-related cues than HC males, whereas females with cocaine dependence showed a greater neural response to stress-related cues than HC females (Potenza et al., 2012). Dong and colleagues found that IGD males had decreased FC among cognitive control-related regions (DLPFC and superior frontal gyrus) compared with HC males during game playing, but this difference did not exist in IGD females and HC females (Dong, Wang et al., 2019). These findings, along with the current findings, indicate that males and females have different neural substrates in developing and maintaining IGD.
At the behavioral level, a nonsignificant interaction effect and a main effect of group on h values were identified in the present study. This finding is consistent with several studies on IGD or internet addiction. Using the PD task, two studies found no difference between individuals with IGD/problematic internet use and controls in the probability discounting rate (Q. Li et al., 2016; Tian et al., 2018). Using the Balloon Analog Risk Test (BART), one study indicated no differences between individuals with IGD and HCs in risk-taking potential (the number of popped balloons) (Ko et al., 2010). On the other hand, two studies suggested greater risk-taking in individuals with IGD, indicated by their more disadvantageous and risky choices compared to HCs using the Iowa gambling task and one risk-taking task (Dong & Potenza, 2016; Yao et al., 2015). According to prospect theory, people take greater risks in relation to smaller amounts of money (Kahneman & Tversky, 1979). In the current version of the PD task, the amounts of probabilistic (risky) options ranged from 11 to 100¥ (≈$1.71–15.51). Thus, the RGU group may have discounted the probabilistic options as steeply as the IGD group, i.e., the two groups took similar risks regarding small amounts of monetary resources. This may be one of the reasons explaining the discrepancy between the current findings and previous findings from several studies. An alternative explanation is the gaming experiences of the control group. In the present study, the control group was composed of RGUs who had similar gaming time as the IGD group but were not addicted. Researchers have reported that individuals with a high level of sensation-seeking trait (i.e., greater desire for risk-taking) are more likely to engage in gaming behavior and develop IGD (Chiu et al., 2004; Mehroof & Griffiths, 2010). Accordingly, the difference in risk-taking tendency between the current RGU group and the IGD group might be minor, especially with regard to small amounts of monetary resources. Studies examining the effects of gaming experiences of control groups and the amounts of risky options on final results are needed. At last, it is important to note that the absence of evidence is not evidence of absence, and the nonsignificant difference between the IGD and RGU groups in probability discounting should be interpreted with caution. Moreover, our fMRI results identified different neural responses associated with risk-taking between IGD and RGU. FMRI has superior sensitivity in detecting deficits among clinical populations in comparison with behavioral measurements (Rose & Donohoe, 2012). We expect future studies to verify the current results.
Why sex differences in risk-taking exist in individuals with IGD and RGUs
The sex differences in risk-taking of IGD and RGUs involve complex interactions between neurobiological and sociocultural factors (Becker et al., 2012, 2017; Fattore et al., 2014). First, males and females differ in sex hormones, which further leads to differences in neurotransmitter dopamine release and delivery in the reward neural circuit from the midbrain regions to the NAcc, dorsal striatum and prefrontal cortex (Bobzean, DeNobrega, & Perrotti, 2014; Jacobs & D'Esposito, 2011; Perry, Westenbroek, & Becker, 2016). Risky decision making is associated with increased dopamine release in the NAcc (Freels, Gabriel, Lester, & Simon, 2020). Researchers found that ovariectomy improved risky decision making among females and estradiol reversed this effect, indicating that ovarian hormones maintain the weaker risk-taking tendency in females than males (Orsini et al., 2021). Thus, the current sex differences in the brain activation of the vmPFC/ACC, putamen and IFG during risky choices might result from sex differences in estradiol and dopamine release.
Second, sociocultural factors also account for sex differences in risk-taking among IGD and RGUs, such as social norms and sex stereotypes. For a long time, a prevalent sex stereotype in most cultures was that males were bolder and more willing to take risks than females (Byrnes et al., 1999; Slovic, 1966). This can be reflected by the fact that most games were designed with strong competition, violence and masculinity to attract males, which further resulted in more male gamers than female gamers (Leonhardt & Overå, 2021). In recent years, the number of female gamers has steadily grown as this sex stereotype has receded (Lopez-Fernandez, Williams, & Kuss, 2019; Royse, Lee, Undrahbuyan, Hopson, & Consalvo, 2007). However, it should be noted that although the number of female gamers is growing, the risk factors and core symptoms are different in males and females, as the current study and previous studies revealed. High risk-taking and decreased neural responses related to the risk/value evaluation and anticipation of reward are the main risk factors for developing and maintaining IGD in males but not in females. Designing and adopting different treatments and prevention strategies for IGD in males and females are necessary and would be more effective.
Strengths
Using the probability discounting task, this study demonstrated a higher risk-taking tendency in male gamers than female gamers from both behavioral and neural evidence (whole-brain activation and DCM analyses). Moreover, only IGD males, but not IGD females, were found to be reduced in the functions of risk/value evaluation of reward at the neural level compared to controls. These findings indicate that a high risk-taking tendency is a risk factor and core symptom of IGD in a sex-specific form. For males, reducing the high risk-taking tendency could be a target for preventing and treating IGD. However, for females, it is crucial to identify their specific risk factors, such as negative emotions. This study fills the gap in information on the behavioral and neural substrates underlying IGD among females and might account for why males are more likely to engage in playing games and be vulnerable to IGD.
Limitations
Two limitations should be noted. First, during the present PD task, the position of the risky and safe options and the corresponding response buttons remained unchanged. This might affect the present results considering that the risky or safe choice was related to the participants' own distinct motor action, although some of this effect could possibly be balanced between the IGD and RGU groups. Future studies with randomized positions of options in the PD task to examine the present results are needed. Second, the causal relationship between the altered neural responses related to risk/value evaluation and IGD in males could not be concluded in the current cross-sectional study. Longitudinal studies to identify this relationship are warranted.
Conclusions
This study identified distinct risk-taking behavior and neural substrates underlying IGD among males and females. First, among recreational gamers, males showed a higher risk-taking tendency and greater neural responses associated with the risk/value evaluation of reward and smaller neural responses associated with cognitive control than females, which contributes to explaining why males are more likely to engage in gaming and be vulnerable to IGD. Second, IGD males showed decreased neural activation associated with the risk/value evaluation of reward compared to RGU males, whereas this decrease did not exist in IGD females. These findings imply that a high risk-taking tendency is a risk factor and core symptom only in IGD males, not in IGD females. There is an urgent need to identify the risk factors for IGD in females. Designing and adopting distinct treatments for IGD and prevention strategies for males and females are necessary and would be more effective.
Funding sources
This work was supported by the cultivation project of the province-leveled preponderant characteristic discipline of Hangzhou Normal University [number 20JYXK025] to Dr. L Wang, the Scientific Research Foundation for Scholars of Hangzhou Normal University [number 2020QDL021] to Dr. L Wang, Zhejiang Provincial Natural Science Foundation of China [number LQ22C090005] to Dr. L Wang, and the Key medical disciplines of Hangzhou. The funding sources had no involvement in any of the manuscript.
Authors' contribution
Dr. L Wang and Dr. Dong collected the research data, conducted the statistical analysis and wrote the manuscript. Dr. Dong designed the study. Mr. Zheng and Mr. M Wang contributed to the data collection and statistical analysis. Dr. Chen, Dr. Du and Dr. Dong contributed to modifying the manuscript. All authors have full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Lingxiao Wang, Email: wanglingxiao@hznu.edu.cn.
Hui Zheng, Email: zh.dmtr@gmail.com.
Min Wang, Email: 905827424@qq.com.
Shuaiyu Chen, Email: 1316289208@qq.com.
Xiaoxia Du, Email: merryxiaoxia@163.com.
Guang-Heng Dong, Email: dongguangheng@hznu.edu.cn.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Amlung, M. , & MacKillop, J. (2014). Clarifying the relationship between impulsive delay discounting and nicotine dependence. Psychology of Addictive Behaviors , 28(3), 761. 10.1037/a0036726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine, B. W. , Delgado, M. R. , & Hikosaka, O. (2007). The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience , 27(31), 8161–8165. 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. B. , McClellan, M. L. , & Reed, B. G. (2017). Sex differences, gender and addiction. Journal of Neuroscience Research , 95(1–2), 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. B. , Perry, A. N. , & Westenbroek, C. (2012). Sex differences in the neural mechanisms mediating addiction: A new synthesis and hypothesis. Biology of Sex Differences , 3(1), 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco, C. , Hasin, D. S. , Petry, N. , Stinson, F. S. , & Grant, B. F. (2006). Sex differences in subclinical and DSM-IV pathological gambling: Results from the national epidemiologic survey on alcohol and related conditions. Psychological Medicine , 36(7), 943–953. [DOI] [PubMed] [Google Scholar]
- Bobzean, S. A. , DeNobrega, A. K. , & Perrotti, L. I. (2014). Sex differences in the neurobiology of drug addiction. Experimental Neurology , 259, 64–74. [DOI] [PubMed] [Google Scholar]
- Borgonovi, F. (2016). Video gaming and gender differences in digital and printed reading performance among 15-year-olds students in 26 countries. Journal of Adolescence , 48, 45–61. 10.1016/j.adolescence.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Byrnes, J. P. , Miller, D. C. , & Schafer, W. D. (1999). Gender differences in risk taking: A meta-analysis. Psychological Bulletin , 125(3), 367–383. 10.1037/0033-2909.125.3.367. [DOI] [Google Scholar]
- Cahill, L. (2006). Why sex matters for neuroscience. Nature Reviews Neuroscience , 7(6), 477–484. [DOI] [PubMed] [Google Scholar]
- Cai, C. , Yuan, K. , Yin, J. , Feng, D. , Bi, Y. , Li, Y. , … Tian, J. (2016). Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain Imaging and Behavior , 10(1), 12–20. [DOI] [PubMed] [Google Scholar]
- Campbell, N. , & Ettorre, E. (2011). Gendering addiction: The politics of drug treatment in a neurochemical world . New York: Palgrave Macmillan. [Google Scholar]
- Cardinal, R. N. (2006). Neural systems implicated in delayed and probabilistic reinforcement. Neural Networks , 19(8), 1277–1301. [DOI] [PubMed] [Google Scholar]
- Chan, R. C. , Shum, D. , Toulopoulou, T. , & Chen, E. Y. (2008). Assessment of executive functions: Review of instruments and identification of critical issues. Archives of Clinical Neuropsychology , 23(2), 201–216. 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Chiu, S. I. , Lee, J. Z. , & Huang, D. H. (2004). Video game addiction in children and teenagers in Taiwan. Cyberpsychol Behav , 7(5), 571–581. 10.1089/cpb.2004.7.571. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1969). Statistical power analysis for the behavioral sciences . New York: Academic Press. [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Cohen, M. X. , & Ranganath, C. (2005). Behavioral and neural predictors of upcoming decisions. Cognitive Affective & Behavioral Neuroscience , 5(2), 117–126. [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Mathias, C. J. , & Dolan, R. J. (2001). Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron , 29(2), 537–545. 10.1016/S0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Diamond, A. (2013). Executive functions. Annual Review of Psychology , 64, 135–168. 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Dong, H. , Wang, M. , Zhang, J. , Zhou, W. , Du, X. , & Potenza, M. N. (2021). Dorsal and ventral striatal functional connectivity shifts play a potential role in internet gaming disorder. Communications Biology , 4(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Lin, X. , Hu, Y. , Xie, C. , & Du, X. (2015). Imbalanced functional link between executive control network and reward network explain the online-game seeking behaviors in Internet gaming disorder. Scientific Reports , 5(5 Suppl 2), 9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Li, H. , Wang, L. , & Potenza, M. N. (2017). Cognitive control and reward/loss processing in Internet gaming disorder: Results from a comparison with recreational Internet game-users. European Psychiatry , 44, 30–38. [DOI] [PubMed] [Google Scholar]
- Dong, G. , Liu, X. , Zheng, H. , Du, X. , & Potenza, M. N. (2019). Brain response features during forced break could predict subsequent recovery in internet gaming disorder: A longitudinal study. Journal of Psychiatric Research , 113, 17–26. 10.1016/j.jpsychires.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Dong, G. , & Potenza, M. N. (2016). Risk-taking and risky decision-making in Internet gaming disorder: Implications regarding online gaming in the setting of negative consequences. Journal of Psychiatric Research , 73(1), 1–8. 10.1016/j.jpsychires.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Dong, G. , Wang, L. , Du, X. , & Potenza, M. N. (2018). Gender-related differences in neural responses to gaming cues before and after gaming: Implications for gender-specific vulnerabilities to internet gaming disorder. Social Cognitive and Affective Neuroscience , 13(11), 1203–1214. 10.1093/scan/nsy084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, G. , Wang, Z. , Wang, Y. , Du, X. , & Potenza, M. N. (2019). Gender-related functional connectivity and craving during gaming and immediate abstinence during a mandatory break: Implications for development and progression of internet gaming disorder. Progress in Neuro-psychopharmacology & Biological Psychiatry , 88, 1–10. 10.1016/j.pnpbp.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Dong, G. , Wang, M. , Zheng, H. , Wang, Z. , Du, X. , & Potenza, M. N. (2020). Disrupted prefrontal regulation of striatum-related craving in Internet gaming disorder revealed by dynamic causal modeling: Results from a cue-reactivity task. Psychological Medicine , 1–13. 10.1017/S003329172000032X. [DOI] [PubMed] [Google Scholar]
- Dong, G. , Zheng, H. , Liu, X. , Wang, Y. , Du, X. , & Potenza, M. N. (2018). Gender-related differences in cue-elicited cravings in Internet gaming disorder: The effects of deprivation. Journal of Behavioral Addictions , 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann, J. B. , & Tamir, D. (2009). Individual differences in risk preference predict neural responses during financial decision-making. Brain Research , 1290, 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt, B. J. , & Robbins, T. W. (2013). From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neuroscience and Biobehavioral Reviews , 37(9), 1946–1954. [DOI] [PubMed] [Google Scholar]
- Fattore, L. , Melis, M. , Fadda, P. , & Fratta, W. (2014). Sex differences in addictive disorders. Frontiers in neuroendocrinology , 35(3), 272–284. [DOI] [PubMed] [Google Scholar]
- Fauth-Bühler, M. , & Mann, K. (2017). Neurobiological correlates of internet gaming disorder: Similarities to pathological gambling. Addictive Behaviors , 64, 349–356. [DOI] [PubMed] [Google Scholar]
- Feng, W. , Ramo, D. E. , Chan, S. R. , & Bourgeois, J. A. (2017). Internet gaming disorder: Trends in prevalence 1998–2016. Addictive Behaviors , 75, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, H. C. , Morgan, P. T. , & Sinha, R. (2014). Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology , 39(6), 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences , 102(27), 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson, P. (2006). How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia , 44(14), 2836–2845. [DOI] [PubMed] [Google Scholar]
- Freels, T. G. , Gabriel, D. B. , Lester, D. B. , & Simon, N. W. (2020). Risky decision-making predicts dopamine release dynamics in nucleus accumbens shell. Neuropsychopharmacology , 45(2), 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , Harrison, L. , & Penny, W. (2003). Dynamic causal modelling. Neuroimage , 19(4), 1273–1302. 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gilman, J. M. , Curran, M. T. , Calderon, V. , Stoeckel, L. E. , & Evins, A. E. (2014). Impulsive social influence increases impulsive choices on a temporal discounting task in young adults. Plos One , 9(7), e101570. 10.1371/journal.pone.0101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher, J. , Hampton, A. N. , & O'Doherty, J. P. (2009). Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cerebral Cortex , 19(2), 483–495. 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ortega, I. , Echeburúa, E. , de Corral, P. , & Polo-López, R. (2015). Pathological gambling: Clinical gender differences. In Sáenz-Herrero M. (Ed.), Psychopathology in women (pp. 713–726). Cham: Springer. [Google Scholar]
- Grant, J. , Mottet, L. , Tanis, J. , Herman, J. L. , Harrison, J. , & Keisling, M. (2010). National transgender discrimination survey report on health and health care. [Google Scholar]
- Ha, Y. , & Hwang, W. (2014). Gender differences in internet addiction associated with psychological health indicators among adolescents using a national web-based survey. International Journal of Mental Health and Addiction , 12(5), 660–669. 10.1007/s11469-014-9500-7. [DOI] [Google Scholar]
- Hare, T. A. , Camerer, C. F. , & Rangel, A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science , 324(5927), 646–648. http://doi.org/0.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare, T. A. , O'doherty, J. , Camerer, C. F. , Schultz, W. , & Rangel, A. (2008). Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience , 28(22), 5623–5630. 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, C. R. , & Jenkins, M. (2006). Gender differences in risk assessment: Why do women take fewer risks than men? Judgment and Decision Making , 1(1), 48–63. http://doi.org/06016/jdm06016.htm. [Google Scholar]
- Haruno, M. , & Kawato, M. (2006). Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. Journal of Neurophysiology , 95(2), 948–959. 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Hori, Y. , Minamimoto, T. , & Kimura, M. (2009). Neuronal encoding of reward value and direction of actions in the primate putamen. Journal of Neurophysiology , 102(6), 3530–3543. 10.1152/jn.00104.2009. [DOI] [PubMed] [Google Scholar]
- Jacobs, E. , & D'Esposito, M. (2011). Estrogen shapes dopamine-dependent cognitive processes: Implications for women's health. Journal of Neuroscience , 31(14), 5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, C. , Zhang, T. , Cai, C. , Bi, Y. , Li, Y. , Yu, D. , … Yuan, K. (2016). Abnormal prefrontal cortex resting state functional connectivity and severity of internet gaming disorder. Brain Imaging and Behavior , 10(3), 719–729. [DOI] [PubMed] [Google Scholar]
- Kable, J. W. , & Glimcher, P. W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience , 10(12), 1625–1633. 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman, D. , & Tversky, A. (1979). Prospect theory: An analysis of decision under risk. Econometrica , 47(2), 263–292. 10.2307/1914185. [DOI] [Google Scholar]
- Kerstin, P. , Peter, B. , & Quartz, S. R. (2006). Neural differentiation of expected reward and risk in human subcortical structures. Neuron , 51(3), 381–390. [DOI] [PubMed] [Google Scholar]
- Kirby, K. N. , & Maraković, N. N. (1996). Delay-discounting probabilistic rewards: Rates decrease as amounts increase. Psychonomic Bulletin & Review , 3(1), 100–104. 10.3758/BF03210748. [DOI] [PubMed] [Google Scholar]
- Kirby, K. N. , Petry, N. M. , & Bickel, W. K. (1999). Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General , 128(1), 78–87. 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Knutson, B. , Fong, G. W. , Adams, C. M. , Varner, J. L. , & Hommer, D. (2001). Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport , 12(17), 3683. [DOI] [PubMed] [Google Scholar]
- Knutson, B. , Taylor, J. , Kaufman, M. , Peterson, R. , & Glover, G. (2005). Distributed neural representation of expected value. Journal of Neuroscience , 25(19), 4806–4812. 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, C. , Hsiao, S. , Liu, G. C. , Yen, J. Y. , Yang, M. J. , & Yen, C. F. (2010). The characteristics of decision making, potential to take risks, and personality of college students with Internet addiction. Psychiatry Research , 175(1), 121–125. 10.1016/j.psychres.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kreek, M. J. , Nielsen, D. A. , Butelman, E. R. , & LaForge, K. S. (2005). Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience , 8(11), 1450–1457. 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Lai, C. M. , Mak, K. K. , Watanabe, H. , Ang, R. P. , Pang, J. S. , & Ho, R. C. (2013). Psychometric properties of the internet addiction test in Chinese adolescents. Journal of Pediatric Psychology , 38(7), 794–807. [DOI] [PubMed] [Google Scholar]
- Lee, D. , Lee, J. , Yoon, K. J. , Kee, N. , & Jung, Y.-C. (2016). Impaired anterior insular activation during risky decision making in young adults with internet gaming disorder. Neuroreport , 27(8), 605–609. [DOI] [PubMed] [Google Scholar]
- Lee, D. , Namkoong, K. , Lee, J. , & Jung, Y. C. (2021). Dorsal striatal functional connectivity changes in internet gaming disorder: A longitudinal magnetic resonance imaging study. Addiction Biology , 26(1), e12868. [DOI] [PubMed] [Google Scholar]
- Legault, M. C. , Liu, H. Z. , & Balodis, I. M. (2021). Neuropsychological constructs in gaming disorders: A systematic review. Current Behavioral Neuroscience Reports , 8(3), 59–76. [Google Scholar]
- Leonhardt, M. , & Overå, S. (2021). Are there differences in video gaming and use of social media among boys and girls?—A mixed methods approach. International Journal of Environmental Research and Public Health , 18(11), 6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Dai, W. , Yang, Z. , Wang, L. , Dai, B. , & Liu, X. (2019). The mediating role of coping styles on impulsivity, behavioral inhibition/approach system, and internet addiction in adolescents from a gender perspective. Frontiers in Psychology , 10, 2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Tian, M. , Taxer, J. , Zheng, Y. , Wu, H. , Sun, S. , & Liu, X. (2016). Problematic internet users' discounting behaviors reflect an inability to delay gratification, not risk taking. Cyberpsychology, Behavior and Social Networking , 19(3), 172–178. 10.1089/cyber.2015.0295. [DOI] [PubMed] [Google Scholar]
- Li, L. , Yu, Q. , Zhang, L. , & Jin, S. (2015). The gender difference on Internet addictive among adolescent: The mediation effect of the differentiation of social and psychological situation in school. Chinese Journal of Clinical Psychology , 23(6), 1044–1048. 10.16128/j.cnki.1005-3611.2015.06.020. [DOI] [Google Scholar]
- Lin, X. , Zhou, H. , Dong, G. , & Du, X. (2015). Impaired risk evaluation in people with internet gaming disorder: fMRI evidence from a probability discounting task. Progress in Neuro-psychopharmacology & Biological Psychiatry , 56, 142–148. [DOI] [PubMed] [Google Scholar]
- Liu, L. , Chen, X. , Cui, J. , Wang, J. , Zhang, Y. , Neumann, D. L. , … Chan, R. C. (2016). Age differences in delay discounting in Chinese adults. Personality and Individual Differences , 90, 205–209. 10.1016/j.paid.2015.11.006. [DOI] [Google Scholar]
- Lopez-Fernandez, O. , Williams, A. J. , & Kuss, D. J. (2019). Measuring female gaming: Gamer profile, predictors, prevalence, and characteristics from psychological and gender perspectives. Frontiers in Psychology , 10, 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehroof, M. , & Griffiths, M. D. (2010). Online gaming addiction: The role of sensation seeking, self-control, neuroticism, aggression, state anxiety, and trait anxiety. Cyberpsychology Behavior & Social Networking , 13(3), 313–316. 10.1089/cyber.2009.0229. [DOI] [PubMed] [Google Scholar]
- Miller, E. K. (2000). The prefontral cortex and cognitive control. Nature Reviews Neuroscience , 1(1), 59–65. [DOI] [PubMed] [Google Scholar]
- Mohr, P. N. , Biele, G. , & Heekeren, H. R. (2010). Neural processing of risk. Journal of Neuroscience , 30(19), 6613–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Santa, M. , Flanagan, J. , & Brady, K. (2014). Ovarian hormones and drug abuse. Current Psychiatry Reports , 16(11), 511. 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, C. A. , McCaul, M. E. , Wong, D. F. , Oswald, L. M. , Zhou, Y. , Brasic, J. , … Ye, W. (2006). Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry , 59(10), 966–974. [DOI] [PubMed] [Google Scholar]
- Orsini, C. A. , Blaes, S. L. , Hernandez, C. M. , Betzhold, S. M. , Perera, H. , Wheeler, A.-R. , … Setlow, B. (2021). Regulation of risky decision making by gonadal hormones in males and females. Neuropsychopharmacology , 46(3), 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio, D. , Luking, K. R. , Anokhin, A. P. , Gotlib, I. H. , Hayden, E. P. , Olino, T. M. , … Barch, D. M. (2016). Revising the BIS/BAS scale to study development: Measurement invariance and normative effects of age and sex from childhood through adulthood. Psychological Assessment , 28(4), 429–442. 10.1037/pas0000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus, F. W. , Ohmann, S. , Von Gontard, A. , & Popow, C. (2018). Internet gaming disorder in children and adolescents: A systematic review. Developmental Medicine and Child Neurology , 60(7), 645–659. 10.1111/dmcn.13754. [DOI] [PubMed] [Google Scholar]
- Paulus, M. P. , Rogalsky, C. , Simmons, A. , Feinstein, J. S. , & Stein, M. B. (2003). Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage , 19(4), 1439–1448. 10.1016/S1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Pawlikowski, M. , Altstötter-Gleich, C. , & Brand, M. (2013). Validation and psychometric properties of a short version of Young's Internet Addiction Test. Computers in Human Behavior , 29(3), 1212–1223. 10.1016/j.chb.2012.10.014. [DOI] [Google Scholar]
- Perry, A. N. , Westenbroek, C. , & Becker, J. B. (2016). Sex differences and addiction. Sex Differences in the Central Nervous System , 129–147. [Google Scholar]
- Peters, J. , & Büchel, C. (2009). Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. Journal of Neuroscience , 29(50), 15727–15734. 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry, N. M. , Rehbein, F. , Gentile, D. A. , Lemmens, J. S. , Rumpf, H. J. , Mößle, T. , … Borges, G. (2014). An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction , 109(9), 1399–1406. [DOI] [PubMed] [Google Scholar]
- Potenza, M. N. , Hong, K.-I. A. , Lacadie, C. M. , Fulbright, R. K. , Tuit, K. L. , & Sinha, R. (2012). Neural correlates of stress-induced and cue-induced drug craving: Influences of sex and cocaine dependence. American Journal of Psychiatry , 169(4), 406–414. 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza, M. N. , Steinberg, M. A. , McLaughlin, S. D. , Wu, R. , Rounsaville, B. J. , & O’Malley, S. S. (2001). Gender-related differences in the characteristics of problem gamblers using a gambling helpline. American Journal of Psychiatry , 158(9), 1500–1505. 10.1176/appi.ajp.158.9.1500. [DOI] [PubMed] [Google Scholar]
- Rademacher, L. , Krach, S. , Kohls, G. , Irmak, A. , Gründer, G. , & Spreckelmeyer, K. N. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage , 49(4), 3276–3285. [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. (2015). The brain's default mode network. Annual Review of Neuroscience , 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Reisner, S. L. , Conron, K. J. , Tardiff, L. A. , Jarvi, S. , Gordon, A. R. , & Austin, S. B. (2014). Monitoring the health of transgender and other gender minority populations: Validity of natal sex and gender identity survey items in a US national cohort of young adults. BMC Public Health , 14(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, E. J. , & Donohoe, G. (2012). Brain vs behavior: An effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophrenia Bulletin , 39(3), 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royse, P. , Lee, J. , Undrahbuyan, B. , Hopson, M. , & Consalvo, M. (2007). Women and games: Technologies of the gendered self. New Media & Society , 9(4), 555–576. [Google Scholar]
- Sausa, L. , Sevelius, J. , Keatley, J. , Iñiguez, J. R. , & Reyes, M. (2009). Policy recommendations for inclusive data collection of trans people in HIV prevention, care & services . San Francisco, CA: Center of Excellence for Transgender HIV Prevention: University of California, San Francisco. [Google Scholar]
- Schultz, W. , Tremblay, L. , & Hollerman, J. R. (2000). Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex , 10(3), 272–283. [DOI] [PubMed] [Google Scholar]
- Seaman, K. L. , Brooks, N. , Karrer, T. M. , Castrellon, J. J. , Perkins, S. F. , Dang, L. C. , … Samanez-Larkin, G. R. (2018). Subjective value representations during effort, probability and time discounting across adulthood. Social Cognitive and Affective Neuroscience , 13(5), 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovic, P. (1966). Risk-taking in children: Age and sex differences. Child Development , 169–176. [Google Scholar]
- Spurrier, M. , & Blaszczynski, A. (2014). Risk perception in gambling: A systematic review. Journal of Gambling Studies , 30(2), 253–276. 10.1007/s10899-013-9371-z. [DOI] [PubMed] [Google Scholar]
- Thirion, B. , Pinel, P. , Mériaux, S. , Roche, A. , Dehaene, S. , & Poline, J.-B. (2007). Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage , 35(1), 105–120. [DOI] [PubMed] [Google Scholar]
- Tian, M. , Tao, R. , Zheng, Y. , Zhang, H. , Yang, G. , Li, Q. , & Liu, X. (2018). Internet gaming disorder in adolescents is linked to delay discounting but not probability discounting. Computers in Human Behavior , 80, 59–66. 10.1016/j.chb.2017.10.018. [DOI] [Google Scholar]
- Vincent, J. L. , Kahn, I. , Snyder, A. Z. , Raichle, M. E. , & Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology , 100(6), 3328–3342. 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viriyavejakul, C. (2008). Recreational gaming behavior of undergraduate students in Thailand . Paper presented at the Society for Information Technology & Teacher Education International Conference . [Google Scholar]
- Wagner, F. A. , & Anthony, J. C. (2007). Male–female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug and Alcohol Dependence , 86(2–3), 191–198. [DOI] [PubMed] [Google Scholar]
- Wang, M. , Dong, G. , Wang, L. , Zheng, H. , & Potenza, M. N. (2018). Brain responses during strategic online gaming of varying proficiencies: Implications for better gaming. Brain and Behavior , 8(8), e01076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Dong, H. , Zheng, H. , Du, X. , & Dong, G. H. (2020). Inhibitory neuromodulation of the putamen to the prefrontal cortex in Internet gaming disorder: How addiction impairs executive control. Journal of Behavioral Addictions , 9(2), 312–324. 10.1556/2006.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Liu, X. , Hu, Y. , Zheng, H. , Du, X. , & Dong, G. (2019). Altered brain functional networks in internet gaming disorder: Independent component and graph theoretical analysis under a probability discounting task. CNS Spectrums , 1–13. 10.1017/S1092852918001505. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Wu, L. , Lin, X. , Zhang, Y. , Zhou, H. , Du, X. , & Dong, G. (2016). Dysfunctional default mode network and executive control network in people with Internet gaming disorder: Independent component analysis under a probability discounting task. European Psychiatry , 34, 36–42. 10.1016/j.eurpsy.2016.01.2424. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Wu, L. , Wang, Y. , Li, H. , Liu, X. , Du, X. , & Dong, G. (2017). Altered brain activities associated with craving and cue reactivity in people with internet gaming disorder: Evidence from the comparison with recreational internet game users. Frontiers in Psychology , 8, 1–12. 10.3389/fpsyg.2017.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Yang, G. , Zheng, Y. , Li, Z. , Qi, Y. , Li, Q. , & Liu, X. (2021). Enhanced neural responses in specific phases of reward processing in individuals with Internet gaming disorder. Journal of Behavioral Addictions , 10(1), 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Zhang, Y. , Lin, X. , Zhou, H. , Du, X. , & Dong, G. (2018). Group independent component analysis reveals alternation of right executive control network in Internet gaming disorder. CNS Spectrums , 23(5), 300–310. 10.1017/S1092852917000360. [DOI] [PubMed] [Google Scholar]
- Weber, E. U. , Blais, A. R. , & Betz, N. E. (2002). A domain‐specific risk‐attitude scale: Measuring risk perceptions and risk behaviors. Journal of Behavioral Decision Making , 15(4), 263–290. 10.1002/bdm.414. [DOI] [Google Scholar]
- Weber, B. J. , & Huettel, S. A. (2008). The neural substrates of probabilistic and intertemporal decision making. Brain Research , 1234, 104–115. 10.1016/j.brainres.2008.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widyanto, L. , & Mcmurran, M. (2004). The psychometric properties of the internet addiction test. Cyberpsychology & Behavior , 7(4), 443. 10.1089/cpb.2004.7.443. [DOI] [PubMed] [Google Scholar]
- Yao, Y. , Wang, L. , Yip, S. W. , Chen, P. , Li, S. , Xu, J. ,… Fang, X. (2015). Impaired decision-making under risk is associated with gaming-specific inhibition deficits among college students with Internet gaming disorder. Psychiatry Research , 229(1–2), 302–309. 10.1016/j.psychres.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Young, K. S. (1998). Caught in the net: How to recognize the signs of internet addiction--and a winning strategy for recovery . New York: John Wiley & Sons, Inc. [Google Scholar]