Abstract
Objective
This study aimed to describe the characteristics of fetal demise after SARS-CoV-2 infections and clarify whether it is associated with clinical severity, placental lesions, or malformations or due to actual fetal infections.
Data Sources
PubMed and Web of Science databases were searched between December 1, 2019, and April 30, 2022.
Study Eligibility Criteria
Cohort, cross-sectional, and case-control studies and case series or case reports describing stillbirths or late miscarriages (ie, pregnancy loss occurring between 14 and 22 weeks of gestation, before and after the onset of labor) from mothers with SARS-CoV-2 infection during pregnancy (demonstrated by at least 1 positive real-time reverse transcription-polymerase chain reaction from nasopharyngeal swabs and/or SARS-CoV-2 placental infection). No language restriction was applied; cases with other causes possibly explaining the fetal demise were excluded.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-analysis Of Observational Studies in Epidemiology guidelines were followed. The quality of the case series and case reports was evaluated using the specific Mayo Clinic Evidence-Based Practice Center tool. Maternal and clinical fetal data and placental and fetal virology and histology findings were collected. Data were summarized with descriptive statistics using the World Health Organization criteria to classify disease severity and fetal-neonatal infections.
Results
Data from 184 mothers and 190 fetuses were analyzed. No clear link to maternal clinical severity or fetal malformation was evident. Approximately 78% of fetal demise cases occurred during the second and third trimesters of pregnancy, approximately 6 to 13 days after the diagnosis of SARS-CoV-2 infection or the onset of symptoms. Most placentas (88%) were positive for SARS-CoV-2 or presented the histologic features of placentitis (massive fibrin deposition and chronic intervillositis) previously observed in transplacentally transmitted infections (85%–91%). Of note, 11 fetuses (5.8%) had a confirmed in utero transmitted SARS-CoV-2 infection, and 114 fetuses (60%) had a possible in utero transmitted SARS-CoV-2 infection.
Conclusion
The synthesis of available data showed that fetal demise generally occurs a few days after the infection with histologic placental inflammatory lesions associated with transplacental SARS-CoV-2 transmission and eventually causing placental insufficiency.
Key words: COVID-19, fetal demise, fetus, loss, malformation, neonate, placenta, pregnancy, transmission, virus
Introduction
In December 2019, the first cases of pneumonia caused by a novel coronavirus (SARS-CoV-2) were reported by the World Health Organization (WHO), and since then, approximately 552,000,000 confirmed cases of COVID-19 have occurred, causing more than 4,300,000 deaths.1
AJOG at a Glance.
Why was this study conducted?
It is unknown whether fetal demise (miscarriage or stillbirth) is associated with clinical severity, placental lesions, or malformations and whether it is due to actual SARS-CoV-2 fetal infection.
Key findings
Fetal demise generally occurred in the second and third trimesters of pregnancy (between 14 and 39 weeks of gestation), approximately 6 to 13 days after the diagnosis of infection or the onset of symptoms, without a link to maternal clinical severity and comorbidities or congenital fetal malformations. Most placentas were positive for SARS-CoV-2 or presented the histologic anomalies previously observed in transplacentally transmitted infections, which causes placental insufficiency. Moreover, 65% of the fetuses had a confirmed or possible in utero transmitted infection.
What does this add to what is known?
This study synthesized the characteristics of fetal demise from women with SARS-CoV-2 infection and helps in understanding the role of SARS-CoV-2 infection in fetal demise.
Although several studies indicate SARS-CoV-2 infection during pregnancy as a risk factor for poor maternal outcomes,2 , 3 the results of large cohorts are discordant regarding the effect on fetal outcomes because of the rarity of negative fetal outcomes, leading to relatively a low power to detect any association. The association of preterm birth, miscarriage, and stillbirth with SARS-CoV-2 infection and COVID-19 remains unclear.4, 5, 6, 7, 8, 9, 10, 11 Starting from 2020, some cases have suggested a link between SARS-CoV-2 infection and pregnancy loss,12 and the rate of stillbirth in women with SARS-CoV-2 infection is estimated to be between 1% and 3%.13, 14, 15, 16, 17, 18 In some cases, SARS-CoV-2 has been isolated in fetal tissues and suspected to be responsible for pregnancy loss through fetal infection.12 , 19
Conversely, transplacental SARS-CoV-2 transmission to live neonates has been well demonstrated,20 and neonatal COVID-19 has been described.21 The WHO has included SARS-CoV-2 among vertically transmissible viruses and issued integrated criteria to diagnose the infection in both neonates and stillborn fetuses.1 Furthermore, the association of chronic intervillositis, trophoblastic necrosis, and perivillous fibrin deposition, so-called placentitis, has been identified as a typical placental reaction to viral infection and observed in both neonates and stillborn fetuses with transplacentally acquired infection.20 , 22 , 23
There is a knowledge gap regarding stillbirths and late miscarriages in pregnant women with SARS-CoV-2 infection: it is unknown whether they are linked to clinical severity, placental lesions, or malformations, and it is also unknown how many of these cases may be due to actual fetal infections. We conducted a systematic review and synthesis of published cases of stillbirths and late miscarriages from women with SARS-CoV-2 infection, describing their characteristics and filling this gap. We aimed to characterize shared clinical features that may be helpful to recognize the role of SARS-CoV-2 infection in negative pregnancy outcomes, as we hypothesized that some fetal demise cases are due to maternal infection.
Methods
Protocol
Before commencing the project, a protocol was established, including the search modalities, eligibility criteria, and all methodological details. Several meetings among the authors were organized. The work was performed using secured files; however, the original articles only provided deidentified data. Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed throughout the project.24
Eligibility and exclusion criteria
We searched for cohort, cross-sectional, and case-control studies and case series or case reports published between December 1, 2019, and April 30, 2022, describing stillborn fetuses or late miscarriages (ie, pregnancy loss occurred between 14 and 22 weeks of gestation, before and after the onset of labor; after 22 weeks of gestation, only intrauterine fetal deaths have been considered) from mothers with SARS-CoV-2 infection during pregnancy, as demonstrated by (1) at least 1 positive real-time reverse transcription-polymerase chain reaction (RT-PCR) from nasopharyngeal swabs and/or (2) placental infection with SARS-CoV-2 (ie, a positive placental RT-PCR, immunostaining, in situ hybridization, or electron microscopy1). All these tests had to be performed according to the WHO or national guidelines.25
No language restriction was applied: non-English publications were examined using Google Translator. We excluded conference abstracts, meta-analysis, cases of women exposed to SARS-CoV-2 but not fulfilling at least one of the two aforementioned eligibility criteria. In addition, we excluded “gray” literature and cases with other causes possibly explaining stillbirth and late miscarriage.26, 27, 28, 29, 30, 31 Duplicate reports were identified and eventually excluded.
Information sources and search strategy
We searched PubMed and Web of Science databases with the following key words or Medical Subject Headings terms: “fetal demise,” “stillbirth,” “miscarriage,” “SARS-CoV-2,” and “COVID-19.” Furthermore, we hand-searched references cited in the eligible manuscripts or review articles on the subject and the authors’ archives. We used the following Boolean string: (((((((Fetal demise AND Covid) OR (Fetal demise AND SARS-CoV-2)) OR (stillbirth AND Covid)) OR (stillbirth AND SARS-CoV-2)) OR (Miscarriage AND Covid)) OR (Miscarriage AND SARS-CoV-2)) OR (Intrauterine death AND Covid)) OR (Intrauterine death AND SARS-CoV-2).
Study selection
Abstracts and, where necessary, full texts of each article were assessed by 2 independent researchers (N.A. and G.R.), following the Meta-analysis Of Observational Studies in Epidemiology guidelines.32 The Consensus-based Clinical Case Reporting Guideline Development (CARE) recommendations, specifically dedicated to case reports and case series, were considered for the evaluation of these types of manuscripts.33 If an article was eligible but reported data on both stillbirths and late miscarriages and living neonates, only data about the former were considered and directly extracted when available. In case of unavailability, or when additional information was needed anyway, the authors were contacted, and at least 2 e-mails were sent 2 weeks apart to the corresponding author. If discrepancies or uncertainties persisted, they were resolved by discussion between the 2 independent researchers, and if no agreement was reached, a third researcher was consulted (A.V.). All articles finally deemed eligible were included in an electronic database (Zotero; version 5.0.65; Roy Rosenzweig Center for History and New Media, Fairfax, VI).
Data collection and extraction
We customized an online data extraction sheet, pilot-tested it on 3 randomly selected manuscripts, and refined it accordingly. Data from included records were extracted independently by 2 investigators (N.A. and G.R.) and cross-verified. If data were missing, they were requested from the corresponding authors as described above. Data were considered lacking if the authors did not provide them after 2 e-mail requests; lacking data were considered as such and not estimated. If discrepancies or uncertainties about data interpretation persisted, they were resolved by discussion between the 2 independent researchers, and if no agreement was reached, a third researcher was consulted (A.V.).
Data synthesis
Data collected included article type; country; number of fetuses; date; maternal characteristics, such as age, number of pregnancies, parity, or singleton or twin pregnancy; gestational age (GA) at SARS-CoV-2 infection; medical history; obesity (defined as a body mass index of ≥30 kg/m2); and any obstetrical complication. Moreover, data about SARS-CoV-2 infection features, vaccination status, hospitalization for COVID-19, and COVID-19 severity were collected. The clinical severity was classified according to the WHO criteria34 based on clinical data accumulated before the occurrence of fetal demise. The criteria were not influenced by pregnancy characteristics, and severity was not upgraded on the basis of the clinical evolution occurring after fetal demise.
In addition, we extracted fetal and placental data: the interval between SARS-CoV-2 infection and stillbirth or late miscarriage diagnosis, fetal sex and growth, and fetal (any tissue) and placental SARS-CoV-2 positivities. Fetal or placental SARS-CoV-2 positivity was interpreted according to the WHO criteria for vertical SARS-CoV-2 transmission: in detail, fetal or placental tissue was considered positive if there was a positive RT-PCR, immunostaining, in situ hybridization, or electron microscopy.1 Clinical chorioamnionitis was considered according to the US National Institute of Child Health and Human Development consensus criteria,35 and where data were available, the diagnosis of histologic chorioamnionitis was also considered. Fetal growth was evaluated using Association des Utilisateurs de Dossiers Informatisés en Pédiatrie, Obstétrique et Gynécologie curves.36 The likelihood (confirmed, possible, or unlikely) of fetal infection was evaluated using the specific WHO criteria (freely available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1).1 Maternal and fetal characteristics were considered as the main outcomes.
Assessment of risk of bias
As we expected most articles to be case reports or case series, we decided to evaluate their methodological quality according to 4 domains (selection, ascertainment, causality, and reporting) using the Mayo Clinic Evidence-Based Practice Center tool, which is specifically dedicated to the evaluation of case report or case series quality.37 Of note, 2 investigators (N.A. and G.R.) independently summarized the results of this evaluation by aggregating the eight binary responses into a 0 to 8 score (the higher the score, the better the quality), and the results were also qualitatively summarized, as previously done.21 If discrepancies or uncertainties persisted, they were resolved by discussion between the 2 researchers (N.A. and G.R.), and if no agreement was reached, a third researcher was consulted (A.V.).
Summary measures
Cumulative estimates of event rates (frequency) were reported as a percentage. The percentage refers to the total number of fetuses, unless otherwise indicated. Continuous data were described as mean (standard deviation); minimum and maximum values were also reported. Excel 2016 online (Microsoft Corporation, Redmond, WA) was used.
Results
Study selection
Abstracts and full texts of each article were assessed by 2 independent researchers after duplicate removal, with discrepancies resolved by a third researcher.
Study characteristics
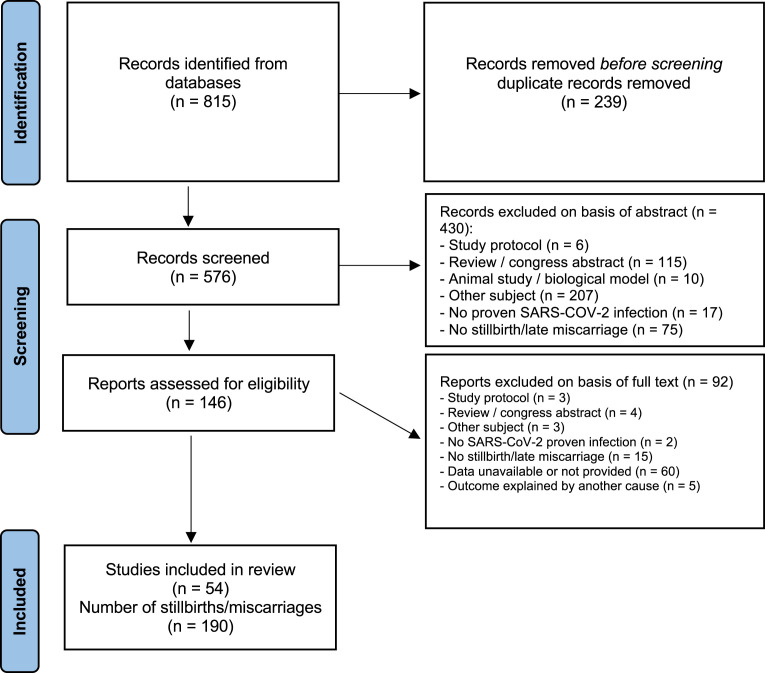
Figure 1 illustrates the project flowchart with included and excluded records (and the reasons for their exclusions). Finally, 54 articles were considered, consisting of 19 case series, 30 case reports, 1 case-control study, and 4 cohort studies, accounting for a total of 184 mothers and 190 fetuses, that is, 166 stillbirths and 24 late miscarriages. Most of the articles were already peer-reviewed.
Figure 1.
PRISMA flowchart for the systematic review
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
Risk of bias of included studies
According to the CARE recommendations, the methodological quality of case reports and case series was estimated as intermediate to good (Table 1 ).
Table 1.
Characteristics of articles included in the systematic review
| First author, year | Article type | Country | Quality score | Overall quality | Publication status | Number of fetuses |
|---|---|---|---|---|---|---|
| Popescu et al,38 2021 | Case report | Romania | 6 | Good | Reviewed | 1 |
| Aminimoghaddam et al,26 2021 | Case report | Iran | 4 | Intermediate | Reviewed | 1 |
| Marinho et al,39 2021 | Case report | Brazil | 6 | Good | Reviewed | 1 |
| Remaeus et al,40 2020 | Case series | Sweden | 4 | Intermediate | Reviewed | 2 |
| Schwartz et al,23 2021 | Case series | United States | 6 | Good | Reviewed | 2 |
| Lokken et al,41 2020 | Case series | United States | 6 | Good | Reviewed | 1 |
| Shmakov et al,42 2022 | Cohort | Russia | NA | NA | Reviewed | 2 |
| Lesieur et al,43 2022 | Case report | France | 6 | Good | Reviewed | 1 |
| Futterman et al,44 2020 | Case report | United States | 5 | Intermediate | Reviewed | 1 |
| Watkins et al,45 2022 | Case series | United States | 5 | Intermediate | Reviewed | 1 |
| Michel et al,46 2021 | Case report | France | 6 | Good | Reviewed | 1 |
| Valk et al,47 2021 | Case report | United States | 5 | Intermediate | Reviewed | 1 |
| Garrido-Pontnou et al,48 2021 | Case series | Spain | 4 | Intermediate | Reviewed | 5 |
| Richtmann et al,49 2020 | Case series | Brazil | 6 | Good | Reviewed | 5 |
| Stonoga et al,50 2021 | Case report | Brazil | 6 | Good | Reviewed | 1 |
| Halici-Ozturk et al,51 2021 | Case series | Turkey | 4 | Intermediate | Reviewed | 5 |
| Hachem et al,52 2020 | Case report | France | 4 | Intermediate | Reviewed | 1 |
| Marton et al,53 2021 | Case report | United Kingdom | 5 | Intermediate | Reviewed | 1 |
| Sadiq et al,54 2021 | Case series | Pakistan | 4 | Intermediate | Reviewed | 1 |
| Hcini et al,55 2021 | Cohort | French Guiana | NA | NA | Reviewed | 7 |
| Verma et al,56 2020 | Case report | United States | 4 | Intermediate | Reviewed | 3 |
| Bouachba et al,57 2021 | Case report | France | 6 | Good | Reviewed | 3 |
| Shanes et al,58 2020 | Case-control study | United States | 5 | Intermediate | Reviewed | 1 |
| Mattar et al,59 2020 | Case series | Singapore | 4 | Intermediate | Reviewed | 1 |
| Hodžić et al,60 2022 | Cohort | Bosnia | 5 | Intermediate | Reviewed | 2 |
| Argueta et al,61 2021 | Case report | United States | 5 | Intermediate | Preprint | 2 |
| Baud et al,12 2020 | Case report | Switzerland | 6 | Good | Reviewed | 1 |
| Rodrigues et al,62 2021 | Case report | Portugal | 6 | Good | Reviewed | 1 |
| Baral et al,63 2021 | Case report | Nepal | 5 | Intermediate | Reviewed | 1 |
| Pulinx et al,64 2020 | Case report | Belgium | 6 | Good | Reviewed | 2 |
| Fernandez et al,65 2022 | Case report | Brazil | 4 | Intermediate | Reviewed | 1 |
| Ferreira et al,66 2022 | Case series | Brazil | 4 | Intermediate | Reviewed | 1 |
| Zaigham et al,67 2022 | Case series | Sweden | 6 | Good | Reviewed | 5 |
| Coté et al,68 2022 | Case report | United States | 5 | Intermediate | Reviewed | 1 |
| Patanè et al,69 2022 | Case report | Italy | 5 | Intermediate | Reviewed | 2 |
| Fitzgerald et al,70 2022 | Case series | Ireland | 5 | Intermediate | Reviewed | 6 |
| Schwartz et al,71 2021 | Case series | United States | 5 | Intermediate | Reviewed | 2 |
| Thomas et al,72 2021 | Case series | United States | 4 | Intermediate | Reviewed | 2 |
| Babal et al,73 2021 | Case report | Slovakia | 5 | Intermediate | Reviewed | 1 |
| Eich et al,74 2022 | Case report | Germany | 4 | Intermediate | Reviewed | 1 |
| Guan et al,75 2022 | Case report | United States | 6 | Good | Reviewed | 1 |
| Mithal et al,76 2022 | Case report | United States | 5 | Intermediate | Reviewed | 1 |
| Sagara et al,77 2022 | Case report | Japan | 5 | Intermediate | Reviewed | 2 |
| Wong et al,78 2022 | Case report | United States | 4 | Intermediate | Reviewed | 1 |
| Bewley et al,79 2021 | Case report | United States | 4 | Intermediate | Reviewed | 1 |
| Borges Charepe et al,30 2022 | Cohort | Portugal | NA | NA | Reviewed | 9 |
| Schwartz et al,22 2022 | Case series | Several | 4 | Intermediate | Reviewed | 31 |
| Huynh et al,80 2022 | Case series | United States | 5 | Intermediate | Reviewed | 18 |
| Shook et al,81 2022 | Case report | United States | 6 | Good | Reviewed | 2 |
| Stenton et al,82 2022 | Case series | United Kingdom | 5 | Intermediate | Reviewed | 29 |
| Dubucs et al,83 2022 | Case series | France | 6 | Good | Reviewed | 7 |
| Di Gioia et al,84 2022 | Case report | Italy | 5 | Intermediate | Reviewed | 1 |
| Konstantinidou et al,85 2022 | Case series | Greece | 6 | Good | Reviewed | 6 |
| Zinserling et al,86 2021 | Case report | Russia | 5 | Intermediate | Reviewed | 1 |
| Total | 5 (0.8) | 190 | ||||
The methodological quality of case reports and case series was evaluated using the Mayo Clinic Evidence-Based Practice Center tool,37 specifically dedicated to evaluation of the quality of these types of articles and shown both as a 0-6 score and as overall qualitative evaluation. The score was applied exactly in the same way to peer reviewed and non-peer reviewed articles. The score was summarized as mean (standard deviation). This tool was not applied to the retrospective cohort studies included in the review.
NA, not applicable.
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
Synthesis of results
Basic obstetrical data are reported in Table 2 . Of note, 42 mothers were multiparous; moreover, obesity, diabetes mellitus, and chorioamnionitis were the most common comorbidities. The following COVID-19 treatments had been provided in a few women: steroids (n=6), remdesivir (n=3), tocilizumab (n=2), and anakinra (n=1).
Table 2.
Basic obstetrical data of reviewed stillbirth and early miscarriage cases
| Variable | Summary statistics | IQR |
|---|---|---|
| Maternal age (y) | 30.5 (6.5) | 15–42 |
| Gravidity | 2.9 (2.6) | 0–14 |
| Nulliparous | 33 (17.9%) | |
| Multiparous | 42 (22.8%) | |
| Singleton | 175 (95.1%) | |
| Hospitalization for COVID-19 | 10 (5.4%) | |
| Comorbidities | ||
| Diabetes mellitus (any type) | 15 (8.1%) | |
| Obesity (body mass index) | 28 (15.2%) | |
| Antiphospholipid syndrome | 1 (0.5%) | |
| Chronic hypertension | 13 (7.0%) | |
| Disseminated intravascular coagulopathy | 4 (2.2%) | |
| Obstetrical cholestasis | 0 (0%) | |
| Preeclampsia | 8 (4.3%) | |
| Clinical chorioamnionitis | 1 (0.5%) | |
| Histologic chorioamnionitis | 14 (7.6%) | |
Data are presented as mean (standard deviation), IQR, or number (percentage).
The percentage refers to the number of pregnant women (n=184). Comorbidities were considered absent if not detailed in the reviewed articles and not declared by authors during e-mail communications with investigators.
IQR, interquartile range.
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
Data regarding SARS-CoV-2 infection are presented in Table 3 . No woman was vaccinated against SARS-CoV-2; GA at the diagnosis of SARS-CoV-2 infection spanned from a minimum of 14.0 weeks to a maximum of 39.2 weeks. Less than 5% of women suffered from severe COVID-19, whereas most women had mild-to-moderate disease. Of note, 4 women had a negative nasopharyngeal RT-PCR, but their infection was confirmed by a positive placental RT-PCR.46 , 82 The viral strain was known only in 19 cases (10.3%).
Table 3.
Main data about SARS-CoV-2 infection in reviewed cases of stillbirth and early miscarriage
| Variable | Summary statistics |
|---|---|
| GA at the diagnosis of SARS-CoV-2 infection (wk) | 27.4 (6.9) |
| GA classes at the diagnosis of SARS-CoV-2 infection | |
| 14–22 wk | 26 (13.7%) |
| 22–32 wk | 52 (27.4%) |
| 32–42 wk | 32 (16.8%) |
| Unknown | 80 (42.1%) |
| Maternal COVID-19 severity | |
| Asymptomatic | 49 (26.6%) |
| Mild | 36 (19.6%) |
| Moderate | 25 (13.6%) |
| Severe | 8 (4.3%) |
| Unknown | 66 (35.9%) |
| Viral strain | |
| α | 10 (5.4%) |
| β | 3 (1.6%) |
| γ | 1 (0.6%) |
| δ | 5 (2.7%) |
| Unknown | 165 (89.7%) |
Data are presented as mean (standard deviation) or number (percentage). The percentage refers to the total number of fetuses (N=190), except that for maternal COVID-19 severity and viral strains where the percentage refers to the number of pregnant women (n=184). GA classes were defined using trimester thresholds.
GA, gestational age.
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
Fetal and placental data are reported in Table 4 . Stillbirths and late miscarriages occurred approximately 6 to 13 days after the confirmation of SARS-CoV-2 infection or the onset of symptoms (only 9 cases (4.7%) were outliers; ie, they had fetal demise occurring more than 15 days from maternal infection). Most stillbirths and late miscarriages occurred during the second trimester of pregnancy. Fetal weight was appropriate for the GA: only 7 fetuses (4%) had a weight z score of <−2. Of note, 23 fetuses (12.1%) were positive for SARS-CoV-2 in at least 1 tissue, and positivity was detected with RT-PCR, immunostaining, and in situ hybridization in 10, 10, and 5 cases, respectively; 132 (69.5%) placentas were positive for SARS-CoV-2 as indicated by RT-PCR, immunostaining, and in situ hybridization in 54, 101, and 35 cases, respectively. Placental histology was examined in 150 cases (79%), and most placentas presented the histologic features previously observed in transplacentally transmitted infections (Table 4). Of note, 3 cases presented with nonlethal congenital anomalies: 1 with hand malformation (shortening of 2 fingers and suspected absence of 2 metacarpal bones), 1 with isolated agenesis of the corpus callosum, and 1 with unilateral kidney agenesis.
Table 4.
Fetal and placental data of reviewed cases of stillbirth and early miscarriage
| Variable | Summary statistics | IQR |
|---|---|---|
| Time between COVID-19 symptoms and stillbirth or miscarriage (d) | 9.5 (6.0–9.5) | 2–77 |
| Time between SARS-CoV-2 infection and stillbirth or miscarriage (d) | 2.5 (0.0–9.3) | 0–70 |
| GA at stillbirth or miscarriage (wk) | 28.37 (22.6–33.6) | 14–41 |
| GA classes at stillbirth or miscarriage | ||
| 14–22 wk | 40 (21.1%) | |
| 22–32 wk | 85 (44.7%) | |
| 32–42 wk | 64 (33.7%) | |
| Unknown | 1 (0.5%) | |
| Fetal characteristics | ||
| Male | 36 (19.0%) | |
| Female | 39 (20.5%) | |
| Unknown sex | 115 (60.5%) | |
| Fetal weight (g) | 1416 (948) | 120–4250 |
| Fetal weight z score | −0.52 (3.17) | −22.10 to 4.77 |
| Fetal SARS-CoV-2 positivity | 23 (12.1%) | |
| Placental characteristics | ||
| Fibrin deposition | 136 (90.7%) | |
| Chronic intervillositis | 128 (85.3%) | |
| Trophoblast necrosis | 96 (64.0%) | |
| Villitis | 19 (12.7%) | |
| Placental SARS-CoV-2 positivity | 132 (88.0%) | |
Data are presented as median (IQR), mean (standard deviation), IQR, or number (percentage). The percentage refers to the total number of fetuses, except that for placental histology where the percentage refers to the total number of placental histologic examinations (N=150). The z score is a dimensionless variable. GA classes were defined using trimester thresholds.
GA, gestational age; IQR, interquartile range.
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
According to the WHO criteria, 11 fetuses (5.8%) had a confirmed in utero transmitted SARS-CoV-2 infection, and 114 fetuses (60.0%) had a possible in utero transmitted SARS-CoV-2 infection; in 18 fetuses (9.5%), the transmission was considered unlikely; lack of data prevented classification in approximately one-quarter of cases. The distribution of the likelihood of SARS-CoV-2 transplacental transmission in the reviewed cases is depicted in Figure 2 .
Figure 2.
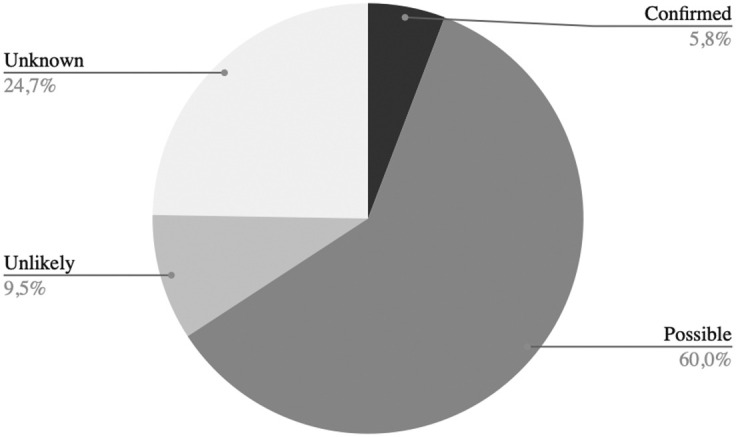
Likelihood of in utero transplacental SARS-CoV-2 transmission in reviewed cases of stillbirth and early miscarriage
Degree of likelihood was evaluated following the WHO criteria.1 When the data required by the WHO definition were unavailable (despite multiple requests to authors of the original articles), the cases were considered as unknown.
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
Comment
Principal findings
Our work synthesized the characteristics of stillbirths and late miscarriages from women with SARS-CoV-2 infection and will aid in understanding the role of SARS-CoV-2 infection in fetal demise. Moreover, we found the following:
-
1.
Most fetal demise cases seemed to occur in the second trimester of pregnancy; they did not selectively occur in patients with a certain COVID-19 severity, age, or obstetrical comorbidity.
-
2.
Fetal demise cases generally occurred a few days after the confirmation of SARS-CoV-2 infection or the onset of symptoms, without evidence of growth retardation.
-
3.
Most placental tissues were positive for SARS-CoV-2 or showed histologic abnormalities (so-called placentitis) already observed in cases of SARS-CoV-2 transplacental transmission. and overall, approximately 65% of cases had a confirmed or possible fetal infection.
Results in the context of what is known
The higher occurrence of fetal demise cases in the second trimester of pregnancy might have been biased by the testing policies that varied between centers and settings; moreover, the active management offered to women in the third trimester of pregnancy may have had an influence. The lack of a link between the clinical severity of COVID-19 and fetal demise might be surprising, although it indicates that several factors can interact and influence fetal outcomes. Stillbirth and transplacental SARS-CoV-2 transmission are both uncommon events (whose rates are between 1% and 3% of pregnancies13, 14, 15, 16, 17, 18 , 87). Therefore, the relatively small population of available cases (with <5% of severe cases) means that the link with maternal clinical severity may go undetected as much larger populations may be needed to capture its effects on such a rare outcome. Conversely, if a bias regarding clinical severity would exist, it would be visible because of more tests being performed in more symptomatic women. At the time of the publication of reviewed cases, no woman was vaccinated. With the current large diffusion of vaccination in the Western world, observation of a larger population of fetal demise cases after SARS-CoV-2 infections may be unlikely, as vaccines are efficacious in preventing maternal infections.88 Unfortunately, vaccine hesitancy has been observed among pregnant women.
As fetal demise cases occurred close to SARS-CoV-2 infection, an important role of this infection should be suspected. Previous biological or observational data seemed to support this role.9 , 12 , 19 Our work must be considered as an additional piece of information obtained with a different technique, that is, the meta-analysis and synthesis of clinical data (eg, maternal and fetal characteristics), to clarify the link between the infection and outcomes as commonly done during outbreaks.
Vertically transmittable infections may result in fetal demise either by placental damage or by direct fetopathy. Our data suggested that placental damage seems to play the main role, as placental infection and inflammation were almost always evident; however, not all fetuses had a confirmed SARS-CoV-2 infection. This distinction is only possible nowadays thanks to the introduction of the WHO criteria for the diagnosis of fetal-neonatal SARS-CoV-2 infection1; however, we should consider that 1 mechanism does not exclude the other and, in some cases, a dual hit (placental damage plus fetal infection) may be involved in fetal demise. Furthermore, the placental damage hypothesis is biologically supported by the observed histologic abnormalities that are associated with SARS-CoV-2 transplacental transmission.20 , 89 , 90 These placental histologic features may eventually impair fetal vascular perfusion,91 cause placental insufficiency, and, as recently demonstrated, lead to fetal hypoxia.22 , 89 These abnormalities (mainly constituted by massive perivillous fibrin deposition and chronic histiocytic intervillositis20 , 89 , 90) constitute the so-called SARS-CoV-2 placentitis92 and have been associated with both vertically transmittable infections93 and pregnancy loss.94 An excessive or dysregulated host inflammatory reaction might be the underlying pathobiological mechanism,95 as it happens for other severe consequences observed in nonpregnant patients with COVID-19.96
Although fetal infection was not unquestionably demonstrated in all fetuses, most reviewed cases were classified as confirmed or possible fetal infection. SARS-CoV-2 can infect the fetus as its receptors are variably expressed in placental and fetal tissues.97 Nonetheless, the occurrence of a fetal infection depends on many factors, and although the placental expression of the angiotensin-converting enzyme 2 receptor increases with GA,98 their fetal expression is less constant and variable from 1 tissue to another.99, 100, 101 The absence of a significant number of congenital malformations can be explained by the timing of COVID-19 occurrence, which was mostly during the late second trimester of pregnancy and was consistent with current knowledge suggesting a low risk of congenital malformations because of SARS-CoV-2 infection.102
Research implications
Some questions remain unanswered and call for specific data. As the studied population was mainly affected by asymptomatic SARS-CoV-2 infection or mild-to-moderate COVID-19, we cannot exclude that fetal demise might occur with different pathobiological mechanisms when a pregnant woman is affected by severe COVID-19 leading to various organ dysfunctions. Similarly, as the viral genome was sequenced in a few cases, we were unable to study the relationship between fetal demise and any viral strain, although preliminary data suggested that certain variants cause more severe disease in pregnant women.103 Further research is needed to clarify whether stillbirths mostly occur in the second late or early third trimester of pregnancy. This might have been influenced by active management once the viability threshold had been reached and can only be clarified by specifically designed international registries.
Strength and limitations
We acknowledge some limitations. There were several missing virological tests of placental and fetal tissues that prevent, in some cases, a definite classification according to the WHO definition. This may be understandable as performing multiple tests may have been unfeasible while the pandemic was profoundly impacting routine care. The WHO definition is complex and based on multiple criteria to avoid misclassifications because of contamination or at least to reduce its likelihood. However, as this was a meta-analysis, we did not have access to raw laboratory results, and we could not completely exclude this possibility.
Similarly, some clinical data, the viral load and the degree of fetal inflammation, were unavailable, preventing further pathobiological considerations regarding the mechanisms underlying fetal demise. Pathologists examining placentas were usually unmasked to maternal clinical data; thus, information bias was theoretically not excluded but very unlikely, given the large number of available observations. The lack of masking was understandable for the pandemic context and the need to reduce the risk of contamination. As we excluded fetal losses occurring in the first trimester of pregnancy, we could not provide definite data about malformations. This would require a similar project focused on early fetal demise cases. We could not exclude a degree of publication bias; however, after more than 2 years of the pandemic, several cases of fetal demise have been reported, and it is unlikely that unusual cases would have been undetected. Although the quality of the reviewed reports was intermediate to good, we should remember that uncontrolled case descriptions and their synthesis are at the bottom of the evidence-based medicine pyramid.37 Thus, we could not provide a demonstration of a causal link between SARS-CoV-2 infection and fetal demise for each reported case, as the available data were based on uncontrolled reports. The outliers presenting a long time between maternal infection and fetal demise were those with a more uncertain link between the 2, but they can be explained with relevant placental damage leading to chronic placental insufficiency. Furthermore, we applied the best methodology available. The Grading of Recommendations, Assessment, Development and Evaluation guidelines discloses the decision-making process based on low-quality evidence in some particular circumstances,104 and the pandemic surely represents an extraordinary situation.47 Over and above this, the availability of more controlled data or larger populations is unlikely, so this remains the best available knowledge.
Conclusions
The synthesis of available data about stillbirths and late miscarriages in mothers with SARS-CoV-2 infection showed that fetal demise occurs mostly in the third trimester of pregnancy and a few days after infection. Most stillbirths and late miscarriages presented with histologic placental abnormalities associated with transplacental SARS-CoV-2 transmission, causing placental insufficiency and eventually fetal hypoxia.
Acknowledgments
We are grateful to the authors of reviewed manuscripts who provided the details needed for the synthesis. We thank Luca Vedovelli, PhD, for his supervision as a professional methodologist.
Footnotes
A.J.V. and D.D.L. contributed equally to this work and should be considered as co-last authors.
N.A. and G.R. contributed equally to this work and should be considered as co-first authors.
The authors report no conflict of interest
This study received no funding.
This study was registered in the International Prospective Register of Systematic Reviews (protocol number: CRD42022365327).
All data generated during this study are included in this published article. Raw data used for the analyses are presented in the original manuscripts or available on request.
Supplementary Data
XXX
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
References
- 1.World Health Organization. https://www.who.int/publications/i/item/WHO-2019-nCoV-mother-to-child-transmission-2021.1; 2019–.
- 2.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight M., Bunch K., Vousden N., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Q., Liu J., Liu Q., et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muin D.A., Neururer S., Falcone V., et al. Antepartum stillbirth rates during the COVID-19 pandemic in Austria: a population-based study. Int J Gynaecol Obstet. 2022;156:459–465. doi: 10.1002/ijgo.13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., Ferre C., Ouyang L., Mohamoud Y., Barfield W., Cox S. Changes and geographic variation in rates of preterm birth and stillbirth during the prepandemic period and COVID-19 pandemic, according to health insurance claims in the United States, April-June 2019 and April-June 2020. Am J Obstet Gynecol MFM. 2022;4 doi: 10.1016/j.ajogmf.2021.100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon E., Cottenet J., Mariet A.S., et al. Impact of the COVID-19 pandemic on preterm birth and stillbirth: a nationwide, population-based retrospective cohort study. Am J Obstet Gynecol. 2021;225:347–348. doi: 10.1016/j.ajog.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okeke E.N., Abubakar I.S., De Guttry R. In Nigeria, stillbirths and newborn deaths increased during the COVID-19 pandemic. Health Aff (Millwood) 2021;40:1797–1805. doi: 10.1377/hlthaff.2021.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Curtis M., Villani L., Polo A. Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106:456. doi: 10.1136/archdischild-2020-320682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasternak B., Neovius M., Söderling J., et al. Preterm birth and stillbirth during the COVID-19 pandemic in Sweden: a nationwide cohort study. Ann Intern Med. 2021;174:873–875. doi: 10.7326/M20-6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stowe J., Smith H., Thurland K., Ramsay M.E., Andrews N., Ladhani S.N. Stillbirths during the COVID-19 pandemic in England, April-June 2020. JAMA. 2021;325:86–87. doi: 10.1001/jama.2020.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baud D., Greub G., Favre G., et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020;323:2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi J., Gong W., Gao Q. Clinical characteristics and outcomes of pregnant women with COVID-19 and the risk of vertical transmission: a systematic review. Arch Gynecol Obstet. 2021;303:337–345. doi: 10.1007/s00404-020-05889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi H., Chiu N.C., Tai Y.L., et al. Clinical features of neonates born to mothers with coronavirus disease-2019: a systematic review of 105 neonates. J Microbiol Immunol Infect. 2021;54:69–76. doi: 10.1016/j.jmii.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soheili M., Moradi G., Baradaran H.R., Soheili M., Mokhtari M.M., Moradi Y. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. 2022;35:5672–5685. doi: 10.1080/14767058.2021.1888923. [DOI] [PubMed] [Google Scholar]
- 16.Yee J., Kim W., Han J.M., et al. Clinical manifestations and perinatal outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. Sci Rep. 2020;10 doi: 10.1038/s41598-020-75096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Medeiros K.S., Sarmento A.C.A., Costa A.P.F., et al. Consequences and implications of the coronavirus disease (COVID-19) on pregnancy and newborns: a comprehensive systematic review and meta-analysis. Int J Gynaecol Obstet. 2022;156:394–405. doi: 10.1002/ijgo.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z., Wang M., Zhu Z., Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2022;35:1619–1622. doi: 10.1080/14767058.2020.1759541. [DOI] [PubMed] [Google Scholar]
- 19.Shende P., Gaikwad P., Gandhewar M., et al. Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum Reprod. 2021;36:899–906. doi: 10.1093/humrep/deaa367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivanti A.J., Vauloup-Fellous C., Prevot S., et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raschetti R., Vivanti A.J., Vauloup-Fellous C., Loi B., Benachi A., De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11:5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz D.A., Avvad-Portari E., Babál P., et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury. Arch Pathol Lab Med. 2022;146:660–676. doi: 10.5858/arpa.2022-0029-SA. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz D.A., Baldewijns M., Benachi A., et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch Pathol Lab Med. 2021;145:517–528. doi: 10.5858/arpa.2020-0771-SA. [DOI] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization Coronavirus disease (COVID-19) technical guidance: laboratory testing for 2019-NCoV in humans. Https://Www.Who.Int/Emergencies/Diseases/Novelcoronavirus- 2019/Technical-Guidance/Laboratory-Guidance Available at:
- 26.Aminimoghaddam S., Nasiri S., Abrari A., Yazdizadeh M., Rashidi R. A case of COVID-19 mortality in a pregnant woman with diabetes ketoacidosis. Med J Islam Repub Iran. 2021;35:139. doi: 10.47176/mjiri.35.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curi B., Sabre A., Benjamin I., Serventi L., Nuritdinova D. Coronavirus infection in a high-risk obstetrical population of the South Bronx, New York. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damar Çakırca T., Torun A., Hamidanoğlu M., et al. COVID-19 infection in pregnancy: a single center experience with 75 cases. Ginekol Pol. 2021 doi: 10.5603/GP.a2021.0118. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Kleinwechter H., Groten T., Schäfer-Graf U., et al. CRONOS-Netzwerk. COVID-19 und Schwangerschaft: Fallserie mit der Komorbidität Diabetes aus der Registerstudie “Covid-19 Related Obstetric and Neonatal Outcome Study” (CRONOS) [COVID-19 and pregnancy] Gynakologe. 2021;54:357–365. doi: 10.1007/s00129-021-04784-7. [German] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges Charepe N., Queirós A., Alves M.J., et al. One year of COVID-19 in pregnancy: a national wide collaborative study. Acta Med Port. 2022;35:357–366. doi: 10.20344/amp.16574. [DOI] [PubMed] [Google Scholar]
- 31.Sunder A., Varghese B., Darwish B., Shaikho N., Rashid M. Impacts and effects of COVID-19 infection in pregnancy. Saudi Med J. 2022;43:67–74. doi: 10.15537/smj.2022.43.1.20210694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stroup D.F. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 33.Gagnier J.J., Kienle G., Altman D.G., et al. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol. 2014;67:46–51. doi: 10.1016/j.jclinepi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. nCoV-clinical-2021.2-eng.pdf; 2019.
- 35.Higgins R.D., Saade G., Polin R.A., et al. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol. 2016;127:426–436. doi: 10.1097/AOG.0000000000001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AUDIPOG. Available at: https://www.audipog.net/Estimation-croissance#formu. Accessed June 12, 2023.
- 37.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popescu D.E., Cioca A., Muresan C., et al. A case of COVID-19 pregnancy complicated with Hydrops fetalis and intrauterine death. Medicina (Kaunas) 2021;57:667. doi: 10.3390/medicina57070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marinho P.S., da Cunha A.J.L.A., Chimelli L., et al. Case report: SARS-CoV-2 mother-to-child transmission and fetal death associated with severe placental thromboembolism. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.677001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remaeus K., Savchenko J., Brismar Wendel S., et al. Characteristics and short-term obstetric outcomes in a case series of 67 women test-positive for SARS-CoV-2 in Stockholm, Sweden. Acta Obstet Gynecol Scand. 2020;99:1626–1631. doi: 10.1111/aogs.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lokken E.M., Walker C.L., Delaney S., et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2020;223:911.e1–911.e14. doi: 10.1016/j.ajog.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shmakov R.G., Prikhodko A., Polushkina E., et al. Clinical course of novel COVID-19 infection in pregnant women. J Matern Fetal Neonatal Med. 2022;35:4431–4437. doi: 10.1080/14767058.2020.1850683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesieur E., Torrents J., Fina F., et al. Congenital infection of severe acute respiratory syndrome coronavirus 2 with intrauterine fetal death: a clinicopathological study with molecular analysis. Clin Infect Dis. 2022;75:e1092–e1100. doi: 10.1093/cid/ciab840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Futterman I., Toaff M., Navi L., Clare C.A. COVID-19 and HELLP: overlapping clinical pictures in two gravid patients. AJP Rep. 2020;10:e179–e182. doi: 10.1055/s-0040-1712978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watkins J.C., Torous V.F., Roberts D.J. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis. Arch Pathol Lab Med. 2021;145:1341–1349. doi: 10.5858/arpa.2021-0246-SA. [DOI] [PubMed] [Google Scholar]
- 46.Michel A.S., De Logiviere V., Schnuriger A., Lefebvre M., Maisonneuve E., Kayem G. Description of a late miscarriage case at 16 weeks of gestation associated with a SARS-CoV-2 infection. J Gynecol Obstet Hum Reprod. 2021;50 doi: 10.1016/j.jogoh.2021.102064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valk J.E., Chong A.M., Uhlemann A.C., Debelenko L. Detection of SARS-CoV-2 in placental but not fetal tissues in the second trimester. J Perinatol. 2021;41:1184–1186. doi: 10.1038/s41372-020-00877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garrido-Pontnou M., Navarro A., Camacho J., et al. Diffuse trophoblast damage is the hallmark of SARS-CoV-2-associated fetal demise. Mod Pathol. 2021;34:1704–1709. doi: 10.1038/s41379-021-00827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richtmann R., Torloni M.R., Oyamada Otani A.R., et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: a case series. Case Rep Womens Health. 2020;27 doi: 10.1016/j.crwh.2020.e00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stonoga E.T.S., de Almeida Lanzoni L., Rebutini P.Z., et al. Intrauterine transmission of SARS-CoV-2. Emerg Infect Dis. 2021;27:638–641. doi: 10.3201/eid2702.203824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halici-Ozturk F., Ocal F.D., Aydin S., et al. Investigating the risk of maternal-fetal transmission of SARS-CoV-2 in early pregnancy. Placenta. 2021;106:25–29. doi: 10.1016/j.placenta.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hachem R., Markou G.A., Veluppillai C., Poncelet C. Late miscarriage as a presenting manifestation of COVID-19. Eur J Obstet Gynecol Reprod Biol. 2020;252:614. doi: 10.1016/j.ejogrb.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marton T., Hargitai B., Hunter K., Pugh M., Murray P. Massive perivillous fibrin deposition and chronic histiocytic intervillositis a complication of SARS-CoV-2 infection. Pediatr Dev Pathol. 2021;24:450–454. doi: 10.1177/10935266211020723. [DOI] [PubMed] [Google Scholar]
- 54.Sadiq H., Sohail I., Nasir F. Maternal and fetal outcomes in pregnant women with COVID-19 infection; a case series of 16 patients at a tertiary care hospital. IBCAST. 2021:464–470. [Google Scholar]
- 55.Hcini N., Maamri F., Picone O., et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2021;257:11–18. doi: 10.1016/j.ejogrb.2020.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma S., Bradshaw C., Auyeung N.S.F., et al. Outcomes of maternal-newborn dyads after maternal SARS-CoV-2. Pediatrics. 2020;146 doi: 10.1542/peds.2020-005637. [DOI] [PubMed] [Google Scholar]
- 57.Bouachba A., Allias F., Nadaud B., et al. Placental lesions and SARS-Cov-2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta. 2021;112:97–104. doi: 10.1016/j.placenta.2021.07.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattar C.N., Kalimuddin S., Sadarangani S.P., et al. Pregnancy outcomes in COVID-19: a prospective cohort study in Singapore. Ann Acad Med Singap. 2020;49:857–869. [PubMed] [Google Scholar]
- 60.Hodžić J., Muračević B., Štimjanin H., Iriškić R., Husika M. Bosnia and Herzegovina. Med Glas (Zenica); 2022. Pregnancy outcomes of COVID-19 positive pregnant women at the Cantonal Hospital Zenica. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Argueta L.B., Lacko L.A., Bram Y., et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience. 2022;25 doi: 10.1016/j.isci.2022.104223. 104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodrigues M.L., Gasparinho G., Sepúlveda F., Matos T. Signs suggestive of congenital SARS-CoV-2 infection with intrauterine fetal death: a case report. Eur J Obstet Gynecol Reprod Biol. 2021;256:508–509. doi: 10.1016/j.ejogrb.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baral G., Shrestha O., Baral R.S. Thrombotic pathology in placenta of COVID positive pregnancy. J Nepal Health Res Counc. 2021;19:206–208. doi: 10.33314/jnhrc.v19i1.3403. [DOI] [PubMed] [Google Scholar]
- 64.Pulinx B., Kieffer D., Michiels I., et al. Vertical transmission of SARS-CoV-2 infection and preterm birth. Eur J Clin Microbiol Infect Dis. 2020;39:2441–2445. doi: 10.1007/s10096-020-03964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernandez Z., Lichs G.G.C., Zubieta C.S., et al. Case report: SARS-CoV-2 gamma isolation from placenta of a miscarriage in midwest, Brazil. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.839389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferreira M.F.C., Pavon J.A.R., Napoleão A.C.B., et al. Clinical and genomic data of SARS-CoV-2 detected in maternal-fetal interface during the first wave of infection in Brazil. Microbes Infect. 2022;24 doi: 10.1016/j.micinf.2022.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaigham M., Gisselsson D., Sand A., et al. Clinical-pathological features in placentas of pregnancies with SARS-CoV-2 infection and adverse outcome: case series with and without congenital transmission. BJOG. 2022;129:1361–1374. doi: 10.1111/1471-0528.17132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coté J.J., Granger P., Mishra A., Sorini G. COVID-19 in a pregnant cystic fibrosis carrier with myasthenia gravis: a case report. Case Rep Womens Health. 2022;34 doi: 10.1016/j.crwh.2022.e00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patanè L., Cadamuro M., Massazza G., et al. Evidence of vertical transmission of SARS-CoV-2 and interstitial pneumonia in second-trimester twin stillbirth in asymptomatic woman. Case report and review of the literature. Am J Obstet Gynecol MFM. 2022;4 doi: 10.1016/j.ajogmf.2022.100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fitzgerald B., O’Donoghue K., McEntagart N., et al. Fetal deaths in Ireland due to SARS-CoV-2 placentitis caused by SARS-CoV-2 alpha. Arch Pathol Lab Med. 2022;146:529–537. doi: 10.5858/arpa.2021-0586-SA. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz D.A., Baldewijns M., Benachi A., et al. Hofbauer cells and COVID-19 in pregnancy. Arch Pathol Lab Med. 2021;145:1328–1340. doi: 10.5858/arpa.2021-0296-SA. [DOI] [PubMed] [Google Scholar]
- 72.Thomas J., Sun Y., Debelenko L. Infrequent placental and fetal involvement in SARS-CoV-2 infection: pathology data from a large medical center. J Dev Biol. 2021;9:45. doi: 10.3390/jdb9040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Babal P., Krivosikova L., Sarvaicova L., et al. Intrauterine fetal demise after uncomplicated COVID-19: what can we learn from the case? Viruses. 2021;13:2545. doi: 10.3390/v13122545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eich M.L., Menter T., Mokwa N.F., Grüttner B., Müller A.M. [Intrauterine fetal demise in extensive SARS-CoV-2-associated placental maternal vascular malperfusion in the setting of SARS-CoV-2 placentitis (severe acute respiratory syndrome coronavirus 2)] Pathologe. 2022;43:135–139. doi: 10.1007/s00292-021-01035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guan M., Johannesen E., Tang C.Y., et al. Intrauterine fetal demise in the third trimester of pregnancy associated with mild infection with the SARS-CoV-2 delta variant without protection from vaccination. J Infect Dis. 2022;225:748–753. doi: 10.1093/infdis/jiac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mithal L.B., Otero S., Simons L.M., et al. Low-level SARS-CoV-2 viremia coincident with COVID placentitis and stillbirth. Placenta. 2022;121:79–81. doi: 10.1016/j.placenta.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sagara A., Yamaguchi M., Mikami Y., Motohara T., Ohba T., Kondoh E. Maternal thrombocytopenia precedes fetal death associated with COVID-19. J Obstet Gynaecol Res. 2022;48:1475–1479. doi: 10.1111/jog.15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong M.J., Bharadwaj S., Lankford A.S., Galey J.L., Kodali B.S. Mechanical ventilation and prone positioning in pregnant patients with severe COVID-19 pneumonia: experience at a quaternary referral center. Int J Obstet Anesth. 2022;49 doi: 10.1016/j.ijoa.2021.103236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bewley D.J., Lee J., Popescu O., Oviedo A. SARS-CoV-2 placental infection in an unvaccinated mother resulting in fetal demise. Cureus. 2021;13 doi: 10.7759/cureus.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huynh A., Goldfarb I., Watkins J., Torous V., Sehn J., Roberts D. SARS-CoV-2 placentitis with intraparenchymal thrombohematomas: association with intrauterine fetal demise, perinatal morbidity, and ultrasonographic findings. Lab Investig. 2022;102(Suppl1):1276–1278. [Google Scholar]
- 81.Shook L.L., Brigida S., Regan J., et al. SARS-CoV-2 placentitis associated with B.1.617.2 (delta) variant and fetal distress or demise. J Infect Dis. 2022;225:754–758. doi: 10.1093/infdis/jiac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stenton S., McPartland J., Shukla R., et al. SARS-COV2 placentitis and pregnancy outcome: a multicentre experience during the alpha and early delta waves of coronavirus pandemic in England. EClinicalMedicine. 2022;47 doi: 10.1016/j.eclinm.2022.101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dubucs C., Groussolles M., Ousselin J., et al. Severe placental lesions due to maternal SARS-CoV-2 infection associated to intrauterine fetal death. Hum Pathol. 2022;121:46–55. doi: 10.1016/j.humpath.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.di Gioia C., Zullo F., Bruno Vecchio R.C., et al. Stillbirth and fetal capillary infection by SARS-CoV-2. Am J Obstet Gynecol MFM. 2022;4 doi: 10.1016/j.ajogmf.2021.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Konstantinidou A.E., Angelidou S., Havaki S., et al. Stillbirth due to SARS-CoV-2 placentitis without evidence of intrauterine transmission to fetus: association with maternal risk factors. Ultrasound Obstet Gynecol. 2022;59:813–822. doi: 10.1002/uog.24906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zinserling V.A., Bornstein S.R., Narkevich T.A., et al. Stillborn child with diffuse SARS-CoV-2 viral infection of multiple organs. IDCases. 2021;26 doi: 10.1016/j.idcr.2021.e01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allotey J., Chatterjee S., Kew T., et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-067696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pratama N.R., Wafa I.A., Budi D.S., Putra M., Wardhana M.P., Wungu C.D.K. mRNA COVID-19 vaccines in pregnancy: a systematic review. PLoS One. 2022;17 doi: 10.1371/journal.pone.0261350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vivanti A.J., Vauloup-Fellous C., Escourrou G., et al. Factors associated with SARS-CoV-2 transplacental transmission. Am J Obstet Gynecol. 2022;227:541–543.e11. doi: 10.1016/j.ajog.2022.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hosier H., Farhadian S.F., Morotti R.A., et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharps M.C., Hayes D.J.L., Lee S., et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. doi: 10.1016/j.placenta.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwartz D.A., Mulkey S.B., Roberts D.J. SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination: clinical-pathologic correlations. Am J Obstet Gynecol. 2022 doi: 10.1016/j.ajog.2022.10.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taweevisit M., Thawornwong N., Thorner P.S. Massive perivillous fibrin deposition associated with placental syphilis: a case report. Pediatr Dev Pathol. 2021;24:43–46. doi: 10.1177/1093526620957523. [DOI] [PubMed] [Google Scholar]
- 94.Cornish E.F., McDonnell T., Williams D.J. Chronic inflammatory placental disorders associated with recurrent adverse pregnancy outcome. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.825075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Benachi A., Rabant M., Martinovic J., et al. Chronic histiocytic intervillositis: manifestation of placental alloantibody-mediated rejection. Am J Obstet Gynecol. 2021;225:662.e1–662.e11. doi: 10.1016/j.ajog.2021.06.051. [DOI] [PubMed] [Google Scholar]
- 96.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Luca D., Sanguinetti M. COVID-19 and perinatology. Springer International Publishing; 2022. Chapter 9. Vertical SARS-CoV-2 transmission. [Google Scholar]
- 98.Gengler C., Dubruc E., Favre G., Greub G., de Leval L., Baud D. SARS-CoV-2 ACE-receptor detection in the placenta throughout pregnancy. Clin Microbiol Infect. 2021;27:489–490. doi: 10.1016/j.cmi.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li M., Chen L., Zhang J., Xiong C., Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One Chan. 2020;15 doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vento-Tormo R., Efremova M., Botting R.A., et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. 2018;563:347–353. doi: 10.1038/s41586-018-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Faure-Bardon V., Isnard P., Roux N., et al. Protein expression of angiotensin-converting enzyme 2, a SARS-CoV-2-specific receptor, in fetal and placental tissues throughout gestation: new insight for perinatal counseling. Ultrasound Obstet Gynecol. 2021;57:242–247. doi: 10.1002/uog.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hernández-Díaz S., Smith L.H., Wyszynski D.F., Rasmussen S.A. First trimester COVID-19 and the risk of major congenital malformations-International Registry of Coronavirus Exposure in Pregnancy. Birth Defects Res. 2022;114:906–914. doi: 10.1002/bdr2.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mosnino E., Bernardes L.S., Mattern J., et al. Impact of SARS-CoV-2 alpha and gamma variants among symptomatic pregnant women: a two-center retrospective cohort study between France and Brazil. J Clin Med. 2022;11:2663. doi: 10.3390/jcm11092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andrews J.C., Schünemann H.J., Oxman A.D., et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66:726–735. doi: 10.1016/j.jclinepi.2013.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
XXX
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.
Alcover. Fetal demises following SARS-CoV-2 infection. Am J Obstet Gynecol 2023.