Abstract
Antigen-specific cytolytic CD4+ T lymphocytes control Mycobacterium tuberculosis infection by secreting cytokines and by killing macrophages that have phagocytosed the pathogen. However, lysis of the latter cells promotes microbial dissemination, and other macrophages engulf the released bacteria. Subsequently, CD4+ T-cell-mediated killing of macrophages goes on, and this persistent process may hamper control of infection, unless regulatory mechanisms maintain a subtle balance between lysis of macrophages by cytolytic CD4+ cells and activation of cytolytic CD4+ cells by infected macrophages. We asked whether inhibitory molecules expressed by CD4+ cytolytic T lymphocytes could play a role in such a balance. To this end, human CD4+ T-cell clones specific for M. tuberculosis were produced that displayed an autologous major histocompatibility complex class II-restricted lytic ability against purified protein derivative (PPD)-pulsed antigen-presenting cells. All T-cell clones expressed CD152 (cytotoxic T-lymphocyte antigen 4 [CTLA-4]) and CD85/leukocyte immunoglobulin-like receptor 1 (LIR-1)/immunoglobulin-like transcript 2 (ILT2) inhibitory receptors, but not CD94 and the killer inhibitory receptor (or killer immunoglobulin-like receptor [KIR]) p58.2. CD3-mediated activation of the clones was inhibited in a redirected killing assay in which CD152 and CD85/LIR-1/ILT2 were cross-linked. Specific antigen-mediated proliferation of the clones was also sharply reduced when CD152 and CD85/LIR-1/ILT2 were cross-linked by specific monoclonal antibody (MAb) followed by goat anti-mouse antiserum. In contrast, blockade of the receptors by specific MAb only increased their proliferation. Production of interleukin 2 (IL-2) and gamma interferon (IFN-γ) by the T-cell clones was also strongly reduced when CD152 and CD85/LIR-1/ILT2 were cross-linked. The lytic activity of the T-cell clones against PPD-pulsed autologous monocytes or Epstein-Barr virus-activated B cells was increased by blockade and decreased by cross-linking of the receptors. These results indicate that CD152 and CD85/LIR-1/ILT2 play a role in the regulation of the antigen-specific activity of CD4+ cytolytic T lymphocytes against PPD-presenting cells.
Intracellular pathogens such as Mycobacterium tuberculosis and Mycobacterium leprae are endowed with the ability to survive and proliferate within macrophage phagosomes (see Fig. 1.25 in reference 15). In these organelles, pathogens are eventually destroyed when phagosomes fuse with primary lysosomes, which release a vast array of hydrolytic enzymes into them. However, several infections in which pathogens are phagocytosed by macrophages allow disease development, because, within the phagosome, permissive conditions develop that not only allow survival of the aggressor, but also permit its proliferation. The pathogen itself contributes to establishing such permissive conditions by impeding fusion between phagosomes and primary lysosomes (12) and by inhibiting vesicular acidification, possibly by exclusion of proton-ATPase, thus hampering the activity of lysosomal enzymes, which occurs at an acidic pH (30).
Given all of the findings presented above, macrophages can process some of the antigen load and present it to T lymphocytes in the context of major histocompatibility complex (MHC) class II molecules. Such cells have been identified in patients with tuberculosis and tuberculoid leprosy as CD4+ cytolytic T lymphocytes (13, 23). They kill infected macrophages via Fas/Fas ligand-mediated mechanisms (29) and by perforin (or granzyme) (18) and granulysin (21) released from the secretory lysosomes of the effector cells. Mycobacteria dismissed from lysed macrophages may have reduced viability (10, 22). However, this mechanism does not induce an efficient immune-mediated control of the disease. On the contrary, following microbial dissemination, other macrophages will engulf the released pathogens (25), thus causing the persistency of the CD4+ T-cell-mediated killing of mycobacterial antigen-presenting cells, which may hamper the pivotal role of macrophages in the control of bacterial infection.
We asked whether the subtle balance between lysis of activated macrophages by cytolytic CD4+ cells and activation of cytolytic CD4+ cells by activated macrophages might be maintained via an autoregulatory mechanism mediated by surface inhibitory molecules expressed on T cells.
CD152 (cytotoxic T lymphocyte antigen 4 [CTLA-4]) and CD85/leukocyte immunoglobulin-like receptor 1 (LIR-1)/immunoglobulin-like transcript 2 (ILT-2) are inhibitory receptors expressed by all T lymphocytes (27, 28). CD152 binds CD80/CD86 molecules on antigen-presenting cells (APCs), thus competing with the coactivation molecule CD28. In addition, its cross-linking triggers phosphatases that dephosphorylate molecules of the CD3–T-cell receptor (TCR) activation cascade (6, 19, 35). We have shown that CD152 inhibits the specific lysis mediated by CD8+ cytolytic T lymphocytes in a clonally distributed fashion (27).
The CD85/LIR-1/ILT2 molecule belongs to the LIR/ILT family and binds to the nonclassical class I HLA-G protein (2), to some alleles of HLA-A and -B loci (11), and to the human cytomegalovirus UL18 gene product, a viral homolog of HLA class I (7). It is a transmembrane molecule with four immunoreceptor tyrosine-based inhibition motifs (ITIMs) in its cytoplasmic domain (8, 9). Tyrosine phosphorylation of ITIMs creates docking sites for the SH2 domain-containing phosphatase SHP-1, which subsequently transduces an inhibitory signal by dephosphorylating and inactivating downstream tyrosine kinases (4).
We have shown that CD85/LIR-1/ILT2 is expressed by all T lymphocytes (28) and that this molecule is detectable on the surface and in the cytoplasm of T lymphocytes by the anti-LIR-1 M402 monoclonal antibody (MAb) and in the cytoplasm by the HP-F1 MAb (28), which is less sensitive for surface immunofluorescence. Biochemical analyses of the protein and the expression of mRNA further proved that CD85/LIR-1/ILT2 is present in all T lymphocytes. Importantly, the same study demonstrated that CD85/LIR-1/ILT2 down-regulates the antigen-specific cytolytic activity of CD8+ T cells (28).
In this study, we focused on human cytolytic CD4+ T cells and investigated the effects of CD152 and CD85/LIR-1/ILT2, blocked or cross-linked, on the M. tuberculosis-specific activity of cytolytic CD4+ T lymphocytes. We generated CD4+ T-cell clones specific for M. tuberculosis that displayed an autologous, MHC class II-restricted, lytic ability against APCs. We blocked or cross-linked the CD152 or CD85/LIR-1/ILT2 receptors by treating T cells with anti-CD152 or anti-CD85/LIR-1/ILT2 MAbs, alone or followed by goat anti-mouse (GAM) antiserum as a cross-linker, respectively. We found that blockade of the receptors increased antigen-mediated proliferation of CD4+ cytolytic clones, whereas their cross-linking significantly decreased the proliferation. Next, we evaluated the lytic activity of the T-cell clones against autologous monocytes and Epstein-Barr virus (EBV)-infected B cells (B-EBV) pulsed with purified protein derivative (PPD) from M. tuberculosis, and we found that blockade of the receptors significantly increased lysis, whereas cross-linking of the receptors decreased it. Likewise, production of interleukin 2 (IL-2) and gamma interferon (IFN-γ) by CD4+ cytolytic clones was strongly reduced by CD152 and CD85/LIR-1/ILT2 cross-linking. These results indicate that CD152 and CD85/LIR-1/ILT2 play a role in the regulation of the antigen-specific lytic activity of CD4+ cytolytic T lymphocytes against PPD APCs.
MATERIALS AND METHODS
Generation of CD4+ T-cell clones.
CD4+ T-cell lines were obtained from five healthy donors. Peripheral blood mononuclear cells (PBMCs), isolated from heparinized venous blood by Ficoll density gradient centifugation, were cultured with PPD from M. tuberculosis (5 μg/ml) in 24-well plates (Costar, Cambridge, Mass.). Human recombinant IL-2 (hrIL-2) (Pepro Tech EC, London, United Kingdom) was added on culture days 2 and 4 at a final concentration of 50 U/ml. CD4+ T-cell lines were selected by repeated restimulation cycles with PPD in the presence of autologous irradiated PBMCs. Clones were generated from specific T-cell lines by plating cells at limiting dilutions of 10 cells and 1 cell/well. Phenotype and monoclonality were assessed with anti-CD3, anti-TCR-αβ, anti-CD4, anti-CD8, and anti-Vβ MAbs (Becton Dickinson, San Jose, Calif.). MHC class II restriction of antigen recognition by the clones was probed by cytotoxicity assays in the presence of anti-MHC class II MAb (clone D1.12; a gift from R. S. Accolla, Unit of Cellular and Molecular Genetics, Advanced Biotechnology Center, Genoa, Italy).
Antibodies to inhibitory TCR.
Anti-CD152 MAb (clone 4F10, γ2a) was from Pharmingen (Hamburg, Germany). Anti-CD94 (clone HP-3B1, γ2a) was provided by Miguel López-Botet (Servicio de Immunologia, Hospital Universitario de la Princesa, Madrid, Spain), and anti-p58.2 (clone GL183, γ1) was from Immunotech (Marseille, France). The anti-CD85/LIR-1/ILT2 MAbs used for immunostaining were M402 (γ1; Immunex, Co., Seattle, Wash.), anti-CD85 (clone GHI/75; Pharmingen), and HP-F1 (provided by Miguel López-Botet). The HP-F1 MAb (γ1) was used as an anti-CD85/LIR-1/ILT2 MAb in proliferation and cytotoxicity assays. Controls of HP-F1- and anti-CD152-mediated effects on proliferation, cytotoxicity, and cytokine production were provided by MAbs specific for irrelevant mock antigens, such as anti-CD8 (clone OKT8) and anti-MHC class I (clone HB82, specific for HLA-A2, γ2b), from the American Type Culture Collection (Rockville, Md.).
Immunophenotyping.
The surface phenotype of T-cell clones was determined by flow cytometric analyses (FACScalibur; Becton Dickinson). The secondary reagent was fluorescein isothiocyanate (FITC)-labeled GAM antiserum (Southern Biotechnology Associates, Birmingham, Ala.). T cells (5 × 104) were incubated with specific MAb for 20 min at 4°C. Cells were washed twice with phosphate-buffered saline (PBS), and the secondary labeled reagent was added. After incubation, cells were washed and fixed with 1% paraformaldehyde. Control cells were stained with the secondary reagent alone.
Cytoplasmic immunostaining.
For the demonstration of intracellular CD85/LIR-1/ILT2 and CD152, T cells were processed as described previously (28). Briefly, cells were fixed for 10 min at 37°C with 4% paraformaldehyde in PBS, washed with PBS, and stained with HP-F1 MAb or anti-CD152 MAb in PBS containing 0.1% saponin. Labeling with secondary MAb was performed by addition of phycoerythrin-conjugated GAM antiserum (Southern Biotechnology Associates), and fluorescence was measured by flow cytometry. As positive controls, antibodies to MHC class I w6/32 (γ2a) and β-tubulin (γ1) were used. As negative controls, antibodies to CD94 (γ2a) and to KIR p-58.2 (γ1) were employed. An additional control was provided by cells stained with the secondary reagent alone.
Proliferation assays.
Proliferation assays were performed by coculture of 104 T cells with 105 autologous irradiated (3,000 rads) PBMCs. Cells were pulsed with PPD from M. tuberculosis at a final concentration of 5 μg/ml in 200 μl of medium. On day 3, 0.5 μCi of [3H]thymidine (specific activity, 5 μCi/mmol; Amersham, Little Chalfont, United Kingdom) was added to the cultures for 8 h. Cells were then harvested and counted in a β-counter. Results are expressed as 103 cpm.
DNA flow cytometry for the evaluation of apoptosis and proliferation.
Cells were harvested and fixed with 70% ethanol for 48 h at −20°C. After washing with PBS, samples were incubated with 30 μg of propidium iodide (PI) per ml (Sigma Chemical Co., St. Louis, Mo.) and 0.5 mg of RNase per ml for 30 min at room temperature and in the dark. Flow cytometric analyses were performed with a FACSCalibur (Becton Dickinson) equipped with a 488-nm laser for PI excitation and optical filters for PI emission fluorescence. At least 10,000 events were acquired from each sample. Frequency distributions of DNA content (DNA histograms) were analyzed for the evaluation of apoptosis and the percentage of cells in the various phases of the cell cycle.
Cytotoxicity assays.
The ability of various MAbs to regulate the cytolytic activity of CD4+ T-cell clones was measured in a conventional 4-h 51Cr-release assay. Briefly, target cells (T) (autologous and allogeneic monocytes or B-EBV cells) were labeled for 1 h with 51Cr (100 μCi/106 cells; Amersham), washed twice with PBS, resuspended in RPMI with 10% fetal calf serum, and plated in triplicate at 5 × 103 cells/well in 96-well U-bottom plates. Effector cells (E) were plated at 5:1 effector/target (E:T) cell ratios. HP-F1 or anti-CD152 MAbs, as well as irrelevant MAbs used as controls, were included in the cytolytic assay. Cross-linking of HP-F1 or CD152 was obtained with a MAb to HP-F1 or to CD152 followed by GAM antiserum (Southern Biotechnology Associates). For redirected killing assays (27), P815 cells (targets) were plated in triplicate in the presence of one of the following MAbs: anti-CD3, HP-F1, anti-CD152, anti-CD4, anti-CD8, or anti-MHC class II. To evaluate a possible inhibitory effect of CD85/LIR-1/ILT2 and CD152, effector cells were pretreated for 20 min at 4°C with the HP-F1 MAb and anti-CD152 MAb. P815 cells were then added to each well together with the stimulatory anti-CD3 MAb. The E:T ratio was 10:1. After 4 h, 100 μl of supernatant was collected from each well and analyzed in a gamma counter for 51Cr release. The percentage of specific lysis was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
In vitro cytokine production assays.
For analyses of cytokine production, culture supernatants were collected from wells at defined time points, and levels of IL-2 and IFN-γ were measured with commercial ELISA kits (Diaclone Research, Besançon, France) according to the manufacturer's recommendations.
Statistical analysis.
Comparisons between values of cytotoxicity, cell proliferation in response to antigen, and cytokine production were performed with Student's t test. P < 0.05 was considered statistically significant.
RESULTS
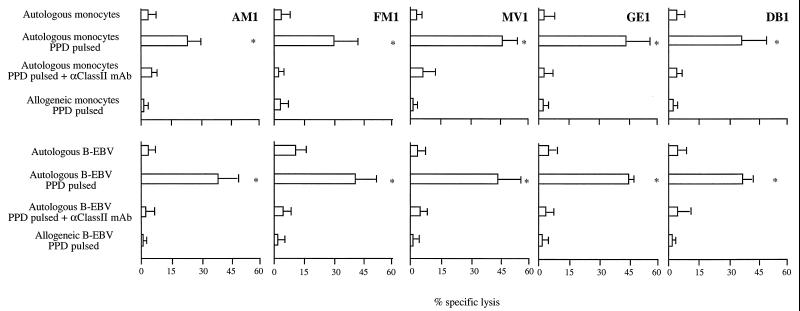
Generation of human cytolytic CD4+ T-cell clones specific for M. tuberculosis. PBMCs were derived from five donors: three positive for the PPD skin test (namely clonesGE1, MV1, and DB1) and two negative (clones AM1 and FM1). Specific cell lines were obtained by repeated cycles of stimulation with PPD from M. tuberculosis in the presence of irradiated autologous feeder cells. All T-cell lines obtained displayed a CD3+ TCR-αβ+ CD4+ phenotype (data not shown). Clones were produced from these T-cell lines by limiting dilution. Clones were able to lyse autologous monocytes and autologous B-EBV cells pulsed with PPD. Their antigen specificity was confirmed by the inability of these clones to lyse autologous target cells not pulsed with PPD and allogeneic cells pulsed with the antigen. One clone from each of the five donors was arbitrarily selected for the study. In Fig. 1, the cytolytic activity of CD4+ T-cell clones against autologous monocytes or B-EBV cells, untreated or PPD pulsed, as well as that against allogeneic target cells is shown. Addition to the cytolytic assay of soluble anti-HLA class II MAb, which impairs presentation of the PPD peptide by HLA class II molecules, significantly inhibited lysis of PPD-pulsed targets (Fig. 1), thus reinforcing the specificity and HLA class II restriction of the lytic function of CD4+ T-cell clones.
FIG. 1.
MHC class II restriction and antigen specificity of cytolytic CD4+ T-cell clones. The lytic activity of five cytolytic CD4+ T-cell clones specific for PPD from M. tuberculosis was assayed against monocytes (upper panel) or EBV-infected B cells (lower panel). Autologous target cells were untreated or previously cultured for 18 h with PPD (“PPD pulsed”). The targets were also tested in the presence of soluble anti-HLA class II MAbs, which impair presentation of PPD antigen by HLA class II molecules to cytolytic CD4+ T cells. Lysis of allogeneic PPD-pulsed target cells did not occur. Asterisks indicate differences that are statistically significant between cytolysis in the presence of PPD and PPD plus anti-HLA class II MAb culture conditions. P < 0.05.
Inhibitory molecules are expressed by M. tuberculosis-specific cytolytic CD4+ T-cell clones.
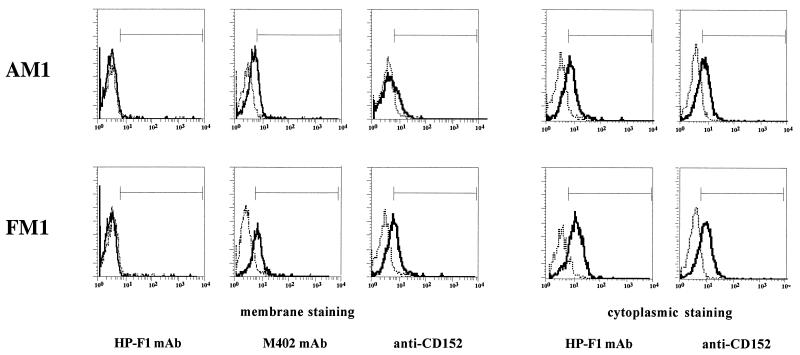
The pattern of expression of inhibitory molecules has been evaluated by surface and cytoplasmic immunofluorescence in the five cytolytic CD4+ T-cell clones selected. Cells were analyzed after 1 week of specific antigen restimulation. CD94 and the killer inhibitory receptor (or killer immunoglobulin-like receptor [KIR]) molecule p58.2 were not detectable on the surface or in the cytoplasm of any clone (data not shown). CD85/LIR-1/ILT2 was demonstrated on the surface of all clones by using the M402 MAb and in the cytoplasm by using M402 and the HP-F1 MAb. Accordingly, all five T-cell clones expressed CD152 on the surface and in the cytoplasm shortly after restimulation, as demonstrated by the anti-CD152 MAb. As an example, immunofluorescence histograms of clones AM1 and FM1 are shown in Fig. 2.
FIG. 2.
CD85/LIR-1/ILT2 and CD152 are expressed by M. tuberculosis-specific cytolytic CD4+ T-cell clones. CD85/LIR-1/ILT2 and CD152 surface and cytoplasmic immunofluorescence histograms of clones AM1 and FM1. CD85/LIR-1/ILT2 is detected by the M402 MAb on the surface and by the M402 (data not shown) and HP-F1 MAbs in the cytoplasm. CD152 shows similar behavior. Dotted lines represent negative controls obtained with isotype-matched irrelevant MAbs.
CD85/LIR-1/ILT2 and CD152 inhibit CD3-mediated activation of cytolytic CD4+ T-cell clones as assessed in a redirected killing assay.
To ascertain the regulatory role of CD85/LIR-1/ILT2 and CD152 in the activation of cytolytic CD4+ T cells via CD3, we performed redirected killing assays in which target cells were murine P815 cells coated with MAbs bound via their Fc portion. P815 cells coated with the murine MAbs are able to cross-link their ligands on the effector cells (explained in reference 27). The anti-CD3 MAb induced activation of cytolytic CD4+ T cells that efficiently lysed the P815 target cells. To evaluate the effect of CD85/LIR-1/ILT2 and CD152 on CD3-mediated activation and lysis, P815 cells were also coated with HP-F1 and anti-CD152, in addition to anti-CD3 MAb. A significant decrease in P815 lysis was observed when HP-F1 and anti-CD152 MAb were added to the assay, compared to lysis observed in the presence of anti-CD3 only. Conversely, there was no effect on lysis when control antibodies (i.e., anti-CD4, anti-CD8, and anti-MHC class II) were bound to P815 cells in addition to anti-CD3 (Table 1). These results indicate that CD85/LIR-1/ILT2 and CD152 mediate inhibitory signals in CD3-activated cytolytic CD4+ T cells.
TABLE 1.
CD85/LIR-1/ILT2 and CD152 inhibit CD3-mediated activation of cytolytic CD4+ T-cell clones in redirected killing assays
| MAb on P815 cells | % of specific lysis with
clonea:
|
|||
|---|---|---|---|---|
| AM1 | FM1 | MV1 | GE1 | |
| None | 2 | 9 | 7 | 4 |
| Anti-CD3 | 34 | 62 | 29 | 38 |
| Anti-CD3 + HP-F1 | 10 | 35 | 6 | 24 |
| Anti-CD3 + anti-CD152 | 11 | 42 | 0 | 21 |
| Anti-CD3 + anti-CD4 | 30 | 64 | NDb | 34 |
| Anti-CD3 + anti-CD8 | ND | 60 | ND | ND |
| Anti-CD3 + anti-MHC class II | 32 | 58 | 28 | 33 |
P815 cells are lysed by cytolytic CD4+ T-cell clones upon coating of the target cells with the indicated MAb. The E:T ratio was 10:1, and lysis was measured in a conventional 4-h 51Cr-release assay.
ND, not done.
CD85/LIR-1/ILT2 and CD152 inhibit antigen-specific proliferation of cytolytic CD4+ T-cell clones.
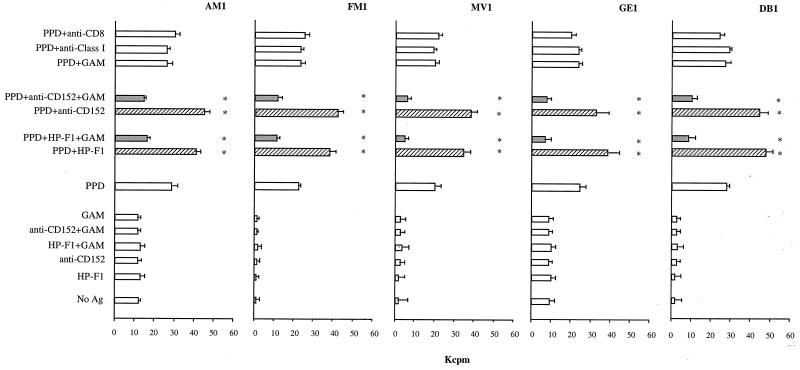
We next investigated the antigen-specific functions of cytolytic CD4+ T-cell clones. The role of CD85/LIR-1/ILT2 and CD152 in PPD-induced restimulation of the M. tuberculosis-specific clones was evaluated. Clones were restimulated by autologous irradiated PBMCs pulsed with PPD. Simultaneous addition in the culture of anti-CD152 MAb or of the anti-CD85/LIR-1/ILT2 MAb HP-F1, both cross-linked by GAM antiserum, inhibited proliferation, as revealed by the sharp reduction, or even absence, of cell clump formation assessed by light microscopy. [3H]thymidine uptake in these samples 4 days after restimulation was significantly lower than that of control samples, with a reduction ranging from 40 to 80% (Fig. 3). On the other hand, addition of non-cross-linked anti-CD152 or HP-F1 MAbs, which block the receptors, led to an increment of PPD-induced proliferation ranging from 30 to 70% (Fig. 3). Controls with GAM antiserum alone, or with irrelevant antibodies used as controls (anti-CD8 and anti-MHC class I), had no effect on PPD-induced proliferation (Fig. 3).
FIG. 3.
CD85/LIR-1/ILT2 and CD152 regulate antigen (Ag)-specific proliferation of cytolytic CD4+ T-cell clones. T-cell clones were restimulated by autologous irradiated PBMCs pulsed with PPD, in the absence or presence of soluble MAbs, alone or cross-linked by GAM antiserum. Four days later, proliferation was measured by [3H]thymidine uptake. Controls were provided by cells cultured with autologous PBMCs not pulsed with PPD, as well as by cells restimulated by PPD-pulsed PBMCs in the presence of GAM antiserum alone or in the presence of two irrelevant MAbs used as controls (i.e., anti-CD8 MAb or anti-MHC class I MAb). Asterisks indicate values that are significantly different for PPD, PPD plus HP-F1, PPD plus HP-F1 plus GAM, PPD plus anti-CD152, and PPD plus anti-CD152 plus GAM culture conditions. P < 0.05. ▨, blocking MAb conditions; ░⃞, cross-linking of MAb specific for the inhibitory receptors.
A follow-up of the cultures over time showed that in samples in which CD85/LIR-1/ILT2 and CD152 were cross-linked at the time of restimulation, proliferation was not restored, but instead the cells started to undergo apoptosis. As an example, DNA-content histograms of clone AM1, measured 10 days after restimulation, are shown in Fig. 4.
FIG. 4.
Cross-linked CD85/LIR-1/ILT2 and CD152 inhibit restimulation and induce delayed cell death. Flow cytometric DNA content analysis was performed on clone AM1 10 days after restimulation carried out in the absence or presence of HP-F1 or anti-CD152, soluble or cross-linked by GAM antiserum. The marker to the left of the G1 peak (sub-G1 region) accounts for apoptotic cells, and the marker to the right of the G1 peak indicates S + G2M cells expressed as a percentage of the viable cells (i.e., S+G2M/G1+ S + G2M).
CD85/LIR-1/ILT2 and CD152 inhibit the specific lysis mediated by PPD-specific CD4+ T-cell clones.
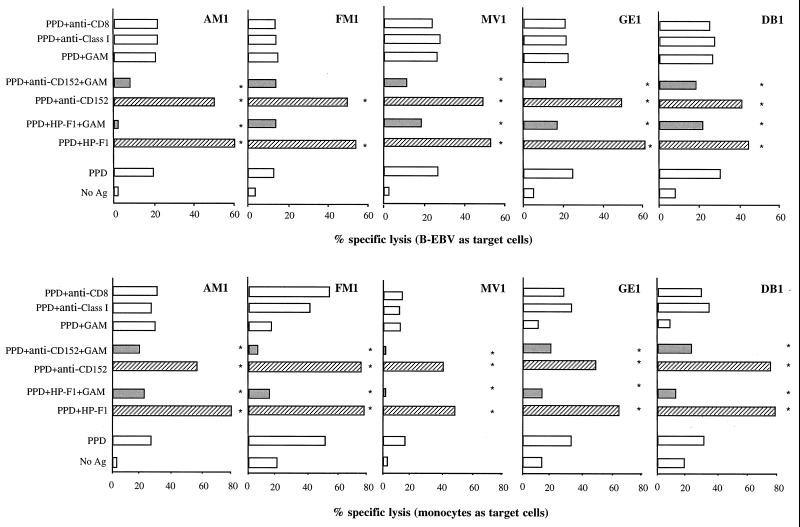
Since PPD-specific CD4+ T-cell clones are able to mediate cytolytic functions, we investigated whether cytotoxicity is modulated by CD85/LIR-1/ILT2 and CD152. When soluble anti-CD152 or HP-F1 MAbs were included in the cytolytic assay, thus blocking the activity of the receptors, a significant increase in lysis by all clones was observed with both B-EBV and monocytes as target cells (Fig. 5). The percentage of increase in lysis ranged between 40 and 170%. On the other hand, when anti-CD152 and HP-F1 were cross-linked with GAM antiserum, a significant reduction in specific lysis was observed in 8 of 10 tests, and it ranged from 25 to 80% (Fig. 5). In control samples, in which GAM antiserum alone or irrelevant MAbs were added to the cytolytic tests, lysis was not affected (Fig. 5).
FIG. 5.
CD85/LIR-1/ILT2 and CD152 regulate the antigen-specific cytotoxic activity of CD4+ T-cell clones. The function of cytolytic CD4+ T-cell clones against autologous antigen-presenting B-EBV cells or monocytes was assayed in a conventional 4-h 51Cr-release assay, carried out in the absence or presence of soluble MAbs, alone or cross-linked by GAM antiserum. Soluble HP-F1 or anti-CD152 MAb significantly increased specific lysis, while the same MAb cross-linked by GAM antiserum reduced lysis. Control samples were treated with GAM antiserum alone or two irrelevant MAbs (i.e., anti-CD8 or anti-MHC class I MAb). Asterisks indicate values that are significantly different for PPD, PPD plus HP-F1, PPD plus HP-F1 plus GAM, PPD plus anti-CD152, and PPD plus anti-CD152 plus GAM culture conditions. P < 0.05. ▨, blocking MAb conditions; ░⃞, cross-linking of MAb specific for inhibitory receptors.
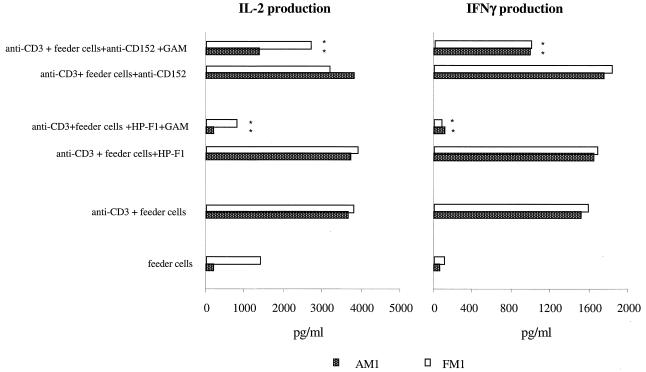
Cross-linked CD85/LIR-1/ILT2 and CD152 molecules down-regulate IL-2 and IFN-γ production by specific cytolytic CD4+ T-cell clones.
IL-2 and IFN-γ have been shown to exert a major role in the proliferation and boosting of cytotoxic function of cytolytic CD4+ T cells (31). Accordingly, levels of IL-2 and IFN-γ secreted by two clones 1 day after OKT3-triggered restimulation were determined (Fig. 6). Whereas feeder cells alone did not stimulate T cells, the presence of OKT3 was sufficient to induce production of IL-2 and IFN-γ. Cross-linking of HP-F1 and anti-CD152 MAb by GAM antiserum led to inhibition of IL-2 production ranging from 30 to 92% and inhibition of IFN-γ production ranging from 20 to 90% (Fig. 6). HP-F1 was more effective than anti-CD152 in the down-regulation of cytokine production. In contrast, the cross-linking of irrelevant MAbs used as controls (i.e., anti-CD8 and anti-MHC class I) did not affect cytokine production (not shown).
FIG. 6.
CD85/LIR-1/ILT2 and CD152 down-regulate IL-2 and IFN-γ production by cytolytic CD4+ T-cell clones. Twenty-four hours after restimulation of CD4+ T-cell clones with OKT3 and irradiated feeder PBMCs, the amount of IL-2 and IFN-γ in the culture medium was measured and expressed as picograms per milliliter. At the time of restimulation, HP-F1 and anti-CD152 were added to the cultures alone (blockade of receptors) or cross-linked by GAM antiserum (cross-linking of the receptors). A negative “restimulation control” was provided by T cells exposed to irradiated autologous feeder PBMCs only, in the absence of OKT3. Asterisks indicate values that are significantly different for anti-CD3, anti-CD3 plus HP-F1, anti-CD3 plus HP-F1 plus GAM, anti-CD3 plus anti-CD152, and anti-CD3 plus anti-CD152 plus GAM culture conditions. P < 0.05.
DISCUSSION
A persistent stimulation of the immune system, such as that exerted by intracellular bacteria (e.g., Mycobacteria) or by some viruses, may lead to a dramatic expansion of specifically reactive T-cell clones that play a major role in the control of chronic infections. When uncontrolled, the cell-mediated arm of the immune defenses could determine a reduction in the diversity of the clonal repertoire due to overwhelming antigen-specific clonal expansion and to the emergence of possible autoagressive clones due to loss of tolerance (17, 32).
It thus appears that the possibly infinite expansion of antigen-specific lymphocyte clones in response to antigens must be contained and finely tuned. Several down-regulatory mechanisms effective to this end have been recently identified. Containment of T-cell clonal expansion in response to antigens occurs via apoptosis (17). A large number of responsive T cells in the clone will die by apoptosis independently of the clearing off of the antigen. A second down-regulatory mechanism is provided by secretion of cytokines (e.g., transforming growth factor β [TGF-β]) that down-regulate the activation or proliferation of antigen-responsive T cells (33). A third, and possibly most efficient, way of containing an uncontrolled clonal expansion of T cells is the killing of those cells that process and present antigens (APCs) to T lymphocytes (3, 14). Strong evidence in favor of this contention has been provided by studies of autoimmune lymphoproliferative syndromes, in which mutations of several molecules involved in the cascade leading to apoptosis (FAS, FAS-ligand, and caspases) have been described (34, 36). It appears that APCs, which are normally killed for the containment of a cell-mediated response, survive and accumulate in the T-cell-dependent areas of peripheral lymphoid tissues where such responses occur (34).
Finally, several groups, including our own, have shown that on the surface and in the cytoplasm of virtually all T lymphocytes, inhibitory molecules are expressed that down-regulate T-cell proliferation and effector functions, such as cytokine production and cytolysis (1, 5, 6, 27, 28). Among these molecules, we have previously described the down-regulatory effects of the CD152 (CTLA-4) and CD85/LIR-1/ILT2 molecules on the function of CD8+ T lymphocytes (27, 28).
In the study presented here, we propose a model system in which a down-regulatory role of such molecules is shown to occur in a subset of T-helper (CD4+) human lymphocytes that exert cytotoxic functions against APCs in an antigen-specific fashion. Thus, we refer to one of the mechanisms of containment of a cell-mediated response outlined above: i.e., the killing of APCs by cytotoxic T cells. Our experiments were conducted with TCR-αβ+/CD4+ human lymphocyte clones generated by stimulation with PPD from M. tuberculosis. These antigen (PPD)-specific CD4+ T-cell clones are cytolytic against targets represented by PPD-pulsed APCs (monocytes and B-EBV cells) in an antigen-specific fashion. The Th1 cytolytic phenotype of these clones was not unexpected according to studies showing that T cells from both tuberculosis-infected and uninfected subjects develop into Th1 effectors after stimulation with PPD (20, 26). It is of note that virus-specific cytolytic CD4+ T cells have also been found to lyse specifically in vitro virus-infected cells in an MHC class II-restricted fashion (24). However, the role of these T-cell clones in the immune defense against virus-infected cells is questioned, and they are unlikely to exert relevant effector functions in vivo (24).
In this study, we have shown that cytolytic PPD-specific CD4+ T-cell clones express sets of molecules that may trigger inhibition of T-cell functions. The receptors CD152 and CD85/LIR-1/ILT2 may be directly blocked by the appropriate MAb; alternatively, by using GAM antiserum as a second reagent, the inhibitory receptors are cross-linked and rendered active. Our previous data on cytotoxic CD8+ T lymphocytes indicate that blockade of these receptors by the appropriate MAb results in increased T-cell-mediated cytolysis, whereas their cross-linking leads to a sharp decrease in such function (27, 28).
Proliferation of the cytotoxic CD4+ T-cell clones investigated herein is also regulated by CD152 and CD85/LIR-1/ILT2 molecules, because their blockade increases T-cell proliferation, which is sharply reduced following their cross-linking. In the latter condition, we also show that T-cell apoptosis is increased and that entry into the cell cycle is reduced.
A functional down-regulation mediated by CD152 and CD85/LIR-1/ILT2 is further supported by cytotoxicity experiments in which syngeneic APC targets pulsed with PPD are challenged by the PPD-specific CD4+ T-cell clones. We found that blockade of CD152 and CD85/LIR-1/ILT2 receptors significantly increased target cell lysis, while their cross-linking decreased it. The functional data also demonstrate that CD85/LIR-1/ILT2 molecules are indeed present on the surface of cytolytic CD4+ T-cell clones, although they are hardly detectable by immunofluorescence with the HP-F1 MAb. Functional assays are generally more sensitive than immunofluorescence assays and may be able to reveal the role of molecules expressed at very low density, as already proven for the CD152 molecule, which is barely found on resting T cells, but is functionally active (1, 5, 6).
Finally, we demonstrate that the release of Th1-type cytokines (IL-2 and IFN-γ) by cytolytic CD4+ T-cell clones is increased following blockade of CD152 and CD85/LIR-1/ILT2 by specific MAbs and is sharply reduced after cross-linking of these receptors by GAM antiserum.
In all functional assays, the addition of anti-CD152 and anti- CD85/LIR-1/ILT2 MAbs in combination does not significantly affect the inhibitory effect.
Some conclusions can be drawn from the data presented above. Mycobacteria are taken up by macrophages, but they survive and proliferate within these cells until the latter present antigens to cytotoxic CD4+ cells. Following this interaction, macrophages may be killed by T cells. This would result in the release of still viable bacteria that could be phagocytosed by other macrophages, thus keeping the infection going. However, this phenomenon is self-limiting, suggesting that there should exist an autoregulatory mechanism to keep the fine balance between lysis of activated macrophages by cytotoxic CD4+ cells and activation of cytotoxic CD4+ cells by infected macrophages. According to the results of this study, we propose that this mechanism might be regulated, at least in part, by CD85/LIR-1/ILT2 and CD152 inhibitory receptors expressed by cytolytic CD4+ T cells.
In a study on the effect of in vivo CD152 blockade by specific MAbs in Mycobacterium bovis BCG-infected mice, enhanced antigen-specific lymphocyte expansion and cytokine production in the draining mediastinal lymph nodes were observed (16). However, the number of viable mycobacteria recovered from the infection sites was not affected, and no enhanced clearance of the infection was eventually observed (16). This could be possibly due, at least in part, to the self-limiting autoregulatory mechanism suggested by the present study.
ACKNOWLEDGMENTS
This work was supported by AIRC and MURST grants to E. Ciccone and C. E. Grossi. A. Merlo is supported by a fellowship from FIRC.
REFERENCES
- 1.Alegre M L, Noel P J, Eisfelder B J, Chuang E, Clark M R, Reiner S L, Thompson C B. Regulation of surface and intracellular expression of CTLA-4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 2.Allan D S, Colonna M, Lanier L L, Churakova T D, Abrams J S, Ellis S A, McMichael A J, Braud V M. Tetrameric complexes of human histocompatibility leukocyte antigen (HLA)-G bind to peripheral blood myelomonocytic cells. J Exp Med. 1999;18:1149–1156. doi: 10.1084/jem.189.7.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Borges L, Cosman D. LIRs/ILTs/MIRs, inhibitory and stimulatory Ig-superfamily receptors expressed in myeloid and lymphoid cells. Cytokine Growth Factor Rev. 2000;11:209–217. doi: 10.1016/s1359-6101(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 5.Brunner M C, Chambers C A, Chan F K, Hanke J, Winoto A, Allison J P. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 6.Chambers C A. The expanding world of co-stimulation: the two-signal model revisited. Trends Immunol. 2001;22:217–223. doi: 10.1016/s1471-4906(01)01868-3. [DOI] [PubMed] [Google Scholar]
- 7.Chapman T L, Heikeman A P, Bjorkman P J. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity. 1999;11:603–613. doi: 10.1016/s1074-7613(00)80135-1. [DOI] [PubMed] [Google Scholar]
- 8.Colonna M, Navarro F, Bellon T, Llano M, Garcia P, Samaridis J, Angman L, Cella M, Lopez-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, Hsu M L. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–281. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 10.Ernst W A, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson D A, Clayberger C, Krensky A M, Leippe M, Bloom B R, Ganz T, Modlin R L. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol. 2000;165:7102–7108. doi: 10.4049/jimmunol.165.12.7102. [DOI] [PubMed] [Google Scholar]
- 11.Fanger N A, Cosman D, Peterson L, Braddy S C, Maliszewski C R, Borges L. The MHC class I binding proteins LIR-1 and LIR-2 inhibit Fc receptor-mediated signaling in monocytes. Eur J Immunol. 1998;28:3423–3434. doi: 10.1002/(SICI)1521-4141(199811)28:11<3423::AID-IMMU3423>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Frehel C, de Chastellier C, Lang T, Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986;52:252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn S, Gehri S, Erb P. Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev. 1995;146:57–79. doi: 10.1111/j.1600-065x.1995.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 14.Ingulli E, Mondino A, Khoruts A, Jenkins M K. In vivo detection of dendritic cell antigen presentation to CD4+ T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janeway C A., Jr . The recognition and effector mechanisms of adaptive immunity. In: Janeway C A, Travers P, Walport H, Capra J D, editors. Immunobiology: the immune system in health and disease. 4th ed. London, United Kingdom: Elsevier Science, Ltd.; 1999. pp. 20–30. [Google Scholar]
- 16.Kirman J, McCoy K, Hook S, Prout M, Delahunt B, Orme I, Frank A, Le Gros G. CTLA-4 blockade enhances the immune response induced by mycobacterial infection but does not lead to increased protection. Infect Immun. 1999;67:3786–3792. doi: 10.1128/iai.67.8.3786-3792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenardo M, Chan F K, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis. Immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 18.Lewinsohn D M, Bement T T, Xu J, Lynch D H, Grabstein K H, Reed S G, Alderson M R. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160:2374–2379. [PubMed] [Google Scholar]
- 19.Marengere L E, Waterhouse P, Duncan G S, Mittrucker H W, Feng G S, Mak T W. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;24:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 20.Mutis T, Cornelisse Y E, Ottenhoff T H. Mycobacteria induce CD4+ T cells that are cytotoxic and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur J Immunol. 1993;23:2189–2195. doi: 10.1002/eji.1830230921. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa M T, Stenger S, Sieling P A, Thoma-Uszynski S, Sabet S, Cho S, Krensky A M, Rollinghoff M, Nunes Sarno E, Burdick A E, Rea T H, Modlin R L. T-cell release of granulysin contributes to host defense in leprosy. Nat Med. 2001;7:174–179. doi: 10.1038/84620. [DOI] [PubMed] [Google Scholar]
- 22.Oddo M, Renno T, Attinger A, Bakker T, MacDonald H R, Meylan P R. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160:5448–5454. [PubMed] [Google Scholar]
- 23.Orme I M, Roberts A D, Griffen J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosisinfection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 24.Oxenius A, Zinkernagel R M, Hengartner H. CD4+ T-cell induction and effector functions: a comparison of immunity against soluble antigens and viral infections. Adv Immunol. 1998;70:313–367. doi: 10.1016/s0065-2776(08)60390-9. [DOI] [PubMed] [Google Scholar]
- 25.Raviglione M C, O'Brien R J. Tuberculosis. In: Fauci A S, Braunwald E, Esselbach K J, Wilson J D, Martin J B, Kasper D L, Hauser S L, Longo D L, editors. Principles of internal medicine. 14th ed. New York, N.Y: McGraw-Hill; 1998. pp. 1004–1015. [Google Scholar]
- 26.Russo D M, Kozlova N, Lakey D L, Kernodle D. Naive human T cells develop into Th1 effectors after stimulation with Mycobacterium tuberculosis-infected macrophages or recombinant Ag85 proteins. Infect Immun. 2000;68:6826–6832. doi: 10.1128/iai.68.12.6826-6832.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saverino D, Tenca C, Zarcone D, Merlo A, Megiovanni A M, Valle M T, Manca F, Grossi C E, Ciccone E. CTLA-4 (CD152) inhibits the specific lysis mediated by human cytolytic T lymphocytes in a clonally distributed fashion. J Immunol. 1999;162:651–658. [PubMed] [Google Scholar]
- 28.Saverino D, Fabbi M, Ghiotto F, Merlo A, Bruno S, Zarcone D, Tenca C, Tiso M, Santoro G, Anastasi G, Cosman D, Grossi C E, Ciccone E. The CD85/LIR-1/ILT2 inhibitory receptor is expressed by all human T lymphocytes and regulates T-cell function. J Immunol. 2000;165:3742–3751. doi: 10.4049/jimmunol.165.7.3742. [DOI] [PubMed] [Google Scholar]
- 29.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994;152:1127–1133. [PubMed] [Google Scholar]
- 30.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 31.Tsukaguchi K, Balaji K N, Boom W H. CD4+ alpha beta T cell and gamma delta T cell responses to Mycobacterium tuberculosis. Similarities and differences in Ag recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786–1796. [PubMed] [Google Scholar]
- 32.Van Parijs L, Abbas A K. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 33.Wahl S M. Transforming growth factor: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Zheng L, Lobito A, Chan F K, Dale J, Sneller M, Yao X, Puck J M, Straus S E, Lenardo M J. Inherited human caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse P, Marengere L E M, Mittrucker H-W, Mak T W. CTLA-4: a negative regulator of T-lymphocyte activation. Immunol Rev. 1996;153:183–207. doi: 10.1111/j.1600-065x.1996.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Wilson J, He J, Xiang L, Schur P H, Mountz J D. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Investig. 1996;98:1107–1113. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]