Abstract
Rationale
The outcomes of survivors of critical illness due to coronavirus disease (COVID-19) compared with non–COVID-19 are yet to be established.
Objectives
We aimed to investigate new disability at 6 months in mechanically ventilated patients admitted to Australian ICUs with COVID-19 compared with non–COVID-19.
Methods
We included critically ill patients with COVID-19 and non–COVID-19 from two prospective observational studies. Patients were eligible if they were adult (age ⩾ 18 yr) and received ⩾24 hours of mechanical ventilation. In addition, patients with COVID-19 were eligible with a positive laboratory PCR test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Measurements and Main Results
Demographic, intervention, and hospital outcome data were obtained from electronic medical records. Survivors were contacted by telephone for functional outcomes with trained outcome assessors using the World Health Organization Disability Assessment Schedule 2.0. Between March 6, 2020, and April 21, 2021, 120 critically ill patients with COVID-19, and between August 2017 and January 2019, 199 critically ill patients without COVID-19, fulfilled the inclusion criteria. Patients with COVID-19 were older (median [interquartile range], 62 [55–71] vs. 58 [44–69] yr; P = 0.019) with a lower Acute Physiology and Chronic Health Evaluation II score (17 [13–20] vs. 19 [15–23]; P = 0.011). Although duration of ventilation was longer in patients with COVID-19 than in those without COVID-19 (12 [5–19] vs. 4.8 [2.3–8.8] d; P < 0.001), 180-day mortality was similar between the groups (39/120 [32.5%] vs. 70/199 [35.2%]; P = 0.715). The incidence of death or new disability at 180 days was similar (58/93 [62.4%] vs. 99/150 [66/0%]; P = 0.583).
Conclusions
At 6 months, there was no difference in new disability for patients requiring mechanical ventilation for acute respiratory failure due to COVID-19 compared with non–COVID-19.
Clinical trial registered with www.clinicaltrials.gov (NCT04401254).
Keywords: SARS-CoV-2, long COVID, critical care, recovery, long-term outcomes
At a Glance Commentary
Scientific Knowledge on the Subject
Few studies have reported the 6-month outcomes of critically ill patients with coronavirus disease (COVID-19), the change in functional outcomes at 6 months compared to preillness levels of functioning, or a comparison with other critically ill patients with acute respiratory failure.
What This Study Adds to the Field
At 6 months after ICU admission, there was no difference in the incidence of new disability, the severity of disability, psychological function, cognitive function, or health-related quality of life in critically ill patients with COVID-19 compared to non–COVID-19 with acute respiratory failure requiring mechanical ventilation. Both patients with COVID-19 and patients with non–COVID-19 reported new disabilities in all domains of functioning, including physical function (e.g., walking or standing for long periods), psychological function (e.g., how emotionally affected they were by their illness), and cognitive function (e.g., concentrating or learning a new task). The global pandemic has highlighted the importance of early detection and screening for new disabilities, functional impairment, and ongoing symptoms for survivors of acute respiratory failure. Survivors with new disability should be referred to appropriate services after hospital discharge to enhance recovery.
Over the past decade, there has been increasing research into the long-term effects of critical illness (1–3). Many survivors of critical illness have long-term impairments in physical, psychological, and cognitive functioning and health-related quality of life (2, 4). There remain, however, important questions about recovery after critical illness because of heterogeneity of the population, differences in baseline functioning, and varying support in intensive care (5, 6).
An emerging, urgent public health problem as a result of the coronavirus disease (COVID-19) pandemic is the long-term effects in survivors of COVID-19 (7). To date, there have been widespread international reports of ongoing symptoms, reduced lung capacity, organ dysfunction, disability, and reduced health-related quality of life in the months after COVID-19 (8–10). In a recent multicenter study, the long-term impairments in patients who were critically ill with COVID-19 were substantial, with more than one-third of survivors reporting new disabilities at 6 months (11). However, the burden of disability and the long-term outcomes of survivors of critical illness after acute respiratory failure due to COVID-19 compared with non–COVID-19 are yet to be established (12–14).
The primary aim of the study was to compare death or new disability of mechanically ventilated patients with acute respiratory failure admitted to Australian ICUs with COVID-19 with non–COVID-19 at 6 months after ICU admission. The secondary aim was to compare functional outcomes of survivors between the two groups. We hypothesized that death or new disability would be worse in mechanically ventilated patients with acute respiratory failure due to COVID-19 than in those without COVID-19.
Methods
Study Design
We included patients critically ill with COVID-19 and non–COVID-19 from two prospective observational studies in Australia with follow-up at 6 months to compare outcomes. The COVID-Recovery study was a registry-embedded, prospective cohort study conducted at 26 ICUs in Australia that enrolled patients with confirmed COVID-19 infection (see Table E1 in the online supplement) (11). The study was performed between March 6, 2020 and April 21, 2021. We included patients without COVID-19 from the PREDICT study, a prospective, multicenter, longitudinal cohort study conducted at six metropolitan ICUs in the state of Victoria, Australia (Table E1) (15). The study was performed between August 2017 and January 2019. Both studies included a waiver of consent for hospital data and an opt-out consent for follow-up at 6 months by telephone.
Patients Included with COVID-19 versus Non–COVID-19
Patients with COVID-19 were eligible if they were adults (age ⩾ 18 yr), had a positive laboratory PCR test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), admitted to the ICU, and received >24 hours of mechanical ventilation. Patients without COVID-19 were eligible if they were adults (age ⩾ 8 yr), had been admitted to the ICU, and received >24 hours of mechanical ventilation because of acute respiratory failure not due to congestive heart failure (the codes used are described in Table E2).
We used a convenient sample size of all available patients. The total study population was the hospital cohort, comprising all patients who were eligible for inclusion in the study (Figure E1). The follow-up cohort was eligible patients with a known outcome at 6 months (i.e., survival with or without disability or death).
Data Collection and Outcomes
Demographic, intervention, and hospital outcome data were obtained for the hospital cohort (15, 16). The follow-up cohort was first contacted by mail for opt-out consent and then followed up by telephone with trained outcome assessors either centrally or at the site. Baseline health and disability in the month before ICU admission were assessed retrospectively at 6 months.
Patient-reported Outcomes
The patient-reported outcome measures were evaluated at 6 months in responders and are detailed in Table E3, including global health with the World Health Organization’s Disability Assessment Schedule (WHODAS 2.0 12L), health status with the EQ-5D-5 level (EQ-5D-5L), anxiety and depression with the Hospital Anxiety and Depression Scale (HADS), screening for post-traumatic stress with the Impact of Events-6, cognitive function with the Montreal Cognitive Assessment-BLIND, and activities of daily living with the Instrumental Activities of Daily Living. Most of these were recommended by the core outcome set for survivors of acute respiratory failure (17).
The WHODAS is reported as a percentage score, where new disability is an increase in WHODAS at 6 months from baseline of 10%. Validated and previously published definitions of mild, moderate, and severe disability were used (4, 18). The domains of the EQ-5D-5L were described as “no problems” and “new problems,” where the score for the specific component at 6 months was higher than at baseline. The EQ-5D-5L utility score and the EQ-5D visual analogue scale were also reported (17, 19).
Significant anxiety was defined as HADS anxiety score of eight or higher and significant depression as HADS depression score of eight or higher (20). The WHODAS work question reported unemployment due to poor health (21). An Impact of Events Scale-6 score of greater than or equal to 1.75 was used to screen for post-traumatic stress (22), fully independent was defined as an Instrumental Activities of Daily Living score of 8 (23), and cognitive dysfunction as a Montreal Cognitive Assessment score of less than 18 (24, 25).
Statistical Analysis
Continuous variables are reported as median and interquartile range and categorical variables as number and percentage. Comparisons between groups were analyzed using Wilcoxon rank-sum or Kruskal-Wallis tests for continuous variables and Fisher exact test for categorical variables. The primary outcome (death or new disability) was compared using a Fisher exact test, and survival to 180 days was compared using a log-rank test and presented as Kaplan-Meier curves. For the secondary outcomes, comparisons of categorical outcomes between the groups were examined using mixed-effect generalized linear models with binomial distribution and identity link and reported as risk difference. Mortality at 180 days was presented in Kaplan-Meier curves and compared with log-rank tests.
For the secondary outcomes, comparisons of categorical outcomes between the groups were examined using mixed-effect generalized linear models with binomial distribution and identity link and reported as risk difference. For continuous outcomes, a mixed-effect quantile model considering a Τ = 0.50, an interior point algorithm, and reported as median difference was used. For the five WHODAS categories, a mixed-effect cumulative logistic model was used and reported as common odds ratio (COR). In all models, the center and the wave of admission (first vs. second) were included as random effects to account for clustering of the data, and 95% confidence intervals (CIs) were reported.
To adjust for potential imbalances between groups that could affect 6-month outcomes, the following covariates were included as fixed effects in the models described above: age, sex, Acute Physiology and Chronic Health Evaluation (APACHE) II score, body mass index, clinical frailty scale, duration of ventilation, use of renal replacement therapy, and need for tracheostomy. These variables were selected a priori and based on clinical relevance only. Whenever available, the models were further adjusted by the baseline value of the outcome of interest (for example, if WHODAS at 6 months was the outcome of interest, WHODAS assessed at baseline was included as an additional confounder). Based on multiple linear regression with 145 patients and eight predictor variables, this study had an 86% power (two-sided P value of 0.05) to detect a partial R2 of 6%.
As a sensitivity analysis, the models above were reassessed by adjusting each patient’s risk of having COVID-19, with the centers included as random effect. The risk of having COVID-19 was determined using a logistic model considering all covariates described above. Because of the number of comparisons, a two-sided P value of less than 0.01 was considered as evidence of statistical significance to adjust for multiplicity. All analyses were performed using R software, version 4.0.2 (R Core Team) (26).
Results
Patients
Between March 6, 2020, and April 21, 2021, 274 critically ill patients with COVID-19 were enrolled in COVID-Recovery from 26 sites in six states of Australia (Figure 1). After exclusions, 120 fulfilled the inclusion criteria of the present study. Of these, 22 were unable to be contacted and were lost to follow-up. Between August 2017 and January 2019, 888 critically ill patients were enrolled in PREDICT in six sites, with 199 patients fulfilling the inclusion criteria of the present study (Figure 1). Of these, 41 were unable to be followed up (13 were assessed at 3 months but opted out of follow-up at 6 months, 15 opted out of 3- and 6-month follow-up, 7 remained in the hospital, and 6 were lost to follow-up).
Figure 1.

Study flowcharts of (A) the original COVID-Recovery study and (B) the original PREDICT study (Predicting the Outcome of Critically Ill Patients). COVID-19 = coronavirus disease; EQ-5D-5L = EQ-5D-5 level; HADS = Hospital Anxiety and Depression Scale; IADL = Instrumental Activities of Daily Living; IES-6 = Impact of Events Scale-6; MoCA-BLIND = Montreal Cognitive Assessment; SPRINT-SARI = Short Period Incidence Study of Severe Acute Respiratory Infection; WHODAS = World Health Organization Disability Assessment Schedule.
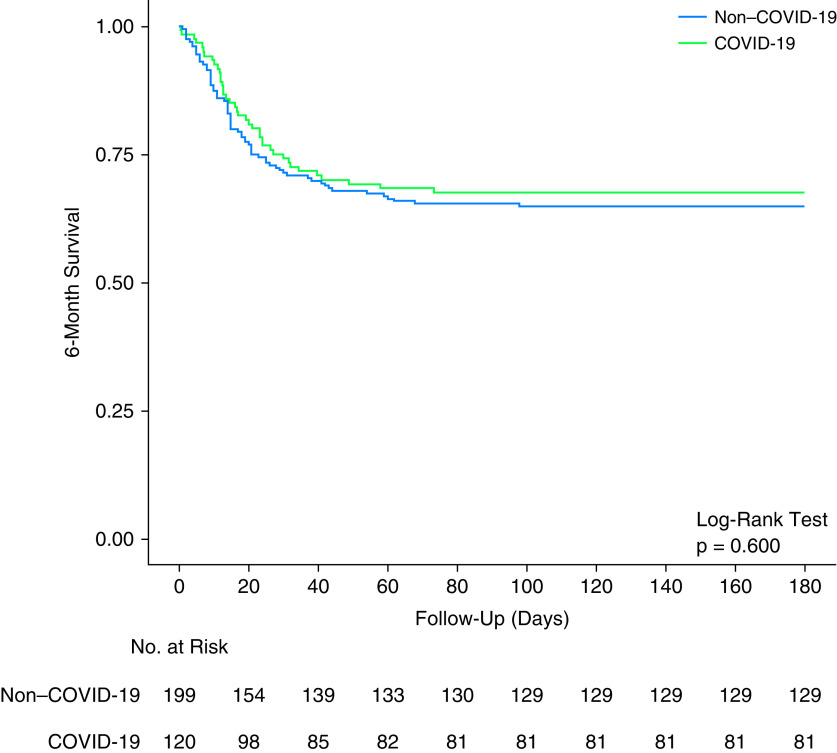
Baseline characteristics of the hospital cohort at hospital admission are shown in Table 1. Overall, median age was 60 (47–69) years, 191 (59.8%) patients were male, and the median APACHE II score was 18 (14–22). Patients with COVID-19 were older, had higher body mass index and clinical frailty scale, lower APACHE II score, higher prevalence of chronic cardiac failure, higher respiratory rate and temperature at baseline, and less often received renal replacement therapy. Although duration of ventilation and ICU and hospital length of stay were longer in patients with COVID-19, mortality was similar between the groups (Table 1 and Figure 2).
Table 1.
Baseline Characteristics and Clinical Outcomes of the Included Patients
| Hospital Cohort |
Follow-Up Cohort* |
|||||
|---|---|---|---|---|---|---|
| COVID-19 (n = 120) | Non–COVID-19 (n = 199) | P Value | COVID-19 (n = 98) | Non–COVID-19 (n = 158) | P Value | |
| Age, yr | 62 (55–71) | 58 (44–69) | 0.019 | 62 (56–71) | 60 (44–69) | 0.016 |
| Male sex | 77 (64.2) | 114 (57.3) | 0.240 | 64 (65.3) | 91 (57.6) | 0.238 |
| APACHE II | 17 (13–20) | 19 (15–23) | 0.011 | 17 (13–20) | 20 (15–23) | 0.009 |
| Body mass index, kg/m2 | 29.5 (25.6–35.4) | 27.2 (22.9–31.6) | <0.001 | 29.5 (25.5–35.1) | 27.1 (22.7–31.7) | 0.002 |
| Coexisting disorders | ||||||
| Diabetes | 42/118 (35.6) | 69/192 (35.9) | 0.999 | 36/96 (37.5) | 54/153 (35.3) | 0.787 |
| Obesity | 39/117 (33.3) | 52/157 (33.1) | 0.999 | 31/95 (32.6) | 43/129 (33.3) | 0.999 |
| Chronic cardiac failure | 25/117 (21.4) | 6/199 (3.0) | <0.001 | 22/95 (23.2) | 4/158 (2.5) | <0.001 |
| Immunosuppression | 13/117 (11.1) | 26/199 (13.1) | 0.724 | 10/95 (10.5) | 21/158 (13.3) | 0.559 |
| Chronic kidney disease | 9/118 (7.6) | 8/199 (4.0) | 0.200 | 8/96 (8.3) | 7/158 (4.4) | 0.272 |
| Chronic pulmonary disease | 10/117 (8.5) | 21/199 (10.6) | 0.696 | 9/96 (9.4) | 20/158 (12.7) | 0.543 |
| Baseline function | ||||||
| EQ-5D-5L utility scale | 1.0 (0.8–1.0) | 0.7 (0.4–1.0) | <0.001 | 1.0 (0.8–1.0) | 0.8 (0.5–1.0) | <0.001 |
| EQ-5D-5L visual analogue scale | 85 (80–95) | 70 (40–85) | <0.001 | 85 (80–95) | 70 (43.8–90) | <0.001 |
| WHODAS score, % | 0.0 (0.0–2.1) | 12.5 (1.6–39.6) | <0.001 | 0.0 (0.0–2.1) | 9.4 (0.0–33.9) | <0.001 |
| Disability | 1/56 (1.8) | 38/92 (41.3) | <0.001 | 1/56 (1.8) | 27/80 (33.8) | <0.001 |
| First 24 h of ICU admission | ||||||
| Vital signs | ||||||
| Heart rate, bpm | 101 (90–112) | 97 (84–111) | 0.108 | 100 (89–110) | 98 (85–114) | 0.537 |
| Respiratory rate, breaths/min | 32 (24–39) | 19 (16–24) | <0.001 | 32 (24–38) | 19 (16–23) | <0.001 |
| Mean arterial pressure, mm Hg | 78 (68–94) | 75 (72–81) | 0.294 | 80 (68–94) | 76 (71–81) | 0.324 |
| Temperature, °C | 38.5 (37.5–39.0) | 36.8 (36.4–37.2) | <0.001 | 38.3 (37.4–38.9) | 36.8 (36.3–37.2) | <0.001 |
| Laboratory tests | ||||||
| PaO2/FiO2, mm Hg | 125 (89–181) | 150 (108–246) | 0.002 | 125 (88–170) | 152 (107–250) | 0.001 |
| 200–300 mm Hg | 17/107 (15.9) | 35/152 (23.0) | 0.122 | 13/89 (14.6) | 26/122 (21.3) | 0.266 |
| 100–200 mm Hg | 50/107 (46.7) | 77/152 (50.7) | 43/89 (48.3) | 62/122 (50.8) | ||
| <100 mm Hg | 30/107 (37.4) | 40/152 (26.3) | 33/89 (37.1) | 34/122 (27.9) | ||
| FiO2 | 0.60 (0.45–0.97) | 0.60 (0.45–1.00) | 0.208 | 0.60 (0.45–0.99) | 0.60 (0.45–1.00) | 0.438 |
| PaCO2, mm Hg | 38 (34–47) | 42 (36–50) | 0.048 | 38 (34–46) | 42 (35–50) | 0.109 |
| pH | 7.40 (7.34–7.46) | 7.31 (7.24–7.40) | <0.001 | 7.40 (7.34–7.47) | 7.30 (7.24–7.40) | <0.001 |
| Support during ICU stay | ||||||
| Renal replacement therapy | 27/116 (23.3) | 52/133 (39.1) | 0.009 | 24/94 (25.5) | 45/107 (42.1) | 0.017 |
| Inotropes and/or vasopressors | 109/116 (94.0) | 120/134 (89.6) | 0.256 | 88/94 (93.6) | 98/108 (90.7) | 0.603 |
| ECMO | 7/116 (6.0) | 17/178 (9.6) | 0.384 | 7/94 (7.4) | 14/145 (9.7) | 0.644 |
| Tracheostomy | 21/116 (18.1) | 17/134 (12.7) | 0.290 | 15/94 (16.0) | 14/108 (13.0) | 0.554 |
| Clinical outcomes | ||||||
| Death or new disability at 6 mo | — | — | — | 58/93 (62.4) | 99/150 (66.0) | 0.583 |
| Duration of ventilation, d | 12.0 (5.0–19.0) | 4.8 (2.3–8.8) | <0.001 | 13.0 (5.0–19.0) | 5.2 (2.4–9.4) | <0.001 |
| ICU length of stay, d | 15.9 (7.6–26.4) | 8.8 (4.4–13.8) | <0.001 | 15.9 (7.5–25.8) | 8.9 (4.3–13.8) | <0.001 |
| Hospital length of stay, d | 22.9 (12.6–40.3) | 18.0 (9.8–30.8) | 0.030 | 22.4 (12.2–40.6) | 17.8 (9.7–30.1) | 0.052 |
| ICU mortality | 36/120 (30.0) | 49/199 (24.6) | 0.299 | 36/98 (36.7) | 49/158 (31.0) | 0.413 |
| Hospital mortality | 38/119 (31.9) | 64/199 (32.2) | 0.999 | 38/98 (38.8) | 64/158 (40.5) | 0.794 |
| 180-d mortality | 39/120 (32.5) | 70/199 (35.2) | 0.715 | 39/98 (39.8) | 70/158 (44.3) | 0.517 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; COVID-19 = coronavirus disease; ECMO = extracorporeal membrane oxygenation; EQ-5D-5L = EQ-5D-5 level; WHODAS = World Health Organization Disability Assessment Schedule.
Data are median (quartile 1–quartile 3) or n (%). Percentages may not total 100 because of rounding.
The follow-up cohort comprises patients who died within 6 months or who were contacted successfully at 6 months.
Figure 2.
Kaplan-Meier curves of 6-month survival in patients with coronavirus disease (COVID-19) and non–COVID-19.
Baseline Function, Disability, and Health Status
At baseline, the patients with COVID-19 had lower median WHODAS scores (0% [0% to 2%] vs. 12% [2% to 40%]; median difference, −12.50 [95% CI, −21.31 to −3.69]; P = 0.006). The prevalence and severity of preexisting disability was lower in patients with COVID-19 (1.8% vs. 41.3%; risk difference, −39.51 [95% CI, −50.12 to −28.72]; P < 0.001; and COR, 0.09 [95% CI, 0.04 to 0.21]; P < 0.010) (Figure 2 and Table E6).
At baseline, the median EQ-5D-5L utility scale was higher in patients with COVID-19 (1.0 [0.8–1.0] vs. 0.7 [0.4–1.0]; median difference, 0.25 [95% CI, 0.14–0.36]; P < 0.001; Figure 3 and Table E6). Across all domains of the EQ-5D-5L, the prevalence of patients reporting no problems was higher in patients with COVID-19.
Figure 3.

World Health Organization Disability Assessment Schedule (WHODAS) score and EQ-5D-5 level (EQ-5D-5L) scale in patients with coronavirus disease (COVID-19) and non–COVID-19. (A) Comparison of WHODAS score at baseline and 6 months (P values from a mixed-effect quantile model considering a Τ = 0.50, an interior point algorithm, and including center as random effect). (B) Proportion of patients developing no (WHODAS < 5%), mild (5% ⩽ WHODAS < 25%), moderate (25% ⩽ WHODAS < 50%), or severe (50% ⩽ WHODAS < 96%) disability. P values for comparisons are shown in Table 2. (C) Comparison of EQ-5D-5L scale at baseline and 6 months (P values from a mixed-effect quantile model considering a Τ = 0.50, an interior point algorithm, and including center as random effect). (D) Proportion of patients not reporting problems with mobility, personal care, usual activities, pain/discomfort, or anxiety/depression. P values for comparisons are shown in Table 2. VAS = visual analogue scale.
Primary Outcome
At 6 months, there was no difference in the incidence of death or new disability between patients with COVID-19 compared with non–COVID-19 acute respiratory failure (58/93 [62.4%] vs. 99/150 [66.0%]; P = 0.583) (Table 1). The baseline characteristics and clinical outcomes of patients lost to follow-up in the study are shown in Table E4. Patients with COVID-19 were older, were more likely to have chronic cardiac failure, and had higher respiratory rate, pH, and temperature at admission to the ICU.
Secondary Outcomes at 6 Months
There was one patient with COVID-19 and six patients without COVID-19 who died after hospital discharge and before 6 months; these patients were not included in the secondary outcomes. The rate of missing data for functional outcomes in survivors at 6 months is shown in Table E5. Before adjustment for confounders, the WHODAS percentage score and the severity of disability were lower in patients with COVID-19 (6% [2% to 17%] vs. 22% [4% to 44%]; median difference, −16.00 [95% CI, −23.48 to −8.47]; P < 0.001; and COR, 0.32 [95% CI, 0.17 to 0.60]; P < 0.001) (Figure 3 and Table 2). However, after adjustment for confounders, the WHODAS percentage score, incidence of new disability, and severity of disability were similar between the groups (P = 0.36, P = 0.65, and P = 0.81, respectively). Development of new disability occurred in each of the domains assessed by the WHODAS for both COVID-19 and non–COVID-19 survivors (Figure E1).
Table 2.
Functional Outcomes at 6 Months
| COVID-19 (n = 98) |
Non–COVID-19 (n = 158) |
Unadjusted Analysis* |
Adjusted Analysis*† |
|||
|---|---|---|---|---|---|---|
| Absolute Difference (95% CI) | P Value | Absolute Difference (95% CI) | P Value | |||
| EQ-5D-5L | ||||||
| Utility score | 0.8 (0.7 to 0.9) (n = 57) | 0.7 (0.5 to 0.9) (n = 88) | MD, 0.06 (−0.02 to 0.15) | 0.140 | MD, −0.05 (−0.15 to 0.06)‡ | 0.390 |
| New problems with mobility§ | 19/56 (33.9) | 18/81 (22.2) | RD, 11.70 (−3.44 to 27.16) | 0.135 | RD, 10.86 (−10.98 to 32.03) | 0.631 |
| New problems with personal care§ | 8/55 (14.5) | 23/81 (28.4) | RD, −13.85 (−27.07 to 0.22) | 0.059 | RD, −7.38 (−30.45 to 8.25) | 0.753 |
| New problems with usual activities§ | 27/55 (49.1) | 36/81 (44.4) | RD, 4.64 (−12.69 to 21.98) | 0.597 | RD, 15.53 (−8.70 to 40.31) | 0.230 |
| New problems with pain/discomfort§ | 24/55 (43.6) | 26/81 (32.1) | RD, 11.54 (−5.13 to 28.20) | 0.173 | RD, 15.87 (−7.25 to 39.00) | 0.197 |
| New problems with anxiety/depression§ | 8/55 (14.5) | 11/81 (13.6) | RD, 0.96 (−11.10 to 13.03) | 0.874 | RD, 3.58 (−14.84 to 20.59) | 0.834 |
| EQ-5D-5L visual analogue scale | 70 (60 to 85) (n = 56) | 70 (50 to 80) (n = 87) | MD, −0.00 (−13.45 to 13.45) | 0.999 | MD, −2.69 (−10.70 to 5.31)‡ | 0.511 |
| Financial distressǁ | 1 (1 to 4) | 1 (1 to 6) | MD, −0.00 (−1.54 to 1.54) | 0.999 | MD, −0.28 (−1.65 to 1.07) | 0.682 |
| Unemployed because of health issues | 5/57 (8.8) | 43/88 (48.9) | RD, −40.09 (−52.30 to −26.65) | <0.001 | RD, −39.87 (−58.60 to −21.14) | <0.001 |
| WHODAS score, % | 6.2 (2.1 to 16.7) (n = 57) | 21.9 (4.2 to 43.8) (n = 88) | MD, −16.00 (−23.48 to −8.47) | <0.001 | MD, −3.19 (−9.94 to 3.55)‡ | 0.355 |
| New disability¶ | 19/54 (35.2) | 29/80 (36.2) | RD, −1.06 (−17.89 to 15.76) | 0.901 | RD, 5.58 (−17.72 to 28.88) | 0.653 |
| Disability | 9/57 (15.8) | 40/88 (45.5) | RD, −29.66 (−44.90 to −14.42) | <0.001 | RD, −13.69 (−37.00 to 9.63)‡ | 0.272 |
| No disability | 27/57 (47.4) | 23/88 (26.1) | COR, 0.32 (0.17 to 0.60)** | <0.001 | COR, 1.14 (0.37 to 3.55)‡** | 0.814 |
| Mild disability | 21/57 (36.8) | 25/88 (28.4) | ||||
| Moderate disability | 6/57 (10.5) | 26/88 (29.5) | ||||
| Severe disability | 3/57 (5.3) | 13/88 (14.8) | ||||
| Complete disability | 0/57 (0.0) | 1/88 (1.1) | ||||
| IADL | 8 (7 to 8) (n = 48) | 8 (6 to 8) (n = 88) | MD, −0.00 (−0.92 to 0.92) | 0.999 | MD, 0.59 (−0.47 to 1.65) | 0.275 |
| Fully independent | 32/48 (66.7) | 45/88 (51.1) | RD, 15.53 (−2.00 to 33.04) | 0.081 | RD, 24.09 (−0.31 to 48.50) | 0.066 |
| HADS anxiety | 2 (0 to 6) (n = 44) | 4 (1 to 9) (n = 59) | MD, −2.00 (−4.65 to 0.66) | 0.143 | MD, −1.09 (−3.16 to 0.97) | 0.302 |
| Anxiety | 8/44 (18.2) | 19/59 (32.2) | RD, −14.02 (−31.35 to 3.30) | 0.112 | RD, −14.29 (−38.97 to 10.40) | 0.286 |
| HADS depression | 2 (1 to 5) (n = 44) | 3 (1 to 7) (n = 58) | MD, −1.00 (−2.63 to 0.63) | 0.233 | MD, 0.11 (−2.04 to 2.27) | 0.917 |
| Depression | 7/44 (15.9) | 10/58 (17.2) | RD, −1.33 (−16.25 to 13.59) | 0.859 | RD, −4.31 (−25.01 to 16.41) | 0.700 |
| IES-R total | 2 (1 to 8) (n = 44) | 1 (0 to 5) (n = 49) | MD, −1.00 (−1.91 to 3.91) | 0.502 | MD, 0.52 (−2.77 to 3.81) | 0.759 |
| Mean score | 0.3 (0.2 to 1.3) (n = 44) | 0.2 (0.0 to 0.8) (n = 49) | MD, 0.17 (−0.32 to 0.65) | 0.502 | MD, 0.09 (−0.46 to 0.63) | 0.759 |
| Post-traumatic stress disorder | 9/44 (20.5) | 7/49 (14.3) | RD, 6.16 (−9.51 to 21.85) | 0.437 | RD, −3.01 (−25.23 to 19.20) | 0.802 |
| MoCA-BLIND | 18 (17 to 21) (n = 34) | 20 (17 to 21) (n = 44) | MD, −2.00 (−4.29 to 0.29) | 0.091 | MD, −0.51 (−3.80 to 2.78) | 0.762 |
| Cognitive dysfunction | 14/34 (41.2) | 13/44 (29.5) | RD, 11.63 (−10.12 to 33.38) | 0.290 | RD, 13.57 (−17.63 to 44.76) | 0.430 |
Definition of abbreviations: CI = confidence interval; COR = common odds ratio; COVID-19 = coronavirus disease; EQ-5D-5L = EQ-5D-5 level; HADS = Hospital Anxiety and Depression Scale; IADL = Instrumental Activities of Daily Living; IES-R = Impact of Events Scale-Revised; MD = median difference; MoCA-BLIND = Montreal Cognitive Assessment; RD = risk difference; WHODAS = World Health Organization Disability Assessment Schedule.
Data are median (quartile 1–quartile 3) or n (%). Percentages may not total 100 because of rounding.
Values greater than 0 indicate a higher score or proportion in patients with COVID-19 than in those with non–COVID-19.
Adjusted for age, sex, APACHE II score, body mass index, clinical frailty score, duration of ventilation, use of renal replacement therapy, and need for tracheostomy and including the center and the wave of admission (first vs. second wave) as random effect.
Further adjusted by the baseline value of the score.
New problems defined when the score of the specific component of EQ-5D-5L at 6 months was higher than at baseline.
On a scale from 1 to 10 (1 would be the lowest level of financial distress).
Calculated as the difference in the score at 6 months and baseline (positive values indicates an increase at 6 months).
Values less than 1 indicate a lower severity in patients with COVID-19 than in those with non–COVID-19.
Before and after adjustment, both EQ-5D-5L utility scale and the EQ-5D visual analogue scale were similar between the groups (Figure 3 and Table 2). After adjustment for confounders, there was no difference in the incidence of new problems between the groups across all domains of the EQ5D-5L (Table 2).
At 6 months, the incidence of anxiety, depression, cognitive dysfunction, or positive screening for post-traumatic stress disorder were all similar between the groups (Table 2). After adjustment for confounders, no difference was found in any of these outcomes (Table 2).
Sensitivity Analysis
The sensitivity analysis is reported in Table E7 and confirms the original findings.
Discussion
Key Findings
In this study of patients mechanically ventilated for acute respiratory failure, we found that the incidence of new disability, the severity of disability, health-related quality of life, psychological function, and cognitive function at 6 months were similar between COVID-19 and non–COVID-19 survivors. Both patients with COVID-19 and patients with non–COVID-19 reported new disabilities in all domains of functioning, including physical function (e.g., walking, standing for long periods), psychological function (e.g., how emotionally affected they were by their illness), and cognitive function (e.g., concentrating, learning a new task).
The global pandemic has highlighted the importance of screening and early identification of new disabilities, functional impairment, and ongoing symptoms for survivors of COVID-19 critical illness (27, 28). This study has shown that screening of premorbid functional status is important for all survivors of critical illness because of acute respiratory failure. The rate of new disability and new problems was similar regardless of a diagnosis of COVID-19.
In a recent study of 478 hospitalized patients with COVID-19 in a single center in France, 142 patients had been critically ill and approximately 50% had been mechanically ventilated (29). Lung computed tomography scan in survivors at 4 months after hospitalization showed abnormalities in 75% who had received invasive ventilation. However, pulmonary function was mostly preserved, and impaired cardiac or kidney function were uncommon. Several recent studies have also described persistent symptoms, weakness, and reduced health-related quality of life after COVID-19 critical illness (30, 31). However, there are very few reports that measured preillness function to determine changes from baseline or compared outcomes to survivors of non–COVID-19 acute respiratory failure requiring mechanical ventilation. This current study shows that although new disability is common in survivors of COVID-19 critical illness, it is not different from the rate of onset of new disability in survivors of non–COVID-19 acute respiratory failure, despite increased duration of mechanical ventilation and increased length of stay in the ICU in survivors of COVID-19.
Poor cognitive function has been reported after COVID-19 in several studies. In a single-center study of 29 survivors of COVID-19, 59–65% had cognitive dysfunction 4 months after hospital discharge (32). Cognitive impairments were associated with the degree of long-term pulmonary dysfunction, increased respiratory symptoms, and D-dimer concentrations during acute illness, and the authors suggested a potential link to restricted oxygen delivery to the brain. Importantly, subjective cognitive complaints had a strong correlation with objectively measured global cognitive impairments. Cognitive impairments have previously been reported in critically ill survivors of non–COVID-19 acute respiratory failure or shock. In a large cohort study, 821 patients were evaluated at 3 months after critical illness, and 40% had cognitive impairment (33). This impairment was still present in >25% of the cohort at 12 months and was described as similar to moderate traumatic brain injury or mild Alzheimer’s disease. In the current study, patient-reported cognitive function was measured, with the finding that cognitive impairment was similar between survivors of COVID-19 and non–COVID-19 acute respiratory failure. Future studies should further explore the link between brain oxygen delivery and cognitive outcomes and therapies that may attenuate the effect of acute respiratory failure on cognitive impairment.
The strengths of this study include its prospective, multicenter design with detailed clinical and functional outcomes. Baseline measures of disability and health-related quality of life enabled evaluation of new disability and changes in health status. The outcome measures include validated, reliable measures of function (17). Several limitations to this study warrant acknowledgment. Patients with COVID-19 who were mechanically ventilated for >24 hours were included to draw comparison with an existing cohort of patients with non–COVID-19 with similar outcomes; however, the patients were not matched, and there were some baseline differences that may affect the results. With patients in the non–COVID-19 group coming from only six metropolitan hospitals, and in particular 64% of patients coming from the same hospital, our comparator group is potentially less diverse. Baseline function was assessed retrospectively at 6 months, which may introduce recall bias. In the absence of daily organ failure data, a further limitation of this study was our inability to accurately measure severity of illness with a reliance on severity markers collected at ICU admission. Also, as a result of the opt-out consent process for follow-up interviews, there were missing functional outcome data for patients who were unable or unwilling to participate. Such missingness reduces our ability to quantify the underlying relationship between COVID-19 and outcome. Finally, the results were from a health system in Australia that was not overloaded, and the long-term outcomes of other regions, including overloaded systems or lower-middle-income countries may be different.
Conclusions
At 6 months, there was no difference in the incidence of new disability, the severity of disability, psychological function, cognitive function, or health-related quality of life in patients with COVID-19 compared with non–COVID-19 with acute respiratory failure requiring mechanical ventilation. The global pandemic has highlighted the importance of early detection and screening for new disabilities, functional impairment, and ongoing symptoms for all survivors of acute respiratory failure.
Acknowledgments
Acknowledgment
This work was completed with thanks to the patients, families, and all staff who contributed to the care of COVID-19 patients in Australian ICUs and the SPRINT-SARI (Short Period Incidence Study of Severe Acute Respiratory Infection) Australia management committee, investigators, and research coordinators. SPRINT-SARI Australia is supported by the Department of Health, Commonwealth of Australia (Standing Deed SON60002733).
COVID-Recovery Study Investigators and the ANZICS Clinical Trials Group members: Alfred Hospital, Melbourne VIC: Meredith Young, Jasmin Board, Phoebe McCracken, Emma-Leah Martin; Austin Hospital, Melbourne VIC: Nicola Burgess; Eastern Health, Melbourne VIC: Kirsty Hearn; Cabrini Hospital: David Brewster, Alyssa Waanders, Shannon Simpson; Canberra Hospital, Canberra ACT; Concord Hospital, Concord NSW: Yasmin de Silva; Epworth Hospital, VIC: Jonathon Barrett, Gabrielle Hanlon; Footscray Hospital, Melbourne VIC: Jenna Lang, Sarah Burleigh, Elisha Killer; Frankston Hospital, Melbourne VIC: Michael Wang; Gold Coast University Hospital, Gold Coast QLD: Lauren O’Connor; John Hunter Hospital, Newcastle NSW; Lauren Thomas; Launceston Hospital, Launceston TAS: Lucy Dennis; Monash Health, Melbourne VIC: Joanna Caruana, Wisam Al-Bassam; Nepean Hospital, Sydney NSW; Princess Alexandra Hospital, Brisbane QLD; Redcliffe Hospital, Brisbane QLD: Morag Shealy; Royal Adelaide Hospital, Adelaide SA: Marianne Chapman, Stephanie O’Connor; Royal Brisbane and Women’s Hospital, Brisbane QLD; Royal Melbourne Hospital, Melbourne, VIC: Janne Sheehan, Emily Alexander; Royal North Shore Hospital, Sydney NSW: Amanda Sukkar, Liesl Davis, Francis Bass, Naomi Hammond, Anne O’Connor, Elizabeth Yarad; Royal Prince Alfred Hospital, Sydney NSW: Richard Totaro, Heidi Buhr, Nazmeen Reddy; St. George Hospital, Sydney NSW: Wendy Chaseling; St. Vincent’s Hospital, Melbourne VIC; St. Vincent’s Hospital, Sydney NSW; Sunshine Hospital, Melbourne VIC: Kelvin Ip; The Prince Charles Hospital, Brisbane QLD: Oystein Tronstad, Alison Mahoney; Westmead Hospital, Sydney NSW: Cadi Fanning, Hariette Esterman, Alexia Kozary, Bronte Scott; and The Frontline ICU Physiotherapy Initiative: Donna Urquhart.
Footnotes
A complete list of the COVID-Recovery Study Investigators and the ANZICS Clinical Trials Group members may be found before the beginning of the References.
Data sharing: Partial data set sharing according to individual requests for data access, including the data dictionary. Requests will be considered by the study’s management committee 12 months after publication. Requests for data sharing to be made to the corresponding author, C.L.H. (carol.hodgson@monash.edu), and A.S.N. (anzicrc@monash.edu).
Supported by a National Health and Medical Research Council Australia Translational Project grant (the PREDICT study [Predicting the Outcome of Critically Ill Patients]) and National Health and Medical Research Council Australia Investigator grant GNT1173271 (C.L.H.). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author Contributions: A.S.N. and C.L.H. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: C.L.H., A.M.H., and A.S.N., with advice from all authors. Acquisition, analysis, or interpretation of data: A.M.H., C.L.H., and A.S.N. Drafting of the manuscript: C.L.H., A.S.N., A.M.H., M.J.B., and A.M.M. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: A.S.N. and M.J.B. Administrative, technical, or material support: A.M.M. and N.J.L. Supervision: C.L.H. and A.S.N.
This version of the article was corrected on Sept 1, 2022 (see https://www.atsjournals.org/doi/10.1164/rccm.v206erratum7).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202110-2335OC on March 8, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
the COVID-Recovery Study Investigators and the ANZICS Clinical Trials Group:
Meredith Young, Jasmin Board, Phoebe McCracken, Emma-Leah Martin, Nicola Burgess, Kirsty Hearn, David Brewster, Alyssa Waanders, Shannon Simpson, Yasmin de Silva, Jonathon Barrett, Gabrielle Hanlon, Jenna Lang, Sarah Burleigh, Elisha Killer, Michael Wang, Lauren O’Connor, Lauren Thomas, Lucy Dennis, Joanna Caruana, Wisam Al-Bassam, Morag Shealy, Marianne Chapman, Stephanie O’Connor, Janne Sheehan, Emily Alexander, Amanda Sukkar, Liesl Davis, Francis Bass, Naomi Hammond, Anne O’Connor, Elizabeth Yarad, Richard Totaro, Heidi Buhr, Nazmeen Reddy, Wendy Chaseling, Kelvin Ip, Oystein Tronstad, Alison Mahoney, Cadi Fanning, Hariette Esterman, Alexia Kozary, and Bronte Scott
References
- 1. Lone NI, Gillies MA, Haddow C, Dobbie R, Rowan KM, Wild SH, et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med . 2016;194:198–208. doi: 10.1164/rccm.201511-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med . 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 3. Geense WW, Zegers M, Peters MAA, Ewalds E, Simons KS, Vermeulen H, et al. New physical, mental, and cognitive problems 1 year after ICU admission: a prospective multicenter study. Am J Respir Crit Care Med . 2021;203:1512–1521. doi: 10.1164/rccm.202009-3381OC. [DOI] [PubMed] [Google Scholar]
- 4. Hodgson CL, Udy AA, Bailey M, Barrett J, Bellomo R, Bucknall T, et al. The impact of disability in survivors of critical illness. Intensive Care Med . 2017;43:992–1001. doi: 10.1007/s00134-017-4830-0. [DOI] [PubMed] [Google Scholar]
- 5. Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med . 2012;186:302–304. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med . 2016;194:147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fraser E. Long term respiratory complications of COVID-19. BMJ . 2020;370:m3001. doi: 10.1136/bmj.m3001. [DOI] [PubMed] [Google Scholar]
- 8. Aucott JN, Rebman AW. Long-haul COVID: heed the lessons from other infection-triggered illnesses. Lancet . 2021;397:967–968. doi: 10.1016/S0140-6736(21)00446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA . 2020;324:1723–1724. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet . 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodgson CL, Higgins AM, Bailey MJ, Mather AM, Beach L, Bellomo R, et al. COVID-Recovery Study Investigators and the ANZICS Clinical Trials Group The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: a prospective cohort study. Crit Care . 2021;25:382. doi: 10.1186/s13054-021-03794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nabavi N. Long COVID: how to define it and how to manage it. BMJ . 2020;370:m3489. doi: 10.1136/bmj.m3489. [DOI] [PubMed] [Google Scholar]
- 13. Cortinovis M, Perico N, Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet . 2021;397:173–175. doi: 10.1016/S0140-6736(21)00039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phillips S, Williams MA. Confronting our next national health disaster – long-haul COVID. N Engl J Med . 2021;385:577–579. doi: 10.1056/NEJMp2109285. [DOI] [PubMed] [Google Scholar]
- 15. Higgins AM, Neto AS, Bailey M, Barrett J, Bellomo R, Cooper DJ, et al. PREDICT Study Investigators Predictors of death and new disability after critical illness: a multicentre prospective cohort study. Intensive Care Med . 2021;47:772–781. doi: 10.1007/s00134-021-06438-7. [DOI] [PubMed] [Google Scholar]
- 16. Burrell AJ, Pellegrini B, Salimi F, Begum H, Broadley T, Campbell LT, et al. Outcomes for patients with COVID-19 admitted to Australian intensive care units during the first four months of the pandemic. Med J Aust . 2021;214:23–30. doi: 10.5694/mja2.50883. [DOI] [PubMed] [Google Scholar]
- 17. Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham CO, III, et al. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med . 2017;196: 1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins AM, Serpa Neto A, Bailey M, Barrett J, Bellomo R, Cooper DJ, et al. The psychometric properties and the minimal clinically important difference for disability assessment using the WHODAS 2.0 in critically ill patients. Crit Care Resusc . 2021;23:103–112. doi: 10.51893/2021.1.OA10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerth AMJ, Hatch RA, Young JD, Watkinson PJ. Changes in health-related quality of life after discharge from an intensive care unit: a systematic review. Anaesthesia . 2019;74:100–108. doi: 10.1111/anae.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sukantarat KT, Williamson RC, Brett SJ. Psychological assessment of ICU survivors: a comparison between the Hospital Anxiety and Depression scale and the Depression, Anxiety and Stress scale. Anaesthesia . 2007;62:239–243. doi: 10.1111/j.1365-2044.2006.04948.x. [DOI] [PubMed] [Google Scholar]
- 21. Hodgson CL, Haines KJ, Bailey M, Barrett J, Bellomo R, Bucknall T, et al. ICU-Recovery Investigators Predictors of return to work in survivors of critical illness. J Crit Care . 2018;48:21–25. doi: 10.1016/j.jcrc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 22. Hosey MM, Leoutsakos JS, Li X, Dinglas VD, Bienvenu OJ, Parker AM, et al. Screening for posttraumatic stress disorder in ARDS survivors: validation of the Impact of Event Scale-6 (IES-6) Crit Care . 2019; 23:276. doi: 10.1186/s13054-019-2553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hopkins RO, Suchyta MR, Kamdar BB, Darowski E, Jackson JC, Needham DM. Instrumental activities of daily living after critical illness: a systematic review. Ann Am Thorac Soc . 2017;14: 1332–1343. doi: 10.1513/AnnalsATS.201701-059SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feng Y, Zhang J, Zhou Y, Chen B, Yin Y. Concurrent validity of the short version of Montreal Cognitive Assessment (MoCA) for patients with stroke. Sci Rep . 2021;11:7204. doi: 10.1038/s41598-021-86615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klil-Drori S, Phillips N, Fernandez A, Solomon S, Klil-Drori AJ, Chertkow H. Evaluation of a telephone version for the Montreal Cognitive Assessment: establishing a cutoff for normative data from a cross-sectional study. J Geriatr Psychiatry Neurol . 2021 doi: 10.1177/08919887211002640. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. Vienna, Austria: R Foundation for Statistical Computing; 2019. R: A language and environment for statistical computing.https://www.R-project.org/ [Google Scholar]
- 27. Castro-Avila AC, Jefferson L, Dale V, Bloor K. Support and follow-up needs of patients discharged from intensive care after severe COVID-19: a mixed-methods study of the views of UK general practitioners and intensive care staff during the pandemic’s first wave. BMJ Open . 2021;11:e048392. doi: 10.1136/bmjopen-2020-048392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute COVID-19 in primary care. BMJ . 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 29. Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, et al. Writing Committee for the COMEBAC Study Group Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA . 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parker AJ, Humbir A, Tiwary P, Mishra M, Shanmugam M, Bhatia K, et al. Recovery after critical illness in COVID-19 ICU survivors. Br J Anaesth . 2021;126:e217–e219. doi: 10.1016/j.bja.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taboada M, Moreno E, Cariñena A, Rey T, Pita-Romero R, Leal S, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth . 2021;126: e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miskowiak KW, Johnsen S, Sattler SM, Nielsen S, Kunalan K, Rungby J, et al. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur Neuropsychopharmacol . 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N Engl J Med . 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]