Abstract
The need for the development of cheap and effective vaccines against pneumococcal disease has necessitated the evaluation of common virulence-associated proteins of Streptococcus pneumoniae as potential vaccine antigens. In this study, we examined the capacity of active immunization with a genetic toxoid derivative of pneumolysin (PdB) and/or a fragment of choline binding protein A (CbpA; also known as PspC, Hic, and SpsA) to protect mice from intraperitoneal challenge with medium to very high doses of a highly virulent capsular type 2 pneumococcal strain, D39. The median survival times for mice immunized with the individual protein antigens in different adjuvant combinations were significantly longer than those for mice that received the respective adjuvants alone. Mice immunized with CbpA alone were significantly better protected than mice immunized with PdB alone. Correspondingly, the median survival times for mice that were immunized with a combination of PdB and CbpA were significantly longer than those for mice that received PdB alone but not significantly different from those that received CbpA alone. Mice immunized with the protein antigens in a mixture of monophospholipid A (MPL) and aluminium phosphate (AlPO4) adjuvants had higher antibody titers than mice that received the antigens in AlPO4 alone. Mice immunized with PdB in MPL plus AlPO4 were also significantly better protected than mice that received PdB in AlPO4 alone.
Streptococcus pneumoniae (the pneumococcus) is a major cause of life-threatening invasive diseases, such as pneumonia, meningitis, and bacteremia, as well as other less serious but highly prevalent infections, such as otitis media and sinusitis. The currently available vaccination strategies against pneumococcal disease, comprising polyvalent pneumococcal capsular polysaccharide (PS) and protein-PS conjugate formulations (6, 12, 13, 36), have some known and potential limitations. These include serotype-specific protection, poor immunogenicity of unconjugated PS in children under 2 years of age, and the possibility of nasopharyngeal replacement carriage by invasive, nonvaccine serotypes in vaccinated individuals (24). Furthermore, protein-PS conjugate vaccines are likely to be expensive, and this may limit their deployment in developing countries, where they are needed most.
We and others have been addressing the aforementioned shortcomings of existing vaccination strategies by investigating the capacities of pneumococcal virulence proteins to elicit non-serotype-dependent protection against disease. So far, the virulence proteins which have shown the greatest potential as vaccine antigens are the thiol-activated toxin pneumolysin (Ply) (5, 26, 28), two choline-binding surface proteins called pneumococcal surface protein A (PspA) (37) and choline-binding protein A (CbpA) (also referred to as PspC, Hic, or SpsA) (8, 15, 19, 32), and a metal-binding lipoprotein called pneumococcal surface antigen A (PsaA) (11). These proteins possess a range of biological activities, indicating that they act at different stages of the pathogenic process. For instance, Ply has both direct cytotoxic and complement activation properties, mediated by different domains within the toxin (5). The cytotoxic property accounts for inhibition of specific and nonspecific immune responses (14, 29), as well as stimulation of the release of inflammatory cytokines from host cells (18). Direct activation of the classical complement pathway is the result of binding of Ply to the Fc region of immunoglobulin G, which also contributes to inflammation and depletes serum opsonic activity (21, 31). PspA interferes with complement activation and slows the clearance of pneumococci from the blood of infected mice (20, 22, 35). It has also been shown to bind lactoferrin (16) and thus may also function by scavenging iron in the nasopharynx. CbpA is structurally related to PspA and mediates adherence to cytokine-activated lung cells, as well as playing a major role in colonization of the nasopharynx in an infant rat model (32). CbpA also specifically binds the secretory component of human secretory immunoglobulin A (17), human factor H (10), and complement component C3 (19, 33). Furthermore, CbpA has recently been shown to interact with the human polymeric immunoglobulin receptor, thereby facilitating invasion of the mucosa (38). PsaA forms part of an ABC-type manganese permease complex (11), and mutations in psaA have been reported to have pleiotropic effects on various pneumococcal functions, including adherence, autolysis, and virulence (3, 9, 23). Immunization with each of these proteins, either singly or in combination, has been shown to elicit a significant level of protection in animal models against one or more S. pneumoniae serotypes (1, 6, 7, 8, 25, 34).
CbpA shares similar structural domains with PspA, and its N-terminal α-helical domain is highly variable in both size and sequence among different strains of S. pneumoniae (8, 15, 19, 32). Brooks-Walter et al. (8) have suggested that the virulence properties of PspA and CbpA may complement each other in the host, a hypothesis supported by their observation that mutagenesis of pspA has a much lesser impact on systemic virulence in S. pneumoniae strains which contain cbpA than in those which lack it. Moreover, they have demonstrated that immunization with purified CbpA elicits protection against sepsis and that the protection is mediated by antibodies cross-reactive with PspA domains. Our previous study (25) demonstrated that immunization with a combination of Ply and PspA provides a higher degree of protection than any of the antigens alone in a mouse intraperitoneal model of infection. In another study, we have shown that while mutagenesis of the cbpA gene of S. pneumoniae D39 has a much smaller effect on virulence in a mouse intraperitoneal model than a mutation in ply, mutagenesis of both genes resulted in significant additive attenuation (4). This suggests that Ply and CbpA might also be an effective vaccine antigen combination, a possibility which is examined in the present study.
MATERIALS AND METHODS
Cloning, expression, and purification of His6-tagged CbpA fusion protein.
The cloning, expression, and purification of the pneumolysin toxoid derivative used in this study (PdB) has been described elsewhere (30). The cloning and expression of the N-terminal fragment of cbpA from the virulent type 2 S. pneumoniae strain D39 (2) was carried out as follows. Oligonucleotides AD12 (5′-TGTGGTGCATGCGACAGAAAACGAAGGAAGTACCCA-3′) and AD13 (5′-CCACATACCGTTTTCTTGTTTCAAGCTTGTTTTTGGAG-3′), incorporating an SphI and an HindIII restriction site (underlined), respectively, were used as primers for high-fidelity PCR amplification of a 1.3-kb fragment from the 5′ end of cbpA from D39 chromosomal DNA. The primers were designed to amplify the region encoding amino acids 1 to 445 of the mature CbpA polypeptide, and the restriction sites were incorporated to allow in-frame cloning of the PCR product into the corresponding restriction sites in the polylinker of the QIAexpress vector pQE31 (Qiagen Inc). The resultant recombinant plasmid was predicted to express an N-terminal His6-tagged truncated CbpA fusion protein incorporating the α-helical and proline-rich regions but lacking the signal peptide and the C-terminal choline-binding domain (8). Correct in-frame fusion of the cbpA fragment into pQE31 was confirmed by automated dye-terminator sequencing of plasmid DNA from a selected clone using the QIAexpress sequencing primer QE1 (5′-GGCGTATCACGAGGCCCTTTCG-3′). The recombinant plasmid was then used to transform the Escherichia coli K-12 expression strain M15 carrying a kanamycin resistance repressor plasmid, pREP4 (Qiagen Inc.). High-level expression of the His6-CbpA fusion protein was achieved by the addition of isopropyl-β-d-thiogalactoside (IPTG) at a final concentration of 2 mM to a Luria-Bertani broth culture containing the expression construct in the presence of 100 μg of ampicillin/ml and 25 μg of kanamycin/ml for 4 h at 37°C with vigorous shaking. The cells were then harvested by centrifugation at 6,000 × g for 10 min and resuspended in lysis buffer (50 mM sodium-phosphate [pH 8.0], 2 M NaCl, 20 mM imidazole). The cells were then lysed in a French pressure cell (SLM Aminco Inc.) at 12,000 lb/in2, and the resultant lysate was centrifuged at 100,000 × g for 1 h. The supernatant was then loaded onto a 2-ml nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen Inc.) previously equilibrated with 5 column volumes of lysis buffer. The resin was then washed with 10 column volumes of 10 mM sodium phosphate–500 mM NaCl (pH 6.0). The protein was eluted with a 30-ml gradient of 0 to 500 mM imidazole in 10 mM sodium phosphate (pH 6.0); 3-ml fractions were collected and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Fractions containing the purified protein were dialyzed against 10 mM sodium phosphate (pH 7.0) to remove the imidazole. The protein was finally resuspended in 50 mM sodium phosphate (pH 7.0), glycerol was added to a final concentration of 50% (vol/vol), and the mixture was stored at −15°C.
Formulation of vaccine antigens.
Antigens, with or without MPL (monophosphoryl lipid A), were adsorbed on AlPO4 as concentrated monobulks (1 mg/ml of AlPO4). The antigen/carrier ratio (by weight) was 10:25 for PdB and 8:25 for the CbpA fragment. The MPL was adsorbed at an MPL/carrier ratio of 10:50 (by weight). All the concentrated monobulks were prepared in 150 mM NaCl. The final vaccines were prepared by mixing the different required monobulks and by adjusting the preparations at 1 mg/ml of AlPO4 with an isotonic solution of AlPO4 at 1 mg/ml. One μg of thimerosal/ml was added as a preservative. The vaccines were prepared at least 1 week before the first injection.
Immunization of mice and analysis of sera.
For each experiment, eight groups of 5- to 6-week-old male BALB/c mice (13 to 15 per group) were immunized intraperitoneally with either AlPO4 alone, MPL plus AlPO4, PdB in AlPO4, PdB in MPL plus AlPO4, CbpA in AlPO4, CbpA in MPL plus AlPO4, a combination of PdB and CbpA in AlPO4, or a combination of PdB and CbpA in MPL plus AlPO4. Each mouse received three doses of 10 μg of each protein antigen in either formulation (AlPO4 or MPL plus AlPO4) at 12- to 14-day intervals, and sera were collected from the mice by retro-orbital bleeding 1 week after the third immunization. The sera were pooled on a group-by-group basis and assayed for PdB- and CbpA-specific antibodies by enzyme-linked immunosorbent assay (ELISA). The sera were also subjected to Western immonoblot analyses using purified PdB, purified CbpA, or whole-cell lysates of S. pneumoniae D39 derivatives as the antigen.
Challenge.
Intraperitoneal-challenge experiments were carried out 2 weeks after the third immunization using a highly virulent capsular type 2 strain (D39). Before challenge, the bacteria were grown at 37°C overnight on blood agar and then inoculated into serum broth consisting of 10% (vol/vol) horse serum in meat extract broth. They were then grown statically for 3 h at 37°C to give approximately 108 CFU/ml. Serotype-specific capsule production was confirmed by Quellung reaction using antisera obtained from Statens Seruminstitut, Copenhagen, Denmark.
For the challenge experiments, groups of immunized BALB/c mice were infected with either 1.3 × 105 or 1.3 × 107 CFU of the challenge strain. The challenge dose was equivalent to approximately 103 or 105 times the 50% lethal dose (LD50), respectively, for BALB/c mice. The mice were closely monitored for 21 days, and the survival time of each mouse was recorded. Differences between the median survival times of groups were analyzed by the Mann-Whitney U test. Differences between the overall survival rates of groups were analyzed by the Fisher exact test.
RESULTS
Purification of His6-tagged CbpA fusion protein.
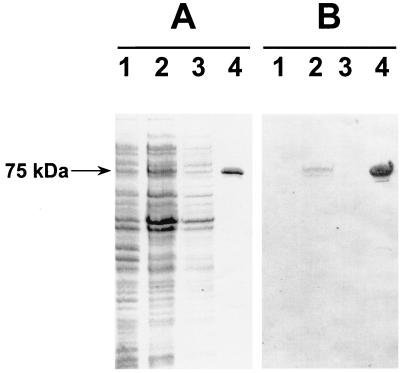
The first step employed in assessing the protective ability of the CbpA fragment involved the purification of the His6-tagged protein by Ni-NTA affinity chromatography. The purified His6-tagged CbpA protein was >95% pure as judged by SDS-PAGE after it was stained with Coomassie brilliant blue R250 (Fig. 1A). The mobility of the purified protein on SDS-PAGE was consistent with a size of 75 kDa, including the His6 tag, which was larger than that predicted from the DNA sequence (50 kDa). However, anomalous migration of CbpA on SDS-PAGE has been observed previously (32). The purified fusion protein also reacted with anti-His6 monoclonal antibody (Roche) in a Western immunoblot assay (Fig. 1B).
FIG. 1.
Purification of His6-tagged truncated CbpA fragment from recombinant E. coli. (A) SDS-PAGE analysis (12% gel) of protein samples from various stages of the purification procedure. Lanes: 1, lysate of recombinant E. coli expression construct before induction; 2, 100,000 × g supernatant of E. coli lysate after a 4-h induction with IPTG before being loaded onto Ni-NTA resin; 3, unbound fraction washed from the resin; 4, purified His6-tagged CbpA fragment (approximate mass, 75 kDa) after elution from Ni-NTA with imidazole. (B) Western immunoblot analysis of corresponding samples in panel A after they were electroblotted onto nitrocellulose. The samples were reacted with anti-His6 monoclonal antibody.
Analysis of sera.
ELISA analysis of pooled sera from groups of mice immunized with PdB or CbpA singly and in combination shows that strong antibody responses were elicited (Table 1). In addition, antigen-specific antibody titers were not diminished when the antigens were administered in combination, indicating that there was no detectable antagonistic effect of combining these antigens. Higher ELISA titers were reproducibly obtained when the antigens (alone or in combination) were administered with MPL plus AlPO4 than when they were administered with AlPO4 alone. The ELISA titers also suggest that CbpA is the more immunogenic antigen when administered alone or in combination with PdB, regardless of the adjuvant used.
TABLE 1.
Antibody titers obtained from mice immunized with PdB and CbpA
| Immunization group | Antibody titer (ELISA)a against:
|
|
|---|---|---|
| PdB | CbpA | |
| Placebo (AlPO4 or MPL + AlPO4) | −b | − |
| PdB-AlPO4 | 11,000 | − |
| PdB-MPL-AlPO4 | 40,000 | − |
| CbpA-AlPO4 | − | 74,000 |
| CbpA-MPL-AlPO4 | − | 140,000 |
| PdB-CbpA-AlPO4 | 24,000 | 67,200 |
| PdB-CbpA-MPL-AlPO4 | 39,000 | 135,000 |
ELISA titers were defined as the reciprocal of the dilution of serum yielding 50% of the maximum A405 above the background.
−, <100.
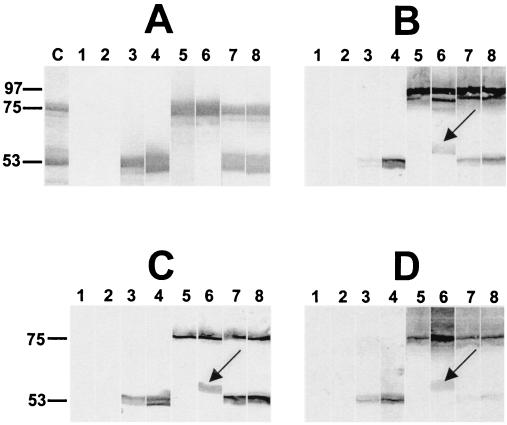
Western immunoblot analysis of the sera also demonstrated antibody responses to each of the purified protein antigens (Fig. 2A). A pneumolysin-specific antibody response was also demonstrated in sera from mice immunized with PdB when whole-cell lysates of S. pneumoniae D39 were used as the antigen (Fig. 2B). The major response of the anti-CbpA serum was directed against CbpA itself (the native protein has an apparent electrophoretic mobility of approximately 100 kDa). This species was absent when a lysate of a CbpA-negative derivative of D39 (4) was used as the antigen (Fig. 2C). However, in addition to CbpA, the anti-CbpA serum showed cross-reactivity with several protein species. One of these (approximately 86 kDa) was presumed to be PspA, as the reactive band was absent when a lysate of a PspA-negative D39 mutant (4) was used as the antigen (Fig. 2D). In addition, a band of approximately 60 kDa was labeled in the D39 lysate (Fig. 2B). It is unlikely that this species is a degradation product of CbpA or PspA, as it was still present when the anti-CbpA serum was reacted with lysates of either the CbpA-negative or the PspA-negative D39 mutant (Fig. 2C and D).
FIG. 2.
Western immunoblot analysis of purified PdB (53 kDa) and His6-tagged CbpA fragment (75 kDa) (A) and of whole-cell lysates of S. pneumoniae D39 (B), isogenic CbpA-negative D39 (4) (C), and isogenic PspA-negative D39 (4) (D) showing specificity of antibody responses to the protein antigens. The proteins were separated by SDS-PAGE and then electroblotted onto nitrocellulose. They were then reacted with sera from the groups of mice immunized with the proteins in different adjuvant combinations. Lane C, Coomassie blue-stained gel showing the relative mobilities of the two purified proteins. Lanes 1 to 8, nitrocellulose membrane strips reacted with sera from mice immunized with AlPO4 adjuvant (lane 1), MPL plus AlPO4 (lane 2), PdB in AlPO4 adjuvant (lane 3), PdB in MPL plus AlPO4 (lane 4), CbpA in AlPO4 adjuvant (lane 5), CbpA in MPL plus AlPO4 (lane 6), a combination of PdB and CbpA in AlPO4 adjuvant (lane 7), and a combination of PdB and CbpA in MPL plus AlPO4 (lane 8). The arrows show a band of approximately 60 kDa cross-reacting with the anti-CbpA serum pool.
Protection studies.
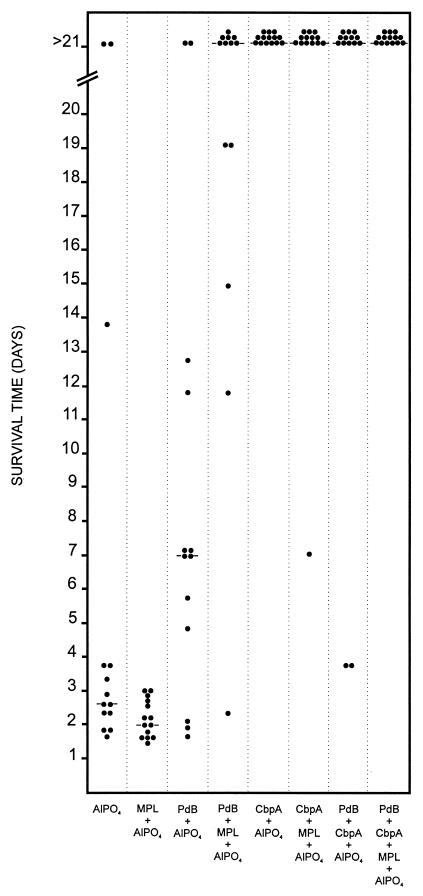
In order to assess the protection afforded by immunization of mice with PdB and the CbpA fragment either singly or in combination, two separate intraperitoneal-challenge experiments were carried out as described in Materials and Methods. Figure 3 shows the results obtained when the mice were challenged with approximately 1.3 × 105 CFU of the highly virulent capsular type 2 pneumococcal strain D39. This challenge inoculum is equivalent to approximately 103 times the LD50 for BALB/c mice. In this experiment, the median survival times for mice that received PdB either in AlPO4 or in MPL plus AlPO4 were significantly longer than those for mice that received the corresponding adjuvants alone (P ≪ 0.001 in both cases). Similarly, mice that received CbpA in either AlPO4 alone or in MPL plus AlPO4 survived significantly longer than those that received the corresponding adjuvants alone (P ≪ 0.001 in both cases). Furthermore, mice that received PdB in MPL plus AlPO4 survived significantly longer than those that received PdB in AlPO4 (P ≪ 0.01). However, mice that received CbpA in MPL plus AlPO4 did not survive significantly longer than those that received CbpA in AlPO4 (Table 2). Mice immunized with a combination of PdB and CbpA in AlPO4 survived significantly longer than those that received PdB alone in AlPO4 (P ≪ 0.001), but mice that received a combination of PdB and CbpA in AlPO4 did not survive significantly longer than mice that received CbpA alone in AlPO4. Interestingly, mice that received a combination of PdB and CbpA in MPL plus AlPO4 did not survive significantly longer than those that received PdB or CbpA alone in MPL plus AlPO4.
FIG. 3.
Survival times for mice after intraperitoneal challenge. Groups of 13 or 14 BALB/c mice were immunized with the indicated antigens and challenged 2 weeks after the third immunization with approximately 1.3 × 105 CFU of the capsular type 2 strain D39. The broken lines denote the median survival time for each group.
TABLE 2.
Statistical comparison of median survival timesa
| Immunization group | Statistical difference (P value) between indicated groups
|
|||||||
|---|---|---|---|---|---|---|---|---|
| AlPO4 | MPL + AlPO4 | PdB + AlPO4 | PdB + MPL + AlPO4 | CbpA + AlPO4 | CbpA + MPL + AlPO4 | PdB + CbpA + AlPO4 | PdB + CbpA + MPL + AlPO4 | |
| AlPO4 | <0.025 | ≪0.001 | ND | ≪0.001 | ND | ≪0.001 | ND | |
| MPL + AlPO4 | <0.001 | ND | ≪0.001 | ND | ≪0.001 | ND | ≪0.001 | |
| PdB + AlPO4 | NS | ND | ≪0.01 | ≪0.001 | ND | ≪0.001 | ND | |
| PdB + MPL + AlPO4 | ND | ≪0.001 | <0.025 | ND | NS | ND | NS | |
| CbpA + AlPO4 | NS | ND | NS | ND | NS | NS | ND | |
| CbpA + MPL + AlPO4 | ND | <0.001 | ND | <0.05 | NS | ND | NS | |
| PdB + CbpA + AlPO4 | <0.025 | ND | <0.01 | ND | NS | ND | NS | |
| PdB + CbpA + MPL + AlPO4 | ND | ≪0.001 | ND | NS | ND | NS | NS | |
Differences in median survival times between groups of mice immunized with the indicated antigen formulations were analyzed using the Mann-Whitney U test (one tailed). P values are indicated for groups challenged with 1.3 × 105 CFU or 1.3 × 107 CFU (in bold) of capsular type 2 strain D39. ND, not determined; NS, not significant.
The overall survival rates of the various groups of mice in the first challenge experiment were compared using the Fisher exact test. The survival rate for mice that received PdB in MPL plus AlPO4 was significantly greater than that for mice that received MPL plus AlPO4 alone (P ≪ 0.005). Likewise, the survival rate for mice that received PdB in MPL plus AlPO4 was significantly greater than that for mice that received PdB in AlPO4 (P < 0.025). In addition, the survival rate for mice that received a combination of PdB and CbpA in MPL plus AlPO4 was significantly greater than that for mice that received PdB in MPL plus AlPO4 (P < 0.025), but there was no significant difference between the survival rates of the former group and mice that received CbpA in MPL plus AlPO4. The survival rate for mice that received CbpA in AlPO4 was significantly greater than that for mice that received either PdB in AlPO4 or AlPO4 adjuvant only (P ≪ 0.005 in both cases). There was also a significant difference between the survival rate of mice immunized with PdB in AlPO4 and that of mice immunized with a combination of PdB and CbpA in AlPO4 (P ≪ 0.005). However, there was no significant difference between the survival rates of mice that received a combination of PdB and CbpA in AlPO4 and those that received CbpA in AlPO4.
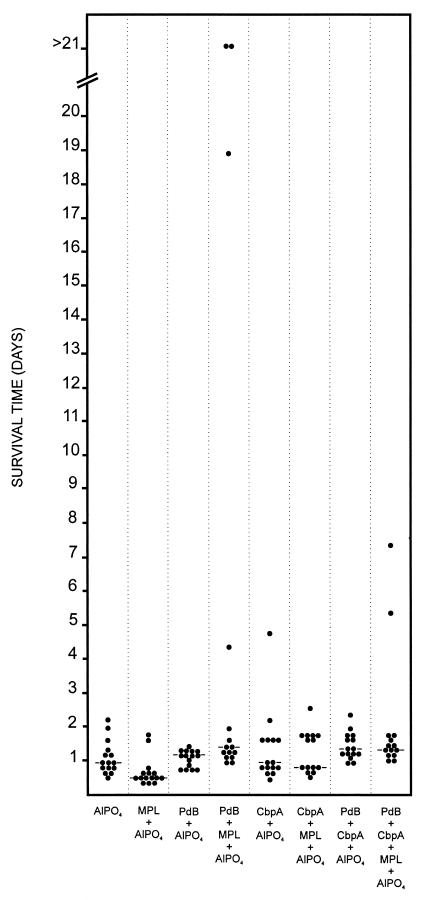
The high protection rate observed for the CbpA fragment alone complicated assessment of whether additive protection could be achieved with the PdB-CbpA combination. Accordingly, a second challenge experiment was carried out using a dose of approximately 1.3 × 107 CFU of S. pneumoniae D39 (equivalent to 105 times the LD50) (Fig. 4). In this experiment, the median survival times of mice that received either PdB or CbpA alone in AlPO4 adjuvant were not significantly different from that of the AlPO4 placebo group, but a significant increase in survival time was observed for the group which received the combination of PdB and CbpA in AlPO4 (P < 0.025) (Table 2). The median survival time for mice that received PdB in MPL plus AlPO4 was significantly better than that for mice that received CbpA in MPL plus AlPO4 (P < 0.05). The median survival times for mice that received either PdB or CbpA in MPL plus AlPO4 were significantly longer than that for mice that received MPL plus AlPO4 alone (P ≪ 0.001 and P < 0.001, respectively), but there was no significant additive protection for the group which received the combination of PdB and CbpA in MPL plus AlPO4. Although the median survival time of mice that received PdB in MPL plus AlPO4 was significantly longer than that for mice that received PdB in AlPO4 alone (P < 0.025), there was no significant difference between the median survival times for mice that received CbpA in AlPO4 and mice that received CbpA in MPL plus AlPO4.
FIG. 4.
Survival times for mice after intraperitoneal challenge. Groups of 14 or 15 BALB/c mice were immunized with the indicated antigens and challenged 2 weeks after the third immunization with approximately 1.3 × 107 CFU of capsular type 2 strain D39. The broken lines denote the median survival time for each group.
DISCUSSION
The pneumococcal proteins Ply, PspA, CbpA, and PsaA have all been shown to contribute to the pathogenesis of pneumococcal disease (3, 8, 26, 27), and studies focusing on the development of cheap and effective vaccines against S. pneumoniae have indicated that certain combinations of these antigens may provide superior non-serotype-dependent protection against S. pneumoniae (1, 6, 7, 8, 25). The interest in CbpA as a potential vaccine antigen arises from the demonstration that immunization with purified CbpA elicits protection against sepsis and that the protection is mediated by antibodies cross-reactive with PspA domains (8). In a previous study (25), we demonstrated that immunization with a combination of Ply and PspA provides a higher degree of protection than either antigen alone in a mouse intraperitoneal model of infection and that the protection is at least in part antibody mediated. As an extension of that work, the present study sought to determine whether any additive protection could be achieved by immunization with a combination of a genetic toxoid derivative of Ply (PdB) and CbpA relative to that achieved with either of these antigens alone. We employed an active immunization-challenge model, as in the previous work (25), but here we tested two adjuvant formulations and two challenge doses of the serotype 2 strain D39.
The results of the intraperitoneal-challenge experiments demonstrate that CbpA alone is highly protective against challenge with 1.3 × 105 CFU of strain D39 (approximately 103 times the LD50). This is consistent with the findings of Brooks-Walter et al. (8), who demonstrated that immunization of mice with a similar fragment (amino acids 1 to 455) derived from D39 CbpA elicited significant protection against intravenous challenge with a heterologous (type 3) strain. However, the capacity to protect against the highly virulent D39 strain was not examined. The present study also demonstrates a significant level of protection in PdB-immunized mice at this challenge dose, in agreement with previous reports (1, 25, 27, 28, 30). Importantly, when antigens were administered with AlPO4 adjuvant, the CbpA fragment elicited stronger protection than PdB; a similar trend was seen when MPL plus AlPO4 was used as the adjuvant, although the difference did not reach statistical significance. Immunization with both CbpA and PdB resulted in superior protection compared with PdB alone but not compared with that imparted by the CbpA fragment alone. Of course, the strength of the protection imparted by CbpA alone would have masked any additive effect that might have been imparted by the combination of antigens. When the challenge dose was increased 100-fold, none of the antigen-adjuvant combinations resulted in significant increases in the overall survival rate. However, when MPL plus AlPO4 was used as the adjuvant, PdB alone, CbpA alone, and the combination of antigens all imparted a significant (albeit modest) increase in median survival time after the high-dose challenge, although there was no additive effect. Notably, when challenged with a high dose of D39, mice that received PdB in the combined adjuvant formulation had a significantly longer median survival time than those that received CbpA in the combined adjuvant formulation. Interestingly, when AlPO4 was used as the adjuvant, only the combination of PdB and CbpA resulted in a significant increase in the median survival time. This result is comparable to that obtained when mice were immunized with a combination of PdB and PspA and challenged with very high doses of D39 or a type 4 serotype strain in our earlier study (25).
We also demonstrated that a mixture of MPL and AlPO4 adjuvants was more effective than AlPO4 adjuvant alone in terms of antibody response to both PdB and CbpA, as judged by ELISA titers. Moreover, for PdB-immunized mice, use of the combined adjuvant resulted in significantly enhanced protection relative to that achieved with AlPO4 alone, as judged by both median survival time and overall survival rate. This was in spite of the fact that the combined adjuvant resulted in a lower level of nonspecific protection in the placebo groups.
This study provides further support for the notion that virulence-associated proteins of S. pneumoniae, and combinations thereof, warrant serious consideration as components of new pneumococcal vaccines. Clearly, the relative strength of the protection imparted by immunization with PdB and CbpA, singly and in combination, needs to be evaluated using a variety of challenge strains. The strength of the protection imparted by the CbpA fragment relative to PdB is particularly encouraging, although this may not necessarily hold when mice are challenged with strains expressing heterologous CbpA types. Moreover, CbpA is not present in a small proportion of pneumococcal strains, although it may still impart a degree of protection against such strains due to cross-reaction of CbpA antibodies with other choline-binding proteins such as PspA, as demonstrated earlier (8) and in this study. Interestingly, a protein of approximately 60 kDa present in D39 and its isogenic CbpA- and PspA-negative mutants was found to cross-react with serum raised against the CbpA fragment (lacking the choline-binding region) used in this study. Because of the cross-reaction of the anti-CbpA serum with other pneumococcal proteins, it is difficult at this stage to assess the level of protection imparted specifically by CbpA. We are in the process of characterizing the 60-kDa protein and evaluating its impact on protection against challenge with various S. pneumoniae strains. A further extension of these studies could include an evaluation of protection afforded by various combinations of PdB, CbpA, PspA, and other promising protein vaccine candidates. Such studies will provide a clearer understanding of which combinations of pneumococcal proteins elicit the most effective non-serotype-dependent protection against S. pneumoniae.
ACKNOWLEDGMENTS
This work was supported by grants from the National Health and Medical Research Council of Australia and the World Health Organization.
REFERENCES
- 1.Alexander J E, Lock R A, Peeters C C A M, Poolman J T, Andrew P W, Mitchell T J, Hansman D, Paton J C. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect Immun. 1994;62:5683–5688. doi: 10.1128/iai.62.12.5683-5688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry A M, Paton J C. Sequence heterogeneity of PsaA, a 37-kDa putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64:5255–5262. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry A M, Paton J C. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000;68:133–140. doi: 10.1128/iai.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulnois G J, Paton J C, Mitchell T J, Andrew P W. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2611–2616. doi: 10.1111/j.1365-2958.1991.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 6.Briles D E, Ades E, Paton J C, Sampson J S, Carlone G M, Huebner R C, Virolainen A, Swiatlo E, Hollingshead S K. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000;68:796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles D E, Hollingshead S, Brooks-Walter A, Nabors G S, Ferguson L, Schilling M, Gravenstein S, Braun P, King J, Swift A. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine. 2000;18:1707–1711. doi: 10.1016/s0264-410x(99)00511-3. [DOI] [PubMed] [Google Scholar]
- 8.Brooks-Walter A, Briles D E, Hollingshead S K. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect Immun. 1999;67:6533–6542. doi: 10.1128/iai.67.12.6533-6542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claverys J-P, Granadel C, Berry A M, Paton J C. Penicillin tolerance in Streptococcus pneumoniae, autolysis and the Psa ATP-binding cassette (ABC) manganese permease. Mol Microbiol. 1999;32:881–883. doi: 10.1046/j.1365-2958.1999.01369.x. [DOI] [PubMed] [Google Scholar]
- 10.Dave S, Brooks-Walter A, Pangburn M K, McDaniel L S. PspC, a pneumococcal surface protein, binds human factor H. Infect Immun. 2001;69:3435–3437. doi: 10.1128/IAI.69.5.3435-3437.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dintilhac A, Alloing G, Granadel C, Claverys J-P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 12.Eskola J. Immunogenicity of pneumococcal conjugate vaccines. Pediatr Infect Dis J. 2000;19:388–393. doi: 10.1097/00006454-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 13.Fedson D S. The clinical effectiveness of pneumococcal vaccination: a brief review. Vaccine. 1999;17(Suppl. 1):S85–S90. doi: 10.1016/s0264-410x(99)00113-9. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante A, Rowan-Kelly B, Paton J C. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect Immun. 1984;46:585–589. doi: 10.1128/iai.46.2.585-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerschmidt S, Talay S R, Brandtzaeg P, Chhatwal G S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschmidt S, Bethe G, Remane P H, Chhatwal G S. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect Immun. 1999;67:1683–1687. doi: 10.1128/iai.67.4.1683-1687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammerschmidt S, Tillig M P, Wolff S, Vaerman J P, Chhatwal G S. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol Microbiol. 2000;36:726–736. doi: 10.1046/j.1365-2958.2000.01897.x. [DOI] [PubMed] [Google Scholar]
- 18.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janulczyk R, Iannelli F, Sjöholm A G, Pozzi G, Björck L. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J Biol Chem. 2000;275:37257–37263. doi: 10.1074/jbc.M004572200. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel L S, Yother J, Vijayakamur M, McGarry L, Guild W R, Briles D E. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA) J Exp Med. 1987;165:381–394. doi: 10.1084/jem.165.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell T J, Andrew P W, Saunders F K, Smith A N, Boulnois G J. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol Microbiol. 1991;5:1883–1888. doi: 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 22.Neeleman C, Geelen S P M, Aerts P C, Daha M R, Mollnes T E, Roord J J, Posthuma G, van Dijk H, Fleer A. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect Immun. 1999;67:4517–4524. doi: 10.1128/iai.67.9.4517-4524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak R, Braun J S, Charpentier E, Tuomanen E. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex PsaA. Mol Microbiol. 1998;29:1285–1296. doi: 10.1046/j.1365-2958.1998.01016.x. [DOI] [PubMed] [Google Scholar]
- 24.Obaro S K. Prospects for pneumococcal vaccination in African children. Acta Trop. 2000;75:141–153. doi: 10.1016/s0001-706x(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 25.Ogunniyi A D, Folland R L, Briles D E, Hollingshead S K, Paton J C. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect Immun. 2000;68:3028–3033. doi: 10.1128/iai.68.5.3028-3033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton J C. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 27.Paton J C. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 1998;6:85–87. doi: 10.1016/s0966-842x(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 28.Paton J C, Andrew P W, Boulnois G J, Mitchell T J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 29.Paton J C, Ferrante A. Inhibition of human polymophonuclear leukocyte respiratory burst, migration and bactericidal activity by the pneumococcal toxin, pneumolysin. Infect Immun. 1983;4l:1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paton J C, Lock R A, Lee C-J, Li J P, Berry A M, Mitchell T J, Andrew P W, Hansman D, Boulnois G J. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect Immun. 1991;59:2297–2304. doi: 10.1128/iai.59.7.2297-2304.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paton J C, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin, pneumolysin. Infect Immun. 1984;43:1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith B L, Hostetter M K. C3 as substrate for adhesion of Streptococcus pneumoniae. J Infect Dis. 2000;182:497–508. doi: 10.1086/315722. [DOI] [PubMed] [Google Scholar]
- 34.Tart R C, McDaniel L S, Ralph B A, Briles D E. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis. 1996;173:380–386. doi: 10.1093/infdis/173.2.380. [DOI] [PubMed] [Google Scholar]
- 35.Tu T A-H, Fulgham R L, McCrory M A, Briles D E, Szalai A J. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun. 1999;67:4720–4724. doi: 10.1128/iai.67.9.4720-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Pneumococcal vaccines: World Health Organization position paper. Can Commun Dis Rep. 1999;25:150–151. [PubMed] [Google Scholar]
- 37.Yother J, Briles D E. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J Bacteriol. 1992;174:601–609. doi: 10.1128/jb.174.2.601-609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J R, Mostov K E, Lamm M E, Nanno M, Shimida S, Ohwaki M, Tuomanen E. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell. 2000;102:827–837. doi: 10.1016/s0092-8674(00)00071-4. [DOI] [PubMed] [Google Scholar]