Abstract
This year marks the 50th anniversary of the uncovering of the Tuskegee syphilis study, when the public learned that the Public Health Service (precursor of the CDC) for 40 years intentionally withheld effective therapy against a life-threatening illness in 400 African American men. In 2010, we learned that the same research group had deliberately infected hundreds of Guatemalans with syphilis and gonorrhea in the 1940s, with the goal of developing better methods for preventing these infections. Despite 15 journal articles detailing the results, no physician published a letter criticizing the Tuskegee study. Informed consent was never sought; instead, Public Health Service researchers deceived the men into believing they were receiving expert medical care. The study is an especially powerful parable because readers can identify the key players in the narrative and recognize them as exemplars of people they encounter in daily life—these flesh-and-blood characters convey the principles of research ethics more vividly than a dry account in a textbook of bioethics. The study spurred reforms leading to fundamental changes in the infrastructure of research ethics. The reason people fail to take steps to halt behavior that in retrospect everyone judges reprehensible is complex. Lack of imagination, rationalization, and institutional constraints are formidable obstacles. The central lessons from the study are the need to pause and think, reflect, and examine one’s conscience; the courage to speak; and above all the willpower to act. History, although about the past, is our best defense against future errors and transgressions.
Keywords: research ethics, racism, vulnerable patient groups, researcher responsibilities, medical history
Contents
The PHS Syphilis Study
Peter Buxtun
The Story Breaks
Why Was the PHS Syphilis Study Undertaken?
The Guatemalan Epilogue
Lessons
The history of medicine is presented as a cavalcade of triumphal breakthroughs leading to marked increases in life expectancy. Advances arise from the ingenuity and industry of innumerable investigators but also depend on millions of patients who selflessly make their bodies available for experimentation. The interaction between investigators and patients is a source of pride but on occasion has also been a reason for shame. Few medical experiments are more ignominious than that conducted by physicians who for 40 years (1932–1972) intentionally withheld effective therapy from hundreds of African American men known to have a life-threatening illness (1).
One of the most disturbing features of this experiment is the realization that it was conducted by the major health arm of the federal government: the Public Health Service (PHS; precursor of the CDC). When the experiment was uncovered in 1972, it was difficult to imagine that the PHS could contain a worse chapter in its history (2). Yet in 2010, we learned that the same group of researchers had deliberately infected hundreds of Guatemalans with syphilis and gonorrhea in the 1940s in the hope of developing a better means of preventing these infections.
The PHS study has its origin with researchers who wanted to study the natural history of untreated syphilis. The site chosen, Macon County, Alabama, had a population of 27,000 in 1932, of whom 82% were African American (1). The PHS sought the cooperation of the nearby Tuskegee Institute, the Black university founded by Booker T. Washington (1856–1915), and made use of the facilities of Andrew Memorial Hospital, located on the campus (3). The study population consisted of 600 Black men: 399 with syphilis and 201 free of the disease who served as control subjects (4). By 1969, at least 28 and perhaps 100 men had died as a direct result of syphilis; despite this knowledge, the government scientists continued the experiment (1, 5).
“In 1932, Macon County was still very much tied to its plantation past,” Britt Rusert avows (Figure 1). “Most of the men selected for the syphilis experiments were poor sharecroppers with little or no formal education who worked under white farmers in a system of debt peonage” (6). The men agreed to participate because the investigators offered them free medical care and burial insurance (1). Informed consent was never sought. On the contrary, PHS researchers deceived the men into believing they were being treated for “bad blood,” a colloquialism for several ailments (1). The term is included in the title of a book by James Jones, Bad Blood: The Tuskegee Syphilis Experiment (1981), regarded as the definitive history of the experiment (7) and “the single most important book ever written in bioethics” (8).
Figure 1.
Unidentified study participant in a cotton field. Reproduced from file of photographs of participants in the Tuskegee syphilis study, National Archives (in public domain).
As an active physician who has spent more than 45 years conducting research on patients and a former journal editor-in-chief who investigated various problems of research ethics and imposed sanctions on researchers for malfeasance, I reflect on how physician-scientists who dedicate their lives to a noble cause can persuade themselves that it is morally acceptable to perform disturbing experiments on unwitting individuals to attain their goals. A more detailed version of this article is available in the online supplement.
The PHS Syphilis Study
The idea for the experiment originated with Dr. Taliaferro Clark, director of the Venereal Disease Division of the PHS (9–11). Dr. Clark was analyzing data from an earlier study when “the thought came to me that the Alabama community offered an unparalleled opportunity for the study of the effects of untreated syphilis” (1). In time, this thought became the Tuskegee Study of Untreated Syphilis in the Negro Male.
The men remained untreated only because the government doctors deliberately withheld therapy over a 40-year period and misled the men into believing that the medications they received (vitamin tonics and aspirin as placebo) were effective against their disease (6) (Figures 2 and 3). When seeking assistance from the principal of the Tuskegee Institute, the surgeon general, Dr. Hugh Cumming (1869–1948), wrote to him in 1932 saying that the study “offers an unparalleled opportunity for carrying on this piece of scientific research which probably cannot be duplicated anywhere else in the world.” Presumably, Dr. Cumming did not intend any irony (12).
Figure 2.
PHS staff members Dr. David Allbritton, nurse Eunice Rivers, and Dr. Walter Edmondson, conducting an annual roundup in Macon County, 1953. On the side of the vehicle, “U.S. Department of Health, Education, and Welfare, Public Health Service” is prominently displayed. Reproduced from National Archives (in public domain). PHS = Public Health Service.
Figure 3.
Dr. Walter Edmondson of the PHS drawing a blood sample from a study participant during an annual roundup in Milstead, Macon County, 1953. Reproduced from National Archives (in public domain). PHS = Public Health Service.
The background knowledge that led to the PHS study came from the Oslo Study of Untreated Syphilis (9). Convinced that available therapy, primarily mercury compounds that had been used since the 16th century, was harmful, Dr. Caesar Boeck withheld treatment from almost 2,000 syphilitic patients between 1890 and 1910 (13). Like tuberculosis, syphilis had been one of the most feared scourges of mankind, estimated to affect 1 in every 10 Americans in the early 20th century (14). Around this time, German investigators made a series of path-breaking discoveries that revolutionized the ability of physicians to manage the disease (15). Therapy was transformed in 1908 when Sahachirō Hata (1873–1938) and Nobelist Paul Ehrlich (1854–1915) discovered an arsenical compound, arsphenamine, which was highly toxic to spirochetes and much less so to humans (15). Arsphenamine was marketed as Salvarsan in 1910; Boeck became quickly convinced of its efficacy and immediately terminated the Oslo study (16).
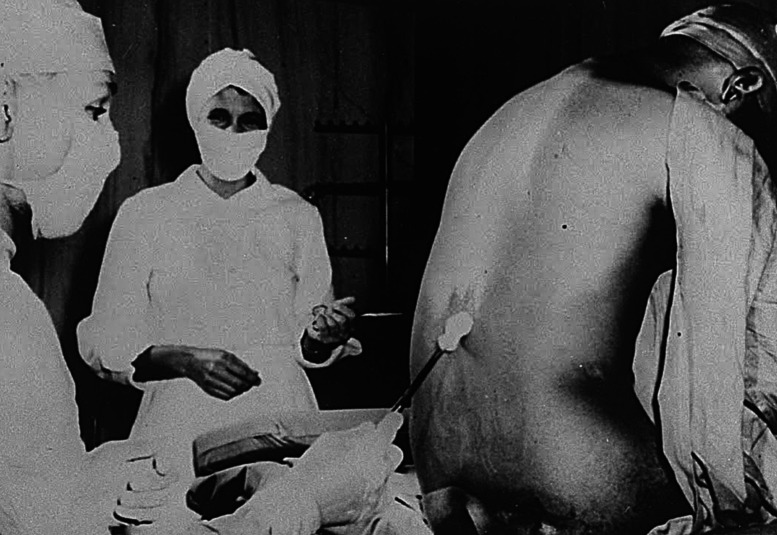
Once PHS investigators had enrolled the Alabama men and obtained baseline measurements, they next decided to check for evidence of neurosyphilis. Dr. Raymond Vonderlehr realized that the men might refuse lumbar puncture if they realized it was solely for diagnostic purposes. “My idea,” he wrote to his collaborators, is that “details of the puncture techniques should be kept from them as far as possible” (1). To entice the men to cooperate, he told them he would give them a special therapy: free “spinal shots,” deceiving them into believing that lumbar punctures were therapeutic (Figure 4) (9).
Figure 4.
Lumbar puncture, 1933. From left: Dr. Jesse J. Peters, nurse Eunice Rivers, and unidentified study participant. Reproduced from National Archives (in public domain).
The final step in data collection was to obtain pathological specimens at autopsy. “As I see it,” another PHS investigator, Dr. Oliver Wenger, wrote to Dr. Vonderlehr, “we have no further interest in these patients until they die” (underlining in original) (17). The surgeon general, Dr. Cumming, stressed this step in a letter to the director of Andrew Hospital: “Since clinical observations are not considered final in the medical world, it is our desire to continue observation on the cases selected for the recent study and if possible to bring a percentage of these cases to autopsy so that pathological confirmation may be made of the disease processes.” PHS investigators feared the enrollees would quit if they knew they would be autopsied. Dr. Wenger wrote to Dr. Vonderlehr, “If the colored population become aware that accepting free hospital care means a postmortem every darkey will leave Macon County” (17).
To coax enrollees into the hospital when they became severely ill, the PHS promised to cover their burial expenses. Given the importance of funeral rites in the cultural life of rural Black persons, this was a particularly strong inducement (9). “The grotesque violation of these men’s bodies,” chides Britt Rusert, “extended even into their death: family members were required to turn over the corpse for an autopsy to secure funeral benefits” (6).
Shortly after commencing his tenure as surgeon general (1936–1948), Dr. Thomas Parran (1892–1968) launched a vigorous campaign to eradicate venereal disease using mass screening and mobile treatment clinics (18). Dr. Parran, who had visited Tuskegee in the early 1930s, is credited for the great strides made by this nationwide campaign (Figure 5). When the mobile unit reached Macon County, PHS staff members alerted local doctors about enrollees and instructed physicians: “He’s under study and not to be treated” (1).
Figure 5.

Thomas Parran, Jr., M.D., sixth U.S. surgeon general (1936–1948), in 1946, the year the Guatemala research commenced (reproduced from Reference 109) (in public domain).
In 1943, Dr. John Heller succeeded Dr. Vonderlehr as director of the Division of Venereal Diseases (1). One year later, penicillin became the therapy of choice for syphilis (19), and in 1947 PHS established rapid treatment centers across the country. There was no discussion of treating the men enrolled in the study. Given the effectiveness of penicillin, PHS scientists insisted that it was all the more urgent for the experiment to continue—it had become a never-again-to-be-repeated opportunity.
Although physician-scientists intentionally withheld penicillin, the experiment was fundamentally flawed because “the vast majority of the patients” had received “effective and undocumented” penicillin “in the happenstance manner while under treatment for other conditions” (20). As such, the study was not one of untreated syphilis, but rather of undertreated syphilis (21).
When Dr. Heller left the Division of Venereal Diseases in 1948, he became director of the National Cancer Institute and, in 1960, president of the Memorial Sloan-Kettering Cancer Center in New York. His years as leader of the PHS study coincided with the introduction of penicillin for syphilis and promulgation of the Nuremberg Code. There is no evidence that the PHS study was ever discussed in the light of the Nuremberg Code (1). When the experiment was brought to public attention in 1972, Dr. Heller shocked the public by telling journalists, “There was no racial side to this. It just happened to be in a black community. I feel this was a perfectly straightforward study, perfectly ethical, with controls” (9). When Dr. Heller died in 1989, the New York Times published a glowing obituary, listing his many accomplishments without mentioning the PHS study (22).
Some believe the PHS experiment was a secret study (6). On the contrary, the first report was published in JAMA in 1936 (23), and PHS researchers issued subsequent papers every 4–6 years until 1973 (24). For those who did not read the entire articles, the titles were sufficient to have aroused suspicion. “The Tuskegee Study of Untreated Syphilis; the 30th Year of Observation” was the title of a 1964 article in Archives of Internal Medicine (25). A 1955 article on autopsy findings communicated that more than 30% of the men had died directly from advanced syphilitic lesions (26). Despite repeated accounts of the ravages of untreated syphilis, appearing in 15 articles in reputable journals spread over 37 years, no physician or scientist from anywhere around the world published a letter or commentary criticizing the ethics of the experiment (21).
Peter Buxtun
In December 1965, Peter Buxtun was hired by the PHS to interview patients with venereal disease. Within a year, the 29-year-old Czech-born psychiatric social worker sent a letter to Dr. William Brown, director of the Division of Venereal Diseases of the CDC, expressing grave moral concerns about the PHS study (1) (Figure 6). The CDC remained silent for months and then invited Buxtun to a meeting in Atlanta. As soon as Buxtun entered the conference room, Dr. John Cutler, a PHS investigator (26, 27), began to harangue him. “He had obviously read my material,” Buxtun recalled, “thought of me as some form of lunatic who needed immediate chastisement and he proceeded to administer it” (1).
Figure 6.

Mr. Peter Buxtun, a 29-year-old social worker, communicated with the CDC about the ethics of the PHS study and subsequently revealed details of the study to a newspaper reporter (reproduced from Reference 110) (in public domain). PHS = Public Health Service.
In November 1968, Buxtun again wrote to Dr. Brown, who showed the letter to Dr. David Sencer, director of the CDC (1966–1977). Realizing they had a problem on their hands, Sencer and Brown convened a blue-ribbon panel in February 1969 to discuss the study (1). CDC scientists presented an overview of the study and said they needed advice on deciding whether to terminate it. Dr. Brown noted that 83 men had shown evidence of syphilis at death, but he personally believed the disease was the primary cause of death in only 7 of them (1).
Dr. Lawton Smith emerged as the leading advocate for continuing the study. He stressed, “You will never have another study like this; take advantage of it,” and boasted that “20 years from now, when these patients are gone, we can show their pictures” (28). (Today one can access the Lawton Smith Lecture Series on a website hosted by the North American Neuro-Ophthalmology Society [29].) Of 17 panelists, only Dr. Gene Stollerman saw the men as patients and believed they had a right to be treated: “You should treat each individual case as such, not treat as a group” (5). The blue-ribbon panel dismissed this objection and continued to refer to the survivors as a group of subjects rather than as individual patients. It was almost as if the words, “399-Alabama-Black-rural-sharecropping-illiterate-men” constituted a single word (21).
Dr. Brown wrote to Peter Buxtun informing him that a blue-ribbon panel had reviewed the experiment and decided against treating the men. Buxtun made no attempt to challenge the panel’s medical authority but asked, “What is the ethical thing to do?” (1). The CDC did not answer him. Buxtun discussed the matter with several law professors, who were sympathetic but offered little encouragement—an illustration of Ian Kershaw’s adage that the road to Auschwitz was paved with indifference (30). Buxtun contacted a journalist, and the story finally broke in the Washington Star on July 25, 1972, and as front-page news in the New York Times the following day (31).
The Story Breaks
The American public found it hard to wrap its mind around the idea that government doctors had been intentionally duping men with a disease as serious as syphilis for 40 years (24). The Afro-American of Baltimore exclaimed, “How condescending and void of credibility are the claims that racial considerations had nothing to do with the fact that 600 [all] of the subjects were black” (1). A number of physicians defended the study, the most spirited defense coming from Vanderbilt’s Rudolph Kampmeier (1898–1990), former president of the American College of Physicians (1967–1968) and editor of the Southern Medical Journal (32). Dr. Kampmeier blasted journalists for raising “a great hue and cry,” chastised them for their “complete disregard for their abysmal ignorance,” and trumpeted that his analysis would “put this ‘tempest in a teapot’ into proper historical perspective” (33).
Dr. Kampmeier considered the insinuation “that treatment was purposefully withheld” from the enrollees as unjust. On the contrary, “the subjects were not deterred from obtaining treatment if they desired it or bothered to get what was available” (33). In his mind, it was the fault of the men that they did not request penicillin as treatment for their syphilitic aortitis: “Since these men did not elect to obtain the treatment available to them, the development of aortic disease lay at the subject’s door and not in the Study’s protocol.” Regarding higher mortality in subjects with syphilis than in control subjects, Dr. Kampmeier coolly observed, “This is not surprising. No one has ever implied that syphilis was a benign infection” (33).
The Department of Health, Education, and Welfare (HEW) announced that it would undertake a review (1). In October 1972, the Ad Hoc Advisory Panel advised that the experiment be terminated and that the men receive immediate medical care. In February and March 1973, Senator Edward Kennedy conducted congressional hearings into the study, which led to the passage of the National Research Act and, in turn, the establishment of institutional review boards, principles of informed consent, and protection of vulnerable populations (5). Legal proceedings against any physician-scientist were never initiated (34).
In 1997, President Clinton finally tendered the government’s apology: “What the United States government did was shameful. . . . To our African American citizens, I am sorry that your federal government orchestrated a study so clearly racist” (35).
Why Was the PHS Syphilis Study Undertaken?
The HEW panel report, issued in April 1973 (36), failed to address two central questions: “Why was the experiment undertaken?” and “Why did it continue for 40 years?” The answers are complex. Insights are gained from examining the beliefs of the PHS investigators who initiated the study, scientific understanding of syphilis (treated and untreated), and prevailing cultural and social forces at the time.
In the early decades of the 20th century, eugenics was a worldwide force and judged to represent cutting-edge biology research (37, 38). PHS study leaders were vocal advocates of eugenic measures (39). Dr. Taliaferro Clark earned his PHS stripes by undertaking eugenics-motivated projects on rural schoolchildren (40). Dr. Clark’s data would later be used by the state of Indiana to select individuals for sterilization. Because of its influence on the future of the “the race,” venereal disease was considered “directly antagonistic to the eugenic ideal” (14). Recognizing its threat to the family, several states enacted eugenic marriage laws, making venereal disease a bar to matrimony.
Racist views were not confined to the postbellum South nor directed solely at the lower echelons of Black society. When 5,000 Black physicians petitioned for membership in the American Medical Association (AMA) in 1939, their application was rejected (41). Not only did the AMA refuse to admit Black physicians as members, but it also did not allow them to attend its annual conferences. This discrimination lasted well into the civil rights era. Between 1944 and 1965, more than a dozen attempts to include Black physicians were rebuffed by the AMA (42, 43). Black physicians consequently founded their own organization, the National Medical Association, which continues to publish its own journal to this day (44, 45). The AMA did not officially desegregate until 1968 (46).
Fast forward to February 23, 2021: JAMA broadcasted a 16-minute podcast with the Twitter headline “No physician is racist, so how can there be structural racism in health care?” The host, Ed Livingston, M.D., a “fulltime editor of JAMA,” dismissed structural racism as “an unfortunate term,” insisting that people are “turned off by the whole structural racism phenomenon,” concluding that “personally, I think taking racism out of the conversation will help” (47). Critics claimed the podcast exposed a culture of systemic racism in medicine. The furor arising from the podcast led to the resignation of Dr. Livingston, and the editor-in-chief, Howard Bauchner, M.D., was placed on administrative leave on March 25 and resigned on June 30 (48).
Assumptions that racial differences are genetic in origin have become embedded within medical practice, with half of White medical students and residents holding false beliefs about biological differences between Black and White individuals (49), which result in undertreatment of pain (among other consequences) (50, 51). For years, researchers have treated race as an innate genetic attribute, whereas the perspective of race as a social construct is now widely embraced (52). The term “structural racism” is used to convey that racism has a systemic basis, embedded in social policy and norms and not simply private prejudices of individuals (53, 54). Structural racism is the common denominator to the PHS experiments, inferior medical care (49–51, 55–57) and increased coronavirus disease (COVID-19) mortality among African Americans (58), and police violence against Black individuals (59).
The Ad Hoc Advisory Panel that investigated the PHS study in 1972 was constrained by the narrowness of the charges HEW gave them (36). The nine-member panel included five Black and four White members, with Broadus Butler, Ph.D. (1920–1996), president of historically Black Dillard University and a former World War II Tuskegee Airman, as chairman (5). Several panel members subsequently claimed that Dr. Butler engaged in a government whitewash (60). Members traveled to Tuskegee and conducted taped interviews with study staff members and participants (5). On their return, the tape was burned at Dr. Butler’s insistence (5, 60). A cover letter to HEW on the front page of the final report of April 28, 1973, contains the statement, “The Chairman specifically abstains from concurrence in this final report” (36). In a private letter, Dr. Butler wrote that the panel had become “advocates,” and had “lost their objectivity” (5). Dr. Butler died without leaving papers to shed light on his actions (5).
Many commentators focus on the failure of PHS researchers to administer penicillin once it became standard therapy. That argument betrays a basic misunderstanding of the purposes of the experiment, as it assumes that satisfactory therapy for syphilis did not exist before 1945 (16). By the 1920s, leading experts had become convinced that Salvarsan-based therapy was effective in decreasing morbidity and mortality (15). The administration of any effective medication, not just penicillin, to the men would have violated the rationale of the experiment, which was to study the natural course of untreated syphilis until death and autopsy.
Another common criticism, failure to obtain informed consent, also obscures the historical facts of the experiment. That informed consent, as we know it today, was not a component of a research protocol in the 1930s does not diminish PHS researchers’ obligations. In 1907 William Osler wrote on “the limits of justifiable experimentation upon our fellow creatures,” emphasizing, “For man absolute safety and full consent are the conditions which make such tests allowable” (61). A more fundamental point is that the Tuskegee men never saw themselves as volunteers in a scientific experiment. They were told and they believed that they were getting free treatment from expert government doctors for a serious disease.
The Guatemalan Epilogue
While accumulating material for her book Examining Tuskegee (2009) (5), historian Susan Reverby traveled to the University of Pittsburgh in 2003 to investigate the stored records of Dr. Thomas Parran. Library staff members informed her that Dr. John Cutler (1915–2003) had donated his research records to the university in 1990 (62, 63). On opening the files, Reverby found almost nothing about the Alabama study but copious records of PHS studies conducted between 1946 and 1948 wherein American physicians deliberately infected hundreds of Guatemalans with syphilis and gonorrhea without their knowledge or consent.
In 2010, Reverby submitted a manuscript to the Journal of Policy History (64), sending a preprint to a former director of the CDC. The information made its way through layers of government to reach the White House. President Obama appointed a commission to investigate the matter, and the results were published in two reports in late 2011 (65, 66).
The ideas that led to the Guatemalan research originated during the second World War. The effect of sexually transmitted disease on military manpower is always a concern in wartime (67), and the United States was experiencing more than half a million new cases of syphilis each year (68). To develop better prophylaxis regimens, PHS investigators drew up plans for an experimental model wherein infection would be induced in healthy subjects. The principal investigator, Dr. Mahoney, began experiments in September 1943.
John Mahoney (1889–1957) graduated from medical school in 1914 and after clinical training joined the PHS and was appointed director of the Venereal Diseases Research Laboratory (progenitor of the CDC) of the U.S. Marine Hospital on Staten Island, New York, in 1929 (67) (Figure 7). The 54-year-old physician-scientist supervised the experiments on federal prisoners, while 28-year-old John Cutler, M.D., assisted by other PHS researchers, conducted on-site work in Terre Haute, Indiana. Dr. Cutler was born in Cleveland in 1915 and graduated from Western Reserve University Medical School in 1941, joining the PHS 1 year later (65, 69) (Figure 8). A total of 241 prisoners participated in the experiments, all of whom were inoculated with Neisseria gonorrhoeae deposited into the end of the penis. Investigators failed to consistently produce infection, and the experiments ended in July 1944 (70).
Figure 7.

John F. Mahoney, M.D. (1889–1957), director of the Venereal Diseases Research Laboratory (progenitor of the CDC) of the U.S. Marine Hospital on Staten Island, New York. Reproduced from the National Library of Medicine (in public domain).
Figure 8.

John C. Cutler, M.D., in 1942, the year the 27-year-old physician joined the PHS. Reproduced from the National Library of Medicine (in public domain). PHS = Public Health Service.
In 1945, Guatemalan physician Dr. Juan Funes spent a 1-year fellowship in the Venereal Diseases Research Laboratory (71). He informed his supervisors that prostitution was legal in Guatemala and that it was also legal for prostitutes to visit men in penal institutions (64). To PHS investigators, Guatemala presented an opportunity to transmute the Terre Haute disappointment into a success (65).
Funding was sought from the NIH, and in March 1946, the first ever study section approved the proposal for “the Guatemalan study dealing with the experimental transmission of syphilis to human volunteers and improved methods of prophylaxis” (72), providing $146,000 in funding (equivalent to $2.1 million today) (73). Study section members included physician-scientists from Harvard, Johns Hopkins, the University of Pennsylvania, and other institutions. In August 1946, Dr. Cutler arrived in Guatemala to conduct the experiments, assisted by other PHS physicians and staff members (65).
The original plan was to induce syphilis in prisoners in Penitenciaría Central through sexual intercourse with infected prostitutes and then test the efficacy of prophylactic regimens. When the American physicians encountered unexpected difficulties, they began to conduct studies on Guatemalan soldiers, inmates in the country’s only mental hospital, and children in the national orphanage (64) (Figures 9 and 10). Because the rate of infection resulting from intercourse with prostitutes was lower than expected (<10%) (10), the NIH-sponsored researchers attempted to artificially inoculate subjects with syphilis, gonorrhea, and chancroid.
Figure 9.
Left: A 25-year-old female patient in Asilo de Alienados (Psychiatric Hospital) in Guatemala was exposed to syphilis once with no record of treatment. Right: A 16-year-old female patient in Asilo de Alienados was exposed to syphilis twice and was treated with penicillin. Records indicate that the patient was “uncooperative.” Reproduced from the National Archives and Records Administration (in public domain).
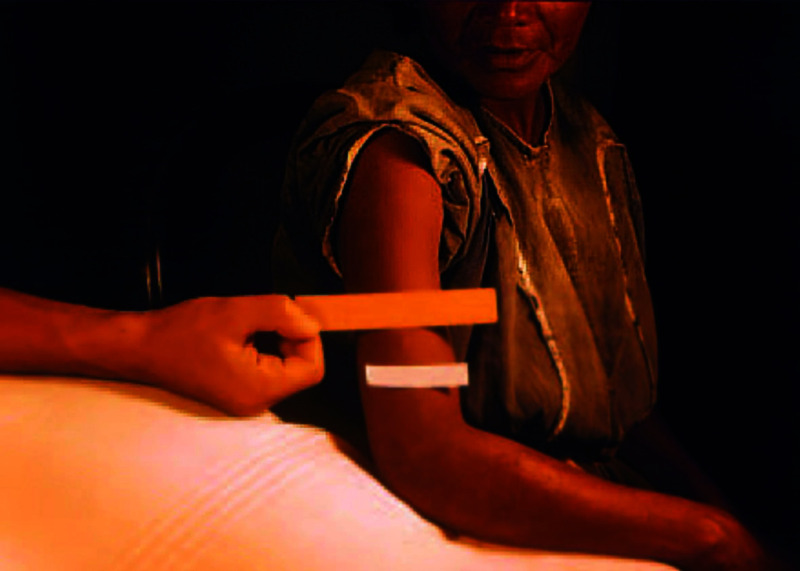
Figure 10.
The injection site of a female psychiatric patient who was exposed to syphilis three times and received some treatment. Reproduced from the National Archives and Records Administration (in public domain).
The investigators’ notebooks contain graphic accounts of steps in these experiments. A physician held the penis of a participant, pulled back the foreskin, and “with some force, rolled the large inoculating swab over the mucosa so as to try to contaminate the entire fossa navicularis” (65). If enrolled prostitutes were uninfected, investigators inoculated women by moistening a cotton-tipped swab with gonorrheal pus, inserting it into the woman’s cervix, and “swabb[ing] it around . . . with considerable vigor” (65).
Only five infections resulted when 93 soldiers engaged in 138 episodes of sexual intercourse with 12 prostitutes (65). A higher rate of transmission, 17.9%, was achieved by inserting an infected pledget under the foreskin (65). To achieve a still higher rate of infection, the PHS physicians used a hypodermic needle to abrade the dorsal surface of a subject’s glans “short of drawing blood” and then covered the abraded area with a pledget soaked in Treponema pallidum, achieving a 91.6% rate of transmission (65). Many participants actively objected. One psychiatric patient “fled the room” after being subjected to penile scarification and was not found for several hours (65).
The American physicians also studied other modes of transmission, including oral ingestion of syphilitic material and inoculating the rectum, urethra, and eyes of participants (65). One psychiatric patient, Berta, had syphilis injected into her arm in February 1948. She developed bumps and skin wasting, and 6 months later Dr. Cutler wrote that Berta appeared as if she was going to die. The same day, August 23, he put gonorrheal pus from a male participant into Berta’s eyes, urethra, and rectum. Her eyes filled up with pus, and four days later Berta died (65).
By December 1948, the NIH-sponsored investigators had intentionally exposed at least 1,308 individuals to syphilis, gonorrhea, and chancroid (65, 74). Of those infected, 61–87% showed evidence of disease, and the majority were not provided adequate treatment (75). Eighty-three deaths were reported (76). The rate of induced infection was unexpectedly low except for experiments involving scarification of participants’ membranes, which Dr. Mahoney considered “beyond the range of natural transmission and [would] not serve as a basis for the study of a locally applied prophylactic agent” (65). Among other considerations, this was a major reason for stopping the experiments.
The Guatemala studies were halted abruptly in December 1948, and none of the experimental findings were published. One motivation for terminating the research was Dr. Parran’s imminent departure from the surgeon general’s office on April 6, 1948. As that date drew near, Dr. Mahoney wrote to Dr. Cutler, “We have lost a very good friend and that it appears to be advisable to get our ducks in line. In this regard we feel that the Guatemala project should be brought to the innocuous stage as rapidly as possible” (63).
Many journalists portray Dr. Cutler as a Dr. Mengele–type scientist acting autonomously. This characterization is wrong. Although Dr. Cutler was the main on-site investigator, the primary supervisor of the Guatemalan experiments, Dr. Mahoney, was kept fully apprised. A few months after the research commenced, Dr. Mahoney wrote encouragingly to Dr. Cutler, “Your show is already attracting rather wide and favorable attention up here. We are frequently asked as to the progress of your work” (65). Dr. Coatney, a PHS investigator, wrote to Dr. Cutler about a conversation he had with the surgeon general: “As you well know, he is very much interested in the project and a merry twinkle came into his [Dr. Parran’s] eye when he said, ‘You know, we couldn’t do such an experiment in this country’” (65).
In June 1943, while submitting plans for the Terre Haute experiments, Dr. Mahoney began experiments into the efficacy of penicillin on syphilis (67). The antibiotic caused rapid and complete disappearance of spirochetal activity in infected men (77). Dr. Mahoney presented the unexpected findings at a meeting of the American Public Health Association in October 1943. The presentation had an electrifying effect on the audience, with one attendee claiming, “This is probably the most significant paper ever presented in the medical field” (67). Penicillin revolutionized the management of sexually transmitted disease. In 1940, the death rate for syphilis was 10.7 per 100,000; in 1950 it fell to 5 per 100,000, and in 1970 it reached 0.2 per 100,000 (14).
As the Guatemalan research was commencing in 1946, Dr. Mahoney was awarded the Lasker Award for his “distinguished service as a pioneer in the treatment of syphilis with penicillin.” Other awardees that year included Karl Landsteiner (1868–1943), discoverer of blood groups and Rhesus factor (78), and Ferdinand Cori (1896–1984), discoverer of the mechanism whereby glycogen is metabolized and resynthesized (78). The Lasker Award is known as the American Nobel Prize because many awardees receive both, as did Landsteiner and Cori. Accordingly, it is not an exaggeration to say that Dr. Mahoney was the preeminent American physician-scientist in 1946. As Dr. Mahoney received the award, the presenter proclaimed, “Your name will be joined in history with that of Paul Ehrlich” (79).
In pursuit of a praiseworthy goal (eradication of sexually transmitted disease), the PHS investigators rationalized to themselves that it was morally acceptable to infect people with the same fearsome disease. In all of the studies—Guatemala, Indiana, and Alabama—vulnerable people were used as a means to further the scientific ends and careers of physicians they trusted.
In their analysis of PHS research in Central America, the Presidential Commission for the Study of Bioethical Issues devoted considerable space to how stringent rules can prevent scientific misconduct. Yet, according to the commission, PHS investigators recognized the existence of such rules—clear evidence that rules provide no substitute for individual conscience. None of the PHS investigators volunteered to serve as subjects in their own experiments. The commission considered self-experimentation “as quaint and irrelevant” (65). As a physician-scientist who has conducted numerous physiological experiments on himself, I believe self-experimentation may prove a stronger deterrent than sets of rules.
The commission assumed—incorrectly—that Dr. Cutler wished to hide the results of the Guatemalan research. Researchers commonly fail to write up the results of experiments that do not produce clear answers. PHS researchers published several papers on the basis of serological studies conducted in Guatemala (80–83) and published other studies on the basis of induction of infection through inoculation (65, 84). If Dr. Cutler had wished to be secretive, he would have destroyed the records rather than donate them to University of Pittsburgh 40 years after completing the work. In donating his files, he may have hoped that future scientists would build on his observations.
A striking feature of the Guatemalan research is that it did not arise through any fault in the chain of command (65). The principal investigator was the most eminent physician-scientist in the United States. The research plan was approved by an NIH study section, which included physician-investigators from the country’s leading medical schools. The surgeon general was enthusiastic about the studies and was kept informed of their progress.
Lessons
Lessons from the PHS experiments are manifold. The Alabama investigation was conducted in an open society, it extended over 40 years, and it resulted in numerous publications in reputable journals read all over the world. The experiment is a story that needs to stay forever on the moral horizons of medical scientists, yet many young investigators know little of its details or lessons (85).
For the final 25 years of the Alabama experiment, the message of the Nuremberg Code had been widely disseminated. Investigators looked on it as “a good code for barbarians” (86), and it had little impact in the United States (87, 88). Bioethicist Arthur Caplan avers that the PHS study is “the single most important event in the rise of bioethics” (8). Reforms arising from the Kennedy congressional hearings led to fundamental changes in the infrastructure of research ethics. Yet it is doubtful that these provisions benefited significantly the segments of society affected by the study: impoverished Black persons.
Some argue that revolutionary changes in research ethics obviate claims by HEW advisory panel members of a government whitewash (5, 60, 89). History is the story of roads taken, and counterfactual history contemplates what might have happened had a different road been ventured. The dominant factor that undergirded the PHS study was racism (90), which was played down to near invisibility in the HEW final report (5). Had society confronted the flagrant evidence of structural racism in 1972 and instituted fundamental reform of social contributors to health, the stark racial disparities of health outcomes exposed by the klieg light of COVID-19 could have been prevented (58, 91); likewise, root reform of law enforcement in 1972 could have prevented the many deaths of Black persons consequent to unlawful police actions (59).
As with many instances of scientific misconduct, senior scientists were fully aware of the nature and magnitude of the PHS irregularities and took no action. Yet when the information was communicated in the lay press, the problem was immediately obvious to the general public. How can it be that problems reported on the front page of the New York Times become clear in retrospect, yet, in the preceding years, extremely accomplished physician-scientists saw no problem? Lack of imagination, rationalization, and institutional constraints are formidable obstacles in such situations.
In Humanity: a Moral History of the Twentieth Century, philosopher Jonathan Glover (92) analyzes several genocides, bringing together ethics and history, and concludes that only moral imagination (the ability to imagine ourselves in the shoes of endangered individuals) can enable us to alter our outlook and take steps to remedy a threatening situation. Many factors deaden moral imagination—groupthink, tribalism, obedience—and prevent us from taking action. Cultivation of moral imagination, Glover contends, holds the best hope of battling against comforting conventional attitudes and official policy, making vivid the destiny of dehumanized individuals, and becoming determined to take action. A succession of physicians worked on the Tuskegee project. If the consciences of new recruits were troubled on being first exposed to the study design, they acted as if they did not notice the peril of the enrollees, looking away and keeping silent. The consciences of these physicians were protected by moral inertia—finding it easier to fall in with the momentum of established routine and policy (92).
When officials are confronted with major sociopolitical problems, they spin themselves. They convince themselves that raising the concern will be futile and may even backfire with worse consequences. The CDC used this argument when trying to persuade Peter Buxtun that the PHS study should not be stopped. The blue-ribbon panel argued that penicillin would be dangerous (28). When the men were eventually treated with penicillin, not a single complication was observed (1).
When morals collide with actions, a common response is to blame the victim—Dr. Kampmeier blamed study participants for failing to request penicillin for aortitis (33). The prefix attached to the study by PHS investigators is a variant of the blame-the-victim tactic. Tuskegee University, founded by former slave Booker T. Washington in 1881, should be celebrated as a milestone in African American history. Instead, each time the Tuskegee study is mentioned, the university and townspeople are touched by a legacy of shame. Rather than besmirching the victims and their descendants, it would be more accurate to label the experiment after the perpetrators: the Public Health Service Study of Partially Treated Syphilis (93).
None of the study scientists wrote articles reflecting on its moral lessons. “No apologies were tendered. No one admitted any wrongdoing,” inveighs James Jones (1). In 1993, Dr. Cutler appeared on the PBS Nova documentary “Deadly Deception” (94). When asked about the Tuskegee men, he declaimed, “It was important that they were supposedly untreated, and it would be undesirable to go ahead and use large amounts of penicillin to treat the disease, because you’d interfere with the study.” He remonstrated, “I was bitterly opposed to killing off the Study for obvious reasons” (95). Regarding the enrollees, he attested, “They served their race very well.”
Dr. Parran served as surgeon general (1936–1948) during the time that penicillin was advocated to treat every American with syphilis—except men in Macon County. He did more than any other person to control sexually transmitted infections (68, 96, 97). He was founding dean of the University of Pittsburgh Graduate School of Public Health (1948–1958), and the school’s main building was named Parran Hall in 1969. In 1972, the American Sexually Transmitted Diseases Association named its highest award in his honor (98). The Pittsburgh school introduced the John C. Cutler Memorial Lecture in Global Health in 2003 to honor another former faculty member. A new dean canceled the lecture series in 2008 because of community sensitivities regarding Dr. Cutler’s role in the Tuskegee research (69). In 2013, American Sexually Transmitted Diseases Association members voted to remove Dr. Parran’s name from its annual award (76); in 2018, his name was stripped from the Pittsburgh Graduate School of Public Health building (99).
There is a common perception that moral judgment is linked to education. Yet the person who stopped the PHS study, Peter Buxtun, had no training in research; he was a social worker and had far less conventional education than the future director of the National Cancer Institute who led the study for years and many surgeons general who had intimate knowledge of it. With characteristic concision, Thomas Jefferson captured the distinction in a letter to his nephew: “An honest heart being the first blessing, a knowing head is the second” (100). Intelligence and education are not enough in human affairs: character and conscience come first. It is tempting to compartmentalize the lessons of the PHS study into those that apply to our actions as researchers and those that apply to our behavior as lay citizens. That would be a mistake because the two blend into each other.
When we look back at the Alabama and Guatemala stories, we fall into the trap of placing ourselves on the side of the angels, of grouping ourselves with the Buxtuns of this world. Hindsight is comforting, but it is also misleading (101). Coping with challenges as they unfold in real time is very different. Only one Peter Buxtun stood up over 40 years. It is more likely that most researchers would have followed in the footprints of Drs. Vonderlehr, Wenger, and Heller and the many other investigators involved.
There is a natural tendency to believe that group effort and cooperation are more effective than the actions of an individual. Correction of the great ills of society has always started in the heart of one individual and thereafter spread to a small group who recognized the same injustice. An especially astute commentator on social affairs, Adam Smith, wrote in 1763, “Slavery has hardly any possibility of it being abolished. . . . [It] has been universall [sic] in the beginnings of society, and the love of dominion and authority over others will probably make it perpetual” (102). A few years later, a 25-year-old deacon, Thomas Clarkson (1760–1846), started a movement that forced British Parliament to pass an act in 1807 abolishing the slave trade (102).
Individuals such as Buxtun and Clarkson who set out to make a difference are usually branded as irrational, soft, or naive. In official and administrative circles, where discussion is performed in the cold language of interests, people who urge intervention on the basis of moral arguments are considered “emotional.”
The reason people fail to take steps to halt behavior that in retrospect everyone judges reprehensible is complex. Scholars have long pondered the question. One of the first to wonder what light the second World War shed on this question was Hannah Arendt (1906–1975). She deconstructed the psychological and moral implications of evil (103). In 1961, she attended the trial of war criminal Adolf Eichmann. Arendt published a controversial book, Eichmann in Jerusalem: A Report on the Banality of Evil (1963). The expression “banality of evil” gave rise to much criticism and misunderstanding. Some saw Arendt as exonerating Eichmann and blaming the victims. When writing early drafts, Arendt was inclined to describe the evil quality of totalitarianism as something utterly “radical” (104). One of her mentors, physician-philosopher Karl Jaspers (1883–1969), argued that such a characterization made Nazism seem somehow unique and thus, in an awful way, “great” (103). As Arendt reflected on the matter, she arrived at the conclusion that evil arises from a simple failure to think.
What struck Arendt when listening to Eichmann was his banality: “his penchant for ‘officialese,’ for stock phrases, for shallow elations, his ‘empty talk,’ his being ‘genuinely incapable of uttering a single sentence that was not a cliché’” (104). She continued, “The longer one listened to him, the more obvious it became that his inability to speak was closely connected with an inability to think, namely to think from the standpoint of somebody else” (104). As Arendt inferred, “The trouble with Eichmann was precisely that so many were like him, and that the many were neither perverted nor sadistic, that they were, and still are, terribly and terrifyingly normal . . . this normality was much more terrifying than all the atrocities put together” (104). In this sense, the evil of the PHS experiments is banal and not radical. Banality does not trivialize evil: it is precisely what makes the behavior so dangerous (105).
Allied to a lack of thinking is a lack of reflection, an examination of conscience—the courage to form a judgment. Peter Buxtun was not afraid to judge and be counted. Today, we are constantly cautioned against being judgmental—not to form a moral opinion about the actions of others (106). Ahead of her time, Arendt saw the dangers of ethical relativism. Writing to Jaspers in 1963, she reflected that “even good and, at bottom, worthy people have, in our time, the most extraordinary fear about making judgments. This confusion about judgment can go hand in hand with fine and strong intelligence, just as good judgment can be found in those not remarkable for their intelligence” (103). For Buxtun, exercising judgment was a matter of moral courage.
When faced with serious injustice in their midst, the real reason people fail to intervene is a lack of willpower. Consider the Rwandan genocide—the most efficient killing spree of the 20th century (107). Across 100 days (April 6 to July 18, 1994), 800,000 Tutsi and politically moderate Hutu were murdered—the equivalent of more than two World Trade Center attacks every day for 100 days. In contrast to the broad support for the United States after September 11, every country turned away when the Tutsi cried out. During the 3 months of the genocide, the U.S. president never once assembled his top policy advisers to discuss the killings (108). After being personally lobbied by Human Rights Watch, Anthony Lake (born 1939), the president’s national security adviser, issued a statement calling on Rwandan military leaders to “do everything in their power to end the violence immediately.”
When Lake was informed 6 years after the genocide that this statement constituted the sum total of official public attempts to shame the Rwandan government, he was stunned: “You’re kidding,” he replied. “That’s truly pathetic” (108). Here is a leader who had acquired a reputation as a person of conscience, who was in a position of enormous power, and yet he failed to act; indeed, he appeared to be unaware that he had not acted. So it is not only medical researchers who fail to act on concerns that seem repellant in retrospect. In all walks of life, people who have reputations for good conscience, who are trained at the highest level, who possess all the facts and know the harmful consequences, and who have the power to act, still fail to act. Instead, they find sound logical reasons to dismiss all the information and decide not to intervene as events unfold in real time.
We must be careful not to use the Alabama and Guatemala research as an opportunity for letting off moralistic steam. Denouncing an injustice, observes Tzvetan Todorov, “constitutes a moral act only at those times when such denunciation is not simply a matter of course and thus involves some personal risk. There is nothing moral in speaking out against slavery today” (105). One can legitimately make moral demands only on oneself. To imagine oneself floating above the fields of Macon County and Guatemala City and wagging an indignant finger at the shades of Dr. Vonderlehr and Dr. Cutler constitutes “moralism.” People who hold themselves up as examples to others are in fact acting immorally, irrespective of how commendable their conduct may otherwise be (105). Hannah Arendt again: “Goodness can exist only when it is not perceived, not even by its author; whoever sees himself performing a good work is no longer good, but at best a useful member of society” (104).
Reflection on the PHS experiments highlights that out of the crooked timber of humanity, nothing entirely straight can be fashioned. Everything we know about the PHS researchers informs us that they were perceived as being decent people who did much good in other parts of their professional lives. Given the actions of Drs. Parran, Mahoney, and Cutler and other esteemed researchers, we need to approach today’s ethical challenges with “fear and trembling” (Kierkegaard’s phrase)—and remember to pause and think, reflect and examine our conscience, and have the courage to speak and, above all, the willpower to act.
Acknowledgments
Acknowledgment
The author thanks Sidney Wolfe, M.D., and Charles Natanson, M.D., for comments on an earlier version of the manuscript.
Footnotes
Supported by National Institute of Nursing Research grant R01-NR016055 and Veterans Administration Research Merit Review Award 1 I01 RX002803-01A1.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202201-0136SO on May 2, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jones JH. Bad blood: the Tuskegee syphilis experiment . New York: Free Press; 1981. p. 1993. [Google Scholar]
- 2. Katz RV, Kegeles SS, Kressin NR, Green BL, James SA, Wang MQ, et al. Awareness of the Tuskegee syphilis study and the US presidential apology and their influence on minority participation in biomedical research. Am J Public Health . 2008;98:1137–1142. doi: 10.2105/AJPH.2006.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dibble EH, Jr, Rabb LA, Ballard RB. John A. Andrew Memorial Hospital. J Natl Med Assoc . 1961;53:103–118. [PMC free article] [PubMed] [Google Scholar]
- 4.Reverby SM. In: The search for the legacy of the USPHS syphilis study. Katz RV, Warren RC, editors. Lanham: Lexington Books; 2013. The “Tuskegee” syphilis study as a “site of memory.”; pp. 29–40. [Google Scholar]
- 5.Reverby SM. Examining Tuskegee: the infamous syphilis study and its legacy. Chapel Hill, NC: University of North Carolina Press; 2009. [Google Scholar]
- 6. Rusert B. “A study in nature”: the Tuskegee experiments and the New South plantation. J Med Humanit . 2009;30:155–171. doi: 10.1007/s10912-009-9086-4. [DOI] [PubMed] [Google Scholar]
- 7. Reverby SM. Enemy of the people/enemy of the state: two great(ly infamous) doctors, passions, and the judgment of history. Bull Hist Med . 2014;88:403–430. doi: 10.1353/bhm.2014.0062. [DOI] [PubMed] [Google Scholar]
- 8. Caplan A. Bad blood: the Tuskegee syphilis experiment. Biosocieties . 2007;2:275–276. [Google Scholar]
- 9. Brandt AM. Racism and research: the case of the Tuskegee syphilis study. Hastings Cent Rep . 1978;8:21–29. [PubMed] [Google Scholar]
- 10. Zenilman J. The Guatemala sexually transmitted disease studies: what happened. Sex Transm Dis . 2013;40:277–279. doi: 10.1097/OLQ.0b013e31828abc1b. [DOI] [PubMed] [Google Scholar]
- 11. Frieden TR, Collins FS. Intentional infection of vulnerable populations in 1946–1948: another tragic history lesson. JAMA . 2010;304:2063–2064. doi: 10.1001/jama.2010.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cumming HS. In: Tuskegee’s truths: rethinking the Tuskegee syphilis study. Reverby SM, editor. Chapel Hill, NC: University of North Carolina Press; 2000. p. 77. [Google Scholar]
- 13. Hem E. Boeck’s sarcoidosis—a centennial. Int J Dermatol . 2000;39:545–549. doi: 10.1046/j.1365-4362.2000.00397.x. [DOI] [PubMed] [Google Scholar]
- 14.Brandt AM. No magic bullet: a social history of venereal disease in the United States since 1880 . New York, NY: Oxford University Press; 1987. [Google Scholar]
- 15.Porter R. The greatest benefit to mankind: a medical history of humanity. New York, NY: W.W. Norton; 1998. [Google Scholar]
- 16. Benedek TG. The “Tuskegee study” of syphilis: analysis of moral versus methodologic aspects. J Chronic Dis . 1978;31:35–50. doi: 10.1016/0021-9681(78)90079-6. [DOI] [PubMed] [Google Scholar]
- 17.Wenger OC. In: Tuskegee’s truths: rethinking the Tuskegee syphilis study. Reverby SM, editor. Chapel Hill, NC: University of North Carolina Press; 2000. pp. 84–85. [Google Scholar]
- 18.Parran T. In: Tuskegee’s truths: rethinking the Tuskegee syphilis study. Reverby SM, editor. Chapel Hill, NC: University of North Carolina Press; 2000. pp. 59–69. [Google Scholar]
- 19. Mahoney JF, Arnold RC, Sterner BL, Harris A, Zwally MR. Penicillin treatment of early syphilis: II. JAMA . 1944;126:63–67. doi: 10.1001/jama.251.15.2005. [DOI] [PubMed] [Google Scholar]
- 20.Lucas JB. In: Tuskegee’s truths: rethinking the Tuskegee syphilis study. Reverby SM, editor. Chapel Hill, NC: University of North Carolina Press; 2000. pp. 107–109. [Google Scholar]
- 21. Reverby SM. More than fact and fiction: cultural memory and the Tuskegee syphilis study. Hastings Cent Rep . 2001;31:22–28. [PubMed] [Google Scholar]
- 22.Fowler G.1989. https://www.nytimes.com/1989/05/06/obituaries/dr-john-r-heller-jr-dies-at-84-headed-national-cancer-institute.html
- 23. Vonderlehr RA, Clark T, Wenger OC, Heller JR Jr. Untreated syphilis in the male Negro: a comparative study of treated and untreated cases. JAMA . 1936;107:856–860. [Google Scholar]
- 24. Caplan AL. Twenty years after: the legacy of the Tuskegee syphilis study. When evil intrudes. Hastings Cent Rep . 1992;22:29–32. [PubMed] [Google Scholar]
- 25. Rockwell DH, Yobs AR, Moore MB., Jr The Tuskegee study of untreated syphilis; the 30th year of observation. Arch Intern Med . 1964;114:792–798. doi: 10.1001/archinte.1964.03860120104011. [DOI] [PubMed] [Google Scholar]
- 26. Peters JJ, Peers JH, Olansky S, Cutler JC, Gleeson GA. Untreated syphilis in the male Negro; pathologic findings in syphilitic and nonsyphilitic patients. J Chronic Dis . 1955;1:127–148. doi: 10.1016/0021-9681(55)90204-6. [DOI] [PubMed] [Google Scholar]
- 27. Olansky S, Harris A, Cutler JC, Price EV. Untreated syphilis in the male Negro; twenty-two years of serologic observation in a selected syphilis study group. AMA Arch Derm . 1956;73:516–522. doi: 10.1001/archderm.1956.01550050094017. [DOI] [PubMed] [Google Scholar]
- 28.Reverby SM, editor. Summary of Ad Hoc Committee to Consider the Tuskegee Study. Tuskegee’s truths: rethinking the Tuskegee syphilis study. Chapel Hill, NC: University of North Carolina Press; 2000. pp. 463–472. [Google Scholar]
- 29.North American Neuro-Ophthalmology Society. 2022. https://library.med.utah.edu/NOVEL/Smith/
- 30.Kershaw I. Hitler, the Germans, and the final solution. New Haven, CT: Yale University Press; 2008. [Google Scholar]
- 31.Heller J. In: Tuskegee’s truths: rethinking the Tuskegee syphilis study. Reverby SM, editor. Chapel Hill, NC: University of North Carolina Press; 2000. pp. 116–117. [Google Scholar]
- 32. Billings FT., Jr Memorial: Rudolph H. Kampmeier. Trans Am Clin Climatol Assoc . 1991;102:lxii–lxv. [PMC free article] [PubMed] [Google Scholar]
- 33. Kampmeier RH. The Tuskegee study of untreated syphilis. South Med J . 1972;65:1247–1251. [PubMed] [Google Scholar]
- 34.Reverby SM, editor. Tuskegee’s truths: rethinking the Tuskegee syphilis study. Chapel Hill, NC: University of North Carolina Press; 2000. [Google Scholar]
- 35.Reverby SM, editor. President William J. Clinton’s remarks. Tuskegee’s truths: rethinking the Tuskegee syphilis study. Chapel Hill, NC: University of North Carolina Press; 2000. pp. 574–577. [Google Scholar]
- 36.U.S. Department of Health, Education, and Welfare, Public Health Service. Final report of the Tuskegee Syphilis Study Ad Hoc Advisory Panel. Washington, DC: U.S. Department of Health, Education, and Welfare, Public Health Service; 1973. [Google Scholar]
- 37.Watson P. The modern mind: an intellectual history of the 20th century. New York, NY: Perennial; 2001. [Google Scholar]
- 38. Lombardo PA. A child’s right to be well born: venereal disease and the eugenic marriage laws, 1913–1935. Perspect Biol Med . 2017;60:211–232. doi: 10.1353/pbm.2017.0029. [DOI] [PubMed] [Google Scholar]
- 39.Friedlander H. The origins of Nazi genocide: from euthanasia to the final solution. Chapel Hill, NC: University of North Carolina Press; 1995. [Google Scholar]
- 40. Lombardo PA, Dorr GM. Eugenics, medical education, and the Public Health Service: another perspective on the Tuskegee syphilis experiment. Bull Hist Med . 2006;80:291–316. doi: 10.1353/bhm.2006.0066. [DOI] [PubMed] [Google Scholar]
- 41.Proctor RN. Racial hygiene: medicine under the Nazis. Cambridge, MA: Harvard University Press; 1998. [Google Scholar]
- 42. Davis RM. Achieving racial harmony for the benefit of patients and communities: contrition, reconciliation, and collaboration. JAMA . 2008;300:323–325. doi: 10.1001/jama.300.3.323. [DOI] [PubMed] [Google Scholar]
- 43. Baker RB, Washington HA, Olakanmi O, Savitt TL, Jacobs EA, Hoover E, et al. African American physicians and organized medicine, 1846–1968: origins of a racial divide. JAMA . 2008;300:306–313. doi: 10.1001/jama.300.3.306. [DOI] [PubMed] [Google Scholar]
- 44. Baker RB, Washington HA, Olakanmi O, Savitt TL, Jacobs EA, Hoover E, et al. Writing Group on the History of African Americans and the Medical Profession Creating a segregated medical profession: African American physicians and organized medicine, 1846–1910. J Natl Med Assoc . 2009;101:501–512. doi: 10.1016/s0027-9684(15)30935-4. [DOI] [PubMed] [Google Scholar]
- 45. Washington HA, Baker RB, Olakanmi O, Savitt TL, Jacobs EA, Hoover E, et al. Writing Group on the History of African Americans and the Medical Profession Segregation, civil rights, and health disparities: the legacy of African American physicians and organized medicine, 1910–1968. J Natl Med Assoc . 2009;101:513–527. doi: 10.1016/s0027-9684(15)30936-6. [DOI] [PubMed] [Google Scholar]
- 46. Baker RB. The American Medical Association and race. Virtual Mentor . 2014;16:479–488. doi: 10.1001/virtualmentor.2014.16.06.mhst1-1406. [DOI] [PubMed] [Google Scholar]
- 47.American Association for the History of Medicine. 2021. https://www.histmed.org/announcements/news/aahm-news-aahm-calls-for-properly-archiving-jamas-podcast-on-structural-racism-for-doctors-3226
- 48. Tanne JH. JAMA editor in chief steps down over controversial structural racism podcast. BMJ . 2021;373:n1433. doi: 10.1136/bmj.n1433. [DOI] [PubMed] [Google Scholar]
- 49. Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A . 2016;113:4296–4301. doi: 10.1073/pnas.1516047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Todd KH, Deaton C, D’Adamo AP, Goe L. Ethnicity and analgesic practice. Ann Emerg Med . 2000;35:11–16. doi: 10.1016/s0196-0644(00)70099-0. [DOI] [PubMed] [Google Scholar]
- 51. Goyal MK, Kuppermann N, Cleary SD, Teach SJ, Chamberlain JM. Racial disparities in pain management of children with appendicitis in emergency departments. JAMA Pediatr . 2015;169:996–1002. doi: 10.1001/jamapediatrics.2015.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Braun L, Wolfgang M, Dickersin K. Defining race/ethnicity and explaining difference in research studies on lung function. Eur Respir J . 2013;41:1362–1370. doi: 10.1183/09031936.00091612. [DOI] [PubMed] [Google Scholar]
- 53. Bailey ZD, Feldman JM, Bassett MT. How structural racism works—racist policies as a root cause of US racial health inequities. N Engl J Med . 2021;384:768–773. doi: 10.1056/NEJMms2025396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hardeman RR, Medina EM, Kozhimannil KB. Structural racism and supporting black lives—the role of health professionals. N Engl J Med . 2016;375:2113–2115. doi: 10.1056/NEJMp1609535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eberly LA, Richterman A, Beckett AG, Wispelwey B, Marsh RH, Cleveland Manchanda EC, et al. Identification of racial inequities in access to specialized inpatient heart failure care at an academic medical center. Circ Heart Fail . 2019;12:e006214. doi: 10.1161/CIRCHEARTFAILURE.119.006214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA . 2019;322:113–114. doi: 10.1001/jama.2019.5774. [DOI] [PubMed] [Google Scholar]
- 57. Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med . 2020;383:874–882. doi: 10.1056/NEJMms2004740. [DOI] [PubMed] [Google Scholar]
- 58. Acosta AM, Garg S, Pham H, Whitaker M, Anglin O, O’Halloran A, et al. Racial and ethnic disparities in rates of COVID-19-associated hospitalization, intensive care unit admission, and in-hospital death in the United States from March 2020 to February 2021. JAMA Netw Open . 2021;4:e2130479. doi: 10.1001/jamanetworkopen.2021.30479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Edwards F, Lee H, Esposito M. Risk of being killed by police use of force in the United States by age, race-ethnicity, and sex. Proc Natl Acad Sci U S A . 2019;116:16793–16798. doi: 10.1073/pnas.1821204116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Washington HA. Medical apartheid: the dark history of medical experimentation on Black Americans from colonial times to the present. New York, NY: Anchor Books; 2006. [Google Scholar]
- 61.Osler W. Transactions of the Congress of American Physicians and Surgeons, Seventh Triennial Session. New Haven, CT: Congress of American Physicians and Surgeons; 1907. The evolution of the idea of experiment in medicine; pp. 1–8. [Google Scholar]
- 62.Reverby SM. In: Bioethics in action. Baylis F, Dreger A, editors. Cambridge, United Kingdom: Cambridge University Press; 2018. “So what?” Historical contingency, activism, and reflections on the studies in Tuskegee and Guatemala; pp. 32–54. [Google Scholar]
- 63.National Archives. 2017. https://www.archives.gov/research/health/cdc-cutler-records/
- 64. Reverby SM. “Normal exposure” and inoculation syphilis: a PHS “Tuskegee” doctor in Guatemala, 1946–1948. J Policy Hist . 2011;23:6–28. [Google Scholar]
- 65.Presidential Commission for the Study of Bioethical Issues 2011https://bioethicsarchive.georgetown.edu/pcsbi/sites/default/files/Ethically%20Impossible%20(with%20linked%20historical%20documents)%202.7.13.pdf.
- 66.Presidential Commission for the Study of Bioethical Issues 2011https://bioethicsarchive.georgetown.edu/pcsbi/sites/default/files/Moral%20Science%20June%202012.pdf.
- 67. Parascandola J. John Mahoney and the introduction of penicillin to treat syphilis. Pharm Hist . 2001;43:3–13. [Google Scholar]
- 68. Hook EW., III Remembering Thomas Parran, his contributions and missteps going forward: history informs us. Sex Transm Dis . 2013;40:281–282. doi: 10.1097/OLQ.0b013e31828abc55. [DOI] [PubMed] [Google Scholar]
- 69.Ove T.2011. https://www.post-gazette.com/opinion/Op-Ed/2011/06/12/The-Next-Page-Before-Tuskegee-the-Guatemala-Experiment-a-Pitt-legend-s-research-is-under-scrutiny/stories/201106120141
- 70. Mahoney JF, Van Slyke CJ, Cutler JC, Blum HL. Experimental gonococcic urethritis in human volunteers. Am J Syph Gonorrhea Vener Dis . 1946;30:1–39. [PubMed] [Google Scholar]
- 71. Walter M. Human experiments: first, do harm. Nature . 2012;482:148–152. doi: 10.1038/482148a. [DOI] [PubMed] [Google Scholar]
- 72. Spector-Bagdady K, Lombardo PA. “Something of an adventure”: postwar NIH research ethos and the Guatemala STD experiments. J Law Med Ethics . 2013;41:697–710. doi: 10.1111/jlme.12080. [DOI] [PubMed] [Google Scholar]
- 73. Zenilman JM. Ethics gone awry: the US Public Health Service studies in Guatemala; 1946–1948. Sex Transm Infect . 2013;89:295–300. doi: 10.1136/sextrans-2012-050741. [DOI] [PubMed] [Google Scholar]
- 74. Reverby SM. Ethical failures and history lessons: the US Public Health Service research studies in Tuskegee and Guatemala Public Health Rev 2012. 34 1 18 26236074 [Google Scholar]
- 75. Reverby SM. Compensation and reparations for victims and bystanders of the U.S. Public Health Service research studies in Tuskegee and Guatemala: who do we owe what? Bioethics . 2020;34:893–898. doi: 10.1111/bioe.12784. [DOI] [PubMed] [Google Scholar]
- 76. Spector-Bagdady K, Lombardo PA. US Public Health Service STD experiments in Guatemala (1946–1948) and their aftermath. Ethics Hum Res . 2019;41:29–34. doi: 10.1002/eahr.500010. [DOI] [PubMed] [Google Scholar]
- 77. Mahoney JF, Arnold RC, Harris A. Penicillin treatment of early syphilis—a preliminary report. Am J Public Health Nations Health . 1943;33:1387–1391. doi: 10.2105/ajph.33.12.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Millar D, Millar I, Millar J, Millar M. The Cambridge dictionary of scientists. Cambridge, United Kingdom: Cambridge University Press; 1996. [Google Scholar]
- 79.Lasker Foundation 2020https://laskerfoundation.org/winners/penicillin-as-a-cure-for-syphilis/.
- 80. Levitan S, Aragon HA, Cutler JC, Funes JM, Portnoy J, Paredes-Luna A. Clinical and serologic studies with reference to syphilis in Guatemala Central America. I. Studies of comparative performance of the Kahn, Kolmer, Mazzini, and VDRL slide tests as carried out in the national orphanage. Am J Syph Gonorrhea Vener Dis . 1952;36:379–387. [PubMed] [Google Scholar]
- 81. Cutler JC, Levitan S, Arnold RC, Portnoy J. Studies on the comparative behavior of various serologic tests for syphilis. II. A report on an observed pattern of entrance into seroreactivity among patients with untreated primary syphilis. Am J Syph Gonorrhea Vener Dis . 1952;36:533–544. [PubMed] [Google Scholar]
- 82. Portnoy J, Galvez R, Cutler JC. Clinical and serologic studies with reference to syphilis in Guatemala, Central America. III. Studies of comparative performance of the Kahn, Kolmer, Mazzini, and VDRL slide tests among leprosy patients. Am J Syph Gonorrhea Vener Dis . 1952;36:566–570. [PubMed] [Google Scholar]
- 83. Funes JM, Cutler JC, Levitan S, Portnoy J, Funes R. Serologic and clinical studies of syphilis in Guatemala, Central America. II. Study of a group of school children in the port of San José [in Spanish] Bol Oficina Sanit Panam . 1953;34:14–18. [PubMed] [Google Scholar]
- 84. Magnuson HJ, Thomas EW, Olansky S, Kaplan BI, De Mello L, Cutler JC. Inoculation syphilis in human volunteers. Medicine (Baltimore) . 1956;35:33–82. doi: 10.1097/00005792-195602000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Jones JH. In: Tuskegee’s truths: rethinking the Tuskegee syphilis study. Reverby SM, editor. Chapel Hill, NC: University of North Carolina Press; 2000. Foreword; pp. xi–xiii. [Google Scholar]
- 86.Katz J. In: The Nazi doctors and the Nuremberg code: human rights in human experimentation. Annas GJ, Grodin MA, editors. New York, NY: Oxford University Press; 1992. The consent principle of the Nuremberg Code: its significance then and now; pp. 227–239. [Google Scholar]
- 87. Caplan AL. Too hard to face. J Am Acad Psychiatry Law . 2005;33:394–400. [PubMed] [Google Scholar]
- 88. Caplan AL. Are existing safeguards adequate? J Calif Alliance Ment Ill . 1994;5:36–38. [PubMed] [Google Scholar]
- 89. Reverby SM. Inclusion and exclusion: the politics of history, difference, and medical research. J Hist Med Allied Sci . 2008;63:103–113. doi: 10.1093/jhmas/jrm030. [DOI] [PubMed] [Google Scholar]
- 90.Katz RV, Warren RC, editors. The search for the legacy of the USPHS syphilis study. Lanham, MD: Lexington Books; 2013. [DOI] [PubMed] [Google Scholar]
- 91. Shiels MS, Haque AT, Haozous EA, Albert PS, Almeida JS, García-Closas M, et al. Racial and ethnic disparities in excess deaths during the COVID-19 pandemic, March to December 2020. Ann Intern Med . 2021;174:1693–1699. doi: 10.7326/M21-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glover J. Humanity: a moral history of the twentieth century. London, United Kingdom: Jonathan Cape; 1999. [Google Scholar]
- 93.Fletcher JC. In: Tuskegee’s truths: rethinking the Tuskegee syphilis study. Reverby SM, editor. Chapel Hill, NC: University of North Carolina Press; 2000. A case study in historical relativism: the Tuskegee (Public Health Service) syphilis study; pp. 276–298. [Google Scholar]
- 94.DiAnni D. Nova. Boston, MA: WGBH; 1993. The deadly deception. [Google Scholar]
- 95.Junod T. In: Tuskegee’s truths: rethinking the Tuskegee syphilis study. Reverby SM, editor. Chapel Hill, NC: University of North Carolina Press; 2000. Deadly medicine; pp. 509–524. [Google Scholar]
- 96. Stoner BP, Marrazzo JM. American Sexually Transmitted Diseases Association and the Thomas Parran Award: past, present, and future. Sex Transm Dis . 2013;40:275–276. doi: 10.1097/OLQ.0b013e31828abc2e. [DOI] [PubMed] [Google Scholar]
- 97. Altman LK. Of medical giants, accolades and feet of clay. New York Times . 2013 https://www.nytimes.com/2013/04/02/health/link-to-ethical-scandals-tarnishes-prestigious-parran-award.html [Google Scholar]
- 98. Zenilman JM. The legacy of sexually transmitted disease research: lessons from Guatemala and Dr. Thomas Parran: the American STD Association Distinguished Career Award Lecture. Sex Transm Dis . 2013;40:901–908. doi: 10.1097/OLQ.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 99. University of Pittsburgh. Parran Hall name disappears after Board of Trustees agrees with chancellor’s recommendation. University Times . 2018 https://www.utimes.pitt.edu/news/parran-hall-name [Google Scholar]
- 100.Pellegrino ED, Thomasma DC. The virtues in medical practice. New York, NY: Oxford University Press; 1993. [Google Scholar]
- 101. Henriksen K, Kaplan H. Hindsight bias, outcome knowledge and adaptive learning. Qual Saf Health Care . 2003;12:ii46–ii50. doi: 10.1136/qhc.12.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hochschild A. Bury the chains: prophets and rebels in the fight to free an empire’s slaves. Boston, MA: Mariner Books; 2005. [Google Scholar]
- 103.Judt T. Reappraisals: reflections on the forgotten twentieth century. London, United Kingdom: Penguin Books; 2008. [Google Scholar]
- 104.Baehr P, editor. The portable Hannah Arendt. New York, NY: Penguin; 2000. [Google Scholar]
- 105.Todorov T. Facing the extreme: moral life in the concentration camps. New York, NY: Owl Books; 1996. [Google Scholar]
- 106.Warnock M. An intelligent person’s guide to ethics. Croydon, United Kingdom: Duckbacks; 1998. [Google Scholar]
- 107.Dallaire R. Shake hands with the devil: the failure of humanity in Rwanda. Cambridge, MA: Da Capo Press; 2004. [Google Scholar]
- 108.Power S. A problem from hell: America and the age of genocide. New York, NY: Harper Perennial; 2002. [Google Scholar]
- 109.ICP. John Albert [accessed 2022 Apr 3]. Available from: . https://www.icp.org/browse/archive/objects/thomas-parran-jr-sixth-surgeon-general-of-the-united-states-new-york
- 110.Becker B. Plagues & people. http://faculty.humanities.uci.edu/bjbecker/plaguesandpeople/lecture10.html