To the Editor:
Mechanical power (MP) and mechanical energy (ME) have been proposed as unifying determinants of ventilator-induced lung injury (VILI). Theoretically, a ventilation strategy can be selected to minimize MP or ME, which should lower the risk for VILI, although this principle still needs to be tested in clinical trials. However, both MP and ME need to be normalized to account for differences in lung size because the energy per unit volume is a key determinant of VILI (1–3). Most typically, lung volume measurements are normalized to predicted body weight (PBW), in both adults and children. However, when considering patients with acute respiratory distress syndrome (ARDS), the “baby lung” concept reinforces that lung volumes, particularly FRC, are further reduced beyond what would be expected from PBW. As such, some suggest normalization of MP and ME to FRC, and in adult patients with ARDS, FRC-normalized MP is more associated with outcome than MP normalized to other parameters (4). However, FRC is often not available for clinical use. Respiratory system static compliance (Crs) may be a readily available surrogate for FRC in adult patients with ARDS, although lung compliance (CL) is more precise and appears to be most proportional to FRC in adults (5). Unfortunately, CL requires esophageal pressure (Pes) measurement.
There are no data in children surrounding methods to normalize lung volumes for the calculation of MP or ME. Such methods are crucial to develop and validate for use in children given developmental differences in lung volumes, FRC, and lung and respiratory system compliance. Errors in estimating ME may inadvertently lead to changes in ventilator management that may harm the patient. Therefore, we investigated differences in normalized ME according to the method of normalization (PBW, Crs, or CL) in children with ARDS to identify whether the method of normalization could produce potentially important differences in the estimate of energy per unit lung volume. We present the analysis for normalized ME; similar results would be expected for the normalization of MP, as MP = ME × respiratory rate.
Methods
Population and settings
We performed a secondary analysis of physiologic data from mechanically ventilated children enrolled in an ongoing randomized clinical trial testing a lung and diaphragm protective ventilation strategy (REDvent [Real-time Effort Driven ventilator management]; NIH/NHLBI grant R01HL124666) (ClinicalTrials.gov identifier NCT 03266016) at Children’s Hospital Los Angeles. The parent REDvent study included children 1 month to 18 years old with ARDS who had no contraindications to the implementation of a lung and diaphragm protective ventilation strategy (6).
Data collection
Physiologic waveforms of Pes, flow, and airway pressure were recorded daily with a series of three inspiratory and expiratory holds to calculate the physiologic parameters described below. To be included in these analyses, patients had to be passive (no respiratory effort detected on Pes) on recordings from 1 of the first 2 study days. Patients were ventilated using Servo I (Maquet), Hamilton G5 (Hamilton Medical), or AVEA (CareFusion) ventilators using pressure-controlled ventilation.
We examined the correlation among ME normalized to Crs (MECrs), ME normalized to CL (MECL), and ME normalized to PBW (MEPBW). To illustrate potential differences among methods of normalization, subjects were divided into quartiles on the basis of the normalization methods, and parameters were compared among quartiles.
Definitions
ME, Crs, and CL are calculated using the following formulas. Static measurements were computed. ME is calculated according to the simplified equation Becher and colleagues proposed (7).
where Vt is tidal volume, PIP is peak inspiratory airway pressure, PEEP is positive end-expiratory pressure, Pplat is plateau pressure, Pesplat is plateau pressure of Pes, and PesPEEP is end-expiratory pressure of Pes.
PBW was calculated using the Moore method (8).
Statistical analysis
Data are expressed as median (interquartile range). A P value of <0.05 was considered to indicate statistical significance on Kruskal-Wallis analysis. Correlation was evaluated using Spearman’s correlation coefficient, and age stratification was performed using age groups of 12 months or less, 12–60 months, and 60 months or more. Analyses were performed using EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (R Foundation for Statistical Computing).
Results
Fifty-seven patients were included. The median age of patients was 82.3 months (interquartile range, 27.5–151.4 mo). There was a very strong correlation between MECrs and MECL, regardless of age group (<12 mo, rs = 0.905; 12–60 mo, rs = 0.92; >60 mo, rs = 0.969) (Figure 1A). However, there was only a modest correlation between MEPBW and MECrs (rs = 0.481; Figure 1B). When stratifying by quartiles of MEPBW compared with MECrs, only 23 of 57 patients (40%) would be classified in the same quartile, and 12 (21%) were more than two quartiles disparate (Table 1). Patients with MEPBW calculated lower than MECrs had higher PIP, higher driving pressure (DP), lower Vt/PBW, and lower Crs/PBW.
Figure 1.
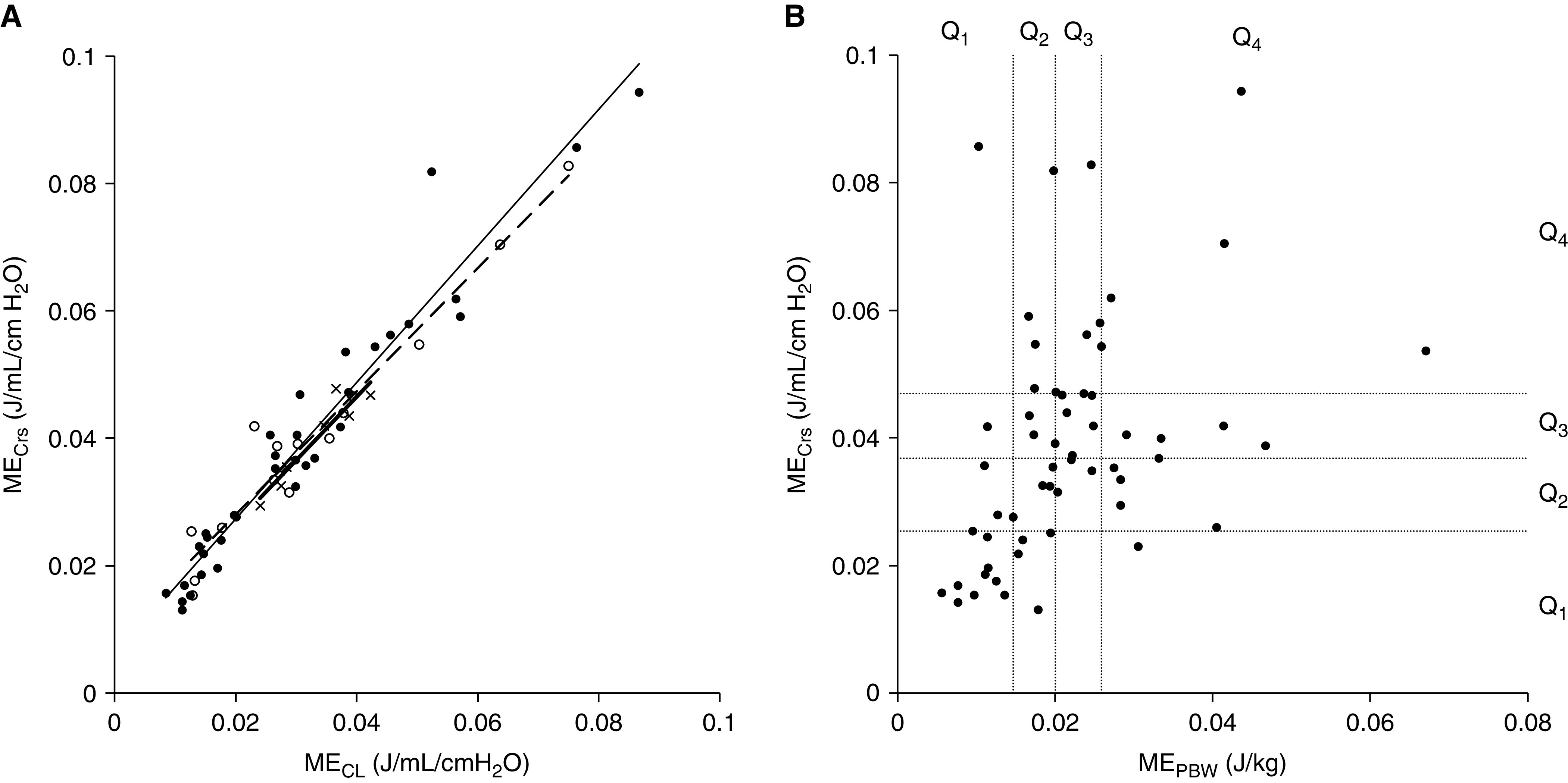
(A) Correlation between mechanical energy (ME) normalized to respiratory system static compliance (MECrs) and ME normalized to lung compliance (MCL) by age group (x/thick line, <12 mo; open circle/dashed line, 12–60 mo; solid circle/normal line, >60 mo). The lines are the regression lines for each age group. MECrs and MECL in these patients were significantly correlated in all age groups. (B) Correlation between MECrs and ME normalized to predictive body weight (MEPBW) and classification of each value grouped using interquartile range. Q1–Q4 on the x- and y-axes represent quartiles of MECrs and MEPBW, respectively. There are some patients whose MECrs and MEPBW differ greatly. Q = quartile.
Table 1.
Characteristics of Subjects by Degrees of Difference between Classification Based on Respiratory System Static Compliance–normalized Mechanical Energy and Classification Based on Predictive Body Weight–normalized Mechanical Energy
| Difference in Quartile | MEPBW < MECrs 2 or More (n = 6) |
MEPBW < MECrs 1 (n = 11) |
MEPBW = MECrs (n = 23) |
MEPBW > MECrs 1 (n = 11) |
MEPBW > MECrs 2 or More (n = 6) |
P Value |
|---|---|---|---|---|---|---|
| Age, mo | 150 (71–183) | 93 (60–143) | 65 (21–168) | 62 (28–105) | 65 (28–151) | 0.762 |
| PBW, kg | 44.8 (21.9–50.4) | 23.8 (14.7–46.9) | 17.6 (10.3–50.1) | 17.0 (12.3–25.7) | 18.8 (9.7–28.0) | 0.601 |
| PIP, cm H2O | 35.5 (33.6–38.5) | 30.1 (27.5–34.5) | 26.3 (22.3–30.0) | 26.5 (23.1–29.8) | 27.0 (26.1–30.2) | 0.009 |
| PEEP, cm H2O | 8.0 (5.8–9.5) | 10.8 (10.2–12.5) | 10.3 (8.7–12.5) | 10.3 (10.1–12.3) | 11.0 (10.0–12.0) | 0.6 |
| DP, cm H2O | 16.9 (15.8–19.1) | 15.8 (12.7–16.6) | 12.9 (8.3–15.9) | 12.7 (11.3–13.3) | 11.3 (10.4–12.0) | 0.013 |
| Vt/PBW, ml/kg | 4.6 (3.4–5.2) | 6.6 (6.0–6.8) | 7.5 (5.0–8.3) | 9.1 (8.3–10.1) | 11.0 (10.5–11.9) | <0.001 |
| Crs/PBW, ml/cm H2O/kg | 0.28 (0.25–0.31) | 0.43 (0.40–0.47) | 0.56 (0.46–0.60) | 0.72 (0.68–0.91) | 0.93 (0.86–1.24) | <0.001 |
| MEPBW, J/kg | 0.017 (0.013–0.018) | 0.020 (0.016–0.024) | 0.019 (0.011–0.024) | 0.022 (0.019–0.031) | 0.029 (0.028–0.033) | 0.006 |
| MECrs, J/ml/cm H2O | 0.057 (0.050–0.076) | 0.044 (0.037–0.055) | 0.033 (0.018–0.047) | 0.035 (0.025–0.039) | 0.032 (0.027–0.035) | 0.004 |
Definition of abbreviations: Crs = respiratory system static compliance; DP = driving pressure; MECrs = mechanical energy normalized to respiratory system static compliance; MEPBW = mechanical energy normalized to predictive body weight; PBW = predictive body weight; PEEP = positive end-expiratory pressure; PIP = peak inspiratory airway pressure.
Characteristics of the subjects whose quartiles changed by one or by two or more and those whose quartiles did not change are shown. MECrs is relatively larger than MEPBW, which correlates with higher PIP, higher DP, lower Vt/PBW, and lower Crs/PBW.
Discussion
Our results demonstrate potentially large differences in ME according to the method of normalization. When ME is normalized to PBW, patients with poor pulmonary compliance, high DP, but low Vt would likely be classified in a lower ME stratum, whereas if ME is normalized to Crs, the same patients would likely be in a higher stratum. This suggests that MEPBW may underestimate the energy loaded per unit volume, especially in patients with low Crs and, possibly, low FRC, as in patients with severe ARDS. For this reason, we believe that ME normalization on the basis of PBW in children is not sufficient to reflect energy per unit lung volume.
The optimal method to normalize ME in children is unknown, and ultimately it will be important to evaluate normalization methods against clinical outcomes and other markers of VILI. Although normalization of ME to CL may be a good surrogate for FRC, the limitations of esophageal manometry, particularly in children (9), make this impractical. Despite differences in chest wall mechanics as a function of age, MECrs and MECL had excellent correlation, regardless of age.
Conclusions
There are major differences in ME calculations according to the method of normalization of lung volume (PBW vs. static compliance). It is likely that MEPBW will underestimate ME, particularly when Vt is low despite high DP and poor compliance. ME normalized to static compliance of the respiratory system should be a focus of investigation in children, particularly to determine if this variable has a relationship with clinically relevant outcomes. This requires routine measurement of Pplat to calculate Crs, which may suggest a need to change our standard practice in children (10).
Footnotes
Supported by Japan Society for the Promotion of Science grant KAKENHI 21K09063 (M.T., principal investigator) and NIH/NHLBI grant R01HL124666 (R.G.K., principal investigator).
Author Contributions: Study concept and design: Y. Ito, R.G.K., and M.T.; acquisition of data: J.C.H. and M.K.; analysis and interpretation of data and drafting of the manuscript: Y. Ito; critical revision and important intellectual contributions: Y. Inata, C.J.L.N., A.K.B., J.C.H., and R.G.K.
Originally Published in Press as DOI: 10.1164/rccm.202111-2641LE on March 22, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med . 2016;42:1567–1575. doi: 10.1007/s00134-016-4505-2. [DOI] [PubMed] [Google Scholar]
- 2. Marini JJ, Jaber S. Dynamic predictors of VILI risk: beyond the driving pressure. Intensive Care Med . 2016;42:1597–1600. doi: 10.1007/s00134-016-4534-x. [DOI] [PubMed] [Google Scholar]
- 3. Gattinoni L, Marini JJ, Collino F, Maiolo G, Rapetti F, Tonetti T, et al. The future of mechanical ventilation: lessons from the present and the past. Crit Care . 2017;21:183. doi: 10.1186/s13054-017-1750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coppola S, Caccioppola A, Froio S, Formenti P, De Giorgis V, Galanti V, et al. Effect of mechanical power on intensive care mortality in ARDS patients. Crit Care . 2020;24:246. doi: 10.1186/s13054-020-02963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med . 2008;178:346–355. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 6. Khemani RG, Hotz JC, Klein MJ, Kwok J, Park C, Lane C, et al. A phase II randomized controlled trial for lung and diaphragm protective ventilation (Real-time Effort Driven VENTilator management) Contemp Clin Trials . 2020;88:105893. doi: 10.1016/j.cct.2019.105893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becher T, van der Staay M, Schädler D, Frerichs I, Weiler N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med . 2019;45:1321–1323. doi: 10.1007/s00134-019-05636-8. [DOI] [PubMed] [Google Scholar]
- 8. Moore DJ, Durie PR, Forstner GG, Pencharz PB. The assessment of nutritional status in children. Nutr Res . 1985;5:797–799. [Google Scholar]
- 9. Hotz JC, Sodetani CT, Van Steenbergen J, Khemani RG, Deakers TW, Newth CJ. Measurements obtained from esophageal balloon catheters are affected by the esophageal balloon filling volume in children with ARDS. Respir Care . 2018;63:177–186. doi: 10.4187/respcare.05685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhalla AK, Klein MJ, Emeriaud G, Lopez-Fernandez YM, Napolitano N, Fernandez A, et al. Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) V.2. Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network Adherence to lung-protective ventilation principles in pediatric acute respiratory distress syndrome: a Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology Study. Crit Care Med . 2021;49:1779–1789. doi: 10.1097/CCM.0000000000005060. [DOI] [PMC free article] [PubMed] [Google Scholar]


