Abstract
Background and aims
Parkinson's disease (PD) is one of the most prevalent neurodegenerative diseases. First-line medications consist of drugs that act by counteracting dopamine deficiency in the basal ganglia. Unfortunately, iatrogenic impulsive-compulsive behaviors (ICBs) can occur in up to 20% of PD patients over the course of their illness. ICBs must be considered multifactorial disorders that reflect the interactions of the medication with an individual's vulnerability and the underlying neurobiology of PD. We aimed to explore the predictive genetic, psychopathological and neurological factors involved in the development of ICBs in PD patients by building a complete model of individual vulnerability.
Methods
The PARKADD study was a case/non-case study. A total of 225 patients were enrolled (“ICB” group, N = 75; “no ICB” group, N = 150), and 163 agreed to provide saliva samples for genetic analysis. Sociodemographic, neurological and psychiatric characteristics were assessed, and genotyping for the characterization of polymorphisms related to dopaminergic and opioid systems was performed.
Results
Factors associated with “ICBs” were younger age of PD onset, personal history of ICB prior to PD onset and higher scores on the urgency and sensation seeking facets of impulsivity. No gene variant was significantly associated, but the association with the opioid receptor mu 1 (OPRM1) rs1799971 polymorphism was close to significance.
Discussion and conclusions
The influence of gene-environment interactions probably exists, and additional studies are needed to decipher the possible role of the opioid system in the development of ICBs in PD patients.
Keywords: Parkinson's disease; disruptive; impulse control, and conduct disorders; addictive behavior; opioid receptor mu 1; predictive model; vulnerability
Introduction
Strong evidence supports the need to initiate pharmacological treatment as soon as the diagnosis of Parkinson's disease (PD) is confirmed, especially when functional impairments are present (Haute-Autorité-de-Santé, 2016; NICE, 2017; Orayj & Lane, 2019; Pirtosek et al., 2020). When treating PD with dopamine replacement therapy, the main goal is to target dopamine receptors in the nigrostriatal pathway to alleviate motor symptoms. However, drug action is rarely limited to one particular region of the brain, and these medications also impact dopamine receptors in the mesocorticolimbic and tuberoinfundibular pathways, leading to specific side effects (Ritter et al., 2020). In particular, iatrogenic impulsive-compulsive behaviors (ICBs), which likely result from hyperactivity in the mesocorticolimbic pathway, can occur in up to 20% of PD patients over the course of their illness (Weintraub & Claassen, 2017).
Some of these induced ICBs relate to daily life reward-driven behaviors such as eating, sexuality, shopping, or gambling, which become excessive and out of control, such that they take the form of impulse control disorders (Ceravolo, Rossi, Del Prete, & Bonuccelli, 2016). Due to their neurobiological, neurocognitive and clinical similarities with substance use disorders, ICBs are also commonly called “behavioral addictions”, even though not all of them are grouped in the “Substance related and addictive disorders” category in the fifth version of the diagnostic and statistical manual of mental disorders (DSM-5) (APA, 2013). Other ICBs that are marked by a more pronounced compulsive dimension have also been observed. In particular, these include obsessive hobbying, hoarding, punding and compulsive medication use in the context of dopaminergic dysregulation syndrome (DDS) (Aoki, Shiraishi, Mikami, & Kamo, 2019; Giovannoni, O'Sullivan, Turner, Manson, & Lees, 2000).
Reflecting dysfunction in both emotional and behavioral regulation, ICBs undoubtedly have a negative impact on patients' health-related quality of life and satisfaction with life and on caregivers' distress (Dujardin & Sgambato, 2020; Erga, Alves, Tysnes, & Pedersen, 2020). Therefore, it is crucial to prevent their occurrence. To address this problem, clinicians have been encouraged to adopt the “P4 medicine” approach (Grall-Bronnec et al., 2018). One of the key stages of P4 medicine is to promote a more systematic comprehensive assessment for better identification of patients at high risk of ICBs.
From a pathophysiological point of view, ICBs must be considered as multifactorial disorders that reflect interactions of the medication with an individual's vulnerability and the underlying neurobiology of PD (Voon et al., 2017). Indeed, ICBs occur under the combined influence of various factors. The most robust findings have suggested a role for male gender, younger age, single marital status, history of psychiatric symptoms or addictive disorders, earlier onset of disease, longer disease duration, rapid eye movement sleep behavior disorder, motor complications, treatment with drugs having a higher selectivity for D3 receptors, higher levodopa equivalent daily dose, immediate release formulations and higher peak dopamine agonist dose (Gatto & Aldinio, 2019; Grall-Bronnec et al., 2018).
Surprisingly, studies assessing impulsivity - whether the impulsivity personality trait as such or the impulse dimension of a specific mental health disorder - in PD patients with ICBs are relatively scarce. On the one hand, the few existing studies on trait impulsivity found higher scores among PD patients with ICBs than among those without ICBs (Isaias et al., 2008; Saez-Francas et al., 2016; Voon et al., 2011) and a link between impulsivity and ICB severity (Marin-Lahoz et al., 2018). On the other hand, studies investigating the link between PD and mental health disorders that include a high level of impulsivity, such as addictive disorders, attention-deficit/hyperactivity disorder (ADHD) and antisocial personality disorder, are even rarer. Some authors suggested that history of cigarettes smoking and drug abuse increased the risk for ICBs (Gatto & Aldinio, 2019). Regarding ADHD, Fan et al. (2020) concluded that PD patients were almost 3 times more likely to exhibit a history of ADHD than controls without PD (Fan et al., 2020). Finally, Gerscheidt et al. (2016) showed that early-onset PD patients with ICBs scored higher on Self-assertive/Antisocial personality style (Gerscheidt et al., 2016).
Genetic factors were more recently identified, with single nucleotide polymorphisms (SNPs) in dopaminergic, glutamatergic, serotoninergic and opioid neurotransmitter systems being potential predictors of ICBs (Gatto & Aldinio, 2019), as already shown for addictive disorders in non-PD subjects (Cilia et al., 2016). However, a comparison between models including either clinical variables or clinical and genetic variables did not demonstrate that the selected genetic variables contribute to better predictions of the development of ICBs in a multivariate analysis (Redensek, Jenko Bizjan, Trost, & Dolzan, 2020).
We therefore aimed to explore the predictive factors involved in the development of ICBs in PD patients by building a complete model of individual vulnerability. For this reason, we did not consider iatrogenic factors.
To the best of our knowledge, the current study is the first attempt to take into account both disease-related factors and psychopathological factors, with a focus on factors associated with addiction vulnerability, including genetic factors that may predispose PD patients to develop iatrogenic ICBs.
Material and methods
The PARKADD (PARK: PARKinson's disease; ADD: behavioral ADDictions) study resulted from the collaboration of specialists in neurology, psychiatry, and pharmacogenetics.
Procedure
The PARKADD (NCT01733199) study was a monocenter hospital-based prospective case/non-case study conducted between October 2012 and March 2017. It was initially designed to assess the factors associated with ICBs in PD patients, associated or not with DDS. For this purpose, patients were divided into three groups based on the presence of ICB and DDS. However, as only one patient was identified as meeting the criteria for DDS, we focused only on the first two groups, namely, PD patients without ICB vs PD patients with ICBs, and the patient having DDS was classified in the latter group.
At the time of construction of the study, only a cross-sectional assessment was planned. Due to a financial opportunity, we were able to add a follow-up to explore the clinical outcomes of patients (especially with regard to PD progression), and certain genetic markers. To this end, participants were contacted by phone at least 12 months after inclusion. Those who agreed received an informed consent form to sign, a set of self-report questionnaires to complete, and a kit to collect a saliva sample. For the present work, only genetic data were used from the follow-up assessment.
Participants
Our intention was to conduct the study in “real-life” conditions, excluding as few patients as possible, regardless of their history. The sample consisted of idiopathic PD patients aged 18 years and over who received PD treatment for at least 6 months. Subjects with deep brain stimulation, cognitive impairment, psychotic symptoms or under guardianship were not included. A total of 225 patients were enrolled: patients with at least one ICB occurring or worsening after the beginning of PD (cases: “ICB” group, N = 75) and patients with no ICB occurring or worsening after the beginning of PD (non-cases: “no ICB” group, N = 150).
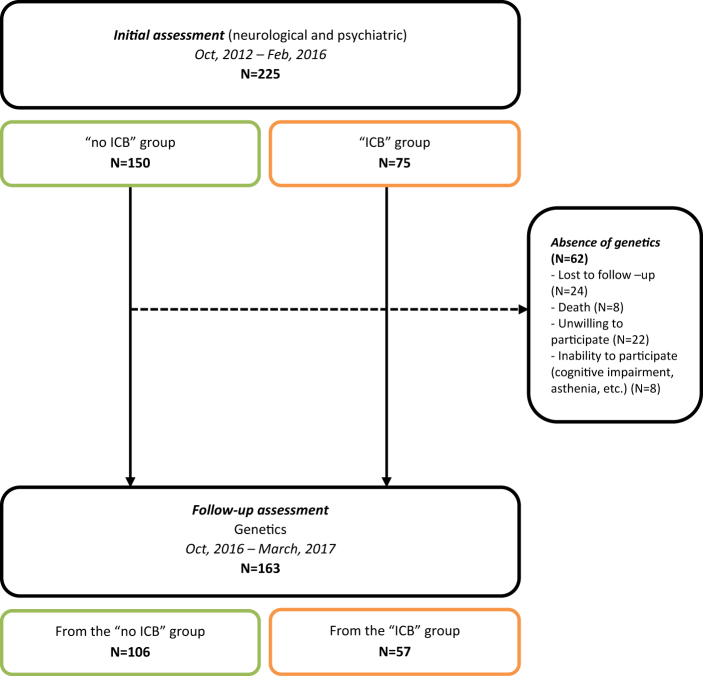
Of the 225 patients enrolled, 62 dropped out from the follow-up; 163 agreed to complete self-rated questionnaires and provide saliva samples, of whom 106 were from the “no ICB” group and 57 were from the “ICB” group at baseline. Participant selection is described in the flow chart provided in Fig. 1.
Fig. 1.
Flow chart of participant selection
The sample for the present analysis consisted only of patients for whom clinical and genetic data were available to be able to model vulnerability to ICBs.
Measures
Sociodemographic characteristics
We collected information about age and sex.
Neurological characteristics
A neurological examination was performed by a movement disorders specialist (TR or PD) and included the Unified Parkinson's Disease Rating Scale (UPDRS) part III (Fahn, Elton, & Members-of-UPDRS-Development-Committee., 1987; Movement-Disorder-Society-Task-Force-on-Rating-Scales-for-Parkinson's-Disease., 2003), Hoehn and Yahr staging (Hoehn & Yahr, 1967), the severity of dyskinesia and their type (chorea, dystonia) as evaluated by the Unified Dyskinesia Rating Scale (UDysRS) (Goetz, Nutt, & Stebbins, 2008), and a collection of various types of data related to PD, including age of onset, duration of the disease, duration of PD treatment, and family history of PD. The Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) was used to exclude patients with cognitive impairment (score <24/30).
Psychiatric characteristics
A face-to-face interview with a trained rater explored the history of addictive disorders: misuse of PD treatment, notably as part of DDS, was assessed with the Giovanni criteria (Giovannoni et al., 2000); personal history of addictive disorders was explored using the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) for substance use disorders (to be exhaustive, we explored the following substances: alcohol, nicotine, medications, and illicit drugs) and the Minnesota Impulsive Disorder Interview (MIDI) (Chamberlain & Grant, 2019) for the disorders regrouped under the label “ICBs” (compulsive buying, pathological gambling, compulsive sexual behavior, binge eating and punding behavior). Regarding personal history of ICBs, two time periods were considered, before and after PD onset. Family history of addictive disorders was also explored. Impulsivity profiles were assessed with the UPPS Impulsive Behavior Scale (Whiteside, Lynam, Miller, & Reynolds, 2005) and by exploring the history of ADHD in childhood (Wender-Utah Rating Scale-Child, WURS-C) (Ward, Wender, & Reimherr, 1993) and in adulthood (Adult ADHD Self-report Scale, ASRS-Screener v1.1) (Kessler et al., 2005). Based on the results of these questionnaires, it was possible to screen for the presence of ADHD in childhood (WURS-C score ≥46/100) and to specify whether ADHD likely persisted in adulthood (WURS-C score ≥46/100 AND at least 4 checkmarks in the darkly shaded area of the ASRS Screener v1.1). The presence of an antisocial personality disorder was diagnosed using the MINI.
Genetics
Saliva samples were sent to the INSERM U894 Center for Psychiatry and Neurosciences (Paris). DNA was extracted according to the protocol provided by the manufacturer of the saliva collection kits (DNA Genotek | Oragene DNA | DNA Saliva Collection | OG-500 Tube). The DNA concentration was measured by spectrophotometry on a Thermo Fisher Scientific NanodropTM 1000 apparatus. DNA concentrations ranged between 5 and 1000 ng μl−1. The DNAs were diluted and aliquoted to a final volume of 100 μl in 96-well plates at a concentration between 5 and 10 ng μl−1.
Genotyping for the characterization of polymorphisms related to the dopaminergic and opioid systems were carried out using quantitative real-time PCR (TaqMan SNP genotyping assay, Life Technologies). A total of 163 DNAs were analyzed. The study focused on 50 SNPs of 15 genes involved in the dopaminergic system (dopamine receptors DRD1, DRD2, DRD3, DRD4 and DRD5, dopamine transporter DAT1/SLC6A3, dopamine beta-hydroxylase DBH, dopa decarboxylase DDC, tyrosine hydroxylase TH), the catabolism of amines (including dopamine) (catechol-O-methyltransferase COMT, monoamine oxidase MAO-A and MAO-B), the opioid system (opioid receptor mu MOR/OPRM1 and opioid receptor kappa KOR/OPRK1), and brain-derived neurotrophic factor (BDNF).
Ethics
The study was approved by the French Research Ethics Committee (CPP) Nantes (inclusion of patients) and Tours (patients' follow-up) ethics committees and conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. Written informed consent was collected from all participants.
Statistical analyses
First, sociodemographic, clinical and genetic characteristics of the whole sample were described by means and standard deviations for continuous variables and by numbers and percentages for categorical variables.
Then, we divided the sample into two groups based on status at inclusion (“no ICB” and “ICB” groups) and compared these groups thanks to bivariate analyses (Chi-square tests or Fisher's tests for qualitative variables, and Student's or Wilcoxon tests for quantitative variables).
Thereafter, we performed a multivariate logistic regression analysis in order to identify the variables that were significantly associated with the “ICB” status, as assessed by the likelihood ratio test. Only variable that were associated with the “ICB” status in bivariate analyses at a P < 0.20 level of significance (with the exception of variables for which the number of patients was zero for a modality in a group because the convergence of the model would be impossible) (Mickey & Greenland, 1989) were integrated as candidates in the model. Then, backward selection was applied using a P < 0.05 level of significance in order to retain only variables that provided significant information in the model. Adjustment for genetic characteristics associated with the “ICB” status in bivariate analyses was maintained in the final multivariate model. The odds ratio and associated 95% confidence interval of the final model were estimated to quantify the strength of the association between the final factors retained and the “ICB” status. Finally, the quality of the model was investigated through the area under the receiver operating characteristic (ROC) curve (ability of the final model to discriminate between the presence or absence of an ICB), and the Hosmer-Lemeshow test (goodness-of-fit of the model).
Finally, due to the high dropout rate, we conducted a sensitivity analysis to assess whether patients who dropped out were different at baseline from those who participated in the follow-up and to confirm the robustness of our results, especially regarding genetics. The two groups were compared on all the variables.
The statistical analyses were carried out with SAS 9.4 statistical software (SAS Institute, Inc.).
Results
Sensitivity analysis
There were no substantial differences in the distribution of baseline characteristics between patients who participated in the follow-up and those who did not (see Appendix, Table A1). Importantly, they did not differ with respect to the presence of at least one ICB occurring or worsening after the beginning of PD, the history of addictive disorders or the level of impulsivity. The only differences involved the severity of PD, which could explain dropouts due to death or inability to participate in follow-up assessments.
Description of the sample used for analysis at the time of inclusion
Sociodemographic characteristics
As shown in Table 1, more than two-thirds (69.3%) of the sample were men. The mean age was 62.5 years (±7.8).
Table 1.
Bivariate analyses: baseline characteristics of patients with and without ICBs (N = 163)
| Total sample (N = 163) | No ICB (N = 106) | ICB (N = 57) | P-value | |
| Mean (sd) or number of patients (%) | ||||
| Sociodemographic | ||||
| Age (years) | 62.5 (7.8) | 63.8 (7.7) | 60.2 (7.4) | 0.0048 |
| Sex (male) | 113 (69.3%) | 72 (67.9%) | 41 (71.9%) | 0.5969 |
| Neurological | ||||
| MMSE score (/30) | 28.5 (1.6) | 28.4 (1.7) | 28.6 (1.6) | 0.4133 |
| Age of PD onset (years) | 55.3 (8.0) | 56.8 (7.6) | 52.5 (8.1) | 0.0012 |
| PD duration (years) | 10.1 (4.4) | 9.8 (4.5) | 10.5 (4.1) | 0.3715 |
| PD treatment duration (years) | 6.9 (4.3) | 6.7 (4.6) | 7.4 (4.0) | 0.3308 |
| Family history of PD (yes) | 36 (22.1%) | 22 (20.8%) | 14 (24.6%) | 0.5764 |
| Hoehn and Yahr stage | 0.9423 | |||
| - 0 | 3 (1.9%) | 2 (1.9%) | 1 (1.8%) | |
| - 1 | 56 (34.6%) | 38 (35.9%) | 18 (32.1%) | |
| - 2 | 60 (37.0%) | 39 (36.8%) | 21 (37.5%) | |
| - ≥ 3 | 43 (26.5%) | 27 (25.5%) | 16 (28.57%) | |
| On dopa UPDRS –III (/108) | 15.2 (10.7) | 15.3 (10.7) | 14.9 (11.0) | 0.8390 |
| On dopa axial sub-score (/32)* | 3.9 (3.8) | 3.9 (3.7) | 3.98 (4.1) | 0.8604 |
| Dyskinesia (presence) | 76 (46.6%) | 51 (48.1%) | 25 (43.9%) | 0.6037 |
| Dyskinesia severity | 0.8 (0.9) | 0.8 (1.0) | 0.7 (0.9) | 0.8118 |
| Dyskinesia type | ||||
| - Chorea | 74 (46.0%) | 50 (47.6%) | 24 (42.9%) | 0.5636 |
| - Dystonia | 17 (16.4%) | 9 (14.1%) | 8 (20.0%) | 0.4257 |
| Psychiatric | ||||
| Family history | ||||
| - at least one substance** use disorder | 97 (59.5%) | 68 (64.2%) | 29 (50.9%) | 0.0997 |
| - at least one ICB*** | 11 (6.8%) | 6 (5.7%) | 5 (8.8%) | 0.5182 |
| Personal history of substance* use disorder before the PD onset | ||||
| - at least one substance use disorder | 29 (17.8%) | 15 (14.2%) | 14 (24.6%) | 0.0975 |
| - nicotine dependence | 24 (15.2%) | 12 (11.7%) | 12 (21.8%) | 0.0898 |
| - alcohol use disorder | 8 (5.6%) | 3 (3.2%) | 5 (10.4%) | 0.1198 |
| - medication use disorder | 0 (0%) | 0 (0%) | 0 (0%) | |
| - illicit drug use disorder | 3 (2.2%) | 1 (1.1%) | 2 (4.4%) | 0.2511 |
| Personal history of ICB*** before the PD onset | ||||
| - at least one ICB*** | 17 (10.4%) | 5 (4.7%) | 12 (21.1%) | 0.0011 |
| - compulsive sexual behavior | 2 (1.4%) | 0 (0%) | 2 (4.3%) | 0.0994 |
| - binge eating | 9 (5.8%) | 2 (1.9%) | 7 (13.5%) | 0.0070 |
| - pathological gambling | 3 (2.0%) | 2 (1.9%) | 1 (2.2%) | 1.0000 |
| - compulsive buying | 4 (2.7%) | 1 (1.0%) | 3 (6.3%) | 0.0967 |
| - punding behavior | 1 (0.7%) | 0 (0%) | 1 (2.2%) | 0.3129 |
| Personal history of substance* use disorder after the PD onset | ||||
| - at least one substance use disorder | 10 (6.1%) | 5 (4.7%) | 5 (8.8%) | 0.3214 |
| - nicotine dependence | 7 (4.4%) | 4 (3.8%) | 3 (5.5%) | 0.6925 |
| - alcohol use disorder | 2 (1.3%) | 0 (0%) | 2 (3.7%) | 0.1199 |
| - medication use disorder | 0 (0%) | 0 (0%) | 0 (0%) | |
| - illicit drug use disorder | 1 (0.7%) | 1 (1.0%) | 0 (0%) | 1.0000 |
| Antisocial personality disorder | 0 (0%) | 0 (0%) | 0 (0%) | |
| Impulsivity dimensions | ||||
| - UPPS-Urgency (/48) | 25.2 (7.0) | 23.6 (5.8) | 28.2 (8.1) | 0.0003 |
| - UPPS-(lack of Premeditation) (/48) | 17.4 (5.0) | 16.9 (4.5) | 18.4 (5.7) | 0.0848 |
| - UPPS-(lack of Perseverance) (/48) | 16.9 (4.6) | 16.5 (4.5) | 17.6 (4.8) | 0.1609 |
| - UPPS-Sensations Seeking (/48) | 22.1 (7.0) | 21.1 (6.6) | 24.0 (7.5) | 0.0128 |
| ADHD | ||||
| - In childhood (WURS-C ≥46/100) | 11 (6.8%) | 4 (3.8%) | 7 (12.3%) | 0.0513 |
| - Persistent in adulthood (WURS-C ≥46/100 AND ASRS ≥4/6) | 6 (3.7%) | 2 (1.9%) | 4 (7.0%) | 0.1847 |
ADHD: attention deficit/hyperactivity disorder; ASRS: adult ADHD self-report scale; ICB: impulsive-compulsive behavior; MMSE: mini-mental state examination; MP: Parkinson's disease; sd: standard deviations; UPDRS: unified Parkinson's disease rating scale; UPPS: UPPS impulsive behavior scale; WURS-C: Wender-Utah Rating Scale-Child.
*: axial sub-score was based on the assessment of speech, facial expression, neck rigidity, arising from chair, gait, postural stability, posture, body bradykinesia; **: “substance” refers to nicotine, alcohol, medication, and illicit drug; ***: “ICB” refers to compulsive sexual behavior, binge eating, pathological gambling, compulsive buying and punding behavior.
Neurological characteristics
PD began on average ten years before inclusion (55.3 years ±8) and was treated on average for 6.9 years (±4.4). A family history of PD was reported for almost one-quarter (22.1%) of the patients.
The majority of the patients had PD stage II (37.0%) without any dyskinesia (53.4%). As expected based on the exclusion criteria, the cognitive state was normal, with a mean MMSE score of 28.5 (±1.6)/30.
Psychiatric characteristics
A family history of substance use disorders was reported by more than half of the sample (59.5%), but a personal history of substance use disorders prior to PD onset was found in only 17.8% of the patients and even less after PD onset (6.1%).
A personal history of ICBs prior to PD onset was noticed in 10.4% of the sample, but the proportion reached 36.2% after PD onset due to the selection of the participants.
Regarding ICBs occurring or worsening after PD onset, binge eating was the most prevalent disorder (N = 22), followed by compulsive sexual behavior (N = 19), pathological gambling (N = 15) and compulsive buying (N = 12). Punding behavior was diagnosed in only 6 patients. A substantial proportion of the patients had more than one ICB (N = 16, 28.1%).
Overall, patients had a low level of impulsivity, as shown in Table 1. Averaged scores on the UPPS questionnaire were low for the 4 dimensions. In addition, almost the entire sample was free of ADHD in childhood (93.3%) or in adulthood (96.3%). No participant was diagnosed with an antisocial personality disorder.
Factors associated with the occurrence or worsening of an ICB after PD onset
Bivariate analyses were conducted to compare sociodemographic, clinical and genetic characteristics between the two groups of patients. The results are shown in Tables 1 and 2.
Table 2.
Bivariate analyses: genetic characteristics of patients with and without ICBs (N = 156)
(among all the SNPs explored, the OPRM1 rs1799971 polymorphism was the only potential candidate for the final multivariate model)
| Genotype | Total sample (N = 156) | No ICB (N = 102) | ICBs (N = 54) | P-value | |
| OPRM1 rs1799971 | AA | 109 (69.9%) | 65 (63.7%) | 44 (81.5%) | 0.0541 |
| AG | 40 (25.6%) | 32 (31.4%) | 8 (14.8%) | ||
| GG | 7 (4.5%) | 5 (4.9%) | 2 (3.7%) |
In the whole sample, the allele frequency was 82.7% for the A allele (258/312) and 17.3% for the G allele (54/312).
The G allele frequency differed across groups: 11.1% in the “ICB” group (12/108) and 20.6% in the “no ICB” group (42/204).
We included in the multivariate logistic regression the 12 variables that were associated with “ICB” at the 0.20 level of significance in the bivariate analyses, namely age, age of PD onset, family history of at least one substance use disorder, personal history of at least one substance use disorder and of at least one ICB before the PD onset, four scores the UPPS Impulsive Behavior Scale, ADHD in childhood and persistent in adulthood, and OPRM1 rs1799971 polymorphism.
After excluding observations with missing data, 156 patients were included in the multivariate analysis. Only four variables were found to be independently associated with the occurrence or worsening of an ICB after PD onset: younger age of PD onset, personal history of ICB prior to PD onset and higher score on the UPPS-P urgency and sensation seeking scales.
The Hosmer-Lemeshow goodness-of-fit test showed that the final model was well calibrated, with P = 0.1714 (P-value >0.05 indicates good model fit), and the area under the ROC curve was 0.77 [0.68; 0.85], showing that the model discriminated well between patients with “no ICB” and patients with “ICBs”.
Table 3 shows the results of the “ICB” model.
Table 3.
Factors associated with the occurring or worsening of an ICB after the PD onset (N = 156)
| Variables | Adjusted OR | [CI95%] | P-value |
| Age of PD onset | 0.94 | [0.89; 0.99] | 0.0139 |
| Personal history of ICB before the PD onset | 4.06 | [1.23; 13.49] | 0.0220 |
| UPPS-Urgency | 1.08 | [1.02; 1.14] | 0.0109 |
| UPPS-Sensations Seeking | 1.06 | [1.00; 1.12] | 0.0361 |
| OPRM1-rs1799971 (absence G vs presence G) | 2.15 | [0.88; 5.28] | 0.0936 |
OR: Odds Ratio; [CI95%]: Confidence Interval of 95%
Discussion
Main results
Our study focused on predictive factors involved in the development of ICBs in PD patients. Several key findings should be highlighted.
First, it is important to note the specific distribution of ICBs in our sample, which is quite similar to that of Jesus et al. (2020) (Jesus et al., 2020). In contrast to some studies (see (Grall-Bronnec et al., 2018) for a review), binge eating was the most frequently observed ICB. This discrepancy could be explained by the lack of consensus on the diagnostic criteria and assessment tools that were used. It can also be assumed that binge eating is underdiagnosed because it is usually not associated with negative consequences for relatives (unlike compulsive sexual behavior or pathological gambling) and is therefore less reported by them. That said, binge eating causes individual distress and negative consequences and must therefore be systematically screened by clinicians.
Second, as expected, we found that a younger age at PD onset was an independent predictor for the occurrence or worsening of an ICD during the course of disease. This result is in line with numerous previous studies (Grall-Bronnec et al., 2018; Smith, Xie, & Weintraub, 2016) and could be intuitively associated with PD duration (Callesen, Weintraub, Damholdt, & Moller, 2014; Pontieri et al., 2015) and treatment duration (Giladi, Weitzman, Schreiber, Shabtai, & Peretz, 2007; Hassan et al., 2011). Interestingly, and in keeping with the findings from Jesus et al. (2020), these two characteristics did not differ between our two groups of patients (Jesus et al., 2020). According to some authors, the crucial role of younger age of PD onset could be explained by greater dopamine transporter deficits, signifying more nigrostriatal dopamine deficiency (Weintraub & Mamikonyan, 2019).
Third, having higher scores on the UPPS urgency and sensation seeking scales appear to be predictive factors of ICBs. Impulsivity was the most assessed personality dimension in studies exploring the link between PD and ICBs (Grall-Bronnec et al., 2018), but to the best of our knowledge, only a few studies have used the UPPS to explore impulsivity among PD patients (Bayard et al., 2016; Grall-Bronnec et al., 2016; Hlavata et al., 2020). One such study concluded that patients with PD had higher impulsivity than controls and that those with impulse control disorders had higher levels of sensation seeking than patients without (Bayard et al., 2016). A study assessing the links between decision-making and impulsivity among healthy volunteers found that high scores on the sensation seeking and urgency facets of impulsivity led to disadvantageous decisions relying on explicit information (Bayard, Raffard, & Gely-Nargeot, 2011). These two facets appear to be closely related to emotion regulation, especially the urgency facet. Thus, according to the “self-medication hypothesis”, maladaptive behaviors such as ICBs could be understood in the context of PD as a way to cope with the experience of negative emotion in the short term (Rochat, Billieux, Gagnon, & Van der Linden, 2018).
Fourth, having a personal history of ICB before PD onset was also identified as a risk factor for the occurrence or worsening of ICBs. This result was rarely reported (Jesus et al., 2020; Olley, Blaszczynski, & Lewis, 2015), perhaps because exploring lifetime ICBs among PD patients is rarely performed. However, an association between a history of addictive disorders and the occurrence of ICBs during the course of PD had been identified by some authors, but mainly regarding alcohol or other substance use disorders (Grall-Bronnec et al., 2018). The occurrence of an ICB could be explained by the “underlying addictive process” (Goodman, 2008). People who had an addiction in the past remain vulnerable and at higher risk of subsequent relapse, even after a long period of abstinence, especially if they are exposed to negative life events, such as a neurodegenerative disease. The revised I-PACE model fits into this perspective by postulating that the person may engage in certain excessive behaviors to relieve negative affects and modify his/her own coping styles over time (Brand et al., 2019).
Finally, no gene variant was significantly associated with the occurrence or worsening of an ICB during the course of PD in our model. However, the OPRM1 rs1799971 polymorphism (absence vs presence of the G allele) was close to significance, as previously found by Cormier-Dequaire et al. (Cormier-Dequaire et al., 2018). The recruitment in their study was quite different from our study, as their participants were free of any history of ICB and only differed by the occurrence of at least one ICB during the course of PD for the cases. In contrast, we decided to include PD patients regardless of their ICB history using “real-life” conditions. This was a pragmatic choice, since ICBs are associated with high prevalence rates in the general population (Calado & Griffiths, 2016; Imperatori et al., 2016; Chamberlain & Grant, 2019) and could therefore affect people prior to PD onset and the initiation of dopaminergic treatment. A history of at least one ICB before PD onset was found in 4.7% of our non-cases. By placing ourselves in this less contrasting situation, we could have speculated it was more challenging to highlight a difference regarding the OPRM1 rs1799971 polymorphism. Post hoc analyses were performed to test this hypothesis (exclusion of patients with a history of at least one ICB before PD onset), but we were unable to demonstrate a significant association between the OPRM1 rs1799971 polymorphism and the occurrence or worsening of an ICB during the course of PD (results not showed).
Strengths and weaknesses
There are several limitations of our study. First, participants were not enrolled strictly at the time when the ICB occurred. However, the clinical interview rigorously assessed the relative chronological course of both PD and ICB. Second, we used self-report measures of impulsivity, with the current period as the reference period, which did not allow us to explore premorbid functioning. Third, 27.6% of the sample dropped out from the follow-up, which could have constituted a source of bias. This was addressed by conducting a sensitivity analysis that concluded that there was no differential loss to follow-up. Finally, we failed to identify any significant association with genetic characteristics, likely due to the relatively small sample size.
However, the strengths of the study compensate for these limitations. Our sample consisted of a broad range of PD patients, including some with a long disease duration. This allowed us to evaluate patients with ICBs that reoccurred or worsened in the course of the disease, in addition to subjects with de novo ICBs. Furthermore, ICBs and other addictive disorders were diagnosed using standardized clinical interviews, which guaranteed the validity of their identification. Finally, the originality of our study relies on the choice of a multiaxial assessment, considering sociodemographic, clinical and genetic characteristics.
Conclusion
Prevention of ICBs in patients with PD is a public health challenge, both in light of the high prevalence of PD in the general population and the high prevalence of ICBs in the specific population suffering from PD. As such, recommendations have been published helping clinicians manage ICBs from a P4 medicine perspective. The first and inescapable step of the strategy is to identify patients who are at high risk for developing ICB early. Predictive medicine could be achieved by encouraging a more systematic comprehensive assessment of patients. It therefore appears essential to guide clinicians in their assessment, emphasizing the clinical elements that should be considered to conclude that an individual is vulnerable. The influence of gene-environment interactions probably exists, and additional studies are needed to decipher the possible role of the opioid system in the development of ICBs in PD patients.
Funding sources
The PARKADD study was supported by a grant from the French National Research Agency (ANR-SAMENTA call for proposal, 2012, decision number: ANR 12 SAMA 013 01). It received an additional funding as part of labeling by the Atlanpole Biotherapies competitiveness cluster.
This research was conducted at the initiative of and coordinated by the Addictology and Psychiatry Department of Nantes University Hospital. Nantes University Hospital is the sponsor of this study. There were no constraints on publishing.
Authors'contribution
Study concept and design: MGB, CVV, JBH, MGL, PD, GCB. Analysis and interpretation of data: MGB, CVV, AV, BS, PD, GCB. Statistical analysis: FF. Obtained funding: MGB, GCB. Study supervision: MGB, GCB. Inclusion of the participants and development of the protocol (selection of the assessment content): MGB, TR, JL, ET, MR, PD, GCB. Drafting of manuscript: MGB, CVV, FF, PD, GCB. Critical revision: TR, AV, BS, JL, ET, MR, JBH, MGL. All authors had full access to all data in the study and take the responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank Nicolas Ramoz and Philip Gorwood (INSERM U894 Center for Psychiatry and Neurosciences, Paris), who performed the genetic analysis.
Appendix
Table A1.
Baseline characteristics of patients who dropped-out and those who participated in the follow-up (N = 225)
| Total sample (N = 225) | Drop-out (N = 62) | Follow-up (N = 163) | p-value | |
| Mean (sd) or number of patients (%) | ||||
| Sociodemographic | ||||
| Age (years) | 62.6 (8.2) | 62.9 (9.2) | 62.5 (7.8) | 0.7857 |
| Sex (male) | 154 (68.4%) | 41 (66.1%) | 113 (69.3%) | 0.6449 |
| Neurological | ||||
| MMSE score ( /30) | 28.3 (1.7) | 27.9 (1.9) | 28.5 (1.6) | 0.0340 |
| Age of PD onset (years) | 54.9 (8.4) | 53.8 (9.3) | 55.3 (8.0) | 0.2502 |
| PD duration (years) | 10 (4.3) | 8.0 (3.0) | 10.1 (4.4) | 0.2561 |
| PD treatment duration (years) | 7.4 (4.9) | 8.7 (5.9) | 6.9 (4.4) | 0.0365 |
| Family history of PD (yes) | 48 (21.3%) | 12 (19.4%) | 36 (22.1%) | 0.6550 |
| Hoehn and Yahr stage | 0.0006 | |||
| - 0 | 4 (1.8%) | 1 (1.6%) | 3 (1.9%) | |
| - 1 | 63 (28.1%) | 7 (11.3%) | 56 (34.6%) | |
| - 2 | 93 (41.5%) | 33 (53.2%) | 60 (37%) | |
| - ≥ 3 | 64 (28.4%) | 21 (33.9%) | 43 (26.5%) | |
| On dopa UPDRS –III (/108) | 17.2 (12.7) | 22.9 (15.8) | 15.2 (10.7) | 0.0008 |
| On dopa axial sub-score (/32)* | 4.6 (4.4) | 6.4 (5.4) | 3.9 (3.8) | 0.0013 |
| Dyskinesia (presence) | 113 (50.2%) | 37 (59.7%) | 76 (46.6%) | 0.0802 |
| Dyskinesia severity | 0.9 (1.1) | 1.2 (1.3) | 0.8 (0.9) | 0.0117 |
| Dyskinesia type | ||||
| - Chorea (presence) | 110 (49.6%) | 36 (59%) | 74 (46%) | 0.0825 |
| - Dystonia (presence) | 23 (17%) | 6 (19.4%) | 17 (16.4%) | 0.6957 |
| Psychiatric | ||||
| Family history | ||||
| - at least one substance** use disorder | 133 (59.1%) | 36 (58.1%) | 97 (59.5%) | 0.8439 |
| - at least one ICB*** | 13 (5.8%) | 2 (3.2%) | 11 (6.8%) | 0.5229 |
| Personal history of substance* use disorder before the PD onset | ||||
| - at least one substance use disorder | 36 (16.0%) | 7 (11.3%) | 29 (17.8%) | 0.2347 |
| - nicotine dependence | 30 (13.7%) | 6 (9.8%) | 24 (15.2%) | 0.3016 |
| - alcohol use disorder | 9 (4.6%) | 1 (1.8%) | 8 (5.6%) | 0.4498 |
| - medication use disorder | 0 (0%) | 0 (0%) | 0 (0%) | |
| - illicit drug use disorder | 3 (1.6%) | 0 (0%) | 3 (1.6%) | 0.5588 |
| Personal history of ICB*** before the PD onset | ||||
| - at least one ICB*** | 19 (8.4%) | 2 (3.2%) | 17 (10.4%) | 0.0825 |
| - compulsive sexual behavior | 2 (1%) | 0 (0%) | 2 (1.4%) | 1.0000 |
| - binge eating | 9 (4.2%) | 0 (0%) | 9 (5.8%) | 0.0647 |
| - pathological gambling | 4 (1.9%) | 1 (1.6%) | 3 (2%) | 1.0000 |
| - compulsive buying | 4 (1.9%) | 0 (0%) | 4 (2.7%) | 0.5799 |
| - punding behavior | 2 (1%) | 1 (1.6%) | 1 (0.7%) | 0.5015 |
| Personal history of substance** use disorder after the PD onset | ||||
| - at least one substance use disorder | 12 (5.3%) | 2 (3.2%) | 10 (6.1%) | 0.5186 |
| - nicotine dependence | 9 (4.1%) | 2 (3.2%) | 7 (4.4%) | 1.0000 |
| - alcohol use disorder | 2 (0.9%) | 0 (0%) | 2 (1.3%) | 1.0000 |
| - medication use disorder | 0 (0%) | 0 (0%) | 0 (0%) | |
| - illicit drug use disorder | 1 (0.5%) | 0 (0%) | 1 (0.7%) | 1.0000 |
| Personal history of ICB*** after the PD onset | ||||
| - at least one ICB*** | 77 (34.2%) | 18 (29%) | 59 (36.2%) | 0.3116 |
| - compulsive sexual behavior | 24 (14%) | 5 (10.2%) | 19 (15.5%) | 0.3704 |
| - binge eating | 28 (15.9%) | 5 (10.2%) | 23 (18.1%) | 0.1987 |
| - pathological gambling | 22 (12.9%) | 6 (12%) | 16 (13.3%) | 0.8134 |
| - compulsive buying | 16 (9.8%) | 4 (8.3%) | 12 (10.3%) | 0.7810 |
| - punding behavior | ||||
| Antisocial personality disorder | 0 (0%) | 0 (0%) | 0 (0%) | |
| Impulsivity dimensions | ||||
| - UPPS-Urgency (/48) | 2572 (7.5) | 27.2 (8.6) | 25.26 (7) | 0.1030 |
| - UPPS-(lack of Premeditation) (/48) | 17.2 (4.9) | 16.5 (4.8) | 17.4 (5) | 0.2281 |
| - UPPS-(lack of Perseverence) (/48) | 17 (4.7) | 17.3 (5) | 16.9 (4.6) | 0.5564 |
| - UPPS-Sensations Seeking (/48) | 21.7 (7.0) | 20.6 (6.9) | 22.1 (7) | 0.1545 |
| ADHD | ||||
| - In childhood (WURS-C ≥46/100) | 13 (5.8%) | 2 (3.2%) | 11 (6.8%) | 0.5229 |
| - Persistent in adulthood (WURS-C ≥46/100 AND ASRS ≥4/6) | 6 (2.7%) | 0 (0%) | 6 (3.7%) | 0.1912 |
ADHD: attention deficit/hyperactivity disorder; ASRS: adult ADHD self-report scale; ICB: impulsive-compulsive behavior; MMSE: mini-mental state examination; MP: Parkinson’s disease; sd: standard deviations; UPDRS: unified Parkinson's disease rating scale; UPPS: UPPS impulsive behavior scale; WURS-C: Wender-Utah Rating Scale-Child.
*: axial sub-score was based on the assessment of speech, facial expression, neck rigidity, arising from chair, gait, postural stability, posture, body bradykinesia; **: “substance” refers to nicotine, alcohol, medication, and illicit drug; ***: “ICB” refers to compulsive sexual behavior, binge eating, pathological gambling, compulsive buying and punding behavior.
Contributor Information
Marie Grall-Bronnec, Email: marie.bronnec@chu-nantes.fr.
Caroline Victorri-Vigneau, Email: caroline.vigneau@chu-nantes.fr.
Tiphaine Rouaud, Email: tiphaine.rouaud@chu-nantes.fr.
Audrey Verholleman, Email: audrey.verholleman@chu-nantes.fr.
Benoit Schreck, Email: benoit.schreck@chu-nantes.fr.
Juliette Leboucher, Email: juliette.leboucher@chu-nantes.fr.
Elsa Thiabaud, Email: elsa.thiabaud@chu-nantes.fr.
Fanny Feuillet, Email: fanny.feuillet@chu-nantes.fr.
Monica Roy, Email: monica.roy@chu-nantes.fr.
Jean-Benoit Hardouin, Email: jean-benoit.hardouin@univ-nantes.fr.
Morgane Guillou-Landreat, Email: rgane77@hotmail.com.
Pascal Derkinderen, Email: pascal.derkinderen@chu-nantes.fr.
Gaëlle Challet-Bouju, Email: gaelle.bouju@chu-nantes.fr.
References
- Aoki, R. , Shiraishi, M. , Mikami, K. , & Kamo, T. (2019). Deterioration of postural deformity in Parkinson's disease patients with punding and hobbyism. Journal of Clinical Neuroscience , 69, 179–183. 10.1016/j.jocn.2019.07.069. [DOI] [PubMed] [Google Scholar]
- APA (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC, USA. [Google Scholar]
- Bayard, S. , Joly, E. , Ghisletta, P. , Rossignol, A. , Herades, Y. , & Geny, C. (2016). A multidimensional approach to impulsivity in Parkinson's disease: Measurement and structural invariance of the UPPS impulsive Behaviour scale. Psychological Medicine , 46(14), 2931–2941. 10.1017/S0033291716001586. [DOI] [PubMed] [Google Scholar]
- Bayard, S. , Raffard, S. , & Gely-Nargeot, M. C. (2011). Do facets of self-reported impulsivity predict decision-making under ambiguity and risk? Evidence from a community sample. Psychiatry Research , 190(2–3), 322–326. 10.1016/j.psychres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Brand, M. , Wegmann, E. , Stark, R. , Muller, A. , Wolfling, K. , & Robbins, T. W. (2019). The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neuroscience and Biobehavioral Reviews , 104, 1–10. 10.1016/j.neubiorev.2019.06.032. [DOI] [PubMed] [Google Scholar]
- Calado, F. , & Griffiths, M. D. (2016). Problem gambling worldwide: An update and systematic review of empirical research (2000–2015). Journal of Behavioral Addictions , 5(4), 592–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callesen, M. B. , Weintraub, D. , Damholdt, M. F. , & Moller, A. (2014). Impulsive and compulsive behaviors among Danish patients with Parkinson's disease: Prevalence, depression, and personality. Parkinsonism & Related Disorders , 20(1), 22–26. 10.1016/j.parkreldis.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Ceravolo, R. , Rossi, C. , Del Prete, E. , & Bonuccelli, U. (2016). A review of adverse events linked to dopamine agonists in the treatment of Parkinson's disease. Expert Opinion on Drug Safety , 15(2), 181–198. 10.1517/14740338.2016.1130128. [DOI] [PubMed] [Google Scholar]
- Chamberlain, S. R. , & Grant, J. E. (2019). Behavioral addictions. In Fontenelle LF Y. M., (Ed.), A transdiagnostic approach to obsessions, compulsions and related phenomena (pp. 401–412). Cambridge: Cambridge University Press. [Google Scholar]
- Cilia, R. , Benfante, R. , Asselta, R. , Marabini, L. , Cereda, E. , & Siri, C. (2016). Tryptophan hydroxylase type 2 variants modulate severity and outcome of addictive behaviors in Parkinson's disease. Parkinsonism & Related Disorders , 29, 96–103. [DOI] [PubMed] [Google Scholar]
- Cormier-Dequaire, F. , Bekadar, S. , Anheim, M. , Lebbah, S. , Pelissolo, A. , & Krack, P. (2018). Suggestive association between OPRM1 and impulse control disorders in Parkinson's disease. Movement Disorders , 33(12), 1878–1886. 10.1002/mds.27519. [DOI] [PubMed] [Google Scholar]
- Dujardin, K. , & Sgambato, V. (2020). Neuropsychiatric disorders in Parkinson's disease: What do we know about the role of dopaminergic and non-dopaminergic systems? Front Neurosci , 14, 25 eCollection 2020. 10.3389/fnins.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erga, A. H. , Alves, G. , Tysnes, O. B. , & Pedersen, K. F. (2020). Impulsive and compulsive behaviors in Parkinson's disease: Impact on quality of and satisfaction with life, and caregiver burden. Parkinsonism & Related Disorders , 78, 27–30. 10.1016/j.parkreldis.2020.07.007. [DOI] [PubMed] [Google Scholar]
- Fahn, S. , Elton, R. L. , & Members-of-UPDRS-Development-Committee (1987). Unified Parkinson’s disease rating scale. In Fahn S., Marsden CD., & Calne DB. (Eds.), Recent developments in Parkinson’s disease (Vol. 2, pp. 153–163). London: Macmillan Health Care Information, Florham Park Macmillan. In M. C. Fahn S., Calne DB. (Ed.), Recent Developments in Parkinson’s Disease. [Google Scholar]
- Fan, H. C. , Chang, Y. K. , Tsai, J. D. , Chiang, K. L. , Shih, J. H. , & Yeh, K. Y. (2020). The association between Parkinson's disease and attention-deficit hyperactivity disorder. Cell Transplantation , 29, 963689720947416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research , 12(3), 189–198. 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gatto, E. M. , & Aldinio, V. (2019). Impulse control disorders in Parkinson's disease. A brief and comprehensive review. Front Neurol , 10, 351 eCollection 2019. 10.3389/fneur.2019.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerscheidt, T. , Majerová, V. , Menšíková, K. , Dušek, L. , Czekóová, K. , & Kotková, P. (2016). Impulse control disorders in young-onset patients with Parkinson's disease: Cross-sectional study seeking associated factors with regard of personal characteristics. Clinical Neurophysiology , 127(e20–e132). [Google Scholar]
- Giladi, N. , Weitzman, N. , Schreiber, S. , Shabtai, H. , & Peretz, C. (2007). New onset heightened interest or drive for gambling, shopping, eating or sexual activity in patients with Parkinson's disease: The role of dopamine agonist treatment and age at motor symptoms onset. Journal of Psychopharmacology , 21(5), 501–506. 10.1177/0269881106073109. [DOI] [PubMed] [Google Scholar]
- Giovannoni, G. , O'Sullivan, J. D. , Turner, K. , Manson, A. J. , & Lees, A. J. (2000). Hedonistic homeostatic dysregulation in patients with Parkinson's disease on dopamine replacement therapies. Journal of Neurology, Neurosurgery, and Psychiatry , 68(4), 423–428. 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz, C. G. , Nutt, J. G. , & Stebbins, G. T. (2008). The unified dyskinesia rating scale: Presentation and clinimetric profile. Movement Disorders , 23(16), 2398–2403. 10.1002/mds.22341. [DOI] [PubMed] [Google Scholar]
- Goodman, A. (2008). Neurobiology of addiction. An integrative review. Biochemical Pharmacology , 75(1), 266–322. 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Grall-Bronnec, M. , Sauvaget, A. , Perrouin, F. , Leboucher, J. , Etcheverrigaray, F. , & Challet-Bouju, G. (2016). Pathological gambling associated with aripiprazole or dopamine replacement therapy: Do patients share the same features? A review. Journal of Clinical Psychopharmacology , 36(1), 63–70. 10.1097/JCP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall-Bronnec, M. , Victorri-Vigneau, C. , Donnio, Y. , Leboucher, J. , Rousselet, M. , & Thiabaud, E. (2018). Dopamine agonists and impulse control disorders: A complex association. Drug Safety , 41(1), 19–75. 10.1007/s40264-017-0590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, A. , Bower, J. H. , Kumar, N. , Matsumoto, J. Y. , Fealey, R. D. , & Josephs, K. A. (2011). Dopamine agonist-triggered pathological behaviors: Surveillance in the PD clinic reveals high frequencies. Parkinsonism & Related Disorders , 17(4), 260–264. 10.1016/j.parkreldis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Haute-Autorité-de-Santé (Ed.), (2016). Guide du parcours de soins . Maladie de Parkinson. [Google Scholar]
- Hlavata, P. , Linhartova, P. , Sumec, R. , Filip, P. , Svetlak, M. , & Balaz, M. (2020). Behavioral and neuroanatomical account of impulsivity in Parkinson's disease. Front Neurol , 10, 1338. 10.3389/fneur.2019.01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn, M. M. , & Yahr, M. D. (1967). Parkinsonism: Onset, progression and mortality. Neurology , 17(5), 427–442. 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Imperatori, C. , Fabbricatore, M. , Vumbaca, V. , Innamorati, M. , Contardi, A. , & Farina, B. (2016). Food addiction: Definition, measurement and prevalence in healthy subjects and in patients with eating disorders. Riv Psichiatr , 51(2), 60–65. [DOI] [PubMed] [Google Scholar]
- Isaias, I. U. , Siri, C. , Cilia, R. , De Gaspari, D. , Pezzoli, G. , & Antonini, A. (2008). The relationship between impulsivity and impulse control disorders in Parkinson's disease. Movement Disorders , 23(3), 411–415. 10.1002/mds.21872. [DOI] [PubMed] [Google Scholar]
- Jesus, S. , Labrador-Espinosa, M. A. , Adarmes, A. D. , Mendel-Del Barrio, C. , Martinez-Castrillo, J. C. , & Alonso-Canovas, A. (2020). Non-motor symptom burden in patients with Parkinson's disease with impulse control disorders and compulsive behaviours: Results from the COPPADIS cohort. Scientific Reports , 10(1), 16893. 10.1038/s41598-020-73756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C. , Adler, L. , Ames, M. , Demler, O. , Faraone, S. , & Hiripi, E. (2005). The world health organization adult ADHD self-report scale (ASRS): A short screening scale for use in the general population. Psychological Medicine , 35(2), 245–256. 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Marin-Lahoz, J. , Pagonabarraga, J. , Martinez-Horta, S. , Fernandez de Bobadilla, R. , Pascual-Sedano, B. , & Perez-Perez, J. (2018). Parkinson's disease: Impulsivity does not cause impulse control disorders but boosts their severity. Front Psychiatry , 9, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey, R. M. , & Greenland, S. (1989). The impact of confounder selection criteria on effect estimation. American Journal of Epidemiology , 129(1), 125–137. 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- Movement-Disorder-Society-Task-Force-on-Rating-Scales-for-Parkinson's-Disease (2003). The unified Parkinson's disease rating scale (UPDRS): Status and recommendations. Movement Disorders , 18(7), 738–750. 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- NICE (2017). Parkinson’s disease in adults. https://www.nice.org.uk/guidance/ng71/resources/parkinsons-disease-in-adults-pdf-1837629189061.
- Olley, J. , Blaszczynski, A. , & Lewis, S. (2015). Dopaminergic medication in Parkinson's disease and problem gambling. Journal of Gambling Studies , 31(3), 1085–1106. 10.1007/s10899-014-9503-0. [DOI] [PubMed] [Google Scholar]
- Orayj, K. , & Lane, E. (2019). Patterns and determinants of prescribing for Parkinson's disease: A systematic literature review. Parkinsons Dis , 2019, 9237181. 10.1155/2019/9237181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirtosek, Z. , Bajenaru, O. , Kovacs, N. , Milanov, I. , Relja, M. , & Skorvanek, M. (2020). Update on the management of Parkinson's disease for general neurologists. Parkinsons Disease , 2020, 9131474. 10.1155/2020/9131474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri, F. E. , Assogna, F. , Pellicano, C. , Cacciari, C. , Pannunzi, S. , & Morrone, A. (2015). Sociodemographic, neuropsychiatric and cognitive characteristics of pathological gambling and impulse control disorders NOS in Parkinson's disease. European Neuropsychopharmacology , 25(1), 69–76. 10.1016/j.euroneuro.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Redensek, S. , Jenko Bizjan, B. , Trost, M. , & Dolzan, V. (2020). Clinical and clinical-pharmacogenetic models for prediction of the most common psychiatric complications due to dopaminergic treatment in Parkinson's disease. The International Journal of Neuropsychopharmacology . 10.1093/ijnp/pyaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter, J. M. , Flower, R. , Henderson, G. , Loke, Y. K. , MacEwan, D. , & Rang, H. P. (2020). Rang and dale's pharmacology (9th ed.). [Google Scholar]
- Rochat, L. , Billieux, J. , Gagnon, J. , & Van der Linden, M. (2018). A multifactorial and integrative approach to impulsivity in neuropsychology: Insights from the UPPS model of impulsivity. Journal of Clinical and Experimental Neuropsychology , 40(1), 45–61. 10.1080/13803395.2017.1313393. [DOI] [PubMed] [Google Scholar]
- Saez-Francas, N. , Marti Andres, G. , Ramirez, N. , de Fabregues, O. , Alvarez-Sabin, J. , & Casas, M. (2016). Clinical and psychopathological factors associated with impulse control disorders in Parkinson's disease. Neurologia , 31(4), 231–238. 10.1016/j.nrl.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , & Weiller, E. (1998). The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry , 59(Suppl 20), 22–33 quiz 34–57. [PubMed] [Google Scholar]
- Smith, K. M. , Xie, S. X. , & Weintraub, D. (2016). Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. Journal of Neurology, Neurosurgery, and Psychiatry , 87(8), 864–870. 10.1136/jnnp-2015-311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon, V. , Napier, T. C. , Frank, M. J. , Sgambato-Faure, V. , Grace, A. A. , & Rodriguez-Oroz, M. (2017). Impulse control disorders and levodopa-induced dyskinesias in Parkinson's disease: An update. Lancet Neurology , 16(3), 238–250. 10.1016/S1474-4422(17)30004-2. [DOI] [PubMed] [Google Scholar]
- Voon, V. , Sohr, M. , Lang, A. E. , Potenza, M. N. , Siderowf, A. D. , & Whetteckey, J. (2011). Impulse control disorders in Parkinson disease: A multicenter case--control study. Annals of Neurology , 69(6), 986–996. 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- Ward, M. F. , Wender, P. H. , & Reimherr, F. W. (1993). The wender Utah rating scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. The American Journal of Psychiatry , 150(6), 885–890. 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Weintraub, D. , & Claassen, D. O. (2017). Impulse control and related disorders in Parkinson's disease. International Review of Neurobiology , 133, 679–717. 10.1016/bs.irn.2017.04.006. [DOI] [PubMed] [Google Scholar]
- Weintraub, D. , & Mamikonyan, E. (2019). Impulse control disorders in Parkinson's disease. The American Journal of Psychiatry , 176(1), 5–11. 10.1176/appi.ajp.2018.18040465. [DOI] [PubMed] [Google Scholar]
- Whiteside, S. P. , Lynam, D. R. , Miller, J. D. , & Reynolds, S. K. (2005). Validation of the UPPS impulsive Behaviour scale: A four model of impulsivity. European Journal of Personality , 19, 559–574. 10.1002/per.556. [DOI] [Google Scholar]