Abstract
Background and Objectives
Few studies have examined care partners’ reactions to their loved ones receiving amyloid-β positron emission tomography (PET) scan results, which can be indicative of Alzheimer’s disease. We explored care partners’ reactions qualitatively, and checked the association of scan results and diagnostic category (dementia vs mild cognitive impairment [MCI]) with care partner anxious and depressive symptoms through quantitative analysis.
Research Design and Methods
Using data from 1,761 care partners in the Caregivers’ Reactions and Experience, a supplemental study of the Imaging Dementia Evidence for Amyloid Scanning study, we applied an exploratory sequential mixed-methods design and examined the reactions of 196 care partners to receiving amyloid PET scan results through open-ended interview questions. Based on the qualitative content analysis, we hypothesized there would be an association of care partners’ depressive (Patient Health Questionnaire-2) and anxious (6-item State–Trait Anxiety Inventory) symptoms with scan results and diagnostic category which we then tested with logistic regression models.
Results
Content analysis of open-ended responses suggests that when scan results follow the care partner’s expectations, for example, elevated amyloid in persons with dementia, care partners report relief and gratitude for the information, rather than distress. Adjusted logistic regression models of survey responses support this finding, with significantly higher odds of anxiety, but not depressive symptoms, among care partners of persons with MCI versus dementia and elevated amyloid.
Discussion and Implications
Care partners of persons with MCI reported distress and had higher odds of anxiety after receiving elevated amyloid PET scan results than care partners of persons with dementia. This has the potential to inform clinical practice through recommendations for mental health screening and referrals.
Keywords: Alzheimer’s disease, Anxiety, Caregiving, Mild cognitive impairment
Background and Objectives
As the global population ages, a growing number of adults live with Alzheimer’s disease (AD) and other forms of cognitive impairment. An estimated 6.2 million Americans over the age of 65 live with AD today, and this number is projected to surpass 13.8 million by 2060 (Alzheimer’s Association, 2021). While there has been a recent boom in pharmaceutical research and diagnostic tools seeking to treat and manage AD and related dementias, less attention has been paid to the care partners of these individuals. Over 11 million unpaid caregivers, often family members, provided approximately 15.3 billion hours of care to individuals with dementia in 2020, representing simultaneously a decline in the number of caregivers and an increase in the hours of care provided (Alzheimer’s Association, 2021). The monetary cost of caregiving was valued at $256.7 billion in 2020, but this figure does not account for the emotional toll or negative mental health outcomes for care partners of individuals with AD.
Care partners for persons with dementia are at increased risk for symptoms of depression and anxiety, as well as generalized anxiety disorder: they fare worse on measures of physical, mental, and emotional health compared to care partners of older adults with physical but no cognitive impairment (Clipp & George, 1993; Cooper et al., 2007; Ma et al., 2018; Pinquart & Sörensen, 2003). Anxiety and depressive symptoms in care partners are significantly associated with certain characteristics of the individual with dementia, particularly dementia severity (García-Alberca et al., 2011). Care partners experience depressive disorders at higher rates than the approximately 15% prevalence of major and minor depression found among community-dwelling older adults in a meta-analysis (Gallo & Lebowitz, 1999): a separate meta-analysis found 22.3% of caregivers had a depressive disorder (Cuijpers, 2005). Another meta-analysis of care partners for persons with dementia found the aggregate prevalence of anxiety measured with various standard instruments was 43.6% among 392 caregivers across four studies (Sallim et al., 2015). Care partners are at increased risk due to their caretaking responsibilities and merit increased attention to identify and mitigate mental health symptoms (Cooper et al., 2007; Joling et al., 2015; Largent & Karlawish, 2019).
As biomarkers become increasingly commonplace tools to diagnose AD, it is vital to understand the experiences of care partners as their loved ones undergo these tests. Amyloid-β PET scans are a clinically useful diagnostic tool to measure amyloid-β plaque levels in the brain, differentiate AD from other causes of dementia, and potentially inform clinical management of individuals with memory impairment (Blennow et al., 2015; Fleisher et al., 2011; Marcus et al., 2014; Rabinovici et al., 2019). While studies to date indicate that patients themselves may not exhibit worsening psychological symptoms after learning their scan results (Lim et al., 2016; Lingler et al., 2020; Taswell et al., 2018), little research has examined the experiences of care partners of persons living with cognitive impairment as they learn these results. Previous work by our team demonstrated that 83% of persons with cognitive impairment and 85% of their care partners correctly reported amyloid-β PET scan results (James et al., 2020). In one small study, receiving scan results also improved acceptance of the disease process in care partners and provided better understanding of their loved one’s condition (Bensaïdane et al., 2016). However, a randomized clinical trial (RCT) found that receiving scan results decreased self-efficacy for coping with mild cognitive impairment (MCI) in care partners (Lingler et al., 2020), demonstrating a potential need for emotional support. Interviews with 70 care partners of individuals without cognitive impairment receiving amyloid PET scans further indicated that care partners’ preferences should be incorporated into diagnostic disclosure guidelines (Largent et al., 2021). Care partners of individuals with cognitive impairment differ in many ways from care partners of those without cognitive impairment, and little is known about their experiences receiving amyloid PET scan results, despite their vital role in patient care. They are often referred to as the “invisible second patient”: they are as valuable to the care team (particularly in research settings) as they are to the person with cognitive impairment, yet their needs are often neglected in the diagnostic and treatment process (Black et al., 2018; Brodaty & Donkin, 2009).
Further investigation is necessary to understand the experiences of care partners of persons with cognitive impairment receiving amyloid-β PET scan results to offer appropriate support. Pharmacological advances in AD treatments will likely increase the frequency with which PET scans are used (Grundman et al., 2013), but knowledge of how care partners react to and make meaning of these results is slim. To address this dearth of knowledge, we utilized an exploratory, sequential mixed-methods research design. Qualitative analysis of open-ended interview responses about experiences receiving scan results informed hypotheses that were then examined through quantitative analyses of survey data about depressive (Patient Health Questionnaire-2 [PHQ-2]) and anxious (six-item State–Trait Anxiety Inventory [STAI-6]) symptoms. The research question guiding our qualitative investigation was: what are care partners’ reactions to their loved one receiving negative (not-elevated) or positive (elevated) amyloid PET scan results? Based on our qualitative findings, we then sought to confirm our hypothesis that among recipients of elevated amyloid PET scan results, care partners to persons with MCI would have stronger negative reactions and poorer emotional health compared to care partners for individuals with dementia using quantitative analysis of standardized survey measures.
Research Design and Methods
Study Design and Population
This study uses baseline data from the prospective cohort study: “Caregivers’ Reactions and Experience, a supplemental study of the Imaging Dementia Evidence for Amyloid Scanning (CARE IDEAS) Study.” The IDEAS study was an observational, open-label, longitudinal cohort study which enrolled a total of 16,008 Medicare beneficiaries with MCI or dementia of uncertain etiology who received an amyloid PET scan. Eligible participants were recruited between February 2016 and September 2017 from 595 dementia care practices and academic medical centers across the United States. Participants received amyloid PET scans reimbursed by Medicare, with the overall goal of understanding how scan results may influence patient-related clinical outcomes (e.g., appropriate drug or treatment changes). All inclusion and exclusion criteria have been reported in previous publications (Rabinovici et al., 2019).
For the CARE IDEAS study, we recruited a subsample of 2,228 IDEAS participants and 1,872 of their care partners from 415 dementia care practices across 40 states. Only respondents with a potential care partner were screened for inclusion in the CARE IDEAS study, as previously reported (Jutkowitz et al., 2020). The aim of the CARE IDEAS study was to further explore the experiences of patients and care partners after learning scan results through a combination of quantitative survey questionnaires with standardized measures and open-ended qualitative questions. This information, integrated with clinical data from the parent IDEAS study, offers an enhanced understanding of the impact of amyloid PET scan results on patient-related clinical outcomes, patient and care partner decision making, care preferences, cognitive status, and emotional well-being after receiving results. The median time elapsed between a patient’s PET scan and participation in the baseline CARE IDEAS survey was 4.5 months. We combined qualitative analysis of the open-ended interview responses provided by care partners with an examination of the association of scan results and level of impairment with anxiety and depressive symptoms among care partners. The CARE IDEAS study was approved by the Institutional Review Board at Brown University (Protocol #1606001534).
Methods of Data Collection
The primary method of data collection was a structured telephone survey questionnaire administered to 1,872 care partners. Care partner questionnaires comprised standard items measuring sociodemographic characteristics, aspects of decision making related to the patients’ preferences, care partner cognitive function, and symptoms of anxiety and depression, as well as assessment of the patient’s ability to function despite memory impairment. Additionally, a subset of care partners was asked five open-ended interview questions as part of the survey and answers were audio-recorded with permission. The open-ended questions concerned what made the care partner aware of problems with the patient’s memory, what the results of the scan mean according to the doctor, how the scan influences plans for the patient’s future, how they hope that the scan will affect the patient’s care and treatment, and how difficult it was to get the diagnosis (see Supplementary Material for open-ended questions included in qualitative analysis). While the fifth question about how difficult it was to get the diagnosis elicited the most information about emotional response to the disclosure of test results, we considered all questions with potentially relevant answers during analysis because participants would at times continue their narrative across questions and repeat important information. The survey firm interviewers did not prompt participants for additional information and the qualitative data are overall more structured and shorter than what semistructured interviews usually would yield.
Qualitative Data Analysis and Sample Selection
Verbatim transcripts of responses were imported into NVivo (Version 12) and analyzed by two authors (E. Bélanger and T. T. Wetle) using qualitative content analysis following the conventional approach described by Hsieh and Shannon (2005). The coding process consisted of reading transcripts closely for each participant, followed by line-by-line coding to derive detailed codes that were then clustered around larger meaningful categories through an iterative process. Preliminary codes were generated from the list of open-ended interview questions (e.g., classifying symptoms as reported in Question 1: memory loss, gait changes, and other indicators), but the majority of analysis was inductive as we captured emergent content from participants’ responses. Regular team meetings were held to discuss the development of the coding book with clear definitions and examples for each code, before applying these to all transcripts. Coding was then compared across researchers by a third coder (J. D’Silva), and any discordance was discussed until consensus was reached. After the qualitative content analysis process had been completed, open-ended responses were stratified by diagnostic category (MCI vs dementia) and scan result (elevated beta-amyloid vs not-elevated) using survey data and further examined using NVivo matrix coding queries to determine how coded content differed across these groups.
Given the large number of participants who answered open-ended questions and relatively short recordings ranging in duration from 1 to 19 min, we conducted analysis in waves to make sure that we reached saturation. We started with two initial randomly selected batches of 50 dyads to develop the coding book, following an exploratory, inductive approach suitable for a relatively novel diagnostic test. After randomly selecting 100 additional responses, the team agreed that saturation had been reached insofar as no novel information was gleaned from the additional transcripts. Of the 200 selected dyads who answered the open-ended questionnaires, 196 care partners from 123 different dementia care practices had audio recordings that were of sufficient quality to be transcribed included in the qualitative analysis. Those with missing responses on relevant quantitative data (N = 9) were retained at this stage including one inconclusive amyloid scan result.
Sequential Mixed-Methods Integration
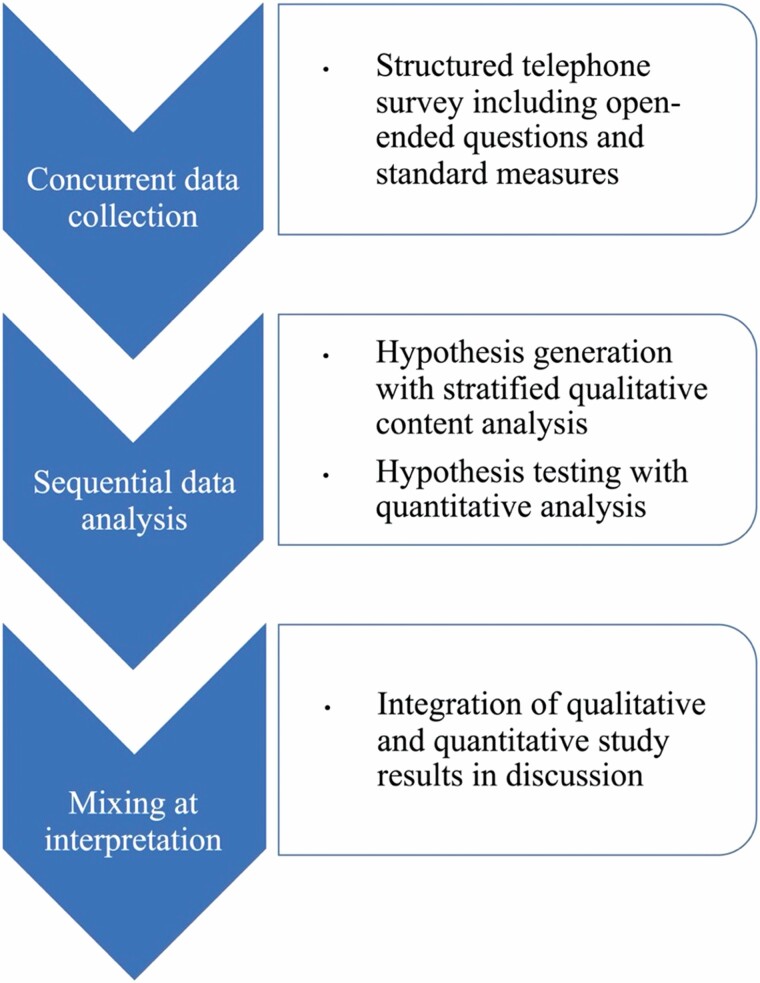
Based on qualitative findings from the open-ended questions along stratifying survey variables, we hypothesized that care partners to persons with MCI have stronger negative emotional reactions to scan results indicating elevated amyloid plaque and therefore higher levels of depressive and anxiety symptoms compared to care partners for persons with dementia with elevated amyloid levels. The qualitative and quantitative measures were collected concurrently (see Figure 1), and analysis was conducted sequentially with qualitative results informing quantitative analysis. Through this mixed-methods design, participants’ experiences informed our findings and grounded our conclusions in a process that was both inductive and deductive. By combining our inductive qualitative conclusions with hypothesis testing through statistical methods, our study offers a considered perspective on a little-researched topic.
Figure 1.
Mixed-Methods Integration.
Quantitative Sample and Study Measures
Our primary dependent variables of interest were symptoms of depression and anxiety. Depressive symptoms were measured using the standard PHQ-2, asking about the frequency of anhedonia, “Little interest or pleasure in doing things?” and dysthymia, “Feeling down, depressed, or hopeless?” during the past 2 weeks on a 4-point Likert scale from “0 = not at all” to “3 = nearly every day.” A score of 3 or more out of 6 has been deemed optimal for the screening of depressive symptoms and would warrant further assessment by a licensed clinician (Kroenke et al., 2003). To capture anxiety levels, care partners were asked to complete the STAI-6 (Marteau & Bekker, 1992), a shorter version of the original 40-item STAI questionnaires (Spielberger et al., 1983) widely used by health researchers. The STAI-6 questionnaire assesses the following six components: “I feel calm,” “I am tense,” “I feel upset,” “I am relaxed,” “I feel content,” and “I am worried,” using a Likert-ranking scale ranging from 1 to 4 representing “not at all,” “somewhat,” “moderately,” and “very much,” in ascending order for each item. After transformation of reversed items, the total sum of responses results in a score range of 6–36. Using this score range, a cutoff point of 1 SD above the mean (mean: 10.19, SD: 3.88; Millar et al., 1995) was used to dichotomize the STAI-6 variable into a categorical binary variable representing two categories for levels of anxiety: “higher anxiety than average” or not. Respondents were excluded from analyses if they had missing values on depressive symptoms (N = 39) or anxiety (N = 28).
Scan results were categorized as either “positive” or “negative” for elevated amyloid-beta deposition in the brain. A negative scan suggests a reduced probability of developing AD and an elevated or positive scan suggests probable AD among those with dementia and increased likelihood of developing AD among those with MCI. While elevated scan results do not definitively confirm a diagnosis of AD or correlate with diagnostic measures for cognitive impairment, they are used as a strong clinical indicator of probable AD that may help revise treatment plans for patients with cognitive impairment of previously uncertain etiology (Blennow et al., 2015; Fleisher et al., 2011; Marcus et al., 2014). Seven care partners were excluded from the quantitative analysis due to missing or inconclusive scans.
To differentiate between preexisting levels of cognitive impairment among patients, we used patients’ diagnostic category as determined by a memory specialist at enrollment into the IDEAS study (i.e., MCI vs dementia), prior to receiving the PET scan (Rabinovici et al., 2019). The key distinction between the two categories relates to the level or severity of cognitive impairment. Persons described as living with MCI exhibit evidence of mild cognitive decline from previous levels in one or more cognitive domains, but their impairment does not interfere with their capacity for independence. Persons with dementia are described as experiencing a significant cognitive decline in one or more domains, with deficits interfering with independence in activities of daily life such as paying bills or managing medication (Albert et al., 2011; American Psychiatric Association, 2013; Jack et al., 2018; McKhann et al., 2011).
We controlled for care partners’ subjective reports of patient everyday cognitive function using the 12-item everyday cognition scale (E-COG-12; Tomaszewski Farias et al., 2011)—a shortened version of the original 39-item E-COG scale (Farias et al., 2008). Both versions of the E-COG scale assess the following six domains relevant to patients’ daily cognition and functioning as observed by their care partner: memory, language, visuospatial abilities, planning, organization, and divided attention (Farias et al., 2008). Care partners reported the person with cognitive impairment’s current performance on common daily activities in these domains compared to 10 years prior. Using a Likert-rating scale between “1 = better or no change” to “4 = consistently much worse” for 12 items, a score ranging from 12 to 48 was obtained. The total score was summed and divided by the number of items completed by the care partner to obtain an overall average score for E-COG-12 ranging from 1 to 4, with higher score indicating worsening impairment in everyday cognition. Finally, we adjusted for important sociodemographic variables that may be associated with the likelihood of exhibiting depressive symptoms and anxiety (García-Alberca et al., 2011; Haley et al., 2004; O’Rourke et al., 2010; Sallim et al., 2015; Watson et al., 2019). These variables included gender (dichotomous: male or female), age (categorical: below 65 years, 65–74 years, 75–84 years, and 85 years and older), race/ethnicity (categorical: non-Hispanic White, Hispanic White, non-Hispanic African American, and Other), and level of education (categorical: secondary or less, some college without degree, bachelor’s degree, and graduate degree). All responses with missing values for covariates (N = 37) were excluded from analyses.
Statistical Analyses
We first examined the levels of anxiety and depressive symptoms of the study sample through descriptive statistics. We then estimated logistic regression models to obtain unadjusted odds ratios (ORs) to understand the relationship between each of the independent variables and the odds of having high levels of anxious and depressive symptoms among care partners. We used multivariable logistic regression analyses to obtain adjusted ORs for the independent associations of scan result (elevated vs not-elevated) and preexisting diagnostic category (dementia vs MCI), controlling for important covariates including age, gender, race/ethnicity, education level, and E-COG-12 score. Finally, we fit an additional multivariable logistic regression model to test an interaction term between amyloid PET scan results and preexisting dementia diagnostic category, which could affect the odds of increased levels of anxious and depressive symptoms among care partners based on care partner expectations of scan results. Following significant interaction results, the same models were estimated among stratified samples of care partners of patients with MCI and dementia to obtain an effect size in each group. Statistical analyses were performed using Stata (StataCorp, 2017).
Results
Qualitative Results
The qualitative subsample comprised 196 care partners (see Table 1), a majority of whom were female, married or living with a domestic partner, and non-Hispanic White. Approximately 85% were above the age of 65, and they were overall highly educated: 34.7% had completed graduate education. They were caring for persons with cognitive impairment with an average E-COG-12 score of 2.3, indicating worsening dependency. At the time of recruitment to the IDEAS study, 24% of participants had dementia.
Table 1.
Descriptive Characteristics of Care Partners in the CARE IDEAS Study
| Characteristic | Qualitative subsample (n = 196) | Quantitative analytical sample (N = 1,761) | ||||
|---|---|---|---|---|---|---|
| n | % | M (SD) | n | % | M (SD) | |
| Age | ||||||
| Below 65 | 30 | 15.3 | 339 | 19.2 | ||
| 65–74 | 93 | 47.4 | 829 | 47.1 | ||
| 75–84 | 66 | 33.7 | 542 | 30.8 | ||
| 85 and older | NRa | 51 | 2.9 | |||
| Gender | ||||||
| Male | 62 | 31.6 | 568 | 32.3 | ||
| Female | 134 | 68.4 | 1193 | 67.7 | ||
| Race/ethnicity | ||||||
| White, non-Hispanic | 186 | 94.9 | 1,655 | 94.0 | ||
| Marital status | ||||||
| Married/domestic partner | 186 | 94.9 | 1,670 | 94.8 | ||
| Education | ||||||
| High school or less | 29 | 14.8 | 25 | 14.4 | ||
| Some college | 55 | 28.1 | 491 | 27.9 | ||
| College degree | 44 | 22.5 | 481 | 27.3 | ||
| Graduate degree | 68 | 34.7 | 536 | 30.4 | ||
| Everyday cognitive function 0–4 | 2.3 (0.7) | 2.2 (0.7) | ||||
| Diagnostic category of person with cognitive impairment | ||||||
| Mild cognitive impairment | 149 | 76.0 | 1,292 | 73.4 | ||
| Dementia | 47 | 24.0 | 469 | 26.6 | ||
| Scan results of person with cognitive impairmentb | ||||||
| Positive: elevated amyloid | 131 | 66.8 | 1,209 | 68.7 | ||
| Negative: not-elevated amyloid | 64 | 32.7 | 552 | 31.4 |
Notes: Percentages may not add to 100% due to missing values. CARE IDEAS Study = Caregivers’ Reactions and Experience, a supplemental study of the Imaging Dementia Evidence for Amyloid Scanning study; SD = standard deviation.
aNot reported because of small cell numbers.
bOne or more scans were inconclusive.
As demonstrated in Table 2, the qualitative data suggest that care partners experienced more difficult emotions when the scan results did not align with their expectations, particularly when a person with mild impairment showed elevated amyloid plaque. Such participants described the scan results as “shocking,” “distressing,” and “devastating,” when learning that the person they were caring for had elevated amyloid plaque, especially when they attributed memory issues to other causes, such as normal aging:
Table 2.
Care Partners’ Emotional Reactions Stratified by Level of Impairment and Scan Results
| Level of impairment | Elevated amyloid | Not-elevated amyloid |
|---|---|---|
| Mild cognitive impairment | “Getting the results, I mean it shocked us … In a way, I think it shocked me more, maybe, than him. I don’t know if he thought about maybe he was going down that road or not. I think he’d mentioned it a couple times, ‘Well, maybe that’s what I have.’ But it seems like it hit me more than him.” “The tests were positive for Alzheimer’s, and the news was devastating.” “I can honestly say that when we received them, we weren’t shocked, because we were expecting it. So it wasn’t a shock. Did it upset us? I think maybe yeah, it does upset you a little bit, because you know what’s coming down the road.” |
“It relieved us, because we were really … Because of the memory loss, we were really afraid that he did have Alzheimer’s, so we were very relieved.” “Well it was negative. I’m not sure. Just knowing that that’s a relief, but looking at other things that might be causing similar issues.” “Well it gives a little more hope that it’s just more of age related, maybe dementia type things that means she won’t go down as fast, compared to maybe Alzheimer’s patients.” |
| Dementia | “Actually, he suspected it and told me that’s what he thought that it was. So when we got the confirmation, it wasn’t a big shock or anything.” “I’m certainly grateful that we’re on that … I’m not grateful we have the diagnosis but that the diagnosis was found sooner than later and that they can continue to treat him and do what we can with some ongoing … at this.” |
“I think the fact that he did not have Alzheimer’s was a big relief because he’s more frightened of Alzheimer’s than he is of arteriosclerosis of the vessels in his brain. So I don’t think the PET (positron emission tomography) scan has really changed his care.” “I don’t know that it’s actually influenced our plans. We still are gonna have to deal with dementia. It’s just that it’s most likely a blood flow situation or something.” |
Note: Bolding and italics added for emphasis.
It was shocking and surprising, because it was something we didn’t expect. We really expected it just to be nothing. Very surreal … I guess. Since there were no changes immediately over the last year, I mean there’s been small changes but nothing that … He’s going to be 81 so at 81 people forget things. (Care partner to person with MCI; elevated amyloid)
Participants reported their emotions toward receiving the scan results in different ways, describing being “very upset” upon learning the “difficult” news, and that it was “a blow to us both.”
Care partners who reported having considered the possibility of AD were less shocked by elevated scan results, even among those with MCI: “It wasn’t that difficult to receive it [scan results], because I had already told him what I suspected it was [laugh]” (care partner to person with MCI; elevated amyloid). Similarly, many care partners of persons with dementia expressed gratitude for the confirmation and ability to plan ahead, knowing that amyloid levels indicated probable AD.
You tend to want to believe that it might be something else, especially since there was no real definitive test up to then, to get a better handle on what might be going on. Now we feel at least that we have more knowledge of what we’re dealing with. (Care partner of person with dementia; elevated amyloid)
For care partners of persons with MCI, receiving news of not-elevated amyloid plaque was welcome news. Relief was a recurring emotion reported among these participants, and was never mentioned in our data by those whose scan results revealed elevated amyloid. “Well, it was very positive and uplifting to know that we’re not facing Alzheimer’s” (care partner of person with MCI; not-elevated amyloid). Not all care partners expressed relief at obtaining not-elevated scan results; many mentioned uncertainty and seeking other causes of progressive memory issues (see Table 2). While a not-elevated scan result provided hope for a perhaps less severe trajectory of decline in cognitive function, many care partners were candid about the likelihood of enduring memory impairment with an unpredictable course of illness:
Well, we will continue our life as we have, he may continue to have memory loss but it won’t be due to Alzheimer’s. There’s 78 types of memory impairment or dementia, so we will just take signs and symptoms as they occur and follow care as we can. It’s a little bit difficult. The PET scan isn’t really … I think in a positive sense you still can have some of the symptoms of Alzheimer’s, but it will not be as severe. (Care partner of person with MCI; not-elevated amyloid)
Quantitative Results
Of the total participants enrolled in the original IDEAS study, 2,228 patients completed the baseline structured telephone interviews for the CARE IDEAS supplement. Of this sample, 1,872 care partners were eligible for analysis; 356 care partners did not complete an interview for various reasons. After excluding those with missing values for independent and dependent variables and all covariates selected for analysis, we restricted our final analytical sample to 94.1% of the eligible care partner population (N = 1,761). The quantitative analytical sample, as seen in Table 1, was very similar to the qualitative subsample.
In this sample, 7.8% of care partners had a score of 3 or above on the PHQ-2, indicating positive screening for depression. In our fully adjusted models (Table 3), the odds of a care partner having clinically significant depressive symptoms was 1.93 (95% confidence interval [CI]: 1.50, 2.49) times higher for each point increase on the patient’s E-COG-12 score. Neither amyloid scan results nor diagnostic category at the time of the scan were significantly associated with the likelihood of depressive symptoms. There was no statistically significant interaction either between scan results or diagnostic category and care partner depressive symptoms (p = .997).
Table 3.
Odds Ratios (ORs) of Depressive Symptoms Among Care Partners (N = 1,761)
| Measure | Unadjusted models | Adjusted modela |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Everyday cognitive function (0–4) | 1.85 (1.46, 2.34) | 1.93 (1.50, 2.49) |
| Diagnostic category of person with cognitive impairment | ||
| MCI | Ref. (—) | Ref. (—) |
| Dementia | 1.02 (0.69, 1.51) | 0.75 (0.49, 1.14) |
| Scan results of person with cognitive impairment | ||
| Elevated amyloid | 1.08 (0.73, 1.57) | 0.99 (0.67, 1.47) |
| Not-elevated amyloid | Ref. (—) | Ref. (—) |
Notes: CI = confidence interval; MCI = mild cognitive impairment.
aAdjusted for age, gender, race/ethnicity, and education.
A total of 19.4% of care partners had higher levels of anxiety than the sample average on their STAI-6 assessment. As can be seen in Table 4, across patients with MCI and dementia, care partners of persons with elevated amyloid PET scan results had an average of 1.46 (95% CI: 1.10, 1.95) times the odds of high anxiety compared to those caring for individuals without elevated scans. After adjusting for relevant covariates there was also a strong, statistically significant relationship between higher anxiety and more severe E-COG-12 score, with a one-point increase in impairment being associated with over twice the odds of having high anxiety levels. There was also a significant interaction between diagnostic category and amyloid scan results (p = .007), demonstrating a different relationship between anxiety and elevated amyloid PET scans among care partners of persons with dementia. Stratified analyses indicated that among care partners of persons with MCI, an elevated PET scan was associated with significantly higher odds of high anxiety (OR: 1.83; 95% CI: 1.29, 2.58), while this was not the case among care partners of persons with dementia. A strong association remained between more severe cognitive impairment (E-COG-12) and high anxiety among our samples of care partners of persons with MCI (OR: 2.29, 95% CI: 1.83, 2.86) and dementia (OR: 1.86, 95% CI: 1.34, 2.57).
Table 4.
Odds Ratios (ORs) of High Anxiety Among Care Partners
| Measure | Full sample N = 1,761 |
Stratified analyses | ||
|---|---|---|---|---|
| Unadjusted model | Adjusted modela | Adjusted modela MCI: N = 1,292 |
Adjusted modela Dementia: N = 469 |
|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Everyday cognitive function (0–4) | 2.20 (1.86, 2.60) | 2.10 (1.76, 2.52) | 2.29 (1.83, 2.86) | 1.86 (1.34, 2.57) |
| Diagnostic category of person with cognitive impairment | ||||
| MCI | Ref. (—) | Ref. (—) | N/A | N/A |
| Dementia | 1.43 (1.11, 1.85) | 0.98 (0.74, 1.30) | N/A | N/A |
| Scan results of person with cognitive impairment | ||||
| Elevated amyloid | 1.53 (1.17, 2.01) | 1.46 (1.10, 1.95) | 1.83 (1.29, 2.58) | 0.77 (0.46, 1.31) |
| Not-elevated amyloid | Ref. (—) | Ref. (—) | Ref. (—) | Ref. (—) |
Notes: CI = confidence interval; MCI = mild cognitive impairment.
aAdjusted for age, gender, race/ethnicity, and education.
Discussion and Implications
In this study, we explored care partners’ reactions to their loved ones receiving amyloid PET scan results. Our qualitative results demonstrated that care partners not expecting elevated amyloid levels reported negative emotions, including shock and distress. These emotions were more commonly mentioned by care partners of persons with MCI than those caring for persons with dementia at the time of the scan. The quantitative results confirmed the hypothesis derived from qualitative findings, whereby elevated amyloid PET scan results were associated with higher levels of anxiety among care partners of persons with MCI, even after controlling for covariates. As anxiety was coded as a distributional cutoff, our categorization of “higher anxiety” is specific to this sample. Importantly, patients’ worsening impairment in everyday cognitive function remained strongly associated with both depressive symptoms and anxiety levels among care partners, even in fully adjusted models.
Prior to this work, little was known about the experiences of care partners of persons with cognitive impairment receiving PET scan results. One study investigated the preferences of individuals with and without cognitive impairment and their care partners for receiving PET scan results, but did not offer PET scans. Participants indicated that they would prefer the results to be presented as a risk assessment, rather than a diagnosis, and that psychosocial support be available after disclosure (Milne et al., 2018). These results resonate with another qualitative study of 29 caregivers about the disclosure of the diagnosis of dementia itself, with participants preferring progressive disclosure, specifically mentioning the possibility of AD or dementia early on in the diagnostic process, to allow patients and families time to process and prepare (Byszewski et al., 2007).
A growing body of literature has been examining the relationship between the disclosure of elevated amyloid levels and the anxious and depressive symptoms of care partners to persons with cognitive impairment. An RCT of 82 dyads of persons with cognitive impairment and their caregivers found no change in depressive or anxious symptoms over time as a factor of receiving amyloid PET scan results (Lingler et al., 2020). However, caregivers to persons with elevated amyloid had significantly higher negative reactions to the scan results, as indicated by standard measures of distress developed for AD genetic screening. Conversely, Bensaïdane and colleagues (2016) found that disclosure of amyloid scan results to a sample of 28 patients with atypical dementia syndrome improved caregiver outcomes, including anxiety and depression, regardless of PET scan result. Another small qualitative investigation found that care partners experienced similar emotional reactions to those expressed in our results. Grill and colleagues (2017) found that eight caregivers of 20 dyads with varying levels of cognitive impairment who learned of elevated amyloid levels expressed sadness or despair, although they were not assessed for depressive or anxiety symptoms. Findings from studies about other AD biomarkers, such as apolipoprotein E4 genotyping, also suggest that persons with more emotional distress before undergoing screening were at increased risk of exhibiting increased depressive and anxious symptoms after results disclosure (Green et al., 2009).
This study had several limitations. We did not conduct an RCT and the absence of a control condition limits our ability to isolate the impact of communicating amyloid scan results on care partner emotional well-being. We also did not measure anxiety or depressive symptoms in care partners prior to the person with cognitive impairment receiving an amyloid PET scan; thus, we are unable to determine whether the scan results increased care partners’ anxious and depressive symptoms or that symptoms differed from baseline. Longitudinal examination of emotional well-being among care partners of patients who receive early amyloid PET scan results is also warranted to determine whether anxious symptoms persist. A prospective cohort of 181 nondepressed spousal care partners for persons with dementia in the Netherlands found that 60% of care partners developed a depressive and/or anxiety disorder over a 24-month follow-up period (Joling et al., 2015), indicating that mental health symptoms develop over time in this population. The aforementioned RCT that provided amyloid scan results to 82 dyads of caregivers and persons with cognitive impairment also confirmed that caregivers developed higher levels of anxiety over time regardless of amyloid PET scan results (Lingler et al., 2020). It is also possible that individuals who were more concerned or anxious about their health were more likely to seek care in a specialized memory clinic and may be more represented in our sample. Our sample contained fewer care partners of persons with dementia than care partners of persons with MCI, which could have reduced our statistical power to detect a significant association between elevated amyloid plaque and anxiety levels in the former group. Our sample was also more highly educated and less racially/ethnically diverse than the overall U.S. population, which may limit the generalizability of our results. Finally, we lacked information about how the amyloid PET scan results were communicated to persons with cognitive impairment and their care partners as part of routine clinical care during the IDEAS parent study, which represents an important area of future research and potential intervention.
Care partners’ reactions to receiving the amyloid PET scan results of a loved one with cognitive impairment had yet to be fully investigated in a larger sample prior to our work, and our results therefore have important implications for research and practice. Care partners who did not expect an elevated amyloid result, particularly those caring for persons with limited cognitive impairment, experienced shock and distress, and anticipating this reaction may help to address the high level of anxiety observed in this population. Amyloid PET scans may become more widely administered as a diagnostic tool to determine eligibility for new pharmacological treatments among persons with memory impairment, increasing the relevance of our results. Moreover, better understanding the potential reactions of care partners can prepare clinicians to fully support care partners throughout the diagnostic process and beyond and should inform referral to appropriate resources (Lingler et al., 2020; Milne et al., 2018). Future studies should explore the impact of supportive interventions to prepare care partners for the possible results of amyloid PET scans. Additionally, clinical practice should prioritize counseling and referrals for care partners who express shock and distress upon learning loved ones’ results to address the high levels of anxiety in this population. Care partners are a vital component of the care team for persons with cognitive impairment, and the emotional toll of caregiving should not be ignored.
Supplementary Material
Contributor Information
Emmanuelle Bélanger, Center for Gerontology and Healthcare Research, Brown University, School of Public Health, Providence, Rhode Island, USA; Department of Health Services, Policy & Practice, Brown University, School of Public Health, Providence, Rhode Island, USA.
Jessica D’Silva, Center for Gerontology and Healthcare Research, Brown University, School of Public Health, Providence, Rhode Island, USA.
Michaela S Carroll, Center for Gerontology and Healthcare Research, Brown University, School of Public Health, Providence, Rhode Island, USA.
Courtney H Van Houtven, Department of Population Health Sciences, Duke University School of Medicine, Durham, North Carolina, USA; Center of Innovation to Accelerate Discovery and Practice Transformation, Durham VA Health Care System, Durham, North Carolina, USA.
Megan Shepherd-Banigan, Department of Population Health Sciences, Duke University School of Medicine, Durham, North Carolina, USA; Center of Innovation to Accelerate Discovery and Practice Transformation, Durham VA Health Care System, Durham, North Carolina, USA.
Valerie A Smith, Department of Population Health Sciences, Duke University School of Medicine, Durham, North Carolina, USA; Center of Innovation to Accelerate Discovery and Practice Transformation, Durham VA Health Care System, Durham, North Carolina, USA.
Terrie T Wetle, Center for Gerontology and Healthcare Research, Brown University, School of Public Health, Providence, Rhode Island, USA; Department of Health Services, Policy & Practice, Brown University, School of Public Health, Providence, Rhode Island, USA.
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (R01AG053934) and by the American College of Radiology Imaging Network and the Alzheimer’s Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American College of Radiology Imaging, or the Alzheimer’s Association.
Conflict of Interest
None declared.
Data Availability
Resources pertaining to this work are accessible on the Brown Digital Repository (https://doi.org/10.26300/c2v6-3v57), which includes a CARE IDEAS codebook, and a PDF file with a description of the software used and syntax used for data cleaning and the final analytical models.
References
- 2021 Alzheimer’s disease facts and figures. (2021). Alzheimer’s & Dementia, 17(3), 327–406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., Gamst, A., Holtzman, D. M., Jagust, W. J., Petersen, R. C., Snyder, P. J., Carrillo, M. C., Thies, B., & Phelps, C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Publishing, Inc. doi: 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Bensaïdane, M. R., Beauregard, J.-M., Poulin, S., Buteau, F.-A., Guimond, J., Bergeron, D., Verret, L., Fortin, M.-P., Houde, M., Bouchard, R. W., Soucy, J.-P., & Laforce, R. (2016). Clinical utility of amyloid PET imaging in the differential diagnosis of atypical dementias and its impact on caregivers. Journal of Alzheimer’s Disease, 52(4), 1251–1262. doi: 10.3233/JAD-151180 [DOI] [PubMed] [Google Scholar]
- Black, B. S., Taylor, H. A., Rabins, P. V., & Karlawish, J. (2018). Study partners perform essential tasks in dementia research and can experience burdens and benefits in this role. Dementia, 17(4), 494–514. doi: 10.1177/1471301216648796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow, K., Mattsson, N., Schöll, M., Hansson, O., & Zetterberg, H. (2015). Amyloid biomarkers in Alzheimer’s disease. Trends in Pharmacological Sciences, 36(5), 297–309. doi: 10.1016/j.tips.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Brodaty, H., & Donkin, M. (2009). Family caregivers of people with dementia. Dialogues in Clinical Neuroscience, 11(2), 217–228. doi: 10.31887/dcns.2009.11.2/hbrodaty [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byszewski, A. M., Molnar, F. J., Aminzadeh, F., Eisner, M., Gardezi, F., & Bassett, R. (2007). Dementia diagnosis disclosure: A study of patient and caregiver perspectives. Alzheimer Disease and Associated Disorders, 21(2), 107–114. doi: 10.1097/WAD.0b013e318065c481 [DOI] [PubMed] [Google Scholar]
- Clipp, E. C., & George, L. K. (1993). Dementia and cancer: A comparison of spouse caregivers. The Gerontologist, 33(4), 534–541. doi: 10.1093/geront/33.4.534 [DOI] [PubMed] [Google Scholar]
- Cooper, C., Balamurali, T. B. S., & Livingston, G. (2007). A systematic review of the prevalence and covariates of anxiety in caregivers of people with dementia. International Psychogeriatrics, 19(2), 175–195. doi: 10.1017/S1041610206004297 [DOI] [PubMed] [Google Scholar]
- Cuijpers, P. (2005). Depressive disorders in caregivers of dementia patients: A systematic review. Aging & Mental Health, 9(4), 325–330. doi: 10.1080/13607860500090078 [DOI] [PubMed] [Google Scholar]
- Farias, S. T., Mungas, D., Reed, B. R., Cahn-Weiner, D., Jagust, W., Baynes, K., & Decarli, C. (2008). The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology, 22(4), 531–544. doi: 10.1037/0894-4105.22.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher, A. S., Chen, K., Liu, X., Roontiva, A., Thiyyagura, P., Ayutyanont, N., Joshi, A. D., Clark, C. M., Mintun, M. A., Pontecorvo, M. J., Doraiswamy, P. M., Johnson, K. A., Skovronsky, D. M., & Reiman, E. M. (2011). Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer’s disease. Archives of Neurology, 68(11), 1404–1411. doi: 10.1001/archneurol.2011.150 [DOI] [PubMed] [Google Scholar]
- Gallo, J. J., & Lebowitz, B. D. (1999). The epidemiology of common late-life mental disorders in the community: Themes for the new century. Psychiatric Services, 50(9), 1158–1166. doi: 10.1176/ps.50.9.1158 [DOI] [PubMed] [Google Scholar]
- García-Alberca, J. M., Lara, J. P., & Berthier, M. L. (2011). Anxiety and depression in caregivers are associated with patient and caregiver characteristics in Alzheimer’s disease. International Journal of Psychiatry in Medicine, 41(1), 57–69. doi: 10.2190/PM.41.1.f [DOI] [PubMed] [Google Scholar]
- Green, R. C., Roberts, J. S., Cupples, L. A., Relkin, N. R., Whitehouse, P. J., Brown, T., Eckert, S. L., Butson, M., Sadovnick, A. D., Quaid, K. A., Chen, C., Cook-Deegan, R., Farrer, L. A., R. E., & VEAL Study Group (2009). Disclosure of APOE genotype for risk of Alzheimer’s disease. The New England Journal of Medicine, 361(3), 245–254. doi: 10.1056/NEJMoa0809578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, J. D., Cox, C. G., Kremen, S., Mendez, M. F., Teng, E., Shapira, J., Ringman, J. M., & Apostolova, L. G. (2017). Patient and caregiver reactions to clinical amyloid imaging. Alzheimer’s & dementia: the journal of the Alzheimer’s Association, 13(8), 924–932. doi: 10.1016/j.jalz.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundman, M., Pontecorvo, M. J., Salloway, S. P., Doraiswamy, P. M., Fleisher, A. S., Sadowsky, C. H., Nair, A. K., Siderowf, A., Lu, M., Arora, A. K., Agbulos, A., Flitter, M. L., Krautkramer, M. J., Sarsour, K., Skovronsky, D. M., Mintun, M. A., & 45-A17 Study Group. (2013). Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Disease and Associated Disorders, 27(1), 4–15. doi: 10.1097/WAD.0b013e318279d02a [DOI] [PubMed] [Google Scholar]
- Haley, W. E., Gitlin, L. N., Wisniewski, S. R., Mahoney, D. F., Coon, D. W., Winter, L., Corcoran, M., Schinfeld, S., & Ory, M. (2004). Well-being, appraisal, and coping in African-American and Caucasian dementia caregivers: Findings from the REACH study. Aging & Mental Health, 8(4), 316–329. doi: 10.1080/13607860410001728998 [DOI] [PubMed] [Google Scholar]
- Hsieh, H.-F., & Shannon, S. E. (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. doi: 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., Holtzman, D. M., Jagust, W., Jessen, F., Karlawish, J., Liu, E., Molinuevo, J. L., Montine, T., Phelps, C., Rankin, K. P., Rowe, C. C., Scheltens, P., Siemers, E., Snyder, H. M., … Silverberg, N. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia, 14(4), 535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, H. J., Van Houtven, C. H., Lippmann, S., Burke, J. R., Shepherd-Banigan, M., Belanger, E., Wetle, T. F., & Plassman, B. L. (2020). How accurately do patients and their care partners report results of amyloid-β PET scans for Alzheimer’s disease assessment? Journal of Alzheimer’s Disease, 74(2), 625–636. doi: 10.3233/JAD-190922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joling, K. J., van Marwijk, H. W. J., Veldhuijzen, A. E., van der Horst, H. E., Scheltens, P., Smit, F., & van Hout, H. P. J. (2015). The two-year incidence of depression and anxiety disorders in spousal caregivers of persons with dementia: Who is at the greatest risk? The American Journal of Geriatric Psychiatry, 23(3), 293–303. doi: 10.1016/j.jagp.2014.05.005 [DOI] [PubMed] [Google Scholar]
- Jutkowitz, E., Van Houtven, C. H., Plassman, B. L., & Mor, V. (2020). Willingness to undergo a risky treatment to improve cognition among persons with cognitive impairment who received an amyloid PET scan. Alzheimer Disease and Associated Disorders, 34(1), 1–9. doi: 10.1097/WAD.0000000000000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K., Spitzer, R. L., & Williams, J. B. W. (2003). The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care, 41(11), 1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- Largent, E. A., Abera, M., Harkins, K., Feldman, S. J., Uhlmann, W. R., Roberts, J. S., Karlawish, J., R. E., & VEAL-SCAN Team (2021). Family members’ perspectives on learning cognitively unimpaired older adults’ amyloid-β PET scan results. Journal of the American Geriatrics Society, 69(11), 3203–3211. doi: 10.1111/jgs.17362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent, E. A., & Karlawish, J. (2019). Preclinical Alzheimer disease and the dawn of the pre-caregiver. JAMA Neurology, 76(6), 631–632. doi: 10.1001/jamaneurol.2019.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, Y. Y., Maruff, P., Getter, C., & Snyder, P. J. (2016). Disclosure of positron emission tomography amyloid imaging results: A preliminary study of safety and tolerability. Alzheimer’s & Dementia, 12(4), 454–458. doi: 10.1016/j.jalz.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Lingler, J. H., Sereika, S. M., Butters, M. A., Cohen, A. D., Klunk, W. E., Knox, M. L., McDade, E., Nadkarni, N. K., Roberts, J. S., Tamres, L. K., & Lopez, O. L. (2020). A randomized controlled trial of amyloid positron emission tomography results disclosure in mild cognitive impairment. Alzheimer’s & Dementia, 16(9), 1330–1337. doi: 10.1002/alz.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, M., Dorstyn, D., Ward, L., & Prentice, S. (2018). Alzheimers’ disease and caregiving: A meta-analytic review comparing the mental health of primary carers to controls. Aging & Mental Health, 22(11), 1395–1405. doi: 10.1080/13607863.2017.1370689 [DOI] [PubMed] [Google Scholar]
- Marcus, C., Mena, E., & Subramaniam, R. M. (2014). Brain PET in the diagnosis of Alzheimer’s disease. Clinical Nuclear Medicine, 39(10), e413–e422; quiz e423–e426. doi: 10.1097/RLU.0000000000000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau, T. M., & Bekker, H. (1992). The development of a six-item short-form of the state scale of the Spielberger State–Trait Anxiety Inventory (STAI). The British Journal of Clinical Psychology, 31(3), 301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R.Kawas, C. H., Klunk, W. E., Koroshetz, W. J., Manly, J. J., Mayeux, R., Mohs, R. C., Morris, J. C., Rossor, M. N., Scheltens, P., Carrillo, M. C., Thies, B., Weintraub, S., & Phelps, C. H. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, K., Jelicic, M., Bonke, B., & Asbury, A. J. (1995). Assessment of preoperative anxiety: Comparison of measures in patients awaiting surgery for breast cancer. British Journal of Anaesthesia, 74(2), 180–183. doi: 10.1093/bja/74.2.180 [DOI] [PubMed] [Google Scholar]
- Milne, R., Bunnik, E., Diaz, A., Richard, E., Badger, S., Gove, D., Georges, J., Fauria, K., Molinuevo, J.-L., Wells, K., Ritchie, C., & Brayne, C. (2018). Perspectives on communicating biomarker-based assessments of Alzheimer’s disease to cognitively healthy individuals. Journal of Alzheimer’s Disease, 62(2), 487–498. doi: 10.3233/JAD-170813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke, N., Kupferschmidt, A. L., Claxton, A., Smith, J. Z., Chappell, N., & Beattie, B. L. (2010). Psychological resilience predicts depressive symptoms among spouses of persons with Alzheimer disease over time. Aging & Mental Health, 14(8), 984–993. doi: 10.1080/13607863.2010.501063 [DOI] [PubMed] [Google Scholar]
- Pinquart, M., & Sörensen, S. (2003). Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging, 18(2), 250–267. doi: 10.1037/0882-7974.18.2.250 [DOI] [PubMed] [Google Scholar]
- Rabinovici, G. D., Gatsonis, C., Apgar, C., Chaudhary, K., Gareen, I., Hanna, L., Hendrix, J., Hillner, B. E., Olson, C., Lesman-Segev, O. H., Romanoff, J., Siegel, B. A., Whitmer, R. A., & Carrillo, M. C. (2019). Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. Journal of American Medical Association, 321(13), 1286–1294. doi: 10.1001/jama.2019.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallim, A. B., Sayampanathan, A. A., Cuttilan, A., & Ho, R. (2015). Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. Journal of the American Medical Directors Association, 16(12), 1034–1041. doi: 10.1016/j.jamda.2015.09.007 [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., & Jacobs, G. A. (1983). Manual for the State–Trait Anxiety Inventory. Consulting Psychologists Press. [Google Scholar]
- StataCorp. (2017). Stata statistical software (Version Release 15) [Computer software]. StataCorp LP. [Google Scholar]
- Taswell, C., Donohue, C., Mastwyk, M., Louey, A. G., Giummarra, J., Robertson, J., Darby, D. G., Masters, C. L., & Rowe, C. C. (2018). Safety of disclosing amyloid imaging results to MCI and AD patients. Mental Health in Family Medicine, 14, 748–756. doi: 10.1016/j.jagp.2017.01.147 [DOI] [Google Scholar]
- Tomaszewski Farias, S., Mungas, D., Harvey, D. J., Simmons, A., Reed, B. R., & Decarli, C. (2011). The measurement of everyday cognition: Development and validation of a short form of the everyday cognition scales. Alzheimer’s & Dementia, 7(6), 593–601. doi: 10.1016/j.jalz.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, B., Tatangelo, G., & McCabe, M. (2019). Depression and anxiety among partner and offspring carers of people with dementia: A systematic review. The Gerontologist, 59(5), e597–e610. doi: 10.1093/geront/gny049 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Resources pertaining to this work are accessible on the Brown Digital Repository (https://doi.org/10.26300/c2v6-3v57), which includes a CARE IDEAS codebook, and a PDF file with a description of the software used and syntax used for data cleaning and the final analytical models.