To the Editor:
For patients with severe acute respiratory distress syndrome (ARDS), receipt of extracorporeal membrane oxygenation (ECMO) (1) may improve survival. During the coronavirus disease (COVID-19) pandemic, the number of patients with COVID-19 referred for ECMO has exceeded the capacity of specialized centers to provide ECMO (2, 3). The outcomes of patients with COVID-19 who are eligible to receive ECMO but do not because of limited health system capacity have not been reported.
Methods
We analyzed prospectively collected clinical data from all consecutive patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who were referred for ECMO to a single center between January 1, 2021, and August 31, 2021. For all referrals, a standardized case report form was used to record patient characteristics (as listed in Table 1) and the result of a multidisciplinary committee’s determination of whether the patient was eligible for ECMO.
Table 1.
Patient Characteristics at the Time of Extracorporeal Membrane Oxygenation Referral and Clinical Outcomes
| Characteristic | Overall (N = 90) | Capacity for ECMO (n = 35) | No Capacity for ECMO (n = 55) | P Value |
|---|---|---|---|---|
| Age, yr | 40.0 (34.0–48.0) | 40.0 (32.0–47.0) | 41.0 (35.0–51.0) | 0.07 |
| Sex, F | 25 (27.8) | 10 (28.6) | 15 (27.8) | 0.89 |
| Body mass index, kg/m2 | 35.0 (30.0–39.0) | 35.0 (31.0–40.0) | 34.0 (32.0–38.0) | 0.40 |
| Comorbidities | ||||
| Hypertension | 22 (24.4) | 10 (28.6) | 12 (21.8) | 0.47 |
| Diabetes mellitus | 16 (17.8) | 4 (11.4) | 12 (22.2) | 0.27 |
| Hyperlipidemia | 3 (3.3) | 0 (0.0) | 3 (5.5) | 0.28 |
| Asthma | 5 (5.6) | 5 (14.3) | 0 (0.0) | 0.004 |
| Obstructive sleep apnea | 3 (3.3) | 2 (5.7) | 1 (1.8) | 0.32 |
| Hypothyroidism | 3 (3.3) | 2 (5.7) | 1 (1.8) | 0.32 |
| Other* | 8 (8.9) | 6 (17.1) | 6 (10.9) | 0.53 |
| Pregnant or postpartum | 4 (4.4) | 2 (5.7) | 2 (3.6) | 0.64 |
| Vasopressors | 37 (41.1) | 11 (31.4) | 26 (47.3) | 0.14 |
| Acute kidney injury | 24 (26.7) | 9 (25.7) | 15 (27.3) | 0.87 |
| Renal replacement therapy | 7 (8.1) | 3 (8.6) | 4 (7.8) | >0.99 |
| Duration of mechanical ventilation, d | 2.0 (1.0–3.0) | 1.0 (1.0–3.0) | 2.0 (1.0–5.0) | 0.03 |
| Ventilator settings | ||||
| Mode | 0.63 | |||
| Volume control | 74 (92.5) | 30 (91.9) | 44 (93.6) | — |
| Pressure control | 5 (6.3) | 2 (6.1) | 3 (6.4) | — |
| Airway pressure release ventilation | 1 (1.3) | 1 (3.0) | 0 (0.0) | — |
| Respiratory rate | 28.0 (24.0–32.0) | 30.0 (26.0–32.0) | 28.0 (22.0–32.0) | 0.29 |
| Vt, ml | 410.0 (360.0–450.0) | 400.0 (350.0–450.0) | 430.0 (385.0–460.0) | 0.20 |
| Plateau pressure, cm H2O | 33.0 (28.0–38.0) | 34.5 (32.0–38.0) | 29.0 (22.0–36.5) | 0.08 |
| FiO2 | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.13 |
| Positive end-expiratory pressure, cm H2O | 14.0 (12.0–16.0) | 15.5 (12.0–18.0) | 14.0 (12.0–15.0) | 0.07 |
| Arterial blood gas | ||||
| pH | 7.31 (7.24–7.38) | 7.30 (7.23–7.35) | 7.31 (7.24–7.38) | 0.62 |
| Partial pressure of carbon dioxide, mm Hg | 57.5 (46.0–68.5) | 57.0 (45.0–66.0) | 60.0 (47.0–69.0) | 0.69 |
| Partial pressure of oxygen, mm Hg | 63.0 (54.0–72.0) | 64.0 (57.0–72.0) | 62.0 (53.0–70.0) | 0.35 |
| Adjunctive therapies | ||||
| Neuromuscular blocking agent | 81 (96.4) | 34 (97.1) | 47 (95.9) | >0.99 |
| Prone positioning | 46 (55.4) | 20 (58.8) | 26 (53.1) | 0.60 |
| Inhaled pulmonary vasodilators | 12 (14.3) | 8 (22.9) | 4 (8.2) | 0.11 |
| Miles from referring to receiving hospital | 165.0 (100.0–243.0) | 163.0 (81.0–211.0) | 171.0 (105.0–278.0) | 0.28 |
| Outcome | ||||
| Died before hospital discharge† | 64 (71.1) | 15 (42.9) | 49 (89.1) | <0.001 |
| Survived to hospital discharge‡ | 23 (25.6) | 18 (51.4) | 5 (9.1) | <0.001 |
| Remains alive in the hospital§ | 3 (3.3) | 2 (5.7) | 1 (1.8) | 0.07 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; IQR = interquartile range.
Data are expressed as median (IQR) or frequency (percentage) unless otherwise indicated. The Wilcoxon rank-sum test, Fisher’s exact test, or the Kruskal-Wallis test was used to examine differences between groups when appropriate. Missingness in observations: mode (n = 10), body mass index (n = 7), ventilator days (n = 2), receipt of renal replacement therapy (n = 4), ventilator mode (n = 10), respiratory rate (n = 19), Vt (n = 18), positive end-expiratory pressure (n = 10), pH (n = 6), PaCO2 (n = 8), PaO2 (n = 4), receipt of prone positioning (n = 7), receipt of neuromuscular blocking agent (n = 6), and receipt of inhaled pulmonary vasodilator (n = 6).
Other comorbidities included (n = 1 for each) Asperger syndrome, polycystic ovarian syndrome, and thyroid cancer status after thyroidectomy in the capacity for ECMO group and chronic atrial fibrillation, congestive heart failure, osteoporosis, seizures, and sickle cell disease in the no capacity for ECMO group.
Median time from referral for ECMO to death was 19.0 days (IQR, 7.0–47.0) among patients for whom the capacity to provide ECMO was available and 7.0 days (IQR, 4.0–12.0) among patients for whom capacity to provide ECMO was unavailable.
Median time from referral for ECMO to discharge from the hospital alive was 36.0 days (IQR, 0.0–72.0) among patients for whom the capacity to provide ECMO was available and 32.0 days (IQR, 28.0–43.0) among patients for whom capacity to provide ECMO was unavailable.
Median time from referral for ECMO to last follow-up among patients who remained alive in the hospital was 40.0 days (IQR, 30.0–55.0) among patients for whom the capacity to provide ECMO was available and 50.0 days (IQR, 50.0–50.0) among patients for whom capacity to provide ECMO was unavailable.
Patients were considered medically eligible for ECMO 1) if criteria for sufficiently severe ARDS, as defined by the EOLIA (Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome) inclusion criteria, were present (1); 2) in the absence of the absolute contraindications of age greater than 60 years, body mass index greater than 55 kg/m2, duration of mechanical ventilation greater than 7 days, irreversible neurologic injury, chronic lung disease, active malignancy, or advanced multiple organ dysfunction; and 3) if they had three or fewer of the relative contraindications of age greater than 50 years, body mass index greater than 45 kg/m2, presence of comorbidities, duration of mechanical ventilation greater than 4 days, presence of acute kidney injury, receipt of vasopressors, duration of hospitalization greater than 14 days, or greater than 4 weeks since COVID-19 diagnosis (3, 4). Contraindications used to determine eligibility were selected by the committee on the basis of published guidance (3), published data on factors associated with death during ECMO for COVID-19 (4), and investigator experience.
After a patient was determined to be medically eligible to receive ECMO, a separate systematic assessment of the health system’s resources was performed to provide ECMO with regard to equipment, personnel, and ICU bed availability. When health system resources were not available, the patient was not transferred to an ECMO center and did not receive ECMO. When health system resources were available, the patient was transferred to an ECMO center. No waiting list was maintained, given the short eligibility window for ECMO after tracheal intubation and the long average duration of ECMO support for patients using existing ECMO resources. For patients transferred to the ECMO center receiving the referral, the ECMO team performed cannulation at the referring facility and transported patients while receiving ECMO. For patients who were transferred to other regional ECMO centers, cannulation was performed after arrival at the receiving facility.
All patients were followed until the time of death or hospital discharge by review of electronic health records or by telephone. Among patients determined to be eligible for ECMO, we compared those for whom health system capacity permitted transfer to receive ECMO at a specialized center with those for whom health system capacity did not permit transfer to receive ECMO with regard to the primary outcome of all-cause in-hospital mortality using Cox proportional hazards regression analysis, adjusting for patient age, the presence of acute kidney injury, and receipt of vasopressors. To determine whether the relationship between the availability of resources to provide ECMO and mortality was modified by changing COVID-19 outcomes over time, we tested for interactions between ECMO availability and the date of each ECMO consult. To examine whether hospital strain modified the relationship between the availability of resources to provide ECMO and mortality, we tested for interactions between ECMO availability and hospital strain, as represented by the 2-week average of COVID-19 hospitalizations and deaths in the region over the study period (5).
Results
Among the 240 patients with COVID-19 referred for ECMO, 26 patients (10.8%) did not complete the referral evaluation, 44 (18.3%) did not meet criteria for severity of lung injury, 80 (33.3%) had one or more absolute contraindications or more than three relative contraindications, and 90 patients (37.5%) were determined to be medically eligible to receive ECMO and were included in this study. The median age of patients was 40 years (interquartile range, 34–48), and 25 (27.8%) were female.
For 35 patients (38.9%), the health system capacity to provide ECMO at a specialized center was available. Of these, 24 patients were cannulated at the referring hospital and transferred to the ECMO center that received the referral, and 11 patients were transferred to another regional ECMO center, of whom 8 were cannulated after arrival and 3 died or developed a contraindication to ECMO after transfer but before cannulation. For 55 patients (61.1%), the health system capacity to provide ECMO at a specialized center was unavailable; none were transferred to an ECMO center, and none received ECMO. Characteristics at the time of referral were similar between patients for whom health system capacity permitted transfer to receive ECMO at a specialized center and patients for whom health system capacity did not permit transfer to receive ECMO (Table 1).
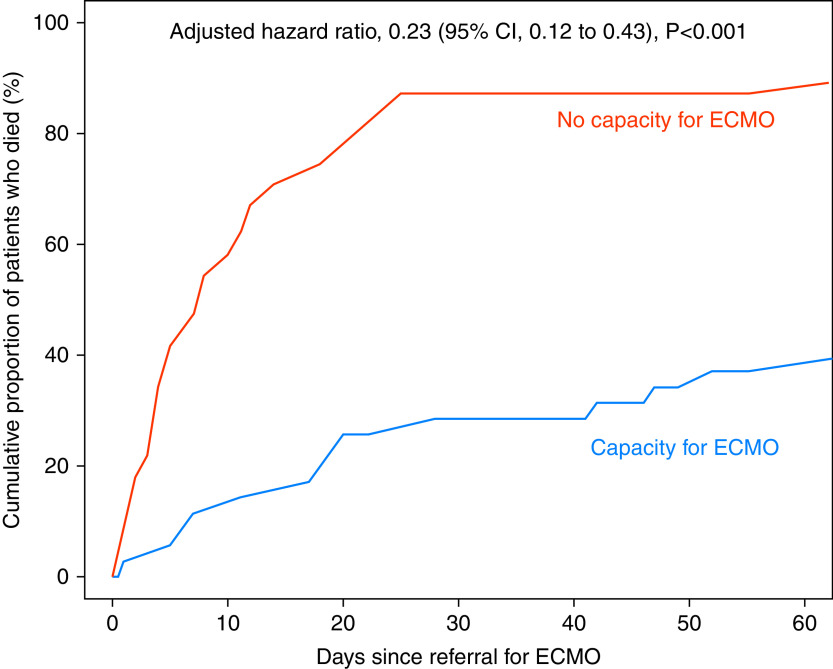
Death before hospital discharge occurred in 15 (42.9%) of the 35 patients for whom health system capacity permitted transfer to receive ECMO at a specialized center compared with 49 (89.1%) of the 55 patients for whom health system capacity did not permit transfer to receive ECMO (adjusted hazard ratio, 0.23; 95% confidence interval, 0.12–0.43; P < 0.001) (Figure 1).
Figure 1.
Cumulative proportion of patients who died before hospital discharge. The cumulative proportion of patients who died before hospital discharge is displayed for the 35 patients for whom the health system capacity to provide extracorporeal membrane oxygenation (ECMO) at a specialized center was available (blue) and the 55 patients for whom the health system capacity to provide ECMO was unavailable (red). Groups were compared using Cox proportional hazards regression, adjusting for age, acute kidney injury, and receipt of vasopressors. CI = confidence interval.
The effect on mortality of health system capacity to provide ECMO was not modified by time as measured by date of ECMO consult (P value for interaction, 0.80) or by hospital strain as measured by the 2-week average number of hospitalizations or deaths in the state over the study period (P values for interaction, 0.87 and 0.99, respectively). The results were similar in sensitivity analyses excluding days with multiple consults.
Discussion
In this cohort of adults with COVID-19, nearly 90% of patients who were eligible for ECMO but did not receive it because of limited health system capacity died before hospital discharge, despite young age and limited comorbidities. The benefits of a life-support therapy can be difficult to estimate. Clinical trials of providing or withholding a life-support therapy may be infeasible, unethical, or limited by selection bias and crossover (1, 6). Periods when resource limitations determine which patients receive life support therapy may act as a natural experiment and provide unique information on the effect of the life support therapies on outcomes. The large difference in survival associated with the availability of health system capacity to provide ECMO at a specialized center in our study suggests that the benefit of ECMO for some patients with severe ARDS because of COVID-19 may be greater than previously understood (1).
Like prior studies (6), this study cannot differentiate between the potential beneficial effects of receiving ECMO and the effects of receiving care at a specialized ECMO center. Unlike prior studies, however, no patients in this study who were transferred to a specialized center survived without receiving ECMO. Moreover, 3 of the 11 patients for whom transfer to a specialized center without cannulation was attempted died of complications arising during transfer. Together, these suggest that the observed difference in outcomes may be more likely to be attributable to receipt of ECMO than to transfer to a specialized center without receiving ECMO. Regardless of whether ECMO itself or care at a specialized center is primary, the observations that 1) lack of health system capacity prevented more than half of eligible patients with COVID-19 from receiving ECMO at a specialized center and 2) the risk of death among those who received ECMO at a specialized center was approximately half that of those who did not may have implications for resource allocation decisions by health systems and policy makers. Additional limitations of this study include its small sample size, conduct in one ECMO referral region, nonrandomized group allocation, and uncertainty of the final outcome for three patients who remain alive and in the hospital.
In conclusion, among patients eligible for ECMO in one referral region, the health system capacity to provide ECMO was available for less than half of patients. Mortality was 90% when the health system capacity to provide ECMO was unavailable, compared with 43% when capacity was available, despite both groups having young age and limited comorbidities. These findings suggest that ECMO provides a significant mortality benefit in the treatment of COVID-19 and that the inability to provide ECMO to all eligible patients because of limited healthcare system resources may be causing potentially preventable deaths.
Acknowledgments
Acknowledgment
The authors acknowledge the contributions of the centers that referred patients for ECMO; the nurses, advanced practice providers, physicians, and staff who cared for the patients; and the members of the multidisciplinary ECMO committee, including Rosemarie Dudenhofer, M.D.; Christina Jelly, M.D.; Katie McPherson, M.D.; Warren Sandberg, M.D., Ph.D.; and Anil Trindade, M.D., all of Vanderbilt University Medical Center in Nashville, Tennessee.
Footnotes
Supported in part by NHLBI grant K23HL153584 (J.D.C.) and in part by NHLBI grant K23HL143053 (M.W.S.).
Author Contributions: Study concept and design: W.D.G., J.W.S., S.A.F., M.B., M.W.S., and J.D.C.; acquisition of data: W.D.G., J.W.S., and S.A.F.; analysis and interpretation of data: W.D.G., J.W.S., S.A.F., J.D.C., and M.W.S.; drafting of the manuscript: W.D.G., M.W.S., and J.D.C.; critical revision of the manuscript for important intellectual content: W.D.G., J.W.S., S.A.F., Y.J.P., M.E.P., C.B., T.W.R., M.B., M.W.S., and J.D.C.
Originally Published in Press as DOI: 10.1164/rccm.202110-2399LE on February 25, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. EOLIA Trial Group, REVA, and ECMONet Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med . 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 2.Farmer B.https://www.npr.org/sections/health-shots/2021/09/06/1033832562/covid-icu-ecmo-life-support-shortage-hospitals.
- 3. Seethala R, Keller SP. Extracorporeal membrane oxygenation resource planning in the setting of pandemic respiratory illness. Ann Am Thorac Soc . 2020;17:800–803. doi: 10.1513/AnnalsATS.202003-233PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal Life Support Organization Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet . 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tracking coronavirus in Tennessee https://www.nytimes.com/interactive/2021/us/tennessee-covid-cases.html.
- 6. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR trial collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet . 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]