Background:
Carpal tunnel syndrome (CTS) is common in patients with transthyretin amyloidosis (ATTR), and many experience residual symptoms and/or develop recurrent disease following routine carpal tunnel release (CTR). An extended CTR with median nerve neurolysis is recommended for thorough nerve decompression. Tissue confirmation of amyloidosis can be performed at the time of CTR with biopsies of the transverse carpal ligament and/or tenosynovium.
Methods:
We describe a retrospective, single-center experience performing an extended CTR technique including unilateral and bilateral cases for 13 consecutive patients (18 wrists) with ATTR and symptomatic median neuropathy at the wrist.
Results:
The mean patient age was 83 (range 67–90) years and 11 (85%) were men. Notable intraoperative findings in all cases included thickened tenosynovium and median nerve epineurium, and adherence of the median nerve to the deep surface of transverse carpal ligament. Pathology findings were positive for amyloidosis from both the transverse carpal ligament and the tenosynovium biopsies in all patients.
Conclusions:
Extended CTR with simultaneous wrist tissue biopsy can be safely performed for ATTR patients with CTS. Characteristic intraoperative findings should increase clinical suspicion for undiagnosed ATTR and prompt performance of biopsy for diagnostic confirmation. Volar wrist tenosynovial biopsy is our preferred tissue for confirmation of ATTR, for patients with and without CTS, given its safety profile and 100% pathological yield in our series.
Takeaways
Question: How reliable are tenosynovium and transverse carpal ligament biopsies for the diagnosis of amyloidosis?
Findings: In our study, the biopsy yield was 100% for tenosynovium and transverse carpal ligament biopsies for patients with amyloidosis.
Meaning: Tenosynovium and transverse carpal ligament biopsies are highly sensitive and specific for amyloidosis. They are safe and easily performed with minimal patient recovery time postoperatively.
INTRODUCTION
Amyloidosis is an infiltrative disorder caused by the extracellular deposition of misfolded endogenous precursor proteins. Although there are multiple types of amyloidosis, the most common is light chain (AL) amyloidosis, a hematologic malignancy caused by deposition of misfolded immunoglobulin light chain fragments produced by a clonal plasma cell population, and transthyretin amyloidosis (ATTR), caused by misfolding of the hepatically derived transport protein transthyretin.1 ATTR can be further subdivided into hereditary ATTR (ATTRh), caused by a gene mutation of the transthyretin protein, and wild-type ATTR (ATTRwt), an idiopathic age-related disorder occurring mostly in men in the absence of a gene mutation.1 Disease-modifying treatments are available for AL and ATTR, and the prognosis without treatment is poor for both types.
ATTR predominantly causes polyneuropathy and cardiomyopathy. Carpal tunnel syndrome (CTS) is common in patients with amyloidosis and is particularly prevalent among those with ATTR. ATTR patients often have severe CTS at the time of diagnosis, which is frequently bilateral and has a high rate of recurrence after carpal tunnel release (CTR).2 In one recent study, 88% (36 of 41) of ATTRwt cardiomyopathy patients screened for routine neuropathy with a neurologist assessment and nerve conduction studies at the time of ATTRwt diagnosis had CTS, compared to 7% of age-matched controls.3 Another cohort analysis showed that 4% of all men with bilateral CTS had ATTRwt cardiomyopathy, which increased to 33% if they also had left ventricular hypertrophy.4
CTS is often an early clinical manifestation of ATTR and, in some patients, can present years before other systemic manifestations such as cardiomyopathy develop.5 ATTR patients often have relatively aggressive CTS, which is frequently bilateral with a rapid rate of progression and a higher rate of recurrence after CTR compared with patients without ATTR.4,6 Therefore, it has been suggested that ATTR patients with CTS should be treated with an extended CTR surgical technique to improve symptoms and to reduce the risk of recurrence.6 Tissue biopsy of the transverse carpal ligament (TCL) and/or volar wrist flexor tendon tenosynovium can be easily performed at the time of the CTR to allow for tissue confirmation of amyloidosis. For patients without CTS, tissue biopsy of the tenosynovium can be performed without CTR from the distal volar forearm to confirm the diagnosis of amyloidosis.
In addition to diagnostic confirmation in patients with suspected ATTR, in our region, amyloidosis biopsy tissue confirmation is required for patients to be eligible for public reimbursement eligibility for the novel ATTR medication tafamidis, a transthyretin stabilizer, which attenuates disease progression and improves survival for this otherwise fatal disease.7 Tafamidis is expensive (list price in Canada of approximately $200,000/year) and not covered by most private medical insurance plans, and therefore, public coverage is the only option for access for most patients. In light of either the high risk (eg, cardiac) or low yield (eg, fat aspirate) of other targeted biopsy sites,8,9 wrist biopsy has become our preferred approach for tissue confirmation of amyloidosis, including patients without an indication for CTR.
The purpose of this report is to describe our center’s experience performing extended CTR, and TCL and tenosynovium biopsies in ATTR patients.
METHODS
Patient Population
This retrospective study includes patients with a confirmed or suspected diagnosis of ATTR referred to our center for evaluation and management over a 1-year period between 2021 and 2022. In recognition of the complex needs of patients with this multisystem disease, amyloidosis patient care is delivered by a multidisciplinary team of clinical specialists that form the Amyloidosis Program of Calgary (APC, University of Calgary, Calgary, Alberta, Canada). The baseline assessment of all patients includes comprehensive neurology evaluation for CTS and other neuropathies or risk factors for neuropathy [including thyroid dysfunction, diabetes mellitus, monoclonal gammopathy of undetermined significance (MGUS), and rheumatoid arthritis], in addition to cardiovascular evaluation. Patients are then referred to a hand and wrist specialist plastic surgeon for consideration of CTR and/or TCL and tenosynovium biopsy as clinically indicated.
Diagnostic criteria for ATTR are based on current consensus guidelines as follows: (1) exclusion of serum and urine monoclonal protein indicating AL amyloidosis; (2) evidence of cardiac ATTR uptake by technetium 99m-pyrophosphare nuclear scintigraphy, or positive biopsy indicating amyloidosis in addition to evidence of ATTR cardiomyopathy by cardiac imaging criteria and/or neuropathy by clinical and electrophysiology testing; and (3) genetic testing for a transthyretin gene mutation to diagnose ATTRh, or the absence of a gene mutation to diagnose ATTRwt.
Surgical Technique
The procedure is performed in the minor surgery clinic without an anesthesiologist, scrub nurse, or surgical assistant. An extended CTR incision is used over the volar proximal palm and distal forearm. (See Video 1 [online], which shows the incision for extended CTR.) Mini-open10 and endoscopic11 approaches are not recommended for patients with amyloidosis as external neurolysis of the nerve is indicated to ensure complete decompression of the median nerve and dissection of the nerve away from the undersurface of the radial TCL is required.
Video 1. This video demonstrates the incision for extended carpal tunnel release.
Local anesthetic with 1:100 000–1:200 000 epinephrine is infiltrated 30 minutes before commencing surgery to maximize vasoconstriction12 and obviate the need for a tourniquet, improving patient comfort.13 The surgeon wears loupe magnification to visualize the median nerve anatomy in detail.
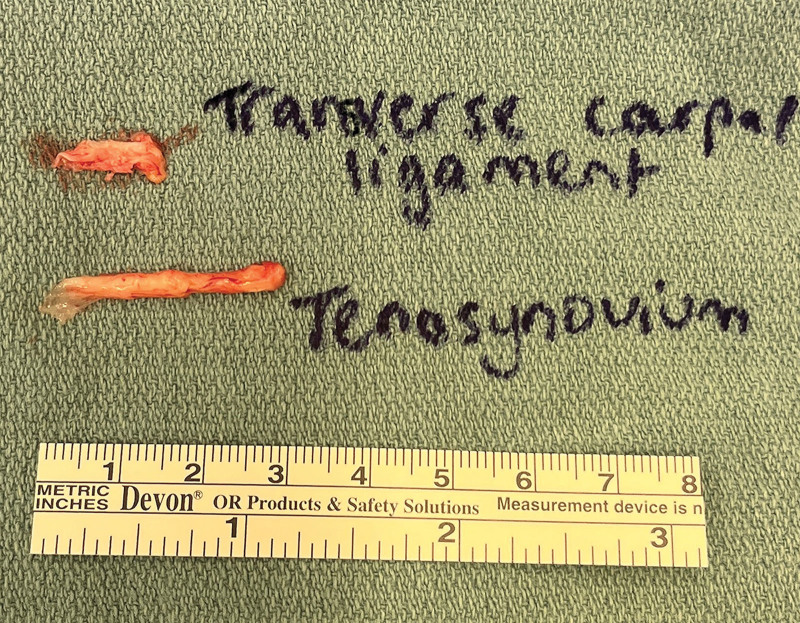
The skin is incised. (See Video 2 [online], which shows the extended CTR with transverse carpal ligament and tenosynovium biopsies.) The palmar fascia is opened. The TCL is divided longitudinally on its ulnar aspect, taking care to avoid injury to the recurrent motor branch of the median nerve.14 A full-thickness biopsy of the TCL is performed from the ulnar TCL, approximately 10 mm in length and 3 mm in width, and sent in formalin for pathological analysis (Fig. 1). The tenosynovium from the flexor tendons crossing the volar wrist, primarily surrounding the more superficial flexor digitorum superficialis tendons compared to the deeper flexor digitorum profundus tendons and the more radially located flexor pollicis longus, is dissected off the tendons, to further facilitate decompression of the median nerve and is sent for pathological analysis.15 Tenosynovium over 20 mm in length can generally be easily harvested during an extended CTR (Fig. 2).
Fig. 1.
Biopsy of the TCL is performed from the ulnar aspect of the TCL. A full-thickness segment of the TCL, approximately 10 mm in length and 3 mm in width, is harvested. Biopsy of the volar wrist flexor tendon tenosynovium is about 20–30 mm in length.
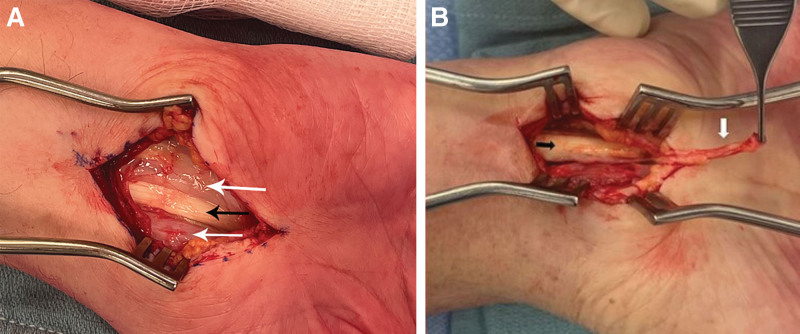
Fig. 2.
The tenosynovium (white arrows) surrounding the volar wrist flexor tendons (black arrows) is elevated from the underlying tendons, shown here held in the forceps.
Video 2. This video demonstrates extended carpal tunnel release with transverse carpal ligament and tenosynovium biopsies.
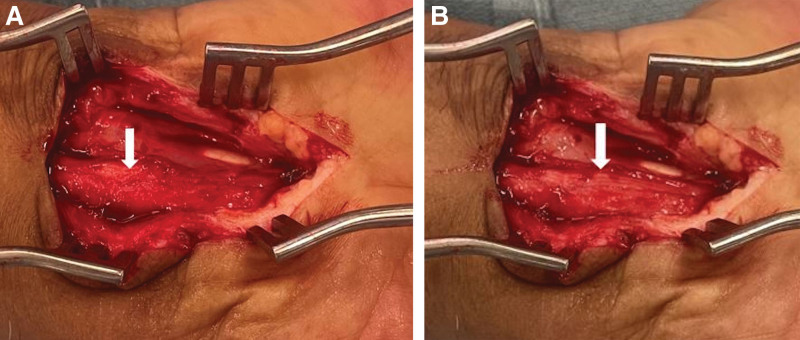
In amyloidosis patients, the epineurium surrounding the median nerve is thick (Figs. 3 and 4A) and requires external neurolysis to release the dense, constricting epineurium to decompress the median nerve until a healthy fascicular pattern is seen (Fig. 4B). (See Video 3 [online], which shows the median nerve appearance following external neurolysis.) In these cases, the median nerve is often adherent to the deep radial surface of the TCL and needs to be dissected away from this area.
Fig. 3.
Patient A: The thick external epineurium of the median nerve is visible here, held in the forceps. The tenotomy scissors are used to perform the external neurolysis, dissecting away the thick, constricting epineurium until a healthy fascicular pattern of the nerve is visualized.
Fig. 4.
Patient B. A, Thick epineurium is seen. B, External neurolysis is performed until the fascicular pattern of the median nerve is visualized, seen here on the ulnar aspect of the nerve, under loupe magnification. In this case, further neurolysis was performed to also decompress the thick, compressive epineurium on the radial aspect of the nerve (not pictured).
Video 3. This video demonstrates the median nerve appearance following external neurolysis.
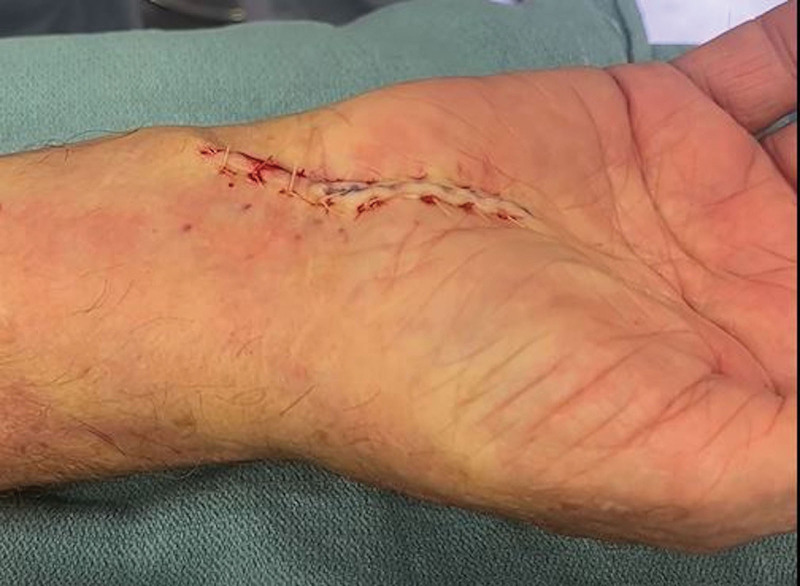
Skin closure is performed with absorbable (eg, 4-0 Monocryl or Vicryl Rapide) or nonabsorbable (eg, 4-0 nylon or Prolene) sutures depending on the patient’s preference (Fig. 5). A nonstick dressing (eg, Jelonet) followed by gauze and a self-adherent wrap (eg, Coban) are placed. (See Video 4 [online], which shows the dressing application following CTR.) Postoperative splinting is not recommended.16 Early active range of motion exercises are encouraged for the fingers and wrist to permit early nerve gliding and to decrease the risk of postoperative scar fibrosis causing recurrent median nerve compression. This also allows patients to regain their grip and pinch strength sooner after surgery than those who are immobilized.17 Finger and wrist flexion and extension range of motion exercises are recommended on an hourly basis, while awake, starting on postoperative day 1.
Fig. 5.
The incision has been sutured with 4-0 dissolving Vicryl Rapide sutures.
Video 4. This video demonstrates the dressing application following carpal tunnel release.
Pathology
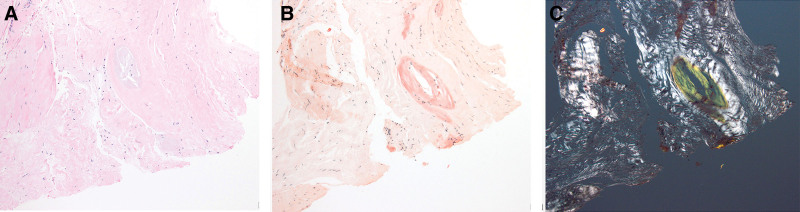
In accordance with routine laboratory protocols, specimens are fixed in 10% neutral buffered formalin for a minimum of 6 hours, beginning immediately after procurement, followed by paraffin embedding. A combination of hematoxylin and eosin and Congo red stains is then assessed. Amyloid deposition is confirmed by the presence of salmon-pink coloration under normal illumination of Congo red-stained sections, with concomitant apple-green birefringence under polarized illumination (Fig. 6).
Fig. 6.
Amyloid deposition. A, Hematoxylin and eosin (100×). B, Congo red (100×). C, Congo red, under polarized light (200×).
Statistical Analysis
Continuous variables are reported as the mean and range, whereas categorical variables are reported as the frequency and percentage of total.
RESULTS
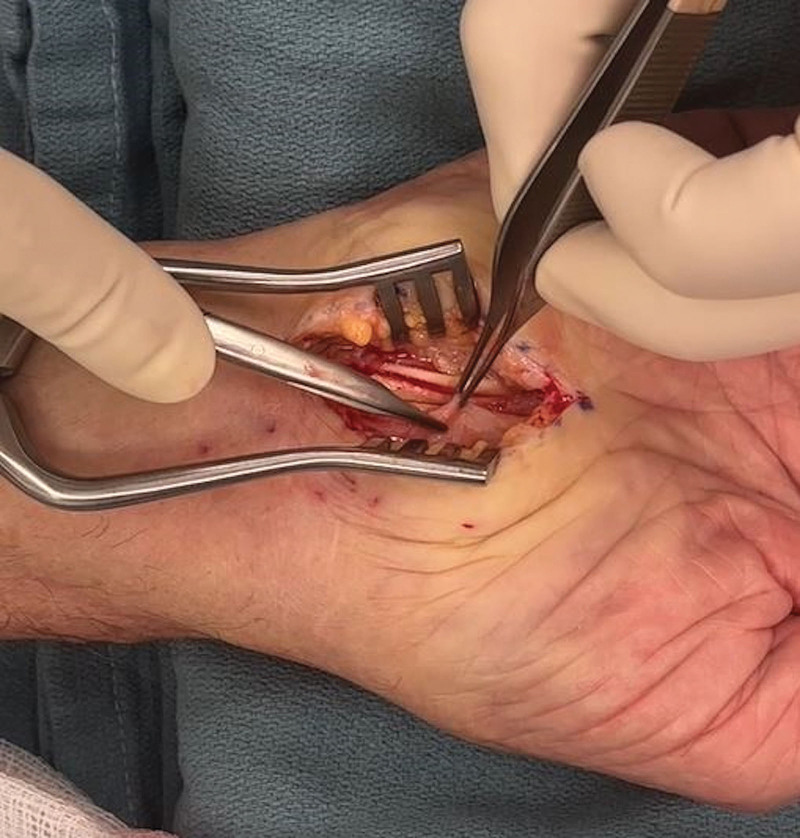
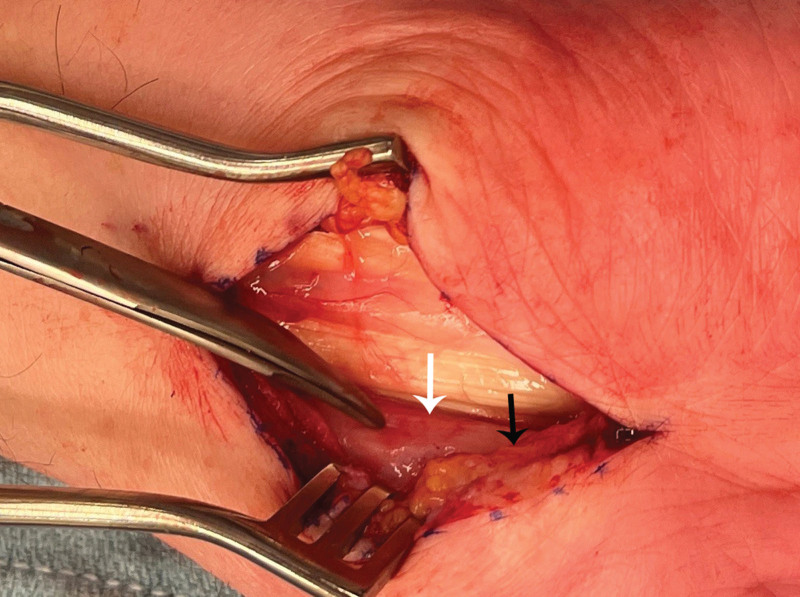
Over a 1-year period, 13 ATTR patients (18 wrists when including those undergoing a simultaneous bilateral procedure) underwent biopsy of the TCL and tenosynovium during CTR for pathological tissue confirmation of amyloidosis (Table 1). The indication for surgery was symptomatic CTS as well as the need for tissue confirmation of amyloidosis for all patients. The mean patient age was 83 (range 67–90) years. The majority of patients were male [n = 11 (85%)]. All patients had the wild-type ATTR subtype. All 13 (100%) patients had a clinical and electrophysiologic diagnosis of CTS, which was bilateral in all cases. Eight (62%) patients had a history of previous CTR, with five (42%) having had previous bilateral CTR. Notable intraoperative findings in all cases included thickened tenosynovium, thickened median nerve epineurium, and median nerve adherence to the deep radial surface of TCL (Fig. 7).
Table 1.
Tissue Confirmation of Amyloidosis in Consecutive Patients Undergoing Tenosynovium and TCL Biopsy
| Patient | Age (y) | Sex | CTR Side | Tenosynovium (±) | TCL (±) | ||
|---|---|---|---|---|---|---|---|
| R | L | R | L | ||||
| 1 | 84 | M | B | + | + | + | + |
| 2 | 81 | M | R | + | N/A | + | N/A |
| 3 | 86 | F | B | + | + | + | + |
| 4 | 87 | M | R | + | N/A | + | N/A |
| 5 | 85 | M | R | + | N/A | + | N/A |
| 6 | 90 | M | B | + | + | + | + |
| 7 | 89 | M | L | N/A | + | N/A | + |
| 8 | 68 | M | B | N/A | N/A | + | + |
| 9 | 85 | M | R | + | N/A | + | N/A |
| 10 | 84 | M | N/A | N/A | + | N/A | N/A |
| 11 | 84 | M | R | + | N/A | + | N/A |
| 12 | 84 | F | R | + | N/A | + | N/A |
| 13 | 67 | M | R | + | N/A | + | N/A |
| 14 | 86 | M | B | + | + | + | + |
B, bilateral; L, left; M, male; F, female; N/A, not applicable; +, positive biopsy; -, negative biopsy; R, right.
Fig. 7.
The median nerve (white arrow) is erythematous and adherent to the deep radial aspect of the divided transverse carpal ligament (black arrow).
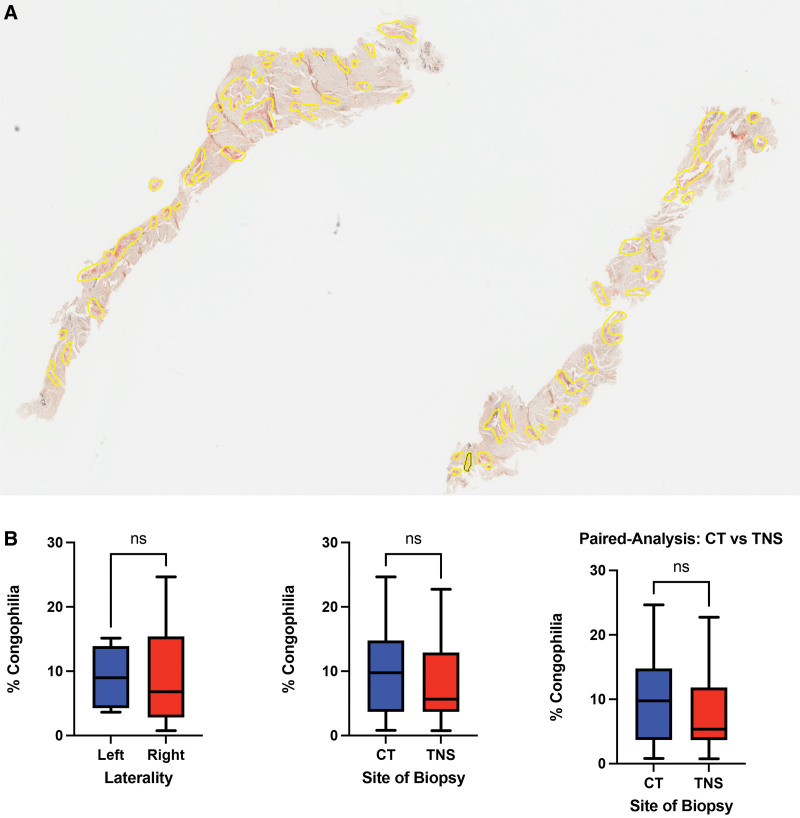
Specimens from both the TCL and the tenosynovium were positive for amyloid in all patients in which both sites were biopsied. To address whether one site or the other might be more informative, we compared the burden of amyloid deposition in the TCL and tenosynovium specimens. To do this, all Congo red sections were digitized (using the Aperio digital imaging system) followed by annotation of affected regions (Fig. 8). We performed concurrent real-time image comparison to the original polarized Congo red slides to confirm the presence of apple-green birefringence. There was no significant difference in amyloid burden in the TCL and tenosynovium specimens.
Fig. 8.
Amyloid deposition. A, Example digitized Congo red stain, with regions of Salmon-pink staining and apple-green birefringence highlighted. B, Box-plots comparing the burden of amyloid deposition by laterality and site. CT, transverse carpal ligament; TNS, tenosynovium; ns, non-significant.
No major complications were noted, including systemic and local (eg, nerve injury, infection, hematoma) complications. One minor complication occurred, a wound dehiscence of the left CTR incision in a patient who had undergone a simultaneous bilateral CTR with wound closure using absorbable sutures (Monocryl) 2 days after surgery. The wound was debrided and resutured using nonabsorbable sutures (nylon) and local anesthetic, and healed uneventfully. Postoperative healing time and time to return to activities have been the same as for patients undergoing standard CTR alone. CTS symptom improvement has been noted in all patients after surgery, with improvements in median nerve sensation and thenar muscle motor strength. We have had no early (symptoms within 1 year of surgery) recurrences. We will follow our patients for recurrent CTS over the next 10 years.
Of our 13 patients who had an extended CTR with wrist biopsies over the 1-year study period, four (31%) underwent simultaneous bilateral release, seven (53%) underwent a right CTR, one (8%) underwent a left CTR, and one (8%) underwent a staged procedure with a left- then right-sided release as per his preference rather than having a simultaneous procedure, with a 7-day interval between procedures. For patients undergoing a unilateral release, the side of the surgery was based on their symptoms, physical examination, and electrodiagnostic findings to determine which wrist to operate upon first. Once recovered, the patients were booked for contralateral CTR if clinically and electrodiagnostically indicated, and repeat tissue biopsies were not performed as pathological confirmation of amyloidosis had been confirmed with the first CTR biopsies. Five (38%) patients presented for a first-time CTR, whereas eight (62%) presented for repeat CTR due to symptom recurrence an average of 10 years after their initial CTR.
Of the 13 patients, five (38%) had hypothyroidism, two (15%) had diabetes mellitus, and six (46%) had polyneuropathy. There were no cases of rheumatoid arthritis or MGUS. Six patients (46%) were anticoagulated for atrial fibrillation. Anticoagulation was held for five patients preoperatively and resumed on postoperative day 1. One patient with a bilateral release continued his apixaban perioperatively with no adverse effects.
Over the 1-year study period, we had one patient (an 84-year-old man) without CTS who underwent tenosynovium biopsy, without CTR. His biopsy confirmed a diagnosis of amyloidosis (Table 1).
DISCUSSION
The principal finding of this report is that all ATTR patients undergoing TCL and tenosynovium biopsy during CTR had biopsy results positive for amyloid deposits. Most patients with ATTR have clinical and electrodiagnostic evidence of median neuropathy at the wrist of variable symptomatic severity. When there is no CTS, tenosynovium biopsy alone performed at the distal forearm, proximal to the TCL, can be considered without the need for CTR. Our report demonstrates that tenosynovial biopsy can be performed safely in this population with a high diagnostic yield and represents an attractive site for amyloidosis biopsy confirmation, given the technical ease of the procedure, the quick recovery for patients, the low risk of complications (particularly serious complications), and the high pathological yield.
ATTR patients with CTS are typically referred for biopsy from either cardiology or neurology. To date, for patients undergoing CTR, we have performed biopsy of both the TCL and the tenosynovium. Since all biopsies were positive for amyloid deposits, a single biopsy would most likely have been adequate, minimizing the pathology resources required to process the specimens. Performing TCL biopsy and tenosynovium biopsy adds approximately 1 and 3 minutes, respectively, to the CTR procedure time. Excision of excess tenosynovium helps decompress the carpal tunnel and should be performed, and this tissue can easily be sent for pathological analysis rather than being discarded. The TCL biopsy does not change the volume of the carpal tunnel contents in the same fashion. Thus, we recommend a tenosynovium biopsy as the preferred wrist biopsy if only one biopsy is performed at the time of CTR and as the sole biopsy in patients who do not require CTR.
Many patients with amyloidosis have bilateral CTS. For patients presenting with median neuropathy at the wrist and amyloidosis, we offer a unilateral or bilateral CTR with TCL and tenosynovium biopsies. The risks, benefits, and postoperative course of surgery are discussed, the patient’s questions are answered, and the patient can choose a bilateral CTR or staged procedures. Time between two CTR procedures for patients choosing a staged approach is determined by the patient when they feel comfortable proceeding with the second side. Earlier nerve decompression surgery results in better pain, sensory, and motor outcomes.18
Risk factors for amyloidosis include elevated age (men over 50 years old and women over 60 years old), male sex, Black race, family history of ATTR amyloidosis, concurrent MGUS or multiple myeloma, atrial fibrillation, spinal stenosis, biceps tendon rupture, and bilateral CTS.19–22 Patients without a known diagnosis of amyloidosis who have bilateral, typically severe CTS and other risk factors listed above should be offered wrist tissue biopsy at the time of CTR.4 CTS recurrence rates are high for patients with amyloidosis; therefore, a history of recurrent bilateral CTS should also prompt consideration of tissue biopsy at the time of repeat CTR.6 If the median nerve is noted to have thickened epineurium, to be adherent to the undersurface of the radial TCL, and/or if the tenosynovium is thicker than usual, biopsies of the TCL and tenosynovium should be considered. An early diagnosis of amyloidosis can allow patients to start treatment to prevent disease morbidity.
Patients in most Canadian provinces require tissue biopsy confirmation of amyloidosis to be eligible for public reimbursement coverage for treatment with tafamidis, an oral transthyretin stabilizer. This medication binds to transthyretin, mitigating further amyloid deposition to reduce cardiovascular morbidity and all-cause mortality in patients with transthyretin amyloid cardiomyopathy.7 Functional capacity and quality of life are improved with tafamidis, which is well tolerated with minimal if any adverse effects.7
Wrist tissue biopsy is simple and fast with minimal added morbidity compared to CTR alone, with a high yield for amyloidosis (100% in our series of patients) and a lower risk of serious complications compared to endomyocardial (cardiac, complication rate 6%), liver, abdominal subcutaneous fat (yield 15% in patients with ATTRwt), rectal (yield 85% in patients with systemic AL amyloidosis), bone marrow (yield 50%–60% in patients with systemic AL amyloidosis), salivary gland (yield 86% in patients with systemic AL amyloidosis), and trigger finger (yield 2% in idiopathic cases) biopsies.8,23–25 The cost of wrist tissue biopsy is low.
After surgery, patients are encouraged to wash their hand with soap and water and to apply Vaseline once or twice daily to keep the wound moist as the incision heals over the upcoming weeks. No activity restrictions are imposed, patients are permitted to use their hand as much as they feel comfortable and are advised to perform finger and wrist range of motion exercises to prevent stiffness. Wound dehiscence is the most common complication following CTS and is managed expectantly or with repeat suture closure.
Atrial fibrillation is common in amyloidosis, and the majority of patients with amyloidosis are anticoagulated to reduce their risk of intracardiac thrombus and stroke.26 To decrease the risk of postoperative hematoma and wound dehiscence, we prefer for the patient’s anticoagulation to be held before their CTR and resumed on postoperative day 1. If the risks of holding the patient’s anticoagulation are deemed higher than the postoperative surgical benefits by the referring physician, anticoagulation can be continued perioperatively and bipolar cautery is used intraoperatively for hemostasis. The TCL and tenosynovium are relatively avascular structures and their harvest for biopsy does not significantly increase the risk of procedure complications including bleeding, infection, pain, or nerve injury.
CONCLUSIONS
Volar wrist tenosynovial biopsy is our preferred biopsy site for confirmation of ATTR given its low morbidity and 100% pathological yield in our series. For patients requiring CTR, the tenosynovium is present at the surgical site, allowing simple harvest without an additional incision, and ideally is excised regardless to decompress the median nerve to decrease the risk of recurrent median nerve compression. For patients without CTS, tenosynovial biopsy can be performed at the distal volar forearm with local anesthesia with minimal risk and minimal postoperative pain.
ACKNOWLEDGMENT
The authors would like to thank Dr. Rob Harrop for his review of this article.
Footnotes
This original article has IRB approval from the University of Calgary, all patients provided informed consent, and followed the Declaration of Helsinki.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Siddiqi OK, Ruberg FL. Cardiac amyloidosis: an update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milandri A, Farioli A, Gagliardi C, et al. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail. 2020;22:507–513. [DOI] [PubMed] [Google Scholar]
- 3.Russell A, Hahn C, Chhibber S, et al. Utility of neuropathy screening for wild-type transthyretin amyloidosis patients. Can J Neurol Sci. 2021;48:607–615. [DOI] [PubMed] [Google Scholar]
- 4.Vianello PF, La Malfa G, Tini G, et al. Prevalence of transthyretin amyloid cardiomyopathy in male patients who underwent bilateral carpal tunnel surgery: the ACTUAL study. Int J Cardiol. 2021;329:144–147. [DOI] [PubMed] [Google Scholar]
- 5.Fosbøl EL, Rørth R, Leicht BP, et al. Association of carpal tunnel syndrome with amyloidosis, heart failure, and adverse cardiovascular outcomes. J Am Coll Cardiol. 2019;74:15–23. [DOI] [PubMed] [Google Scholar]
- 6.Eroğlu A, Sari E, Topuz AK, et al. Recurrent carpal tunnel syndrome: evaluation and treatment of the possible causes. World J Clin Cases. 2018;6:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 8.Quarta CC, Gonzalez-Lopez E, Gilbertson JA, et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J. 2017;38:1905–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellikka PA, Holmes DR, Edwards WD, et al. Endomyocardial biopsy in 30 patients with primary amyloidosis and suspected cardiac involvement. Arch Intern Med. 1988;148:662–666. [PubMed] [Google Scholar]
- 10.Bai J, Kong L, Zhao H, et al. Carpal tunnel release with a new mini-incision approach versus a conventional approach, a retrospective cohort study. Int J Surg. 2018;52:105–109. [DOI] [PubMed] [Google Scholar]
- 11.Uchiyama S, Itsubo T, Nakamura K, et al. Current concepts of carpal tunnel syndrome: Pathophysiology, treatment, and evaluation. J Orthop Sci. 2010;15:1–13. [DOI] [PubMed] [Google Scholar]
- 12.Mckee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalonde D. Wide awake local anaesthesia no tourniquet technique (WALANT). BMC Proc. 2015;9:A81. [Google Scholar]
- 14.Mitchell R, Chesney A, Seal S, et al. Anatomical variations of the carpal tunnel structures. Can J Plast Surg 2009;17:e3–e7. [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly JP, Hanna M, Sperry BW, et al. Carpal tunnel syndrome: a potential early, red-flag sign of amyloidosis. J Hand Surg Am. 2019;44:868–876. [DOI] [PubMed] [Google Scholar]
- 16.Graham B, Peljovich AE, Afra R, et al. The American Academy of Orthopaedic Surgeons evidence-based clinical practice guideline on: management of carpal tunnel syndrome. J Bone Joint Surg Am. 2016;98:1750–1754. [DOI] [PubMed] [Google Scholar]
- 17.Cook AC, Szabo RM, Birkholz SW, et al. Early mobilization following carpal tunnel release: a prospective randomized study. J Hand Surg (British Eur Vol). 1995;20;228–230. [DOI] [PubMed] [Google Scholar]
- 18.Masud M, Rashid M, Malik SA, et al. Does the duration and severity of symptoms have an impact on relief of symptoms after carpal tunnel release? J Brachial Plex Peripher Nerve Inj. 2019;14:E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott KL, Conley CR, Renfree KJ. Histopathologic evaluation of flexor tenosynovium in recurrent carpal tunnel syndrome. Plast Reconstr Surg. 2019;143:169–175. [DOI] [PubMed] [Google Scholar]
- 20.Sperry BW, Reyes BA, Ikram A, et al. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018;72:2040–2050. [DOI] [PubMed] [Google Scholar]
- 21.Sood RF, Kamenko S, McCreary E, et al. Diagnosing systemic amyloidosis presenting as carpal tunnel syndrome: a risk nomogram to guide biopsy at time of carpal tunnel release. J Bone Joint Surg Am. 2021;103:1284–1294. [DOI] [PubMed] [Google Scholar]
- 22.Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition inpatientswith heart failure and preserved ejection fraction. JACC Hear Fail. 2014;2:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisniowski B, Wechalekar A. Confirming the diagnosis of amyloidosis. Acta Haematol. 2020;143:312–321. [DOI] [PubMed] [Google Scholar]
- 24.Sperry BW, Khedraki R, Gabrovsek A, et al. Cardiac amyloidosis screening at trigger finger release surgery. Am J Cardiol. 2021;160:96–98. [DOI] [PubMed] [Google Scholar]
- 25.Rowe K, Pankow J, Nehme F, et al. Gastrointestinal amyloidosis: review of the literature. Cureus. 2017;9:e1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitrani LR, De Los Santos J, Driggin E, et al. Anticoagulation with warfarin compared to novel oral anticoagulants for atrial fibrillation in adults with transthyretin cardiac amyloidosis: comparison of thromboembolic events and major bleeding. Amyloid. 2021;28:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]