Abstract
Reactive oxygen species (ROS) are thought to be involved in intracellular signaling, including activation of the transcription factor NF-κB. We investigated the role of NADPH oxidase in the NF-κB activation pathway by utilizing knockout mice (p47phox−/−) lacking the p47phox component of NADPH oxidase. Wild-type (WT) controls and p47phox−/− mice were treated with intraperitoneal (i.p.) Escherichia coli lipopolysaccharide (LPS) (5 or 20 μg/g of body weight). LPS-induced NF-κB binding activity and accumulation of RelA in nuclear protein extracts of lung tissue were markedly increased in WT compared to p47phox−/− mice 90 min after treatment with 20 but not 5 μg of i.p. LPS per g. In another model of lung inflammation, RelA nuclear translocation was reduced in p47phox−/− mice compared to WT mice following treatment with aerosolized LPS. In contrast to NF-κB activation in p47phox−/− mice, LPS-induced production of macrophage inflammatory protein 2 in the lungs and neutrophilic lung inflammation were not diminished in these mice compared to WT mice. We conclude that LPS-induced NF-κB activation is deficient in the lungs of p47phox−/− mice compared to WT mice, but this abnormality does not result in overt alteration in the acute inflammatory response.
Proinflammatory stimuli such as endotoxin (lipopolysaccharide [LPS]), tumor necrosis factor α (TNF-α), and interleukin 1β (IL-1β) are potent triggers for the NF-κB activation pathway (2, 23). The same stimuli also elicit an oxidative cellular stress response (6, 8, 30). A role for reactive oxygen species (ROS) as mediators of cellular events or as secondary messengers has been proposed (22), but little supportive whole-animal data exist. A number of potential mechanisms could account for the intracellular effects of ROS, including modulation of calcium flux and protein phosphorylation (29) or alterations in protein conformation and function (17), but the exact pathway remains to be defined.
NF-κB regulates gene expression of many proinflammatory mediators, including cytokines (TNF-α and IL-6), CXC chemokines (IL-8; gro α, β, and γ; macrophage inflammatory protein-2 [MIP-2]; and keratinocyte-derived cytokine [KC]) and enzymes (inducible nitric oxide synthase and COX) (3). Gene expression of these molecules is related to and may be the cause of acute neutrophilic inflammation and the systemic inflammatory response syndrome (23). Heterodimeric NF-κB is retained in the cytoplasm by binding to members of the inhibitory (IκB) family. The key step in NF-κB activation is the inducible phosphorylation of N-terminal serines (Ser 32 and 36) of IκB-α. Phosphorylation targets IκB-α for ubiquitination of N-terminal lysine residues, which marks IκB-α for 28S proteasome degradation, allowing nuclear translocation and DNA binding by the free NF-κB heterodimer (10). The DNA binding avidity of NF-κB can be modulated by changes in the cellular redox state (12).
NADPH oxidase is the main cellular source of ROS in mononuclear and granulocytic leukocytes (1). The key role of NADPH oxidase in host defense is illustrated by the immune deficiency syndrome chronic granulomatous disease, which is an autosomal or X-linked deficiency in NADPH oxidase that causes recurrent life-threatening infections and tissue granuloma formation (28). Mouse knockout models of X-linked (24) and autosomal (p47phox) defects in the NADPH oxidase system have been developed (18). We examined the effect of treatment with intraperitoneal (i.p.) and aerosolized LPS on activation of NF-κB in p47phox knockout mice (p47phox−/−) with defective NADPH oxidase function compared to that in C57/B6 wild-type (WT) control mice. Following treatment with LPS, p47phox−/− mice exhibited reduced nuclear NF-κB binding activity in lung tissue and reduced immunoreactive RelA in nuclear protein extracts. These alterations in the NF-κB activation pathway, however, were not associated with alterations in the development of neutrophilic alveolitis or MIP-2 production in response to treatment with either i.p. or aerosolized LPS.
MATERIALS AND METHODS
Materials.
Escherichia coli LPS (serotype 055:B5) was obtained from Sigma (St. Louis, Mo.). The double-stranded consensus NF-κB motif 5′-GATCGAGGGGA-CTTTCCCTAAAAGC-3′, used in electrophoretic mobility shift assays (EMSA), was obtained from Stratagene (La Jolla, Calif.). [γ-32P]ATP was obtained from NEN-Dupont (Boston, Mass.), and T4 kinase and T4 kinase buffer used for oligonucleotide labeling were obtained from New England Biolabs (Beverly, Mass.). Antibodies to RelA (also called p65) and p50 used in performing EMSA supershifts and Western blots were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Double-stranded oligonucleotide Oct-1 was used as a nonspecific probe in the EMSA and was obtained from Promega (Madison, Wis.). Enzyme-linked immunosorbent assay (ELISA) kits used for cytokine measurements were purchased from R&D, Minneapolis, Minn.
Animal model.
Homozygous p47phox−/− mice and littermate controls were generated as described previously (18). These mice have been backcrossed 10 generations into the C57/B6 strain and are derived from a single lineage. Mice were housed in filtered air cages. They were fed standard, autoclavable chow pellet diet and had free access to sterile water. Adult mice (between 6 and 10 weeks of age) weighing between 20 and 30 g were used for all experiments.
Lyophilized Esherichia coli LPS was suspended in sterile saline and administered to adult mice as a single i.p. injection at doses of 5 or 20 μg/g of body weight. Animals were sacrificed 90 min after i.p. injections. In other experiments, mice were exposed to aerosolized LPS by placing them within a sealed container. LPS was suspended in sterile saline and delivered as a continuous aerosol with a driving flow rate of 8 liters/min by using a small-volume nebulizer (Resigard II; Marquest Medical, Englewood, Colo.). The concentration of aerosolized LPS used was 0.1 mg/ml, and all aerosol treatments were given over a standardized 30-min interval. Following treatment, mice were returned to sterile cages and were sacrificed 4 h later.
Mice were euthanized by carbon dioxide inhalation as recommended by the Panel on Euthanasia of the American Veterinary Medical Association. Lung lavage fluid and tissue samples were collected after death. Mouse tracheas were cannulated with a 20-gauge blunt-tip needle attached to a 1-ml syringe, and the lungs were lavaged with sterile pyrogen-free physiological saline until a total lavage volume of 3 ml was collected. Lungs were harvested by surgical resection, and tissues were flash frozen in liquid nitrogen and stored at −70°C.
Quantification of neutrophilic lung infiltration.
Neutrophilic lung inflammation was measured as total and differential cell counts in the lung lavage fluid. Total cell counts were determined by using a grid hemocytometer. Differential cell counts were obtained by staining a cytocentrifuge slide preparation with a modified Wright's stain (Diff-Quik; Baxter, Miami, Fla.) and counting 300 to 400 cells in a cross-section.
Extraction of nuclear proteins from tissue samples.
Tissue nuclear proteins were extracted from whole-lung tissue by the method of Deryckere and Gannon (13). Briefly, 50 to 100 mg of tissue was mechanically homogenized in liquid nitrogen, to which 4 ml of buffer A (150 mM NaCl, 10 mM HEPES [pH 7.9], 0.6% [vol/vol] NP-40, 0.2 M EDTA, 0.1 M phenylmethylsulfonyl fluoride) was added. The homogenate was transferred to a 15-ml Falcon tube and centrifuged at 850 × g in a tabletop centrifuge for 30 s to remove cellular debris. The supernatant was then transferred to a 50-ml Falcon tube and incubated on ice for 5 min prior to being centrifuged for 10 min at 3,500 × g. Supernatant was collected as a cytoplasmic extract. The pellet was resuspended in 300 μl of buffer B (sterile water, 25% [vol/vol] glycerol, 20 mM HEPES [pH 7.9], 5 M NaCl, 1 M MgCl2, 0.2 M EDTA,0.1 M phenylmethylsulfonyl fluoride, 1 M dithiothreitol, 10 mg of benzamidine per ml, 1 mg of pepstatin per ml, 1 mg of leupeptin per ml, 1 mg of aprotinin per ml) and incubated on ice for 30 min. Following centrifugation at 14,000 rpm in an Eppendorf microcentrifuge for 2 min, the supernatant was collected as the nuclear extract and frozen at −70°C. Protein concentrations in nuclear and cytoplasmic extracts were determined by using the Bradford assay (9).
Oligonucleotide labeling.
Oligonucleotides were labeled with a double-stranded consensus sequence NF-κB and [γ-32P]ATP. The reaction was catalyzed with T4 polynucleotide kinase and incubated in 10× kinase buffer at 37°C for 45 min. The reaction was terminated by being heated at 65°C for 10 min. Labeled oligonucleotide was column purified on Sephadex G-25 columns (Amersham Pharmacia Biotech). The radioactivity of the labeled probe was assayed with a Beckman LS6500 multipurpose scintillation counter and measured as cpm per microliter of probe.
EMSA.
Five micrograms of nuclear protein was incubated with binding buffer on ice for 30 min (specific antibodies for p50 and RelA were added for supershift studies). Labeled oligonucleotide (approximately 100,000 cpm) was then added, and samples were incubated at room temperature for 1 h. Specificity of binding was ascertained by cold competition with an excess of unlabeled NF-κB oligonucleotides, and nonspecific competition was assessed by incubation with an excess of a double-stranded unlabeled Oct-1 probe. Protein-DNA complexes were separated from free DNA probe by electrophoresis with a 6% polyacrylamide gel. Gels were dried under vacuum on Whatman paper in a Bio-Rad gel dryer and exposed to autoradiographic film for 12 to 36 h at −70°C with intensifying screens.
Western blot analysis.
Nuclear and cytoplasmic proteins from tissue extracts were quantitated by the Bradford assay, and 25 to 50 μg of protein was mixed with an equal volume of 2× sample buffer (containing 0.1% sodium dodecyl sulfate [SDS] and 2-mercaptoethanol) and boiled for 5 min. Denatured proteins were separated by electrophoresis on an SDS-polyacrylamide gel along with molecular weight markers and standards. Proteins were transferred to an Immobilon-P (Millipore, Bedford Mass.) membrane in a mixture of 25 mM Tris base, 192 mM glycine, and 5% (vol/vol) methanol (pH 8.2) at 100 V for 1 h. Nonspecific binding was blocked by soaking the membrane in phosphate-buffered saline (PBS)–5% nonfat dried milk–0.05% Tween 20 overnight at 4°C. Immunoreactive proteins were detected by incubating the filter with specific antibodies to RelA (Santa Cruz Biotechnology, Inc.) for 1 h at room temperature with constant agitation. Nonspecific binding was washed away by rinsing the filter in PBS containing 0.05% Tween 20. The filters were incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Santa Cruz Biotechnology) diluted 1:5,000 in Blotto (Tween–PBS–5% nonfat dried milk) for an hour at room temperature with constant agitation. The filter was washed three times for 10 min with Tween-PBS. To develop the image, filters were treated with Renaissance Western blot luminescent reagent (NEN DuPont) for 5 min and exposed to Biomax film for 3 to 10 min.
ELISA for cytokine production.
Cytokine assays were performed with cytoplasmic extracts derived from whole-lung homogenates. MIP-2 was assayed according to the manufacturer's instructions with a commercially available ELISA (R&D Systems).
Statistical analysis.
Statistical analyses were performed with GraphPad InStat version 3.01 for Windows NT (GraphPad Software, San Diego Calif.) by using the unpaired t test and unpaired analysis of variance test.
RESULTS
NF-κB activation in nuclear extracts of lung and liver tissue following i.p. injection of LPS.
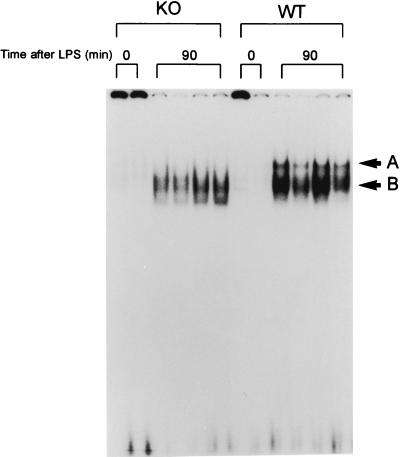
Mice were treated with 5 or 20 μg of i.p. LPS per g and sacrificed 90 min later. Subsequently, nuclear proteins were extracted from lung and liver tissue. Previous studies with mice have shown that NF-κB binding in lung and liver is near maximum at 90 min after i.p. LPS (5). As shown in Fig. 1, NF-κB binding activity in nuclear protein extracts from lung tissue of WT and p47phox−/− mice was minimal in the absence of LPS treatment. There was induction of NF-κB activation in lung nuclear protein extracts from WT and p47phox−/− mice 90 min after treatment with i.p. LPS at a dose of 20 μg/g (bands A and B). Band A was prominently induced only in WT mice. This band was found by antibody supershifts to contain RelA/p50 heterodimers (data not shown). Band B represents p50 homodimers, since this complex supershifted with p50 antibodies but not with RelA antibodies (not shown). This distinction is important, since RelA, but not p50, contains a C-terminal transactivation domain (4).
FIG. 1.
EMSA showing NF-κB activation in four p47phox−/− (knockout [KO]) and four WT mice 90 min after high-dose i.p. LPS (20 μg/g). Nuclear extracts from two untreated WT and two untreated p47phox−/− mice demonstrated minimal NF-κB binding. Lung NF-κB binding was increased in both WT and p47phox−/− mice, but band A, which represents RelA/p50 heterodimers, was increased primarily in WT mice.
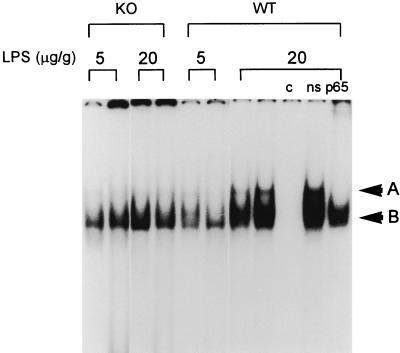
Figure 2 shows NF-κB binding in lung nuclear protein extracts 90 min after low-dose i.p. LPS (5 μg/g) and high-dose i.p. LPS (20 μg/g). After low-dose i.p. LPS, NF-κB binding was predominantly composed of p50 homodimers (band B) and was similar in intensity in p47phox−/− and WT mice. Following high-dose i.p. LPS, lungs from WT mice demonstrated increased RelA/p50 heterodimer binding (band A) compared to p47phox−/− mice treated with high-dose i.p. LPS or either group of mice treated with low-dose i.p. LPS. Increasing the dose of i.p. LPS from 5 to 20 μg/g resulted in increased NF-κB activation in WT mice; however, no increase in NF-κB activation was found in p47phox−/− mice. These findings indicate that p47phox−/− mice were unable to maximally activate NF-κB after treatment with high-dose i.p. LPS. In liver tissue, NF-κB DNA binding activity was induced in WT and p47phox−/− mice by treatment with i.p. LPS (low and high doses). There were no detectable differences in NF-κB binding activity in liver nuclear extracts from p47phox−/− and WT mice after either dose of LPS (data not shown).
FIG. 2.
EMSA of nuclear protein extracts from lungs obtained 90 min after treatment with 5 and 20 μg of i.p. LPS per g showing NF-κB activation in p47phox−/− (knockout [KO]) compared to WT animals. After 5 μg of i.p. LPS per g, NF-κB binding is similar in WT and p47phox−/− mice (predominantly band B). At 20 μg of i.p. LPS per g, band A is induced in WT mice, but NF-κB binding in p47phox−/− mice shows a similar pattern to that seen after low-dose LPS. Specificity of protein binding for NF-κB is shown by cold (c) and nonspecific (ns) competition.
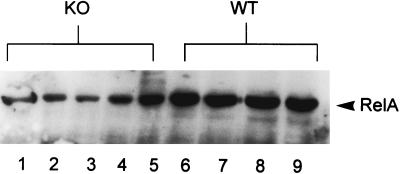
To confirm differences in RelA nuclear translocation, Western blots for RelA were performed with nuclear protein extracts obtained from whole lung tissue following i.p. LPS and probed for RelA. Figure 3 demonstrates that p47phox−/− mice had reduced amounts of nuclear RelA in response to high-dose i.p. LPS (20 μg/g) compared to WT mice. Nuclear RelA was undetectable in untreated p47phox−/− and WT mice (not shown). These results confirm the EMSA findings that p47phox−/− mice have deficient NF-κB activation in lungs after treatment with high-dose i.p. LPS.
FIG. 3.
Western blot for RelA in nuclear extracts obtained from lung tissues harvested 90 min following treatment with 20 μg of i.p. LPS per g. Results for p47phox−/− (knockout [KO]) mice are shown in lanes 1 to 5, and those for WT mice are shown in lanes 6 to 9. p47phox−/− animals had reduced levels of nuclear RelA compared to WT mice.
Nuclear NF-κB activity and RelA translocation after treatment with aerosolized LPS.
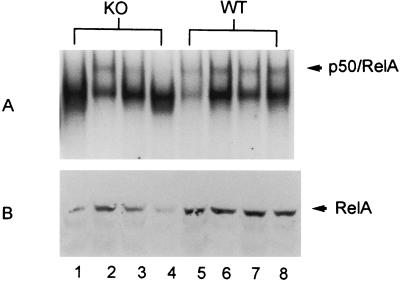
LPS (0.1 mg/ml [wt/vol]) was delivered to mice placed within a sealed container via nebulization at a flow rate of 8 liters/min over 30 min. Mice were sacrificed 4 h after treatment, and lung tissue was preserved for determination of NF-κB binding activity in nuclear extracts and nuclear RelA translocation. We used a 4-h time point for these studies because previous studies showed that both NF-κB activation and neutrophilic influx are present in the lungs 4 h after LPS is inhaled (26). In the present studies, there was marked variability in intensity of the RelA/p50 band in p47phox−/− mice treated with inhaled LPS, with some animals approximating WT levels of NF-κB activation and others having decreased levels (Fig. 4A). This degree of variability was not evident in the control animals. Overall, DNA binding of the RelA/p50 heterodimer band was reduced in most p47phox−/− animals compared to WT controls, but this did not reach statistical significance when assessed by densitometry (Fig. 5). As assessed by Western blotting, WT animals had greater amounts of nuclear RelA than p47phox−/− animals following treatment with inhaled LPS (Fig. 4B).
FIG. 4.
NF-κB activation and nuclear RelA in p47phox−/− (knockout [KO]) and WT mice 4 h following treatment with aerosolized LPS. Results for p47phox−/− animals are shown in lanes 1 to 4, and those for WT animals are shown in lanes 5 to 8. (A) EMSA showing that p47phox−/− mice have a more variable response to inhaled LPS than WT mice. (B) Western blot showing nuclear RelA is reduced in p47phox−/− mice compared to WT mice.
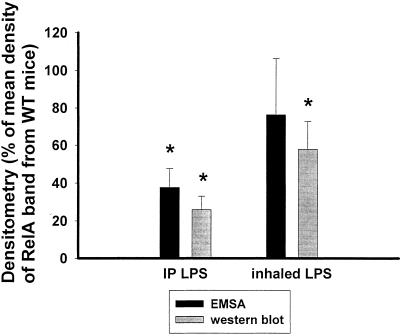
FIG. 5.
Densitometry of the RelA-containing band by EMSA and nuclear RelA detection by Western blots obtained from p47phox−/− and WT mice following i.p. (IP) and inhaled-LPS treatment. Densitometry values are depicted as a percentage of the mean density of bands from WT mice in the same experiments (and on the same blots). Error bars represent the standard error of the mean. Following i.p. LPS, RelA nuclear translocation and DNA binding were significantly reduced in knockout mice (P < 0.001). After aerosolized LPS, RelA/p50 DNA binding was not statistically decreased in p47phox−/− mice, but nuclear RelA was reduced in p47phox−/− mice (P < 0.05). n = 16 for p47phox−/− mice, and n = 15 for WT mice with i.p. LPS. n = 5 in each group for inhaled LPS.
Differences between WT and p47phox−/− mice in LPS-induced NF-κB binding in the lungs (RelA/p50 heterodimers) and nuclear RelA in the lungs was assessed by densitometry of bands on EMSA and Western blots (Fig. 5). The density of the RelA/p50 heterodimer band (band A) is reported from four separate experiments in which 16 p47phox−/− and 15 WT mice were treated with high-dose (20 μg/g) LPS. After high-dose i.p. LPS, the RelA/p50 heterodimer binding activity and RelA concentration in nuclear extracts from p47phox−/− mice were decreased compared to those in WT mice (P < 0.001). Following inhaled-LPS treatment, RelA/p50 heterodimer binding was not statistically different between WT and p47phox−/− mice; however, RelA translocation was found to be decreased in p47phox−/− mice compared with control mice (P < 0.05).
CXC chemokine production and recruitment of neutrophils into the airspace following treatment with LPS.
To evaluate the effects of altered NF-κB activation in p47phox−/− mice, levels of the NF-κB-dependent chemokine MIP-2 were measured in lung tissue homogenates. Baseline MIP-2 levels in the lungs were barely detectable in WT mice, but were higher in p47phox−/− mice (Table 1). Following high-dose i.p. LPS (20 μg/g), the MIP-2 concentration was higher in p47phox−/− mice than in WT mice, but this difference did not reach statistical significance (Table 1). Four hours after LPS was inhaled, MIP-2 concentrations in lung tissue homogenates were increased in p47phox−/− mice compared with those in WT mice (Table 1). In these studies, alterations in LPS-induced NF-κB binding and RelA nuclear concentration in p47phox−/− mice did not correlate with levels of MIP-2 in lung tissue, indicating that MIP-2 regulation may occur through a non-NF-κB-dependent mechanism in these mice.
TABLE 1.
Levels of MIP-2 in lung tissue
| Mouse group | Level of MIP-2 (pg/ml)a
|
||
|---|---|---|---|
| Untreated | i.p. LPS | Aerosol LPS | |
| WT | 11 ± 1 | 4,255 ± 1,087 | 329 ± 96 |
| p47phox−/− | 131 ± 53 | 8,294 ± 2,162 | 2,918 ± 689 |
| P | 0.06 | 0.1 | <0.01 |
Results are means ± standard errors (n = 5 to 7 in each group).
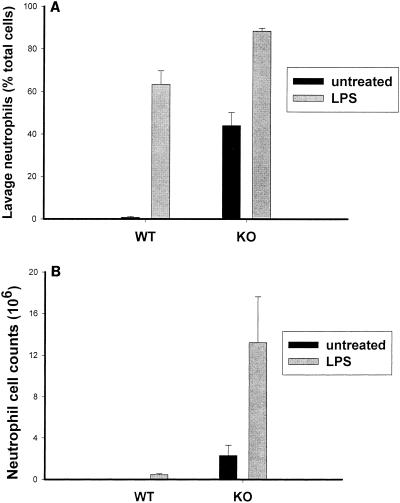
We also examined the effect of LPS treatment on the evolution of neutrophilic lung inflammation by measuring total and differential cell counts in lung lavage fluid obtained from mice following aerosolized and i.p. LPS treatments. Baseline lavages obtained from untreated p47phox−/− animals showed neutrophil levels in excess of that seen in WT animals (n = 7 or 8; P < 0.001) (Fig. 6). Untreated WT mice have few neutrophils in lung lavage (1% ± .4% of total cells), but p47phox−/− mice have a substantial neutrophilia (37% ± 8% of total lavage cells). Four hours after aerosol administration of LPS, both WT and p47phox−/− mice had increases in the number of neutrophils in lung lavage; however, the percentage of neutrophils in lung lavage was significantly higher in 47phox−/− mice than in WT mice (P < 0.05) (Fig. 6). The increased neutrophilic lung inflammation in p47phox−/− mice correlated with increased MIP-2 concentration in lung homogenates in these animals, but contrasted with NF-κB activation and RelA nuclear translocation. At 24 h after treatment with 20 μg of i.p. LPS per g, no significant increase in lung lavage neutrophil counts from baseline was found in WT or p47phox−/− mice, but neutrophil counts were significantly higher in p47phox−/− mice than in WT mice (not shown). Therefore, in NADPH oxidase-deficient mice, LPS-induced NF-κB activation is not a good indicator of the inflammatory response. Impaired NF-κB activation in these mice does not translate to decreased chemokine expression or diminished neutrophilic alveolitis.
FIG. 6.
Neutrophilic lung inflammation depicted as percentage of total cells in lung lavage (A) or as the total number of lavage cells (B). Lung lavage was performed with untreated WT and p47phox−/− (knockout [KO]) mice (black bars) and in both groups 4 h after treatment with aerosolized LPS (gray bars). Results are presented as means ± standard errors. n = 5 in each treatment group. n = 7 for untreated controls.
DISCUSSION
Multiple in vivo and in vitro studies examining the effects of oxidant exposure (7, 27) or addition of antioxidants such as N-acetylcysteine (4) and pyrolidinedithiocarbamate (20) suggest that the intracellular redox state may modulate NF-κB activity, although the exact mechanism is presently unknown. We found a dose-dependent effect of LPS on NF-κB activation in p47phox−/− and WT mice. Low-dose i.p. LPS induced similar NF-κB activation in p47phox−/− and WT mice. In contrast, with high-dose i.p. LPS, a disparity became evident with attenuated binding of RelA/p50 heterodimers in p47phox−/− mice. This suggests that either ROS are not required in the NF-κB activation pathway following low-dose LPS, or p47phox−/− mice are able to compensate for a defect in the NADPH oxidase system by engaging alternate mechanisms of ROS production in response to low-dose LPS; however, our finding of altered NF-κB activation and decreased nuclear RelA translocation in p47phox−/− mice following high-dose i.p. LPS indicates that maximal activation of the NF-κB signal transduction pathway in the lungs by i.p. LPS is dependent on ROS formation through the NADPH oxidase pathway. Our findings are consistent with a previous report of blunted NF-κB activation in liver tissue in p47phox−/− mice exposed to ethanol in a model of alcoholic liver disease (25). In that model, free radical production was shown to be deficient in p47phox−/− mice compared to that in WT controls (19).
The NADPH oxidase pathway is the major means of superoxide generation in neutrophilic leukocytes and macrophages (1). Mice that lack p47phox have reduced capacity to generate superoxide via the NADPH oxidase pathway, but retain the mechanism to do so via alternate pathways (18). The exact contribution of phagocytic NADPH oxidase to lung ROS production is not clearly defined. While originally described in neutrophils and macrophages (1), NADPH oxidase has subsequently been defined in a variety of nonphagocytes, including smooth muscle cells, synoviocytes and chondrocytes (1), endothelial cells (16), and epithelial cells (15, 31). However, the relative concentrations and activities of NADPH oxidase in each of these cell types have not been examined.
NF-κB increases recruitment of inflammatory effector cells by up-regulating gene expression of CXC chemokines (23). Our finding that NF-κB DNA binding did not correlate with inflammatory end points such as neutrophil lung infiltration and chemokine production is interesting and suggests that other regulatory factors may be more important than NF-κB in regulating neutrophilic alveolitis in these experimental settings. These data suggest that DNA binding activity as detected on EMSA is not a good predictor of the severity of inflammation in all situations.
Lack of NADPH oxidase results in airspace neutrophilia in untreated mice as well as in an exaggerated neutrophilic influx following an inflammatory stimulus. Other studies have shown that mice lacking the gp91 subunit have increased neutrophilic infiltration following exposure to Aspergillus fumigatus (21) and Listeria (14). Similarly, p47phox−/− mice exposed to thioglycolate or Mycobacterum tuberculosis exhibited a profound neutrophilia, exceeding that found in WT controls (11, 24). Neutrophils obtained from gp91−/− and p47phox−/− mice have been shown to have diminished pathogen killing compared to WT phagocytes (21, 24). In infection models, it is possible that failure to clear pathogens results in persistence of the inflammatory signals, causing exuberant neutrophilia in NADPH oxidase-deficient animals. It is also possible that these NADPH oxidase knockout mice have exaggerated neutrophil influx in response to a given inflammatory stimulus. Treatment of NADPH oxidase knockout mice with both viable and heat-inactivated Aspergillus fumigatus results in heightened pulmonary neutrophil recruitment compared with that in controls (21). This finding, along with ours, suggests that impaired pathogen clearance is not the sole stimulus for increased neutrophil recruitment in these mice.
In summary, we have shown that the NADPH oxidase pathway in mice is important for maximal activation of NF-κB following treatment with LPS. This defect in NF-κB activation does not translate to diminished production of MIP-2 or decreased neutrophil recruitment to the lungs of p47phox−/− mice. Apparently, neutrophils are recruited by a non-NF-κB-dependent mechanism in these mice.
ACKNOWLEDGMENTS
This work was supported by the U.S. Department of Veterans Affairs and grant no. HL 61419 from the National Heart Lung and Blood Institute, National Institutes of Health, Bethesda, Md.
REFERENCES
- 1.Babior B. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 2.Baeuerle P, Henkel T. Function and activation of NF-kB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell T, Christman J. The role of nuclear factor-kB in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell T S, Blackwell T R, Holden E P, Christman B W, Christman J W. In vivo antioxidant treatment suppresses nuclear factor-kB activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- 5.Blackwell T S, Yull F E, Chen C-L, Venkatakrishnan A, Blackwell T R, Hicks D J, Lancaster L H, Christman J W, Kerr L D. Multi-organ NF-kB activation in a transgenic mouse model of systemic inflammation. Am J Respir Crit Care Med. 2000;162:1095–1101. doi: 10.1164/ajrccm.162.3.9906129. [DOI] [PubMed] [Google Scholar]
- 6.Bohler T, Waiser J, Hepburn H, Gaedeke J, Lehmann C, Hanibach P, Budde K, Neumayer H H. TNF-α and IL-1α induce apoptosis in subconfluent rat mesangial cells. Evidence for the involvement of hydrogen peroxide and lipid peroxidation as second messengers. Cytokine. 2000;12:986–991. doi: 10.1006/cyto.1999.0633. [DOI] [PubMed] [Google Scholar]
- 7.Bonizzi G, Piette J, Merville M P, Bours V. Cell type-specific role for reactive oxygen species in nuclear factor-kappaB activation by interleukin-1. Biochem Pharmacol. 2000;59:7–11. doi: 10.1016/s0006-2952(99)00290-7. [DOI] [PubMed] [Google Scholar]
- 8.Bowie A, O'Neil L. Oxidative stress and nuclear factor κB activation. A re-assessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Christman J, Lancaster L, Blackwell T. Nuclear factor-kB: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med. 1998;24:1131–1138. doi: 10.1007/s001340050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper A M, Segal B H, Frank A A, Holland S M, Orme I M. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect Immun. 2000;68:1231–1234. doi: 10.1128/iai.68.3.1231-1234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton T, Shertzer H, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 13.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA binding proteins from animal tissues. BioTechniques. 1994;16:405. [PubMed] [Google Scholar]
- 14.Dinauer M, Deck M, Unanue E. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J Immunol. 1997;158:5581–5583. [PubMed] [Google Scholar]
- 15.Geiszt M, Kopp J B, Varnal P, Leto T. Identification of Renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorlach A, Brandes R P, Nguyen K, Amidi M, Dehgani F, Busse R. A gp91 phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Hutter D, Greene J. Influence of the cellular redox state on NF-kB regulated gene expression. J Cell Physiol. 2000;183:45–52. doi: 10.1002/(SICI)1097-4652(200004)183:1<45::AID-JCP6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 18.Jackson S, Gallin J, Holland S. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono H, Rusyn I, Bradford B U, Yin M, Dikalova A, Kadiiska M B, Connor H D, Mason R P, Segal B H, Holland S M, Thurman R G. Identification of the source of oxidants in alcoholic liver disease: studies in knockout mice. J Clin Investig. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer M, Schreck R, Baeuerle P. H2O2 and antioxidants have opposite effects on activation of NF-κB and AP-1 in intact cells: AP-1 as secondary antioxidant responsive factor. EMBO J. 1993;12:2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgenstern D E, Gifford M A, Li L L, Doerschuk C M, Dinauer M C. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med. 1997;185:207–218. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 23.Pahl H. Activators and target genes of Rel/NF-kB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 24.Pollock J D, Williams D A, Gifford M A, Li L L, Du X, Fisherman J, Orkin S H, Doerschuk C M, Dinauer M C. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 25.Rusyn I, Yamashina S, Segal B H, Schoonhoven R, Holland S M, Cattley R C, Swenberg J A, Thurman R G. Oxidants from NADPH oxidase are involved in triggering cell proliferation in the liver due to peroxisome proliferators. Cancer Res. 2000;60:4798–4803. [PubMed] [Google Scholar]
- 26.Sadikot, R. T., E. D. Jansen, T. R. Blackwell, O. Zoia, F. E. Yull, J. W. Christman, and T. S. Blackwell. High dose dexamethasone accentuates NF-kB activation in endotoxin-treated mice. Am. J. Respir. Crit. Care Med., in press. [DOI] [PubMed]
- 27.Schreck R, Rieber P, Baeuerle P. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kB transcription factor and HIV-1. EMBO J. 1997;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal B H, Leto T L, Gallin J I, Malech H L, Holland S M. Genetics, biochemistry, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki Y, Forman H, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 30.Tolando R, Jovanovic A, Brigelius-Flohe R, Ursini F, Maiorino M. Reactive oxygen species and pro-inflammatory cytokine signaling in endothelial cells: effects of selenium supplementation. Free Radic Biol Med. 2000;28:979–986. doi: 10.1016/s0891-5849(00)00183-0. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Youngson C, Wong V, Yeger H, Dinauer M C, Vega-Saenz de Miera E, Rudy B, Cutz C. NADPH oxidase and hydrogen peroxide sensitive K+ channel may function as an oxygen sensor complex in airway chemoreceptors and small cell lung carcinoma cell lines. Proc Natl Acad Sci USA. 1996;93:13182–13187. doi: 10.1073/pnas.93.23.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]