ABSTRACT
In the present study, population pharmacokinetic (PK) analysis was performed based on meropenem data from a prospective study conducted in 114 critically ill patients with a wide range of renal functions and various disease conditions. The final model was a one-compartment model with linear elimination, with creatinine clearance and continuous renal replacement therapy affecting clearance, and total bodyweight impacting the volume of distribution. Our model is a valuable addition to the existing meropenem population PK models, and it could be particularly useful during implementation of a therapeutic drug monitoring program combined with Bayesian forecasting. Based on the final model developed, comprehensive Monte Carlo simulations were performed to evaluate the probability of target attainment (PTA) of 16 different dosing regimens. Simulation results showed that 2 g administered every 8 h with 3-h prolonged infusion (PI) and 4 g/day by continuous infusion (CI) appear to be two empirical dosing regimens that are superior to many other regimens when both target attainment and potential toxicity are considered and renal function information is not available. Following a daily CI dose of 6 g or higher, more than 30% of the population with a creatinine clearance of <60 mL/min is predicted to have neurotoxicity. With the availability of institution- and/or unit-specific meropenem susceptibility patterns, as well as an individual patient’s renal function, our PTA results may represent useful references for physicians to make dosing decisions.
KEYWORDS: ICU patients, meropenem dose regimen, target attainment analysis, population pharmacokinetics
INTRODUCTION
Meropenem is a carbapenem antibiotic that has antimicrobial activity against Gram-positive and Gram-negative pathogens, such as Pseudomonas aeruginosa and Acinetobacter spp., as well as anaerobes. Meropenem has a short half-life (~1 h under normal renal function), shows minimal plasma protein binding (<2%), and mainly undergoes renal elimination, with 60 to 80% of the drug being excreted in urine as unchanged drug (1, 2). As with other β-lactam antibiotics, meropenem displays time-dependent killing, and the best pharmacokinetic/pharmacodynamic (PK/PD) predictor is the percentage of the time interval during which the unbound drug concentration exceeds the MIC for the causative pathogen (i.e., % fT>MIC). The traditional PK/PD target for meropenem is 40% fT>MIC; this cutoff value was defined based on in vitro and in vivo animal studies (3). For severe infections, such as in critically ill patients, the PK/PD target of 100% fT>MIC (i.e., fCtrough>MIC) has been advocated because of its association with a favorable clinical outcome (4). In addition, a more aggressive PK/PD target of 100% fT>4×MIC (i.e., fCtrough>4×MIC) has been proposed to achieve maximal killing while avoiding the emergence of antimicrobial resistance (5, 6).
Due to its broad spectrum of antimicrobial activity, meropenem is frequently used for the empirical treatment of severe infections in critically ill patients. Studies have shown that the standard meropenem dosing regimens may lead to insufficient PK exposure and/or suboptimal PK/PD target attainment in a large proportion of intensive care unit (ICU) patients (7, 8). Critically ill patients with augmented renal clearance and/or with infections due to less-susceptible pathogens are a particular concern due to increased risk of treatment failure with subtherapeutic antibiotic concentrations. To improve the rational use of meropenem in critically ill patients, great effort has been made to characterize its PK and probability of target attainment (PTA) in this special population, with several population PK studies of meropenem being reported in the past decade (9–15). However, many of the aforementioned population PK and PTA studies have the limitations of small sample size (8, 12), inclusion of only certain subpopulations (7, 11), and/or the retrospective nature of the work (16), leading to uncertain predictability and generalizability of the reported population PK models. Indeed, a recent study conducted by Yang et al. evaluated the adequacy and predictive capabilities of 14 published meropenem PK models (17), and their results showed that none of the models could adequately describe meropenem PK data collected in their center. This highlights the need to develop a robust population PK model for meropenem. In the present study, we prospectively evaluated meropenem PK and target attainment in a relatively large and heterogeneous ICU population with various conditions (acute kidney injury, sepsis, septic shock, etc.), as well as a wide range of renal function (ranging from renal impairment requiring continuous renal replacement therapy [CRRT] to augmented renal clearance). The aims of the present study were (i) to perform population PK modeling to quantitatively characterize meropenem disposition in the study population, (ii) to identify the potential significant covariates that can explain meropenem PK variability among patients, (iii) to perform comprehensive simulations to evaluate the PTA of meropenem over a wide MIC range following different dosing regimens, and (iv) finally, to provide empirical dosing recommendations based on the modeling and simulation results.
RESULTS
Patient demographics.
In this present meropenem study, 130 critically ill patients, including 10 patients undergoing CRRT, were enrolled. The major reasons for ICU admission (only n ≥ 10 are listed) included respiratory disorders (n = 41) and infections and infestations (n = 30), as well as surgical and medical procedures (n = 13). The median age of the study subjects was 63 years, with male subjects accounting for 53% of the population. The median creatinine clearance, calculated with Cockcroft-Gault formula using total body weight (CLCR,TBW), was 87 mL/min (interquartile range [IQR] = 50 to 128 mL/min). Among the enrolled subjects, 38% had acute kidney injury, 47% had sepsis, 29% had septic shock, and 54% were on mechanical ventilation. The detailed information of patient demographic and clinical characteristics is in Table 1. Patients received meropenem for the following indications: pneumonia (n = 25), sepsis (n = 19), septic shock (n = 12), respiratory tract infection (n = 10), and several other types of infections or diseases (n ≤ 5 for each). The most common meropenem dosing regimens used were 1,000 mg Q8h 3-h infusions (n = 26), 500 mg Q6h 3-h infusions (n = 19), and 500 mg Q8h 3-h infusions (n = 7).
TABLE 1.
Clinical characteristics of study patients receiving meropenem therapy (n = 130)
| Characteristic | Median (IQR) or n (%) |
|---|---|
| CLCR,TBW (mL/min) | 87 (50–128) |
| CLCR,LBW (mL/min) | 56 (33–86) |
| Total body wt (kg) | 85 (69–107) |
| Lean body wt (kg) | 57 (46–66) |
| Age (yrs) | 63 (53–71) |
| Sex | 54 females (47), 60 males (53) |
| Race | 9 Black/African American (8), 104 white (91), 1 multiple (1) |
| Ethnicity | 112 not Hispanic (98), 2 not reported (2) |
| Acute kidney injury | 43 (38) |
| Sepsis | 54 (47) |
| Septic shock | 33 (29) |
| Hepatic function | 17 normal (15), 42 not normal (37), 55 missing (48) |
| SOFA | 7 (4–9) |
| Mechanical ventilation | 61 (54) |
Population PK modeling.
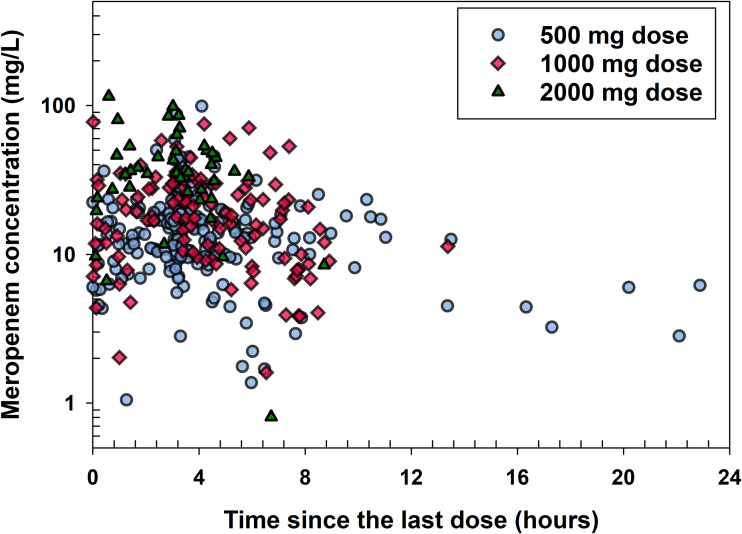
A total of 370 meropenem plasma concentrations from the 112 enrolled subjects were included in the population PK analysis. The number of concentrations ranged from 1 to 8 across all patients. Sample collection times were also random for each subject. Figure 1 shows the observed meropenem plasma concentrations over time since last dose (time past start of infusion of last dose).
FIG 1.
Observed meropenem plasma concentrations versus time since the last dose stratified by the doses.
A one-compartment model with zero-order input and first-order elimination process was found to be the best structural model to characterize meropenem PK in the study population. Covariate analysis identified the following significant covariates: creatinine clearance calculated with the Cockcroft-Gault formula using total body weight (CLCR,TBW) on the renal clearance of meropenem (CLR), CRRT on meropenem total clearance (CLT), and total bodyweight (TBW) on the volume of distribution (V). We evaluated both lean bodyweight (LBW) and total bodyweight (TBW) when we calculated CLCR. However, CLCR,LBW on CLR did not provide a substantial improvement in objective function or unexplained interindividual variability (IIV) over CLCR,TBW on CLR. LBW is more difficult to calculate for clinical application. Therefore, CLCR,TBW on CLR was chosen in the final model. Various other covariates, such as mechanical ventilation and mortality/severity of disease (SOFA) scores, were evaluated, and none of them had significant impact on CL and V of meropenem. In the final model, IIV terms were estimated for both CLT and V. A combined proportional and additive error model best described the unexplained residual variability. The estimates of the meropenem PK parameters from the final model are provided in Table 2. The estimated CLT of meropenem was 5.28 L/h in study subjects not receiving CRRT and 3.98 L/h while receiving CRRT. The volume of distribution per 85 kg total bodyweight was estimated to be 35.1 L.
TABLE 2.
Parameter estimates of the final meropenem population PK modela
| Parameter | Unit | Population estimate | RSE (%) | Bootstrap median | Bootstrap nonparametric 95% CI |
|---|---|---|---|---|---|
| CLR | L/h per 87 mL/min CLCR,TBW | 3.69 | 12.1 | 3.71 | 2.96–4.51 |
| CLCRRT | L/h | 2.39 | 29.2 | 2.39 | 1.09–3.96 |
| CLNR | L/h | 1.59 | 25.2 | 1.55 | 0.87–2.33 |
| CLT,nonCRRT | L/h | 5.28 | 5.27 | 4.76–5.80 | |
| CLT,CRRT | L/h | 3.98 | 3.98 | 2.84–5.34 | |
| V | L/85 kg TBW | 35.1 | 10.3 | 34.0 | 27.8–42.9 |
| IIV CL | % | 47.1 | 27.0 | 46%b | 33–60% |
| IIV V | % | 58.9 | 55.6 | 57% | 18–84% |
| Corr (CL,V) | % | 58.7 | 43.8 | 60% | –5.9–100% |
| CVCP | % | 31.6 | 16.0 | 31% | 25–37% |
| SDCP | mg/L | 0.209 | 231.7 | 0.18 | 0.002–2.05 |
CLR, renal clearance; CLCRRT, clearance by CRRT; CLNR, nonrenal clearance; CLT,CRRT, total body clearance in subjects on CRRT; CLT,nonCRRT, total body clearance in subjects not on CRRT; V, volume of distribution; CVCP, proportional residual error; SDCP, additive residual error; TBW, total body weight; RSE, relative standard error.
The IIV term relates to the total body clearance CLT.
As shown in Table 2, the relative standard error (RSE) was below 30% for each of the estimated PK parameters, suggesting that they were estimated with good precision.
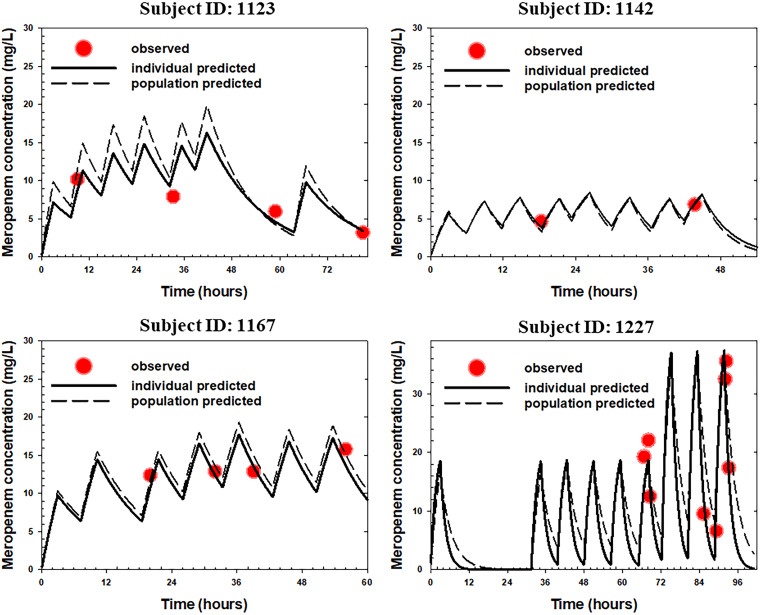
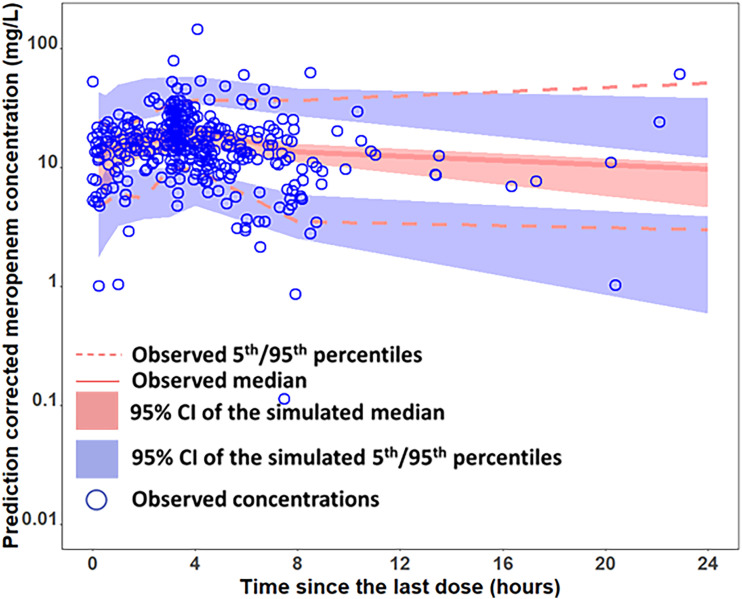
The time courses of observed versus model predicted plasma concentrations of meropenem in four representative ICU patients are provided in Fig. 2, which clearly showed good agreement between the model predicted meropenem concentrations and observed concentrations at different time points across different dosing regimens. The prediction-corrected visual predictive check (pc-VPC) plot, as presented in Fig. 3, shows that the observed median and 5th/95th percentiles fall within the 95% confidence intervals of the simulated median and 5th/95th percentiles, indicating that the prediction-corrected concentrations were well predicted by the final model. Figure S1 in the supplemental material shows additional goodness-of-fit plots, with the population-predicted and individual-predicted concentrations versus the observed concentrations being presented in Fig. S1A and B, respectively, and the conditional weighted residuals versus time and the conditional weighted residuals versus population-predicted concentrations in Fig. S1C and D, respectively. As shown in these plots, the data were uniformly distributed around the line of identity or zero line without bias, indicating that the final model characterized meropenem PK adequately at both population and individual levels without significant bias in the model fit.
FIG 2.
Time course of individual observed (symbols) versus individual/population predicted (solid/dash lines) meropenem plasma concentrations in four representative ICU patients receiving meropenem standard of care.
FIG 3.
Prediction-corrected visual predictive check (pc-VPC) of the final meropenem model.
PTA analysis.
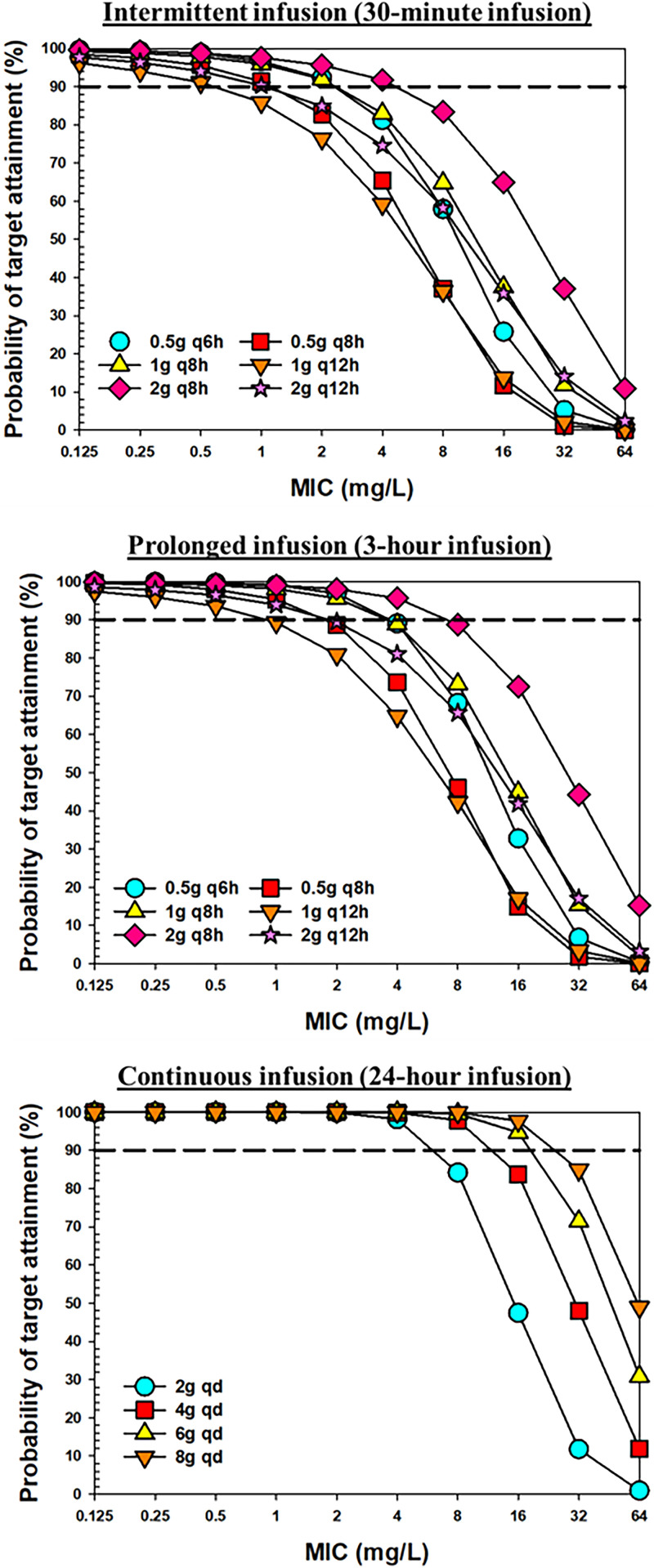
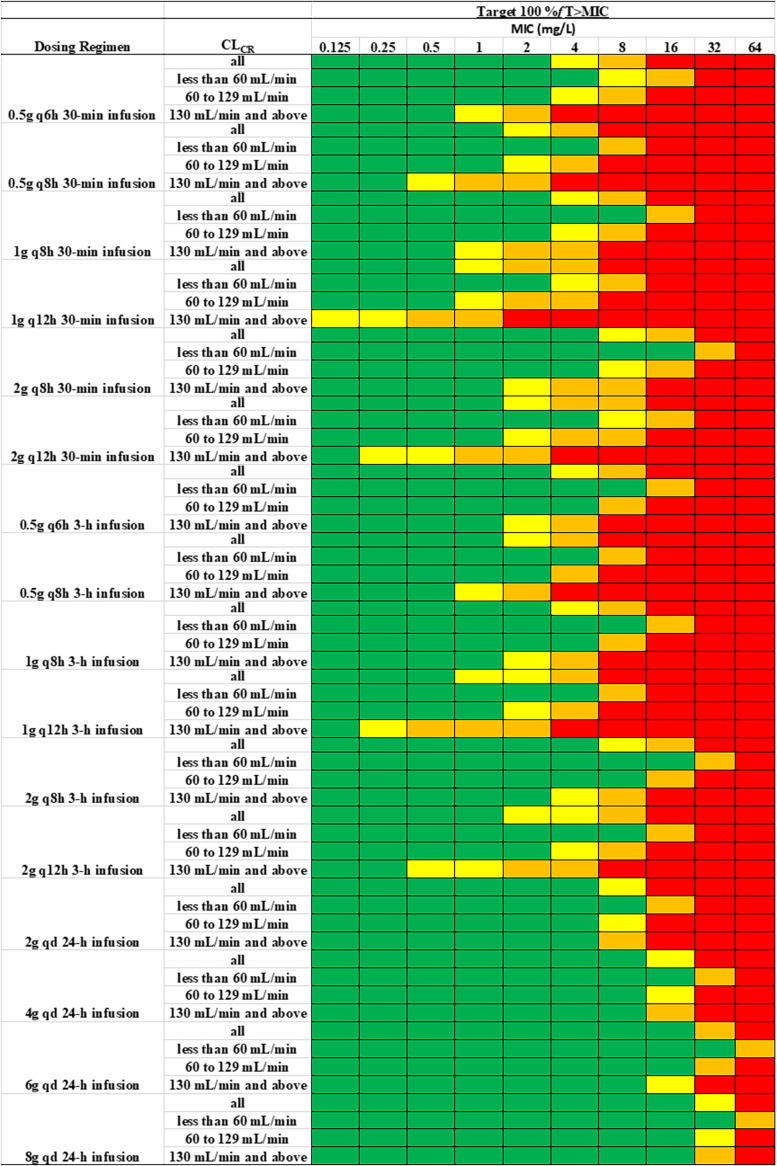
Based on the PK parameters estimated from the final model, comprehensive Monte Carlo simulations were performed to evaluate the PTA of meropenem in critically ill patients following various dose regimens using three different PK/PD targets, namely, 40% fT>MIC, 100% fT>MIC, and 100% fT>4×MIC. The PTA versus MIC profiles for different dosing regimens with intermittent infusion (II), prolonged infusion (PI), or continuous infusion (CI) are presented in Fig. 4 and Fig. S2. A heatmap of PTA of meropenem over a wide MIC range with 16 different dosing regimens in all subjects, as well as subjects stratified by renal function, is shown in Fig. 5 and Fig. S3. A PTA of ≥90% was coded with green color to indicate satisfactory target attainment. Table 3 and Table S1 list the breakpoint values that can be reached with various meropenem dosing regimens. For the 2-g daily dose scenario, we simulated data under five different dosing regimens, including the 0.5-g Q6h 0.5-h infusion, 0.5-g Q6h 3-h infusion, 1-g Q12h 0.5-h infusion, and 1-g Q12h 3-h infusion, as well as the 2-g-per-day continuous infusion. As shown in Table 3 and Fig. 5, with a target of 100% fT>MIC, the dosing regimen of 0.5 g Q6h administered as 0.5-h infusions could reach breakpoints of 4, 2, and 0.5 mg/L in critically patients with CLCR values of <60 mL/min, 60 to 129 mL/min, and ≥130 mL/min, respectively. As shown in Table S1, with a target of 40% fT>MIC, following the same dosing regimen, the breakpoint values increased to 8, 8, and 4 mg/L in patients with renal impairment, normal renal function and augment renal function, respectively. With a target of 100% fT>4×MIC, meropenem 0.5 g Q6h given as 0.5-h infusions did not reach the EUCAST clinical breakpoint of 2 mg/L against Enterobacterales, P. aeruginosa, and Acinetobacter baumannii in any population. With a target of 100% fT>MIC and following the same dosing regimen (0.5 g Q6h) but with prolonged 3-h infusions, the breakpoints that could be reached were 8, 4, and 1 mg/L in critically patients with CLCR values of <60 mL/min, 60 to 129 mL/min, and ≥130 mL/min, respectively; these values are double those achieved for the same target with 0.5 g Q6h administered as 0.5-h infusions.
FIG 4.
PTA of meropenem versus MIC following different dosing regimens with the target of 100% fT>MIC for regimens with intermittent 30-min infusion (upper panel), 3-h prolonged infusion (middle panel), and 24-h continuous infusion (lower panel).
FIG 5.
Heatmap of PTA of meropenem at different MIC values following different dosing regimens in all subjects as well as subjects with different renal functions, with the PK/PD targets of 100% fT>MIC. Color coding: green, PTA ≥ 90%; yellow, PTA 80 to 89%; orange, PTA 50 to 79%; red, PTA < 50%.
TABLE 3.
Predicted meropenem breakpoints following different dosing regimens in all subjects, as well as in subjects with different renal functions
| Dosing regimen | CLCR (mL/min) | Meropenem breakpoint (mg/L)a | PPNb (%) |
|---|---|---|---|
| 0.5 g, Q6h, 30-min infusion | All | 2 | 0.14 |
| <60 | 4 | 0.37 | |
| 60–129 | 2 | 0.04 | |
| ≥130 | 0.5 | 0.04 | |
| 0.5 g, Q8h, 30-min infusion | All | 1 | 0.02 |
| <60 | 4 | 0.03 | |
| 60–129 | 1 | 0.02 | |
| ≥130 | 0.25 | 0.00 | |
| 1 g, Q8h, 30-min infusion | All | 2 | 0.65 |
| <60 | 8 | 1.83 | |
| 60–129 | 2 | 0.13 | |
| ≥130 | 0.5 | 0.12 | |
| 1 g, Q12h, 30-min infusion | All | 0.5 | 0.06 |
| <60 | 2 | 0.17 | |
| 60–129 | 0.5 | 0.00 | |
| ≥130 | <0.125 | 0.00 | |
| 2 g, Q8h, 30-min infusion | All | 4 | 5.38 |
| <60 | 16 | 12.72 | |
| 60–129 | 4 | 2.65 | |
| ≥130 | 1 | 1.00 | |
| 2 g, Q12h, 30-min infusion | All | 1 | 1.16 |
| <60 | 4 | 3.40 | |
| 60–129 | 1 | 0.15 | |
| ≥130 | 0.125 | 0.20 | |
| 0.5 g, Q6h, 3-h infusion | All | 2 | 0.19 |
| <60 | 8 | 0.60 | |
| 60–129 | 4 | 0.00 | |
| ≥130 | 1 | 0.00 | |
| 0.5 g, Q8h, 3-h infusion | All | 1 | 0.02 |
| <60 | 4 | 0.06 | |
| 60–129 | 2 | 0.00 | |
| ≥130 | 0.5 | 0.00 | |
| 1 g, Q8h, 3-h infusion | All | 2 | 0.98 |
| <60 | 8 | 2.72 | |
| 60–129 | 4 | 0.22 | |
| ≥130 | 1 | 0.16 | |
| 1 g, Q12h, 3-h infusion | All | 0.5 | 0.08 |
| <60 | 4 | 0.20 | |
| 60–129 | 1 | 0.02 | |
| ≥130 | 0.125 | 0.04 | |
| 2 g, Q8h, 3-h infusion | All | 4 | 7.61 |
| <60 | 16 | 17.63 | |
| 60–129 | 8 | 4.04 | |
| ≥130 | 2 | 1.28 | |
| 2 g, Q12h, 3-h infusion | All | 1 | 1.56 |
| <60 | 8 | 4.40 | |
| 60–129 | 4 | 0.41 | |
| ≥130 | 0.25 | 0.04 | |
| 2 g, daily continuous infusion | All | 4 | 0.47 |
| <60 | 8 | 1.37 | |
| 60–129 | 4 | 0.08 | |
| ≥130 | 4 | 0.04 | |
| 4 g, daily continuous infusion | All | 8 | 5.89 |
| <60 | 16 | 13.83 | |
| 60–129 | 8 | 3.13 | |
| ≥130 | 8 | 0.72 | |
| 6 g, daily continuous infusion | All | 16 | 15.32 |
| <60 | 32 | 30.03 | |
| 60–129 | 16 | 11.52 | |
| ≥130 | 8 | 2.92 | |
| 8 g, daily continuous infusion | All | 16 | 24.41 |
| <60 | 32 | 39.57 | |
| 60–129 | 16 | 22.39 | |
| ≥130 | 16 | 7.56 |
That is, the highest MIC at which ≥90% of the subjects achieve targets. The target is 100% fT>MIC.
PPN, predicted possibility of neurotoxicity.
Compared to 0.5 g Q6h, the same daily dose but with longer dosing interval (1 g Q12h) resulted in much lower breakpoint coverage, this was consistently observed regardless of whether administration was by II or PI. On the other hand, the breakpoints reached were much higher following continuous infusion of the same daily dose (i.e., 2 g/day CI). For example, for a target of 100% fT>MIC the breakpoints were 16, 8, and 8 mg/L in patients with renal impairment, normal renal function, and augmented renal function, respectively. The detailed results of other dosing regimens can be found in Fig. 4 and 5 and Fig. S2 and S3, as well as Table 3 and Table S1.
It has been reported that there is 50% risk of developing neurotoxicity or nephrotoxicity when meropenem concentrations were higher than 64.2 or 44.5 mg/L, respectively (18). Based on this information, we estimated percentage of subjects with Ctrough exceeding these two thresholds and the corresponding probability of meropenem-induced toxicities following various dosing regimens (Table 3 and see Table S2). Overall, a low toxicity risk is expected when meropenem is given II or PI in subjects with creatinine clearance >60 mL/min. The predicted neurotoxicity or nephrotoxicity incidence is >15% in all populations in those two high daily CI dose regimens (i.e., 6 g/day CI and 8 g/day CI). For subjects with renal function at <60 mL/min, meropenem-inducted toxicity is expected to be high in not only ≥4 g daily CI doses but also in 2-g Q8h II and PI dose regimens.
DISCUSSION
Based on meropenem PK data collected from 114 critically ill patients from two ICU sites, population PK analysis was performed, and a one-compartment model with linear elimination was found to best characterize meropenem disposition in the study population. In our final model, the estimated typical values of CL and V of meropenem are 5.28 L/h (per 87 mL/min CLCR) and 35.1 L (per 85 kg body weight), respectively, with creatinine clearance and CRRT affecting CL and total body weight having impact on V. There are numerous meropenem population PK reports available in the literature, and the estimated parameters, especially CL, vary substantially among different reports (7, 9, 10, 12, 14, 19); this is likely due to the differences across selected populations. For example, Gijsen et al. reported meropenem volume of distribution (37.9 L) that is similar to our estimate, but a much higher clearance (13.7 L/h) (7), which is not surprising since they only enrolled critically ill patients with preserved or increased renal function. On the other hand, Ulldemolins et al. also reported a similar estimate of volume of distribution (33 L) but a lower clearance (3.68 L/h) (10), which could be explained by the fact that only critically ill patients with septic shock who were on CRRT were included in their study. Our model estimated total clearance for patients on CRRT was 3.98 L/h.
Many meropenem population PK studies reported that renal function was a significant covariate on meropenem elimination (13–16, 20, 21); this was confirmed in our analysis. This is expected since meropenem is a hydrophilic compound undergoing extensive renal elimination. In addition to renal function, we also evaluated various other covariates and identified two additional important covariates, namely, body weight and CRRT. This is in line with some of the prior meropenem population PK reports, albeit not all since many studies failed to identify these covariates likely due to small sample size and/or lack of patients with CRRT in their study population (13, 19, 21, 22). While the inclusion of those three significant covariates did explain a considerable fraction of the variability, the remaining unexplained variability is still high, as reflected by the 47% IIV for CLT and 59% IIV for V. These unexplained high variabilities likely are caused by the underlying dynamic and complex pathophysiological changes whose net impact on meropenem PK is difficult to evaluate (23). The remaining unexplained high intersubject variability of meropenem parameters was also consistently reported in many other meropenem population PK reports (9, 12, 15, 21). The fact that meropenem PK variability remains high, even after incorporation of covariates, indicates the importance of performing therapeutic drug monitoring (TDM) for individualized therapy. For ICUs with β-lactam TDM implemented, an attractive strategy of model-informed precision dosing (24), which combines sparse PK data obtained from TDM with Bayesian forecasting using published population PK models, could be applied to optimize dosing regimens in real time for individual patients. Successful Bayesian forecasting relies on robust existing population PK models. We believe that our meropenem population PK model, which was established based on a large population covering a wide range of renal function and various disease conditions, is a valuable addition to the existing meropenem population PK models. We look forward to the usage and external validation by other research groups, especially those planning to implement a TDM program combined with Bayesian forecasting, to verify the applicability and generalizability of our model.
After the final model was established, we performed comprehensive simulation to evaluate the PTA of 16 different dosage regimens of meropenem. Our results indicated that none of the Q12h dosing regimens, regardless of II or PI, evaluated in our analysis can reach target attainment at MIC of 2 mg/L (i.e., EUCAST PK/PD susceptible breakpoint for meropenem) when the target of 100% fT>MIC is used. Following the same dose and dosing interval, PI can provide higher MIC coverage than II. Among all II and PI dosing regimens evaluated, only one dosing regimen, namely, 2-g Q8h 3-h infusion, can reach the breakpoint of 2 mg/L in patients with augmented renal function with the target of 100% fT>MIC. In contrast to the routinely recommended dose adjustment in renally impaired patients, the dose increase in patients with augmented renal function is not standard practice. Our simulation highlights the importance of identifying the subpopulation of patients with augmented renal function and prescribing more aggressive dose regimens as needed.
Compared to PI, CI provides even higher breakpoint coverage. For example, following 6-g daily dose, meropenem 6-g/day CI reaches breakpoint of 16 mg/L with target 100% fT>MIC, which is 4-fold higher than the breakpoint of 4 mg/L reached following 2-g Q8h II or PI. Compared to 6-g/day CI, a higher dose of 8-g/day CI results in essentially same breakpoints as 6-g/day CI does, except for the subjects with augmented renal function. Imani et al. retrospectively reviewed the TDM data of piperacillin, meropenem, or flucloxacillin from 378 patients, and their result indicated that there is 50% risk of developing neurotoxicity or nephrotoxicity when meropenem concentrations were higher than 64.2 or 44.5 mg/L, respectively (18). We calculated the percentage of the simulated population with Ctrough exceeding these two thresholds. Following a daily CI dose of 6 g or higher, meropenem-induced neurotoxicity and nephrotoxicity are expected in more than 15 and 25% of the total population, respectively. For subjects with a creatinine clearance of <60 mL/min, >30% neurotoxicity and >40% nephrotoxicity are expected following daily a CI dose of 6 g or higher. As these thresholds were defined in a retrospective study, their robustness and reliability remain unclear. Based on our simulation results, 2-g Q8h 3-h infusion, which can reach the EUCAST PK/PD sensitive breakpoint of 2 mg/L even in patients with augmented renal function, and 4-g/day CI, which can reach the EUCAST PK/PD resistant breakpoint of 8 mg/L even in patients with augmented renal function, appear to be two empirical dosing regimens that are superior to many other regimens when both target attainment and potential toxicity are considered, even without the availability of MIC or renal function information. If creatinine clearance information is available, our breakpoint table (Table 3 and see Table S1) and predicted toxicity incidence table (Table 3 and see Table S2) could be used for further dose adjustment.
For meropenem CI regimen, we would like to point out that it should not be continuously infused over 24 h. Fawaz et al. evaluated the stability of meropenem in infusion bags and their results showed that meropenem concentrations dropped to 90% of the initial concentration at 7.4 and 5.7 h at 22 and 33°C, respectively (25). Carlier et al. reported dose-dependent stability where 10- and 20-mg/mL solutions were stable for 12 h in 0.9% sodium chloride at 25°C, while the 40-mg/mL solutions were stable for a maximum of 8 h (26). Based on these stability reports, we recommend the replacement of the infusion bag every 6 h for CI regimens.
Even though we evaluated three different PD targets, we mainly discussed empirical dosing recommendations based on the target of 100% fT>MIC rather than 40% fT>MIC or 100% fT>4×MIC for several reasons. The target of 40% fT>MIC was defined based on in vitro and in vivo animal studies, which may not be suitable for critically ill patients. In addition, it has been reported that critically ill patients with severe infections have microcirculatory alterations which can result in impaired tissue distribution and accordingly a lower percentage of fT>MIC at the target site (27). Therefore, the target of 40% fT>MIC may not be adequate for critically ill patients. On the other hand, the target of 100% fT>4×MIC is aggressive and its impact on clinical outcomes is uncertain at this point. Using this target, very high doses will be needed for those less susceptible pathogens, which is unrealistic after factoring in the risk of toxicity.
Our study has the following strengths. First, as noted earlier, it is a prospective study carried out in a relatively large population with various renal function and disease conditions. Second, this study used an opportunistic sampling strategy, in which both sample collection time and number of samples per patient were random among subjects. With this strategy, even with sparse PK samples, a robust population PK model can be established. Our opportunistic sampling strategy is fundamentally different from TDM, in which only Cmax and/or Ctrough are collected for each patient, and accordingly the data are usually not suitable for population PK model development. Third, in our PTA simulation we evaluated 16 different dosing regimens covering various types of scenarios. With the availability of institution- or unit-specific meropenem susceptibility patterns (i.e., historic MIC distributions), physicians could select empirical dose regimens based on the heat map and breakpoint table that we provided.
Our study has a number of limitations. First, most patients enrolled in the study were treated with 3 h PI. As the infusion time masked the distribution phase, the peripheral compartment could not be captured. Accordingly, our final model was a one-compartment model instead of the two-compartment model that has been reported in several studies. However, the lack of a peripheral compartment, which may result in a different shape of the curve in the first few hours after the start of the infusion and an underestimated Cmax, is anticipated to have a smaller impact on target attainment evaluation of meropenem which exhibits time-dependent killing (where % fT>MIC matters) compared to the impact it would have on an antibiotic with concentration-dependent killing (where Cmax/MIC matters). Second, adverse effect data were not collected in our study. As a result, we could not evaluate the reliability of those thresholds proposed by Imani et al. (18). Further investigations are warranted in this area.
In conclusion, a population PK model was successfully developed to characterize meropenem disposition in critically ill patients. Comprehensive Monte Carlo simulation results showed that 2 g Q8h 3-h PI and 4-g/day CI approaches appear to be superior to other regimens when both target attainment and potential toxicity are considered and individual covariate information is not available. Creatinine clearance, body weight, and CRRT were identified to be significant covariates. Our model is a valuable addition to the existing meropenem population PK models, and it could be particularly useful during the implementation of a TDM program combined with Bayesian forecasting. With the availability of institution- and/or unit-specific meropenem susceptibility patterns, as well as an individual patient’s renal function, our PTA results may represent useful references for physicians to make dosing decisions.
MATERIALS AND METHODS
Subjects and study design.
This prospective, opportunistic, open-label population PK study was carried out at the University of Iowa and Vanderbilt University Medical Center. Adult ICU patients prescribed intravenous meropenem as the standard of care were eligible to participate. The main exclusion criteria were patients who were on intermittent dialysis or extracorporeal membrane oxygenation, receiving probenecid, or who were pregnant at the time of enrollment. The protocol was approved by the appropriate IRB at each study site prior to the start of the study. Consent to participate was obtained from each subject prior to any study-related procedures. The dosing of meropenem was based on the manufacturer’s recommendations. Since this was an opportunistic study, the number of samples per subject and sample collection time varied among subjects.
Bioanalytical methods.
The concentrations of meropenem in human plasma were determined using a fully validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (28). Briefly, the extraction of meropenem and 2H6-meropenem (internal standard [IS]) was accomplished by a simple protein precipitation with 4× volume of acetonitrile. Chromatographic separation of analytes was achieved using a stepwise gradient elution with a Phenomenex Kinetex C18 column (2.6 μm by 50 mm by 2.1 mm). Meropenem and IS were monitored using MS/MS with a turbo ion spray source in positive multiple reaction monitoring mode. The m/z ratios of precursor ion and product ion of meropenem were 384.3 and 147.1, respectively, while the corresponding m/z ratios for the IS were 390.3 and 147.1. The assay was linear from 0.1 to 150 mg/L for meropenem. Inter- and intraday precisions and bias of the quality control (QC) samples were within a ±15% range. Based on the dilution integrity test that was validated (up to 4× QC high) (28), samples with analyte concentrations exceeding the upper limit of quantitation, were reassayed after dilution. The plasma samples were stored at −80°C until sample analysis. The long-term storage stability at −80°C was 6 months, and all samples were analyzed within this period.
Population pharmacokinetic model development.
All meropenem PK data were analyzed simultaneously using the nonlinear mixed-effects modeling approach with NONMEM software (version 7.4.3; Icon Development Solutions, Ellicott City, Maryland), and the first-order conditional estimation method with interaction (FOCE+I) was used to estimate the typical values and the variability of the PK parameters. Graphical analyses were performed using SigmaPlot 13.0 (Systat Software, San Jose, CA) and R (version 4.1.3).
During the model building process, both one- and two-compartment models were evaluated, which were parameterized in terms of clearance (CL), volume of distribution in each compartment (V), and intercompartmental clearance (Q, for the two-compartment model) between the peripheral compartment and the central compartment. Since meropenem was administered by prolonged intravenous infusions, drug administration was modeled as a zero-order process. The interindividual variability (IIV) of the PK parameters for meropenem was estimated using an exponential model. To evaluate the residual variability (RV) of the meropenem model, several different error models, including an additive error model, a proportional error model, and a combined additive and proportional error model, were examined.
Various covariates, including age, sex, total body weight, and lean body weight based on equations developed by Janmahasatian et al. (29), estimated creatinine clearance using Cockcroft-Gault equation, presence or absence of CRRT, mortality/severity of disease scores (SOFA), sepsis/septic shock, mechanical ventilation, and acute kidney injury (AKI), were evaluated in the analysis. Exploratory analysis was performed first, and only those covariates with a clear trend on the PK parameters were evaluated further in the formal covariate model testing. The formal covariate analysis included a forward addition step where only covariates that decreased the objective function value by more than 3.84 (1 df at P < 0.05) were selected and a backward elimination step where only covariates that produced an increase in the objective function value of >6.63 (1 df at P < 0.01) were retained in the model. In addition to the statistical measures described above, the principles of the full covariate model approach, which considers prior biological knowledge about covariate effects, were also applied. Therefore, covariates that were part of a predefined base set of covariates (total body weight, lean body weight, estimated creatinine clearance, and the presence or absence of CRRT) were not required to meet all statistical criteria in order to be included in the model.
The final model was selected based on the objective function values, the precision of the estimated parameters, and goodness-of-fit plots, as well as the biological and physiological plausibility of the parameter estimates. The Akaike information criterion (AIC) was used for comparing rival nonhierarchical models and a likelihood ratio test was used to compare rival hierarchical models where a decrease in the NONMEM objective function value of 3.84 points was necessary for statistically significant improvement in model performance (P < 0.05, df = 1). The adequacy of the final model was examined using a prediction-corrected visual predictive check (pc-VPC).
Probability of target attainment analysis.
Based on the PK parameters from the final meropenem model, Monte Carlo simulations were performed using NONMEM (version 7.4.3; Icon Development Solutions, Ellicott City, MD) to evaluate the probabilities of target attainment (PTA) for different dosing regimens of meropenem. In total, 16 different dosing regimens were evaluated: 0.5 g Q6h or Q8h with intermittent infusions (II; 30 min) or prolonged infusions (PI; 3 h), 1 g Q8h or Q12h with II or PI, and 2 g Q8h or Q12h with II or PI, as well as continuous infusion (CI) with daily doses of 2, 4, 6, or 8 g meropenem. For each dosing scenario, 5,700 virtual subjects were simulated, and the concentration-time profile at steady state was obtained for each virtual subject. The unbound (free) concentrations were predicted using literature reported protein binding of 2% for meropenem. Since the data sets for the Monte Carlo simulations were based on the final population PK model, the covariate distributions reflected those in the study population, and the correlations between covariates were preserved.
Based on the Monte Carlo simulation results, the PTA was calculated for each meropenem dosing regimen over a wide MIC range with the following three PK/PD targets: 40% fT>MIC, 100% fT>MIC, and 100% fT>4×MIC. Target attainment was considered successful if PTA >90%. The MIC values covered in our analysis ranged from 0.125 to 64 mg/L; these values span the published MIC distributions of meropenem to pathogens commonly seen in the ICU environment. The PK/PD breakpoint for each dosing regimen was defined as the highest MIC with a PTA of at least 90%.
In addition to PTA for antibacterial effect, the probability of meropenem-induced toxicity was also evaluated. Based on the thresholds of 64.2 mg/L on neurotoxicity and 44.5 mg/L on nephrotoxicity that were proposed by Imani et al. based on retrospective TDM data (18), the percentage of subjects with Ctrough values exceeding these thresholds were estimated, and the corresponding probability of meropenem-induced toxicity was calculated.
ACKNOWLEDGMENTS
This study was supported by the Division of Microbiology and Infectious Diseases, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, through Vaccine and Treatment Evaluation Unit contract HHSN272200800008C.
We thank EMMES, the University of Iowa VTEU study team, and the Vanderbilt University VTEU study team for their efficient and conscientious management of this study.
Footnotes
Supplemental material is available online only.
Contributor Information
Guohua An, Email: guohua-an@uiowa.edu.
Patricia Winokur, Email: patricia-winokur@uiowa.edu.
REFERENCES
- 1.Drusano GL, Hutchison M. 1995. The pharmacokinetics of meropenem. Scand J Infect Dis Suppl 96:11–16. [PubMed] [Google Scholar]
- 2.Wiseman LR, Wagstaff AJ, Brogden RN, Bryson HM. 1995. Meropenem: a review of its antibacterial activity, pharmacokinetic properties, and clinical efficacy. Drugs 50:73–101. 10.2165/00003495-199550010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–12. 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Study D, DALI Study . 2014. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Aziz MH, Lipman J, Roberts JA. 2017. Identifying “at-risk” patients for sub-optimal beta-lactam exposure in critically ill patients with severe infections. Crit Care 21:283. 10.1186/s13054-017-1871-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51:1725–1730. 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gijsen M, Elkayal O, Annaert P, Van Daele R, Meersseman P, Debaveye Y, Wauters J, Dreesen E, Spriet I. 2022. Meropenem target attainment and population pharmacokinetics in critically ill septic patients with preserved or increased renal function. Infect Drug Resist 15:53–62. 10.2147/IDR.S343264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crandon JL, Ariano RE, Zelenitsky SA, Nicasio AM, Kuti JL, Nicolau DP. 2011. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med 37:632–638. 10.1007/s00134-010-2105-0. [DOI] [PubMed] [Google Scholar]
- 9.Burger R, Guidi M, Calpini V, Lamoth F, Decosterd L, Robatel C, Buclin T, Csajka C, Marchetti O. 2018. Effect of renal clearance and continuous renal replacement therapy on appropriateness of recommended meropenem dosing regimens in critically ill patients with susceptible life-threatening infections. J Antimicrob Chemother 73:3413–3422. 10.1093/jac/dky370. [DOI] [PubMed] [Google Scholar]
- 10.Ulldemolins M, Soy D, Llaurado-Serra M, Vaquer S, Castro P, Rodriguez AH, Pontes C, Calvo G, Torres A, Martin-Loeches I. 2015. Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother 59:5520–5528. 10.1128/AAC.00712-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niibe Y, Suzuki T, Yamazaki S, Suzuki T, Takahashi N, Hattori N, Nakada TA, Oda S, Ishii I. 2020. Population pharmacokinetic analysis of meropenem in critically ill patients with acute kidney injury treated with continuous hemodiafiltration. Ther Drug Monit 42:588–594. 10.1097/FTD.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 12.Mattioli F, Fucile C, Del Bono V, Marini V, Parisini A, Molin A, Zuccoli ML, Milano G, Danesi R, Marchese A, Polillo M, Viscoli C, Pelosi P, Martelli A, Di Paolo A. 2016. Population pharmacokinetics and probability of target attainment of meropenem in critically ill patients. Eur J Clin Pharmacol 72:839–848. 10.1007/s00228-016-2053-x. [DOI] [PubMed] [Google Scholar]
- 13.Muro T, Sasaki T, Hosaka N, Umeda Y, Takemoto S, Yamamoto H, Kamimura H, Higuchi S, Karube Y. 2011. Population pharmacokinetic analysis of meropenem in Japanese adult patients. J Clin Pharm Ther 36:230–236. 10.1111/j.1365-2710.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- 14.Ehmann L, Zoller M, Minichmayr IK, Scharf C, Huisinga W, Zander J, Kloft C. 2019. Development of a dosing algorithm for meropenem in critically ill patients based on a population pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents 54:309–317. 10.1016/j.ijantimicag.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Sjovall F, Alobaid AS, Wallis SC, Perner A, Lipman J, Roberts JA. 2018. Maximally effective dosing regimens of meropenem in patients with septic shock. J Antimicrob Chemother 73:191–198. 10.1093/jac/dkx330. [DOI] [PubMed] [Google Scholar]
- 16.Minichmayr IK, Roberts JA, Frey OR, Roehr AC, Kloft C, Brinkmann A. 2018. Development of a dosing nomogram for continuous-infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J Antimicrob Chemother 73:1330–1339. 10.1093/jac/dkx526. [DOI] [PubMed] [Google Scholar]
- 17.Yang N, Wang J, Xie Y, Ding J, Wu C, Liu J, Pei Q. 2022. External evaluation of population pharmacokinetic models to inform precision dosing of meropenem in critically ill patients. Front Pharmacol 13:838205. 10.3389/fphar.2022.838205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imani S, Buscher H, Marriott D, Gentili S, Sandaradura I. 2017. Too much of a good thing: a retrospective study of beta-lactam concentration-toxicity relationships. J Antimicrob Chemother 72:2891–2897. 10.1093/jac/dkx209. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 64:142–150. 10.1093/jac/dkp139. [DOI] [PubMed] [Google Scholar]
- 20.Lan J, Wu Z, Wang X, Wang Y, Yao F, Zhao BX, Wang Y, Chen J, Chen C. 2022. Population pharmacokinetics analysis and dosing simulations of meropenem in critically ill patients with pulmonary infection. J Pharm Sci 111:1833–1842. 10.1016/j.xphs.2022.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Jaruratanasirikul S, Thengyai S, Wongpoowarak W, Wattanavijitkul T, Tangkitwanitjaroen K, Sukarnjanaset W, Jullangkoon M, Samaeng M. 2015. Population pharmacokinetics and Monte Carlo dosing simulations of meropenem during the early phase of severe sepsis and septic shock in critically ill patients in intensive care units. Antimicrob Agents Chemother 59:2995–3001. 10.1128/AAC.04166-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhaese SAM, Roberts JA, Carlier M, Verstraete AG, Stove V, De Waele JJ. 2018. Population pharmacokinetics of continuous infusion of piperacillin in critically ill patients. Int J Antimicrob Agents 51:594–600. 10.1016/j.ijantimicag.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Landersdorfer CB, Nation RL. 2021. Key challenges in providing effective antibiotic therapy for critically ill patients with bacterial sepsis and septic shock. Clin Pharmacol Ther 109:892–904. 10.1002/cpt.2203. [DOI] [PubMed] [Google Scholar]
- 24.Keizer RJ, Ter Heine R, Frymoyer A, Lesko LJ, Mangat R, Goswami S. 2018. Model-informed precision dosing at the bedside: scientific challenges and opportunities. CPT Pharmacometrics Syst Pharmacol 7:785–787. 10.1002/psp4.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fawaz S, Barton S, Whitney L, Swinden J, Nabhani-Gebara S. 2019. Stability of meropenem after reconstitution for administration by prolonged infusion. Hosp Pharm 54:190–196. 10.1177/0018578718779009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlier M, Stove V, Verstraete AG, De Waele JJ. 2015. Stability of generic brands of meropenem reconstituted in isotonic saline. Minerva Anestesiol 81:283–287. [PubMed] [Google Scholar]
- 27.Varghese JM, Jarrett P, Wallis SC, Boots RJ, Kirkpatrick CM, Lipman J, Roberts JA. 2015. Are interstitial fluid concentrations of meropenem equivalent to plasma concentrations in critically ill patients receiving continuous renal replacement therapy? J Antimicrob Chemother 70:528–533. 10.1093/jac/dku413. [DOI] [PubMed] [Google Scholar]
- 28.D’Cunha R, Bach T, Young BA, Li P, Nalbant D, Zhang J, Winokur P, An G. 2018. Quantification of cefepime, meropenem, piperacillin, and tazobactam in human plasma using a sensitive and robust liquid chromatography-tandem mass spectrometry method, part 1: assay development and validation. Antimicrob Agents Chemother 62:e00861-18. 10.1128/AAC.00861-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. 2005. Quantification of lean bodyweight. Clin Pharmacokinet 44:1051–1065. 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01312-22-s0001.pdf, PDF file, 0.7 MB (713.1KB, pdf)