Abstract
Rationale
GM-CSF (granulocyte–macrophage colony–stimulating factor) has emerged as a promising target against the hyperactive host immune response associated with coronavirus disease (COVID-19).
Objectives
We sought to investigate the efficacy and safety of gimsilumab, an anti–GM-CSF monoclonal antibody, for the treatment of hospitalized patients with elevated inflammatory markers and hypoxemia secondary to COVID-19.
Methods
We conducted a 24-week randomized, double-blind, placebo-controlled trial, BREATHE, at 21 locations in the United States. Patients were randomized 1:1 to receive two doses of intravenous gimsilumab or placebo 1 week apart. The primary endpoint was all-cause mortality rate at Day 43. Key secondary outcomes were ventilator-free survival rate, ventilator-free days, and time to hospital discharge. Enrollment was halted early for futility based on an interim analysis.
Measurements and Main Results
Of the planned 270 patients, 225 were randomized and dosed; 44.9% of patients were Hispanic or Latino. The gimsilumab and placebo groups experienced an all-cause mortality rate at Day 43 of 28.3% and 23.2%, respectively (adjusted difference = 5% vs. placebo; 95% confidence interval [−6 to 17]; P = 0.377). Overall mortality rates at 24 weeks were similar across the treatment arms. The key secondary endpoints demonstrated no significant differences between groups. Despite the high background use of corticosteroids and anticoagulants, adverse events were generally balanced between treatment groups.
Conclusions
Gimsilumab did not improve mortality or other key clinical outcomes in patients with COVID-19 pneumonia and evidence of systemic inflammation. The utility of anti–GM-CSF therapy for COVID-19 remains unclear.
Clinical trial registered with www.clinicaltrials.gov (NCT 04351243).
Keywords: gimsilumab, GM-CSF, SARS-CoV-2, COVID-19, acute respiratory distress syndrome
At a Glance Commentary
Scientific Knowledge on the Subject
GM-CSF (granulocyte–macrophage colony–stimulating factor) has emerged as a promising target against the hyperactive host immune response associated with coronavirus disease (COVID-19). It is unclear whether GM-CSF inhibition is a safe and effective treatment for COVID-19 pneumonia.
What This Study Adds to the Field
This study demonstrated that GM-CSF inhibition with gimsilumab did not improve mortality or other key clinical outcomes in patients with COVID-19 pneumonia and evidence of systemic inflammation. The utility of anti–GM-CSF therapy for COVID-19 remains unclear.
Coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a global pandemic. At the start of the pandemic, approximately 20% of patients with COVID-19 developed severe disease that required hospitalization and oxygen support (1). Many severe cases appeared to be driven by a host hyperactive immune response consisting of myeloid cell-mediated organ damage and excessive inflammation (2–5).
GM-CSF (granulocyte–macrophage colony–stimulating factor), a myeloid cell growth factor and proinflammatory cytokine, has been proposed as a key mediator of COVID-19 hyperinflammation (6–10). Immunomodulation with monoclonal antibodies (mAbs) against GM-CSF or its receptor (GM-CSFR) has been under investigation as a strategy for treating COVID-19 in several large randomized controlled trials (6–8). GM–CSF-targeting agents have been studied previously in autoimmune conditions, with statistically significant benefits demonstrated in phase 2 trials for rheumatoid arthritis and giant cell arteritis (11, 12). In small open-label COVID-19 studies, treatment with anti–GM-CSF or anti–GM-CSFR mAbs was correlated with decreased mortality and improvement in other clinical outcomes versus contemporaneous external control groups (13, 14). GM-CSF inhibition has also shown benefits across multiple preclinical models with hyperinflammatory pathologies, including cytokine release syndrome (15, 16), sepsis (17), and acute lung injury (18, 19).
We conducted a pivotal, randomized, double-blind, placebo-controlled clinical trial, BREATHE, to assess gimsilumab, a fully human anti–GM-CSF mAb, for patients with hypoxemia and elevated inflammatory markers due to COVID-19 pneumonia. The primary study objective was to evaluate the effect of gimsilumab on mortality. Key secondary objectives were to assess requirements for ventilation and hospitalization. Some of the results of this study have been previously reported in the form of an abstract (20) and on ClinicalTrials.gov (21).
Methods
Design and Oversight
BREATHE was a multicenter, adaptive, double-blind, randomized, placebo-controlled, parallel-group study in patients hospitalized with COVID-19 demonstrating hypoxemia and evidence of systemic inflammation. The efficacy and safety of intravenous treatment with gimsilumab versus placebo were evaluated over a 24-week period. Patients were enrolled at 21 hospitals in the United States. The study was designed and overseen by Kinevant Sciences, the study sponsor, in collaboration with an academic Steering Committee. A sponsor-funded contract research organization supported trial conduct. An independent Data Monitoring Committee (DMC) periodically reviewed trial data and conducted a prespecified interim analysis on August 26, 2020, using data from 100 patients with 6 weeks of follow-up. This interim analysis demonstrated futility on the primary endpoint, prompting the DMC to investigate potential efficacy signals while considering trial modifications and termination. Trial enrollment was ultimately halted on October 12, 2020, before achieving the planned sample size. All randomized patients were then followed until the end of the study.
The trial protocol, statistical analysis plan (SAP), and a list of committee members and site investigators are provided in the online supplement. The study was reviewed and agreed upon by the U.S. Food and Drug Administration and a central and/or local Institutional Review Board for each clinical site. The trial was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. The Steering Committee and sponsor assembled the manuscript. All authors assume full responsibility for the accuracy and completeness of the reported data.
Patients
BREATHE included adult patients hospitalized for COVID-19 pneumonia who had 1) laboratory-confirmed SARS-CoV-2 infection; 2) serum CRP (C-reactive protein) ⩾50 mg/L or ferritin ⩾1,000 ng/ml based on local laboratory measurements; 3) radiographic evidence of bilateral infiltrates; and 4) clinical evidence of substantial hypoxemia (defined as requiring ⩾4 L supplemental O2 to attempt to maintain ⩾92% SpO2, or measured or imputed [22, 23] PaO2/FiO2 ⩽300 mm Hg) or acute respiratory distress syndrome (ARDS) per the Berlin definition (see online supplement) (24). Patients were excluded if they had evidence of multiorgan failure or had received mechanical ventilation for >72 hours. The full list of entry criteria is described in the Protocol. Administration of biologic anticytokine/anticomplement agents, recombinant GM-CSF, mesenchymal stem cells, and Janus kinase inhibitors was prohibited. Treatment with corticosteroids and antiviral agents was allowed. Written or electronic informed consent was obtained from each patient or from a legally authorized representative before study participation.
Procedures
Patients were randomized 1:1 to receive gimsilumab or placebo on Day 1 of the study. Follow-up began at randomization. Randomization was performed using a validated interactive voice/web response system and stratified based on two categories of patient clinical status at baseline: 1) mild ARDS or lesser extent of hypoxemia; and 2) moderate or severe ARDS. Although the intent of the Protocol was to use the strict Berlin criteria, operationalization of this stratification was difficult as there were concerns around ventilator accessibility and ventilator-associated detrimental outcomes during the pandemic. These circumstances led some investigators to apply a more liberal adaptation of the Berlin criteria where patients with moderate or severe hypoxemia were classified into the moderate/severe ARDS stratum regardless of ventilation status (25, 26). Randomization software could temporarily close a trial stratum to ensure 40–60% of patients were enrolled in each category; however, enrollment was adequately balanced without the need for pausing.
Gimsilumab 400 mg or placebo saline was administered intravenously on Day 1, and gimsilumab 200 mg or placebo saline was administered intravenously on Day 8. The Day 8 dose was omitted if the patient was discharged or no longer required supplemental oxygen. The dosing regimen rationale is described in the Protocol, Section 2.2. All site staff, patients, and caregivers were blinded to treatment assignment, except an unblinded pharmacist who prepared the study treatments.
While hospitalized, patients were assessed daily until discharge or Week 6 and then at Weeks 12 and 24. If discharged, telephone follow-up calls were conducted at Weeks 2, 3, 4, 5, 6, 12, and 24. In-hospital assessments included the seven-point ordinal scale (27), National Early Warning Score (28), Sequential Organ Failure Assessment score (29), supplemental oxygen requirements, and ventilation support requirements. Phlebotomy for centrally assessed laboratory measurements was performed on Days 1, 4, 8, and day of discharge from the hospital. A nasopharyngeal swab for central assessment of SARS-CoV-2 viral load was taken on Days 1, 2, 9, and day of discharge. Telephone follow-up visits recorded the seven-point ordinal scale score, concomitant medications, and adverse events (AEs).
Outcomes
The primary endpoint was the rate of all-cause mortality at Day 43. Key secondary endpoints were 1) the proportion of patients who survived and were free of invasive ventilation at Day 29; 2) the number of invasive ventilator-free days through Day 29 (nonsurvivors were assigned zero ventilator-free days); and 3) the time to hospital discharge (nonsurvivors were considered never discharged). The final selection of key secondary endpoints occurred in a Protocol Amendment on June 12, 2020, before the interim analysis. Other secondary and exploratory endpoints included changes from baseline in National Early Warning Score, Sequential Organ Failure Assessment score, and inflammatory markers.
Safety endpoints included AEs, physical examinations, vital signs, electrocardiograms, and laboratory tests. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) v23.0.
Statistical Analysis
At a two-sided significance level of 0.05, we calculated that a sample size of 135 patients per arm would have approximately 80% power to detect a significant absolute difference in an all-cause mortality rate of 15% versus 30% for gimsilumab and placebo. Both the safety population and the primary population for efficacy analysis (modified intent-to-treat population) consisted of all patients who received at least one dose of double-blind study medication.
The primary endpoint analysis was based on the difference in mortality rate between treatment groups at Day 43 and was performed using the Mantel-Haenszel test stratified by baseline clinical status (mild ARDS/lesser extent of hypoxemia or moderate/severe ARDS). Time-to-event methods were used to perform a supplementary analysis of the primary outcome. Multiple imputation, which used a prediction model to generate plausible values for missing data and then combined the variance of each generated dataset to calculate a test statistic, was used to incorporate missing values into the statistical inference for the binary mortality and ventilator-free survival endpoints (see SAP section 4.8.1.6). Analytical methods of other endpoints, methods for subgroup and sensitivity analyses, and the prespecified endpoint testing hierarchy are described in the SAP; 95% confidence intervals (CIs) and two-sided P values are presented. The interim analysis assessed superiority and futility using the primary analysis methodology, with a statistical α penalty of 0.001. Stopping guidelines were predefined in SAP section 5.2.
All analyses were performed using SAS software, version 9.4. The core features of the SAP were finalized before the interim analysis.
Results
Patient Characteristics
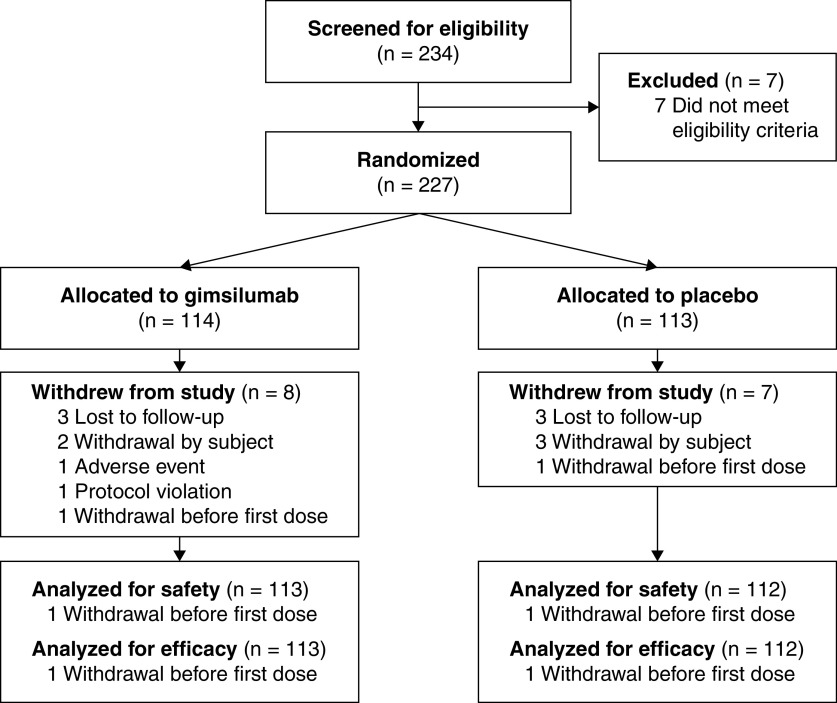
BREATHE randomized patients between April 15, 2020, and October 12, 2020. Enrollment was halted before achieving the target sample size of 270 patients. The final patient follow-up occurred on April 1, 2021. Two hundred and thirty-four patients were screened, and 227 patients were randomized; 225 patients received at least one dose of study drug, comprising the safety and modified intent-to-treat populations (Figure 1) (113 patients received gimsilumab, and 112 received placebo). Almost all (96.2%) screened patients were randomized and treated. In the modified intent-to-treat population, 8 (3.6%) patients withdrew (4 in the gimsilumab group; 4 in the placebo group) and 13 (5.8%) patients died (7 in the gimsilumab group; 6 in the placebo group) before receiving the second drug dose on Day 8. Across the entire follow-up period, 13 (5.7%) patients in the modified intent-to-treat population withdrew, and 63 (28.0%) died. A total of 106 (93.8%) gimsilumab-treated patients and 106 (94.6%) placebo-treated patients completed the study through Week 24.
Figure 1.
Patient flow through BREATHE.
Demographics and baseline characteristics were generally well-balanced across treatment groups and reflected a racially/ethnically diverse patient population (Table 1). 44.9% of patients were Hispanic or Latino, with more Hispanic/Latino patients present in the gimsilumab group. 44.4% of patients were categorized in the milder clinical stratum, and 55.6% of patients were categorized in the more severe stratum. Inflammatory markers were elevated, with a median CRP of 86.0 mg/L and a median ferritin of 1,104.5 ng/ml. 30.7% of patients were treated with low-flow supplemental oxygen, 50.2% were treated with noninvasive positive pressure ventilation or high-flow oxygen support, and 19.1% required invasive mechanical ventilation on the day of dosing. One patient demonstrated the serologic possibility of undiagnosed autoimmune pulmonary alveolar proteinosis as evaluated by anti–GM-CSF autoantibody measurements at baseline.
Table 1.
Demographics and Baseline Characteristics of the Modified Intent-to-Treat Population
| Gimsilumab (N = 113) | Placebo (N = 112) | |
|---|---|---|
| Age (yr), mean (SD) | 59.9 (14.7) | 60.4 (14.3) |
| <65, n (%) | 70 (61.9) | 65 (58.0) |
| ⩾65, n (%) | 43 (38.1) | 47 (42.0) |
| Sex, n (%) | ||
| Male | 73 (64.6) | 81 (72.3) |
| Female | 40 (35.4) | 31 (27.7) |
| Hispanic or Latino, n (%) | 59 (52.2) | 42 (37.5) |
| Race, n (%) | ||
| American Indian or Alaska Native | 0 (0) | 2 (1.8) |
| Asian | 5 (4.4) | 6 (5.4) |
| Black or African American | 16 (14.2) | 29 (25.9) |
| White | 61 (54.0) | 52 (46.4) |
| Multiple | 1 (0.9) | 0 |
| Other | 15 (13.3) | 15 (13.4) |
| Unknown | 15 (13.3) | 8 (7.1) |
| Body mass index (kg/m2), mean (SD) | 33.1 (7.8) | 31.9 (8.4) |
| Days from symptom onset to randomization, median (min, max) | 10.0 (0, 38) | 10.0 (1, 32) |
| Medical history, n (%) | ||
| Chronic obstructive pulmonary disease | 6 (5.3) | 11 (9.8) |
| Coronary artery disease | 11 (9.7) | 7 (6.3) |
| Diabetes mellitus | 27 (23.9) | 20 (17.9) |
| Hypertension | 51 (45.1) | 53 (47.3) |
| Hyperlipidemia | 34 (30.1) | 33 (29.5) |
| Obesity | 30 (26.5) | 30 (26.8) |
| Clinical status stratum, n (%) | ||
| Mild ARDS or lesser extent of hypoxemia* | 50 (44.2) | 50 (44.6) |
| Moderate or severe ARDS* | 63 (55.8) | 62 (55.4) |
| Requiring invasive ventilation, n (%)† | 21 (18.6) | 20 (17.9) |
| Requiring intensive care unit, n (%) | 66 (58.4) | 63 (56.3) |
| Worst seven-point ordinal scale score on the day of dosing, n (%) | ||
| 4. Hospitalized, requiring supplemental oxygen | 37 (32.7) | 32 (28.6) |
| 5. Hospitalized, on noninvasive ventilation or high-flow oxygen devices | 54 (47.8) | 59 (52.7) |
| 6. Hospitalized, on invasive mechanical ventilation or ECMO | 22 (19.5) | 21 (18.8) |
| NEWS, mean (SD) | 6.1 (2.3) | 5.8 (3.0) |
| SOFA Score, mean (SD) | 2.1 (2.9) | 2.2 (3.2) |
| Anti–GM-CSF autoantibody measurements (μg/ml), n (%)‡ | ||
| <5 μg/ml | 83 (73.5) | 86 (76.8) |
| ⩾5 μg/ml | 1 (0.9) | 0 (0) |
| Unknown | 29 (25.7) | 26 (23.2) |
| Laboratory measurements, median (IQR)§ | ||
| C-reactive protein (mg/L) | 90.0 (100.0) | 85.0 (91.0) |
| Ferritin (ng/ml) | 1,165.0 (946.0) | 968.0 (1,154.0) |
| D-Dimer (mg FEU/L) | 1.21 (1.48) | 1.04 (1.27) |
| Lactate dehydrogenase (U/L) | 392.0 (207.0) | 411.0 (205.0) |
| Procalcitonin (ng/ml) | 0.18 (0.49) | 0.19 (0.64) |
| Troponin I (μg/L) | 0.0075 (0.0110) | 0.0055 (0.0155) |
| Alanine aminotransferase (U/L) | 43.5 (40.0) | 32.5 (50.5) |
| Alkaline phosphatase (U/L) | 81.0 (37.0) | 70.0 (42.0) |
| Aspartate aminotransferase (U/L) | 42.0 (34.0) | 40.0 (35.0) |
| Creatine kinase (U/L) | 87.0 (188.0) | 101.0 (218.0) |
| Estimated glomerular filtration rate (ml/min/SA) | 94.0 (46.0) | 83.5 (49.5) |
| N-terminal ProB-type natriuretic peptide (pmol/L) | 23.0 (74.0) | 18.4 (71.4) |
| Lymphocyte count (×109/L) | 0.7 (0.5) | 0.7 (0.5) |
| Leukocyte count (×109/L) | 10.1 (5.3) | 9.9 (5.5) |
| Neutrophil count (×109/L) | 8.7 (6.1) | 8.8 (5.3) |
| Platelet count (×109/L) | 292.5 (147.0) | 272.0 (170.0) |
| SARS-CoV-2 viral load (log copies/ml) | 5.0 (2.0) | 5.0 (3.0) |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; ECMO = extracorporeal membrane oxygenation; FEU = fibrinogen equivalent unit; GM-CSF = granulocyte–macrophage colony–stimulating factor; IQR = interquartile range; NEWS = National Early Warning Score; SA = surface area 1.73 m2; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SOFA = Sequential Organ Failure Assessment.
Represents stratum selected by site investigator at randomization. See METHODS for limitations around stratification definitions.
Data represents status at the time of dosing.
An anti–GM-CSF autoantibody measurement ⩾5 μg/ml is consistent with an early phenotype of pulmonary alveolar proteinosis.
Measurements were derived from a central laboratory and may have differed from the local laboratory results used at study screening.
Although clinical management recommendations were rapidly changing throughout the study, concomitant medication use was generally similar between treatment groups (Table 2). Approximately 10% fewer patients in the gimsilumab group received systemic corticosteroids relative to the placebo group during the study. Dexamethasone use was similar across groups. Eleven (9.7%) patients in the gimsilumab group and 10 (8.9%) patients in the placebo group received a prohibited medication due to rapid decline. Fifty (44.2%) patients in the gimsilumab group and 53 (47.3%) patients in the placebo group did not receive the Day 8 drug dose, most commonly because of hospital discharge.
Table 2.
Medication Use Before and During the Study
| Gimsilumab (N = 113) | Placebo (N = 112) | |
|---|---|---|
| Before study* | ||
| Systemic corticosteroids, n (%) | 22 (19.5) | 29 (25.9) |
| Dexamethasone or dexamethasone sodium phosphate | 16 (14.2) | 10 (8.9) |
| Methylprednisolone or methylprednisolone sodium succinate | 8 (7.1) | 19 (17.0) |
| Prednisone | 3 (2.7) | 7 (6.3) |
| Remdesivir, n (%) | 34 (30.1) | 28 (25.0) |
| Azithromycin, n (%) | 41 (36.3) | 35 (31.3) |
| Hydroxychloroquine, n (%) | 5 (4.4) | 6 (5.4) |
| Immunosuppressants, n (%) | 5 (4.4) | 6 (5.4) |
| Convalescent plasma, n (%) | 3 (2.7) | 1 (0.9) |
| During study | ||
| Received Day 8 gimsilumab or placebo dose, n (%) | 63 (55.8) | 59 (52.7) |
| At least one concomitant medication | 113 (100.0) | 112 (100.0) |
| Systemic corticosteroids, n (%) | 93 (82.3) | 104 (92.9) |
| Dexamethasone or dexamethasone sodium phosphate | 60 (53.1) | 58 (51.8) |
| Methylprednisolone or methylprednisolone sodium succinate | 39 (34.5) | 43 (38.4) |
| Prednisone | 18 (15.9) | 24 (21.4) |
| Antithrombotic agents, n (%) | 110 (97.3) | 111 (99.1) |
| Remdesivir, n (%) | 55 (48.7) | 59 (52.7) |
| Azithromycin, n (%) | 30 (26.5) | 43 (38.4) |
| Immunosuppressants, n (%) | 17 (15.0) | 19 (17.0) |
| Immunoglobulin, n (%) | 13 (11.5) | 15 (13.4) |
| Hydroxychloroquine, n (%) | 4 (3.5) | 5 (4.5) |
| Convalescent plasma, n (%) | 0 (0) | 1 (0.9) |
| Prohibited medications, n (%) | 11 (9.7) | 10 (8.9) |
| Anakinra | 3 (2.7) | 5 (4.5) |
| Eculizumab | 1 (0.9) | 0 (0) |
| Filgrastim | 0 (0) | 1 (0.9) |
| Mesenchymal stem cells | 1 (0.9) | 0 (0) |
| Tocilizumab | 8 (7.1) | 9 (8.0) |
Started and stopped before the first dose of the study drug.
Efficacy
The results of the primary and key secondary efficacy analyses are shown in Table 3. There was no significant difference between the gimsilumab and placebo groups in the rate of all-cause mortality at Day 43 (adjusted difference = 0.05 vs. placebo, 95% CI [−0.06 to 0.17], P = 0.377). The gimsilumab and placebo groups experienced a 28.3% and 23.2% mortality rate by Day 43, respectively. Three (2.7%) patients in the gimsilumab group and two (1.8%) in the placebo group had missing Day 43 mortality data. The time-to-death analysis, which included all data through the end of the study, did not demonstrate a significant difference between groups, and overall mortality rates were nearly equal (hazard ratio, 1.1; 95% CI [0.7–1.8], log-rank P = 0.777) (Figure 2).
Table 3.
Analyses of the Primary and Key Secondary Outcomes in the Modified Intent-to-Treat Population
| Proportion/Mean Difference Analyses | |||||
|---|---|---|---|---|---|
| Gimsilumab (N = 113) | Placebo (N = 112) | Adjusted Difference | 95% CI | P value | |
| All-cause mortality at Day 43, proportion (n) | 0.283 (32) | 0.232 (26) | 0.05 | (−0.06 to 0.17) | 0.377 |
| Alive and off invasive ventilation at Day 29, proportion (n) | 0.708 (80) | 0.696 (78) | 0.02 | (−0.09 to 0.14) | 0.691 |
| Mechanical ventilator-free days by Day 29, median (first quartile, third quartile) | 29.0 (2, 29) | 29.0 (4, 29) | 0.0 | NA | 0.479 |
| Time-to-Event Analyses | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gimsilumab (N = 113) |
Placebo (N = 112) |
Hazard ratio | 95% CI | Log-rank P value | |||||
| Number of patients with event | Percentage of patients with event | Median number of days to event | Number of patients with event | Percentage of patients with event | Median number of days to event | ||||
| All-cause mortality* | 32 | 28.3 | NR | 31 | 27.7 | NR | 1.1 | (0.7 to 1.8) | 0.777 |
| Hospital discharge† | 78 | 69.0 | 13.0 | 79 | 70.5 | 15.0 | 0.9 | (0.7 to 1.3) | 0.727 |
Definition of abbreviations: CI = confidence interval; NA = not assessed; NR = not reached.
Multiple imputation was used to handle missing data for the binary endpoint analyses.
Includes all mortality data through the end of the study at Week 24.
Nonsurvivors, along with patients who had not been discharged by the end of the study, were censored at Week 24; patients who withdrew early were censored at the last visit.
Figure 2.
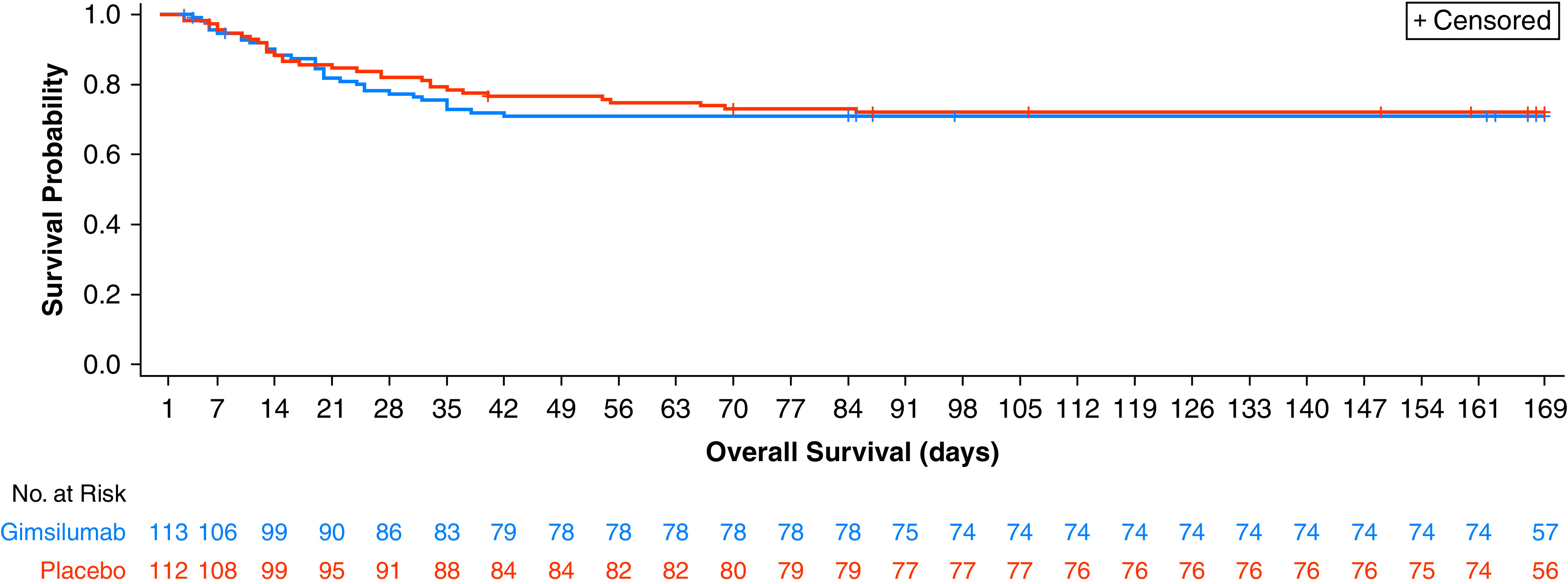
Kaplan-Meier plot of all-cause mortality.
There were no differences between the gimsilumab and placebo groups in any of the key secondary endpoints. The gimsilumab group experienced a Day 29 ventilator-free survival rate of 70.8%, while the placebo group experienced a rate of 69.6% (adjusted difference = 0.02; 95% CI [−0.09 to 0.14]; P = 0.691]. The mean number of ventilator-free days by Day 29 was 19.6 (SD, 12.7) and 20.1 (SD, 12.6) in the gimsilumab and placebo groups, respectively. Both groups had a median of 29.0 ventilator-free days by Day 29 (P = 0.479), indicating that the majority of patients were not treated with invasive ventilation during this study period. The gimsilumab group required a median of 13.0 days to hospital discharge, while the placebo group required 15.0 days (hazard ratio, 0.9; 95% CI [0.7–1.3]; log-rank P = 0.727). Sensitivity analyses for the primary and key secondary endpoints did not achieve statistical significance.
Analyses of other endpoints, including laboratory markers and cytokine levels, are shown in Table E1 in the online supplement. There were generally no differences across treatment groups in most of these endpoints. Both groups demonstrated rapid reductions in inflammatory marker levels within the first week after baseline. The gimsilumab group showed an increase in GM-CSF levels over the study period, likely due to GM-CSF binding by circulating gimsilumab. Although most cytokine analyses showed no differences between groups, levels of MCP-1, which is a cytokine thought to be downstream of GM-CSF signaling, demonstrated numerically greater reductions from baseline in the gimsilumab versus the placebo group.
Most prespecified subgroup analyses showed no differences between treatment groups in the primary and key secondary endpoints (Figures E1–E3). However, in the subgroup of patients invasively ventilated at the time of dosing (n = 41), patients treated with gimsilumab demonstrated an increase in ventilator-free survival rate at Day 29 relative to those treated with placebo (nominal P value = 0.017), with other endpoints showing numerical benefits that did not reach significance (Table E2). Post hoc analyses demonstrated no clear differences between the gimsilumab and placebo arms in the primary and key secondary endpoints when subgroups were analyzed by steroid usage, dexamethasone usage, age ⩾70 years old, CRP quartiles, and ferritins quartiles.
Safety
Safety results, shown in Table 4 and Tables E3 and E4, include all data through the 24-week observational period. Rates of AEs, grade 3–5 AEs, and serious AEs (SAEs), as well as those events deemed to be related to study drug by the site investigator, were balanced between treatment groups. There were two infusion-related reactions in the gimsilumab group. Most AEs of interest that could have been related to gimsilumab’s mechanism of action, including hematopoietic disturbances and liver toxicity, were generally comparable across groups. No cases of pulmonary alveolar proteinosis were reported. The gimsilumab group appeared to have a small elevation in SAEs classified under the infections MedDRA system organ class, although the placebo group had slightly higher rates of SAEs in the renal and cardiac system organ classes.
Table 4.
Summary of Adverse Events Observed in the Safety Population
| Gimsilumab (N = 113) | Placebo (N = 112) | |
|---|---|---|
| Total AEs, n | 393 | 501 |
| Total SAEs, n | 113 | 117 |
| Total deaths, n | 32 | 31 |
| Patients with at least one of the following, n (%) | ||
| AE | 84 (74.3) | 77 (68.8) |
| AE deemed related to study drug | 14 (12.4) | 13 (11.6) |
| Grade 3–5 AE | 48 (42.5) | 47 (42.0) |
| Grade 3–5 AE deemed related to study drug | 6 (5.3) | 4 (3.6) |
| SAE | 47 (41.6) | 45 (40.2) |
| SAE deemed related to study drug | 4 (3.5) | 6 (5.4) |
| AE leading to study drug discontinuation | 3 (2.7) | 1 (0.9) |
| AE classified under Infections and Infestations SOC | 34 (30.1) | 29 (25.9) |
| SAE classified under Infections and Infestations SOC* | 22 (19.5) | 15 (13.4) |
| Septic shock | 7 (6.2) | 6 (5.4) |
| Sepsis | 5 (4.4) | 0 (0.0) |
| Pneumonia | 3 (2.7) | 3 (2.7) |
| Pneumonia pseudomonal | 2 (1.8) | 1 (0.9) |
| SAE classified under Renal and Urinary Disorders SOC* | 6 (5.3) | 11 (9.8) |
| Acute kidney injury | 3 (2.7) | 9 (8.0) |
| Renal failure | 2 (1.8) | 0 (0.0) |
| SAE classified under Cardiac Disorders SOC* | 5 (4.4) | 8 (7.1) |
| Atrial fibrillation | 3 (2.7) | 3 (2.7) |
| Pulseless electrical activity | 1 (0.9) | 2 (1.8) |
| Hematopoietic cytopenia | 12 (10.6) | 12 (10.7) |
| Abnormal liver enzyme test | 8 (7.1) | 10 (8.9) |
| Pulmonary alveolar proteinosis | 0 (0.0) | 0 (0.0) |
| Infusion-related reaction | 2 (1.8) | 0 (0.0) |
Definition of abbreviations: AE = adverse event; MeDRA = Medical Dictionary for Regulatory Activities; SAE = serious adverse event; SOC = system organ class as defined by MedDRA.
Table includes all available data through the 24-wk observational period.
Includes only SAEs by MedDRA Preferred Term that appeared in >1 patient in either treatment arm.
Discussion
Here, we report a randomized, double-blind, placebo-controlled trial of the GM-CSF inhibitor gimsilumab for the treatment of patients with COVID-19 pneumonia. The study population was comprised of ethnically diverse, older, and/or obese patients with multiple comorbidities and elevated inflammatory marker values (risk factors that have all been associated with poor outcomes). Almost all patients received systemic steroids and anticoagulants during the study, and we allowed entry of patients who had been receiving mechanical ventilation for ⩽72 hours. In this population, gimsilumab did not meet the primary objective of reducing mortality. The key secondary objectives of reducing ventilation and hospitalization requirements were also not met.
Our inflammatory marker thresholds, CRP ⩾50 mg/L or ferritin ⩾1,000 ng/ml, are higher than the entry criteria of all other GM-CSF inhibitor trials, exceeding thresholds that have been proposed as predictive markers for hyperimmune activation and poor outcomes (30). This trial design was based on the idea that there exists hypoinflammatory and hyperinflammatory ARDS subphenotypes, which induce differential responses to immunomodulatory treatment (31–33). Derived from GM-CSF’s postulated mechanism as a proinflammatory mediator in COVID-19, BREATHE sought to test the hypothesis that administering anti–GM-CSF therapy to only patients with systemic inflammation would yield positive outcomes.
The study results do not support the use of anti–GM-CSF treatment in patients with hypoxemia and elevated CRP or ferritin secondary to COVID-19. Given that positive results were reported from other GM-CSF inhibitor trials in COVID-19, this insight helps hone the patient population potentially eligible for this treatment class (34–37). COVID-19 trials of agents targeting different cytokines (IL-6, IL1β) have obtained negative results with similar inflammatory biomarker thresholds as BREATHE (38, 39), although interestingly, corticosteroids may be more efficacious in patients experiencing significant inflammation (40). Importantly, levels of GM-CSF, as well as several proinflammatory cytokines such as IL-1β and tumor necrosis factor, did not appear to be elevated in most patients in our cohort. This result may indicate that fulminant cytokine release is not a prominent feature of many patients with COVID-19, providing a biological explanation for the negative results of our trial and previous trials of anticytokine agents. It is also possible that elevated CRP and ferritin did not select for the patient population that would respond best to anticytokine therapy and that more precise biomarkers of inflammation are needed to stratify patients before immunomodulation.
The study’s negative results could have also been due to its broad entry criteria, given that we included patients with earlier stages of lung injury as well as all stages of ARDS. This design reflected our hypothesis that anti–GM-CSF therapy would benefit both milder populations and more severe populations by preventing or blocking deleterious inflammation. Based on our subgroup analyses, it seems that gimsilumab did not benefit most patients. However, patients who were invasively ventilated at dosing demonstrated a suggestion of improvement from gimsilumab in ventilator-free survival. Interestingly, it has been previously speculated that anti–GM-CSF therapy would be most likely to benefit more progressed patients, potentially because pulmonary GM-CSF antiviral action has been exhausted and pathogenic GM–CSF-mediated myeloid cell recruitment has been activated (6, 8). GM-CSF may also be part of a ventilator-induced inflammatory cascade that can perpetuate lung injury. Similar to our results, dexamethasone showed greater efficacy in ventilated patients (41). Our subgroup analyses are subject to type I error, but future trials of anti–GM-CSF therapies in COVID-19 and related disease areas (e.g., sepsis and all-cause ARDS) might consider the inclusion of critically ill ventilated patients, particularly as this population carries a high mortality risk with few treatment options.
Our data provide important context to results from large randomized controlled trials of other anti–GM-CSF and anti–GM-CSFR mAbs for COVID-19 (34–37). The OSCAR (Otilimab in Severe COVID-19 Related Disease) trial (not yet peer-reviewed) of anti–GM-CSF otilimab (N = 806) did not achieve statistical significance on the primary endpoint of Day 28 ventilator-free survival but demonstrated evidence of benefit in the prespecified subgroup of patients ⩾70 years old, which is a population considered to be at high risk for severe COVID-19 (34). OSCAR had similarly broad lung injury-based entry criteria as BREATHE, including patients on high-flow oxygen (⩾15 L/min) as well as mechanical ventilation, and also required CRP or ferritin to be above the upper limit of normal. The phase 2 portion of a phase 2/3 randomized controlled trial of anti–GM-CSFR mavrilimumab demonstrated improvement in ventilator-free survival versus placebo in the cohort of hospitalized nonventilated patients (N = 116) (35). A proof-of-concept phase 2 trial of anti–GM-CSF namilumab and anti-tumor necrosis factor–α infliximab in patients with COVID-19 pneumonia and CRP ⩾40 mg/L demonstrated a significant reduction in CRP concentration with namilumab, but not infliximab, relative to standard of care (N = 146) (37). Lastly, the LIVE-AIR trial of anti–GM-CSF lenzilumab enrolled hospitalized patients before ventilation (N = 520), had no biomarker-based entry criteria, and demonstrated a significant improvement versus placebo on the primary endpoint of Day 28 ventilator-free survival (36). Interestingly, this study used a larger dose regimen (three 600 mg infusions over 24 h) than all other trials (36). Despite these positive results, the FDA recently declined the Emergency Use Authorization Request for lenzilumab (42). Differences in trial results could be related to differing entry criteria, geographies, mAb pharmacokinetics, or chance variation. Overall, the potential benefit of GM-CSF inhibition in COVID-19 remains unclear. Dose, timing, and patient selection will continue to be key considerations as future studies are conducted and findings of ongoing studies are reported.
Finally, the safety profile of gimsilumab was encouraging given that many patients were receiving systemic corticosteroids and thus susceptible to adverse drug effects. Our results corroborate the generally benign safety profile observed in previous trials of anti–GM-CSF and anti–GM-CSFR mAbs for other conditions (11).
Limitations
First, we designed the study in March 2020 with limited available data on the clinical course and event rates of COVID-19. Thus, the power analysis was based on a large mortality rate difference that may have been overly optimistic in the context of the smaller effect sizes later observed with dexamethasone (41) and tocilizumab (43). Second, almost half of the study patients did not receive the second drug dose at Day 8, mostly due to significant improvements in clinical status by that time. This result suggests that the most critical window of therapeutic opportunity lies in these first few days, likely making dose regimens with large between-dose intervals less useful. Third, the emergency nature of the pandemic created issues with protocol violations such that five patients did not have available mortality data at Day 43, and 21 patients received a study-prohibited medication as salvage treatment. Fourth, although randomization was used, there were some imbalances in baseline characteristics and medication usage that potentially skewed study results, including fewer Hispanic/Latino patients and more patients who received systemic corticosteroids in the placebo group. Lastly, standards of care changed during the trial and beyond. For example, the use of tocilizumab, which was a prohibited medication in the study, as well as dexamethasone, has become widespread. Trial data may become less representative of common practice as care routines continue to evolve amid the changing landscape of the pandemic.
Conclusions
This double-blind, randomized, placebo-controlled clinical trial did not demonstrate benefit favoring GM-CSF inhibition over placebo in patients with hypoxemia and elevated inflammatory markers secondary to COVID-19. Strengths of the study include the robust randomized controlled design with hard clinical endpoints, the stringent hyperinflammatory entry criteria, the use of a central laboratory, the racially/ethnically representative patient cohort, and the relatively large sample size across many sites. As the use of corticosteroids and IL-6 inhibition continues to increase, the optimal combination and timing of antiinflammatory agents to treat COVID-19 remain uncertain. More studies may be needed to understand the biological mechanism of GM-CSF in COVID-19 and to determine if there is a therapeutic role for GM-CSF inhibition in this disease. In the context of the totality of data reported by trials of this class, GM-CSF inhibition in COVID-19 remains unclear for select subgroups but unlikely of broad general benefit in hospitalized patients.
Acknowledgments
Acknowledgment
The authors thank the patients and caregivers for their participation in the BREATHE study. The authors thank all site investigators and staff for their support in conducting the study. The authors thank the contract research organization Parexel for its important role. The authors continue to be inspired by the courage and resilience of the medical community and society at large in the face of this global pandemic.
Footnotes
Supported by Kinevant Sciences (a wholly owned subsidiary of Roivant Sciences).
Author Contributions: Study conception and design: G.J.C., F.M.L., R.L.G., K.S.M., T.S.W., T.W.R., D.M., S.S., L.S., and S.L. Trial execution and patient recruitment: G.J.C., F.M.L., R.L.G., K.S.M., T.S.W., T.W.R., D.M., S.B., R.J., A.H.C., M.K.G., G.M.L., K.A., R.M., J.M.D., M.P.T., C.W.S., J.A.A., S.G.D., S.G., S.S., L.S., and S.L. Manuscript drafting for important intellectual content: G.J.C., F.M.L., R.L.G., K.S.M., T.S.W., T.W.R., D.M., S.S., L.S., and S.L.
This version of the article was corrected on Sept 15, 2022 (see https://www.atsjournals.org/doi/full/10.1164/rccm.v206erratum9).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202108-1859OC on March 15, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA . 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2. Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol . 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med . 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 4. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant . 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med . 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lang FM, Lee KMC, Teijaro JR, Becher B, Hamilton JA. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol . 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta P, Porter JC, Manson JJ, Isaacs JD, Openshaw PJM, McInnes IB, et al. Therapeutic blockade of granulocyte macrophage colony-stimulating factor in COVID-19-associated hyperinflammation: challenges and opportunities. Lancet Respir Med . 2020;8:822–830. doi: 10.1016/S2213-2600(20)30267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonaventura A, Vecchié A, Wang TS, Lee E, Cremer PC, Carey B, et al. Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies. Front Immunol . 2020;11:1625. doi: 10.3389/fimmu.2020.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev . 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thwaites RS, Sanchez Sevilla Uruchurtu A, Siggins MK, Liew F, Russell CD, Moore SC, et al. ISARIC4C investigators Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol . 2021;6:eabg9873. doi: 10.1126/sciimmunol.abg9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamilton JA. GM-CSF in inflammation. J Exp Med . 2020;217:e20190945. doi: 10.1084/jem.20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cid MC, Unizony S, Pupim L, Fang F, Pirrello J, Ren A, et al. Mavrilimumab (anti GM-CSF receptor α monoclonal antibody) reduces time to flare and increases sustained remission in a phase 2 trial of patients with giant cell arteritis [abstract] Arthritis Rheumatol . 2020;72:L06. [Google Scholar]
- 13. De Luca G, Cavalli G, Campochiaro C, Della-Torre E, Angelillo P, Tomelleri A, et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol . 2020;2:e465–e473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Temesgen Z, Assi M, Shweta FNU, Vergidis P, Rizza SA, Bauer PR, et al. GM-CSF neutralization with lenzilumab in severe COVID-19 pneumonia: a case-cohort study. Mayo Clin Proc . 2020;95:2382–2394. doi: 10.1016/j.mayocp.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood . 2019;133:697–709. doi: 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tugues S, Amorim A, Spath S, Martin-Blondel G, Schreiner B, De Feo D, et al. Graft-versus-host disease, but not graft-versus-leukemia immunity, is mediated by GM-CSF-licensed myeloid cells. Sci Transl Med . 2018;10:eaat8410. doi: 10.1126/scitranslmed.aat8410. [DOI] [PubMed] [Google Scholar]
- 17. Khameneh HJ, Isa SABM, Min L, Nih FW, Ruedl C. GM-CSF signalling boosts dramatically IL-1 production. PLoS One . 2011;6:e23025. doi: 10.1371/journal.pone.0023025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puljic R, Benediktus E, Plater-Zyberk C, Baeuerle PA, Szelenyi S, Brune K, et al. Lipopolysaccharide-induced lung inflammation is inhibited by neutralization of GM-CSF. Eur J Pharmacol . 2007;557:230–235. doi: 10.1016/j.ejphar.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 19. De Alessandris S, Ferguson GJ, Dodd AJ, Juss JK, Devaprasad A, Piper S, et al. Neutrophil GM-CSF receptor dynamics in acute lung injury. J Leukoc Biol . 2019;105:1183–1194. doi: 10.1002/JLB.3MA0918-347R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gottlieb RL, Lang FM, Criner GJ, et al. Anti-GM-CSF monoclonal antibody gimsilumab improved ventilator-free survival, decreased hospitalization length, and prevented NT-proBNP rise in invasively ventilated patients with hyperinflammatory COVID-19 pneumonia: a subgroup analysis from the BREATHE trial suggests neurohormonal role for GM-CSF inhibition. Circulation . 2021;144:A14310. [Google Scholar]
- 21.A Study to assess the efficacy and safety of gimsilumab in subjects with lung injury or acute respiratory distress syndrome secondary to COVID-19 (BREATHE) 2021. https://clinicaltrials.gov/ct2/show/NCT04351243
- 22. Brown SM, Grissom CK, Moss M, Rice TW, Schoenfeld D, Hou PC, et al. NIH/NHLBI PETAL Network Collaborators Nonlinear imputation of Pao2/Fio2 from Spo2/Fio2 among patients with acute respiratory distress syndrome. Chest . 2016;150:307–313. doi: 10.1016/j.chest.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown SM, Duggal A, Hou PC, Tidswell M, Khan A, Exline M, et al. National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) Prevention and Early Treatment of Acute Lung Injury (PETAL) Network Nonlinear imputation of PaO2/FIO2 From SpO2/FIO2 among mechanically ventilated patients in the ICU: a prospective, observational study. Crit Care Med . 2017;45:1317–1324. doi: 10.1097/CCM.0000000000002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA . 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 25. Chertoff J. High-flow oxygen, positive end-expiratory pressure, and the berlin definition of acute respiratory distress syndrome: are they mutually exclusive? Am J Respir Crit Care Med . 2017;196:396–397. doi: 10.1164/rccm.201701-0005LE. [DOI] [PubMed] [Google Scholar]
- 26. Matthay MA, Thompson BT, Ware LB. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? Lancet Respir Med . 2021;9:933–936. doi: 10.1016/S2213-2600(21)00105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/
- 28.Royal College of Physicians. National Early Warning Score (NEWS) - standardising the assessment of acute-illness severity in the NHS. London, England: Royal College of Physicians; 2012. [Google Scholar]
- 29. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med . 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 30. Caricchio R, Gallucci M, Dass C, Zhang X, Gallucci S, Fleece D, et al. Temple University COVID-19 Research Group Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis . 2021;80:88–95. doi: 10.1136/annrheumdis-2020-218323. [DOI] [PubMed] [Google Scholar]
- 31. Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, et al. Irish Critical Care Trials Group Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med . 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sinha P, Delucchi KL, Thompson BT, McAuley DF, Matthay MA, Calfee CS, NHLBI ARDS Network Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med . 2018;44:1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ranjeva S, Pinciroli R, Hodell E, Mueller A, Hardin CC, Thompson BT, et al. Identifying clinical and biochemical phenotypes in acute respiratory distress syndrome secondary to coronavirus disease-2019. EClinicalMedicine . 2021;34:100829. doi: 10.1016/j.eclinm.2021.100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel J, Beishuizen A, Ruiz XB, et al. 2021. https://www.medrxiv.org/content/10.1101/2021.04.14.21255475v1.full.pdf
- 35. Pupim L, Wang TS, Hudock K, et al. Mavrilimumab improves outcomes in phase 2 trial in non-mechanically-ventilated patients with severe COVID-19 pneumonia and systemic hyperinflammation. Ann Rheum Dis . 2021;80:198–199. [Google Scholar]
- 36. Temesgen Z, Burger CD, Baker J, Polk C, Libertin CR, Kelley CF, et al. Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial. Lancet Respir Med . 2021;10:237–246. doi: 10.1016/S2213-2600(21)00494-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fisher BA, Veenith T, Slade D, Gaskell C, Rowland M, Whitehouse T, et al. Namilumab or infliximab compared with standard of care in hospitalised patients with COVID-19 (CATALYST): a randomised, multicentre, multi-arm, multistage, open-label, adaptive, phase 2, proof-of-concept trial. Lancet Respir Med . 2021;10:255–266. doi: 10.1016/S2213-2600(21)00460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med . 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caricchio R, Abbate A, Gordeev I, Meng J, Hsue PY, Neogi T, et al. CAN-COVID Investigators Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA . 2021;326:230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen H, Xie J, Su N, Wang J, Sun Q, Li S, et al. Corticosteroid therapy is associated with improved outcome in critically ill patients with COVID-19 with hyperinflammatory phenotype. Chest . 2021;159:1793–1802. doi: 10.1016/j.chest.2020.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med . 2020:133. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humanigen, Inc. 2021. https://ir.humanigen.com/English/news/news-details/2021/FDA-has-declined-Humanigens-Emergency-Use-Authorization-EUA-Request-for-Lenzilumab-in-Hospitalized-COVID-19-Patients/default.aspx
- 43. Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA . 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]