Abstract
Introduction
Predictors of the effectiveness of immune checkpoint inhibitor (ICI) monotherapy in previously treated patients with non-small cell lung cancer (NSCLC) remain ill-defined. We investigated whether the Glasgow prognostic score (GPS) could serve as such predictors.
Methods
Eighty patients treated with pembrolizumab or atezolizumab monotherapy as second- or subsequent-line therapy for NSCLC were retrospectively reviewed, and the associations between GPS, body mass index (BMI), and each of progression-free survival (PFS) and overall survival (OS) were assessed.
Results
The median follow-up period was 11.1 months. Patients with a BMI ≥20.4 kg/m<sup>2</sup> had significantly longer PFS and OS (3.7 and 22.2 month, respectively) than did those with a BMI <20.4 kg/m<sup>2</sup> (2.2 and 11.5 months, respectively). Patients with a GPS of 0 had a significantly longer PFS (6.6 months) than did those with a GPS of 1 (2.2 months, p = 0.002) and 2 (1.8 months, p = 0.029). Patients with a GPS of 0 also had a significantly longer OS (22.2 month) than did those with a GPS of 1 (9.2 months, p = 0.002) and 2 (4.7 months, p = 0.002). Notably, the GPS, BMI, and clinical stage were independent predictors of PFS, while the GPS and performance status were independent predictors of OS. The response rate of patients with a GPS of 0 was significantly higher than that of patients with a GPS of 1–2 (26.2% vs. 7.9%, p = 0.03).
Conclusion
The GPS is an independent predictor of PFS and OS in patients with NSCLC who received second- or subsequent-line pembrolizumab or atezolizumab monotherapy.
Keywords: Non-small cell lung cancer, Glasgow prognostic score, Pembrolizumab, Atezolizumab
Introduction
Lung cancer is one of the most fatal malignancies worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers [2], with most patients diagnosed at an advanced stage. Patients with NSCLC frequently experience weight loss and systemic inflammatory response, which can lead to cancer-associated cachexia [3, 4]. The body mass index (BMI) has been used as a prognostic indicator for various cancers [5, 6, 7]. Additionally, the Glasgow prognostic score (GPS) is a systemic inflammatory response-based evaluation system that relies on serum C-reactive protein (CRP) and albumin levels, and is also a measure of nutritional status [3]. The GPS was initially developed by Forrest et al. [8] as a prognostic indicator for patients with advanced NSCLC; subsequently, many studies found that the GPS is an independent predictor of survival in patients with various cancers, including NSCLC [9, 10, 11, 12].
Immune checkpoint inhibitors (ICIs) that target programmed cell death 1 (PD-1) or programmed death-ligand 1 (PD-L1) have been gaining attention as novel therapeutic agents for patients with NSCLC [13, 14]; notably, it was previously reported that the GPS could be a significant predictor of progression-free survival (PFS) and overall survival (OS) in patients receiving such treatments [12]. In addition to PD-L1 expression from tumor cells, diabetes and immune-related adverse events have previously been reported as predictors of ICIs treatment response [15, 16]. However, most previous studies investigated the relationship between the GPS and efficacy of several types of ICIs, in patients who had undergone various treatment lines for NSCLC [12, 17]; conversely, few studies have evaluated the relationship between the GPS and efficacy of pembrolizumab or atezolizumab monotherapy, as second- or subsequent-line treatment for NSCLC. Additionally, previous studies found that the BMI was a prognostic factor in patients with various types of cancer [5] and that it correlated with the effectiveness of ICI therapies against solid tumors including NSCLC [18]. However, it remains unclear whether the GPS can be an independent predictor of PFS and OS following ICI monotherapy after adjusting for BMI. Thus, we performed this study to assess whether the GPS could predict the effectiveness of second- or subsequent-line pembrolizumab or atezolizumab monotherapy in patients previously treated for NSCLC.
Materials and Methods
Patients
We retrospectively evaluated 85 patients with advanced or metastatic NSCLC, who had received pembrolizumab or atezolizumab monotherapy as the second- or subsequent-line treatment between March 2017 and January 2022. Pretreatment evaluation data of tumor PD-L1 expression were unavailable in five of the subjects; hence, 80 patients were ultimately included in the analysis. The data collection cut-off date was May 2022. The pathological diagnosis of NSCLC was classified according to the 2015 World Health Organization Classification of Tumors, and the disease stage was assessed using version 8.0 of the Tumor-Node-Metastasis classification system. The eligibility criteria were as follows: (1) histologically or cytologically confirmed NSCLC, (2) unresectable stage III/IV disease or postoperative recurrence, and (3) pretreatment evaluation of tumor PD-L1 expression. None of the patients had received any ICIs prior to pembrolizumab or atezolizumab monotherapy, and each was confirmed to have died or else was censored at the end of the study. The disease stage at diagnosis was based on physical examination, chest radiography, thoracic and abdominal computed tomography, brain computed tomography or magnetic resonance imaging, and bone scintigraphy or 18F-fluorodeoxyglucose positron emission tomography. Data on patient backgrounds and treatment responses to pembrolizumab or atezolizumab were collected retrospectively from electronic medical records. This study was reviewed and approved by the Kitasato University Medical Ethics Organization (B21-095) and permitted the use of the opt-out method in lieu of written informed consent.
GPS System and BMI Cut-Off
Serum CRP and albumin levels were measured either on the day of the initiation of pembrolizumab or atezolizumab monotherapy or 1 day before. The GPS was categorized into three groups as follows: A GPS of 0 denoted CRP <1.0 mg/dL in conjunction with an albumin level ≥3.5 mg/dL, a score of 1 denoted either CRP elevation or albumin decrease alone, and a score of 2 indicated CRP ≥1.0 mg/dL in conjunction with an albumin level <3.5 mg/dL. The BMI was calculated before the start of pembrolizumab or atezolizumab treatment and was defined as the weight (kg) divided by height (m) squared. The patients were stratified into low versus high BMI groups using receiver operating characteristic analysis for OS.
Assessment of PD-L1 Expression
PD-L1 expression in formalin-fixed tumor specimens was evaluated using a commercially available immunohistochemistry kit for detecting PD-L1 (22C3 pharmDx assay; Dako North America, Agilent, Santa Clara, CA, USA). Biopsy specimens obtained at the time of lung cancer diagnosis were collected from the institutional archives. PD-L1 expression (i.e., membranous staining) was quantified as the proportion of positive cells among the tumor cells and tumor-infiltrating immune cells.
Treatment and Response Evaluation
Pembrolizumab (200 mg) or atezolizumab (1,200 mg) was administered every 3 weeks until the disease progressed, unacceptable toxicity was observed, or the patient refused treatment. Tumor response was evaluated as the best overall response and maximum tumor shrinkage; radiographic responses were evaluated using the Response Evaluation Criteria in Solid Tumors version 1.1. The ICI monotherapy agent as well as prior and subsequent-line treatment regimens were selected at the discretion of the attending physician.
Statistical Analysis
We used the χ2 test and Welch's t-test for categorical and continuous variables, respectively. PFS was defined as the interval between ICI monotherapy initiation and disease progression or death. OS was defined as the interval between the initiation of ICI monotherapy and death; patients who were alive were censored on the date of the last follow-up. The Kaplan–Meier method was used to estimate survival, with differences analyzed using the log-rank test. Cox proportional hazards models with stepwise regression were applied to identify factors predictive of PFS and OS; the results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs), and the variables included all patient background such as sex, age, performance status, tumor histology, clinical stage at diagnosis, mutation status (EGFR and EML4-ALK), BMI, GPS, ICI regimen, smoking status, tumor proportion score, and treatment line. Statistical significance was set at a p value ≤0.05. All statistical analyses were performed using the SPSS software version 28.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Patient Characteristics
Eighty patients with advanced NSCLC who received pembrolizumab or atezolizumab monotherapy as a second- or subsequent-line treatment between March 2017 and January 2022 were included in the final analysis; their basic characteristics are shown in Table 1. The median age was 70.5 years, and 56 (70%) of the patients were male. Fifty-three patients (66%) had an Eastern Cooperative Oncology Group performance status (PS) score of 0 or 1, and most (85%) had stage IV disease at disease diagnosis. PD-L1 tumor expression data were available for all patients; 28 of them (35%) had PD-L1 expression in ≥50% of their tumor cells. Driver oncogene mutation data were available for 73 patients (91.3%). The median BMI was 21.3 kg/m2, and the median CRP and albumin levels were 0.89 mg/dL and 3.8 mg/dL, respectively. The optimal BMI cut-off value at the initiation of ICI treatment was 20.4 kg/m2, which had an area under the receiver operating characteristic curve of 0.60 with a sensitivity of 74.3% and specificity of 47.0%. Of the 80 patients, 42 (53%), 18 (22%), and 20 (25%) had GPS values of 0, 1, and 2, respectively; their characteristics according to these scores are shown in Table 2. There were significant differences (p < 0.05) in BMI between those with GPS 0 versus those with scores of 1–2. The median number of ICI cycles was three (range, 1–74).
Table 1.
Basic characteristics of the patients (N = 80)
| Sex | |
|---|---|
| Female/Male | 24/56 |
| Age in years, median (range) | 70.5 (44–86) |
| ECOG performance status score | |
| 0–1/2–3 | 53/27 |
| Histology | |
| Squamous/non-squamous | 19/61 |
| Clinical stage at diagnosis | |
| III, IV | 68 |
| Recurrent | 12 |
| Mutation (EGFR/ALK) | |
| Wildtype/unknown | 58 |
| Mutant | 22 |
| Body mass index, kg/m2 | |
| Median (range) | 21.3 (16.5–32.2) |
| Laboratory data, median (range) | |
| C-reactive protein, mg/dL | 0.89 (0.03–11.5) |
| Albumin, g/dL | 3.8 (1.8–4.6) |
| Glasgow prognostic score | |
| 0/1/2 | 42/18/20 |
| Regimen | |
| Pembrolizumab/atezolizumab | 49/31 |
| Administered cycles of pembrolizumab | |
| Median (range) | 3 (1–74) |
| Administered cycles of atezolizumab | |
| Median (range) | 3 (1–40) |
| Smoking status | |
| Current or former/never | 62/18 |
| Tumor proportion score | |
| <50%/≥50% | 52/28 |
| Treatment line | |
| Second/third or subsequent | 39/41 |
ECOG, Eastern Cooperative Oncology Group.
Table 2.
Characteristics of patients categorized by GPS
| GPS: 0 (n = 42) | GPS: 1–2 (n = 38) | p value | |
|---|---|---|---|
| Age in years, median (range) | 73 (45–83) | 70 (44–86) | 0.64 |
| Sex | |||
| Male/female | 26/16 | 30/8 | 0.10 |
| Performance status | |||
| 0–1/2–3 | 31/11 | 22/16 | 0.13 |
| Histology | |||
| Squamous/non-squamous | 9/33 | 10/28 | 0.44 |
| Clinical stage at diagnosis | |||
| III or IV/recurrence | 35/7 | 33/5 | 0.66 |
| Mutation (EGFR/ALK) | |||
| Wildtype or unknown/mutant | 27/15 | 31/7 | 0.084 |
| Body mass index (kg/m2), median (range) | 22.6 (16.5–32.2) | 20.8 (16.5–27.0) | 0.14 |
| High (≥20.4)/low (<20.4) | 30/12 | 21/17 | 0.015 |
| Regimen | |||
| Pembrolizumab/atezolizumab | 28/14 | 21/17 | 0.30 |
| Smoking status | |||
| Current or former smoker/never smoker | 32/10 | 30/8 | 0.77 |
| TPS | |||
| <50%/≥50% | 24/18 | 28/10 | 0.12 |
| Treatment line | |||
| Second/third or subsequent | 22/20 | 17/21 | 0.49 |
GPS, Glasgow prognostic score; TPS, tumor proportion score.
Response and Survival
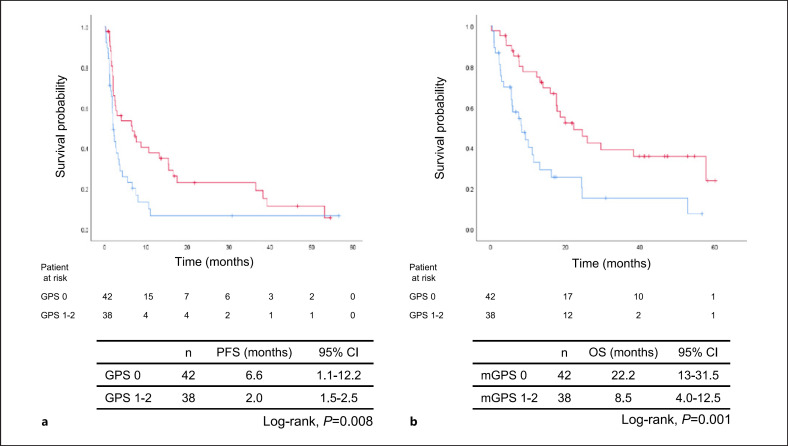
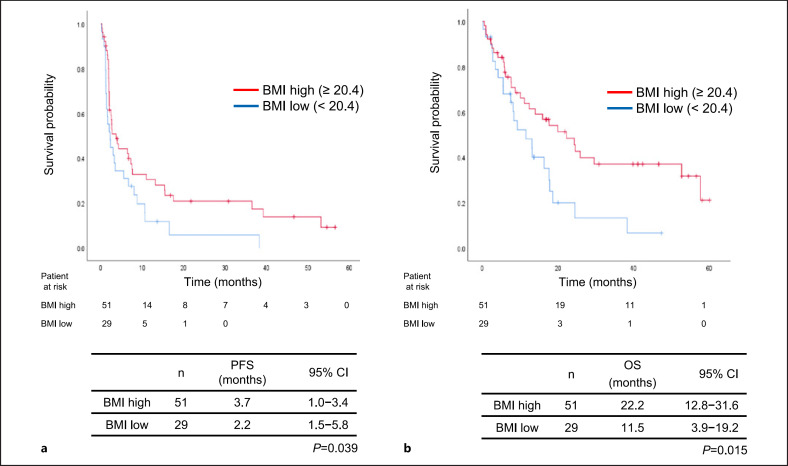
The objective tumor response rates are shown in Table 3. One patient had a complete response to ICI monotherapy, whereas 13 had a partial response; as such, the overall response rate was 17.5% (95% CI: 5.4–29.6). The response rate of patients with a GPS of 0 (26.2%) was significantly higher than that of patients with a GPS of 1–2 (7.9%, p = 0.03). The median follow-up period for all patients was 11.1 months (range, 0.1–60.1 months). The median PFS was 2.7 months (95% CI: 1.5–3.9 months) while the median OS was 16.3 months (95%: CI 11.5–21.0 months). Patients with a GPS of 0 (6.6 months) had significantly more favorable PFS than did those with a GPS of 1-2 (2.0 months, p = 0.008) (Fig. 1a). Furthermore, patients with a GPS of 0 had a significantly more favorable OS (22.2 months) than did those with a GPS of 1-2 (8.5 months, p = 0.001) (Fig. 1b). Additionally, patients with a BMI ≥20.4 kg/m2 exhibited significantly more favorable PFS and OS than did those with a BMI <20.4 kg/m2 (Fig. 2a, b). Univariate analyses demonstrated significant associations between PFS and each of BMI and GPS (Table 4). Multivariate analyses demonstrated that PFS was associated with clinical stage at diagnosis (HR 2.62 [95% CI: 1.20–5.73], p = 0.015), BMI (HR 0.56 [95% CI: 0.33–0.95], p = 0.031), and GPS (HR 1.45 [95% CI: 1.09–1.94], p = 0.012, Table 4). Furthermore, univariate analyses of OS demonstrated significant associations between OS and each of PS and GPS, while multivariate analyses demonstrated that OS was significantly associated with PS (HR 1.99 [95% CI: 1.09–3.62], p = 0.024) and GPS (HR 1.49 [95% CI: 1.07–2.05], p = 0.017, Table 5).
Table 3.
Responses of patients to immune checkpoint inhibitor therapy according to their GPS
| All patients (n = 80) | GPS: 0 (n = 42) | GPS: 1–2 (n = 38) | |
|---|---|---|---|
| Complete response | 1 | 1 | 0 |
| Partial response | 13 | 10 | 3 |
| Stable disease | 20 | 12 | 8 |
| Progressive disease | 42 | 17 | 25 |
| Not evaluable | 4 | 2 | 2 |
| Response rate (95% CI) | 17.5 (5.4–29.6) | 26.2 (12.2–40.2) | 7.9 (1.0–16.5) |
| p = 0.03 |
CI, confidence interval; GPS, Glasgow prognostic score.
Fig. 1.
Kaplan-Meier plots showing (a) progression-free survival (PFS) and (b) overall survival (OS) according to Glasgow prognostic score (GPS); CI: confidence interval.
Fig. 2.
Kaplan-Meier plots showing (a) progression-free survival (PFS) and (b) overall survival (OS) according to the body mass index (BMI; ≥20.4 vs. <20.4 kg/m2); CI: confidence interval.
Discussion
We found that a low GPS (i.e., a CRP <1.0 mg/dL and albumin >3.5 mg/dL) before the initiation of pembrolizumab or atezolizumab monotherapy, was significantly associated with delayed disease progression and death in patients with advanced NSCLC, who had received prior treatment after adjusting for potential confounding covariates, including BMI. Additionally, patients with a low GPS experienced significantly more favorable response rates. To our knowledge, this is the first study to assess the clinical value of GPS in terms of predicting survival while adjusting for BMI, among previously treated patients with NSCLC, who received pembrolizumab or atezolizumab monotherapy. While inflammatory indices including the GPS, modified GPS, and CRP-to-albumin ratio have been shown to be significant prognostic predictors in patients with cancer [19, 20, 21], Takamori et al. [12] revealed that the GPS was a more robust prognostic factor than either the modified GPS or the CRP-to-albumin ratio in patients with NSCLC who were treated with ICIs. Our findings were consistent with theirs, and affirmed the value of the GPS for patients with NSCLC who are treated with ICIs. Importantly, the GPS is derived from serum CRP and albumin concentrations that can be obtained during routine in-patient blood sampling, and is thus easy to acquire.
Hypoalbuminemia has previously been shown to be a significant indicator of poor prognosis in patients with cancer [22, 23]. A previous study showed that albumin binds to prostaglandin E2, which mediates the downregulation of macrophage-derived tumor necrosis factor-α and is involved in immunosuppression [24, 25, 26, 27], and it was notably reported that administering human albumin solution inhibits prostaglandin E2 activation, resulting in improved immune function [26]. Regarding serum CRP, several previous studies have shown that increased serum CRP levels are associated with the upregulation of proinflammatory cytokines and impaired functioning of T lymphocytes and macrophages [28, 29]. The upregulated proinflammatory cytokines contribute to tumor invasiveness and maintenance of tumor growth [30]. Based on the above, GPS, which relies on serum CRP and albumin levels, is a firm prognostic factor in NSCLC patients treated with ICIs.
Several previous studies revealed that the BMI was significantly correlated with the OS of patients with NSCLC who were treated with second- or subsequent-line ICIs, as patients with high BMIs experienced more favorable outcomes [18, 31, 32]. While our multivariate analysis revealed that the BMI tended to be associated with OS, the correlation was not significant in our analysis like it was in previous study [18]. The reason for this inconsistency may be the small number of patients in our study as well as the influence of previous treatments, given that the ICI in our study was administered as a second- or subsequent-line treatment.
Previous studies found that activation of the molecular pathways involved in the pathogenesis of cachexia can impair the immune system's antitumor function [33, 34, 35]. Moreover, inhibiting cytokine signaling, which is implicated in the development of cachexia, has been shown to strengthen the antitumor immune function [36, 37], and the combined blockade of specific pro-cachexia mediators plus the PD-1/PD-L1 axis, reportedly has synergistic effects [38, 39]. Administering anti-cachexia agents in combination with ICIs might improve efficacy of the latter, in patients with cancer who exhibit cachexia. Accordingly, further research of both serum markers and the tumor microenvironment in patients with NSCLC who are cachexic ought to identify the underlying factors that can lead to ICI treatment failure.
PS has previously been identified as a potent prognostic factor in patients with lung cancer [40, 41]. Our study also found that PS was a significant prognostic factor, suggesting that our study population is representative of patients with NSCLC in general. While most doctors are hesitant to administer ICIs to patients with NSCLC having a poor PS, they do not take serum albumin and CRP levels into consideration. The PS is a subjective index scoring system that is useful for evaluating the patient's general well-being; in contrast, the GPS is an objective index scoring system that can be used to classify patients based on a combination of albumin and CRP measures. The two scoring systems not only offer great value but can also complement each other in terms of predicting OS.
There were limitations in our study. First, it was of a retrospective design and comprised a small number of patients from a single institution; as such, our results cannot be considered definitive without additional validation. Second, only patients with advanced NSCLC who had received ICI monotherapy were included, suggesting that our findings should be validated prospectively in patients treated with combination chemotherapy plus ICI regimens and pembrolizumab monotherapy regimen as a first-line setting, that have been approved in clinical practice worldwide. Additionally, patients who received nivolumab monotherapy were not included in this study.
In conclusion, our findings showed that the GPS is an independent predictor for PFS and OS in patients with NSCLC, who received pembrolizumab and atezolizumab monotherapy as a second- or subsequent-line treatment. The GPS can be determined objectively and conveniently and is thus valuable for clinical assessment prior to treatment. A larger study is warranted to determine whether our results can be generalized to other second-line ICI-treated patient populations, and whether the GPS can be an additional differentiating factor in future clinical trials of ICIs.
Statement of Ethics
This study was reviewed and approved by the Kitasato University Medical Ethics Organization (B21-095) and permitted the use of the opt-out method in lieu of written informed consent.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author Contributions
Masashi Kasajima and Satoshi Igawa conceived the study and participated in its design and coordination. Hiroya Manaka, Kaori Yamada, Yuki Akazawa, Hideaki Manabe, Yuri Yagami, Hiroki Yamamoto, Hiroki Ito, and Nobuki Kaizuka performed data curation. Masashi Kasajima and Satoshi Igawa performed statistical analyses and interpretation. Yoshiro Nakahara, Takashi Sato, Hisashi Mitshufuji, Masanori Yokoba, Masaru Kubota, Jiichiro Sasaki, and Katsuhiko Naoki supervised the study. Masashi Kasajima, Satoshi Igawa, and Katsuhiko Naoki drafted the text.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to the staff members of the Department of Respiratory Medicine, Kitasato University School of Medicine, for their suggestions and assistance.
Funding Statement
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
References
- 1.Howlader N, Forjaz G, Mooradian MR, Kong CY, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67:257–262. doi: 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- 4.Proctor MJ, Talwar D, Balmar SM, O'Reilly DS, Foulis AK, Horgan PG, et al. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103:870–876. doi: 10.1038/sj.bjc.6605855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, et al. Impact of sarcopenia in patients with advanced non-small cell lung cancer treated with PD-1 inhibitors a preliminary retrospective study. Sci Rep. 2019;9:2447. doi: 10.1038/s41598-019-39120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors when overweight becomes favorable. J Immunother Cancer. 2019;7:57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichihara E, Harada D, Inoue K, Sato K, Hosokawa S, Kishino D, et al. The impact of body mass index on the efficacy of anti-PD-1/PD-L1 antibodies in patients with non-small cell lung cancer. Lung Cancer. 2020;139:140–145. doi: 10.1016/j.lungcan.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–1030. doi: 10.1038/sj.bjc.6601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitani S, Taniguchi H, Sugiyama K, Masuishi T, Honda K, Narita Y, et al. The impact of the Glasgow Prognostic Score on survival in second-line chemotherapy for metastatic colorectal cancer patients with BRAF V600E mutation. Ther Adv Med Oncol. 2009:11. doi: 10.1177/1758835918820298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crozier JEM, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC. Relationship between emergency presentation, systemic inflammatory response, and cancer-specific survival in patients undergoing potentially curative surgery for colon cancer. Am J Surg. 2009;197:544–549. doi: 10.1016/j.amjsurg.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment Glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol. 2012;7:655–662. doi: 10.1097/JTO.0b013e318244ffe1. [DOI] [PubMed] [Google Scholar]
- 12.Takamori S, Takada K, Shimokawa M, Matsubara T, Fujishita T, Ito K, et al. Clinical utility of pretreatment Glasgow prognostic score in non-small-cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2021;152:27–33. doi: 10.1016/j.lungcan.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Zhou Y, Tang L, Peng X, Jiang H, Wang G, et al. Immune-checkpoint inhibitors as the first line treatment of advanced non-small cell lung cancer A meta-analysis of randomized controlled trials. J Cancer. 2019;10:6261–6268. doi: 10.7150/jca.34677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Man S, Sun R, Li Z, Wu Y, Zuo D. Recent advances and challenges of immune checkpoint inhibitors in immunotherapy of non-small cell lung cancer. Int Immunopharmacol. 2020;85:106613. doi: 10.1016/j.intimp.2020.106613. [DOI] [PubMed] [Google Scholar]
- 15.Jacobi O, Landman Y, Reinhorn D, Icht O, Sternschuss M, Rotem O, et al. The relationship of diabetes mellitus to efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer Oncology. 2021;99:555–561. doi: 10.1159/000516671. [DOI] [PubMed] [Google Scholar]
- 16.Sonehara K, Tateishi K, Araki T, Komatsu M, Yamamoto H, Koizumi T, et al. The role of immuneRelated adverse events in prognosis and efficacy prediction for patients with non-small cell lung cancer treated with immunotherapy a retrospective clinical analysis: Oncology. 2021;99:271–279. doi: 10.1159/000511999. [DOI] [PubMed] [Google Scholar]
- 17.Imai H, Kishikawa T, Minemura H, Yamada Y, Ibe T, Yamaguchi O, et al. Pretreatment Glasgow prognostic score predicts survival among patients with high PD-L1 expression administered first-line pembrolizumab monotherapy for non-small cell lung cancer. Cancer Med. 2021;10:6971–6984. doi: 10.1002/cam4.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai H, Naito E, Yamaguchi O, Hashimoto K, Iemura H, Miura Y, et al. Pretreatment body mass index predicts survival among patients administered nivolumab monotherapy for pretreated non-small cell lung cancer. Thorac Cancer. 2022;13:1479–1489. doi: 10.1111/1759-7714.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930–1935. doi: 10.1038/bjc.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takamori S, Toyokawa G, Shimokawa M, Kinoshita F, Kozuma Y, Matsubara T, et al. The C-reactive protein/albumin ratio is a novel significant prognostic factor in patients with malignant pleural mesothelioma a retrospective multi-institutional study. Ann Surg Oncol. 2018;25:1555–1563. doi: 10.1245/s10434-018-6385-x. [DOI] [PubMed] [Google Scholar]
- 21.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 22.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival a systematic review of the epidemiological literature. Nutr J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miura K, Hamanaka K, Koizumi T, Kitaguchi Y, Terada Y, Nakamura D, et al. Clinical significance of preoperative serum albumin level for prognosis in surgically resected patients with non-small cell lung cancer comparative study of normal lung, emphysema, and pulmonary fibrosis. Lung Cancer. 2017;111:88–95. doi: 10.1016/j.lungcan.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Petersen CE, Ha CE, Bhagavan NV. Structural insights into human serum albumin-mediated prostaglandin catalysis. Protein Sci. 2002;11:538–545. doi: 10.1110/ps.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.China L, Maini A, Skene SS, Shabir Z, Sylvestre Y, Colas RA, et al. Albumin counteracts immune-suppressive effects of lipid mediators in patients with advanced liver disease. Clin Gastroenterol Hepatol. 2018;16:738.e7–747.e7. doi: 10.1016/j.cgh.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choe WH, Baik SK. Prostaglandin E2 -mediated immunosuppression and the role of albumin as its modulator. Hepatology. 2015;61:1080–1082. doi: 10.1002/hep.27644. [DOI] [PubMed] [Google Scholar]
- 27.Arroyo V, Moreau R. Tying up PGE2 with albumin to relieve immunosuppression in cirrhosis. Nat Med. 2014;20:467–469. doi: 10.1038/nm.3553. [DOI] [PubMed] [Google Scholar]
- 28.Nozoe T, Matsumata T, Sugimachi K. Preoperative elevation of serum C-reactive protein is related to impaired immunity in patients with colorectal cancer. Am J Clin Oncol. 2000;23:263–266. doi: 10.1097/00000421-200006000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canna K, Hilmy M, McMillan DC, Smith GW, McKee RF, McArdle CS, et al. The relationship between tumour proliferative activity, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Colorectal Dis. 2008;10:663–667. doi: 10.1111/j.1463-1318.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 31.Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6:512–518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takada K, Takamori S, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, et al. Serum markers associated with treatment response and survival in non-small cell lung cancer patients treated with anti-PD-1 therapy. Lung Cancer. 2020;145:18–26. doi: 10.1016/j.lungcan.2020.04.034. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand F, Montfort A, Marcheteau E, Imbert C, Gilhodes J, Filleron T, et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun. 2017;8:2256. doi: 10.1038/s41467-017-02358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, et al. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016;24:672–684. doi: 10.1016/j.cmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, et al. CXCR1- or CXCR2- modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun. 2019;10:4016. doi: 10.1038/s41467-019-11869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rautela J, Dagley LF, de Oliveira CC, Schuster IS, Hediyeh-Zadeh S, Delconte RB, et al. Therapeutic blockade of activin-A improves NK cell function and antitumor immunity. Sci Signal. 2019;12:eaat7527. doi: 10.1126/scisignal.aat7527. [DOI] [PubMed] [Google Scholar]
- 37.Tinoco R, Carrette F, Barraza ML, Otero DC, Magaña J, Bosenberg MW, et al. PSGL-1 is an immune checkpoint regulator that promotes T cell exhaustion. Immunity. 2016;44:1190–203. doi: 10.1016/j.immuni.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Xu J, Yan X, Jin K, Li W, Zhang R. Targeting interleukin-6 (IL-6) sensitizes anti-PD-L1 treatment in a colorectal cancer preclinical model. Med Sci Monit. 2018;24:5501–5508. doi: 10.12659/MSM.907439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR, et al. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116:1361–1369. doi: 10.1073/pnas.1812266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capewell S, Sudlow MF. Performance and prognosis in patients with lung cancer. The Edinburgh Lung Cancer Group. Thorax. 1990;45:951–956. doi: 10.1136/thx.45.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620–623. doi: 10.1097/JTO.0b013e3181d2dcd9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.