Abstract
Pulmonary vein isolation (PVI) is the cornerstone of atrial fibrillation ablation. Radiofrequency ablation has been the most common source of energy used to achieve PVI until now. In recent years, cryoballoon ablation has gained popularity due to its ability to perform PVI in a ‘single-shot’ fashion. In both cases (radiofrequency and cryoablation), the main limitation is their inability to achieve durable lesions without causing collateral damage to adjacent structures. In contrast, pulsed electric field (PEF) ablation is a non-thermal energy source that causes cell apoptosis by applying an electric current to the tissue. Lesions created by a field of energy seem to be more contiguous than traditional ablation, and the risk of damage to adjacent tissues is largely avoided due to the properties of the tissues and electrical fields. In recent years, new catheters capable of delivering PEF have been developed and are now undergoing clinical testing. In this article, we describe a complete solution for PVI: a single multielectrode catheter with 3D mapping capabilities that can deliver PEF in a single-shot PVI fashion with targeting beyond the pulmonary veins.
Keywords: Atrial fibrillation, atrial fibrillation ablation, electroporation, globe pulsed field system, novel technologies, pulsed field ablation
Catheter ablation has emerged as an effective strategy for the treatment of atrial fibrillation (AF), even becoming a first-line option for selected patients.1 Pulmonary vein isolation (PVI) is the cornerstone of this procedure. Ablation beyond PVI is often practised, but scientific evidence from randomized clinical trials is still controversial.2–8
Point-by-point radiofrequency (RF) ablation has been, by far, the most common source of energy used to achieve PVI to date. In the past 15 years, cryoablation has gained popularity among operators mainly because of its ability to perform PVI in a ‘single shot’ using a balloon, thus removing the technical skills required for point-by-point RF ablation.9–11 Cryoballoon is faster but limited to the vein antra, whereas RF is time consuming but allows for patient-tailored ablation beyond PVI. Both energy sources appear equally effective, with clinical trials showing no difference in outcome.9,12 Both technologies can also cause collateral damage to adjacent structures,12 with phrenic nerve and oesophageal injury occurring in up to 5.0–10.0% and 0.1–0.5% of patients, respectively.13,14
Electroporation or pulsed electric field (PEF) ablation is a non-thermal energy source that causes cell apoptosis by applying an electric current to the tissue, creating microscopic pores that destabilize cell membranes in seconds.15 One advantage is its ability to ablate cardiac tissue while avoiding damage to adjacent structures, such as the phrenic nerve or oesophagus.16 One possible explanation for these findings is that the ablation threshold of myocardium is lower than other tissues, such as the vascular smooth muscle or myelin; however, PEF does not destroy the extracellular matrix, allowing nerves and oesophageal tissue to regenerate rapidly.17–19
In recent years, several systems designed to deliver PEF have been developed and are now undergoing clinical testing. Some systems offer large-footprint ablation catheters, which can isolate veins rapidly but lack mapping. Others allow for point-by-point ablation with mapping but are not single-shot options.
In this article, we describe a novel technology that offers several potential advantages. It has a single, multielectrode catheter with 3D mapping capabilities and is able to deliver RF or PEF in a single-shot PVI fashion but is also able to target beyond the pulmonary veins.
The Globe® catheter: Mapping and ablation all in one
The Globe® Pulsed Field Mapping and Ablation System (Kardium Inc., Burnaby, BC, Canada) is a 30 mm array consisting of 16 ribs with a total of 122 electrodes that can map intracardiac electrograms, pace, measure tissue contact, measure temperature and deliver RF energy. The latest generation of the Globe catheter also allows for the delivery of PEF ablation.
Each rib has between seven and nine electrodes, the sizes of which range from 9.0 to 13.0 mm2. The interelectrode distance along the ribs is only 0.8 mm, with 1.3–1.8 mm between the ribs (Figure 1).
Figure 1: A: The Globe® array with 16 ribs and 122 electrodes; B: The Globe array at the antrum of each pulmonary vein.
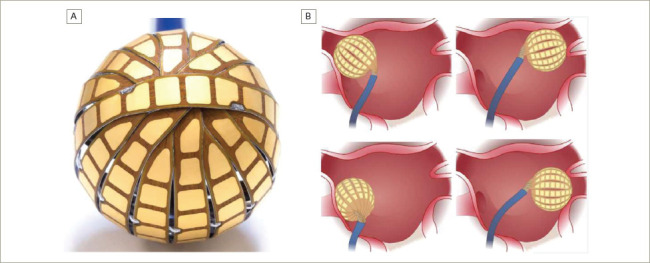
Figure from Kottkamp et al.20
Each electrode can even be individualized to target temperature so that lower temperatures may be targeted in sensitive regions, such as the posterior wall. The temperature sensor is positioned 0.025 mm directly behind each electrode.20,21
The catheter can be inserted into the left atrium through a specifically designed deflectable sheath (23 Fr in its old version, now reduced to 19 Fr). In the left atrium, the coiled ribs fan out to form a spherical array with a diameter of 30 mm (Figure 1).
When delivering RF, each electrode can be individually activated and combined with other electrodes (up to 24 at a time) to deliver RF simultaneously with an energy of 5–10 W, allowing for single-shot PVI, given the large size of the array.
Anatomical 3D mapping is performed by the array using specifically designed software called the Globe Positioning System (Figure 2). Three pairs of electrodes are applied externally to the patient, and low-magnitude electrical fields are generated between each electrode pair, creating an electrical gradient between them. These fields are measured at the 122 electrodes on the catheter and at the electrodes on a reference coronary sinus catheter. By combining these measurements with the known 3D shape of the array, the system calculates the position of the catheter with a high degree of accuracy.20,21
Figure 2: The Globe® Positioning System, 3D, high-definition map with the Globe positioned in the target areas for ablation.
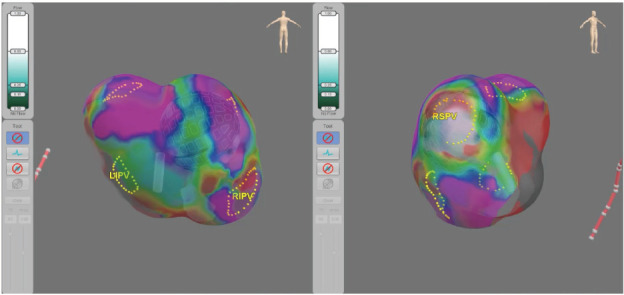
LIPV = left inferior pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
The electrical information obtained from the 122 electrodes is also used to create many different types of electroanatomic maps, including local activation time mapping, voltage mapping and other standard maps. However, the system offers a few unique mapping solutions, as described below (Figure 3 and Figure 4).
Figure 3: The Globe® catheter with the FLOW™ and CONTACT™ maps (white = no contact for both maps). Below, the Globe positioned in the target vein. The red line indicates the electrograms chosen for ablation.
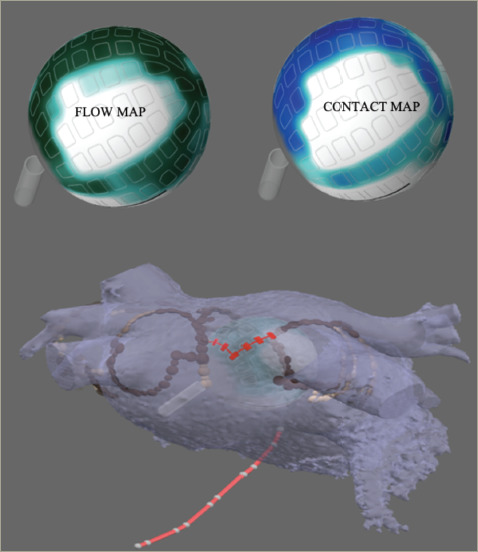
Figure from Kottkamp et al.20
Figure 4: FLOW™ and voltage maps before and after isolation of the left superior pulmonary vein. The FLOW™ map shows no contact in blue and good contact in green. In the voltage map, a black line is drawn to isolate the vein in a single shot. The blue electrograms are outside the ablation line, whereas the red electrograms are inside. In the right panel, we can see how the red electrograms disappear after the radiofrequency ablation, in contrast to the blue ones.
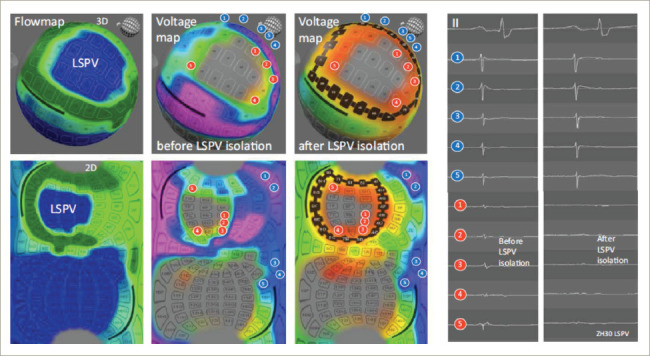
Figure from Kottkamp et al.20
LSPV = left superior pulmonary vein.
The FLOW™ anatomical map
Using the temperature sensor of each of the 122 electrodes, the FLOW™ anatomical map gives us information about the electrode contact with the atrial wall. The contact between the tissue and the electrodes is determined by measuring the convective cooling by blood. A current is applied to the electrode sensor and the rate of cooling measured indicates whether the electrode is in contact or instead is being cooled by the blood. With this information, a map where green indicates good contact and blue no contact is displayed. This helps the operator to select the electrodes that are best suited for ablation at a specific position (Figure 3 and Figure 4).
The CONTACT™ map
The CONTACT™ map is displayed using the information obtained by the temperature sensors. A current is applied to the electrode sensors, but, in this case, the Globe system measures the rate of heating. If heating occurs quickly, the catheter is likely to be in contact as opposed to an electrode that heats slowly because of blood flow. Both maps, FLOW and CONTACT, can be used to obtain information about the electrode contact with the left atrial wall (Figure 3 and Figure 4).
The voltage map
The Globe system can also create a voltage map using the electrogram amplitude, which, unlike the voltage maps generated by other systems, is continuously updated as ablation proceeds (Figure 4 and Figure 5). Therefore, one can visualize ‘real-time’ electrogram abatement.
Figure 5: Voltage map and electrograms before and after the isolation of the right superior pulmonary vein. The voltage map automatically shows the amplitude of the intracardiac electrograms over a portion of the cardiac cycle, with purple indicating high voltage, red low or no voltage and grey no contact. We can see how the electrograms disappear after pulsed electrical field ablation.
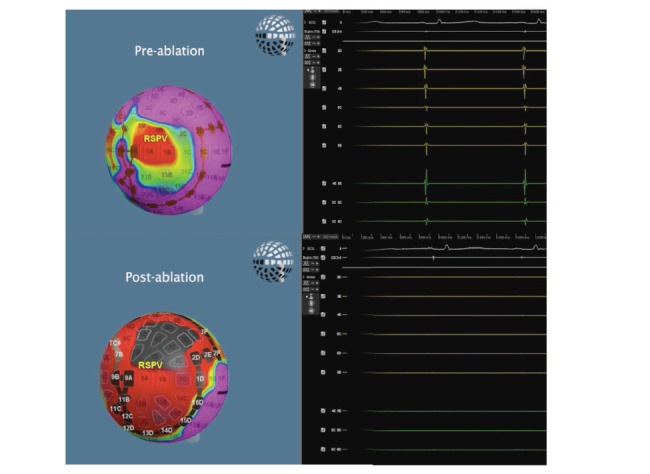
RSPV = right superior pulmonary vein.
The WAVE™ map
The WAVE™ map shows real-time propagation of the electrical activity across the atrium. It is created by measuring the voltage amplitude at each electrode and is continuously updated, thus allowing isolation to be determined in real time during ablation.
Once the catheter is positioned in one of the pulmonary veins, the operator can use the CONTACT map and the FLOW map to select the electrodes that are in contact with the tissue. An isolation line is drawn around the pulmonary vein; then, the RF is delivered through each of the electrodes selected (Figure 4). PVI can be confirmed by the WAVE map, which monitors the voltage map changes, and by the loss of the pulmonary vein electrograms.
When delivering RF, the system uses non-irrigated temperature-controlled ablation. The electrodes are covered with a heparinized coating to avoid char formation. The temperature is controlled independently on each electrode, thus allowing ablation time to be set individually. A temperature setpoint is selected for each electrode, and the system then automatically controls the amount of power delivered to each electrode to maintain the setpoint temperature of individual electrodes. The system can use up to 24 electrodes simultaneously with an energy of 5–10 W, allowing a wide footprint for PVI isolation. The design of the catheter also allows other triggers beyond the pulmonary vein, such as the posterior wall, to be targeted.
Preclinical and clinical data with the Globe® radiofrequency catheter
Kottkamp et al. published the first preclinical data experience of PVI with the Globe catheter in eight canines.20 The device was deployed in the left atrium, and 19 pulmonary veins were targeted using FLOW mapping and fluoroscopy, 18 of which were successfully isolated. The authors reported excellent electrogram quality due to the posterior shielding of the electrodes to minimize far-field detection. Isolation was confirmed using the WAVE map, the voltage map, the intrapulmonary vein electrograms or demonstrating exit block by pacing from the electrodes. After the procedure, lesions were found to be contiguous and transmural, and there was no evidence of brain, kidney or heart embolism in any animal.20
Kottkamp et al. then published the first human experience, the Global AF study, in which the Globe catheter was used to perform PVI in 60 patients.21 Initially, the system could ablate using a maximum of 16 electrodes at a time; however, in the last 34 patients treated, 24 electrodes were used simultaneously, allowing for single-shot ablation. The temperature set in the initial group was between 57°C and 61°C, but later it was increased to 65°C. Of the 234 PVIs attempted, 99.1% (n=232) were isolated successfully.21 For the single-shot group, all of the 136 pulmonary veins were successfully isolated (100.0%). In this last group, the procedure time was 128.0 ± 31.0 minutes, the left atrium time was 87.0 ± 29.0 minutes and the ablation time was 25.8 ± 9.0 minutes.
In the cohort of single-shot ablation (n=34), freedom from AF at 12 months off antiarrhythmics was 75.5%. In the 18 patients treated with the lower temperature, freedom from AF/atrial tachycardia was only 39.0% (p=0.02).
In terms of complications, two patients had pericardial tamponade. One was device related (during the array insertion due to an over-advanced sheath), but the other occurred after the transseptal puncture.21
Ablation was stopped in 28 patients because of oesophageal temperature rise. In two patients with high oesophageal temperatures (41.2°C and 42.8°C, respectively), endoscopy revealed minor oesophageal erosions.21
The new Globe® generation: The Globe® Pulsed Field Mapping and Ablation System
Thanks to a new design, the Globe system now uses PEFs as its primary source of energy. The spherical shape of the Globe has been retained, but the catheter size has been reduced to 16 Fr, with a 19 Fr outside diameter sheath. The unique design of the spherical Globe catheter array combined with a specified ‘GlobePulse’ PEF waveform allows for the isolation of a pulmonary vein in only 3 seconds and with a single shot.
PEF can be delivered in multiple ways: in a unipolar (from the catheter tip to a return electrode on the skin of the patient) or bipolar fashion (between adjacent electrodes) and using a monophasic or biphasic waveform. The Globe pulsed field system delivers PEF as a bipolar and biphasic pulse train over about 3 seconds. The advantage of bipolar and biphasic delivery is that it can reduce the recruitment of skeletal muscle and minimize electrolytic microbubble formation, thus reducing the risk of twitching or movement of the patient and, potentially, the risk of silent cerebral embolism.
Is pulsed electrical field ablation the way to go?
Several characteristics make electroporation or PEF ablation an attractive alternative to conventional RF or cryoablation. Typically, electroporation is delivered in several trains, each consisting of several pulses, in a repetitive series. The width of each pulse is normally measured in nanoseconds or milliseconds, so one delivery of a train of pulses can be delivered in a fraction of a second, thus making the energy delivery ultrarapid.15,22
Moreover, as lesions are created by a field of energy, they seem to be more contiguous than traditional ablation lesions, although tissue depth may not be superior to RF. Furthermore, these electrical fields can reach the tissue even if the catheter has suboptimal contact force (although some contact is required).15,22
The most promising advantage of PEF ablation over conventional thermal ablation is its ability to reduce damage to collateral structures such as the oesophagus and phrenic nerve. One theory for this advantage is that cardiac tissue has a much lower threshold for electrical field damage than nerves or smooth muscle cells.17 However, another observation is that PEF kills tissues via apoptosis, so the extracellular matrix and vasculature are not destroyed. This allows tissues such as those of the oesophagus and myelinated nerves to regenerate very rapidly, even if damaged. Cardiac myocytes, however, are terminally differentiated and therefore do not regenerate.
These proposed advantages have been demonstrated in many preclinical evaluations.23–25 In the clinical realm, the PULSED AF pilot study demonstrated 100% PVI with no serious adverse events, including no change in oesophageal temperature or phrenic nerve injury in 38 patients using the Medtronic Pulsed Select Field Ablation System.26 In the IMPULSE (ClinicalTrials.gov identifier: NCT03700385) and PEFCAT (ClinicalTrials.gov identifier: NCT03714178) trials, the Farapulse Irreversible Electroporation System was used for the treatment of paroxysmal AF and cavotricuspid isthmus-dependent atrial flutter.27 Primary safety endpoints were achieved with no adverse events other than tamponade in one patient in the IMPULSE cohort.27 There was no oesophageal lesions or phrenic nerve damage.27 For persistent AF, the PersAFOn study (Feasibility study of the FARAPULSE endocardial multi-ablation system in the treatment of persistent atrial fibrillation; ClinicalTrials.gov identifier: NCT04170621) also tested the Farapulse PFA system in 25 patients with persistent AF who received PVI + posterior wall isolation + cavotricuspid isthmus.28 First-pass PVI was achieved in all patients (100%, 96/96 pulmonary veins), with a median of 22 minutes elapsing between the first and last ablations. Invasive remapping performed in 22 patients at 82 days (interquartile range 76–90 days) confirmed PVI durability in 82 of 85 pulmonary veins (96%) and 19 of 22 patients (86%). There were no instances of pulmonary vein stenosis, stroke or transient ischaemic attack, phrenic nerve injury or atrio-oesophageal fistula. Oesophagogastroduodenoscopy was performed in 21 patients at a median of 3 days (interquartile range 1–5 days) post-procedure, and no oesophageal injury was observed.
The Globe® pulsed field ablation system: Human experience
The first-in-human clinical experience with the Globe pulsed field system was recently presented in a non-randomized, single-centre trial.29 Eleven patients with paroxysmal and persistent AF underwent PVI, which resulted in 100% first-pass isolation (44/44 pulmonary veins), with no major acute complications. The mean procedure time was 88 ± 20 minutes, with a total PFA delivery time of 24 ± 5 seconds. Four patients underwent oesophagogastroduodenoscopy, and no lesions were reported.29
The potential advantage that the novel Kardium Globe catheter offers over the other technologies is that this single, multi-electrode contact mapping and ablation catheter combines the benefits of single-tip catheters with the simplicity of balloon catheters. It is able to isolate the veins in one shot within 3 seconds and also permits tailored ablation beyond the pulmonary veins, such as the posterior wall. These features, in addition to its mapping capabilities and the high-quality resolution of the intracardiac signals and electrograms, make the Globe pulsed field ablation system a potentially attractive solution for rapid and efficient PVI. Ultimately, future clinical studies will determine the efficacy and safety of the system, as well as the durability of PVI.
Conclusions
The Globe pulsed field ablation system offers a unique solution, combining mapping capabilities with the ability to deliver PEF ablation in a single-shot fashion to isolate the pulmonary veins. On-going studies will determine whether this technology has the potential to change AF ablation by making PVI procedures faster, simpler, safer and more efficient.
Funding Statement
Support: No funding was received in the publication of this article.
Footnotes
Review process: Double-blind peer review.
Compliance with ethics: This article involves a review of the literature and did not involve any studies with human or animal subjects performed by any of the authors.
Data availability: Data sharing is not applicable to this article as no datasets were generated or analysed during the writing of this article.
Authorship: The named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
References
- 1.Calkins H, Hindricks G, Cappato R. et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma A, Jiang CY, Betts TR. et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 3.Marrouche NF, Wilber D, Hindricks G. et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation. JAMA. 2014;311:498. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 4.Wilber DJ, Pappone C, Neuzil P. et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation. JAMA. 2010;303:333. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 5.Stabile G, Bertaglia E, Senatore G. et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: A prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J. 2006;27:216–21. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 6.Lee JM, Shim J, Park J. et al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol. 2019;5:1253–61. doi: 10.1016/j.jacep.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Thiyagarajah A, Kadhim K, Lau DH. et al. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2019;12:e007005. doi: 10.1161/CIRCEP.118.007005. [DOI] [PubMed] [Google Scholar]
- 8.Bai R, di Biase L, Mohanty P. et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2016;13:132–40. doi: 10.1016/j.hrthm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Kuck KH, Brugada J, Furnkranz A. et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–45. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 10.Andrade JG, Khairy P, Guerra PG. et al. Efficacy and safety of cryoballoon ablation for atrial fibrillation: A systematic review of published studies. Heart Rhythm. 2011;8:1444–51. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 11.Andrade JG. Cryoballoon ablation for pulmonary vein isolation. J Cardiovasc Electrophysiol. 2020;31:2128–35. doi: 10.1111/jce.14459. [DOI] [PubMed] [Google Scholar]
- 12.Andrade JG, Champagne J, Dubuc M. et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring. Circulation. 2019;140:1779–88. doi: 10.1161/CIRCULATIONAHA.119.042622. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh A, Patel NJ, Pant S. et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010. Circulation. 2013;128:2104–12. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 14.Mansour M, Lakkireddy D, Packer D. et al. Safety of catheter ablation of atrial fibrillation using fiber optic-based contact force sensing. Heart Rhythm. 2017;14:1631–6. doi: 10.1016/j.hrthm.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Verma A, Asivatham SJ, Deneke T. et al. Primer on pulsed electrical field ablation. Circ Arrhythm Electrophysiol. 2021;14:e010086. doi: 10.1161/CIRCEP.121.010086. [DOI] [PubMed] [Google Scholar]
- 16.Reddy VY, Koruth J, Jais P. et al. Ablation of atrial fibrillation with pulsed electric fields. JACC Clin Electrophysiol. 2018;4:987–95. doi: 10.1016/j.jacep.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez FD, Reddy VY, Viswanathan R. et al. Emerging technologies for pulmonary vein isolation. Circ Res. 2020;127:170–83. doi: 10.1161/CIRCRESAHA.120.316402. [DOI] [PubMed] [Google Scholar]
- 18.Koruth JS, Kuroki K, Kawamura I. et al. Pulsed field ablation versus radiofrequency ablation: Esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol. 2020;13:e008303. doi: 10.1161/CIRCEP.119.008303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Driel VJHM, Neven K, van Wessel H. et al. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015;12:1838–44. doi: 10.1016/j.hrthm.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Kottkamp H, Moser F, Rieger A. et al. Global multielectrode contact mapping plus ablation with a single catheter: Preclinical and preliminary experience in humans with atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28:1247–56. doi: 10.1111/jce.13310. [DOI] [PubMed] [Google Scholar]
- 21.Kottkamp H, Hindricks G, Ponisch C. et al. Global multielectrode contact-mapping plus ablation with a single catheter in patients with atrial fibrillation: Global AF study. J Cardiovasc Electrophysiol. 2019;30:2248–55. doi: 10.1111/jce.14172. [DOI] [PubMed] [Google Scholar]
- 22.Verma MS, Terricabras M, Verma A. The cutting edge of atrial fibrillation ablation. Arrhythm Electrophysiol Rev. 2021;10:101–7. doi: 10.15420/aer.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howard B, Haines DE, Verma A. et al. Characterization of phrenic nerve response to pulsed field ablation. Circ Arrhythm Electrophysiol. 2022;15:e010127. doi: 10.1161/CIRCEP.121.010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart MT, Haines DE, Verma A. et al. Intracardiac pulsed field ablation: Proof of feasibility in a chronic porcine model. Heart Rhythm. 2019;16:754–64. doi: 10.1016/j.hrthm.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Stewart MT, Haines DE, Miklavčič D. et al. Safety and chronic lesion characterization of pulsed field ablation in a porcine model. J Cardiovasc Electrophysiol. 2021;32:958–69. doi: 10.1111/jce.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma A, Boersma L, Haines DE. et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: The PULSED AF Pilot Trial. Circ Arrhythm Electrophysiol. 2022;15:e010168. doi: 10.1161/CIRCEP.121.010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy VY, Neuzil P, Koruth JS. et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–26. doi: 10.1016/j.jacc.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 28.A Safety and Feasibility Study of the FARAPULSE Endocardial Ablation System to Treat Paroxysmal Atrial Fibrillation. ClinicalTrials.govClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03714178 Identifier: NCT03714178. Available at: (accessed 13 December 2022)
- 29.Reddy VY, Koruth JS, Petru J. et al. First-in-human clinical experience of a “single-shot” map-and-ablate multielectrode spherical array pulsed field ablation catheter to isolate pulmonary veins. Heart Rhythm. 2022;19((Suppl.)):S142–3. [Google Scholar]


