Abstract
Rationale
Diaphragm dysfunction is frequently observed in critically ill patients with difficult weaning from mechanical ventilation.
Objectives
To evaluate the effects of temporary transvenous diaphragm neurostimulation on weaning outcome and maximal inspiratory pressure.
Methods
Multicenter, open-label, randomized, controlled study. Patients aged ⩾18 years on invasive mechanical ventilation for ⩾4 days and having failed at least two weaning attempts received temporary transvenous diaphragm neurostimulation using a multielectrode stimulating central venous catheter (bilateral phrenic stimulation) and standard of care (treatment) (n = 57) or standard of care (control) (n = 55). In seven patients, the catheter could not be inserted, and in seven others, pacing therapy could not be delivered; consequently, data were available for 43 patients. The primary outcome was the proportion of patients successfully weaned. Other endpoints were mechanical ventilation duration, 30-day survival, maximal inspiratory pressure, diaphragm-thickening fraction, adverse events, and stimulation-related pain.
Measurements and Main Results
The incidences of successful weaning were 82% (treatment) and 74% (control) (absolute difference [95% confidence interval (CI)], 7% [−10 to 25]), P = 0.59. Mechanical ventilation duration (mean ± SD) was 12.7 ± 9.9 days and 14.1 ± 10.8 days, respectively, P = 0.50; maximal inspiratory pressure increased by 16.6 cm H2O and 4.8 cm H2O, respectively (difference [95% CI], 11.8 [5 to 19]), P = 0.001; and right hemidiaphragm thickening fraction during unassisted spontaneous breathing was +17% and −14%, respectively, P = 0.006, without correlation with changes in maximal inspiratory pressure. Serious adverse event frequency was similar in both groups. Median stimulation-related pain in the treatment group was 0 (no pain).
Conclusions
Temporary transvenous diaphragm neurostimulation did not increase the proportion of successful weaning from mechanical ventilation. It was associated with a significant increase in maximal inspiratory pressure, suggesting reversal of the course of diaphragm dysfunction.
Clinical trial registered with www.clinicaltrials.gov (NCT 03096639) and the European Database on Medical Devices (CIV-17-06-020004).
Keywords: diaphragm weakness, weaning, mechanical ventilation, ventilator-induced diaphragmatic dysfunction
At a Glance Commentary
Scientific Knowledge on the Subject
Ventilator-induced diaphragm dysfunction (VIDD) is frequently observed in patients with difficult weaning and prolonged mechanical ventilation. There is currently no established strategy to directly treat or reverse VIDD.
What This Study Adds to the Field
Although bilateral phrenic nerve stimulation did not increase the proportion of successful weaning from mechanical ventilation compared with the standard of care, it resulted in substantial improvements in inspiratory pressure generation capacity without major safety issues. In the absence of previous clinical data, these findings suggest that diaphragm pacing could be effective in mitigating diaphragm dysfunction in patients who are difficult to wean from mechanical ventilation.
Mechanical ventilation (MV) is the most frequently used life-saving technique in ICUs and is required by 20–40% of ICU patients on a daily basis (1). It is, however, associated with complications such as ventilator-induced lung injury (2) and pulmonary infection (3). Animal experiments have also led to the description of ventilator-induced diaphragmatic lesions and weakness, so called ventilator-induced diaphragmatic dysfunction (VIDD) (4). Critically ill patients with difficult weaning from MV often present with diaphragm dysfunction (5–9), defined as an impaired capacity of the diaphragm to produce negative intrathoracic inspiratory pressure. The underlying mechanisms can include, but are not limited to, contractile weakness. Multiple causes beyond MV can affect the diaphragm in ICU patients, hence the wider concept of “critical illness–associated diaphragm weakness” (10). Preventing or reversing this phenomenon could reduce the duration of MV, resulting in lower mortality in difficult-to-wean patients (11) and lower total inpatient costs (12).
There is currently no established strategy to directly treat or reverse critical illness associated diaphragmatic abnormalities in critically ill patients. Experimental data recorded in animals suggest that keeping the diaphragm active during MV could be useful (13), and limited clinical data have suggested the usefulness of inspiratory muscle training to facilitate weaning from prolonged MV (14). Animal studies also suggest that superimposing diaphragm pacing during MV could mitigate experimental VIDD (15, 16). In humans, the ability of diaphragm pacing to correct profound diaphragm atrophy in the absence of diaphragm denervation has been demonstrated in MV-dependent quadriplegic patients (17).
The present study aimed to investigate the effects of diaphragm pacing with bilateral phrenic nerve stimulation at the bedside (temporary transvenous diaphragm neurostimulation [TTDN]) (15) on weaning outcome in difficult-to-wean patients. We hypothesized that TTDN would result in a higher rate of weaning success and shortened duration of MV in relation to improved maximal inspiratory pressure (MIP).
The data included in this article have been presented in part at the American Thoracic Society International Conference (May 14–19, 2021, virtual event, thematic poster presentation), the European Respiratory Society International Congress (September 5–9, 2020, virtual event, oral presentation), and the 33rd Annual Congress of the European Society of Intensive Medicine (“ESICM LIVES 2020,” December 6–9, 2020, virtual event, oral presentation).
The study protocol, published before completion of the study (18), and three major amendments (see Table E3 in the online supplement) were approved at all sites by institutional review boards or ethical committees depending on local regulations.
Methods
Study Design and Setting
In this multicenter, randomized, controlled study, participants were enrolled from 20 ICUs and long-term weaning units in France and Germany. The study was designed by a steering committee (Table E2) in collaboration with the sponsor and conducted in compliance with the Declaration of Helsinki. All patients/legally authorized representatives gave written informed consent before enrollment. The study was conducted from September 2017 to January 2020 and was publicly registered before the first enrollment (ClinicalTrials.gov ID: NCT 03096639; European Database on Medical Devices: CIV-17-06-020004).
Participants
Adults (⩾18 yr of age) were considered for inclusion in the study if they had been on invasive MV (intubation or tracheotomy) for >96 hours and satisfied protocol-defined readiness-to-wean criteria (see the across-centers standardized weaning protocol in Figure E2) (19) but had failed at least two attempts at ventilator liberation (failed spontaneous breathing trial, extubation with subsequent reintubation within 48 h). The main exclusion criteria were as follows: current extracorporeal membrane oxygenation, failed weaning from MV because of current hypervolemia as determined by the clinicians in charge, clinically overt congestive heart failure, anatomical features preventing left subclavian vein catheterization, history of congenital heart disease, current neuromuscular blockade treatment, preexisting neuromuscular disease potentially affecting respiratory muscles, pleural effusions occupying more than one-third of the pleural space on either side on chest X-ray, body mass index (BMI) of ⩾40 kg/m2, known/suspected phrenic nerve paralysis, presence of any electrical device (implanted or external) with the potential to interact/interfere with the TTDN system, bacteremia, current hemodynamic instability (need for vasopressors), current sepsis/septic shock, terminal illness with an estimated life expectancy of <6 months or not committed to full care, known/suspected pregnancy, lactating, actively participating in another clinical study pertaining to MV weaning, and vulnerable populations.
Randomization and Masking
The study was open label, with neither patients nor investigators blinded to treatment arm or primary/secondary outcomes, with the exception of diaphragm ultrasound measurements (performed at a subset of sites and analyzed centrally in a blinded manner) and the clinical event adjudication committee for serious adverse events (AEs). Patients were randomly assigned to the control or treatment group in a 1:1 ratio with variable block size within each center, with allocation concealment by means of a centralized web-based electronic data capture system (Syncrony, Version 2018.01.02; Syntactx Technologies).
Procedures
Control and intervention groups
Both groups were treated according to a standardized weaning protocol that was the same for all centers (see Figure E2). Daily weaning-readiness screening was followed, when appropriate, by a spontaneous breathing trial (SBT), with zero pressure support and zero end-expiratory pressure (20). Patients who passed the SBT were extubated (or, if tracheotomized, not reconnected to the ventilator). Patients who failed the SBT were put back on initial ventilator settings. Planned postextubation noninvasive ventilation was allowed in patients with risk factors of postextubation respiratory failure (“prophylactic noninvasive ventilation”) (21). Unplanned noninvasive ventilation was strongly discouraged.
Intervention (treatment) group
In the treatment group, a dedicated central-venous catheter was placed for TTDN through a minimally invasive bedside procedure. This system (Lungpacer Diaphragm Pacing Therapy System, Lungpacer Medical, Inc.) included an intravenous multielectrode stimulating catheter (see LIVE catheter in Figure E1) inserted into the left subclavian vein using Seldinger’s technique, checked by chest X-ray, and connected to a cart-mounted control unit with a touchscreen user interface. A mapping procedure before each treatment session ensured adequate capture of both phrenic nerves and determined the stimulation thresholds at which visible or manually palpable diaphragm contractions appeared in response to the electrical impulses. During TTDN, the intensity was increased to the maximal level tolerated by the patient. Stimulation pulses had an intensity of ⩽13.5 mA and a duration of 200–300 μs and were delivered at frequencies of 4 Hz (mapping procedure) and 15 Hz (treatment). Stimulation duration, frequency, and intensity were based on the settings used in animal studies on mitigation of VIDD (15, 16) and were known to provoke fused diaphragmatic contractions in humans (22). In this regard, and in the absence of animal data to rely on regarding stimulation-induced muscle fatigue or nerve damage, the stimulation frequency was chosen at the low end of the possible spectrum for the sake of safety. Pacing sessions consisted of four sets of 10 or six sets of 10 consecutive stimulations administered manually in synchrony with the ventilator. Each set was separated from the following by <1 minute. Two to three sessions (for a total of 120 stimulations) per day were conducted daily for up to 30 days and were stopped when patients successfully passed the SBT and were extubated. If extubation occurred before completion of the 30-day period, the catheter was kept in place for 48 hours, in case weaning was not successful.
In each group, standardized measurement of MIP, a volitional evaluation of global inspiratory muscle function, was performed before randomization using a one-way valve (23) and then every 72 hours and on the day of extubation. At the same time, but not simultaneously, a standardized diaphragmatic ultrasound was performed in a subset of patients while they were spontaneously breathing without ventilatory assistance to measure diaphragm thickness and calculate the thickening fraction as described elsewhere (24), and images were analyzed by an independent blinded reviewer.
Outcomes
The primary efficacy endpoint was the cumulative incidence of successful weaning by Day 30 in both groups. In nontracheotomized patients, the time point of successful weaning was defined as the time after which reintubation had not been necessary for 48 hours. In tracheotomized patients, the time point of successful weaning was defined as the time after which patients had been kept separated from the ventilator for 24 hours and not reconnected to it in the following 48 hours.
Secondary efficacy endpoints were as follows: the number of days from baseline to removal from MV as a result of successful weaning or Day 30, whichever came first; reinstatements of MV by Day 30; difference between groups in MIP changes from baseline to last available measurement; change in MIP over time; rate of MIP change per day from baseline to last available measurement; 30-day survival; changes in diaphragmatic thickening fraction from baseline to last available measurement; changes in rapid shallow breathing index (RSBI); and proportion of patients being tracheostomized. Data collection for MIP, RSBI, and diaphragm thickness ceased at successful weaning.
Safety endpoints included AEs in each group. Stimulation-related pain was evaluated using a visual analog scale ranging from 0 (no pain) to 10 (maximum pain).
Cumulative incidence of discharge from the ICU and hospital from baseline was an exploratory endpoint.
Statistical Analysis
In a prespecified list, eight baseline characteristics (Table 1) were statistically compared between groups, and differences (P < 0.05) were explored as covariates or strata variables in statistical models or in subgroups defined by the median value.
Table 1.
Baseline Characteristics of All Participants in Intent-to-Treat and Modified Intent-to-Treat Populations
| Variables | ITT (n = 57) | mITT (n = 43) | Control (n = 55) |
|---|---|---|---|
| Age, yr, mean ± SD* | 63.8 ± 12.3 | 63.6 ± 11.1 | 65.3 ± 11.3 |
| Sex, n (%)* | |||
| F | 25 (44) | 19 (44) | 23 (42) |
| M | 32 (56) | 24 (56) | 32 (58) |
| Body mass index, kg/m2, mean ± SD*† | 28 ± 6 | 27 ± 6 | 25 ± 5 |
| Smoking, n (%) | |||
| Previous | 15 (28) | 13 (31) | 18 (33) |
| Current | 20 (37) | 17 (41) | 23 (43) |
| Never | 19 (35) | 12 (29) | 13 (24) |
| Medical history, n (%) | |||
| Diabetes | 16 (28) | 10 (23) | 11 (20) |
| Chronic lung disease | — | — | — |
| COPD* | 23 (42) | 19 (46) | 27 (49) |
| State after lung reduction surgery | 3 (5) | 2 (5) | 6 (11) |
| Chronic cardiac disease | — | — | — |
| Coronary artery disease | 11 (19) | 6 (14) | 9 (16) |
| Congestive heart failure | 6 (11) | 4 (9) | 2 (4) |
| Peripheral artery disease | 6 (11) | 4 (9) | 5 (9) |
| Arrhythmia | 19 (33) | 13 (30) | 17 (31) |
| Valve disorders—stenosis and insufficiency | 13 (23) | 8 (19) | 8 (15) |
| Hypertension | 30 (53) | 21 (49) | 33 (60) |
| Hypercholesteremia | 13 (23) | 10 (23) | 10 (18) |
| Reason for invasive mechanical ventilation, n (%)* | |||
| COPD exacerbation | 8 (14) | 6 (14) | 8 (15) |
| ARDS | 23 (40) | 17 (40) | 19 (35) |
| Pneumonia | 8 (14) | 7 (16) | 13 (24) |
| Trauma | 2 (4) | 2 (5) | 0 (0) |
| Surgical | 9 (16) | 7 (16) | 8 (15) |
| Other | 7 (12) | 4 (9) | 7 (13) |
| Baseline characteristics | |||
| SOFA score, mean ± SD* | 5 ± 3 | 5 ± 3 | 4 ± 3 |
| Duration of mechanical ventilation, days, mean ± SD* | 27 ± 18 | 26 ± 18 | 29 ± 23 |
| Tracheostomy, n (%)* | 31 (54) | 22 (51) | 30 (55) |
| Ventilator settings, mean ± SD | |||
| PEEP, cm H2O | 7 ± 2 | 7 ± 2 | 6 ± 1 |
| Pressure support, cm H2O | 13 ± 4 (n = 36) | 13 ± 4 (n = 27) | 13 ± 4 (n = 35) |
| Respiratory variables, mean ± SD | |||
| Baseline MIP, cm H2O*† | 27 ± 15 | 25 ± 12 | 33 ± 14 |
| Right side diaphragm thickening fraction, mean % ± SD | |||
| B-mode ultrasound | 24 ± 22 (n = 10) | 24 ± 22 (n = 10) | 25 ± 23 (n = 10) |
| M-mode ultrasound† | 24 ± 19 (n = 17) | 24 ± 19 (n = 15) | 42 ± 27 (n = 17) |
| Right side end expiratory thickness, mm, mean ± SD | |||
| B-mode ultrasound | 2.1 ± 0.6 (n = 10) | 2.1 ± 0.6 (n = 10) | 2.0 ± 0.7 (n = 10) |
| M-mode ultrasound | 2.4 ± 1.0 (n = 17) | 2.4 ± 1.1 (n = 15) | 2.2 ± 0.9 (n = 17) |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; COPD = chronic obstructive pulmonary disease; ITT = intent to treat; MIP = maximal inspiratory pressure; mITT = modified ITT; PEEP = positive end-expiratory pressure; SOFA = sequential organ failure assessment.
Control = standard of care for difficult and prolonged mechanical ventilation weaning. Treatment = temporary transvenous diaphragm neurostimulation.
These variables were prespecified in the statistical analysis plan to be compared at baseline and, if statistically significant at P = 0.05, were evaluated as covariates or strata variables.
Statistically significant difference in comparison between treatment (ITT and mITT) and control comparison.
Successful weaning was analyzed using a competing risk, time-to-event model with death before weaning as the competing risk. Cumulative incidences for successful weaning and death before weaning were compared at Day 30 using Gray’s test. The average numbers of days on MV until successful weaning or Day 30 was compared using bootstrapping (25). The number of days on MV for those who died before weaning was imputed as if the patient was not successfully weaned by Day 30. The change from baseline to last available measure for respiratory variables (including ultrasound-derived indices) was compared using the two-sample t test. Change in MIP at 8, 15, 22, and 30 days was analyzed using a mixed model, with the last observation carried forward such that patients who were successfully weaned, died, or discontinued the study at earlier time points were represented at later time points. A mixed model was used to incorporate the correlation expected among the repeated measures measured in a patient. Day-30 survival was compared using Kaplan-Meier methods, and the number and percentage of patients with AEs were compared using Fisher’s exact test.
The intent-to-treat (ITT) population was defined by the randomization. Primary and secondary efficacy endpoints were evaluated using the modified intent-to-treat (mITT) population (the same as the ITT population for the control group but excluding patients for whom TTDN was not achieved in the treatment group). Safety endpoints were analyzed in the ITT population.
There were no reliable prior estimates on which to base the sample size. Although the study was not powered to test statistical significance for a minimally clinically relevant effect size, a sample size of 92 patients (46 patients per group) would result in 80.5% power for the primary efficacy endpoint, assuming 80% (treatment) and 60% (control) patients were successfully weaned at Day 30. It was, therefore, expected that 110 patients (55 patients per group) would be sufficient to provide initial treatment effect estimates (18).
SAS Version 9.4 (Cary SAS Institute) was used for statistical analyses.
Results
Participants
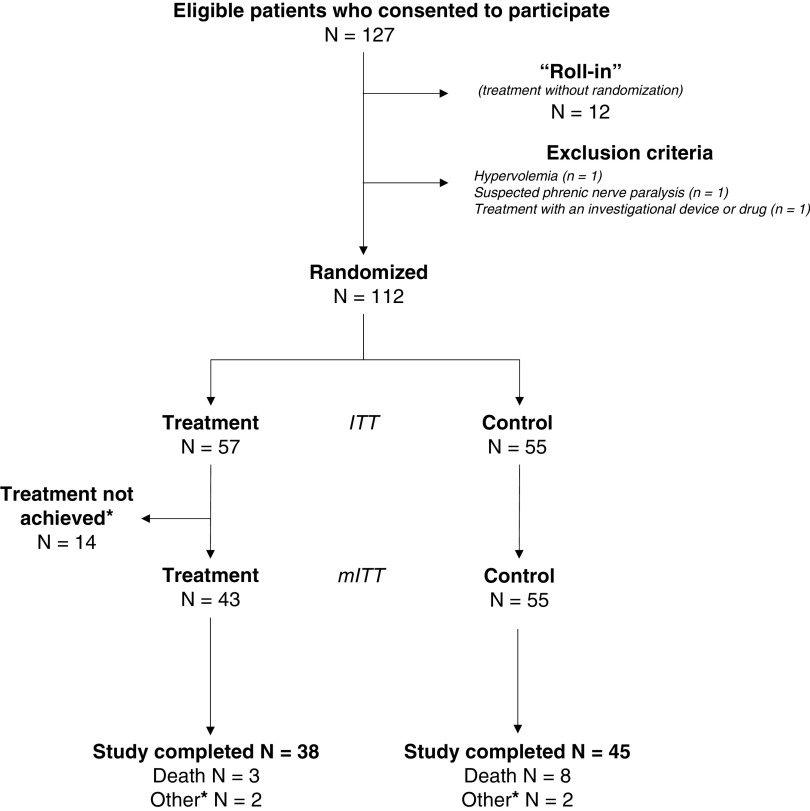
Overall, 127 patients were eligible for participation, 12 were treated but neither randomized nor included in the analyses (“roll-in” patients), 3 met exclusion criteria, and 112 were randomized (57 to the treatment group and 55 to the control group) (Figure 1). All control patients were included in the mITT population; in the treatment group, 14 patients were excluded from the mITT population (guidewire or LIVE catheter could not be inserted [7/14], or pacing therapy could not be delivered [7/14]). The number of patients enrolled at each center during the study period is presented in Table E4.
Figure 1.
Study flow chart. Of the 127 eligible patients who consented to the study, 12 were not randomized and therefore were not included in the study. They were part of a “roll-in” process intended to familiarize the investigators with the technology. As such, for these patients, the catheter was inserted, and the therapy was delivered for training purposes, but they were not included in the study. Fifty-five patients were randomized to the control group, and 57 to the treatment group. All patients who were randomized to the control group qualified for the modified intent-to-treat (mITT) population; 45 of these patients completed the 30-day follow-up, 8 died before the 30-day follow-up, and 2 did not complete the study for other reasons (one patient was a screening failure, and the other patient was transferred to another hospital and withdrew from the study upon transfer). Treatment = temporary transvenous diaphragm neurostimulation. *Of the 57 patients of the treatment group, the therapy could not be achieved in 14 patients; therefore, these patients did not qualify for the mITT population. The reasons for not achieving therapy were the following: 1) The catheter was inserted and correctly positioned, but the diaphragm could not be stimulated (n = 7); 2) the catheter was not inserted in 4 patients (guidewire could not be passed [n = 2], presence of different catheter already in place [n = 1], or vessel damage because of radiotherapy [n = 1]); 3) no attempt (patient withdrew consent [n = 1] or screening failure due to a body mass index of >40 [n = 1]); and 4) the catheter was inserted but removed on the same day because of resistance and thrombus in the vessel (n = 1). These patients were followed for adverse events 48 hours after the initial attempt to place the catheter or last contact with the catheter. The remaining 43 patients were evaluable for the primary effectiveness analysis (mITT), 38 completed the Day 30 follow-up, 3 died before the 30th day, and 2 did not complete the study for other reasons (one patient was withdrawn by the investigator and the second chose to withdraw from the study due to pain associated with stimulation).
Baseline characteristics did not differ in each group, except for BMI and MIP (Table 1). The main reason for intubation was acute respiratory distress syndrome (37.5% overall). The mean ± SD durations of MV before inclusion were 27 ± 18 days (treatment) and 29 ± 23 days (control). Approximately half of the patients already had a tracheostomy at inclusion.
Catheter Placement and Treatment Delivery
The catheter was in place for a median duration of 10 days (range = 2–33). The median number of stimulations delivered (per patient per day) was 72 (range = 24–114) in two to three separate sessions. Thirty-four (79%) patients received >50% of protocol-required stimulations.
Successful Weaning and MV Duration
The mean ± SD duration of MV from baseline to successful weaning or Day 30 was 12.7 ± 9.9 days (treatment) and 14.1 ± 10.8 days (control), respectively (difference [95% confidence interval (CI)], −1.4 [−5.6, 2.7], P = 0.498) (Table 2). The cumulative incidences for successful weaning were 82% (treatment) and 74% (control) (difference [95% CI], 7.4% [−10% to 25%], P = 0.586). The use of noninvasive ventilation during the 48-hour period postextubation to determine successful weaning was 62% (treatment) and 55% (control) (P = 0.639). The cumulative incidences for death before successful weaning were 3% (treatment) and 9% (control) (difference [95% CI], −7% [−16%, 3%], P = 0.191) (Table 2). Primary and secondary outcomes (per protocol analysis) are shown in Table E5.
Table 2.
Primary and Secondary Outcomes
| Outcome | mITT | Control | Absolute Difference, % (95% CI) | P Value |
|---|---|---|---|---|
| Successful weaning and days on mechanical ventilation (Day 30, mITT) | ||||
| Cumulative incidence for successful weaning, %* | 82 | 74 | 7 (−10 to 25) | 0.586 |
| Use of NIV in the 48-h period post extubation, % | 62 | 55 | 7 (−16 to 29) | 0.639 |
| Cumulative incidence for death before successful weaning, % | 3 | 9 | −7% (−16 to 3) | 0.191 |
| Days on mechanical ventilation from baseline to successful weaning or Study Day 30, mean ± SD† | 12.7 ± 9.9 | 14.1 ± 10.8 | −1.4 (−5.6 to 2.7) | 0.498 |
| Number of patients placed back on mechanical ventilation within study period after successful weaning | 3 | 4 | NA | NA |
| Number of patients with tracheostomy placed during the study period | 2 | 5 | NA | NA |
| Cumulative incidence for first successful SBT, % | 86 | 80 | 7 (−9 to 23) | 0.607 |
| Cumulative incidence for ICU discharge, % | 43 | 37 | 6 (−14 to 27) | 0.600 |
| Cumulative incidence for hospital discharge, % | 5 | 8 | −3 (−13 to 7) | 0.587 |
| Respiratory (mITT) | ||||
| Change in MIP from baseline to last available measure, cm H2O, mean ± SD† | 16.6 ± 16.7 | 4.8 ± 17.4 | 11.8 (5 to 19) | 0.001 |
| Rate of change in MIP per day from baseline to last available measure, cm H2O, mean ± SD† | 2.6 ± 3.2 | 1.4 ± 3.8 | 1.2 (−0 to 3) | 0.100 |
| Change in RSBI from baseline to last available measure, breaths/min/L, mean ± SD† | −40.9 ± 72.8 | −18.0 ± 61.1 | −23.0 (−51 to 5) | 0.102 |
| Rate of change in RSBI per day from baseline to last available measure, breaths/min/L, mean ± SD† | −7.2 ± 16.0 | −4.9 ± 13.6 | −2.3 (−8 to 4) | 0.450 |
| Change in right-side diaphragm thickening fraction to last available measure, %† | ||||
| B-mode ultrasound, mean ± SD | −1.3 ± 15.1 (n = 10) | −13.7 ± 22.9 (n = 10) | 12.4 (−6 to 31) | 0.171 |
| M-mode ultrasound, mean ± SD | 16.6 ± 26.8 (n = 15) | −13.6 ± 30.4 (n = 17) | 30.2 (9 to 51) | 0.006 |
| Change in end expiratory thickness, mm | ||||
| B-mode ultrasound, mean ± SD | −0.2 ± 0.3 (n = 10) | 0.2 ± 0.8 (n = 10) | −0.4 (−1.0 to 0.2) | 0.162 |
| M-mode ultrasound, mean ± SD | −0.5 ± 1.2 (n = 15) | 0.0 ± 1.0 (n = 17) | −0.5 (−1.3 to 0.3) | 0.230 |
| Survival (Day 30, ITT), %† | 93 | 85 | 8 (−4 to 20) | 0.216 |
Definition of abbreviations: CI = confidence interval; ITT = intent to treat; MIP = maximal inspiratory pressure; mITT = modified ITT; NA = not applicable; NIV = noninvasive ventilation; SBT = spontaneous breathing trial; RSBI = rapid shallow breathing index.
Control = standard of care for difficult and prolonged mechanical ventilation weaning. Treatment = temporary transvenous diaphragm neurostimulation.
Primary outcome.
Secondary outcome.
Adjusting for differences in baseline BMI or MIP did not affect the main outcome (Table E6). However, the relationships between baseline MIP and successful weaning (P = 0.007) and between BMI and successful weaning (P = 0.048) were statistically significant (Table E6). Cumulative incidence for successful weaning and imputed average days on MV within subgroups defined by baseline MIP and BMI are available in Tables E7 and E8, respectively.
Respiratory Variables
The differences in MIP changes from baseline to last available measurement were statistically significant: +16.6 cm H2O (treatment) and +4.8 cm H2O (control) (difference [95% CI], 11.8 cm H2O [5–19], P = 0.001) (Table 2). When adjusted for differences in baseline BMI and baseline MIP, the difference between groups was maintained at 8 (1–15) cm H2O, P = 0.023.
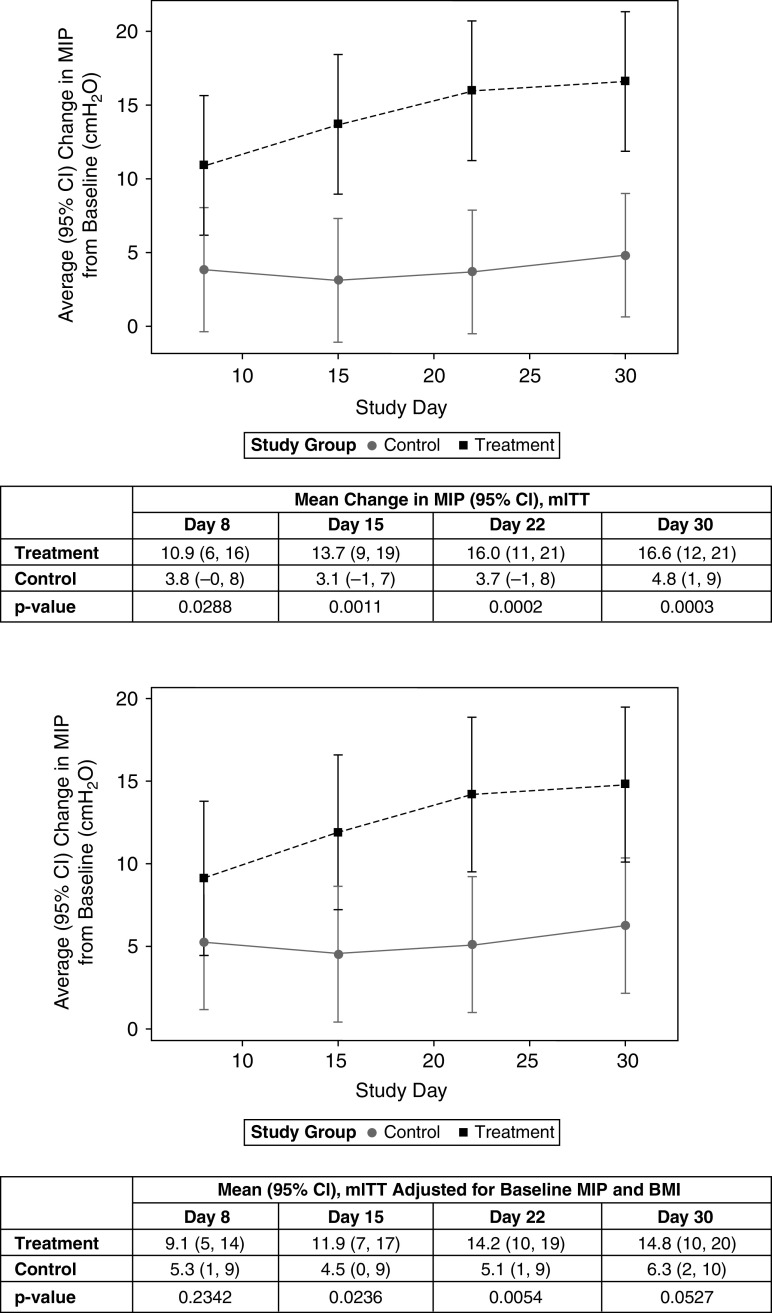
Richmond Agitation–Sedation Scale scores at the first and last available MIP evaluations are reported in Table E9. The change in MIP over time differed significantly between groups by Day 8 (+10.9 cm H2O [treatment] and +3.8 cm H2O [control]; difference [95% CI], 7 cm H2O [1–14], P = 0.029) (Figure 2). The difference between groups was statistically significant at each subsequent follow-up and increased over time (Figure 2, top). Adjusting for baseline differences in MIP and BMI, the average change in MIP reached statistical significance at Day 15 (+11.9 cm H2O [treatment] and +4.5 cm H2O [control]; difference [95% CI], 7 cm H2O [1–14], P = 0.024), with statistical significance maintained at subsequent time points (Figure 2, bottom).
Figure 2.
Time course of maximal inspiratory pressure (MIP). Control = standard of care for difficult and prolonged mechanical ventilation weaning. Treatment = temporary transvenous diaphragm neurostimulation. Top: change in MIP with last observation carried forward. For independent variables, randomized group P < 0.001; study day P = 0.071; and randomized group × study day interaction P = 0.089. Bottom: change in MIP with last observation carried forward. For independent variables, randomized group P = 0.019, study day P = 0.069, baseline MIP P = 0.0006, baseline BMI P = 0.562, and randomized group × study day interaction P = 0.0881. BMI = body mass index; CI = confidence interval; mITT = modified intent to treat.
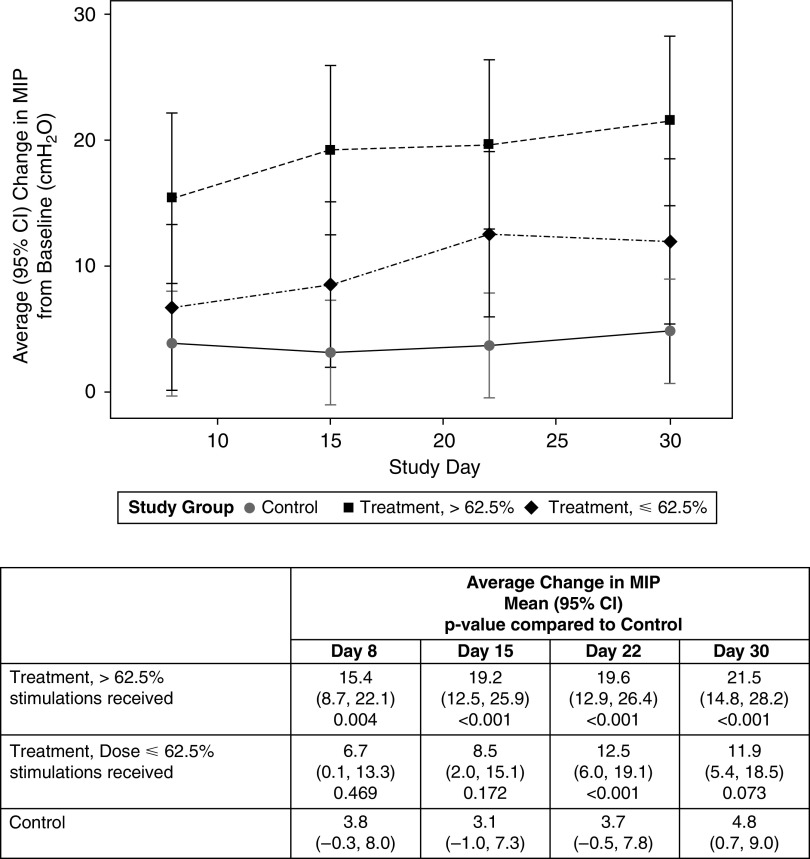
There was a stimulation dose relationship with MIP change (Figure 3): The change from baseline was lower for a lower stimulation dose (⩽62.5% stimulations: +6.7 cm H2O by Day 8, +11.9 cm H2O by Day 30) than for a higher stimulation dose (>62.5% stimulations: 15.4 cm H2O by Day 8, +21.5 cm H2O by Day 30). Compared with the control group, the MIP change was significantly higher on all occasions for the higher dose and on Day 22 and Day 30 for the lower dose (Figure 3).
Figure 3.
Dose relationship between stimulation “dose” and maximal inspiratory pressure (MIP). For study group with treatment divided into two groups on the basis of the median proportion of protocol required stimulations received, P < 0.001, study day P = 0.025, and study group × study interaction P = 0.108. CI = confidence interval.
Ultrasound was performed at eight sites and evaluable for analysis in a total of 34 patients. The change in right side diaphragm thickening fraction to last available measure with M-mode ultrasound was significantly higher in the treatment group (+16.6% [treatment] vs. −13.6% [control], P = 0.006). There was no correlation between MIP change and the change in diaphragm thickening fraction (r ⩽ 0.18, P ⩾ 0.456) for B- or M-mode measurements. Measurement variability of diaphragm thickening fractions is provided in Table E10.
Survival at 30 Days
There was no difference in survival at Day 30 between groups (93% [treatment] and 85% [control]; difference [95% CI], 8% [−4%, 20%], P = 0.216).
Safety and Tolerability
There was no difference between groups regarding device- or procedure-related serious AEs (Table E11).
In the treatment group, median (interquartile range) pain assessment by 36 patients in the mITT population (84%) during at least one diaphragm pacing session was 0 (interquartile range = 0, 3). Pain exceeded 4 in at least one session in 16/36 patients (44%) reporting pain, and 9% of the sessions during which pain assessment was reported.
Exploratory Outcome
By Day 30, 43% (treatment) and 37% (control) of the patients were discharged from the ICU (difference [95% CI], 6% [−14 to 27], P = 0.599).
Discussion
In a population of difficult-to-wean patients who had received invasive MV for about 1 month, TTDN combined with standard management did not increase the proportion of successful weaning, compared with standard treatment alone. However, TTDN was associated with an increase in MIP that was significantly greater in the treatment group than in the control group, suggesting that TTDN could interfere with diaphragm function. Furthermore, TTDN appeared feasible, as 75% of the patients in the treatment group could successfully receive the therapy, which was well tolerated.
Difficult weaning from MV is associated with increased mortality and morbidity (8, 24). It increases health care costs (1) and uses ICU resources. This makes prompt weaning a major goal of intensive care medicine. Standardized protocols including reduced sedation, early screening for weaning readiness, and daily SBTs improve the weaning process (26, 27). One study suggested that diuretics to correct fluid overload can reduce weaning duration (28). Several other studies suggest that this is also the case for inspiratory muscle training (14, 29–31). However, inspiratory muscle training is difficult to generalize, because it involves patient cooperation and requires time-intensive physiotherapy resources that may not be systematically available. Diaphragm pacing should be devoid of these limitations. Diaphragm pacing through phenic stimulation is known to be capable of reversing profound diaphragm disuse atrophy in quadriplegic patients (provided that there is no denervation), to the point of allowing ventilator weaning (17). This justifies investigating diaphragm pacing for difficult MV weaning in ICU patients, where diaphragmatic atrophy is frequent. Our findings suggest that TTDN is feasible.
The improvement in MIP in the treatment group at Day 8 was similar to that reported with inspiratory muscle training (29). However, in contrast to nonselective inspiratory muscle training, TTDN induces contractions of the diaphragm only, which led us to reason that the observed MIP changes most likely reflect selective diaphragmatic improvement. The dose–effect relationship between phrenic stimulation and MIP that we observed supports this assumption and strongly argues against possible investigator bias. Notably, MIP improved with TTDN, despite a substantial proportion of patients not receiving the full stimulation dose as per protocol, because of logistical reasons (e.g., therapy scheduling conflicts and limited staff coverage) and the nonautomated manner of TTDN delivery. The increase in MIP that we observed in stimulated patients might have reflected improved diaphragm function in relation with increased diaphragm contractile strength derived from diaphragm reconditioning in response to phrenic stimulation (supported by the difference in right hemidiaphragm thickening fraction between the treatment group and the control group). However, diaphragm reconditioning could also have improved diaphragm function through increased motor neuron recruitment in relation with training-related reductions in the spinal or supraspinal inhibitory inputs sent by muscle afferents (32–36). However, despite improvement in MIPs, TTDN did not result in improved clinical outcomes. In previous studies, inspiratory muscle training increased both MIP and the proportion of weaned patients compared with control (31). A possible explanation for this difference, compared with our study findings, is that TTDN might not have yielded sufficient MIP improvement to result in statistically improved clinical outcomes in this underpowered study. We cannot rule out that a higher “stimulation dose” or higher stimulation frequencies may have led to different outcomes. The basis for this daily stimulation regimen was provided by the published literature about diaphragm pacing in tetraplegic patients (17) and respiratory muscle strengthening (29, 37).
Weaning failure is multifactorial in nature, and weaning outcome depends heavily on the respiratory muscle load–capacity balance (but also on other, e.g., cardiac, factors) (9). Improving diaphragm function with TTDN is bound to improve this balance and should, therefore, contribute in turn to improve weaning outcome. However, the results of the present study suggest that an intervention targeting the diaphragm alone might not be sufficient to achieve this result. This prompts for studies evaluating the combination of TTDN with tailored load-reducing therapeutic strategies.
Despite concerns related to central venous catheterization by means of the subclavian route, which was required for phrenic nerve stimulation, the rate of AEs, including hemothorax and pneumothorax, did not differ significantly between groups. Although stimulation-related pain was observed during TTDN, overall, the treatment was well tolerated.
Some limitations to our study provide possible explanations for the finding that TTDN did not increase the proportion of successful weaning. First, although the prevalence of diaphragmatic dysfunction is high in patients who have been mechanically ventilated for ⩾7 days (7), the inclusion of patients who were unlikely to benefit from diaphragm pacing because of weaning failure risk factors independent of diaphragm function might have diluted the effect of the intervention. Second, the population studied was very heterogeneous regarding the duration of MV at baseline. Third, in the absence of reliable prior information to estimate effect and compute sample size, optimal statistical power had to be forgone to ensure reasonable feasibility, which may have contributed to its negative outcome. Fourth, only 79% of the patients in the treatment group received >50% of the target number of stimulations (number of stimulations per session times number of sessions), which may have contributed to the lack of a significant difference in the primary endpoint observed between groups. Fifth, it is likely that there was interindividual variability regarding the level of activation of the diaphragm, because the physical signs used to determine thresholds would have depended on a patient’s morphology. It is, therefore, not possible to be certain, at the individual level, that the diaphragm was stimulated enough for positive effects to occur. At the group level, however, the fact that there was a significant increase in MIP in the treatment group and not in the control group is in favor of the treatment. This highlights the importance of finding ways to characterize the degree of diaphragm activation objectively in response to TTDN. Sixth, the implementation of a standardized weaning protocol in centers that did not use one before the study may have improved outcomes in the control group, making any difference between the control and treatment groups more difficult to discern. Seventh, only 0.19 patients were randomized per ICU per month over the study period, with an important heterogeneity between centers (Table E4). This suggests the existence of an inclusion bias at some of the centers.
Our data suggest that TTDN might provide a valuable strategy to improve diaphragm function in noncooperative patients and settings with reduced human critical care resources. These results, and the limitations of our study described earlier, could be of value for the design and planning of future studies assessing TTDN in difficult-to-wean patients: Such studies should focus on homogenous patient populations; ideally exclude patients with a proportionally long duration of MV; avoid mixing tracheotomized and nontracheotomized patients; and, if possible, be sham controlled. Our data could also provide the basis for sample and effect size estimates in future studies. Some of these aspects have already been incorporated in an ongoing clinical study (clinical trial registered with www.clinicaltrials.gov [NCT 03783884]).
Conclusions
In this difficult-to-wean patient population, TTDN did not increase the proportion of successful weaning from MV. It was, however, associated with increased MIPs, most likely in line with improved diaphragm function, and it did not pose major safety issues. Further studies are ongoing to establish the place of TTDN in difficult-to-wean patients.
Acknowledgments
Acknowledgment
The authors acknowledge all study participants for their contribution to advancing the knowledge of the effects of temporary transvenous diaphragm neurostimulation on weaning outcome from mechanical ventilation and respiratory muscle function. Additionally, the authors acknowledge the members of the RESCUE-2 study group (see Table E1) for their contributions to the design, conduct, and analysis of data for this clinical study. Andrew Lane, Ph.D. (Lane Medical Writing), provided medical writing and editorial assistance in accordance with the European Medical Writers Association guidelines and Good Publication Practice.
Footnotes
A list of the RESCUE-2 Study Group Investigators may be found in Table E1 in the online supplement.
Supported by Lungpacer Medical, Inc.
Author Contributions: The academic authors (M.D., M.G.d.A., and T.S.) wrote the first draft of the manuscript and verified the underlying data. All authors critically reviewed and approved the manuscript and are accountable for its accuracy and integrity. M.D. had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. T.N. conducted and is responsible for the data analysis.
Data are available from the Corresponding Author upon reasonable request.
This article has an online supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202107-1709OC on February 2, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
the RESCUE-2 Study Group Investigators:
François Beloncle, Pierre-Yves Olivier, Marie Lemerle, Pierre Asfar, Alain Mercat, Katharina Böllinger, Marc Giesa, Carmen Garcia, Till Jacobi, Nikolas Lambiris, Felix Machleid, Panagiotis Pergantis, Bastian Grube, Damien Roux, Santiago Freita Ramos, Noemie Zucman, Louis Marie Dumont, Laura Federici, Marc Amouretti, Jean-Damien Ricard, Didier Dreyfuss, Jakob Wittenstein, Andreas Güldner, Max Ragaller, Peter Spieth, Christopher Uhlig, Lars-Olav Harnisch, Frank Bloos, Daniel O. Thomas-Rüddel, Gérald Chanques, Mathieu Capdevila, Yassir Aarab, Fanny Garnier, Vincent Brunot, Kada Klouche, Valérie Moulaire, Philippe Corne, Fernand Macone, François Durand, Charles Hugo Marquette, Julie Delemazure, Julien Mayaux, Elise Morawiec, Alexandra Monnier, Hassene Rahmani, Louise-Marie Jandeaux, Antoine Studer, Julie Helms, and Raphaël Clere-Jehl
References
- 1. Wunsch H, Kramer A, Gershengorn HB. Validation of intensive care and mechanical ventilation codes in Medicare data. Crit Care Med . 2017;45:e711–e714. doi: 10.1097/CCM.0000000000002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med . 2014;370:980. doi: 10.1056/NEJMc1400293. [DOI] [PubMed] [Google Scholar]
- 3. Timsit J-F, Esaied W, Neuville M, Bouadma L, Mourvllier B. Update on ventilator-associated pneumonia. F1000 Res . 2017;6:2061. doi: 10.12688/f1000research.12222.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med . 2004;169:336–341. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- 5. Dres M, Goligher EC, Dubé B-P, Morawiec E, Dangers L, Reuter D, et al. Diaphragm function and weaning from mechanical ventilation: an ultrasound and phrenic nerve stimulation clinical study. Ann Intensive Care . 2018;8:53. doi: 10.1186/s13613-018-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dres M, Demoule A. Diaphragm dysfunction during weaning from mechanical ventilation: an underestimated phenomenon with clinical implications. Crit Care . 2018;22:73. doi: 10.1186/s13054-018-1992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dres M, Dubé B-P, Mayaux J, Delemazure J, Reuter D, Brochard L, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med . 2017;195:57–66. doi: 10.1164/rccm.201602-0367OC. [DOI] [PubMed] [Google Scholar]
- 8. Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med . 2016;42:853–861. doi: 10.1007/s00134-015-4125-2. [DOI] [PubMed] [Google Scholar]
- 9. Dres M, Rozenberg E, Morawiec E, Mayaux J, Delemazure J, Similowski T, et al. Diaphragm dysfunction, lung aeration loss and weaning-induced pulmonary oedema in difficult-to-wean patients. Ann Intensive Care . 2021;11:99. doi: 10.1186/s13613-021-00886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med . 2017;43:1441–1452. doi: 10.1007/s00134-017-4928-4. [DOI] [PubMed] [Google Scholar]
- 11. Béduneau G, Pham T, Schortgen F, Piquilloud L, Zogheib E, Jonas M, et al. WIND (Weaning according to a New Definition) Study Group the REVA (Réseau Européen de Recherche en Ventilation Artificielle) Network. Epidemiology of weaning outcome according to a new definition. The WIND study. Am J Respir Crit Care Med . 2017;195:772–783. doi: 10.1164/rccm.201602-0320OC. [DOI] [PubMed] [Google Scholar]
- 12. Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med . 2005;33:1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 13. Powers SK, DeCramer M, Gayan-Ramirez G, Levine S. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care . 2008;12:191. doi: 10.1186/cc7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vorona S, Sabatini U, Al-Maqbali S, Bertoni M, Dres M, Bissett B, et al. Inspiratory muscle rehabilitation in critically ill adults. A systematic review and meta-analysis. Ann Am Thorac Soc . 2018;15:735–744. doi: 10.1513/AnnalsATS.201712-961OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masmoudi H, Coirault C, Demoule A, Mayaux J, Beuvin M, Romero N, et al. Can phrenic stimulation protect the diaphragm from mechanical ventilation-induced damage? Eur Respir J . 2013;42:280–283. doi: 10.1183/09031936.00045613. [DOI] [PubMed] [Google Scholar]
- 16. Reynolds SC, Meyyappan R, Thakkar V, Tran BD, Nolette M-A, Sadarangani G, et al. Mitigation of ventilator-induced diaphragm atrophy by transvenous phrenic nerve stimulation. Am J Respir Crit Care Med . 2017;195:339–348. doi: 10.1164/rccm.201502-0363OC. [DOI] [PubMed] [Google Scholar]
- 17. Le Pimpec-Barthes F, Gonzalez-Bermejo J, Hubsch J-P, Duguet A, Morélot-Panzini C, Riquet M, et al. Intrathoracic phrenic pacing: a 10-year experience in France. J Thorac Cardiovasc Surg . 2011;142:378–383. doi: 10.1016/j.jtcvs.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 18. Evans D, Shure D, Clark L, Criner GJ, Dres M, de Abreu MG, et al. Temporary transvenous diaphragm pacing vs. standard of care for weaning from mechanical ventilation: study protocol for a randomized trial. Trials . 2019;20:60. doi: 10.1186/s13063-018-3171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boles J-M, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J . 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 20. Sklar MC, Burns K, Rittayamai N, Lanys A, Rauseo M, Chen L, et al. Effort to breathe with various spontaneous breathing trial techniques. A physiologic meta-analysis. Am J Respir Crit Care Med . 2017;195:1477–1485. doi: 10.1164/rccm.201607-1338OC. [DOI] [PubMed] [Google Scholar]
- 21. Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J . 2017;50:1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 22. Adler D, Gottfried SB, Bautin N, Mirkovic T, Schmidt M, Raux M, et al. Repetitive magnetic stimulation of the phrenic nerves for diaphragm conditioning: a normative study of feasibility and optimal settings. Appl Physiol Nutr Metab . 2011;36:1001–1008. doi: 10.1139/h11-095. [DOI] [PubMed] [Google Scholar]
- 23. Caruso P, Friedrich C, Denari SD, Ruiz SA, Deheinzelin D. The unidirectional valve is the best method to determine maximal inspiratory pressure during weaning. Chest . 1999;115:1096–1101. doi: 10.1378/chest.115.4.1096. [DOI] [PubMed] [Google Scholar]
- 24. Tuinman PR, Jonkman AH, Dres M, Shi Z-H, Goligher EC, Goffi A, et al. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Intensive Care Med . 2020;46:594–605. doi: 10.1007/s00134-019-05892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall/CRC; 1994. [Google Scholar]
- 26. Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med . 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 27. Girard TD, Kress JP, Fuchs BD, Thomason JWW, Schweickert WD, Pun BT, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet . 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 28. Mekontso Dessap A, Roche-Campo F, Kouatchet A, Tomicic V, Beduneau G, Sonneville R, et al. Natriuretic peptide-driven fluid management during ventilator weaning: a randomized controlled trial. Am J Respir Crit Care Med . 2012;186:1256–1263. doi: 10.1164/rccm.201205-0939OC. [DOI] [PubMed] [Google Scholar]
- 29. Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care . 2011;15:R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bissett BM, Leditschke IA, Neeman T, Boots R, Paratz J. Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax . 2016;71:812–819. doi: 10.1136/thoraxjnl-2016-208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. da Silva Guimarães B, de Souza LC, Cordeiro HF, Regis TL, Leite CA, Puga FP, et al. Inspiratory muscle training with an electronic resistive loading device improves prolonged weaning outcomes in a randomized controlled trial. Crit Care Med . 2021;49:589–597. doi: 10.1097/CCM.0000000000004787. [DOI] [PubMed] [Google Scholar]
- 32. Triscott S, Gordon J, Kuppuswamy A, King N, Davey N, Ellaway P. Differential effects of endurance and resistance training on central fatigue. J Sports Sci . 2008;26:941–951. doi: 10.1080/02640410701885439. [DOI] [PubMed] [Google Scholar]
- 33. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev . 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 34. Chang Y-J, Hsu M-J, Chen S-M, Lin C-H, Wong AMK. Decreased central fatigue in multiple sclerosis patients after 8 weeks of surface functional electrical stimulation. J Rehabil Res Dev . 2011;48:555–564. doi: 10.1682/jrrd.2010.03.0038. [DOI] [PubMed] [Google Scholar]
- 35. Nielsen J, Kagamihara Y. Differential projection of the sural nerve to early and late recruited human tibialis anterior motor units: change of recruitment gain. Acta Physiol Scand . 1993;147:385–401. doi: 10.1111/j.1748-1716.1993.tb09515.x. [DOI] [PubMed] [Google Scholar]
- 36. Nielsen JB, Morita H, Wenzelburger R, Deuschl G, Gossard J-P, Hultborn H. Recruitment gain of spinal motor neuron pools in cat and human. Exp Brain Res . 2019;237:2897–2909. doi: 10.1007/s00221-019-05628-6. [DOI] [PubMed] [Google Scholar]
- 37. Martin AD, Joseph A-M, Beaver TM, Smith BK, Martin TD, Berg K, et al. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit Care Med . 2014;42:e152–e156. doi: 10.1097/CCM.0b013e3182a63fdf. [DOI] [PMC free article] [PubMed] [Google Scholar]