To the Editor:
A central question in coronavirus disease 2019 (COVID-19)-associated hypoxemia management remains how to optimally provide and escalate respiratory support. Numerous studies have investigated the potential impact of “early intubation;” together, these suggest there is neither benefit nor harm with this approach (1). However, as observational studies without protocols for when to initiate invasive mechanical ventilation (IMV), all are likely subject to indication bias. Clinicians are left, therefore, with little evidence on which to base decisions about when and in whom IMV initiation is appropriate.
In this study, we sought to evaluate the outcomes of patients supported with high-flow nasal cannula (HFNC) for increasing periods of time. We hypothesized that after a certain amount of time on HFNC, the odds of HFNC failure (defined as intubation or death without intubation by Study Day 28) would increase. Such results may assist clinicians and patients in decisions about the use and timing of IMV.
Methods
We performed a secondary analysis of data collected for a randomized controlled meta-trial of awake prone positioning in COVID-19 acute respiratory failure requiring support with HFNC (2). In the meta-trial, HFNC was initiated at the maximal tolerated flow, and the fraction of inspired oxygen (FiO2) was titrated to maintain a peripheral arterial oxygen saturation (SpO2) between 90% and 95%. Predefined criteria for intubation were provided at the meta-trial level.
Our cohort consisted of control group patients, those not subjected to protocolized prone positioning. From this full group who received HFNC for any duration of time (⩾0 d), we created 14 nonmutually exclusive, nested cohorts (patients who received HFNC for ⩾1 day, patients who received HFNC for ⩾2 days, etc., up through patients who received HFNC for ⩾14 days). The primary outcome was HFNC failure, defined as the initiation of IMV or death (before IMV), within 28 days of trial enrollment. No patients were lost to follow-up.
We used numbers and percentages to describe patient characteristics. We then evaluated unadjusted HFNC failure rates in each cohort for all patients and subgroups defined by age, sex, body mass index (BMI), and degree of hypoxemia at study enrollment. Finally, we constructed a series of 15 multivariable logistic regression models to estimate the adjusted probability of HFNC failure for each cohort separately. For each model, covariables were determined a priori based on clinical significance and included: age, sex, BMI, respiratory rate and SpO2:FiO2 ratio at the time of study enrollment, number of comorbidities, and HFNC device (Optiflow or Airvo 2 [Fisher and Paykel Healthcare Ltd.] vs. Precision Flow [Vapotherm]). Continuous covariables were modeled linearly. Furthermore, each component of the composite outcome (initiation of IMV or death without IMV), patients treated with different HFNC devices, and patients without do-not-intubate orders were examined separately as sensitivity analyses.
Statistical analyses were performed with R version 3.6.3.
Results
Our full cohort consisted of 557 patients with an average age of 60.7 ± 14.0 years, of whom 65.7% were male. The majority (80.4%) had moderate to severe hypoxemia (SpO2:FiO2 < 190) on study enrollment, and 46.1% experienced HFNC failure by Study Day 28.
Unadjusted rates of HFNC failure varied by time on HFNC in a U-shaped manner (Figure 1A). The lowest rate of failure was 28.1% experienced by those using ⩽6 days of HFNC (vs. 46.1% for the full cohort and 41.0% for those using HFNC for ⩽14 d). Similar trends were observed in all sensitivity analyses and most subgroups (Table 1). Exceptions included patients ⩽85 years old (with stably higher failure risk for all HFNC duration cohorts) and with BMI < 25 (who experienced a largely increasing failure risk with sequentially longer durations of HNFC), although subgroup inferences are limited by sample size.
Figure 1.
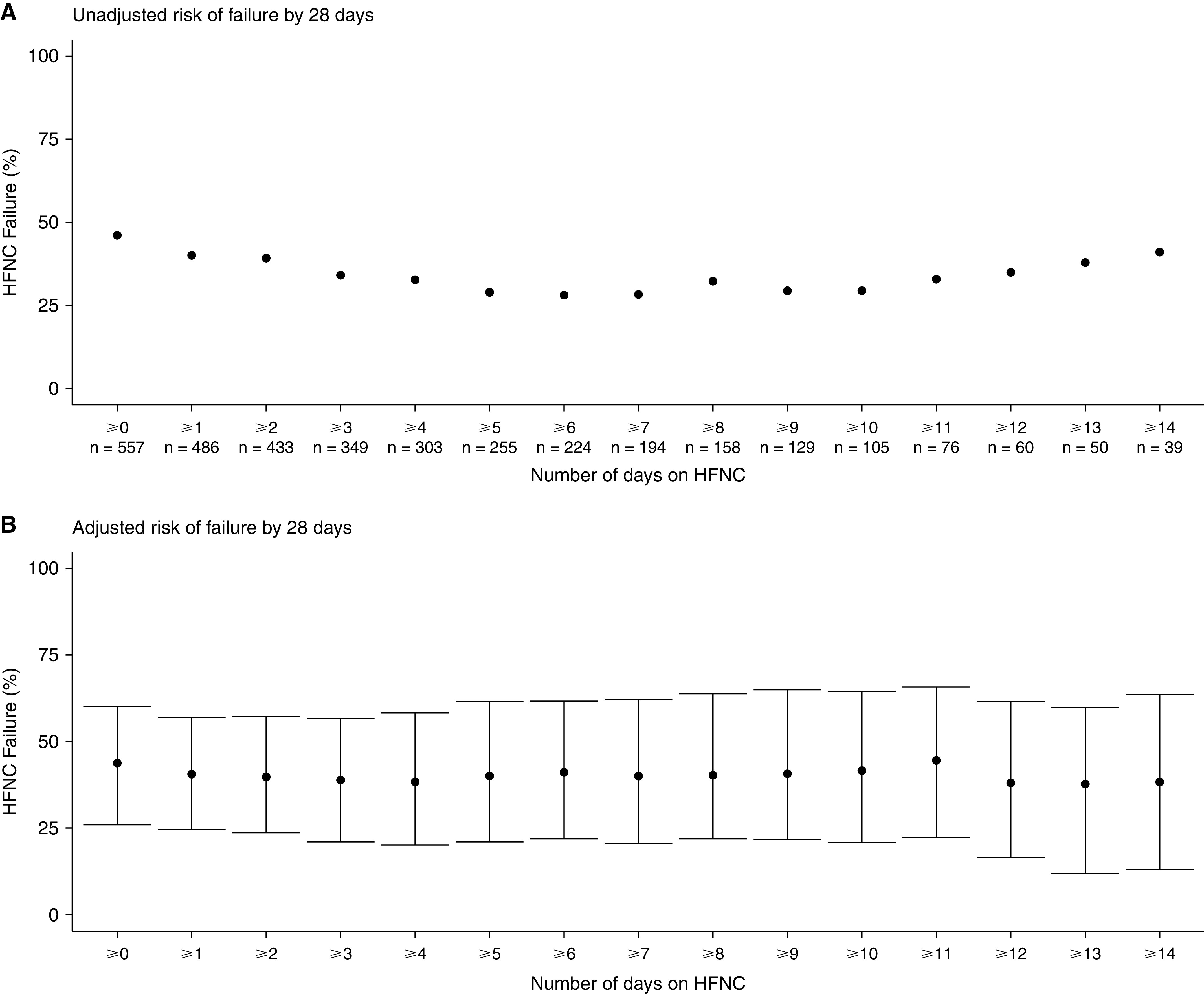
Crude and adjusted proportions of high-flow nasal cannula (HFNC) failure by to-date duration of use. Failure of HFNC is a composite outcome of either initiation of invasive mechanical ventilation or death without mechanical ventilation by Study Day 28. (A) Shows the crude risk of failure in the whole population, according to increasingly longer durations of respiratory support with HFNC, with the minimal duration of exposure and the number of patients in each nested cohort represented on the x-axis. (B) Shows the adjusted probability of HFNC failure by to-date duration of use. Point estimates of failure are based on regression modeling adjusted for age, sex, body mass index, respiratory rate, and SpO2 at time of study enrollment, and the number of comorbidities, with 95% confidence intervals denoted by the error bars. SpO2 = peripheral arterial oxygen saturation.
Table 1.
Failure of High-Flow Nasal Cannula, according to Exposure Duration and Patients’ Characteristics
| Nested Cohorts, n (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFNC, d | ⩾0 | ⩾1 | ⩾2 | ⩾3 | ⩾4 | ⩾5 | ⩾6 | ⩾7 | ⩾8 | ⩾9 | ⩾10 | ⩾11 | ⩾12 | ⩾13 | ⩾14 |
| Patients (n) | 557 | 486 | 433 | 349 | 303 | 255 | 224 | 194 | 158 | 129 | 105 | 76 | 60 | 50 | 39 |
| Treatment failure | |||||||||||||||
| All patients | 257 (46) | 195 (40) | 170 (39) | 119 (34) | 99 (33) | 74 (29) | 63 (28) | 55 (28) | 51 (32) | 38 (29) | 31 (30) | 25 (33) | 21 (35) | 19 (38) | 16 (41) |
| Age,* yr | |||||||||||||||
| <50 | 40/110 (36) |
26/93 (28) |
19/77 (25) |
15/64 (23) |
11/53 (21) |
10/49 (20) |
9/43 (21) |
8/39 (21) |
8/33 (24) |
7/27 (26) |
6/21 (29) |
5/14 (36) |
4/12 (33) |
4/11 (36) |
3/9 (33) |
| 50–64 | 97/215 (45) |
72/188 (38) |
64/169 (38) |
43/135 (32) |
37/118 (31) |
27/101 (27) |
23/87 (26) |
20/75 (27) |
18/60 (30) |
13/50 (26) |
10/41 (24) |
8/28 (29) |
6/21 (29) |
6/18 (33) |
5/11 (45) |
| 65–84 | 114/221 (52) |
91/194 (47) |
82/177 (46) |
56/141 (40) |
46/124 (37) |
32/97 (33) |
26/86 (30) |
22/72 (31) |
20/57 (35) |
15/46 (33) |
12/38 (32) |
10/30 (33) |
9/24 (38) |
7/18 (39) |
7/17 (41) |
| ⩾85 | 6/10 (60) |
6/10 (60) |
5/9 (56) |
5/9 (56) |
5/8 (62) |
5/8 (62) |
5/8 (62) |
5/8 (62) |
5/8 (62) |
3/6 (50) |
3/5 (60) |
2/4 (50) |
2/3 (67) |
2/3 (67) |
1/2 (50) |
| Sex | |||||||||||||||
| Male | 174/366 (48) |
131/318 (41) |
114/281 (41) |
78/224 (35) |
65/192 (34) |
48/161 (30) |
43/144 (30) |
38/124 (31) |
36/101 (36) |
25/82 (30) |
19/66 (29) |
14/45 (31) |
13/35 (37) |
11/29 (38) |
10/22 (45) |
| Female | 83/191 (43) |
64/168 (38) |
56/152 (37) |
41/125 (33) |
34/111 (31) |
26/94 (28) |
20/80 (25) |
17/70 (24) |
15/57 (26) |
13/47 (28) |
12/39 (31) |
11/31 (35) |
8/25 (32) |
8/21 (38) |
6/17 (35) |
| BMI,† kg/m2 | |||||||||||||||
| <25 | 39/84 (46) |
29/71 (41) |
27/63 (43) |
18/46 (39) |
16/37 (43) |
14/34 (41) |
13/28 (46) |
11/22 (50) |
11/21 (52) |
8/16 (50) |
7/15 (47) |
6/12 (50) |
6/11 (55) |
5/9 (56) |
4/7 (57) |
| 25–29 | 84/181 (46) |
65/161 (40) |
58/147 (39) |
41/125 (33) |
35/114 (31) |
26/97 (27) |
22/91 (24) |
19/80 (24) |
18/64 (28) |
13/52 (25) |
12/43 (28) |
9/28 (32) |
7/22 (32) |
7/20 (35) |
6/15 (40) |
| 30–34 | 67/138 (49) |
53/122 (43) |
44/105 (42) |
29/83 (35) |
25/75 (33) |
18/62 (29) |
14/52 (27) |
12/43 (28) |
11/34 (32) |
8/30 (27) |
6/25 (24) |
5/18 (28) |
4/12 (33) |
3/10 (30) |
3/9 (33) |
| ⩾35 | 30/80 (38) |
20/68 (29) |
16/58 (28) |
12/47 (26) |
10/36 (28) |
7/28 (25) |
5/21 (24) |
5/20 (25) |
5/17 (29) |
3/12 (25) |
2/9 (22) |
1/7 (14) |
1/6 (17) |
1/4 (25) |
1/3 (33) |
| SpO2:FiO2 | |||||||||||||||
| <190 | 233/448 (52) |
174/383 (45) |
149/346 (43) |
100/273 (37) |
81/242 (33) |
60/203 (30) |
55/185 (30) |
50/163 (31) |
46/135 (34) |
35/110 (32) |
28/90 (31) |
22/66 (33) |
18/51 (35) |
16/43 (37) |
14/34 (41) |
| ⩾190 | 24/109 (22) |
21/103 (20) |
21/87 (24) |
19/76 (25) |
18/61 (30) |
14/52 (27) |
8/39 (21) |
5/31 (16) |
5/23 (22) |
3/19 (16) |
3/15 (20) |
3/10 (30) |
3/9 (33) |
3/7 (43) |
2/5 (40) |
| Patient characteristics | |||||||||||||||
| DNI‡ | 45 (8) | 43 (9) | 38 (9) | 38 (11) | 37 (12) | 34 (13) | 33 (15) | 31 (16) | 28 (18) | 26 (20) | 22 (21) | 17 (22) | 13 (22) | 11 (22) | 9 (23) |
| Comorbidities§ | |||||||||||||||
| 0 | 265 (48) | 226 (46) | 204 (47) | 161 (46) | 141 (47) | 117 (46) | 101 (45) | 88 (45) | 71 (45) | 59 (46) | 45 (43) | 34 (45) | 29 (48) | 26 (52) | 18 (46) |
| 1 | 172 (31) | 155 (32) | 137 (32) | 109 (31) | 92 (30) | 75 (29) | 67 (30) | 56 (29) | 48 (30) | 37 (29) | 33 (31) | 24 (32) | 15 (25) | 10 (20) | 9 (23) |
| 2 | 96 (17) | 85 (17) | 76 (18) | 65 (19) | 57 (19) | 50 (20) | 45 (20) | 39 (20) | 30 (19) | 25 (19) | 20 (19) | 15 (20) | 13 (22) | 11 (22) | 10 (26) |
| 3 | 24 (4) | 20 (4) | 16 (4) | 14 (4) | 13 (4) | 13 (5) | 11 (5) | 11 (6) | 9 (6) | 8 (6) | 7 (7) | 3 (4) | 3 (5) | 3 (6) | 2 (5) |
| Outcomes | |||||||||||||||
| Initiated on IMV | 223 (40) | 161 (33) | 139 (32) | 88 (25) | 68 (22) | 44 (17) | 36 (16) | 28 (14) | 26 (16) | 15 (12) | 12 (11) | 11 (14) | 10 (17) | 10 (20) | 9 (23) |
| Died | 132 (24) | 117 (24) | 105 (24) | 74 (21) | 62 (20) | 50 (20) | 42 (19) | 38 (20) | 35 (22) | 31 (24) | 25 (24) | 19 (25) | 15 (25) | 13 (26) | 10 (26) |
| Died without intubation | 34 (6) | 34 (7) | 31 (7) | 31 (9) | 31 (10) | 30 (12) | 27 (12) | 27 (14) | 25 (16) | 23 (18) | 19 (18) | 14 (18) | 11 (18) | 9 (18) | 7 (18) |
| Initiated on NIV | 110 (20) | 70 (14) | 51 (12) | 37 (11) | 31 (10) | 19 (7) | 18 (8) | 14 (7) | 14 (9) | 8 (6) | 8 (8) | 6 (8) | 6 (10) | 6 (12) | 6 (15) |
| Weaned off HFNC | 300 (54) | 291 (60) | 263 (61) | 230 (66) | 204 (67) | 181 (71) | 161 (72) | 139 (72) | 107 (68) | 91 (71) | 74 (70) | 51 (67) | 39 (65) | 31 (62) | 23 (59) |
Definition of abbreviations: BMI = body mass index; DNI = do-not-intubate orders; HFNC = high-flow nasal cannula; IMV = invasive mechanical ventilation; NIV = noninvasive ventilation; SpO2:FiO2 = ratio of peripheral arterial oxygen saturation (SpO2) to the fraction of inspired oxygen (FiO2) (a value of 190 is equivalent to a ratio of partial pressure of arterial oxygen [PaO2] to FiO2 of 150 mm Hg [5]).
All outcomes are reported as cumulative incidences by Study Day 28. By that time frame, all patients who did not die and were not intubated were successfully weaned off HFNC.
Age was missing for one patient.
Body mass index was missing for a total of 74 (13.3%) patients.
This number combines patients who had do-not-intubate orders at enrollment and those whose ceiling of care was lowered after enrollment.
Prospectively defined and collected comorbidities were chronic heart disease, chronic lung disease, chronic kidney disease, severe liver disease, diabetes, and active malignancy.
After adjustment, we were not able to detect a similar pattern in the probability of failure, which, instead, appeared to remain stable even as time on HFNC progressed (Figure 1B). This stability was also seen for each component of the composite outcome of HFNC failure individually, with both HFNC device types and in patients without do-not-intubate orders.
Discussion
We found that crude rates of HFNC failure assumed a U-shape as a function of time to date using HFNC; specifically, only 1 in every 3.5 patients receiving HFNC for ⩽6 days experienced failure while rates were higher among patients receiving any (1 in every 2 patients) and ⩽14 days (1 in every 2.5 patients) of HFNC. However, after adjustment for patient characteristics, the probability of HFNC failure did not vary with the duration of previous support with HFNC.
Together these findings suggest that higher failure rates among cohorts inclusive of short-duration HFNC users likely reflect the fact that sicker patients received IMV early on. And while HFNC failure rates eventually increase after 10 days of receiving HFNC, this association is likely driven more by disease course and patient characteristics rather than any intrinsic harm associated with longer HFNC use itself. Moreover, there is no subgroup in which HFNC use for any amount of time up to 2 weeks is associated with odds of failure of >50%.
To our knowledge, this is the first study to evaluate the utility of continued HFNC stratified by duration of use to date in patients with COVID-19. Existing real-world evidence has suggested the rate of HFNC failure varies widely across patient groups (generally, ∼30–60% [3]). Understanding patient risk based on prior duration of HFNC will help clinicians prognosticate.
This study’s strengths include its diverse international population and the use of a standard strategy recommending when to initiate IMV, thereby limiting indication bias. Limitations include: the potential for residual confounding (as patient subgroups did experience different HFNC duration-associated failure rates); an inability to account for preenrollment HFNC use duration, although this was short as median time from hospital admission to enrollment was 1.0 (interquartile range, 0.4–1.5) day (2); potential barriers to generalizability as our cohort includes only patients enrolled in clinical trials; and a nonquantitative assessment of the pattern of failure rates across cohorts.
The role of HFNC in COVID-19-associated hypoxemia over the course of disease remains incompletely understood (4). Clinicians should take comfort, however, that continued use of HFNC even for patients already receiving it for 14 days does not appear futile.
Footnotes
Supported by UHealth Data Analytics Research Team (H.B.G.); Open AI Inc (I.P., S.E., Y.P., and E.T.); Rice Foundation (J.L. and D.V.); Projet Hospitalier de Recherche Clinique Interrégional, Appel d'Offre 2020, Groupement Interrégional de Recherche Clinique et d'Innovation Grand Ouest, Association pour la Promotion à Tours de la Réanimation Médicale, Fond de dotation du CHRU de Tours (S.E., Y.P., and E.T.); and Fisher & Paykel Healthcare Ltd (I.P., S.E., Y.P., and E.T.).
Author Contributions: H.B.G. conceived this study; I.P. and E.T. performed the statistical analyses; H.B.G. and I.P. drafted the manuscript; Y.P., M.I.-E., D.V., B.M., O.R., S.E., J.G.L., and J.L. reviewed the manuscript and contributed important intellectual content. All authors approved the final version of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202111-2509LE on February 17, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care . 2021;25:121. doi: 10.1186/s13054-021-03540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ehrmann S, Li J, Ibarra-Estrada M, Perez Y, Pavlov I, McNicholas B, et al. Awake Prone Positioning Meta-Trial Group Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med . 2021;9:1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crimi C, Pierucci P, Renda T, Pisani L, Carlucci A. High-flow nasal cannula and COVID-19: a clinical review. Respir Care . 2022;67:227–240. doi: 10.4187/respcare.09056. [DOI] [PubMed] [Google Scholar]
- 4. Ospina-Tascón GA, Calderón-Tapia LE, García AF, Zarama V, Gómez-Álvarez F, Álvarez-Saa T, et al. HiFLo-Covid Investigators Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a randomized clinical trial. JAMA . 2021;326:2161–2171. doi: 10.1001/jama.2021.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB, National Institutes of Health, National Heart, Lung, and Blood Institute ARDS Network Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest . 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]


